Summary
Epidemiological studies reveal associations between obesity/metabolic syndrome and mood disorders. We assessed behavioural changes in rats fed diets enriched in fat and fructose in different proportions and correlated the observed alterations with biochemical changes induced by the diets. Three groups of rats were used as follows: control (C) animals fed regular rat chow, rats fed high‐fat diet (HF) and rats fed high‐fat and high‐fructose diet (HFHF). HF and HFHF animals were also given a 10% fructose solution as drinking water. Behavioural and biochemical parameters were determined. Anxiety was measured by the open‐field and the social interaction test. Depression‐like behaviour was evaluated by the forced swimming test. The object recognition test was utilized to assess effects on memory. Diet‐exposed animals displayed signs of anxiety in the open‐field (HF rats had reduced central time; HFHF rats had reduced number of central entries) and in the social interaction test (decreased time of interaction in HF group). In the forced swimming test, the immobility time was prolonged in the HFHF group. While different measures of anxiety scores correlated with visceral adiposity and dyslipidemia, results from both social interaction and forced swimming tests were significantly associated with lipid peroxidation, which in turn also correlated with the metabolic parameters. The experimental diets did not affect the object recognition memory. Both experimental diets induced metabolic derangements in rats and provoked similar anxiety‐ and depression‐like behaviours. Lipid peroxidation seems to play a role in translating diet‐induced metabolic alterations into behavioural disorders.
Keywords: anxiety‐like behaviour, depression‐like behaviour, high‐fat diet, high‐fat high‐fructose diet, lipid peroxidation, rats
Metabolic syndrome is a socially important disorder of energy utilization and storage, comprising visceral obesity, dyslipidemia, arterial hypertension and impaired glucose tolerance as a result of insulin resistance. Approximately 20–25% of the world's adult population is affected (IDF consensus 2006). Metabolic syndrome is a widely acknowledged risk factor for the development of type 2 diabetes and cardiovascular diseases. It is, however, also increasingly associated with the development of psychiatric disorders (Simon et al. 2006). Epidemiological studies reveal connections between obesity and depression (McElroy et al. 2004). Obesity has been found to increase the risk of depression, and depression was found to be predictive for developing obesity. Likewise, obesity has been related to anxiety disorders although the nature of this link is less clear (Gariepy et al. 2010; Lykouras & Michopoulos 2011). Metabolic syndrome is widely linked to cognitive impairment in humans (Elias et al. 2005; Yates et al. 2012). The relationships between obesity and mental function have been recently reviewed by Yamada‐Goto et al. (2013).
Experimental models of metabolic syndrome are useful tools to study the pathogenesis and therapy of the condition. Most of the models utilize diet manipulation as the unhealthy diet with increased caloric intake is considered to be one of the main causes of metabolic syndrome in humans (Gajda et al. 2007; Panchal & Brown 2011). Rat models of diet‐induced obesity have confirmed the development of behavioural and cognitive changes (Yamada‐Goto et al. 2013).
Cognitive effects of energy‐dense diets have been studied in animals mainly by assessing spatial memory (Jurdak et al. 2008; McNay et al. 2010), while other types of memory, for example recognition memory, have received relatively little attention. Studies exploring anxiety have reported controversial results. According to Murphy and Mercer (2013), both anxiogenic and anxiolytic effects have been observed in rodents exposed to high‐fat and/or high‐sugar diets. Animals of different strains have been tested in experimental conditions that differed also in age and gender, type of diets, behavioural measures, etc. – all these variables possibly contributing to the conflicting data reported (Souza et al. 2007; Buchenauer et al. 2009; Anderson et al. 2013; Lalanza et al. 2014; Warneke et al. 2014).
The association between metabolic syndrome and mood disorders in experimental animals has been less widely studied despite the epidemiological evidence for such a connection. Few studies, if any, have looked for correlations between behavioural measures and metabolic parameters in diet‐manipulated animals.
Given the wide variability in the published literature reporting effects of unhealthy foods on behavioural outcomes, in the present work we aimed to assess the potential effect of two Western‐type diets on animal behaviour. The diets were enriched in saturated animal fat and fructose as these constituents are known for their deleterious health potential. They were added to the regular rat chow in relatively moderate amounts in order to achieve a closer resemblance of modern humans' diet, as opposed to the commonly reported diets that are extremely high in fat or sugar and are thus less relevant to real‐life conditions (Wilson et al. 2007; Panchal & Brown 2011). On the other hand, the diets we used contained different proportions of lard and fructose (although they had similar energy values), allowing for a possibility to differentiate between effects according to diet composition – predominantly rich in fat and predominantly rich in fructose. The questions that we asked were whether the metabolic effects of the diets would differ between each other and whether these effects would be accompanied by behavioural changes. We were also interested to see whether there would be any correlations between the two groups of measures – metabolic and behavioural, as a possible mechanism of interrelationship. Psychomotor tests for anxiety, depression‐like state and recognition memory were performed on rats exposed to the experimental diets, and the results were correlated with somatic and biochemical parameters characterizing the metabolic syndrome, including a measure of oxidative stress.
Materials and methods
Experimental animals
The study was performed on 36 male Wistar rats aged 6 weeks with an average body weight of 219–220 g. The animals were provided by the Vivarium of Varna Medical University. They were kept at a temperature of 20–25°C and 12‐h light–dark cycle (with lights on at 7 a.m.) and had free access to food and water.
The rats were allocated in three groups of 12 animals each: a control group (C), a group fed predominantly high‐fat (HF) diet and a group fed predominantly high‐fructose (HFHF) diet. They were collectively housed, six rats per cage. The duration of the study was 8 weeks.
Diets
The rats from the control group were fed the standard rat chow and were given plain water to drink. With each 100 g food consumed, the animals had a caloric intake of 279 kcal. According to the producer's recipe, the chow contains 20.48 g protein (29% kcal), 3 g fat (10% kcal), carbohydrates (61% kcal) in the form of starch (38.3 g) and sugars (4.32 g) per 100 g. We used the chow utilized in the Vivarium for the normal animal feeding, purchased from the Bulgarian forage producer TopMix.
The rats from the HF group had a diet enriched in lard (20% w/w), and their caloric intake was 403 kcal/100 g food, the lard providing 45% kcal.
The rats from the HFHF group were fed a diet with lard (17% w/w) and fructose (17% w/w) added to the standard rat chow, and their caloric intake was 405 kcal/100 g food. The lard provided 38% kcal, and the added fructose accounted for 20% kcal as compared to 6% from simple sugars in the regular chow and 4% in the HF diet.
Both HF and HFHF rats received also 10% fructose in the drinking water, thus consuming additional 40 kcal/100 ml.
Body weight was measured three times weekly. Food (in grams) and water (in ml) consumption per six rats in a cage was measured daily.
Insulin tolerance test
The insulin tolerance test (ITT) was used to assess insulin sensitivity. It was performed at the end of the experiment. Four‐hour‐fasted animals were injected i.p. with regular crystalline insulin (0.75 UI/kg) in saline. Blood glucose was measured using a glucometer ACCU‐CHEK Performa. Blood samples were taken by incision of the distal tail immediately before insulin injection (time 0 min) and at the 30th, 60th and 90th min.
At the end of the experimental period, blood was taken from the sublingual veins of the animals under ether anaesthesia. The blood was centrifuged, and the serum was refrigerated at −20°C until tested.
The rats were euthanized by cervical dislocation, and the livers and the right‐side retroperitoneal fat pads (as a sample of visceral adiposity) were dissected out and weighed. Liver and fat indices were calculated as a ratio of the organ weight to the body weight.
