Abstract
Purpose
The optimal indications for omitting adjuvant radiation therapy (RT) after breast-conserving surgery are still controversial in ductal carcinoma in situ (DCIS) of the breast. The purpose of this study was to validate the role of postoperative RT in DCIS patients aged ≤50 years and with tumor margin widths of <1 cm, both of which have been proven to be high-risk features for recurrence in cohorts not receiving RT.
Methods
Using two multicenter retrospective studies on DCIS, a pooled analysis was performed among patients aged ≤50 years and with margin widths <1 cm. All patients underwent breast-conserving surgery. Two hundred thirty-two patients received postoperative RT, while 54 did not. The median follow-up period was 77 months (range, 2–190 months) and 70 months (range, 5–166 months) in the patients who received RT and those who did not, respectively.
Results
The patients who received RT had larger tumors (p<0.001), higher nuclear grade (p<0.001), closer margin width (p<0.001), and negative estrogen receptor expression (p=0.010) compared with those who did not receive RT. During the follow-up period, there were 17 ipsilateral breast tumor recurrences (IBTRs) as follows: invasive carcinoma in 10 patients and DCIS in seven. In the univariate analysis, the treatment with RT and human epidermal growth factor receptor 2 (HER2) status were significant risk factors for IBTR. The 7-year IBTR rates with and without postoperative RT were 3.6% and 13.1%, respectively (p=0.008). HER2-positive tumors had a higher IBTR rate than the HER2-negative tumors (7-year rate, 13.6% vs. 3.9%; p=0.003).
Conclusion
Postoperative RT following breast-conserving surgery significantly reduced the 7-year IBTR rate in the DCIS patients aged ≤50 years and with margin widths <1 cm. HER2 positivity was associated with increased IBTR in these patients.
Keywords: Age factors, Ductal carcinoma in situ, Margins of excision, Radiotherapy
INTRODUCTION
The optimal treatment for ductal carcinoma in situ (DCIS) of the breast has been controversial. Although randomized controlled trials have already proven the benefit of postoperative radiation therapy (RT) to the whole breast after breast-conserving surgery (BCS) [1,2,3,4], a considerable portion of patients undergo local excision alone without RT [5,6]. In general, patients of younger ages, those with high-grade tumors, and/or those with close/involved resection margins are more likely to receive adjuvant RT; however, the characteristics of low-risk patients in whom RT can be safely omitted have yet to be established.
Several prospective studies on the omission of adjuvant RT after BCS were conducted in low-risk DCIS patients, who were defined as low-risk on the basis of tumor size and nuclear grade [7,8,9]. However, even those patients with small tumors of ≤2.5 cm in size of low-to-intermediate grade had a significant recurrence risk as follows: the 12-year ipsilateral breast event rate was 14.4% in a study by the Eastern Cooperative Oncology Group (ECOG) [7], and the 10-year local recurrence rate was 15.6% in a study by the Dana-Farber group [8]. Moreover, a randomized controlled trial by the Radiation Therapy Oncology Group (RTOG) reported in early results that the 7-year local failure rate was significantly reduced from 6.7% without RT to 0.9% with RT in a patient population similar to that of the ECOG study [9]. Therefore, it appears that risk factors other than tumor size and nuclear grade are necessary to define optimal indications for omitting adjuvant RT.
Given the consistent increase in the crude incidence rate of DCIS in Korea [10], the omission of adjuvant RT in some patients has become an issue of growing concern; while at the same time, a significant number of patients are also undergoing RT [11]. Recently, the Korean Radiation Oncology Group (KROG) conducted a multicenter retrospective study addressing this issue [12]. According to our previous analysis, patients aged ≤50 years and with margin widths of <1 cm had a high risk of recurrence without adjuvant RT after BCS. For these patients, however, whether adjuvant RT can reduce the recurrence risk has not yet been proven.
In this study, we compared the recurrence risk between DCIS patients aged ≤50 years and with margin widths of <1 cm who were treated with or without adjuvant RT, using a pooled analysis of two multicenter retrospective studies.
METHODS
Study population
Two multicenter retrospective studies on DCIS were conducted by the KROG; these were KROG 11-04 and 16-02. The Institutional Review Board approved these studies (numbers: 11-252 for KROG 11-04 and H-1603-027-747 for KROG 16-02), and waived the requirement for obtaining informed consent. KROG 11-04 was designed to evaluate the role of tumor bed boost in addition to whole breast RT after BCS [13], and KROG 16-02 was designed to evaluate recurrence outcomes after omission of the whole breast RT following BCS [12]. Among the patients enrolled in these two studies, those aged ≤50 years and with margin widths of <1 cm were selected for the pooled analysis. Patients from KROG 11-04 (RT group) were compared with those from KROG 16-02 (no-RT group) in terms of patient and tumor characteristics, as well recurrence outcomes.
