Summary
Ductal carcinoma in situ (DCIS) is a heterogeneous disease that has been investigated less extensively than invasive breast cancer. Women with DCIS are mainly treated with conservative surgery almost exclusively followed by radiotherapy. However, as radiation treatment is not always effective, the search for biomarkers capable of identifying DCIS lesions that could progress to invasive cancer is ongoing. Although conventional biomarkers have been thoroughly studied in invasive tumours, little is known about the role played by androgen receptor (AR), widely expressed in DCIS. A series of 42 DCIS patients treated with quadrantectomy and radiotherapy were followed for a period of up to 95 months. Of these, 11 had recurrent DCIS or progressed to invasive cancer. All tumours were analysed for clinical pathological features. Conventional biomarkers and androgen receptor expression were determined by immunohistochemistry. Our results showed that AR was higher in tumours of relapsed patients than non‐relapsed patients (P value: 0.0005). Conversely, oestrogen receptor (ER) was higher, albeit not significantly, in non‐relapsed patients than in relapsed patients. AR/ER ratio was considerably different in the two subgroups (P value: 0.0033). Area under the curve (AUC) values were 0.85 for AR and 0.80 for the AR/ER ratio. These preliminary results highlight the potentially important role of both AR and the AR/ER ratio as prognostic markers in DCIS.
Keywords: AR and AR/ER ratio, ductal carcinoma in situ, prognosis
Breast cancer is the most frequent malignancy and the second cause of death from cancer in women (Globocan 2012). It is detected as an invasive ductal lesion in up to 80% of patients and less frequently (up to 20%) as an in situ ductal lesion. The high incidence of invasive breast cancer and its aggressiveness have led to intensive research to better identify biological profiles corresponding to different prognostic risks that could provide useful information to plan tailored therapy. The discovery of biological subgroups of lesions such as triple negative (oestrogen receptor [ER]‐, progesterone receptor [PgR]‐, human epidermal growth factor receptor 2 [HER2]‐) or triple unfavourable (ER‐, PgR‐, Ki67 > 20%) (Rocca et al. 2014; Amadori et al. 2014) has helped to better define disease aggressiveness and to identify tumours responding differently to conventional treatments (Rocca et al. 2014).
In recent years, we have witnessed the intensification of research into in situ breast disease. The substantial increase in the detection of small tumours (up to 20%) by screening programmes has emphasized the importance of this diagnostic tool. However, close examination of results reveals that a large number of breast cancer cases have been overestimated due to an excess of diagnoses of proliferative lesions of indeterminate evolution, inducing women to undergo unnecessary surgical treatment (Welch & Passow 2014). This limiting aspect of screening programmes, supported by results from important studies (Kroenke 2014; Weedon‐Fekjær et al. 2014), has led to greater research efforts to define the biological profiles of early lesions and to identify markers capable of predicting malignant evolution.
In Italy, women with in situ breast disease are mainly treated by either total mastectomy in the event of extensive (multifocal) ductal carcinoma in situ (DCIS), with or without sentinel node biopsy (normally only performed for high risk lesions), or conservative surgery followed by radiotherapy for full therapeutic efficacy, according to AIOM guidelines for breast neoplasms. However, radiation treatment is not always effective and causes psychological problems in patients with long‐term toxicity. It also represents a heavy economic burden for the National Health System (Cutuli et al. 2014; Correa et al. 2010). In most centres, treatment depends on the grade and extent of the DCIS. A localized focus of low‐grade DCIS may only require needle‐localized excision, with no further treatment if the margins are clear.
