Abstract
Objective
To evaluate the efficacy and safety of ixekizumab alone or with concomitant conventional disease-modifying antirheumatic drugs (cDMARDs) versus placebo in patients with active psoriatic arthritis (PsA) as part of a SPIRIT-P1 subgroup analysis (NCT01695239).
Methods
Patients were stratified by cDMARD use (concomitant cDMARDs use (including methotrexate) or none (past or naïve use)) and randomly assigned to treatment groups (ixekizumab 80 mg every 4 weeks (IXEQ4W) or every 2 weeks (IXEQ2W) or placebo). Efficacy was evaluated versus placebo at week 24 by the American College of Rheumatology criteria (ACR20/50/70), modified total Sharp score and Health Assessment Questionnaire-Disability Index (HAQ-DI). Safety was assessed according to cDMARD status.
Results
Regardless of concomitant cDMARD usage, ACR20, ACR50 and ACR70 response rates were significantly higher versus placebo with IXEQ4W and IXEQ2W. The proportion of patients achieving HAQ-DI minimal clinically important difference was significantly higher versus placebo with IXEQ4W with concomitant cDMARD use and IXEQ2W, regardless of concomitant cDMARD use. Treatment-emergent adverse events (AE) were more frequent versus placebo for either ixekizumab-dosing regimen, regardless of concomitant cDMARD use. Serious AEs were not higher versus placebo, regardless of concomitant cDMARD use.
Conclusion
Ixekizumab treatment improved measures of disease activity and physical function in patients with active PsA relative to placebo, when used with or without concomitant cDMARD therapy.
Keywords: psoriatic arthritis, dmards (synthetic), methotrexate, dmards (biologic)
Key messages.
What is already known about this subject?
Patients with psoriatic arthritis are clinically managed with biologic agents and concomitant conventional disease-modifying antirheumatic drugs (cDMARDs).
Limited evidence exists demonstrating the efficacy of biologic agents when used alone or with concomitant cDMARDs, and there is limited treatment guidance currently available regarding the use of combination therapy.
What does this study add?
This subset analysis of SPIRIT-P1 demonstrated that ixekizumab, a high-affinity monoclonal antibody that selectively targets interleukin (IL)-17A, improved measures of disease activity and physical function in patients with active psoriatic arthritis at 24 weeks relative to placebo, regardless of concomitant cDMARD use.
How might this impact on clinical practice?
The findings from this study offer clinicians additional insights on the utility of employing an anti-IL-17A targeting agent, as represented by ixekizumab, either alone or with concomitant cDMARD use, including methotrexate, in biologic-naïve patients with active psoriatic arthritis.
The evidence provided herein has clinical relevance as the therapeutic options for the treatment of psoriatic arthritis continue to expand and treatment guidelines evolve.
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory arthritis that is often associated with psoriasis and other extra-articular manifestations including joint inflammation (peripheral and/or axial) and structural joint damage.1 Quality of life in patients with PsA is poor and worse than the quality of life in patients with psoriasis alone.2 Abundant evidence indicates that the proinflammatory cytokine interleukin (IL)-17A promotes joint inflammation and damage in PsA.1 Ixekizumab is a high-affinity monoclonal antibody that selectively targets IL-17A and has been demonstrated to improve PsA disease activity and physical function, and inhibit structural progression of joint damage in biologic-naïve patients with active PsA.3 4
Treatment of patients with PsA with biologics with concomitant conventional disease-modifying antirheumatic drugs (cDMARDs) is common, but no specific recommendations have been released by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) due to insufficient evidence.5 Prior studies of tumour necrosis factor inhibitors (TNFi)6–14 and an IL-17A inhibitor15 16 indicate that clinical efficacy is achieved with biologics alone or biologics with concomitant methotrexate (MTX), a commonly used cDMARD. In addition, PsA registry studies report minimal or no additional improvement in patients with PsA who have been treated with TNFis and concomitant cDMARDs versus TNFis alone.17–20 In the SPIRIT-P1 study, 64% of patients used cDMARDs at baseline.3 Here, we evaluated the efficacy and safety of ixekizumab relative to placebo when used alone or with concomitant cDMARDs during the double-blind treatment period of the SPIRIT-P1 study.