Biochemistry
Serum lipids
Serum triglycerides (TGs), total cholesterol and HDL cholesterol were measured on a spectrophotometer AURIUS 2021 (Cecil Instruments Ltd.) using colorimetric kits of HUMAN, Linear Chemicals S.L., Barcelona, Spain.
Serum insulin
The levels of serum insulin were measured by sandwich ELISA (Rat Insulin ELISA Kit of Shibayagi Co., Ltd. 1062‐1, Ishihara, Shibukawa, Gunma Pref., 377‐0007, Japan). In short, the assay includes incubation of the sample and biotin‐conjugated anti‐insulin in monoclonal anti‐insulin‐coated wells. After reaction with horseradish peroxidase‐conjugated streptavidin, a substrate chromogen reagent was added. The absorbance of the coloured product formed, proportional to insulin concentration, was measured spectrophotometrically at 450 nm.
Oxidative stress
Thiobarbituric acid reactive substances (TBARS) were determined as a marker of oxidative stress in serum. The method measures spectrophotometrically the colour obtained as a result of the reaction of thiobarbituric acid with the lipid peroxides (Ohkawa et al. 1979).
Behavioural tests
After 8 weeks of diet exposure, behavioural tests were performed. They were conducted during the light phase of the day under the normal luminescent illumination of the room. The tests were held in three consecutive days in the order given below. The open‐field test and the social interaction test were performed during the first day, and the novel object recognition test and the Porsolt test were carried out during the next 2 days each. In all behavioural tests, the scores were taken by experimenters experienced in these tasks, who were blind to the group allocation of the rats.
Open‐field test
The open‐field (OF) test is a common measure of exploratory behaviour and general locomotion in rats. In addition, the time spent in the central area and the number of entries into the central area were measured as inverse indices of anxiety (Todd et al. 2009). The OF was an open square box (100 × 100 × 40 cm), painted white, with the floor divided into 25 equal‐size (20 × 20 cm) squares. Each animal was placed in the centre of the uniformly lit arena, and its behaviour was observed in silence for 5 min. The locomotion was measured by counting the number of lines crossed. The time spent in the central zone referred to the time that the animal explored the central nine squares, and the central entries were counted every time the rat placed all the four paws into the central zone. The floor was wiped wet with a 70% ethanol after each animal.
Social interaction test
The social interaction (SI) test is a validated method to measure the level of anxiety in rats (File & Hyde 1978). It was performed in the OF arena. Each rat was tested with an unknown partner fed the same diet and with similar weight (difference of no more than 10%) under conditions of high light and familiar arena, known to generate moderate levels of anxiety. The rats were placed simultaneously in the opposite corners of the arena. Their behaviour was observed for 5 min, and the time of social interaction was recorded for each rat. As active interactions, sniffing, grooming, following and crawling under or over the partner were considered. Boxing, wrestling and biting were considered signs of aggressiveness and were not included in the time of social interaction. The passive contact (sitting or lying with bodies in contact) was not considered social interaction.
Novel object recognition test
The novel object recognition (NOR) test is used as a screening tool for recognition memory. It is based on the spontaneous preference of rodents to novelty, allowing them to discriminate a novel object from a familiar one (Ennaceur & Delacour 1988). It can test working, short‐term or long‐term memory depending on the time elapsed between the training and the testing trial (Carlini 2011). The test was performed in a rectangular wooden arena, which was a separated part of the OF arena, 60 × 60 cm, surrounded by 40‐cm walls. In this way, the animals were already familiar with the arena, making a special habituation session unnecessary. The training session was performed with two identical objects (ceramic cubes painted white and measuring 10 cm each side) placed symmetrically. The position of the objects was alternated randomly to prevent object/place preference. Exploration, defined as orienting the animal's nose towards the object from a distance of 1 cm or less, actively approaching and sniffing the object, climbing on the object (but not sitting on it), was measured for 3 min. Between the animals, the arena and the objects were wiped wet with 70% ethanol. During the test session, performed 5 h after the training session, the animal was placed in the arena with one familiar and one novel object (a ceramic pyramid painted white, measuring 10 cm each side) and was allowed to explore them for 3 min. The times spent in exploration of the novel object (B) and the familiar object (A) were recorded. The discrimination ratio was calculated as the time that a rat spent exploring the novel object relative to the total time of exploration during the test session B/(A + B) and was used as a measure of recognition memory. The inter‐trial delay of 5 h was selected to assess short‐term (<24 h) memory.
Porsolt test
The forced swimming test, originally developed by Porsolt (1979) for antidepressant drugs screening, is also used as a model for a depression‐like phenotype in rodents. Each rat was placed in a glass cylinder (17 cm diameter and 60 cm height) full of water (25°C) up to a depth of 30 cm, so that the animal could not touch the bottom with his tail or feet. The rat behaviour was observed. As a rule, the animal is actively struggling at the beginning in an attempt to escape. After a while, the rat acquires an immobile posture with minimum movements necessary to keep him floating. This immobility is regarded as a state of negative mood or behavioural despair that is considered relevant to human depression (Bogdanova et al. 2013; Carter & Shieh 2015; Hoffman 2016). The immobility time recoded for 3 min was taken as a measure of depression‐like behaviour. No pretest was performed.
Statistical analysis
Results are presented as means ± SEM. The data were analysed by one‐way anova. Insulin tolerance test was assessed by two‐way anova with a column factor ‘diet’ and a row factor ‘time’. Bonferroni's multiple comparison test was used to assess differences between groups. Post‐test for linear trend was also performed. Correlation analysis was applied to test associations between different parameters. A level of P < 0.05 was considered significant. GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA) statistical software was used.
Ethical approval statement
The study was ethically approved by the Commission of Foods Safety in the Bulgarian Ministry of Agriculture and Foods and was conducted in agreement with the National Policies and the Council Directive (2010/63/EU).
Results
Biological parameters
The biological characteristics of the condition induced by HF and HFHF diet are presented in Table 1. One‐way anova revealed significant main effect of diets on the total food, fluid and caloric intake for the entire period. Although experimental groups consumed less food than the control one (F(2,3) = 2386, P < 0.0001) (with HFHF rats having higher food intake than HF, according to Bonferroni's post‐test), they drank more fluids (F(2,3) = 34.01, P = 0.0087), with only HFHF group being significantly different from the control. Diet‐fed rats also had higher total caloric intake from food and drinks combined (F(2,3) = 81.37, P = 0.0024). The post‐test for linear trend was significant for food (P < 0.001) and for fluid and energy intake (P < 0.01).
Table 1.
Biological parameters of rats from the control group (C), rats fed a high‐fat diet plus 10% fructose solution (HF) and rats fed a high‐fat, high‐fructose diet plus 10% fructose solution (HFHF)
| Parameters | C | HF | HFHF |
|---|---|---|---|
| Total food intake (g/group)*** | 8803 ± 16 | 5131 ± 56††† | 5420 ± 43†††,‡ |
| Total fluid intake (ml/group) ** | 11760 ± 324 | 14158 ± 364 | 16122 ± 427† |
| Combined total caloric intake from food and fluid intake (kcal/group)** | 24597 ± 48 | 26340 ± 85† | 28388 ± 351†† |
| Initial body weight (g) | 219.2 ± 2.14 | 220 ± 1.23 | 219.5 ± 2.12 |
| Final body weight (g) | 348.8 ± 8.35 | 351.5 ± 8.75 | 360.8 ± 6.34 |
| Fat pad (retroperitoneal) weight (g)** | 2.31 ± 0.26 | 4.43 ± 0.45† | 5.51 ± 0.75†† |
| Fat index (fat weight/body weight ×103)** | 6.61 ± 0.75 | 12.18 ± 1.04† | 15 ± 1.85††† |
**P < 0.01, ***P < 0.001 (one‐way anova); † P < 0.05, †† P < 0.01, ††† P < 0.001 vs. the control group; ‡ P < 0.05 vs. the HF group (Bonferroni's multiple comparison test). (n = 2 groups for food intake, fluid intake and caloric intake, n = 6 rats for fat weight and fat index, n = 10–12 rats for body weight).