A multigene expression assay to predict local recurrence risk of ductal carcinoma in situ of the breast was recently developed (Solin et al. 2013; Raldow et al. 2016). The Oncotype DX DCIS test is a grading system that provides a prognostic score based on a patient's gene expression profile, and is comparable to histological grading. However, many oncologists using the Oncotype DX DCIS assay are not aware of its limitations, especially with regard to cost‐effectiveness (Lagios & Silverstein 2014). Furthermore, as the test does not include a number of important predictive factors such as age, margin width, extent of DCIS, nuclear grade or necrosis, the likelihood of its accuracy is poor in the majority of patients. Thus, there is still no one test that can reliably estimate the risk of local recurrence in DCIS patients. At present, the ability to identify DCIS that is likely to recur or progress to invasive breast cancer is still limited, and there is also a lack of level 1 evidence supporting the omission of adjuvant radiotherapy in selected low‐risk cases who could potentially be treated by complete local excision (Amichetti & Vidali 2012).
Steroid hormone receptors, cell proliferation index and other important tumour markers are conventionally used to study the biological profiles of invasive tumours. Androgen receptors (AR), albeit widely expressed in breast cancer with uncertain significance on the basis of the co‐expression of hormonal receptors (Selim et al. 2002), still require further investigation in DCIS (Lari & Kuerer 2011; Hanley et al. 2008). In a recent study, we evaluated the real prognostic impact of standard biomarkers (ER, PgR, Ki67 and HER2) and AR on DCIS treated with surgery only (Tumedei et al. 2015).
As in clinical practice DCIS patients are treated almost exclusively with surgery and radiotherapy, we decided to investigate the predictive role of specific markers, especially AR, on the clinical outcome in this population.
Materials and methods
This retrospective study was carried out on a series of 42 patients diagnosed with DCIS between 2000 and 2009 during screening at the Cancer Prevention Unit and operated on in the Breast Surgical Unit of Morgagni‐Pierantoni Hospital, Forlì. Eleven patients relapsed and were matched (1:3) for age and nuclear grade with non‐relapsed patients enrolled in the same period.
All patients were aged ≥18 years (range 38–77 years), had a histological diagnosis of DCIS treated with quadrantectomy and radiotherapy and were followed up for 95 months. Both unifocal and multifocal tumours were included. Multifocality is a pathologic feature defined as more than one distinct focus of DCIS, with at least 5 mm of intervening healthy tissue confined to a single quadrant of the breast. The size of the largest focus was recorded in the event of multifocal DCIS. Patients with infiltrating carcinoma (IC) at diagnosis were excluded.
Recurrent disease in patients was defined as a DCIS or IC lesion occurring more than 12 months after surgery. The original haematoxylin‐ and eosin‐stained sections were reviewed by two pathologists responsible for selecting pathological inclusions representative of tumour tissue and for the analysis of clinical pathological features, such as nuclear grade, the presence of comedonecrosis and margin status. The resection margin status was reported as positive when DCIS was present at the inked or cauterized edge of the specimen and negative if there was no DCIS within 2 mm of the inked margin, as recommended by the most recent guidelines endorsed by the Society of Surgical Oncology (SSO), the American Society for Radiation Oncology (ASTRO) and the American Society of Clinical Oncology (ASCO) (Morrow et al. 2016). The final margin status (positive or negative) refers to the resection margin status of the first surgical specimen.
Biomarker determination
Tumour material obtained during surgery was fixed in neutral‐buffered formalin and embedded in paraffin. Four‐micron sections were mounted on positive‐charged slides for each patient (Bio Optica, Milan, Italy). Biomarker determinations were performed according to European Quality Assurance guidelines (Geurts‐Moespot et al. 2000). Immunostaining for conventional biomarkers and AR expression was performed using the Ventana Benchmark XT staining system (Ventana Medical Systems, Tucson, AZ, USA) with Optiview DAB Detection Kit (Ventana Medical Systems). ER, PgR, Ki67 (Leica, Novocastra, Newcastle, UK), HER2 (Dako, Carpinteria, CA, USA) and AR (SP107, Cell Marque, Ventana Medical Systems) antibodies were used. For ER, PgR, Ki67 and HER2 detection, tissue sections were incubated for 60 min with antibody diluted in antibody diluent (1:80, 1:40, 1:100 and 1:350, respectively) with background reducing components (Dako Corporation, Carpinteria). AR antibody prediluted by the supplier was used. Sections were incubated for 16 min and automatically counterstained with haematoxylin II (Ventana Medical Systems).