Materials and methods
Study design
We analysed efficacy, physical function and safety data from the 24-week double-blind, active and placebo-controlled period of the SPIRIT-P1 trial of ixekizumab.3 At study initiation, patients were randomised, and received either ixekizumab 80 mg every 4 weeks (IXEQ4W), ixekizumab 80 mg every 2 weeks (IXEQ2W), adalimumab 40 mg every 2 weeks (ADA) or placebo, all administered subcutaneously. ADA was the active reference arm, but ADA results were not included in this report.3 In ixekizumab treatment groups, patients received an initial 160 mg dose of ixekizumab. Patients were stratified by cDMARD usage (current use (must have been treated for at least 12 weeks prior to baseline and on a stable dosage for at least 8 weeks), past use (no current cDMARD use, with past use occurring more than 8 weeks prior to study baseline) or naïve to cDMARDs (no past use of cDMARDs)) prior to randomisation. Patients who were taking concomitant medications, including cDMARDs, were required to remain on the same background treatment regimen, unless required to stop, modify or change concomitant medication for safety reasons, through the 24-week double-blind treatment period, or if they were designated an inadequate responder and required rescue therapy at week 16.
Participants
Enrolled patients were biologic-naïve and fulfilled the Classification Criteria for Psoriatic Arthritis21; had ≥3 of 68 tender joints and ≥3 of 66 swollen joints; had either ≥1 PsA-related hand or foot erosion on centrally read X-rays or C reactive protein >6 mg/L; and active or documented personal history of plaque psoriasis. All patients provided written informed consent. The study was registered as SPIRIT-P1 on ClinicalTrials.gov (NCT01695239).
Efficacy, physical function and safety assessments
PsA disease activity was measured by the proportion of patients achieving the American College of Rheumatology (ACR) 20, ACR50 and ACR70 responses at week 2422; change from baseline in van der Heijde modified total Sharp score (mTSS) at week 2423; change from baseline in the Health Assessment Questionnaire-Disability Index (HAQ-DI) at week 24; and also by calculation of the proportion of patients achieving minimal clinically important difference (MCID) in the HAQ-DI.24 25 Safety was assessed by the proportion of patients experiencing treatment-emergent adverse events (TEAE), serious adverse events (AE) and discontinuations due to AE.
Statistical analyses
Efficacy analyses were conducted on the intent-to-treat population, defined as all randomly assigned patients and on subgroups defined by concomitant cDMARD (MTX, ciclosporin, leflunomide or sulfasalazine) or MTX usage. Analysis subgroups were defined as those receiving ixekizumab with concomitant cDMARDs, ixekizumab with concomitant MTX or ixekizumab alone (either cDMARD-naïve or past use). Subgroup analyses of ACR20 response rate and mTSS assessments in patients subdivided by concomitant cDMARD use were prespecified analyses, while all other analyses presented here were post-hoc analyses. Safety analyses were conducted on the safety population, defined as all randomly assigned patients who received at least one dose of study medication, and grouped by the defined analysis subgroups. Treatment by subgroup interaction was tested at the significance level of 0.10 using either a logistic regression model for categorical data or an analysis of covariance (ANCOVA) model for continuous data, as appropriate. Within each subgroup, Fisher’s exact test was used for treatment comparisons of categorical endpoints and AE data, and an ANCOVA model was used for comparisons of mTSS and HAQ-DI data. All comparisons were relative to the placebo group. Missing values were imputed by non-responder imputation for categorical endpoints, linear extrapolation for mTSS change from baseline and last observation carried forward for HAQ-DI data.
Results
The SPIRIT-P1 patient population was previously described by Mease and colleagues.3 In the patient population treated with concomitant cDMARDs at baseline, 83.8%–85.5% of patients were treated with MTX. Patient demographics and characteristics were similar across subgroups (table 1).
Table 1.
