Summary
End‐to‐end anastomosis in the treatment for bile duct injury during laparoscopic cholecystectomy has been associated with stricture formation. The aim of this study was to experimentally investigate the effect of oral tamoxifen (tmx) treatment on fibrosis, collagen content and transforming growth factor‐β1, ‐β2 and ‐β3 expression in common bile duct anastomosis of pigs. Twenty‐six pigs were divided into three groups [sham (n = 8), control (n = 9) and tmx (n = 9)]. The common bile ducts were transected and anastomosed in the control and tmx groups. Tmx (40 mg/day) was administered orally to the tmx group, and the animals were euthanized after 60 days. Fibrosis was analysed by Masson's trichrome staining. Picrosirius red was used to quantify the total collagen content and collagen type I/III ratio. mRNA expression of transforming growth factor (TGF)‐β1, ‐β2 and ‐β3 was quantified using real‐time polymerase chain reaction (qRT‐PCR). The control and study groups exhibited higher fibrosis than the sham group, and the study group showed lower fibrosis than the control group (P = 0.011). The control and tmx groups had higher total collagen content than the sham group (P = 0.003). The collagen type I/III ratio was higher in the control group than in the sham and tmx groups (P = 0.015). There were no significant differences in the mRNA expression of TGF‐β1, ‐β2 and ‐β3 among the groups (P > 0.05). Tmx decreased fibrosis and prevented the change in collagen type I/III ratio caused by the procedure.
Keywords: bile duct injury, bile duct stenosis, bile duct wound healing, collagen, tamoxifen, transforming growth factor‐β
Cholecystectomy is one of the most frequently performed general surgical procedures worldwide (Pofahl & Pories 2003). Currently, nearly 90% of cholecystectomies are performed laparoscopically, which has increased the incidence of bile duct injuries from 0.1 to 0.2% in open cholecystectomy to 0.4–0.6% in video laparoscopy (de Santibanes et al. 2006; Linhares et al. 2011). Patients with acute cholecystitis had twice the risk of sustaining biliary lesion(s) (Tornqvist et al. 2016). Such injuries have an impact on healthcare costs (Van de Sande et al. 2003; Pottakkat et al. 2007; Alkhaffaf & Decadt 2010) and are among the leading causes of litigation against surgeons (de Reuver et al. 2007a; de Reuver et al. 2008; Scurr et al. 2010; Berney 2012).
Surgical reconstruction is the only option in patients with loss of biliary continuity (Mercado‐Diaz et al. 2008; Helmy et al. 2011). If bile duct injury is detected during surgery, end‐to‐end anastomosis could be considered a sufficient treatment (de Reuver et al. 2007b). The small calibre of the common bile duct hinders the anastomosis procedure and favours stenosis, which is usually secondary to the inflammatory process and fibrosis (Starling & Abrantes 2003). A previous study showed that rapamycin did not alter the levels of hydroxyproline, a major component of collagen, in an experimental pig model of biliary tract anastomosis (Kahn et al. 2005). Despite great clinical importance, there are few studies on the pharmacological prevention of bile duct stenosis.
Basic and translational wound healing research on the biliary tree lags significantly behind similar studies on the skin and gastrointestinal tract. This is at least partly attributable to the lack of easy access to the biliary tract for study purposes (Demetris et al. 2006). In certain keloid fibroblasts an increase in type I procollagen gene expression was observed (Uitto et al. 1985). Extensive subepithelial fibrosis and excessive collagen deposition suggest the involvement of collagen type I in human postintubation tracheal stenosis (Correa Reis et al. 2012). Several studies have shown that the changes in collagen contents are primarily characterized by a significant alteration in collagen type I/III ratio (Si et al. 2002; Junge et al. 2007). A previous study showed a significant increase in collagen type I/III ratio in a model of urethral stricture in pigs (Sievert et al. 2012). Transforming growth factor (TGF)‐β1 and ‐β2 have been associated with fibrogenic conditions, whereas TGF‐β3 was shown to decrease fibrosis and scarring (Mikulec et al. 2001; Karaca et al. 2013). High expression of TGF‐β1 is related to chronic inflammation, extracellular matrix over deposition and scar contracture of the bile duct caused by bile (Geng et al. 2005). Wound contraction has been shown to be important in the development of biliary tract stenosis (Demetris et al. 2006).
Tamoxifen (tmx), a synthetic non‐steroidal anti‐oestrogen, is the endocrine treatment of choice for all stages of breast cancer (Osborne 1998). It may also be effective in the treatment for fibrotic diseases, such as hypertrophic scars and keloids (Gragnani et al. 2010), encapsulating peritoneal sclerosis (Guest 2009), retroperitoneal fibrosis (Costanzi et al. 2008), fibrosing mediastinitis, sclerosing cervicitis (Savelli et al. 1997) and recurrent desmoid tumours (Gwynne‐Jones et al. 2005; Ohashi et al. 2006). Its antifibrotic property is mainly via downregulation of TGF‐β1 (Gragnani et al. 2010; Delle et al. 2012).