Biomarker expression was semiquantitatively quantified as the percentage of the immunopositive tumour cells. All samples were evaluated by two independent observers; a disagreement >10% of positive cells for the different markers, observed in about 20% of cases, was resolved by consensus after joint review using a multihead microscope. The conventional biomarkers (ER, PgR, Ki67 and HER2) were classified on the basis of the most recent St. Gallen and ASCO‐CAP guidelines (2013) (Goldhirsch et al. 2013; Wolff et al. 2013). With regard to AR expression, an arbitrary cut‐off was considered because there are no validated guidelines for the analysis of this biomarker. Tissue presenting at least 10% of positive tumour cells was considered positive independently of the staining intensity (weak, moderate, strong). The AR/ER ratio was also determined. We used the AR value to determine the ratio value for ER‐negative cases.
Statistical analysis
Frequency tables were performed for all categorical variables. Continuous variables were presented using median and range. Chi‐square test or Fisher's exact test were used to evaluate the relationship between clinical characteristics considered as categorical quantities and the relapse status, as appropriate. Nonparametric ranking statistics (median test) were used to analyse the relationship between median age, biomarker values and relapse status. The accuracy of single or combined biomarkers, considered as continuous variables to evaluate the differences in diagnostic accuracy, was measured using the area under the curve (AUC). In the receiver operating characteristic (ROC) curves, true positive rates (sensitivity) were plotted against false positive rates (1‐specificity) for all classification points.
Recurrence‐free survival was estimated using Kaplan–Meier method. The prognostic role of biomarkers with regard to the survival endpoints was analysed using Cox proportional hazard models. Given the co‐linearity issues between AR, ER and the AR/ER ratio, separate models were performed. Departures from the proportional hazard assumption were assessed on the basis of Schoenfeld residuals. All P values were based on two‐sided testing, and values lower than 0.05 were considered statistically significant. Statistical analysis was carried out using STATA/MP 14.0 for Windows (Stata Corp LP, College Station, TX, USA).
Ethical approval
The study protocol was reviewed and approved by the Medical Scientific Committee of IRST IRCCS and the Ethical Committees of Area Vasta Romagna, Italy (approval no. 1166). Written informed consent was obtained from patients to use biological material for research purposes.
Results
Forty‐two patients were submitted to surgery and radiotherapy. Eleven patients relapsed between 2 and 7 years after the first diagnosis: six with DCIS, three with IC and two with both DCIS and IC histologies. Of the 42 DCIS, three (7%) were classified as well differentiated tumours (G1), 19 (45%) as moderately differentiated (G2) and 20 (48%) as poorly differentiated tumours (G3) (Table 1). Thirty‐one (74%) were unifocal tumours, and 11 (26%) were multifocal tumours (Table 1). Thirty‐six (86%) lesions showed negative surgical margins, and six (14%) showed positive margins, the extent of invasion ranging from 0.5 to 2 mm. Only four (9.5%) DCIS showed comedonecrosis (Table 1).
Table 1.