Baseline demographics and disease characteristics of the patients according to concomitant cDMARD or MTX use at baseline subdivided by treatment group
| Concomitant treatment | cDMARD | MTX | None (cDMARD-naïve or past use at baseline) | ||||||
| PBO, n=69 | IXEQ4W, n=68 | IXEQ2W, n=63 | PBO, n=59 | IXEQ4W, n=57 | IXEQ2W, n=53 | PBO, n=37 | IXEQ4W, n=39 | IXEQ2W, n=40 | |
| Age (years), mean (SD) | 51.0 (12.5) | 49.1 (10.0) | 49.3 (12.8) | 51.3 (12.8) | 49.9 (10.3) | 50.1 (12.7) | 49.9 (12.1) | 49.1 (10.4) | 50.5 (12.4) |
| Male, n (%) | 32 (46.4) | 29 (42.6) | 27 (42.9) | 25 (42.4) | 24 (42.1) | 23 (43.4) | 16 (43.2) | 16 (41.0) | 21 (52.5) |
| Weight (kg), mean (SD) | 84.8 (19.7) | 83.5 (19.5) | 81.0 (16.7) | 84.87 (20.1) | 84.58 (20.0) | 81.88 (15.8) | 81.8 (19.6) | 88.9 (28.0) | 82.6 (18.8) |
| Time since PsA diagnosis (years), mean (SD) | 6.1 (7.4) | 5.6 (5.8) | 7.4 (7.4) | 6.1 (7.6) | 5.1 (5.3) | 7.8 (7.8) | 6.8 (5.8) | 7.3 (7.3) | 7.0 (9.1) |
| Tender joint count (68 joints), mean (SD) | 19.5 (12.8) | 20.6 (13.3) | 21.4 (15.1) | 20.6 (13.4) | 20.5 (13.8) | 21.1 (15.6) | 18.6 (13.4) | 20.3 (14.4) | 21.8 (12.4) |
| Swollen joint count (66 joints), mean (SD) | 10.9 (7.7) | 10.8 (7.4) | 11.8 (7.6) | 11.3 (8.1) | 10.8 (7.9) | 11.4 (6.0) | 10.1 (6.4) | 12.6 (9.4) | 12.5 (6.7) |
| HAQ-DI total score, mean (SD) | 1.19 (0.60) | 1.25 (0.54) | 1.23 (0.55) | 1.21 (0.60) | 1.21 (0.53) | 1.25 (0.57) | 1.09 (0.62) | 1.22 (0.56) | 1.09 (0.59) |
| van der Heijde mTSS, mean (SD) | 18.9 (32.2) | 21.2 (36.9) | 17.3 (32.7) | 17 (28.6) | 21 (38.4) | 19.2 (35.2) | 15.3 (21.6) | 15.7 (23.9) | 11.9 (21.4) |
The study was not designed to test equivalence or non-inferiority of treatment with ixekizumab alone versus treatment with ixekizumab combined with cDMARDs.
cDMARD, conventional disease-modifying antirheumatic drugs; HAQ-DI, Health Assessment Questionnaire-Disability Index; IXEQ2W, 80 mg ixekizumab once every 2 weeks; IXEQ4W, 80 mg ixekizumab once every 4 weeks; mTSS, modified total Sharp score; MTX, methotrexate; n, number of patients; PBO, placebo; PsA, psoriatic arthritis.
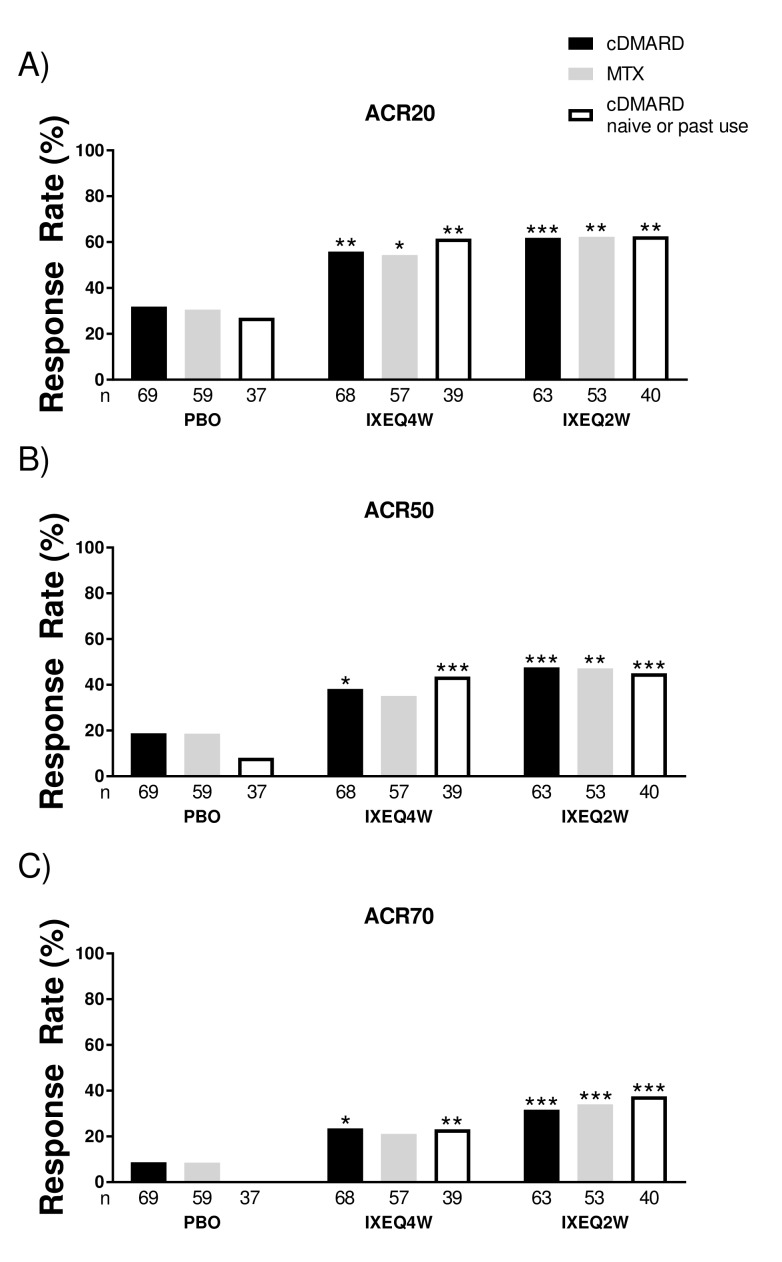
No interaction in treatment-by-cDMARD use was observed in the study (P>0.1). Patients treated with either IXEQ4W or IXEQ2W had significantly higher ACR20, ACR50 and ACR70 response rates at 24 weeks compared with placebo, regardless of concomitant cDMARD use (figure 1A–C). IXEQ4W with concomitant MTX use had significantly higher ACR20 response rates and numerically higher, but not statistically significant, ACR50 and ACR70 response rates relative to placebo (figure 1). ACR response rates for IXEQ2W with concomitant MTX use were consistent with those reported relative to placebo for IXEQ2W with concomitant cDMARD use.
