Abstract
Objectives
To describe the diagnostic spectrum, arthritis persistency and clinical outcomes after 2 years in patients with inflammatory arthritis (IA) of less than 16 weeks’ duration.
Methods
Data from the Norwegian Very Early Arthritis Clinic, a 2-year longitudinal observational study of adults with IA of ≤16 weeks’ duration, were used. Exclusion criteria were arthritis due to crystal deposits, trauma, osteoarthritis and septic arthritis. In all patients who had any follow-up information (population A), clinical diagnoses and persistency of arthritis were described. For patients with 2-year follow-up (population B), we also studied other clinical outcomes (disease activity, pain, fatigue, functional disability and health-related quality of life).
Results
In population A (n=1017) median (25th–75th percentile) duration of joint swelling was 35.0 (13.0–66.5) days, mean (SD) age 45.7 (14.8) years, 55.2% were females and 17.8% anticitrullinated protein antibodies positive. The most common final diagnoses were undifferentiated arthritis (UA) (41.7%), rheumatoid arthritis (RA) (24.1%) and reactive arthritis (18.1%). After 2 years, the arthritis had resolved in 59% of the patients. The remaining 41.0% had persistent disease defined by disease modifying antirheumatic drug (DMARD) use (32.1%) or persistent joint swelling without DMARD use (8.9%). In population B (n=669), all clinical outcomes improved significantly (P<0.001). Baseline joint pain and fatigue were similar across diagnoses.
Conclusions
Among 1017 patients with IA of ≤16 weeks’ duration, UA was the most common diagnosis after 2 years, and less than one-fourth were diagnosed with RA. Arthritis resolved without DMARDs in the majority of the patients. All clinical parameters improved significantly over a 2-year course.
Keywords: epidemiology, outcomes research, early rheumatoid arthritis, synovitis
Key messages.
What is already known about this subject?
Early arthritis might represent a large spectrum of diseases including both resolving and persistent outcomes.
What does this study add?
This study is the first to investigate the whole range of diagnostic outcomes in a large unselected cohort of very early inflammatory arthritis.
The arthritis resolved in almost 60% of the patients with very early arthritis and less than one-fourth of the patients were diagnosed with rheumatoid arthritis.
How might this impact on clinical practice?
Our results will contribute to inform patients and healthcare providers about prognosis in very early arthritis, which seems better than in former studies.
Introduction
Recent-onset inflammatory arthritis (IA) may represent a broad range of diseases, from mild self-limiting arthritis to chronic disease associated with substantially reduced function and quality of life. Various early arthritis clinics (EACs) have provided valuable information about the presentation, disease course and outcome of early IA.1 However, the time span used to define the arthritis as ‘early’ is not clearly defined, and different EACs use different time boundaries, mostly less than 1 year, but with a variation between 3 months and up to 3 years.2 3 Most studies select patients with emphasis on identifying rheumatoid arthritis (RA) early and thus include patients with quite long disease duration or exclude patients who present with monoarthritis or with a definitive diagnosis other than RA.4–11 Nevertheless, a large number of patients who present with recent-onset IA have undifferentiated arthritis (UA), and it is recommended to consider all possible causes of arthritis.12
Few studies have examined the full spectrum of diagnostic outcomes in an unselected cohort of patients with very early IA. Arthritis persistency and other clinical measurements, including patient-reported outcomes are also insufficiently studied in this setting. The aim of this study was to describe the diagnostic spectrum, arthritis persistency and other clinical outcomes after 2 years in patients with IA of very short duration (≤16 weeks) in a large unselected multicentre study.
Methods
Study population and data collection
Six rheumatology departments participated to enrol patients in the Norwegian Very Early Arthritis Clinic (NOR-VEAC) study.13 This first phase of the NOR-VEAC study (inclusion 2004–2010) consisted of a 2-year observational prospective unselected cohort of patients (age 18–75 years) with ≥1 swollen joint(s) as assessed by the treating rheumatologist and ≤16 weeks’ patient-reported duration of joint swelling. The rheumatology departments established a dedicated track for receiving patients with early arthritis within 2 weeks, and primary care physicians in the area were trained by their local rheumatology department to recognise arthritis early and asked to refer all patients directly, to minimise doctor’s delay and to ensure inclusion of a large proportion of patients with recent-onset IA.