Pathological evaluation
Pathological information on tumor size, nuclear grade, resection margin involvement, margin width, estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status was collected. ER, PR, and HER2 status was determined by immunohistochemical staining. Resection margin status was defined as clear (no tumor on inked margin) or involved. Margin width was grouped into ≥2 or <2 mm, according to recent consensus guidelines [14].
Treatment
All patients underwent BCS. In the RT group, the whole breast was treated with postoperative RT, up to a median dose of 50.4 Gy (range, 45.0–50.4 Gy) with 1.8–2.0 Gy/fraction. Tumor bed boost was determined at the discretion of the attending radiation oncologists. The median dose of tumor bed boost was 12.6 Gy (range, 9–20 Gy). Tamoxifen was administered to patients with hormone receptor-positive tumors.
Statistical analysis
The KROG 11-04 study enrolled patients who were treated between 1995 and 2006, and the KROG 16-02 study enrolled patients treated between 2000 and 2010. The median follow-up periods were 77 months (range, 2–190 months) in the RT group (from KROG 11-04) and 70 months (range, 5–166 months) in the no-RT group (from KROG 16-02), respectively.
Ipsilateral breast tumor recurrence (IBTR) rate was estimated from the date of surgery by using the Kaplan-Meier method. To evaluate risk factors for IBTR, a log-rank test was used for univariate analysis. A chi-square test was used for comparison among categorical variables. Factors with a p-value of <0.05 were considered statistically significant. All analyses were performed in PASW Statistics for Windows, version 18.0 (SPSS Inc., Chicago, USA).
RESULTS
Patient and tumor characteristics
A total of 286 patients were selected for the pooled analysis, 232 in the RT group and 54 in the no-RT group. The median age was 43 years (range, 19–50 years). Patient and tumor characteristics were compared between the two groups in Table 1. Patients receiving RT had tumors with larger size (p<0.001), higher nuclear grade (p<0.001), involved resection margins (p=0.034), closer margin width (p<0.001), negative ER expression (p=0.010), and negative PR expression (p=0.001), compared with those who did not receive RT. The distribution of HER2 status was significantly different between the two groups. When patients with unknown or equivocal HER2 status were excluded; however, there was no difference in the distribution of HER2-positive or negative tumors (p=0.636).
Table 1. Patient and tumor characteristics.
| Variable | No-RT group (n = 54) No. (%) | RT group (n = 232) No. (%) | p-value |
|---|---|---|---|
| Age (yr) | 0.950 | ||
| ≤ 40 | 20 (37) | 87 (38) | |
| > 40 | 34 (63) | 145 (63) | |
| Tumor size (cm) | < 0.001 | ||
| ≤1 | 38 (70) | 70 (30) | |
| >1 | 16 (30) | 142 (61) | |
| Unknown | 0 | 20 (9) | |
| Nuclear grade | < 0.001 | ||
| 1 | 31 (57) | 62 (27) | |
| 2 | 14 (26) | 89 (38) | |
| 3 | 5 (9) | 47 (20) | |
| Unknown | 4 (7) | 34 (15) | |
| Margin involvement | 0.034 | ||
| Negative | 54 (100) | 214 (92) | |
| Positive | 0 | 18 (8) | |
| Margin width (mm) | < 0.001 | ||
| <2 | 13 (24) | 146 (63) | |
| ≥2 | 41 (76) | 86 (37) | |
| ER | 0.010 | ||
| Negative | 2 (4) | 26 (11) | |
| Positive | 50 (93) | 170 (73) | |
| Unknown | 2 (4) | 36 (16) | |
| PR | 0.001 | ||
| Negative | 2 (4) | 41 (18) | |
| Positive | 50 (93) | 154 (66) | |
| Unknown | 2 (4) | 37 (16) | |
| HER2 | 0.004 | ||
| Negative | 33 (61) | 107 (46) | |
| Equivocal | 4 (7) | 38 (16) | |
| Positive | 15 (28) | 41 (18) | |
| Unknown | 2 (4) | 46 (20) | |
| Tamoxifen | 0.167 | ||
| No | 15 (28) | 92 (40) | |
| Yes | 39 (72) | 137 (59) | |
| Unknown | 0 | 3 (1) |
RT=radiation therapy; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
Treatment outcomes and prognostic factors for ipsilateral breast tumor recurrence
During the follow-up period, there were 17 IBTRs (invasive carcinoma in 10 and DCIS in seven). In the RT group, 10 IBTRs were observed, and the median time to IBTR was 87 months (range, 26–190 months). In the no-RT group, seven IBTRs were observed, and the median time to IBTR was 49 months (range, 36–98 months).