Patient characteristics
| Characteristics | Overall (n = 42) | Non‐relapsed (n = 31) | Relapsed (n = 11) | P a |
|---|---|---|---|---|
| Median age, years (range) | 57 (38‐77) | 56 (42‐76) | 57 (38‐77) | 0.8410 |
| No. (%) | No. (%) | No. (%) | ||
| Nuclear grade | ||||
| 1 | 3 (7.1) | 3 (9.6) | 0 (0.0) | 0.8740 |
| 2 | 19 (45.2) | 14 (45.2) | 5 (45.5) | |
| 3 | 20 (47.6) | 14 (45.2) | 6 (54.5) | |
| Radiological presentation | ||||
| Microcalcifications (M) | 37 (88.1) | 28 (90.3) | 9 (81.8) | 0.353 |
| Opacity (O) | 3 (7.1) | 2 (6.5) | 1 (9.1) | |
| O + M | 2 (4.8) | 1 (3.2) | 1 (9.1) | |
| Tumor size (mm) | ||||
| ≤5 | 10 (23.8) | 9 (29.0) | 1 (9.1) | 0.3200 |
| >5 and ≤10 | 10 (23.8) | 9 (29.0) | 1 (9.1) | |
| >10 and ≤20 | 10 (23.8) | 6 (19.4) | 4 (36.4) | |
| >20 and ≤30 | 6 (14.3) | 3 (9.7) | 3 (27.3) | |
| >30 | 6 (14.3) | 4 (12.9) | 2 (18.2) | |
| Comedonecrosis | ||||
| Yes | 4 (9.5) | 2 (6.5) | 2 (18.2) | 0.2560 |
| No | 38 (90.5) | 29 (93.5) | 9 (81.8) | |
| Histological focality | ||||
| Unifocal | 31 (73.8) | 23 (74.2) | 8 (72.7) | 1.0000 |
| Multifocal | 11 (26.2) | 8 (25.8) | 3 (27.3) | |
| Margin status | ||||
| Negative | 36 (85.7) | 27 (87.1) | 9 (81.8) | 0.6220 |
| Positive | 6 (14.3) | 4 (12.9) | 2 (18.2) | |
The P value was obtained by comparing the value of the markers in relapsed patients with that of non‐relapsed patients.
No differences in age, tumour size, nuclear grade, focality (unifocal vs. multifocal), margin status (positive vs. negative) and type of DCIS (comedo vs. non‐comedo) were found between relapsed and non‐relapsed patients (Table 1).
AR and conventional biomarkers were analysed in the entire case series (Figure 1a–e). HER2 immunostaining was not feasible in six patients due to insufficient FFPE material (Figure 1e). The evaluation of ER, PgR, Ki67 and HER2 did not bring to light significant differences between relapsed and non‐relapsed patients (Table 2). Our findings revealed that AR expression (Figure 1a) was significantly higher in relapsed patients than in non‐relapsed patients (P value: 0.0005). Conversely, the expression of oestrogen receptors (ER) (Figure 1b) was higher, albeit not significantly (P value: 0.2342), in non‐relapsed patients than in those who relapsed. Seven patients (four relapsed and three non‐relapsed) were negative for ER expression.
Figure 1.
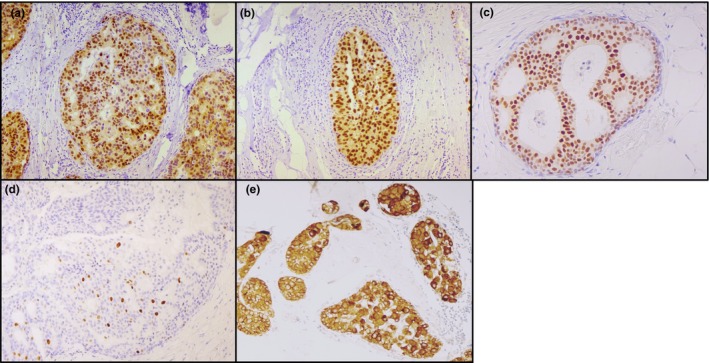
DCIS case positive for (a) AR expression, (b) ER expression, (c) PgR expression, (d) Ki67 expression, (e) HER2 expression. All 10× magnification.
Table 2.