Exclusion criteria at baseline were established diagnosis of any inflammatory rheumatic disease or recurrent unspecified arthritis during the 6 months preceding the onset of the current episode, as well as if the joint swelling was deemed as due to trauma, mechanical joint lesions, osteoarthritis, crystal arthropathies or septic arthritis. If any of these diagnoses were made during follow-up, the patient was excluded from further follow-up.
Patients not attending prescheduled study visits were contacted by a study nurse by telephone. If a patient wished to withdraw from the study follow-up, the reason for this decision was recorded, as well as if the patient still had swollen joints. All patients were treated according to good clinical practice, and a specific treatment protocol was not included. All patients signed an informed consent.
Patient data were collected by rheumatologists and trained study nurses, and the patients reported health status by questionnaires at baseline and then after 3, 6, 12 and 24 months. Registration included age, sex, weight, height, level of education, smoking and coffee drinking habits and patient-reported duration of joint swelling. PROs were measured with standardised questionnaires including joint pain, fatigue and patient’s global assessment on visual analogue scales (VAS), the Norwegian versions of the Health Assessment Questionnaire Disability Index (HAQ-DI)14 and Medical Outcomes Study Short Form-36 (SF-36).15 16 The assessor performed 68 swollen joint counts (SJCs) (including hip joints if the rheumatologist suspected arthritis) and 28 tender joint counts (TJCs) and reported assessor’s global assessment on a VAS, as well as treatment (disease modifying antirheumatic drug (DMARD) use, systemic and intra-articular glucocorticoids, as well as other medication). Erythrocyte sedimentation rate (ESR) and C reactive protein (CRP) levels were determined at the local laboratories. No standard diagnostic procedures were required, but anticitrullinated protein antibodies (ACPA), rheumatoid factor (RF), HLA-B27, serum uric acid, joint fluid analyses, microbial tests or ultrasound were all performed at the discretion of the treating rheumatologist as clinically indicated. However, ACPA, assessed by the anticyclic citrullinated peptide 2 test (Inova Diagnostics, San Diego, California, USA (886 patients), and Phadia, Freiburg, Germany (113 patients)), as well as immunoglobulin M and immunoglobulin A RF (inhouse ELISA) were analysed post hoc for research purposes from stored biobank sera.
The final clinical diagnoses were made by the treating rheumatologist and coded according to the WHO International Classification of Diseases, 10th revision, based on clinical judgement and classification criteria available at the time. Fulfilment of any criteria was not mandatory to make a diagnosis.
To report the diagnostic spectrum of IA and persistency versus resolution of arthritis (main outcomes), we included all patients with any follow-up information (population A), whereas only patients with a visit at 2 years (population B) were included in the analyses of other clinical outcomes.
Outcome measures
The final clinical diagnoses made by the treating rheumatologist were used to study the spectrum of recent-onset IA. Persistency of arthritis was defined as DMARD use and/or joint swelling at last contact. The definition includes patients with persistent joint swelling despite DMARD, patients with persistent joint swelling and no DMARD and patients in remission on DMARD. Resolution of arthritis was defined as no joint swelling at last contact and no DMARD use. Patients with temporary DMARD use were classified as no-DMARD users if they had used DMARDs for ≤4 weeks or were observed for ≥1 year after DMARD cessation. Any persistent glucocorticoid use among the no-DMARD users was recorded. If a patient dropped out of the study before 2 years, the last outcome information was used in a last observation carried forward manner for the main outcomes. This approach was chosen to include all diagnostic outcomes and to prevent bias towards patients with persistent arthritis, as the main reason for leaving the study early was no joint symptoms (population A).
We studied several outcome measures at baseline and after 2 years (population B). HAQ-DI includes 20 questions and assesses functional disability on a score from 0 (no disability) to 3 (completely disabled). SF-36 is a generic questionnaire of health-related quality of life with 36 questions, which can be presented as physical and mental component summary scores (PCS and MCS, respectively), both ranging from 0% (lowest or worst possible level of functioning) to 100% (highest or best possible level of functioning). Fatigue, joint pain and patient’s and assessor’s global assessment are presented as millimetre on a VAS from 0 mm to 100 mm (100 worst health). Disease Activity Score 28 (DAS28) is a composite disease activity measure, developed for RA, combining 28 TJCs, 28 SJCs, ESR and patient’s global assessment.