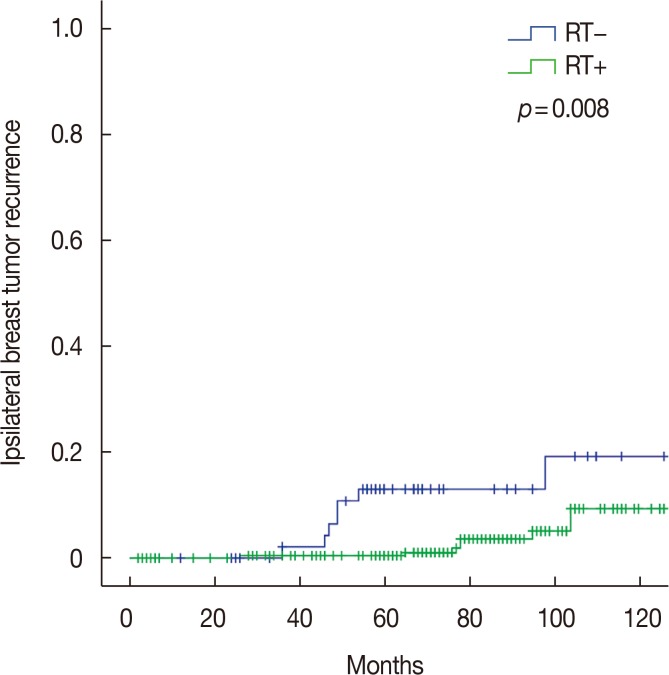
The results of univariate analysis for IBTR are shown in Table 2. Age, tumor size, nuclear grade, resection margin involvement, margin width, and ER or PR status were not correlated with IBTR. HER2-positive tumors had a higher IBTR rate than HER2-negative tumors (7-year rate, 13.6% vs. 3.9%, respectively; p=0.003). The 7-year IBTR rates of the RT and no-RT groups were 3.6% and 13.1%, respectively (p=0.008) (Figure 1).
Table 2. Univariate analysis for ipsilateral breast tumor recurrence.
| Variable | No. of patients | 7-yr IBTR rate (%) | p-value |
|---|---|---|---|
| Age (yr) | 0.389 | ||
| ≤ 40 | 107 | 6.4 | |
| > 40 | 179 | 4.9 | |
| Tumor size (cm) | 0.653 | ||
| ≤1 | 108 | 6.1 | |
| >1 | 158 | 5.5 | |
| Unknown | 20 | 0.0 | |
| Nuclear grade | 0.975 | ||
| 1 | 93 | 3.8 | |
| 2 | 103 | 4.0 | |
| 3 | 52 | 12.6 | |
| Unknown | 38 | 6.2 | |
| Margin involvement | 0.340 | ||
| Negative | 268 | 5.8 | |
| Positive | 18 | 0.0 | |
| Margin width (mm) | 0.534 | ||
| <2 | 159 | 5.4 | |
| ≥2 | 127 | 5.4 | |
| ER | 0.278 | ||
| Negative | 28 | 10.6 | |
| Positive | 220 | 5.3 | |
| Unknown | 38 | 3.0 | |
| PR | 0.299 | ||
| Negative | 43 | 4.3 | |
| Positive | 204 | 6.3 | |
| Unknown | 39 | 2.9 | |
| HER2* | 0.003 | ||
| Negative | 140 | 3.9 | |
| Positive | 56 | 13.6 | |
| Tamoxifen | 0.597 | ||
| No | 107 | 3.8 | |
| Yes | 176 | 6.5 | |
| Unknown | 3 | 0.0 | |
| RT | 0.008 | ||
| No | 54 | 13.1 | |
| Yes | 232 | 3.6 |
IBTR=ipsilateral breast tumor recurrence; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; RT=radiation therapy.
*Ninety patients with unknown or equivocal HER2 status were excluded.