Considering that collagen and TGF‐β1 play a pivotal role in wound healing, we suggest that the inhibition of fibrosis by tmx may interfere with the healing process of biliary tract anastomosis. In an attempt to explore the mechanisms underlying the effects of oral tmx in this process, we studied common bile duct end‐to‐end anastomosis in pigs treated with tmx. Fibrosis, total collagen, collagen type I/III ratio and TGF‐β1, ‐β2 and ‐β3 mRNA expression in the anastomotic tissue were analysed 60 days after surgery.
Methods
Animals
Male pigs (Sus scrofa domesticus; Large White breed; n = 26) with a mean weight of 27.02 kg (standard deviation ± 5.14) were housed at the Farm School of the Veterinary Faculty of Fluminense Federal University, Cachoeiras de Macacu, RJ, Brazil. The animals were divided into three groups: sham (n = 8), control (n = 9) and tmx (n = 9). To avoid potential bias due to the anti‐oestrogenic effect of tmx, only male pigs were used with the following proportion of castrated: sham (87.5%), control (66.7%) and tmx (55.6%). They were kept under a 12/12‐h day/night cycle, fed a standard pig chow and provided ad libitum access to water. The experimental protocols were approved by the Ethics Committee on Animal Use (Reg. No. 444) of Fluminense Federal University, Rio de Janeiro, Brazil.
Ethical approval statement
The experimental protocols were approved by the Ethics Committee on Animal Use (Reg. No. 444) of Fluminense Federal University, Rio de Janeiro, Brazil.
Anaesthesia and analgesia
All animals were premedicated with intramuscular injections of 5 mg/kg ketamine (Ketamina Agener 10%®, União Química Farmacêutica Nacional, São Paulo, Brazil), 0.5 mg/kg midazolam (Dormire®, Cristália, São Paulo, Brazil) and 0.05 mg/kg acepromazine (Acepran 1%®, Vetnil, São Paulo, Brazil) according to the estimated weights. After sedation, the animals were weighed on a digital scale (Lucastec, São Paulo, Brazil). The animals were preoxygenated with 100% oxygen and anesthetized with 4 mg/kg propofol (Provine 1%®, Meizler Biopharma, São Paulo, Brazil), and blood samples were collected for biochemical analysis. After endotracheal intubation, epidural block was performed with 0.5 mg/kg bupivacaine (Neocaína®, Cristália) and 0.1 mg/kg morphine (Sulfato de morfina®, Hipolabor, Minas Gerais, Brazil). Anaesthesia was maintained with sevoflurane (Sevocris®, Cristália) diluted in 100% oxygen (100 ml/kg/min). Meloxicam (0.4 mg/kg; Maxicam 2%®, Ourofino, São Paulo, Brazil) administered subcutaneously was used for postoperative analgesia for 4 days. Tramadol 2 mg/kg (Cloridrato de tramadol®, União Química Farmacêutica Nacional) was administered only to animals that showed signs of discomfort or pain postoperatively.
Surgical procedures
Under aseptic conditions, the peritoneal cavity of each animal was accessed using a right subcostal incision, and then, the cystic artery and duct were dissected, ligated and transected. After gallbladder removal, the hepatoduodenal ligament was incised. The common bile duct was identified and dissected (Figure 1a). The external diameter of the bile duct was measured with a digital calliper (Digimess, São Paulo, Brazil), followed by transection only in the control and tmx groups (Figure 1b). Continuity was re‐established by standardized end‐to‐end anastomosis with continuous (running) stitches (polypropylene 6‐0, Ethicon®, São José dos Campos, SP, Brazil) (Figure 1c,d). All anastomoses were performed by the same surgeon. The abdominal musculoaponeurotic layer was closed using simple running stitches with a polyglycolic acid suture (vicryl 0 B Braun®, Rubi, Spain). The skin was sutured using a continuous suture (nylon 3‐0, Bioline®, Anápolis, GO, Brazil). All animals were maintained on standard chow diet and water was provided ad libitum until the day of euthanasia.
Figure 1.
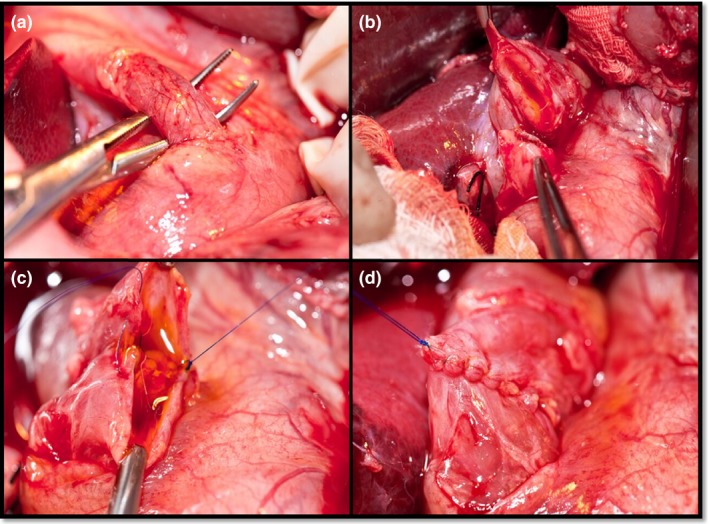
Steps of surgical procedure. a. Dissected common bile duct. b. Transected common bile duct. c. Posterior common bile duct anastomosis. d. Anastomosis final aspect.