Figure 1.
ACR response rates at 24 weeks in patients treated with PBO, IXEQ4W or IXEQ2W alone or in combination with cDMARDs or MTX. The proportions of patients achieving ACR20 (A), ACR50 (B) and ACR70 (C) are shown. The study was not designed to test equivalence or non-inferiority of treatment with ixekizumab alone versus treatment with ixekizumab in combination with cDMARDs. ACR20/50/70, 20%/50%/70% American College of Rheumatology response; cDMARD, conventional disease-modifying antirheumatic drugs; PBO, placebo; IXEQ4W, 80 mg ixekizumab once every 4 weeks; IXEQ2W, 80 mg ixekizumab once every 2 weeks; n, number of patients; MTX, methotrexate. *P<0.05, **P<0.01, ***P<0.001, all versus PBO.
Progression of structural damage, as measured by change from baseline in mTSS at 24 weeks, was significantly less in patients treated with IXEQ4W or IXEQ2W with concomitant cDMARD or MTX use and IXEQ2W alone when compared with the placebo groups (table 2). In patients treated with IXEQ4W alone, change from baseline in mTSS at 24 weeks was numerically lower than placebo (0.25 vs 0.57, respectively), but not statistically significant.
Table 2.
Structural disease progression at 24 weeks in patients treated with PBO, IXEQ4W or IXEQ2W alone or in combination with cDMARD or MTX
| Concomitant treatment |
cDMARD | MTX | None (cDMARD-naïve or past use at baseline) |
||||||
| PBO, n=58 | IXEQ4W, n=63 | IXEQ2W, n=60 | PBO, n=48 | IXEQ4W, n=52 | IXEQ2W, n=49 | PBO, n=35 |
IXEQ4W, n=37 | IXEQ2W, n=37 | |
| mTSS LSM change from baseline at week 24 (SE) | 0.44 (0.100) |
0.11 (0.096)* |
0.11 (0.098)* |
0.52 (0.135) |
0.13 (0.121)* |
0.14 (0.114)* |
0.57 (0.157) |
0.25 (0.153) |
0.03 (0.153)* |
The study was not designed to test equivalence or non-inferiority of treatment with ixekizumab alone versus treatment with ixekizumab with concomitant cDMARDs.
*P<0.05 versus PBO.
cDMARD, conventional disease-modifying antirheumatic drugs; IXEQ2W, 80 mg ixekizumab once every 2 weeks; IXEQ4W, 80 mg ixekizumab once every 4 weeks; LSM, least squares mean; mTSS, van der Heijde modified total Sharp score; MTX, methotrexate; n, number of patients; PBO, placebo.