Statistical analyses
Descriptive methods were applied to describe the whole range of diagnostic outcomes, as well as persistency versus resolution of arthritis and other outcomes. For continuous measures, means with SD were calculated for variables that were normally distributed and medians with 25th and 75th percentiles for variables that were not. For categorical variables, numbers and valid percentages were used. χ2 test was used to compare persistency of arthritis between groups. Paired samples t-test was used to compare disease characteristics at baseline and after 2 years. One-way analysis of variance was used to analyse whether clinical measures differed between the diagnostic groups. IBM SPSS Statistics V.23 was used for the statistical analyses.
Results
Patient characteristics
One thousand and seventeen patients (population A) were included in the current analyses, of whom 669 (65.8%) had a 2-year follow-up visit (population B) (figure 1). Furthermore, 73 patients had information from a phone call at 2 years. The reasons for not completing the study are listed in figure 1. In population A, duration of joint swelling before inclusion (median (25th–75th percentile)) was 35.0 (13.0–66.5) days, mean (SD) age 45.7 (14.8) years, 55.2% were females and 23.0% ACPA and/or RF positive. Presentation as monoarthritis, oligoarthritis (2–4 swollen joints) and polyarthritis (≥5 swollen joints) had approximately the same frequency, 30.8%, 36.4% and 32.8%, respectively. The completers (population B) were more often women (58.9%), ACPA and/or RF positive (28.2%) and had longer median duration of joint swelling (41 (18–72) days). Additional baseline characteristics for populations A and B are reported in table 1.
Figure 1.
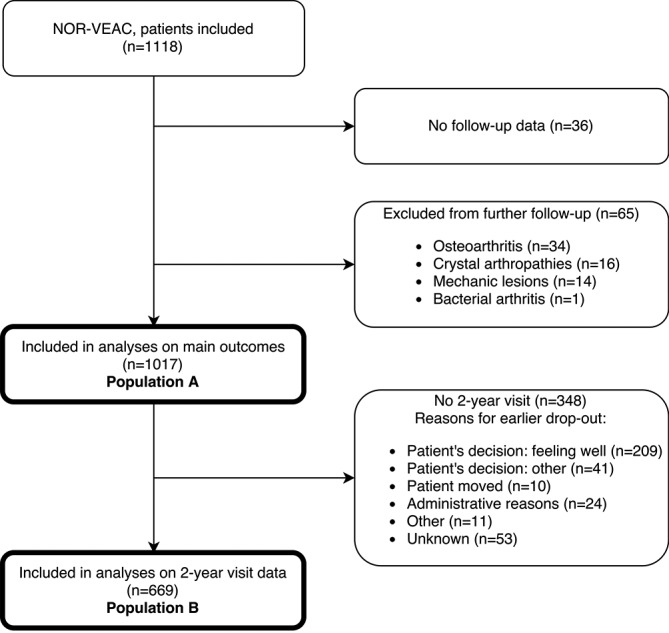
Patient selection and reasons for loss to follow-up. NOR-VEAC, Norwegian Very Early Arthritis Clinic.
Table 1.