Marker expression in tumour cells of relapsed and non‐relapsed patients
| Markers | Overall (n = 42) bMedian (range) | Non‐relapsed (n = 31) bMedian (range) | Relapsed (n = 11) bMedian (range) | P a |
|---|---|---|---|---|
| AR | 60 (0–100) | 40 (0–95) | 80 (40–100) | 0.0005 |
| ER | 80 (0–98) | 80 (0–98) | 40 (0–95) | 0.2342 |
| PgR | 40 (0–90) | 40 (0–80) | 0 (0–90) | 0.0869 |
| Ki67 | 5 (3–25) | 5 (3–25) | 7.5 (5–20) | 0.6936 |
| AR/ER ratio | 0.82 (0–95) | 0.67 (0–90) | 2.5 (0.44–95) | 0.0033 |
| HER2 staining intensity | Overall (n = 36) No. (%) | Non‐relapsed (n = 25) No. (%) | Relapsed (n = 11) No. (%) | P |
|---|---|---|---|---|
| 0 (absent) | 10 (27.8) | 9 (36.0) | 1 (9.1) | 0.2340 |
| 1+ (weak) | 6 (16.7) | 4 (16.0) | 2 (18.2) | |
| 2+ (moderate) | 8 (22.2) | 6 (24.0) | 2 (18.2) | |
| 3+ (strong) | 12 (33.3) | 6 (24.0) | 6 (54.5) |
The P value was obtained by comparing the value of the markers in relapsed patients with that of non‐relapsed patients.
Median values of the % of immunopositive tumour cells.
AR, androgen receptor; ER, oestrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
The AR/ER ratio value was higher (P value: 0.0033) in relapsed patients than in non‐relapsed patients (Table 2).
For AR/ER ratio, the best cut‐off value of 1.1 showed an 81% accuracy in predicting in situ relapse or progression to invasive carcinoma. Moreover, considering the variables separately, AUC values were 0.85 (95% CI: 0.73–0.97) for AR, 0.62 (95% CI: 0.40–0.84) for ER, 0.70 (95% CI: 0.46–0.93) for PgR and 0.80 (95% CI: 0.65–0.96) for the AR/ER ratio, with no significant difference between AR and the AR/ER ratio (P value: 0.4170; Table 3).
Table 3.
Area under the curve (AUC) values of markers in tumour cells
| Markers | Overall AUC (95% CI) |
|---|---|
| AR | 0.85 (0.73–0.97) |
| ER | 0.62 (0.40–0.84) |
| PgR | 0.70 (0.46–0.93) |
| Ki67 | 0.55 (0.30–0.81) |
| AR/ER ratio | 0.80 (0.65–0.96) |
AUC, area under the curve; AR, androgen receptor; ER, oestrogen receptor; PgR, progesterone receptor.