Final body weights did not differ over the study. Retroperitoneal fat weight (F(2,15) = 9.572, P = 0.0021) and fat index (F(2,15) = 10.72, P = 0.0013) increased significantly over 8 weeks. Bonferroni's multiple comparison test revealed significant differences between C and HF and between C and HFHF. The post‐test for linear trend was significant for both measures (P < 0.01).
Biochemical parameters
The biochemical parameters of diet‐fed rats are presented in Table 2. There was a significant effect of diets on serum triglycerides (F(2,30) = 17.66, P < 0.0001, linear trend P < 0.0001), total cholesterol (F(2,30) = 8.082, P = 0.0014, linear trend P < 0.001) and serum TBARS (F(2,33) = 11.46, P = 0.0002, linear trend P < 0.001). The effect on serum insulin was of borderline significance (F(2,28) = 2.505, P = 0.0988, linear trend P < 0.05). There was no significant effect on HDL cholesterol.
Table 2.
Biochemical parameters of rats from the control group (C), rats fed a high‐fat diet plus 10% fructose solution (HF) and rats fed a high‐fat, high‐fructose diet plus 10% fructose solution (HFHF)
| Parameters | C | HF | HFHF |
|---|---|---|---|
| Serum triglycerides [mmol/l]*** | 1.127 ± 0.10 | 3.03 ± 0.25†† | 3.84 ± 0.48††† |
| Total cholesterol** | 1.63 ± 0.09 | 2.09 ± 0.09† | 2.22 ± 0.13†† |
| HDL cholesterol | 0.66 ± 0.04 | 0.67 ± 0.05 | 0.64 ± 0.03 |
| Serum insulin [ng/ml]* | 1.957 ± 0.16 | 1.931 ± 0.17 | 2.807 ± 0.38 |
| Serum TBARS*** | 4.411 ± 0,66 | 10.35 ± 1.04††† | 10.50 ± 1.27††† |
**P < 0.01, ***P < 0.001, *P = 0.099 (one‐way anova); † P < 0.05, †† P < 0.01, ††† P < 0.001 vs. the control group (Bonferroni's multiple comparison test; n = 10–12).
Serum TBARS correlated significantly with fat index and serum TGs in both groups (Table 3):
Table 3.
Correlations between serum TBARS and metabolic parameters
| Fat index | Serum TGs | |||
|---|---|---|---|---|
| Pearson r | P | Pearson r | P | |
| HF group | 0.7850 | 0.0025 | 0.7788 | <0.0001 |
| HFHF group | 0.7673 | 0.0036 | 0.8752 | <0.0001 |
Insulin tolerance test
The results from the ITT are presented in Figure 1. There were no significant differences in the pre‐insulin values (time 0 min) of blood glucose. A significant effect of diets on blood glucose in response to insulin was observed (two‐way anova F(2,118) = 17.29, P < 0.0001).
Figure 1.
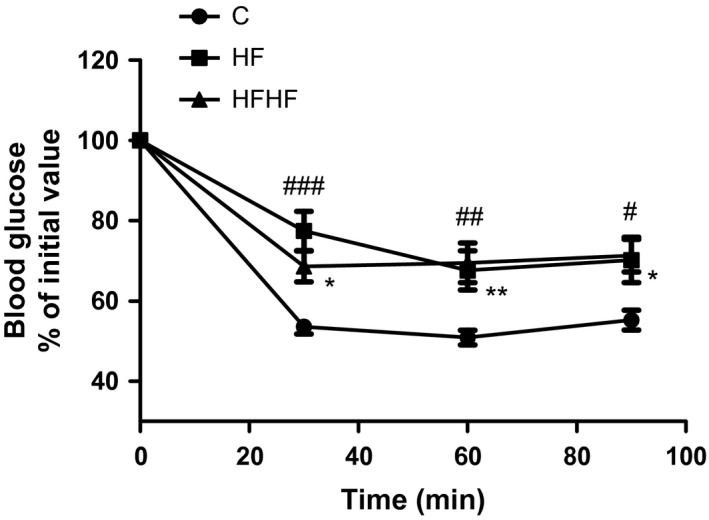
Insulin tolerance test. Results are presented as per cent of initial blood glucose values of at time 0 – before insulin (0.75 UI/kg) injection, and at the 30th, 60th and 90th min thereafter. C – control animals fed regular rat chow and plain water; HF – rats fed a high‐fat diet and 10% fructose solution; HFHF – rats fed a high‐fat high‐fructose diet and 10% fructose solution. Values are mean ± SEM (n = 10–12). Bonferroni's multiple comparison test for HF: # P < 0.05, ## P < 0.01, ### P < 0.001; for HFHF: *P < 0.05, **P < 0.01, vs. the control group.
Open‐field behaviour
General locomotion was not affected by diet manipulation: one‐way anova F(2,33) = 0.0377, P = 0.963.
For the time spent in the central zone (Figure 2a), one‐way anova revealed significant effect of diets: F(2,31) = 4.855, P = 0.0152. The post‐test for linear trend was significant (P < 0.05).
Figure 2.
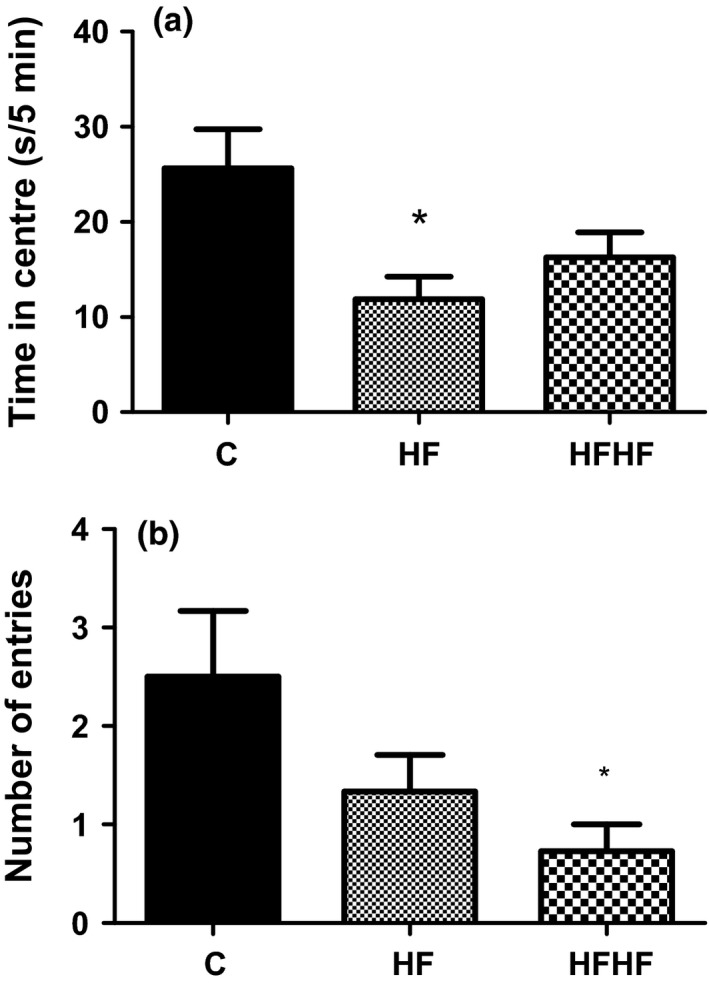
Open‐field behaviour. (a) Time spent in the central area. (b) Number of entries into central squares. C –control animals fed regular rat chow and plain water; HF – rats fed a high‐fat diet and 10% fructose solution; HFHF – rats fed a high‐fat, high‐fructose diet and 10% fructose solution. Values are mean ± SEM (n = 10–12) *P < 0.05 vs. controls (Bonferroni's multiple comparison test).