Figure 1. Ipsilateral breast tumor recurrence curves according to the receipt of postoperative radiation therapy (RT).
Subgroup analysis of patients with small tumors of low-to-intermediate grade
Subgroup analysis was performed in 150 patients with small tumors (≤2.5 cm) of low-to-intermediate grade with clear resection margins. Patient and tumor characteristics are given in Table 3. In this analysis, patients receiving RT had tumors that were larger in size (p=0.002), of higher nuclear grade (p=0.003), and had closer margin width (p<0.001). Although ER or PR expression was not different between the RT and no-RT groups, tamoxifen was more frequently given to patients in the no-RT group (p=0.028). On univariate analysis, treatment with RT was the only prognostic factor affecting IBTR. The 7-year IBTR rates in the RT and no-RT groups were 0% and 11.7%, respectively (p=0.012).
Table 3. Patient and tumor characteristics among patients with small tumors of low-to-intermediate grade.
| Variable | No-RT group (n = 41) No. (%) | RT group (n = 109) No. (%) | p-value |
|---|---|---|---|
| Age (yr) | 0.455 | ||
| ≤ 40 | 17 (41) | 38 (35) | |
| > 40 | 24 (59) | 71 (65) | |
| Tumor size (cm) | 0.002 | ||
| ≤1 | 31 (76) | 51 (47) | |
| >1 | 10 (24) | 58 (53) | |
| Nuclear grade | 0.003 | ||
| 1 | 27 (66) | 42 (39) | |
| 2 | 14 (34) | 67 (61) | |
| Margin width (mm) | < 0.001 | ||
| <2 | 7 (17) | 71 (65) | |
| ≥2 | 34 (83) | 38 (35) | |
| ER | 0.335 | ||
| Negative | 1 (2) | 4 (4) | |
| Positive | 39 (95) | 95 (87) | |
| Unknown | 1 (2) | 10 (9) | |
| PR | 0.115 | ||
| Negative | 1 (2) | 9 (8) | |
| Positive | 39 (95) | 89 (82) | |
| Unknown | 1 (2) | 11 (10) | |
| HER2 | 0.134 | ||
| Negative | 28 (68) | 61 (56) | |
| Equivocal | 4 (10) | 18 (17) | |
| Positive | 7 (17) | 12 (11) | |
| Unknown | 2 (5) | 18 (17) | |
| Tamoxifen | 0.028 | ||
| No | 9 (22) | 45 (41) | |
| Yes | 32 (78) | 64 (59) |
RT=radiation therapy; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
Subgroup analysis according to margin width and HER2 expression
When a subgroup analysis was performed in patients with margin widths <2 mm, the 7-year IBTR rates with or without RT were 4.2% and 18.2%, respectively (p=0.061). For those with margin widths from ≥2 mm to 1 cm, the corresponding figures were 2.5% and 11.4%, respectively (p=0.101). According to the HER2 expression, postoperative RT was significantly associated with a lower 7-year IBTR rate in HER2-negative patients (1.8% vs. 10.3%, p=0.009). However, this statistical significance was lost in HER2-positive patients, for whom the 7-year IBTR rate was 10.0% with RT and 21.4% without RT (p=0.103).
DISCUSSION
In this pooled analysis of two multicenter retrospective studies, adjuvant RT after BCS significantly reduced the 7-year IBTR rate from 13.1% to 3.6% in DCIS patients aged ≤50 years and with margin widths <1 cm. In addition, HER2 positivity was associated with increased IBTR in these patients.
The optimal indications for omitting adjuvant RT after BCS for DCIS have been controversial. In the previous KROG 16-02 study in patients who underwent BCS without adjuvant RT, age and margin width were significant prognostic factors affecting the rate of recurrence [12]. When combined with these two risk factors, patients aged ≤50 years and with margin widths of <1 cm had a 7-year locoregional recurrence rate of 13.1%, which appears to be high enough to warrant a recommendation of adjuvant RT. However, in the absence of a control group, it was not clear whether adjuvant RT could reduce the recurrence risk. In the present study, we compared the outcomes of these patients (the no-RT group) with those of patients with the same risk factors who received RT in the KROG 11-04 study (the RT group) by using a pooled analysis.