ROC curve analysis for AR and the AR/ER ratio is shown in Figure 2. In Cox univariate models, an increase in AR and in AR/ER ratio was related to an increased risk of recurrence (5%, P value 0.003, for AR and 2%, P value 0.015, for AR/ER) (Table 4). In addition, multivariate analysis identified AR as an independent prognostic factor showing a hazard ratio (HR) of 1.06 (95% CI: 1.01–1.11; Table 4). The recurrence‐free survival curve showed that 75% of patients were recurrence‐free after 5 years (Figure 3).
Figure 2.
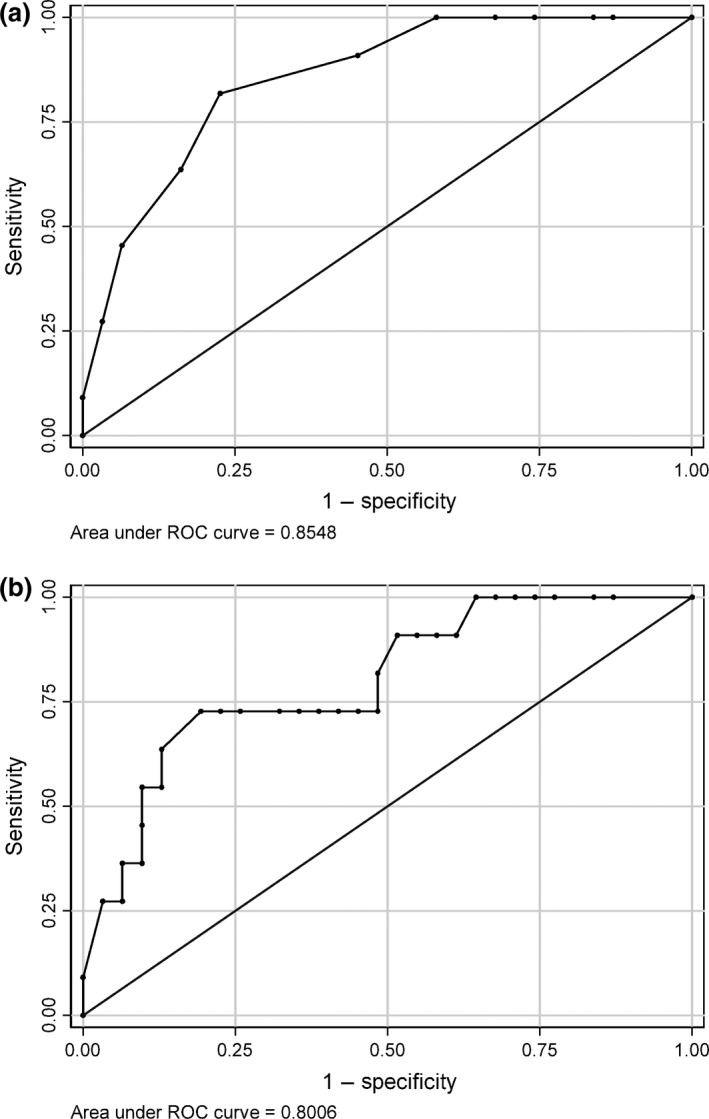
ROC curve for (a) AR and (b) the AR/ER ratio.
Table 4.
Cox regression models
| Variables | Univariate model | Multivariate model | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| AR | 1.05 | 1.02–1.09 | 0.003 | 1.06 | 1.01–1.11 | 0.023 |
| ER | 0.99 | 0.97–1.01 | 0.072 | – | – | – |
| PgR | 0.99 | 0.96–1.01 | 0.205 | – | – | – |
| AR/ER ratio | 1.02 | 1.01–1.03 | 0.015 | 1.00 | 0.98–1.02 | 0.936 |
AR, androgen receptor; ER, oestrogen receptor; PgR, progesterone receptor; HR, hazard ratio; CI, confidence interval.
Figure 3.
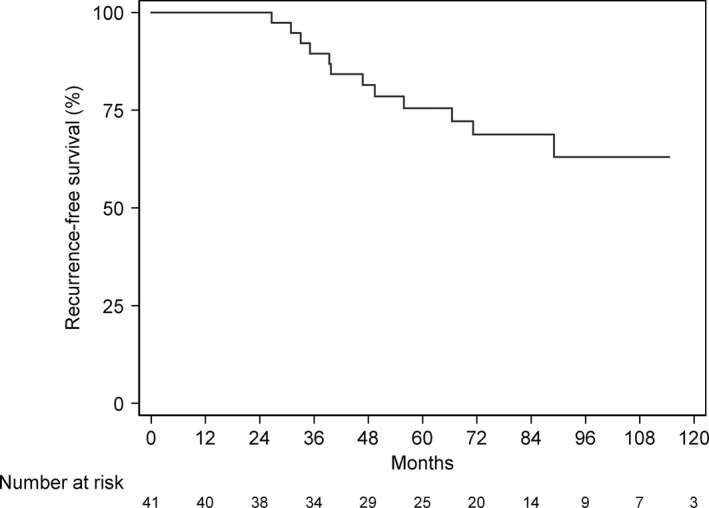
Recurrence‐free survival curve.
Discussion
DCIS is a paradigm of tumour heterogeneity, and only a small number of cases recur or progress into IC. Although clinical pathological features have always been used to establish the type of treatment, their correlation with recurrence has yet to be clarified. For example, positive margin status after surgery for invasive breast cancer tends to increase the rate of recurrence, whereas this has not been proven for DCIS (Wood 2013).
Recent guidelines endorsed by ASCO, ASTRO and SSO suggest that a positive margin, defined as ink on DCIS, is associated with a significant increase in breast tumour recurrence (Morrow et al. 2016). In contrast, Klein et al. (2015) reported that the presence of close or positive margins was not always associated with an increased risk of chest wall recurrence in women with DCIS treated by mastectomy.