Tmx administration
From the next day after surgery, 40 mg/day tmx citrate (Sandoz®, Cambé, PR, Brazil) was orally administrated to the tmx group for 60 days.
Euthanasia protocol
Euthanasia‐anaesthesia protocol
The pigs were euthanized 60 days after surgery. They were premedicated with intramuscular injection of 5 mg/kg ketamine, 0.5 mg/kg midazolam and 0.05 mg/kg acepromazine, based on the estimated weights. After sedation, the animals were weighed on a digital scale. Following this, they were pre‐oxygenated with 100% oxygen, anaesthetized with 4 mg/kg propofol, and blood samples collected again for biochemical analysis. After endotracheal intubation, anaesthesia was maintained with sevoflurane diluted in 100% oxygen (100 ml/kg/min).
Euthanasia‐surgical protocol
A midline incision was performed, and the adhesions were broken. The biliary tract was dissected, and its external diameter was measured upstream of the scarred area of biliary anastomosis in the control and tmx groups with a digital calliper (Digimess). In the sham group, the bile duct was measured in the area that corresponds to the anastomotic scar in the control and tmx groups. The common bile duct was resected, opened longitudinally and divided into two halves. The posterior half was submerged in 10% buffered formalin and sent for histopathological examination and collagen analysis. The scarred area of the anterior wall of the common bile duct was collected for the analysis of TGF‐β 1, ‐β2 and ‐β3 expression. After tissue sample harvesting, 10% potassium chloride was infused until complete cessation of heartbeat.
Histopathological analysis
Tissue samples of the common bile duct were fixed in 10% buffered formalin (pH 7.2) for 24 h. Following fixation, these tissues were processed for paraffin embedding and 5‐μm‐thick longitudinal sections were prepared. All tissue samples were stained with haematoxylin and eosin, Masson's trichrome and picrosirius red. The sections stained with haematoxylin and eosin were initially analysed by an experienced pathologist. Fibrosis of the scar area was detected and analysed by Masson's trichrome staining in the tmx and control groups. In the sham group, fibrosis was analysed in the middle of the tissue fragment. Histological classification of fibrosis was made according to the criteria described in Table 1.
Table 1.
Classification of scores according to the percentage of fibrosis
| Fibrosis percentage | Scores |
|---|---|
| 0% | 0 |
| 1–20% | 1 |
| 21–40% | 2 |
| 41–60% | 3 |
| 61–80% | 4 |
| 81–100% | 5 |
Histomorphometric analysis of collagen content
Picrosirius red was used to detect and quantify the total collagen content in the scar area of the longitudinal section of the common bile duct wall. The photomicrographs captured at ×100 magnification were obtained using a digital camera coupled to a microscope (Nikon E200; Eclipse, Tokyo, Japan). The surface density of total collagen was evaluated using the point‐counting method as described previously (Felix‐Patricio et al. 2015). Briefly, a 99‐point grid was superimposed over the images using the grid tool of Image J software (ImageJ 1.37; National Institutes of Health, NIH, Bethesda, MD, USA), and the points touching the collagen fibres were counted with the cell counter tool. The number of points touching the collagen was multiplied by 100 and divided by 99 to correct for the 99 points used as test system. The percentage of the analysed photomicrographs was considered as the mean of surface density of collagen for each sample: eight images of sham group and nine images for both tmx and control groups.
Collagen types I and III were analysed on slides stained with picrosirius red under polarized light, and photomicrographs of the scar area were captured under ×200 magnification using a digital camera (Olympus DP70, Tokyo, Japan) coupled to a microscope (Olympus BX51, Tokyo, Japan). The percentage of collagen area was determined by colour‐based segmentation method using the Image‐Pro Plus image analysis software (version 4.5.0.29z; Media Cybernetics, Rockville, USA), as described previously (Felix‐Patricio et al. 2015). Briefly, type III and I collagen appeared as green‐ and red‐coloured fibres, respectively (Junqueira et al. 1982), and the surface area was calculated at different moments. The histogram tool was used after colour segmentation of the image based on automatic counting of the percentage of pixels with the same colour. This percentage represents the surface density of red‐ or green‐coloured areas, which indicates the surface density of collagen type I or III in the common bile duct. The results were expressed as the ratio of the area of collagen type I to that of III.