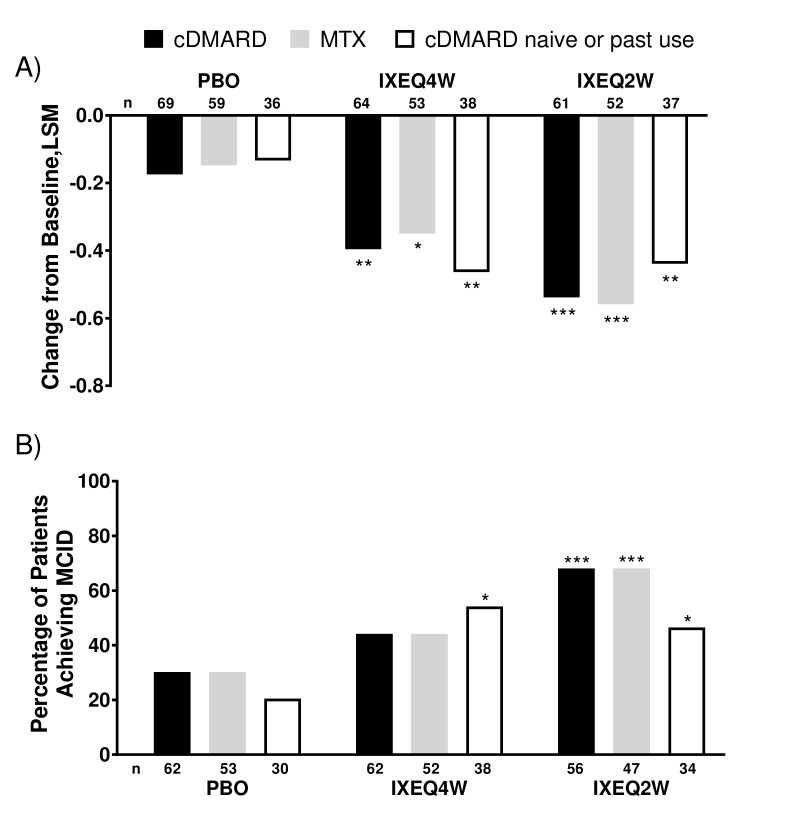
Improvements in physical function, as assessed by HAQ-DI, showed that patients treated with either IXEQ4W or IXEQ2W had significantly greater improvement than placebo regardless of concomitant cDMARD or MTX use (figure 2A). The proportion of patients who reached a HAQ-DI MCID (improvement in HAQ-DI total score ≥0.35 from baseline) was significantly higher than placebo for IXEQ4W alone and IXEQ2W, regardless of concomitant cDMARD or MTX use (figure 2B). Although not statistically significant, treatment with IXEQ4W with concomitant cDMARDs or MTX use had a numerically higher proportion of patients who achieved HAQ-DI MCID relative to placebo.
Figure 2.
HAQ-DI change from baseline and proportion of patients achieving MCID after 24 weeks in patients treated with PBO, IXEQ4W or IXEQ2W alone or in combination with cDMARDs or MTX. (A) LSM changes from baseline of HAQ-DI. (B) Proportion of patients achieving MCID. The study was not designed to test equivalence or non-inferiority of ixekizumab alone versus treatment with ixekizumab in combination with cDMARDs. cDMARD, conventional disease-modifying antirheumatic drugs; HAQ-DI, Health Assessment Questionnaire-Disability Index; IXEQ2W, 80 mg ixekizumab once every 2 weeks; IXEQ4W, 80 mg ixekizumab once every 4 weeks; LSM, least squares mean; MCID, minimal clinically important difference; MTX, methotrexate; n, number of patients; PBO, placebo. *P<0.05, **P<0.01, ***P<0.001, all versus PBO.
Overall, a numerically higher proportion of patients who received IXEQ4W or IXEQ2W experienced at least one TEAE when compared with patients receiving placebo, regardless of concomitant cDMARD or MTX use (table 3). In the groups with significantly higher frequencies of TEAEs reported, injection site reactions were the most frequently reported TEAEs (IXEQ4W+cDMARD: 11.8%, n=8; IXEQ2W+cDMARD: 12.7%, n=8), but this is consistent with previous SPIRIT-P1 reports for the overall treatment groups.3 Mild TEAEs were significantly higher versus placebo for IXEQ4W with concomitant cDMARD or MTX use. The incidence of moderate TEAEs, severe TEAEs, serious TEAEs and TEAEs leading to discontinuation was similar versus placebo across all treatment groups, regardless of concomitant cDMARD or MTX use.
Table 3.