For the number of entries into the central squares (Figure 2b), there was a significant effect of diets: F(2,34) = 3.511, P = 0.0418. The post‐test for linear trend was significant (P < 0.05).
Social interaction behaviour
One‐way ANOVA revealed a significant effect of diets on social interaction (Figure 3): F(2,33) = 5.987, P = 0.006.
Figure 3.
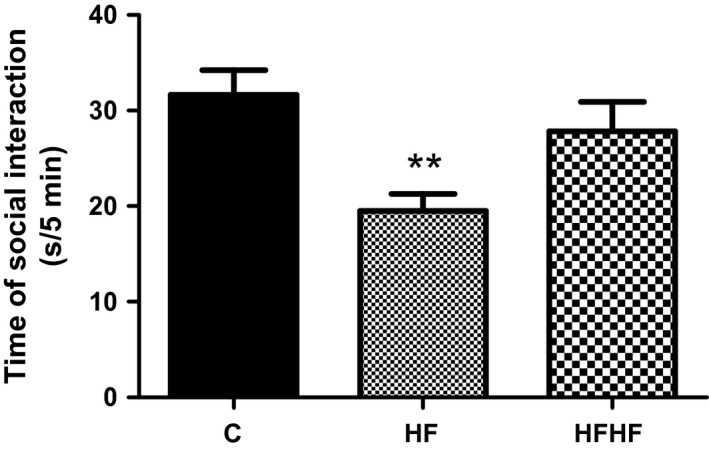
Social interaction behaviour. C – control animals fed regular rat chow and plain water; HF – rats fed a high‐fat diet and 10% fructose solution; HFHF – rats fed a high‐fat, high‐fructose diet and 10% fructose solution. Values are mean ± SEM (n = 10–12). **P < 0.01 (Bonferroni's multiple comparison test).
Behaviour in Porsolt test
One‐way anova revealed a significant effect of diets on the immobility time (Figure 4): F(2,33) = 4.119, P = 0.0259. The post‐test for linear trend was significant (P < 0.01).
Figure 4.
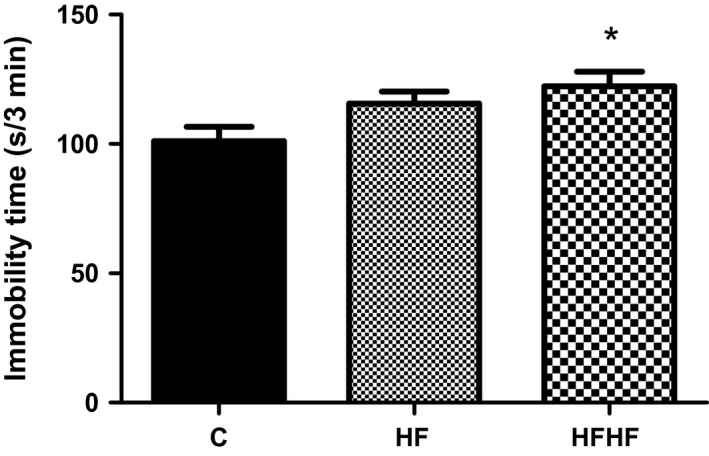
Forced swimming test behaviour. C – control animals fed regular rat chow and plain water; HF – rats fed a high‐fat diet and 10% fructose solution; HFHF – rats fed a high‐fat, high‐fructose diet and 10% fructose solution. Values are mean ± SEM (n = 10–12), *P < 0.05 (Bonferroni's multiple comparison test).
Behaviour in the novel object recognition test
One‐way anova revealed no significant effect of diets on the discrimination ratio (Figure 5): F(2,35) = 0.6201, P = 0.5441.
Figure 5.
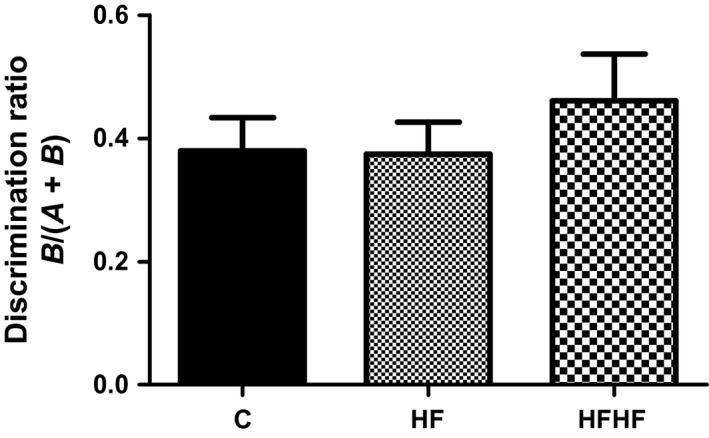
Behaviour in the novel object recognition test. C – control animals fed regular rat chow and plain water; HF – rats fed a high‐fat diet and 10% fructose solution; HFHF – rats fed a high‐fat, high‐fructose diet and 10% fructose solution. Values are mean ± SEM (n = 12).
Correlations of the results from behavioural tests
The correlations between the observed behavioural changes and biological or biochemical parameters in the HF and the HFHF group are presented in Table 4.
Table 4.
Correlations between behavioural changes and biological or biochemical parameters
| Behaviour | Fat index | Serum TGs | TBARS | ||||
|---|---|---|---|---|---|---|---|
| Pearson r | P | Pearson r | P | Pearson r | P | ||
| HF group | Time spent in SI | −0.7526 | 0.005 | −0.4534 | 0.039 | −0.657 | 0.0005 |
| OF central entries | −0.4406 | n.s. | −0.2666 | n.s. | −0.3458 | n.s. | |
| OF central time | −0.3757 | n.s. | −0.5173 | 0.023 | −0.3822 | n.s. | |
| Immobility time in PT | −0.067 | n.s. | 0.3157 | n.s. | 0.4325 | 0.039 | |
| HFHF group | Time spent in SI | −0.4671 | n.s. | −0.0146 | n.s. | −0.4567 | 0.032 |
| OF central entries | −0.5778 | 0.049 | −0.3203 | n.s. | −0.3446 | n.s. | |
| OF central time | −0.2164 | n.s. | −0.2445 | n.s. | −0.2345 | n.s. | |
| Immobility time in PT | −0.001 | n.s. | 0.3559 | n.s. | 0.5916 | 0.004 | |
In the HF group, the time of social interaction was negatively correlated with fat index, serum TGs and serum TBARS. The OF central time was also negatively correlated with serum TGs, and the inverse correlation with serum TBARS was marginally significant: Pearson r (44) = −0.3822, P = 0.072. There was a positive correlation between the immobility time in Porsolt test and serum TBARS.
In the HFHF group, the time of social interaction was inversely correlated with serum TBARS and the number of central OF entries was inversely correlated with fat index. The immobility time was positively correlated with serum TBARS. The correlation between immobility time and serum TGs was of borderline significance: Pearson r(44) = 0.3559, P = 0.095.