Quantitative reverse transcription polymerase chain reaction (qRT‐PCR) of TGF‐β1, β2 and ‐β3
Total RNA from the scarred common bile duct was isolated using Trizol reagent (Invitrogen, CA, USA), and 1 μg of total RNA was reverse‐transcribed to cDNA using a superscript III kit (Invitrogen). The mRNA expression was evaluated using real‐time PCR with specific primers for β‐actin (ACTB) as the reference gene (Table 2). The products were amplified using the Applied Biosystems‐StepOne® real‐time PCR system (Life Technologies Corp., CA, USA) with GoTaq® qPCR master mix (Promega, WI, USA). The cycle parameters were as follows: 95°C for 2 min, followed by 40 cycles at 95°C for 15 s and 60°C for 60 s. The relative mRNA expression [2ΔΔcycle threshold (Ct)] was calculated by comparing the Ct between groups after correcting for the reference ACTB gene. The expression of the ACTB gene was stable for the tissue and experimental groups. The efficiency of each reaction was calculated using serial dilution, and it varied between 95 and 105%. Each sample was measured in duplicate, and the results are expressed relative to the values of the sham group, which was set to 1. The purity of PCR products was monitored by analysing the melting curves.
Table 2.
Proteins and respective primer sequences
| Protein | Primer sequence | |
|---|---|---|
| TGF‐β1 | Forward | 5′ CGATTAAGGTGGACAGAGGACTG 3′ |
| Reverse | 5′ AATGAATGGTGGACAGACACAGG 3′ | |
| TGF‐β2 | Forward | 5′ AAATCGACATGCCGCCCTTC 3′ |
| Reverse | 5′ AAGACTCTGAACTCTGCCTTCAC 3′ | |
| TGF‐β3 | Forward | 5′ CAAGACAAAGTCCCAGAACTGTAC 3′ |
| Reverse | 5′ GGGAACTACCTTACCTGGATTTTC 3′ | |
| ACTB | Forward | 5′ TCCAGAGGCGCTCTTCCA 3′ |
| Reverse | 5′ CGCACTTGATCGAGTTGA 3′ | |
ACTB, β‐actin.
Biochemical parameters
Blood samples were collected on the days of surgery and euthanasia. Total bilirubin, direct bilirubin (DB), indirect bilirubin (IB), aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), γ‐glutamyl transpeptidase (GGT) and albumin (ALB) levels were analysed.
Statistical analysis
Data were analysed using the statistical analysis software (sas ®) system, version 6.11 (SAS Institute, Inc., Cary, NC, USA). Kruskal–Wallis test was used to analyse differences among the groups followed by post hoc Dunn's multiple comparison tests. The differences in diameter of the common bile duct, weight and biochemical parameters between surgery and necropsy were assessed using the Wilcoxon signed‐rank test. The proportion of castrated pigs among the groups was compared using Fisher's exact test. A P‐value <0.05 was considered significant.
Results
Twenty‐six pigs from the sham (n = 8), control (n = 9) and tmx (n = 9) groups were euthanized on postoperative day 60. There was no significant difference (P = 0.43) in the proportion of castrated pigs among the groups. Clinical signs of bile duct stenosis were not observed in any animal in the postoperative observation period.
Histopathological analysis
Fibrosis score (median and interquartile range) was 0.5 (0 to 1) in the sham group, 4 (3 to 5) in the control group and 2 (1 to 3) in the tmx group. A significant difference in fibrosis (P = 0.011) among experimental groups was found. It was identified that the sham group exhibited significantly lower fibrosis than the control and tmx groups, and the tmx group was significantly lower than the control group (Figure 2).
Figure 2.

Comparison of fibrosis in common bile duct tissue in the sham (n = 8), control (n = 9) and tmx (n = 9) groups. Control and tmx groups presented higher fibrosis than the sham group, and the tmx group exhibited lower fibrosis than the control group (P = 0.011).
Total collagen analysis
A significant difference in total collagen content was observed (P = 0.003) between the groups. The control and tmx groups had significantly higher total collagen levels than the sham group. There was no significant difference between the control and tmx groups (Figure 3).
Figure 3.

Comparison of total collagen content in common bile duct tissue in sham (n = 8), control (n = 9) and tmx (n = 9) groups. The control and tmx groups showed higher total collagen levels than the sham group (P < 0.05), with no significant difference between the control and tmx groups.
Collagen type I/III ratio
There was a significant difference in collagen type I/III ratio (P = 0.015) among the groups. The control group showed a significantly higher collagen type I/III ratio than the sham and tmx groups (Figure 4). There was no significant difference between the sham and tmx groups (Figure 5).
Figure 4.

Picrosirius staining under polarized light (a. sham group. b. control group. c. tmx group) showing decreased, increased and decreased collagen type I/III ratios, respectively. Bar = 100 μm.
Figure 5.

Comparison of collagen type I/III ratio in common bile duct tissue in sham (n = 8), control (n = 9) and tmx (n = 9) groups. The control group showed a significantly higher collagen type I/III ratio than the sham and tmx groups (P < 0.05). No significant difference was observed between the sham and tmx groups. Ο: outlier of the control group *: outlier of the tmx group.
Quantification of mRNA expression of TGF‐β1, ‐β2 and ‐β3
There was no significant difference in the mRNA expression of TGF‐β1, ‐β2 and ‐β3 between the groups (Figure 6).
Figure 6.

Comparison of transforming growth factor (TGF)‐β1, ‐β2 and ‐β3 mRNA expression in common bile duct tissue of sham (n = 8), control (n = 9) and tmx (n = 9) groups. The results shown are relative to those of sham group. There was no difference among the groups (P > 0.05). Ο: outlier of TGF‐β2 in the sham and tmx groups.