Demographics and disease characteristics at baseline
| Population A n=1017 |
Population B n=669 |
|
| Demographics | ||
| Age, years | 45.7±14.8 | 47.9±14.5 |
| Females, n (%) | 561 (55.2) | 394 (58.9) |
| BMI, kg/m2 | 25.6±4.3 | 25.8±4.2 |
| Current daily smoker, n (%) | 292 (29.0) | 202 (30.3) |
| Ever smoker, n (%) | 613 (60.8) | 419 (62.9) |
| Coffee ≥5 cups/day, n (%) | 247 (24.6) | 169 (25.5) |
| Education, college/university, n (%) | 451 (44.5) | 287 (42.9) |
| Disease characteristics | ||
| Duration of joint swelling in days | 35.0 (13.0–66.5) | 41.0 (18.0–72.0) |
| ACPA positive, n (%) | 178 (17.8) | 148 (22.3) |
| RF positive, n (%) | 177 (17.7) | 153 (23.0) |
| ACPA and/or RF positive, n (%) | 230 (23.0) | 187 (28.2) |
| ESR, mm | 24.0 (12.0–47.0) | 26.0 (13.0–47.0) |
| CRP, mg/L | 16.0 (5.0–45.0) | 15.7 (5.0–43.0) |
| Joint pain VAS, mm | 53.0 (30.3–71.0) | 54.0 (34.0–72.0) |
| Fatigue VAS, mm | 41.5 (10.0–67.0) | 45.0 (12.0–67.0) |
| Morning stiffness >1 hour, n (%) | 508 (56.5) | 349 (59.0) |
| Patient’s global VAS, mm | 53.0 (35.0–70.0) | 55.0 (37.0–73.0) |
| Assessor’s global VAS, mm | 34.0 (22.0–50.0) | 36.0 (23.0–52.0) |
| SF-36, physical components summary score | 32.8 (25.5–39.8) | 32.1 (24.8–39.0) |
| SF-36, mental components summary score | 49.5 (40.2–57.2) | 48.9 (40.4–56.9) |
| HAQ-DI | 0.75 (0.25–1.25) | 0.88 (0.38–1.38) |
| 28 Tender joint count | 2.0 (1.0–4.0) | 2 (1.0–5.0) |
| 68 Swollen joint count | 2.0 (1.0–6.0) | 3 (1.0–7.0) |
| DAS28 | 4.1 (3.2–5.0) | 4.3 (3.4–5.1) |
Continuous data are presented as median (25th–75th percentile) or mean±SD, counts as numbers and valid percentages.
ACPA, anticitrullinated protein antibodies; BMI, body mass index; CRP, C reactive protein; DAS28, Disease Activity Score 28; ESR, erythrocyte sedimentation rate; HAQ-DI, Health Assessment Questionnaire Disability Index; population A, patients with any follow-up time; population B, patients with data at the 2-year follow-up visit; RF, rheumatoid factor; SF-36, Short-Form 36; VAS, visual analogue scale.
Disease spectrum and persistency of arthritis
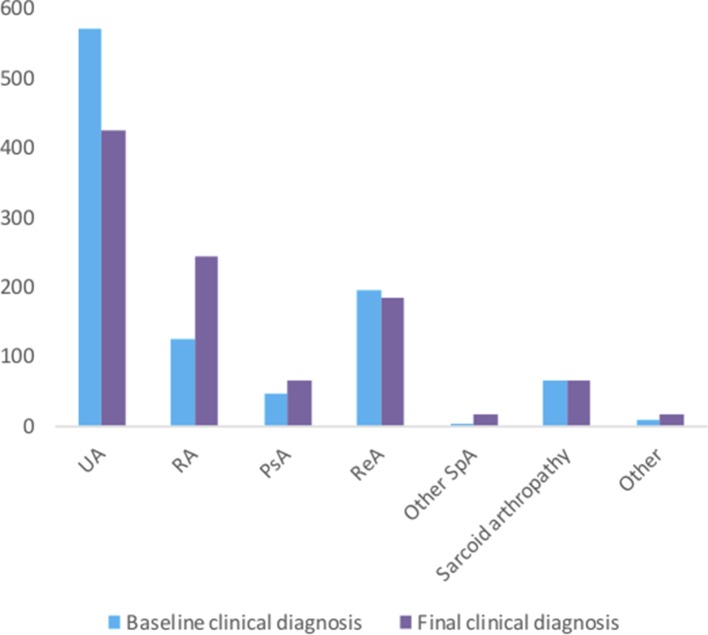
At baseline, 55.9% of the patients (n=569) were classified as having UA. During follow-up, this was reduced to 41.7% (n=424), but UA was still the most common diagnosis at the last follow-up. Other common final clinical diagnoses were RA (24.1% (n=245)), reactive arthritis (18.1% (n=184)), psoriatic arthritis (6.4% (n=65)) and sarcoid arthropathy/Löfgren’s syndrome (6.6% (n=67)) (figure 2).
Figure 2.
Distribution of baseline and final clinical diagnoses. PsA, psoriatic arthritis; RA, rheumatoid arthritis; ReA, reactive arthritis; other SpA, other spondyloarthritides: ankylosing spondylitis (n=7), axial spondyloarthritis (n=4), inflammatory bowel disease-associated arthrtitis (n=5); other: Sjögren syndrome (n=2), systemic lupus erythematosus (n=1), polymyalgia rheumatic (n=4), polyarteritis nodosa (n=1), autoimmune hepatitis (n=1), Lyme disease (n=1), paramalign arthritis (n=1), multiple myeloma (n=1), enthesopathy (n=2), remitting seronegative symmetrical synovitis with pitting oedema syndrome (n=1) and not specified (n=1); UA, undifferentiated arthritis.