Discussion
Diet‐induced metabolic syndrome and behavioural changes
The present study revealed that Wistar rats exposed to two similar hypercaloric diets developed a condition close to the metabolic syndrome in humans. We used diets supplemented with moderate proportions of saturated fat and fructose rather than very high‐fat or very high‐fructose diets, so as to more closely mimic the unhealthy Western diet (Wilson et al. 2007; Kosari et al. 2012; Crescenzo et al. 2014). Although rats are known to exhibit preference to fat and sucrose (Tordoff et al. 2008), the animals from our experimental groups consumed less amounts of food compared to controls, probably as a result of a satiety effect due to the high lipid content of the food and/or because of stimulation of gut hormones (Samra 2010). They, however, drank more fructose solution compared to water consumption of control rats and had a higher total caloric intake. Body weight did not differ across the groups at the end of the experiment. The lack of body weight gain is not uncommon in similar experimental settings, especially when young rats are used and regardless of the increased visceral fat (Souza et al. 2007; Couturier et al. 2010; Crescenzo et al. 2014; Warneke et al. 2014). The diet‐induced metabolic syndrome in our study was verified by the presence of the following symptoms found both in the HF and in the HFHF groups according to the requirements of IDF (2006) to make the diagnosis in humans: increased visceral adiposity, elevated serum TGs and insulin resistance. Serum insulin was elevated in the HFHF group only with borderline significance. Thus, the two experimental groups had similar diet‐induced metabolic derangements, despite the general trend for a stronger metabolic impact of the HFHF diet. The measure of lipid peroxidation was also significantly increased in both experimental groups.
We demonstrated that rats fed the HF and HFHF diet displayed altered behaviours suggesting elevated levels of anxiety and depression‐like states, as evidenced by the results from the OF, SI and Porsolt tests. Although the results were not equally conclusive for each diet and each behaviour, the significant linear trends in most of the behaviours tested imply that both experimental groups were affected by mood and anxiety changes.
There were significant associations between metabolic parameters and anxiety‐related behaviours, pointing to possible pathophysiological links with the exposure to high‐energy diets. Further, measures of both anxiety and depression‐like behaviours correlated significantly with the marker of oxidative stress; in its turn, lipid peroxidation was positively correlated with the metabolic effects of the diets.
Despite the epidemiological data pointing to associations between diet‐induced obesity and psychiatric disorders in humans, experimental studies that examine behavioural deviations in rats with diet‐induced metabolic alterations have reported inconsistent results, especially as far as anxiety is concerned.
Diet‐induced anxiety
To assess anxiety, we used the OF central zone time and entries, as well as the social interaction test. In both tests, the results pointed to increased anxiety in the diet‐manipulated rats with preserved locomotion and lack of difference in motor activity between experimental groups. Published studies addressing the issue have yielded contradictory results. As reviewed by Murphy and Mercer (2013), both high‐sugar and high‐fat diets have resulted in opposite changes in levels of anxiety.
Buchenauer et al. (2009) used methods similar to ours and described anxiety‐like behaviour in Fischer 344 male rats with high‐fat diet‐induced obesity. Increased anxiety in this study was demonstrated in the hole‐board test. Surprisingly, in the social interaction test, the diet‐made obese rats had an increased time of social interaction explained by the authors with the increased aggressive behaviour. In our study, the rats with metabolic syndrome did not display signs of aggressiveness. Souza et al. (2007) found anxiety‐like behaviour in male Wistar rats consuming diet enriched in sucrose in the light–dark paradigm and Anderson et al. (2013) observed greater anxiety in rats fed high‐fat/high‐fructose diet in the elevated plus maze. On the contrary, Lalanza et al. (2014) examined the behavioural effects of cafeteria diet on young adult male and female Sprague Dawley rats by the elevated plus maze and the social interaction test and concluded that the diet lowers anxiety‐like behaviour but only in the female rats. Gender differences were also found by Warneke et al. (2014), who observed low level of anxiety in the high‐energy‐diet‐fed female Sprague Dawley rats, while the male rats showed increased anxiety. However, in a recent study of Marwitz et al. (2015), young male Sprague Dawley rats exposed to dietary conditions similar to ours displayed increased number of entries and time spent in the OF centre, and on the other hand, Sivanathan et al. (2015) found female Long Evans rats fed high‐fat diet to develop anxiety.
It should be noted that in many of the studies demonstrating reduced anxiety with diet manipulation, the rats were given foods referred to as ‘cafeteria’ or ‘comfort’ diet, containing bacon, cheese, biscuits, chocolate, condensed milk, etc. (Lalanza et al. 2014; Warneke et al. 2014; McNeilly et al. 2015). This type of palatable diet seems to be more consistently, but not always, associated with anxiolytic effects (Souza et al. 2007) and, conversely, withdrawal from cafeteria diet, with increased anxiety (Murphy & Mercer 2013). Obviously, central mechanisms involving factors distinct from purely metabolic and/or triggered by them, such as dopamine, serotonin, opioids and cannabinoids in brain structures related to emotion and motivation, are implicated in craving for pleasurable foods (Kirkham 2009; Murphy & Mercer 2013; Yamada‐Goto et al. 2013). On the other hand, the terms ‘cafeteria’, ‘comfort’ or ‘palatable’ diet are not precisely defined in the literature, and they are often referred to as ‘Western‐type’ diet and should be carefully interpreted as synonymous, as they can have quite different meanings, such as lard‐ and dextrose‐enriched diet (Marwitz et al. 2015) or just ‘high‐fat’ diet (McNeilly et al. 2015).
Overall, studies examining the link between diet and anxiety, including ours, cannot be easily compared as they differ in many ways – diet composition, rat strains, gender and/or age of animals, behavioural tests used to assess anxiety, etc.
Our results support the line of evidence of increased anxiety induced by either high‐fat, high‐sugar or mixed diets. They disagree with the generalization made by Murphy and Mercer (2013) in their review that ‘high fat is anxiolytic while diets high in sugar may have more anxiogenic characteristics’. It is possible that the combined nature of diets in our experiment precluded the manifestation of such a ‘rule’ or even reversed it, as has been shown also by Anderson et al. (2013). Alternatively, the validity of such a general conclusion might be questioned, as evidenced by the results reporting anxiety following exposure of rats to high‐fat diet (Buchenauer et al. 2009; Can et al. 2012; Sivanathan et al. 2015). We might thus suggest that anxiety‐generating foods in these and other studies utilizing sugar, or fat and sugar supplementation, act through their energetic overload and the common metabolic and/or biochemical consequences that they induce, rather than through the diet composition itself. Supporting this are our present results showing significant correlations between visceral adiposity and elevated TGs with measures of anxiety in both the HF and HFHF groups.
It is also noteworthy that anxiety scores from the social interaction test from both experimental groups correlated with the products of lipid peroxidation despite only mild and insignificant anxiety effect observed in the HFHF rats. These findings suggest a potential link between diet‐induced lipid peroxidation and anxiety.
Diet‐induced depression‐like behaviour
Plenty of evidence demonstrates the common occurrence of mood disorders and obesity. A growing amount of research suggests that metabolic abnormalities stemming from central obesity may be responsible for the increased incidence of depression. Alterations in glucocorticoids, adipose tissue‐derived hormones, insulin and inflammatory signalling are the possible mechanisms discussed (Hryhorczuk et al. 2013). In mice with diet‐induced obesity, Sharma and Fulton (2013) demonstrated depressive‐like phenotype (increased immobility in the forced swimming test) associated with plasticity‐related changes in reward circuitry. Our results are consistent with these findings given the increased visceral adiposity of the rats in the experimental groups, although direct connection could not be demonstrated. Thus, the alternative possibility that immobility might be a consequence of a reduced intake of proteins from the diets enriched in fat and fructose becomes less likely.