In our study, the presence of positive margins did not affect the prognosis of DCIS patients given that re‐excision was performed in almost all cases (5 of 6 patients) and that the histology of the second specimens showed no trace of DCIS. Thus, biomarkers capable of identifying aggressive tumours must be sought.
Limited information is available about AR status in DCIS, and its prognostic and predictive significance in invasive tumours is still very much open to debate (Castellano et al. 2010; Cochrane et al. 2014). A recent study by Cochrane et al. (2014) concluded that an AR/ER ratio ≥2 was an independent predictor of disease‐free and disease‐specific survival. In particular, the authors suggested that a high AR/ER ratio may influence breast cancer response by increasing the risk of tamoxifen failure.
Given that the majority of patients are also treated with adjuvant radiotherapy, the impact of which remains to be defined in DCIS, we decided to focus our study on this specific population. Interesting results were obtained on two hormonal markers. We previously observed a prognostic value for ER which improved when the marker was combined with AR. In particular, the AR/ER ratio was highly accurate in predicting disease recurrence (AUC 0.92), identifying aggressive tumours with a potentially malignant evolution (Tumedei et al. 2015).
Our retrospective pilot study was carried out on a series of DCIS patients characterized by a biological profile including variables such as hormonal receptors and cell proliferation, treated with surgery and radiotherapy and followed up for a maximum of 95 months.
We are aware that the sample size is quite small, but the case series is highly homogeneous and well characterized, with a long‐term follow‐up, and the non‐relapsed patients were matched correctly on the bases of the main clinical pathological features.
Relapse was observed in those whose primary lesions showed high androgen receptor expression. In our study, both AR and the AR/ER ratio showed high accuracy in predicting DCIS relapse. In addition, these markers have the advantage of being determined by immunohistochemistry, a widely used laboratory method that is less expensive than clinical instrumental determinations and multigene expression assays such as Oncotype DX DCIS (Raldow et al. 2016).
These findings are new in this subset of patients and in line with the results that were recently published by our group on a series of patients with biologically similar tumours treated with surgery alone, without radiotherapy (Tumedei et al. 2015). These similar results led us to hypothesize that radiotherapy fails to improve the therapeutic result of surgery by eliminating androgen receptor‐rich tumours and that other tailored therapies based on anti‐androgenic drugs could be considered.
Conclusions
Our preliminary results suggest that both AR and the AR/ER ratio play an important role in identifying DCIS that is likely to relapse as DCIS or progress to IC and could thus provide valuable biological information when planning therapy. Confirmation of these findings in a larger case series would help to establish these biomarkers as a cost‐effective, reproducible and easy‐to‐perform tool to predict DCIS recurrence.
Conflicts of interest
The authors have declared no competing interests.
Funding source
This work was partially funded internally by our institute (project no. L3P12) and by the Italian Ministry of Health project no. GR‐2009‐1594885.
Acknowledgements
The authors would like to thank Veronica Zanoni for linguistic support.
References
- Amadori D., Serra P., Bravaccini S., et al (2014) Differences in biological features of breast cancer between Caucasian (Italian) and African (Tanzanian) populations. Breast Cancer Res. Treat. 145, 177–183. [DOI] [PubMed] [Google Scholar]
- Amichetti M. & Vidali C. (2012) Radiotherapy after conservative surgery in ductal carcinoma in situ of the breast: a review. Int. J. Surg. Oncol. 2012, 635404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano I., Allia E., Accortanzo V., et al (2010) Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res. Treat. 124, 607–617. [DOI] [PubMed] [Google Scholar]
- Cochrane D.R., Bernales S., Jacobsen B.M., et al (2014) Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 16, R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa C., McGale P., Taylor C., et al (2010) Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J. Natl. Cancer Inst. Monographs 41, 162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutuli B., Bernier J. & Poortmans P. (2014) Radiotherapy in DCIS, an underestimated benefit. Radiother. Oncol. 112, 1–8. [DOI] [PubMed] [Google Scholar]
- Geurts‐Moespot J., Leake R., Benraad T.J., et al (2000) Twenty years of experience with the steroid receptor external quality assessment program ‐ the paradigm for tumour biomarker EQA studies. On behalf of the EROTC Receptor and Biomarker Study Group. Int. J. Oncol. 17, 13–22. [DOI] [PubMed] [Google Scholar]
- Globocan (2012) Breast Cancer, Estimated Incidence, Mortality and Prevalence Worldwide in 2012. IARC, Section of Cancer Surveillance. http://globocan.iarc.fr/old/FactSheets/cancers/breast-new.asp [Accessed 25 Nov 2014].