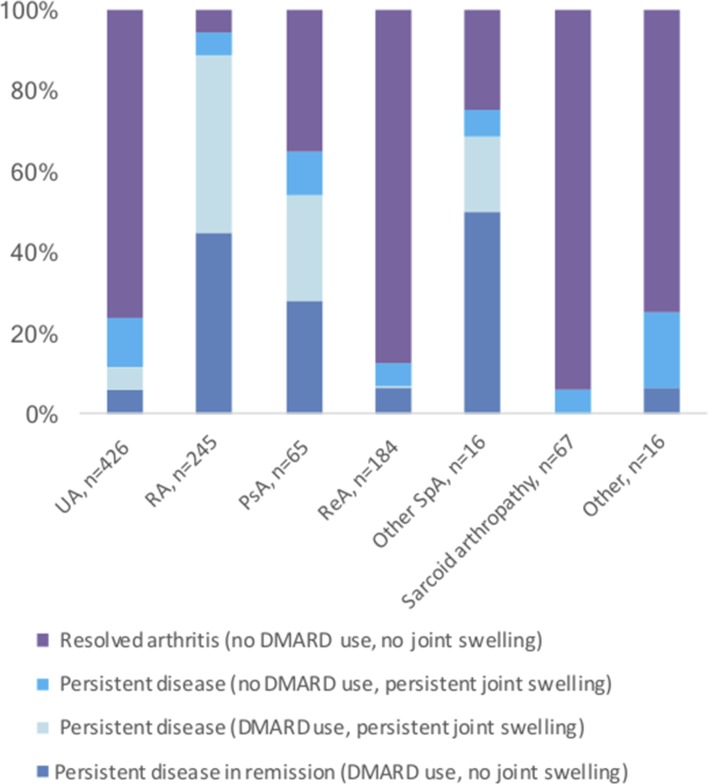
After 2 years, 417 patients (41.0%) had persistent disease: 326 (32.1%) defined by DMARD use (175 (17.2%) in remission) and 91 (8.9%) by persistent joint swelling without DMARD use. The arthritis resolved without DMARDs in the remaining 600 (59.0%) patients. In this group with resolution of arthritis, 21 (3.5%) had temporary DMARD use (median (25th–75th percentile) duration 101 (69.5–292.0) days), 348 (58.0%) had received non-steroidal anti-inflammatory drugs, 254 (42.3%) intra-articular and 205 (34.2%) systemic glucocorticoids, including 21 (3.5%) patients who were still on systemic glucocorticoids at last contact. The outcomes according to final clinical diagnoses are shown in figure 3. A final clinical diagnosis of sarcoid arthropathy, reactive arthritis and UA carried the best prognoses, with resolution of arthritis without DMARDs in 94.0%, 87.5% and 76.2%, respectively.
Figure 3.
Outcome according to final clinical diagnosis. DMARD, disease-modifying antirheumatic drug; PsA, psoriatic arthritis; RA, rheumatoid arthritis; ReA, reactive arthritis; SpA, spondyloarthritis; UA, undifferentiated arthritis.
Nevertheless, among patients with chronic inflammatory joint diseases, such as psoriatic arthritis, other spondyloarthritides and RA, some of the patients also experienced resolution of arthritis, 35.3%, 25.0% and 5.7%, respectively. Patients presenting with polyarthritis developed persistent disease more often than patients with oligoarthritis or monoarthritis (65.6%, 33.2% and 24.0%, respectively) (P<0.001).
Clinical outcomes after 2 years
Among the 669 patients with a 2-year visit (population B), 242 (36.2%) had a final clinical diagnosis of UA, 217 (32.4%) had RA, 55 (8.2%) had psoriatic arthritis, 97 (14.5%) had reactive arthritis, 37 (5.5%) had sarcoid arthropathy, 14 (2.1%) had other spondyloarthritides and 7 (1.0%) had other diagnoses. Characteristics of population B at baseline and after 2 years are shown in tables 1 and 2, respectively. Baseline joint pain, fatigue and patient’s global assessment were similar across all diagnostic groups (P=0.147, P=0.677 and P=0.116, respectively), whereas HAQ-DI, SF-36 PCS and SF-36 MCS, assessor’s global VAS as well as all disease activity measures (DAS28, SJC, TJC, ESR and CRP) varied between groups (P≤0.035). At 2 years, there were significant differences between the diagnostic groups in all of the above variables except SF-36 MCS. For all clinical parameters, there was a significant overall improvement (P<0.001) after 2 years.