Metabolic syndrome is associated with systemic insulin resistance, and brain insulin resistance plays a significant role, at least as a factor perpetuating the vicious cycle between food intake and obesity. There are also data providing evidence that central insulin resistance in obesity is potentially linked to increased risk of depression and anxiety. Grillo et al. (2007) designed an insulin receptor lentiviral vector which they injected into the third ventricle of rats to selectively decrease the expression of insulin receptors in the hypothalamus. Treated rats developed features of metabolic syndrome. The authors discovered that down‐regulation of hypothalamic insulin receptors adversely affected hippocampus‐dependent plasticity and performance of tasks (Grillo et al. 2011a). The rats treated with the lentivirus showed increased immobility time in the forced swim test, decreased sucrose preference and anxiety‐like behaviour (Grillo et al. 2011b).
In our study, insulin resistance was present in both the HF and HFHF groups and serum insulin tended to increase in the HFHF group. Insulin levels were not, however, associated with the altered behaviours. Instead, longer immobility times in both experimental groups were found to correlate positively with the lipid peroxidation, which is known for its potential to contribute to insulin resistance (Ceriello & Motz 2004).
Oxidative stress – more than a separate pathway in metabolic syndrome and anxiety/mood disorders?
Metabolic syndrome and depression appear to share many common pathological mechanisms (McIntyre et al. 2007). Among these, oxidative stress is frequently implicated with each of these disorders separately, but rarely together.
Oxidative stress is proposed as a persistent pathogenic factor mediating the processes from cellular overload of free fatty acids and glucose in over‐nutrition to appearance of insulin resistance and vice versa – the increased insulin, free fatty acids and/or glucose levels can increase the production of reactive oxygen species, thereby worsening both insulin action and insulin secretion (Ceriello & Motz 2004). Hopps et al. (2010) suggest that oxidative stress be classified as a component of the metabolic syndrome. On the other hand, there is evidence showing that anxiety and depression are associated with oxidative stress (Bouayed et al. 2009; Grases et al. 2014). Antidepressant drugs have been demonstrated to restore markers of stress‐induced oxidative damage, behaviourally manifested by changes in the forced swimming test and sucrose preference test (Zafir et al. 2009). Unhealthy Western‐type diet and obesity are among the possible factors considered to contribute to oxidative stress. Zhang et al. (2005) show that high dietary fat causes NADPH oxidase‐associated oxidative stress in rat cerebral cortex. Feeding mice with high‐fat diet impairs hippocampal neurogenesis through increased lipid peroxidation and decreased BDNF (Park et al. 2010). In rats, high‐fat‐diet‐induced metabolic disturbances were associated with brain oxidative dysfunction manifested by low NO levels and MDA accumulation (Amin et al. 2011).
Few studies examine the link between these two lines of evidence at behavioural level. Souza et al. (2007) report anxiety induced by sucrose‐enriched diet, accompanied by obesity phenotype and increased protein oxidation in the frontal cortex.
In our study, diet‐induced oxidative stress seems to be involved in connecting the corresponding metabolic and behavioural changes. Both anxiety (reduced social interaction) and depression indices were associated with elevated serum lipid peroxides, and this was the most consistent finding integrating the altered behaviours with the metabolic derangements. Even those measures of anxiety and depression that were not themselves significantly different from controls correlated with TBARS. At the same time, strong correlations were demonstrated between lipid peroxidation and visceral fat and serum triglycerides as the markers of metabolic syndrome. This double interconnection of lipid peroxidation with anxiety/depression scores on the one hand and with the metabolic alterations on the other strengthens the notion that it might function as a bridging mechanism between these two diet‐induced syndromes.
One weakness of our study is that we did not use other parameters to characterize the oxidative status and lipid peroxidation was only measured in serum but not in brain. Increased oxidative stress markers have been reported in serum or plasma of patients with different phenotypes of mental disorders (Smaga et al. 2015). Besides, in animal behavioural models, peripheral markers have been shown to parallel those in brain (Hovatta et al. 2010). Bouayed et al. (2007) described a positive relationship between the peripheral oxidative status and anxiety in mice. Thus, we can infer that the peripheral and possibly the associated brain oxidative imbalance induced by HF and HFHF diets in our experiment could explain, at least in part, the observed behavioural changes. To our knowledge, this is the first study to directly correlate diet‐induced increase in lipid peroxidation with behavioural signs of anxiety and depression in a model of metabolic syndrome in the rat.
Metabolic syndrome and cognitive impairment
A large body of evidence – epidemiological/clinical and experimental – has shown association between obesity, metabolic syndrome and/or type 2 diabetes with memory and learning deficits. Insulin resistance and oxidative stress have mostly been postulated as the biological mechanisms (Freeman et al. 2014). It has been shown that hippocampus is the structure most vulnerable to damage by highly caloric diets (Marwitz et al. 2015). The hippocampus is considered to be more important for spatial than for recognition memory (Broadbent et al. 2004), although this is a matter of debate (Cohen et al. 2013). Spatial memory as hippocampus‐dependent has been widely studied under conditions of dietary manipulation with high fat, high sugar or both (Jurdak et al. 2008; McNay et al. 2010). Recognition memory has also been reported to be adversely affected by high‐fat diets in mice (Cohen et al. 2013). In rats, non‐spatial memory in the context of diet‐induced obesity/metabolic disorders has been less explored or has been found unaffected (Kosari et al. 2012). Few papers report diet‐induced impairment in novel object recognition in the rat (Jurdak & Kanarek 2009; Marwitz et al. 2015).
Our experiments with the NOR test gave no results despite the metabolic disturbances and the evidence of oxidative stress produced by diets. Although it has been suggested that non‐spatial memory might require a longer term feeding rats high‐energy diet to produce disruption compared to spatial memory (Kanoski & Davidson 2010), our 8‐week diet intervention was not sufficient to reveal memory deficits, unlike the results reported by Marwitz et al. (2015) and Jurdak and Kanarek (2009). A possible explanation for the lack of dietary effect in our study might be the longer, 5‐h delay period between the training and test sessions compared to the 1‐h period used by the cited authors. Alternatively, our results could indicate that recognition memory may not be as sensitive as spatial memory to diet intervention, as shown by the study of Kosari et al. 2012: both longer duration of high‐fat feeding (12 weeks) and shorter inter‐trial interval (1 h) did not disrupt object recognition, while producing spatial deficits in the Y‐maze.
Conclusion
The results from the present study demonstrate that combined diets enriched in saturated fat and fructose induced a condition in rats a condition which is consistent with the metabolic syndrome in humans, and which was accompanied by behavioural alterations. Only insignificant differences could be detected between the two diets in metabolic and behavioural aspects, implying that diets of similarly high energetic density can be similarly deleterious for brain functions. Both anxiety and depression‐like behaviours were associated with the marker of lipid peroxidation, which in turn correlated with the metabolic effects of diets, suggesting that it could play a role in translating diet‐induced metabolic changes into behavioural disorders. Thus, oxidative stress could be implicated as a factor interrelating with both the endocrine and inflammatory signals thought to be involved in mediating such a connection (Moulton et al. 2015). Further work would be necessary to elucidate the role of oxidative imbalance – both in periphery and in brain structures relevant to metabolic/behavioural functions, in the link between these diet‐induced syndromes.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding source
The study was funded with personal sources and supported by the Medical University of Varna, Bulgaria.