According to many retrospective studies comparing RT and no-RT groups, clinical and pathological risk factors were significantly different between the two groups, and patients with high-risk features were more likely to receive adjuvant RT [5,6]. In the present study, treatment with RT was associated with larger tumor size, higher nuclear grade, involved resection margin, closer margin width, ER negativity, and PR negativity. In addition, we analyzed a high-risk study population, given the patient selection criteria of age of ≤50 years and margin width of <1 cm, who had much higher recurrences than the others in our previous study [12]. Despite this imbalance and the high-risk features of the population, the RT group achieved a significantly lower 7-year IBTR rate than the no-RT group. Moreover, considering that all included patients were aged ≤50 years, it is to be expected that they will have longer follow-up periods, in which a significant portion will have recurrences. Moreover, it is well established that in Korea, the age at diagnosis of breast cancer (including DCIS) is much younger than that in Western countries, with a median age at diagnosis of just 50 years in 2013 [10]. Therefore, adjuvant RT after BCS should be given to these selected patients.
Recently, a consensus guideline suggested ≥2 mm as an adequate margin in DCIS patients undergoing BCS plus whole-breast RT [14]. However, an optimal margin width has not been suggested for those undergoing excision alone. Regarding this issue, Tadros et al. [15] studied rates of locoregional recurrence according to margin width and treatment with RT; they found that adjuvant RT significantly reduced the risk of locoregional recurrence in patients with margin widths of <2 mm, but there was no statistically significant difference in outcomes in those with margin widths of ≥2 mm, regardless of RT treatment. In our study, a subgroup analysis was performed in a similar fashion. There was a trend toward reduced IBTR rate with adjuvant RT in patients with margin widths of <2 mm; however, this effect was reduced in those with margin widths ranging from 2 mm to 1 cm. However, the 7-year IBTR rate in patients with margin widths ranging from 2 mm to 1 cm, and not receiving RT was 11.4%, which appears to be high enough to warrant a recommendation of adjuvant RT. Future studies are needed in which patient age is taken into account to suggest an optimal margin width for DCIS patients who may not require adjuvant RT after BCS.
As previously mentioned, prospective studies defined low-risk patients as those with small tumors of ≤2.5 cm in size of low-to-intermediate grade [7,8,9]. In addition, a margin width of at least 3 mm or 1 cm was required in these studies, whereas patients with smaller margin widths were selected in our study. Although comparison with aforementioned prospective studies is not possible because of the differences in margin width, a subgroup analysis was performed that included only patients with tumors of ≤2.5 cm of low-to-intermediate grade and with clear resection margins. In this analysis, patient and tumor characteristics were still different between the RT and no-RT groups, with patients in the RT group more likely to have high-risk features. However, the 7-year IBTR rate of the no-RT group was significantly higher than that of the RT group (11.7% vs. 0%, respectively). Patients enrolled in the RTOG 9804 study were stratified by age (<50 yr vs. ≥50 yr), margin width (negative vs. 3–9 mm vs. ≥10 mm), and other characteristics. Although the early results of the RTOG study did not report the outcomes according to the predefined stratification, a subsequent subgroup analysis would be helpful to define low-risk patients in whom adjuvant RT can be safely omitted, in conjunction with our results.
HER2 overexpression is an established risk factor for recurrence of invasive breast cancer [16]. For DCIS, however, the relevance of HER2 overexpression as a prognostic factor has not been thoroughly investigated. Kerlikowske et al. [17] observed a 2-fold higher risk of DCIS recurrence in patients with HER2-positive tumors when treated with BCS alone. However, they included DCIS with 2+ staining in the HER2 positive tumors. By contrast, Han et al. [18] performed in situ hybridization in all cases with equivocal (2+) staining, and only cases with HER2 gene amplification were included in the HER2-positive tumors. They noted that HER2-positive DCIS had a higher local recurrence rate (5-year rate, 35% vs. 16%) in patients treated with BCS alone, but no difference in recurrence rate was found between HER2-positive and HER2-negative tumors (6% vs. 6%) in patients treated with BCS followed by RT. In our study, when the 90 patients with unknown or equivocal HER2 status were excluded, HER2 positivity was associated with a significantly higher IBTR rate. According to the HER2 expression, adjuvant RT significantly reduced IBTR in HER2-negative patients, but not in HER2-positive patients. However, the 7-year IBTR rate in patients who did not receive RT was >10% regardless of HER2 expression. Therefore, further studies are needed to refine the indication and/or omission of adjuvant RT according to HER2 status.
This study has several limitations, most of which arise from the retrospective study design and small patient numbers. First, there may be biases that potentially influence treatment selection. Second, owing to the limited number of events, multivariate analysis was not performed, and DCIS and invasive recurrences were not separately analyzed. Finally, the follow-up period varied between the two groups because this study is a pooled analysis of two studies conducted at different periods.