Table 2.
Disease characteristics at 2 years
| Population B n=699 |
|
| ESR, mm | 9.0 (5.0–16.0) |
| CRP, mg/L | 2.0 (1.0–6.0) |
| Joint pain VAS, mm | 14.0 (2.0–34) |
| Fatigue VAS, mm | 22.0 (2.0–52.0) |
| Morning stiffness >1 hour, n (%) | 105 (20.5) |
| Patient’s global VAS, mm | 14.0 (2.0–34.0) |
| Assessor’s global VAS, mm | 4.0 (1.0–12.0) |
| SF-36, physical components summary score | 45.3 (34.9–53.4) |
| SF-36, mental components summary score | 53.1 (44.4–57.7) |
| HAQ-DI | 0.13 (0.00–0.63) |
| 28 Tender joint count | 0.0 (0.0–1.0) |
| 68 Swollen joint count | 0.0 (0.0–1.0) |
| DAS28 | 2.0 (1.4–2.8) |
Continuous data are presented as median (25th–75th percentile), counts as numbers and valid percentages. CRP, C reactive protein; DAS28, Disease Activity Score 28; ESR, erythrocyte sedimentation rate; HAQ-DI, Health Assessment Questionnaire Disability Index; population B, patients with 2-year follow-up time; SF-36, Short Form-36; VAS, visual analogue scale.
Discussion
This is, as far as we know, the first study to describe the whole range of diagnostic outcomes in a large unselected cohort of very early IA. More than 40% of the patients in NOR-VEAC ended up with a clinical diagnosis of UA, whereas 32.1% were diagnosed with chronic inflammatory joint disease (among which 24.1% with RA). These findings are in accordance with a Swedish incidence study of inflammatory joint diseases, pointing at a high incidence of non-RA IA.17 In contrast, other EACs normally have larger focus on RA in their selection criteria, with higher proportion of RA as a consequence.18 Our findings underline the importance of careful consideration of all differential diagnoses in unselected patients with IA, especially before application of the 2010 American College of Rheumatology/European League Against Rheumatism (EULAR) classification criteria for RA.19
The arthritis resolved without DMARDs in the majority of our patients (59%). This proportion is somewhat higher than other EACs, which have reported a resolution of arthritis in less than 40% of the patients.2 4 11 Early identification of arthritis, prompt referral of all patients by general practitioners, wide diagnostic inclusion criteria and a mandatory requirement of ≤16 weeks’ duration for inclusion are the most probable reasons for this difference. Nevertheless, a large diversity in how to define resolution of arthritis/self-limiting disease/sustained remission is also a major issue that makes comparison difficult.4 20–23
We found significant improvement of all clinical parameters after 2 years. Our study further demonstrated that joint pain, fatigue and patient’s global assessment did not distinguish between patients with different IA diagnoses at baseline.
In this real-life observational study, the use of diagnostic tools, classification criteria and treatment guidelines were left to the discretion of the rheumatologist. This might be a limitation when it comes to comparing the results with other EAC studies. Another limitation of our study is the loss to follow-up. Persistency versus resolution of arthritis was defined by only one time-point. This approach was chosen because we wanted the analyses to also include patients who discontinued the study because they were feeling well. Recurrence of arthritis in some patients classified as having resolved arthritis, and resolution of arthritis in some patients classified as having persistent arthritis, cannot be excluded.
Most of the published data that the 2016 update of the EULAR recommendations for the management of early arthritis were based on involved studies in patients with early RA, rather than specific studies of early arthritis.18 24 Moreover, the term ‘early’ was not defined in the inclusion criteria for the systematic reviews informing this update.18 25 Other important early arthritis cohorts may not be representative for patients with very early arthritis, as patients with the shortest disease duration are not included,5 6 9 10 the mean disease duration is long4 7 11 or disease onset is defined as observed by the rheumatologist.8 The very short duration of joint swelling in NOR-VEAC is one of the major strengths of our study. With the established need for early referral and treatment, ideally within 3 months of symptom onset,18 it is essential to study such patients. NOR-VEAC is also among the largest cohorts of early arthritis, and it has a multicentre design.