Comparative common bile duct diameter, weight and biochemical parameter variations (Δ) between surgery and necropsy in the three groups
No significant difference in common bile duct diameter in the absolute delta was observed among the groups (P > 0.05) (Tables 3 and 4). In addition, no significant difference in weight and biochemical parameters in absolute delta was observed among the groups (P > 0.05) (Table 4).
Table 3.
Common bile duct diameter in millimetres at surgery and necropsy of the sham, control, and tmx groups
| Surgery | Necropsy | |||
|---|---|---|---|---|
| Median | IQR | Median | IQR | |
| Sham | 7.27 | 6.40–11.45 | 9.75 | 8.9–14.0 |
| Control | 10.3 | 6.88–11.74 | 12.3 | 9.7–14.3 |
| Tmx | 10.1 | 8.83–11.0 | 12.0 | 8.6–13.4 |
IQR, interquartile range; Tmx, tamoxifen.
Table 4.
Variations (Δ) of the weight, common bile duct diameter and biochemical parameters, between surgery and necropsy in the three groups. Median ± interquartile range
| Delta | Sham Group (n = 8) | Control Group (n = 9) | Tmx Group (n = 9) | P‐value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |||||
| Weight (kg) | 15.5 | 11.8 | −16.8 | 14.9 | 10.2 | −15.5 | 11.2 | 11.2 | −14.7 | 0.30 |
| Common bile duct diameter (mm) | 2.22 | 1.64 | −2.90 | 1.39 | −0.09 | −3.38 | 1.74 | 1.74 | −4.12 | 0.72 |
| Aspartate transaminase (IU/l) | 5.00 | −4.00 | −11.75 | 4.00 | −6.00 | −7.00 | 3.00 | 3.00 | −7.50 | 0.68 |
| Alanine transaminase (IU/l) | 2.50 | −4.00 | −16.25 | 12.00 | 7.00 | −19.5 | 13.00 | 13.00 | −16.5 | 0.34 |
| γ‐Glutamyl transpeptidase (IU/l) | −0.50 | −4.25 | −2.50 | −2.00 | −10.00 | −2.50 | −2.00 | −2.00 | −2.00 | 0.87 |
| Albumin (g/dl) | 0.005 | −0.710 | −0.563 | −0.050 | −0.415 | −0.250 | −0.110 | −0.110 | −0.190 | 0.97 |
| Alkaline phosphatase (IU/l) | −8.0 | −15.8 | −62.8 | 25.0 | −6.0 | −70.5 | 37.0 | 37.0 | −100.5 | 0.65 |
| Total bilirubin (mg/dl) | 0.030 | −0.013 | −0.093 | 0.080 | 0.060 | −0.085 | 0.030 | 0.030 | −0.105 | 0.68 |
| Direct bilirubin (mg/dl) | 0.000 | −0.018 | −0.010 | 0.000 | −0.010 | −0.005 | 0.000 | 0.000 | −0.000 | 0.77 |
| Indirect bilirubin (mg/dl) | 0.020 | −0.025 | −0.093 | 0.080 | 0.055 | −0.080 | 0.030 | 0.005 | −0.100 | 0.66 |
Tmx, tamoxifen; IQR, interquartile range.
Discussion
Despite the current improvements in surgical techniques, postoperative stricture of the biliary tract remains a challenge and a major cause of morbidity and mortality. In this study, we aimed to investigate the effect of 40 mg/day oral tmx treatment for 60 days on fibrosis, collagen content and mRNA expression of TGF‐β1, ‐β2 and ‐β3 in common bile duct anastomosis in an experimental pig model. We observed that tmx decreased fibrosis and prevented the change in collagen type I/III ratio. Our group previously studied longitudinal incisions of the bile duct in pigs treated orally with tmx at a dose of 20 mg/day for 30 days. Tmx reduced the expression of α‐SMA in the healing tissue, suggesting a decrease in myofibroblast number (Siqueira et al. 2013). In a previous study in a rat model, it was reported that the antifibrotic effects of tmx are associated with reduced TGF‐β1 levels, which in turn is related to the reduction in postoperative adhesion in a dose‐ and time‐dependent manner (Karaca et al. 2013). Therefore, in the present study, we decided to use a higher dose for a longer duration.
Briefly, histopathological analysis showed that the bile ducts of animals with anastomoses (tmx and control groups) exhibited higher fibrosis than the sham group. In addition, pigs treated with tmx demonstrated a lower amount of fibrosis than the control group. These data suggest that tmx exerted an antifibrotic effect on bile duct anastomosis in the studied animals.
Inflammatory process and fibrosis are important factors associated with common bile duct stenosis (Starling & Abrantes 2003). Extensive subepithelial fibrosis was observed in postintubation human tracheal stenosis (Correa Reis et al. 2012). Our study suggests that the antifibrotic effect of tmx could decrease bile duct anastomotic stricture.
The total collagen content was higher in the control and tmx groups than in the sham group. However, there was no difference between the control and tmx groups. These data showed that transection and anastomosis of the common bile duct increased the total collagen content, while tmx showed no effect on total collagen.