References
- Amin K.A., Kamel H.H., Eltawab M.A.A. (2011) The relation of high fat diet, metabolic disturbances and brain oxidative dysfunction: modulation by hydroxy citric acid. Lipids Health Dis. 10, 74 https://doi.org/10.1186/1476-511x-10-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.A., Qin B., Canini F., Poulet L. & Roussel A.M. (2013) Cinnamon counteracts the negative effects of a high fat/high fructose diet on behavior, brain insulin signaling and Alzheimer‐associated changes. PLoS ONE 8, e83243 https://doi.org/10.1371/journal.pone.0083243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanova O.V., Kanekar S., D'Anci K.E. & Renshaw P.F. (2013) Factors influencing behavior in the forced swim test. Physiol. Behav. 118, 227–239. https://doi.org/10.1016/j.physbeh.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouayed J., Rammal H., Younos C. & Soulimani R. (2007) Positive correlation between peripheral blood granulocyte oxidative status and level of anxiety in mice. Eur. J. Pharmacol. 564, 146–149. [DOI] [PubMed] [Google Scholar]
- Bouayed J., Rammal H. & Soulimani R. (2009) Oxidative stress and anxiety. Relationship and cellular pathways. Oxid. Med. Cell. Longev. 2, 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent N.J., Squire L.R. & Clark R.E. (2004) Spatial memory, recognition memory, and the hippocampus. Proc. Natl Acad. Sci. USA 101, 14515–14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchenauer T., Behrendt P., Bode F.J. et al (2009) Diet‐induced obesity alters behavior as well as serum levels of corticosterone in F344 rats. Physiol. Behav. 98, 563–569. [DOI] [PubMed] [Google Scholar]
- Can Ö.D., Ulupınar E., Özkay Ü.D., Yegin B. & Öztürk Y. (2012) The effect of simvastatin treatment on behavioral parameters, cognitive performance, and hippocampal morphology in rats fed a standard or a high‐fat diet. Behav. Pharmacol. 23, 582–592. https://doi.org/10.1097/FBP.0b013e328356c3f2. [DOI] [PubMed] [Google Scholar]
- Carlini V.P. (2011) Object recognition In: The Object Recognition Task: A New Proposal for the Memory Performance Study, pp. 27–41 (ed Phuong Cao T.), InTech; ISBN: 978‐953‐307‐222‐7. Available from: https://www.intechopen.com/books/object-recognition/the-object-recognition-task-a-new-proposal-for-the-memory-performance-study [Google Scholar]
- Carter M., Shieh J. (2015) Guide to Research Techniques in Neuroscience (Second Edition), eBook ISBN: 9780128005972, Paperback ISBN: 9780128005118, Academic Press Elsevier; [Google Scholar]
- Ceriello A. & Motz E. (2004) Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler. Thromb. Vasc. Biol. 24, 816–823. [DOI] [PubMed] [Google Scholar]
- Cohen S.J., Munchow A.H., Rios L.M., Zhang G., Ásgeirsdóttir H.N. & Stackman R.W. Jr (2013) The rodent hippocampus is essential for nonspatial object memory. Curr. Biol. 23, 1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier K., Batandier C., Awada M. et al (2010) Cinnamon improves insulin sensitivity and alters the body composition in an animal model of the metabolic syndrome. Arch. Biochem. Biophys. 501, 158–161. https://doi.org/10.1016/j.abb.2010.05.032. [DOI] [PubMed] [Google Scholar]
- Crescenzo R., Bianco F., Coppola P. et al (2014) Fructose supplementation worsens the deleterious effects of short‐term high‐fat feeding on hepatic steatosis and lipid metabolism in adult rats. Exp. Physiol. 99, 1203–1213. https://doi.org/10.1113/expphysiol.2014.079632. [DOI] [PubMed] [Google Scholar]
- Elias M.F., Elias P.K., Sullivan L.M., Wolf P.A. & D'Agostino R.B. (2005) Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiol. Aging 26, 11–16. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. & Delacour J. (1988) A new one‐trial test for neurobiological studies of memory in rats. 1. Behavioral data. Behav. Brain Res. 31, 47–59. [DOI] [PubMed] [Google Scholar]
- File S.E. & Hyde J.R.G. (1978) Can social interaction be used to measure anxiety? Br. J. Pharmacol. 62, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman L.R., Haley‐Zitlin V., Rosenberger D.S. & Granholm A.C. (2014) Damaging effects of a high‐fat diet to the brain and cognition: A review of proposed mechanisms. Nutr. Neurosci. 17, 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajda A., Pellizzon M., Ricci M., Ulman E. (2007) Diet‐induced metabolic syndrome in rodent models. Animal Lab News. www.researchdiets.com/OSD/DIDM/metabolic.htm.
- Gariepy G., Nitka D. & Schmitz N. (2010) The association between obesity and anxiety disorders in the population: a systematic review and meta‐analysis. Int. J. Obes. 34, 407–419. [DOI] [PubMed] [Google Scholar]
- Grases G., Colom M.A., Fernandez R.A., Costa‐Bauzá A. & Grases F. (2014) Evidence of higher oxidative status in depression and anxiety. Oxid. Med. Cell. Longev. 2014, 430216 https://doi.org/10.1155/2014/430216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo C.A., Tamashiro K.L., Piroli G.G. et al (2007) Lentivirus‐mediated downregulation of hypothalamic insulin receptor expression. Physiol. Behav. 92, 691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo C.A., Piroli G.G., Junor L. et al (2011a) Obesity/hyperleptinemic phenotype impairs structural and functional plasticity in the rat hippocampus. Physiol. Behav. 105, 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo C.A., Piroli G.G., Kaigler K.F., Wilson S.P., Wilson M.A. & Reagan L.P. (2011b) Downregulation of hypothalamic insulin receptor expression elicits depressive‐like behaviors in rats. Behav. Brain Res. 222, 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K.L. (2016) Modeling Neuropsychiatric Disorders in Laboratory Animals, eBook ISBN: 9780081001066, Hardcover ISBN: 9780081000991, Woodhead Publishing Elsevier. [Google Scholar]
- Hopps E., Noto D., Caimi G. & Averna M.R. (2010) A novel component of the metabolic syndrome: the oxidative stress. Nutr. Metab. Cardiovasc. Dis. 20, 72–77. [DOI] [PubMed] [Google Scholar]
- Hovatta I., Juhila J. & Donnera J. (2010) Oxidative stress in anxiety and comorbid disorders. Neurosci. Res. 68, 261–275. [DOI] [PubMed] [Google Scholar]
- Hryhorczuk C., Sharma S. & Fulton S.E. (2013) Metabolic disturbances connecting obesity and depression. Front. Neurosci. 7, 177 https://doi.org/10.3389/fnins.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDF (2006) The IDF consensus worldwide definition of the metabolic syndrome.