In summary, among patients with IA of ≤16 weeks’ duration, UA was the most common diagnosis after 2 years, 24.1% were diagnosed with RA and 6.4% with psoriatic arthritis. The large proportion of non-RA IA underlines the importance to consider wide diagnostic possibilities in very early arthritis. The arthritis resolved without DMARDs in the majority of the patients. We believe our results will contribute to inform patients with early arthritis about their prognosis, which seems better than in former studies.
Acknowledgments
We would like to thank all the participating patients, nurses, laboratory workers and clinicians in the NOR-VEAC study group who made the study possible.
Footnotes
Contributors: ESN, GHB, TKK, EL and MDM were involved in study design, interpretation of the data and drafting of the work. ESN, GHB, TKK, OB, AJH, HN, CT and MDM contributed to acquisition of the data. ESN conducted statistical analyses. All authors revised the manuscript critically and approved the final version of the manuscript.
Funding: This work was supported by The Norwegian Extra Foundation for Health and Rehabilitation through EXTRA funds and the Norwegian Rheumatism Association, as well as the South-Eastern Norway Regional Health Authority.
Competing interests: TKK has received fees for speaking and/or consulting from AbbVie, Biogen, BMS, Boehringer Ingelheim, Celltrion, Eli Lilly, Epirus, Janssen, Merck-Serono, MSD, Mundipharma, Novartis, Oktal, Orion Pharma, Hospira/Pfizer, Roche, Sandoz and UCB.
Patient consent: Obtained.
Ethics approval: Regional Committee for Medical and Health Research Ethics (REC).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Hazes JM, Luime JJ. The epidemiology of early inflammatory arthritis. Nat Rev Rheumatol 2011;7:381–90. doi:10.1038/nrrheum.2011.78 [DOI] [PubMed] [Google Scholar]
- 2.Cader MZ, Filer A, Hazlehurst J, et al. . Performance of the 2010 ACR/EULAR criteria for rheumatoid arthritis: comparison with 1987 ACR criteria in a very early synovitis cohort. Ann Rheum Dis 2011;70:949–55. doi:10.1136/ard.2010.143560 [DOI] [PubMed] [Google Scholar]
- 3.Jansen LM, van Schaardenburg D, van der Horst-Bruinsma IE, et al. . One year outcome of undifferentiated polyarthritis. Ann Rheum Dis 2002;61:700–3. doi:10.1136/ard.61.8.700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Rooy DP, van der Linden MP, Knevel R, et al. . Predicting arthritis outcomes-what can be learned from the Leiden early arthritis clinic? Rheumatology 2011;50:93–100. doi:10.1093/rheumatology/keq230 [DOI] [PubMed] [Google Scholar]
- 5.Combe B, Benessiano J, Berenbaum F, et al. . The ESPOIR cohort: a ten-year follow-up of early arthritis in France: methodology and baseline characteristics of the 813 included patients. Joint Bone Spine 2007;74:440–5. doi:10.1016/j.jbspin.2007.06.001 [DOI] [PubMed] [Google Scholar]
- 6.Symmons DP, Silman AJ. The Norfolk Arthritis Register (NOAR). Clin Exp Rheumatol 2003;21:S94–9. [PubMed] [Google Scholar]
- 7.Marcos J, Waimann C, Dal Pra F, et al. . General characteristics of an early arthritis cohort in Argentina. Rheumatology 2011;50:110–6. doi:10.1093/rheumatology/keq220 [DOI] [PubMed] [Google Scholar]
- 8.de Hair MJ, Lehmann KA, van de Sande MG, et al. . The clinical picture of rheumatoid arthritis according to the 2010 American College of Rheumatology/European League Against Rheumatism criteria: is this still the same disease? Arthritis Rheum 2012;64:389–93. doi:10.1002/art.33348 [DOI] [PubMed] [Google Scholar]
- 9.Bykerk VP, Jamal S, Boire G, et al. . The Canadian Early Arthritis Cohort (CATCH): patients with new-onset synovitis meeting the 2010 ACR/EULAR classification criteria but not the 1987 ACR classification criteria present with less severe disease activity. J Rheumatol 2012;39:2071–80. doi:10.3899/jrheum.120029 [DOI] [PubMed] [Google Scholar]