Safety overview after 24 weeks according to concomitant cDMARD or MTX use at baseline, subdivided by treatment
| Concomitant treatment | cDMARD | MTX | None (cDMARD-naïve or past use at baseline) | ||||||
| PBO, n=69 | IXEQ4W, n=68 | IXEQ2W, n=63 | PBO, n=59 | IXEQ4W, n=57 | IXEQ2W, n=53 | PBO, n=37 | IXEQ4W, n=39 | IXEQ2W, n=39 | |
| Treatment-emergent AE, n (%) | 30 (43.5) | 42 (61.8)* | 40 (63.5)* | 27 (45.8) | 36 (63.2) | 34 (64.2) | 20 (54.1) | 29 (74.4) | 27 (69.2) |
| Mild | 15 (21.7) | 28 (41.2)* | 23 (36.5) | 13 (22.0) | 25 (43.9)* | 18 (34.0) | 12 (32.4) | 15 (38.5) | 18 (46.2) |
| Moderate | 13 (18.8) | 13 (19.1) | 14 (22.2) | 12 (20.3) | 10 (17.5) | 13 (24.5) | 8 (21.6) | 11 (28.2) | 7 (17.9) |
| Severe | 2 (2.9) | 1 (1.5) | 3 (4.8) | 2 (3.4) | 1 (1.8) | 3 (5.7) | 0 | 3 (7.7) | 2 (5.1) |
| Serious AE, n (%) | 2 (2.9) | 3 (4.4) | 4 (6.3) | 1 (1.7) | 2 (3.5) | 0 | 0 | 3 (7.7) | 3 (7.7) |
| AE leading to discontinuation, n (%) | 2 (2.9) | 1 (1.5) | 4 (7.5) | 2 (3.4) | 1 (1.8) | 4 (7.5) | 0 | 1 (2.6) | 0 |
The study was not designed to test equivalence or non-inferiority of treatment with ixekizumab alone versus treatment with ixekizumab combined with cDMARDs.
*P<0.05 versus PBO.
AE, adverse events; cDMARD, conventional disease-modifying antirheumatic drugs; IXEQ2W, 80 mg ixekizumab once every 2 weeks; IXEQ4W, 80 mg ixekizumab once every 4 weeks; MTX, methotrexate; n, number of patients; PBO, placebo.
Discussion
In biologic-naïve patients with PsA, ixekizumab demonstrated efficacy relative to placebo, regardless of concomitant cDMARD use. Improvements were observed relative to placebo in disease activity, inhibition of structural progression of disease and physical function in patients treated with ixekizumab, regardless of concomitant cDMARD use. Furthermore, the overall safety profile was consistent with previous ixekizumab reports in patients with psoriasis and PsA across treatment subgroups.3 26 27
Reports from PsA randomised controlled trials (RCTs) for TNFis6–14 and an IL-17A inhibitor15 16 support our results. All of these studies, including our results from patients treated with ixekizumab, indicate clinical efficacy with biologics relative to placebo, regardless of concomitant cDMARD use. In addition, results directly comparing the efficacy of biologics alone with biologics with concomitant cDMARD use are available from analyses of PsA registries, which also support the results we observed in patients treated with ixekizumab. Studies of the Danish biologics registry (DANBIO), which contained 764 patients with PsA, found that clinical improvements, as reflected by ACR20 responses, were associated with biologic treatment with concomitant MTX use, but not for other outcome measures, including ACR50 and ACR70.18 Furthermore, analyses of the Norwegian longitudinal observational study on disease-modifying antirheumatic drugs (NOR-DMARD), which contained 440 patients with PsA, observed no significant difference in clinical outcomes with and without concomitant MTX use for ACR20, ACR50 and ACR70 responses.17
The findings of this study, with support from other RCTs, indicate that ixekizumab and other biologics are clinically effective in PsA, regardless of concomitant cDMARD or MTX use through 24 weeks of treatment. The European League Against Rheumatism (EULAR) recommendations include the use of TNFis with concomitant cDMARDs, but EULAR was unable to recommend IL-12/23 and IL-17 inhibitor use with concomitant cDMARDs due to a lack of published evidence.28 In contrast, GRAPPA noted in their 2015 treatment recommendations that based on available study results, concomitant therapy does not yield additional improvements beyond those achieved with biologics alone.5 Acknowledging these guidelines, we note that this study did not compare the efficacy of using an IL-17A antagonist alone with use with concomitant cDMARDs, but instead provides clinicians with additional insights on the utility of using an IL-17A antagonist, as represented by ixekizumab, either alone or with concomitant cDMARDs, including MTX, in biologic-naïve patients with active PsA.