- Goldhirsch A., Winer E.P., Coates A.S., et al (2013) Panel members: personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 24, 2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley K., Wang J., Bourne P., et al (2008) Lack of expression of androgen receptor may play a critical role in transformation from in situ to invasive basal subtype of high‐grade ductal carcinoma of the breast. Hum. Pathol. 39, 386–392. [DOI] [PubMed] [Google Scholar]
- Klein J., Kong I., Paszat L., et al (2015) Close or positive resection margins are not associated with an increased risk of chest wall recurrence in women with DCIS treated by mastectomy: a population‐based analysis. Springerplus 4, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K. (2014) Are the harms of false‐positive screening test results minimal or meaningful? JAMA 174, 961–963. [DOI] [PubMed] [Google Scholar]
- Lagios M.D. & Silverstein M.J. (2014) Risk of recurrence of ductal carcinoma in situ by oncotype DX technology: some concerns. Cancer 120, 1085. [DOI] [PubMed] [Google Scholar]
- Lari S.A. & Kuerer H.M. (2011) Biological markers in DCIS and risk of breast recurrence: a systematic review. J. Cancer 1, 232–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow M., Zee K.J.V., Solin L.J., et al (2016) American society of clinical oncology consensus guideline on margins for breast‐conserving surgery with whole‐breast irradiation in ductal carcinoma in situ . Pract. Radiat. Oncol. 6, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raldow A. C., Sher D., Chen A. B., Recht A., Punglia R. S. (2016) Cost effectiveness of the oncotype dx dcis score for guiding treatment of patients with ductal carcinoma in situ. J. Clin. Oncol. 12, pii: JC0678532. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Rocca A., Bravaccini S., Scarpi E., et al (2014) Benefit from anthracyclines in relation to biological profiles in early breast cancer. Breast Cancer Res. Treat. 144, 307–318. [DOI] [PubMed] [Google Scholar]
- Selim A.G., El‐Ayat G. & Wells C.A. (2002) Androgen receptor expression in ductal carcinoma in situ of the breast: relation to estrogen and progesterone receptors. J. Clin. Pathol. 55, 14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solin L.J., Gray R., Baehner F.L., et al (2013) A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J. Natl Cancer Inst. 105, 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumedei M.M., Silvestrini R., Ravaioli S., et al (2015) Role of androgen and estrogen receptors as prognostic and potential predictive markers of ductal carcinoma in situ of the breast. Int. J. Biol. Markers 30, 425–428. [DOI] [PubMed] [Google Scholar]
- Weedon‐Fekjær H., Romundstad P.R. & Vatten L.J. (2014) Modern mammography screening and breast cancer mortality: population study. BMJ 348, g3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch H.G. & Passow H.J. (2014) Quantifying the benefits and harms of screening mammography. JAMA 174, 448–454. [DOI] [PubMed] [Google Scholar]
- Wolff A.C., Hammond M.E., Hicks D.G., et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline update. J. Clin. Pathol. 31, 3997–4013. [DOI] [PubMed] [Google Scholar]
- Wood W.C. (2013) Close/positive margins after breast‐conserving therapy: additional resection or no resection? Breast 22(Suppl 2), S115–S117. [DOI] [PubMed] [Google Scholar]