- 10.Reneses S, Pestana L, Fernandez-Suarez A, et al. . A recent onset inflammatory polyarthritis register in Spain: factors that predict remission. Scand J Rheumatol 2007;36:378–85. doi:10.1080/03009740701286748 [DOI] [PubMed] [Google Scholar]
- 11.Quinn MA, Green MJ, Marzo-Ortega H, et al. . Prognostic factors in a large cohort of patients with early undifferentiated inflammatory arthritis after application of a structured management protocol - Quinn - 2003 - Arthritis & Rheumatism - Wiley Online Library. Arthritis Rheum 2003;48:3039–45. doi:10.1002/art.11269 [DOI] [PubMed] [Google Scholar]
- 12.Machado P, Castrejon I, Katchamart W, et al. . Multinational evidence-based recommendations on how to investigate and follow-up undifferentiated peripheral inflammatory arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis 2011;70:15–24. doi:10.1136/ard.2010.130625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mjaavatten MD, Haugen AJ, Helgetveit K, et al. . Pattern of joint involvement and other disease characteristics in 634 patients with arthritis of less than 16 weeks' duration. J Rheumatol 2009;36:1401–6. doi:10.3899/jrheum.081217 [DOI] [PubMed] [Google Scholar]
- 14.Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: dimensions and practical applications. Health Qual Life Outcomes 2003;1:20 doi:10.1186/1477-7525-1-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loge JH, Kaasa S, Hjermstad MJ, et al. . Translation and performance of the Norwegian SF-36 Health Survey in patients with rheumatoid arthritis. I. Data quality, scaling assumptions, reliability, and construct validity. J Clin Epidemiol 1998;51:1069–76. doi:10.1016/S0895-4356(98)00098-5 [DOI] [PubMed] [Google Scholar]
- 16.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 17.Söderlin MK, Börjesson O, Kautiainen H, et al. . Annual incidence of inflammatory joint diseases in a population based study in southern Sweden. Ann Rheum Dis 2002;61:911–5. doi:10.1136/ard.61.10.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hua C, Daien CI, Combe B, et al. . Diagnosis, prognosis and classification of early arthritis: results of a systematic review informing the 2016 update of the EULAR recommendations for the management of early arthritis. RMD Open 2017;3:e000406 doi:10.1136/rmdopen-2016-000406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aletaha D, Neogi T, Silman AJ, et al. . 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;2010:1580–8. doi:/10.1136/ard.2010.138461 [DOI] [PubMed] [Google Scholar]
- 20.Harrison BJ, Symmons DP, Brennan P, et al. . Natural remission in inflammatory polyarthritis: issues of definition and prediction. Br J Rheumatol 1996;35:1096–100. doi:10.1093/rheumatology/35.11.1096 [DOI] [PubMed] [Google Scholar]
- 21.Biliavska I, Stamm TA, Martinez-Avila J, et al. . Application of the 2010 ACR/EULAR classification criteria in patients with very early inflammatory arthritis: analysis of sensitivity, specificity and predictive values in the SAVE study cohort. Ann Rheum Dis 2013;72:1335–41. doi:10.1136/annrheumdis-2012-201909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yiannopoulos G, Daoussis D, Melissaropoulos K, et al. . Evolution of undifferentiated arthritis: a ten-year experience from the early arthritis clinic of a tertiary care hospital. Clin Exp Rheumatol 2015;33:341–6. [PubMed] [Google Scholar]
- 23.Visser H, le Cessie S, Vos K, et al. . How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis Rheum 2002;46:357–65. doi:10.1002/art.10117 [DOI] [PubMed] [Google Scholar]
- 24.Combe B, Landewe R, Daien CI, et al. . 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis 2016. doi:10.1136/annrheumdis-2016-210602 [DOI] [PubMed] [Google Scholar]
- 25.Daien CI, Hua C, Combe B, et al. . Non-pharmacological and pharmacological interventions in patients with early arthritis: a systematic literature review informing the 2016 update of EULAR recommendations for the management of early arthritis. RMD Open 2017;3:e000404 doi:10.1136/rmdopen-2016-000404 [DOI] [PMC free article] [PubMed] [Google Scholar]