These results suggest that efficacy is achieved with ixekizumab relative to placebo when given with or without concomitant cDMARDs, but it has limitations. First, these data are subset analyses of the SPIRIT-P1 trial, which was designed to compare treatments with the placebo group alone; thus patients were not randomised to receive or not receive concomitant cDMARDs.3 In addition, the stratification of patients by cDMARD status at baseline balanced these characteristics across treatment arms, but did not ensure balance of these characteristics within treatment arms. Hence, this study design does not allow for making robust comparisons between patients treated with and without concomitant cDMARDs. Despite these limitations, abundant evidence exists from RCTs6–13 15 16 for other PsA biologic therapies indicating that efficacy is achieved with or without concomitant cDMARD use. These studies are in alignment with our findings that ixekizumab is effective relative to placebo in PsA, regardless of cDMARD use. In addition, due to the short-term nature of the period in this study, the impact of concomitant therapy on long-term outcomes and duration of response was not investigated. Such an investigation would be informative but would need to be undertaken as part of a long-term registry study or RCT.
In summary, ixekizumab effectively reduced the severity of PsA symptoms, inhibited structural damage and improved physical function in patients relative to placebo, regardless of concomitant cDMARD use. The safety profile of ixekizumab with or without concomitant cDMARD use is consistent with previous reports in patients with PsA and psoriasis.3 26 27
Acknowledgments
The authors would like to acknowledge Brian S Comer, a full-time employee of Eli Lilly and Company, for writing and editorial assistance with the manuscript.
Footnotes
Contributors: PJM, CLS were involved in the conception and design of the clinical study. PJM, CLS, CL were involved in the acquisition of the data. All authors were involved in the analysis and interpretation of the data. All authors were involved in the drafting and revision of the manuscript. C-YL was involved in the statistical analysis.
Funding: This study was funded and sponsored by Eli Lilly and Company.
Competing interests: LCC: consultant for AbbVie, Celgene, Janssen, Sun Pharma, Pfizer, UCB, MSD, Novartis, Eli Lilly and Company, Amgen, BMS; grant/research support from AbbVie, Janssen. MK: consultant for Eli Lilly and Company. AG: consultant/advisory board agreements with Janssen, Celgene, Bristol Myers Squibb, Beiersdorf, AbbVie, UCB, Novartis, Incyte, Pfizer, Eli Lilly and Company, XenoPort, Crescendo Bioscience, Aclaris, Amicus, Reddy Labs, Valeant, Dermira, Allergan, CSL Behring, Merck, Sun Pharmaceutical Industries; research/educational grants from Janssen, Incyte. CLS, CHL and C-YL are employees and stockholders of Eli Lilly and Company. PJM: consultant for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Genentech, Janssen, Eli Lilly and Company, Novartis, Pfizer, UCB Pharma, Sun; grant/research support from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Genentech, Janssen, Eli Lilly and Company, Novartis, Pfizer, UCB Pharma, Sun; speakers bureau for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Genentech, Janssen, Pfizer, UCB Pharma.
Ethics approval: The protocol was approved by each site’s institutional review board or ethics committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Lilly provides access to relevant anonymised patient level data from studies on approved medicines and indications as defined by the sponsor specific information on www.clinicalstudydatarequest.com. For details on submitting a request, see the instructions provided at www.clinicalstudydatarequest.com.