In a pig model of common bile duct healing, the collagen content per volume showed an increase in collagen for the first 14 postoperative days (Laursen et al. 2007). The percentage of collagen area was used to evaluate changes in collagen content in benign biliary strictures in dogs (Geng et al. 2005). It was concluded that TGF‐β1 might cause prolonged healing of bile duct trauma, overdeposition of extracellular matrix, cicatrix contracture and stenosis of the anastomotic stoma. The qualitative characteristics of the deposited collagen are important for normal biliary tract anastomosis.
To specifically assess the quality of the deposited collagen, we determined the collagen type I/III ratio and found the ratio to be higher in the control group than in the sham and tmx groups. No difference was found between the sham and tmx groups. These findings showed that the 60‐day treatment with tmx 40 mg/day decreased collagen type I/III ratio in the tmx group and prevented the change in collagen type proportion caused by common bile duct anastomosis. These findings clearly show the qualitative effect of tmx on collagen deposition.
Type I collagen has been hypothesized as the basis of hypertrophic (Cheng et al. 2011) and mature burn scar formation (Ozog et al. 2013). Contracted wounds are unique in their type I collagen (Ehrlich and Hunt, 2012), which has also been demonstrated in keloids (Ehrlich et al. 1994). Alterations in the collagen type I/III ratio are hallmark indicators of skin injury. A higher collagen type I/III ratio suggests a stiffer granulation tissue where collagen type I is the predominant type, whereas a lower ratio suggests a more compliant, less stiff granulation tissue, which corresponds to previous quantitative analyses on wound contraction and granulation volume fractions (Monaghan et al. 2014). Wound contraction is important in the development of biliary tract stenosis (Demetris et al. 2006). In our study, pigs in the tmx group had a lower collagen type I/III ratio. Thus, we suggest that untreated animals have stiffer collagen and more rigid anastomoses, which predisposes stenoses. Considering that the bile duct anastomosis is formed in a circular fashion, excessive wound contraction can cause centripetal narrowing. This may result in stricture with upstream biliary ductal dilatation and obstructive jaundice. Therefore, we suggest that tmx probably induced the formation of more compliant anastomoses, thereby reducing the chances of stenosis.
No difference in the mRNA expression of TGF‐β1, ‐β2 and ‐β3 was observed between the groups. This was probably attributable to the duration of treatment (60 days).
In particular, TGF‐β1 appears to be involved in the initiation and completion of the tissue repair process (Mikulec et al. 2001, 2001). In a previous study, high expression of TGF‐β1 was observed in the stenotic bile duct tissue of 23 patients (Geng et al. 2008).
The weight, common bile duct diameter and biochemical parameter variations (Δ) were not significantly different among the groups. This shows that the groups progressed similarly, and these parameters were not sources of bias in the main analysis.
The present results should be interpreted in the light of the potential limitations of the study. Because we addressed biliary wound healing process after transection and anastomosis at a 2‐month follow‐up time point, it is unknown whether tmx exerts a more long‐lasting action. Further studies are needed to describe the time course of this modulatory action of tmx. In addition, as our aim was to specifically investigate fibrosis, collagen content and TGF‐β1, ‐β2 and ‐β3 expression during biliary wound healing process, we did not obtain an overview of the entire wound healing process, including the activation of the TGF‐β‐Smad signalling pathway. Although a more comprehensive approach would be desirable, the present results are straightforward and provide novel evidence for the modulatory role of tmx in fibrosis and collagen type I/III ratio in biliary wound healing.
Conclusion
The main observation of this study is that tmx decreased fibrosis and prevented the change in collagen type I/III ratio caused by common bile duct anastomosis in a pig model. Further experimental and clinical studies must be carried out to investigate the potential impact of using tmx as a preventive agent against stenosis of the common bile duct anastomosis.