- Iemolo A., Blasio A., St Cyr S.A. et al (2013) CRF‐CRF1 receptor system in the central and basolateral nuclei of the amygdala differentially mediates excessive eating of palatable food. Neuropsychopharmacol. J. 38, 2456–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurdak N. & Kanarek R.B. (2009) Sucrose‐induced obesity impairs novel object recognition learning in young rats. Physiol. Behav. 96, 1–5. https://doi.org/10.1016/j.physbeh.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Jurdak N., Lichtenstein A.H. & Kanarek R.B. (2008) Diet‐induced obesity and spatial cognition in young male rats. Nutr. Neurosci. 11, 48–54. [DOI] [PubMed] [Google Scholar]
- Kanoski S.E. & Davidson T.L. (2010) Different patterns of memory impairments accompany short‐ and longer‐term maintenance on a high‐energy diet. J. Exp. Psychol. Anim. Behav. Process. 36, 313–319. [DOI] [PubMed] [Google Scholar]
- Kirkham T.C. (2009) Cannabinoids and appetite: food craving and food pleasure. Int. Rev. Psychiatry 12, 163–171. [DOI] [PubMed] [Google Scholar]
- Kosari S., Badoer E., Nguyen J.C., Killcross A.S. & Jenkins T.A. (2012) Effect of western and high fat diets on memory and cholinergic measures in the rat. Behav. Brain Res. 235, 98–103. https://doi.org/10.1016/j.bbr.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Lalanza J.F., Caimari A., del Bas J.M. et al (2014) Effects of a Post‐Weaning Cafeteria Diet in Young Rats: Metabolic Syndrome. Reduced Activity and Low Anxiety‐Like Behaviour. PLoS ONE. 9, e85049 https://doi.org/10.1371/journal.pone.0085049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykouras L. & Michopoulos J. (2011) Anxiety disorders and obesity. Psychiatriki 22, 307–313. [PubMed] [Google Scholar]
- Marwitz S.E., Woodie L.N. & Blythe S.N. (2015) Western‐style diet induces insulin insensitivity and hyperactivity in adolescent male rats. Physiol. Behav. 151, 147–154. https://doi.org/10.1016/j.physbeh.2015.07.023. [DOI] [PubMed] [Google Scholar]
- McElroy S.L., Kotwal R., Malhotra S., Nelson E.B., Keck P.E. & Nemeroff C.B. (2004) Are mood disorders and obesity related? A review for the mental health professional. J. Clin. Psychiatry 65, 634–651. [DOI] [PubMed] [Google Scholar]
- McIntyre R.S., Soczynska J.K., Konarski J.Z. et al (2007) Should depressive syndromes be reclassified as “metabolic syndrome type II”? Ann. Clin. Psychiatry 19, 257–264. [DOI] [PubMed] [Google Scholar]
- McNay E.C., Ong C.T., McCrimmon R.J., Cresswell J., Bogan J.S. & Sherwin R.S. (2010) Hippocampal memory processes are modulated by insulin and high‐fat‐induced insulin resistance. Neurobiol. Learn. Mem. 93, 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly A.D., Stewart C.A., Sutherland C. & Balfour D.J.K. (2015) High fat feeding is associated with stimulation of the hypothalamic‐pituitary‐adrenal axis and reduced anxiety in the rat. Psychoneuroendocrinology 52, 272–280. [DOI] [PubMed] [Google Scholar]
- Moulton C.D., Pickup J.C. & Ismail K. (2015) The link between depression and diabetes: the search for shared mechanisms. Lancet Diabetes Endocrinol. 3, 461–471. [DOI] [PubMed] [Google Scholar]
- Murphy M. & Mercer J.G. (2013) Diet‐Regulated Anxiety. Int. J. Endocrinol. 2013, 701967 https://doi.org/10.1155/2013/701967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N. & Yagi K. (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358. [DOI] [PubMed] [Google Scholar]
- Panchal S. & Brown L. (2011) Rodent models for metabolic syndrome research. J. Biomed. Biotechnol. 2011, 351982; https://doi.org/10.1155/2011/351982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.R., Park M., Choi J., Park K.Y., Chung H.Y. & Lee J. (2010) A high‐fat diet impairs neurogenesis: involvement of lipid peroxidation and brain‐derived neurotrophic factor. Neurosci. Lett. 482(3), 235–239. https://doi.org/10.1016/j.neulet.2010.07.046. [DOI] [PubMed] [Google Scholar]
- Porsolt R. (1979) D. Animal model of depression. Biomedicine 30, 139–140. [PubMed] [Google Scholar]
- Samra R.A. (2010) Fats and Satiety. Fat Detection In: Taste, Texture, and Post Ingestive Effects, (eds Montmayeur J.P. & le Coutre J.), Boca Raton (FL): CRC Press/Taylor & Francis; Available from: https://www.ncbi.nlm.nih.gov/books/NBK53541/ [PubMed] [Google Scholar]
- Sharma S. & Fulton S. (2013) Diet‐induced obesity promotes depressive‐like behaviour that is associated with neural adaptations in brain reward circuitry. Int. J. Obes. (Lond) 37(3), 382–389. https://doi.org/10.1038/ijo.2012.48. [DOI] [PubMed] [Google Scholar]
- Simon G.E., Von Korff M., Saunders K. et al (2006) Association between obesity and psychiatric disorders in the US adult population. Arch. Gen. Psychiatry 63, 824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanathan S., Thavartnam K., Arif S., Elegino T. & McGowan P.O. (2015) Chronic high fat feeding increases anxiety‐like behaviour and reduces transcript abundance of glucocorticoid signalling genes in the hippocampus of female rats. Behav. Brain Res. 286, 265–270. https://doi.org/10.1016/j.bbr.2015.02.036. [DOI] [PubMed] [Google Scholar]
- Smaga I., Niedzielska E., Gawlik M. et al (2015) Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol. Rep. 67, 569–580. [DOI] [PubMed] [Google Scholar]
- Souza C.G., Moreira J.D., Siqueira I.R. et al (2007) Highly palatable diet consumption increases protein oxidation in rat frontal cortex and anxiety‐like behavior. Life Sci. 81, 198–203. [DOI] [PubMed] [Google Scholar]
- Todd D.G., Dao D.T. & Kovacsics C.E. (2009) The open field test mood and anxiety related phenotypes in mice. Neuromethods 42, 1–20. [Google Scholar]
- Tordoff M.G., Alarcon L.K. & Lawler M.P. (2008) Preferences of 14 rat strains for 17 taste compounds. Physiol. Behav. 95, 308–332. https://doi.org/10.1016/j.physbeh.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warneke W., Klaus S., Fink H., Langley‐Evans S.C. & Voigt J. (2014) The impact of cafeteria diet feeding on physiology and anxiety‐related behaviour in male and female Sprague‐Dawley rats of different ages. Pharmacol. Biochem. Behav. 116, 45–54. [DOI] [PubMed] [Google Scholar]
- Wilson C.R., Tran M.K., Salazar K., Young M.E. & Taegtmeyer H. (2007) Western diet, but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem. J. 406, 457–467. https://doi.org/10.1042/BJ20070392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada‐Goto N., Katsuura G., Nakao K. (2013) Mental Function and Obesity In: Functional Brain Mapping and the Endeavor to Understand the Working Brain, (eds Signorelli R. & Chirchiglia D.), InTech; https://doi.org/10.5772/56228. Available from: https://www.intechopen.com/books/functional-brain-mapping-and-the-endeavor-to-understand-the-working-brain/mental-function-and-obesity [Google Scholar]
- Yates K.F., Sweat V., Yau P.L., Turchiano M.M. & Convit A. (2012) Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arterioscler. Thromb. Vasc. Biol. 32, 2060–2067. https://doi.org/10.1161/ATVBAHA.112.252759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafir A., Ara A. & Banu N. (2009) Invivo antioxidant status: a putative target of antidepressant action. Prog. Neuropsychopharmacol. Biol. Psychiatry 33(2), 220–228. https://doi.org/10.1016/j.pnpbp.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Zhang X., Dong F., Ren J., Driscoll M.J. & Culver B. (2005) High dietary fat induces NADPH oxidase‐associated oxidative stress and inflammation in rat cerebral cortex. Exp. Neurol. 191, 318–325. [DOI] [PubMed] [Google Scholar]