References
- 1.de Vlam K, Gottlieb AB, Mease PJ. Current concepts in psoriatic arthritis: pathogenesis and management. Acta Derm Venereol 2014;94:627–34. 10.2340/00015555-1833 [DOI] [PubMed] [Google Scholar]
- 2.Rosen CF, Mussani F, Chandran V, et al. Patients with psoriatic arthritis have worse quality of life than those with psoriasis alone. Rheumatology 2012;51:571–6. 10.1093/rheumatology/ker365 [DOI] [PubMed] [Google Scholar]
- 3.Mease PJ, van der Heijde D, Ritchlin CT, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 2017;76:79–87. 10.1136/annrheumdis-2016-209709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, Lu J, Allan BW, et al. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin-17A. J Inflamm Res 2016;9:39–50. 10.2147/JIR.S100940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–71. 10.1002/art.39573 [DOI] [PubMed] [Google Scholar]
- 6.Antoni C, Krueger GG, de Vlam K, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis 2005;64:1150–7. 10.1136/ard.2004.032268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gladman DD, Mease PJ, Ritchlin CT, et al. Adalimumab for long-term treatment of psoriatic arthritis: forty-eight week data from the adalimumab effectiveness in psoriatic arthritis trial. Arthritis Rheum 2007;56:476–88. 10.1002/art.22379 [DOI] [PubMed] [Google Scholar]
- 8.Kavanaugh A, Antoni C, Krueger GG, et al. Infliximab improves health related quality of life and physical function in patients with psoriatic arthritis. Ann Rheum Dis 2006;65:471–7. 10.1136/ard.2005.040196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kavanaugh A, Krueger GG, Beutler A, et al. Infliximab maintains a high degree of clinical response in patients with active psoriatic arthritis through 1 year of treatment: results from the IMPACT 2 trial. Ann Rheum Dis 2007;66:498–505. 10.1136/ard.2006.058339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kavanaugh A, McInnes IB, Mease P, et al. Clinical efficacy, radiographic and safety findings through 5 years of subcutaneous golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of a randomised, placebo-controlled trial (the GO-REVEAL study). Ann Rheum Dis 2014;73:1689–94. 10.1136/annrheumdis-2013-204902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kavanaugh A, McInnes IB, Mease PJ, et al. Clinical efficacy, radiographic and safety findings through 2 years of golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of the randomised, placebo-controlled GO-REVEAL study. Ann Rheum Dis 2013;72:1777–85. 10.1136/annrheumdis-2012-202035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mease PJ, Gladman DD, Ritchlin CT, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52:3279–89. 10.1002/art.21306 [DOI] [PubMed] [Google Scholar]
- 13.Mease PJ, Kivitz AJ, Burch FX, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 2004;50:2264–72. 10.1002/art.20335 [DOI] [PubMed] [Google Scholar]
- 14.Mease PJ, Fleischmann R, Deodhar AA, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 2014;73:48–55. 10.1136/annrheumdis-2013-203696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;386:1137–46. 10.1016/S0140-6736(15)61134-5 [DOI] [PubMed] [Google Scholar]
- 16.van der Heijde D, Landewé RB, Mease PJ, et al. Brief report: secukinumab provides significant and sustained inhibition of joint structural damage in a phase III study of active psoriatic arthritis. Arthritis Rheumatol 2016;68:1914–21. 10.1002/art.39685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fagerli KM, Lie E, van der Heijde D, et al. The role of methotrexate co-medication in TNF-inhibitor treatment in patients with psoriatic arthritis: results from 440 patients included in the NOR-DMARD study. Ann Rheum Dis 2014;73:132–7. 10.1136/annrheumdis-2012-202347 [DOI] [PubMed] [Google Scholar]
- 18.Glintborg B, Østergaard M, Dreyer L, et al. Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor α therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheum 2011;63:382–90. 10.1002/art.30117 [DOI] [PubMed] [Google Scholar]
- 19.Mease PJ, Collier DH, Saunders KC, et al. Comparative effectiveness of biologic monotherapy versus combination therapy for patients with psoriatic arthritis: results from the Corrona registry. RMD Open 2015;1:e000181 10.1136/rmdopen-2015-000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glintborg B, Gudbjornsson B, Krogh NS, et al. Impact of different infliximab dose regimens on treatment response and drug survival in 462 patients with psoriatic arthritis: results from the nationwide registries DANBIO and ICEBIO. Rheumatology 2014;53:2100–9. 10.1093/rheumatology/keu252 [DOI] [PubMed] [Google Scholar]
- 21.Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. 10.1002/art.21972 [DOI] [PubMed] [Google Scholar]
- 22.Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727–35. 10.1002/art.1780380602 [DOI] [PubMed] [Google Scholar]
- 23.van der Heijde D, Sharp J, Wassenberg S, et al. Psoriatic arthritis imaging: a review of scoring methods. Ann Rheum Dis 2005;64(Suppl 2):ii61–4. 10.1136/ard.2004.030809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fries JF, Spitz P, Kraines RG, et al. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. 10.1002/art.1780230202 [DOI] [PubMed] [Google Scholar]
- 25.Kwok T, Pope JE. Minimally important difference for patient-reported outcomes in psoriatic arthritis: health assessment questionnaire and pain, fatigue, and global visual analog scales. J Rheumatol 2010;37:1024–8. 10.3899/jrheum.090832 [DOI] [PubMed] [Google Scholar]
- 26.Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med 2012;366:1190–9. 10.1056/NEJMoa1109997 [DOI] [PubMed] [Google Scholar]
- 27.Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015;386:541–51. 10.1016/S0140-6736(15)60125-8 [DOI] [PubMed] [Google Scholar]
- 28.Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. 10.1136/annrheumdis-2015-208337 [DOI] [PubMed] [Google Scholar]