Conflict of interest
None.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
Acknowledgements
We express our heartfelt gratitude to Ary Nascimento Bassous (Antônio Pedro University Hospital, Fluminense Federal University) for photographing the surgical procedures. We thank Waldemar Silva Costa (Urogenital Research Unit, State University of Rio de Janeiro, Rio de Janeiro, Brazil) for the photomicrographic documentation of polarized light on the picrosirius‐stained slides. The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- Alkhaffaf B. & Decadt B. (2010) 15 years of litigation following laparoscopic cholecystectomy in England. Ann. Surg. 251, 682–685. [DOI] [PubMed] [Google Scholar]
- Berney C.R. (2012) Major common bile duct injury and risk of litigation: a surgeon's perspective. Am. J. Surg. 204, 800–802. [DOI] [PubMed] [Google Scholar]
- Cheng W., Yan‐hua R., Fang‐gang N. & Guo‐an Z. (2011) The content and ratio of type I and III collagen in skin differ with age and injury. Afr. J. Biotechnol. 10, 2529–2528. [Google Scholar]
- Correa Reis J.G., Takiya C.M., Lima Carvalho A. et al (2012) Myofibroblast persistence and collagen type I accumulation in the human stenotic trachea. Head Neck 34, 1283–1293. [DOI] [PubMed] [Google Scholar]
- Costanzi S., Zoli A., Ferraro P.M., Danza F.M. & Ferraccioli G.F. (2008) A paraneoplastic retroperitoneal fibrosis resistant to corticosteroids treated with tamoxifen. Clin. Nephrol. 70, 172–175. [DOI] [PubMed] [Google Scholar]
- Delle H., Rocha J.R., Cavaglieri R.C., Vieira J.M. Jr, Malheiros D.M. & Noronha I.L. (2012) Antifibrotic effect of tamoxifen in a model of progressive renal disease. J. Am. Soc. Nephrol. 23, 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetris A.J., Lunz J.G. 3rd, Specht S. & Nozaki I. (2006) Biliary wound healing, ductular reactions, and IL‐6/gp130 signaling in the development of liver disease. World J. Gastroenterol. 12, 3512–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H.P., Desmouliere A., Diegelmann R.F., Cohen I.K., Compton C.C., Garner W.L., Kapanci Y. & Gabbiani G. (1994) Morphological and immunochemical differences between keloid and hypertrophic scar. Am. J. Pathol. 145, 105–113. [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H.P. & Hunt T.K. (2012) Collagen organization critical role in wound contraction. Adv. Wound Care 1, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix‐Patricio B., De Souza D.B., Gregorio B.M., Costa W.S. & Sampaio F.J. (2015) How to quantify penile corpus cavernosum structures with histomorphometry: comparison of two methods. Biomed. Res. Int. 2015, 832156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Z.M., Yao Y.M., Liu Q.G., Niu X.J. & Liu X.G. (2005) Mechanism of benign biliary stricture: a morphological and immunohistochemical study. World J. Gastroenterol. 11, 293–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Z.M., Zheng J.B., Zhang X.X., Tao J. & Wang L. (2008) Role of transforming growth factor‐beta signaling pathway in pathogenesis of benign biliary stricture. World J. Gastroenterol. 14, 4949–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gragnani A., Warde M., Furtado F. & Ferreira L.M. (2010) Topical tamoxifen therapy in hypertrophic scars or keloids in burns. Arch. Dermatol. Res. 302, 1–4. [DOI] [PubMed] [Google Scholar]
- Guest S. (2009) Tamoxifen therapy for encapsulating peritoneal sclerosis: mechanism of action and update on clinical experiences. Perit. Dial. Int. 29, 252–255. [PubMed] [Google Scholar]
- Gwynne‐Jones D.P., Theis J.C., Jeffery A.K. & Hung N.A. (2005) Long‐term follow‐up of a recurrent multifocal desmoid tumour treated with tamoxifen: a case report. J. Orthop. Surg. (Hong Kong) 13, 174–177. [DOI] [PubMed] [Google Scholar]
- Helmy A.A., Hamad M.A., Aly A.M. et al (2011) Novel technique for biliary reconstruction using an isolated gastric tube with a vascularized pedicle: a live animal experimental study and the first clinical case. Ann. Surg. Innov. Res. 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge K., Klinge U., Rosch R. et al (2007) Improved collagen type I/III ratio at the interface of gentamicin‐supplemented polyvinylidenfluoride mesh materials. Langenbecks Arch. Surg. 392, 465–471. [DOI] [PubMed] [Google Scholar]
- Junqueira L.C., Montes G.S. & Sanchez E.M. (1982) The influence of tissue section thickness on the study of collagen by the Picrosirius‐polarization method. Histochemistry 74, 153–156. [DOI] [PubMed] [Google Scholar]
- Kahn D., Spearman C.W., Mall A. et al (2005) Effect of rapamycin on the healing of the bile duct. Transplant. Proc. 37, 832–833. [DOI] [PubMed] [Google Scholar]
- Karaca T., Gozalan A.U., Yoldas O., Bilgin B.C. & Tezer A. (2013) Effects of tamoxifen citrate on postoperative intra‐abdominal adhesion in a rat model. Int. J. Surg. 11, 68–72. [DOI] [PubMed] [Google Scholar]
- Laursen H.B., Thorsoe H.J., Oxlund H. et al (2007) Choledocho‐choledochostomy: the natural history of healing in pigs. J. Hepatobiliary Pancreat. Surg. 14, 498–502. [DOI] [PubMed] [Google Scholar]
- Linhares B.L., Magalhaes Ada G., Cardoso P.M., Linhares Filho J.P., Pinho J.E. & Costa M.L. (2011) Bile duct injury following cholecystectomy. Rev. Col. Bras. Cir. 38, 95–99. [DOI] [PubMed] [Google Scholar]
- Mercado‐Diaz M.A., Ramirez‐Morales R., Medinilla‐Cruz M.A. & Poucel‐Sanchez M.F. (2008) Transhepatic transanastomotic stents for bile duct injuries. Long‐term evolution. Cir. Cir. 76, 219–223. [PubMed] [Google Scholar]
- Mikulec A.A., Hanasono M.M., Lum J., Kadleck J.M., Kita M. & Koch R.J. (2001) Effect of tamoxifen on transforming growth factor β1 production by keloid and fetal fibroblasts. Arch. Facial Plast. Surg. 3, 111–114. [DOI] [PubMed] [Google Scholar]
- Monaghan M., Browne S., Schenke‐Layland K. & Pandit A. (2014) A collagen‐based scaffold delivering exogenous microrna‐29B to modulate extracellular matrix remodeling. Mol. Ther. 22, 786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T., Shigematsu N., Kameyama K. & Kubo A. (2006) Tamoxifen for recurrent desmoid tumor of the chest wall. Int. J. Clin. Oncol. 11, 150–152. [DOI] [PubMed] [Google Scholar]
- Osborne C.K. (1998) Tamoxifen in the treatment of breast cancer. N. Engl. J. Med. 339, 1609–1618. [DOI] [PubMed] [Google Scholar]
- Ozog D.M., Liu A., Chaffins M.L. et al (2013) Evaluation of clinical results, histological architecture, and collagen expression following treatment of mature burn scars with a fractional carbon dioxide laser. JAMA Dermatol. 149, 50–57. [DOI] [PubMed] [Google Scholar]
- Pofahl W.E. & Pories W.J. (2003) Current status and future directions of geriatric general surgery. J. Am. Geriatr. Soc. 51, S351–S354. [DOI] [PubMed] [Google Scholar]
- Pottakkat B., Sikora S.S., Kumar A., Saxena R. & Kapoor V.K. (2007) Recurrent bile duct stricture: causes and long‐term results of surgical management. J. Hepatobiliary Pancreat. Surg. 14, 171–176. [DOI] [PubMed] [Google Scholar]
- de Reuver P.R., Rauws E.A., Bruno M.J., et al (2007a) Survival in bile duct injury patients after laparoscopic cholecystectomy: a multidisciplinary approach of gastroenterologists, radiologists, and surgeons. Surgery 142, 1–9. [DOI] [PubMed] [Google Scholar]
- de Reuver P.R., Busch O.R., Rauws E.A., Lameris J.S., van Gulik T.M. & Gouma D.J. (2007b) Long‐term results of a primary end‐to‐end anastomosis in peroperative detected bile duct injury. J. Gastrointest. Surg. 11, 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Reuver P.R., Dijkgraaf M.G., Gevers S.K., Gouma D.J.& Group B.S. (2008) Poor agreement among expert witnesses in bile duct injury malpractice litigation: an expert panel survey. Ann. Surg. 248, 815–820. [DOI] [PubMed] [Google Scholar]
- de Santibanes E., Palavecino M., Ardiles V. & Pekolj J. (2006) Bile duct injuries: management of late complications. Surg. Endosc. 20, 1648–1653. [DOI] [PubMed] [Google Scholar]
- Savelli B.A., Parshley M. & Morganroth M.L. (1997) Successful treatment of sclerosing cervicitis and fibrosing mediastinitis with tamoxifen. Chest 111, 1137–1140. [DOI] [PubMed] [Google Scholar]
- Scurr J.R., Brigstocke J.R., Shields D.A. & Scurr J.H. (2010) Medicolegal claims following laparoscopic cholecystectomy in the UK and Ireland. Ann. R. Coll. Surg. Engl. 92, 286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Z., Bhardwaj R., Rosch R., Mertens P.R., Klosterhalfen B. & Klinge U. (2002) Impaired balance of type I and type III procollagen mRNA in cultured fibroblasts of patients with incisional hernia. Surgery 131, 324–331. [DOI] [PubMed] [Google Scholar]
- Sievert K.D., Selent‐Stier C., Wiedemann J. et al (2012) Introducing a large animal model to create urethral stricture similar to human stricture disease: a comparative experimental microscopic study. J. Urol. 187, 1101–1109. [DOI] [PubMed] [Google Scholar]
- Siqueira O.H., Herani Filho B., Paula R.E. et al (2013) Tamoxifen decreases the myofibroblast count in the healing bile duct tissue of pigs. Clinics (Sao Paulo) 68, 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling S.V. & Abrantes W.L. (2003) Common bile duct injury: ligation and cholecystojejunostomy as surgical option. Rev. Col. Bras. Cir. 30, 244–246. [Google Scholar]
- Tornqvist B., Waage A., Zheng Z., Ye W. & Nilsson M. (2016) Severity of acute cholecystitis and risk of iatrogenic bile duct injury during cholecystectomy, a population‐based case‐control study. World J. Surg. 40, 1060–1067. [DOI] [PubMed] [Google Scholar]
- Uitto J., Perejda A.J., Abergel R.P., Chu M.L. & Ramirez F. (1985) Altered steady‐state ratio of type I/III procollagen mRNAs correlates with selectively increased type I procollagen biosynthesis in cultured keloid fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 82, 5935–5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Sande S., Bossens M., Parmentier Y. & Gigot J.F. (2003) National survey on cholecystectomy related bile duct injury–public health and financial aspects in Belgian hospitals–1997. Acta Chir. Belg. 103, 168–180. [DOI] [PubMed] [Google Scholar]