In conclusion, postoperative RT following BCS significantly reduced the 7-year IBTR rate in DCIS patients aged ≤50 years and with margin widths of <1 cm; therefore RT should be given to these selected patients. In addition, HER2 positivity was associated with increased IBTR in these patients.
Footnotes
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Wärnberg F, Garmo H, Emdin S, Hedberg V, Adwall L, Sandelin K, et al. Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS Trial. J Clin Oncol. 2014;32:3613–3618. doi: 10.1200/JCO.2014.56.2595. [DOI] [PubMed] [Google Scholar]
- 2.Donker M, Litière S, Werutsky G, Julien JP, Fentiman IS, Agresti R, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma in situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol. 2013;31:4054–4059. doi: 10.1200/JCO.2013.49.5077. [DOI] [PubMed] [Google Scholar]
- 3.Cuzick J, Sestak I, Pinder SE, Ellis IO, Forsyth S, Bundred NJ, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21–29. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher B, Dignam J, Wolmark N, Mamounas E, Costantino J, Poller W, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16:441–452. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 5.Rakovitch E, Nofech-Mozes S, Narod SA, Hanna W, Thiruchelvam D, Saskin R, et al. Can we select individuals with low risk ductal carcinoma in situ (DCIS)? A population-based outcomes analysis. Breast Cancer Res Treat. 2013;138:581–590. doi: 10.1007/s10549-013-2455-8. [DOI] [PubMed] [Google Scholar]
- 6.Van Zee KJ, Subhedar P, Olcese C, Patil S, Morrow M. Relationship between margin width and recurrence of ductal carcinoma in situ: analysis of 2996 women treated with breast-conserving surgery for 30 years. Ann Surg. 2015;262:623–631. doi: 10.1097/SLA.0000000000001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solin LJ, Gray R, Hughes LL, Wood WC, Lowen MA, Badve SS, et al. Surgical excision without radiation for ductal carcinoma in situ of the breast: 12-year results from the ECOG-ACRIN E5194 Study. J Clin Oncol. 2015;33:3938–3944. doi: 10.1200/JCO.2015.60.8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong JS, Chen YH, Gadd MA, Gelman R, Lester SC, Schnitt SJ, et al. Eight-year update of a prospective study of wide excision alone for small low- or intermediate-grade ductal carcinoma in situ (DCIS) Breast Cancer Res Treat. 2014;143:343–350. doi: 10.1007/s10549-013-2813-6. [DOI] [PubMed] [Google Scholar]
- 9.McCormick B, Winter K, Hudis C, Kuerer HM, Rakovitch E, Smith BL, et al. RTOG 9804: a prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J Clin Oncol. 2015;33:709–715. doi: 10.1200/JCO.2014.57.9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Min SY, Kim Z, Hur MH, Yoon CS, Park EH, Jung KW, et al. The basic facts of Korean breast cancer in 2013: results of a nationwide survey and breast cancer registry database. J Breast Cancer. 2016;19:1–7. doi: 10.4048/jbc.2016.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang JK, Kim MS, Jang WI, Seo YS, Kim HJ, Cho CK, et al. The clinical utilization of radiation therapy in Korea between 2009 and 2013. Radiat Oncol J. 2016;34:88–95. doi: 10.3857/roj.2016.01753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim K, Jung SY, Shin KH, Kim JH, Han W, Lee HB, et al. Recurrence outcomes after omission of postoperative radiotherapy following breast-conserving surgery for ductal carcinoma in situ of the breast: a multicenter, retrospective study in Korea (KROG 16-02) Breast Cancer Res Treat. 2017;162:77–83. doi: 10.1007/s10549-017-4111-1. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Choi DH, Park W, Ahn SD, Kim SS, Ha SW, et al. Influence of boost radiotherapy in patients with ductal carcinoma in situ breast cancer: a multicenter, retrospective study in Korea (KROG 11-04) Breast Cancer Res Treat. 2014;146:341–345. doi: 10.1007/s10549-014-3025-4. [DOI] [PubMed] [Google Scholar]
- 14.Morrow M, Van Zee KJ, Solin LJ, Houssami N, Chavez-MacGregor M, Harris JR, et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. J Clin Oncol. 2016;34:4040–4046. doi: 10.1200/JCO.2016.68.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tadros AB, Smith BD, Shen Y, Lin H, Krishnamurthy S, Lucci A, et al. Ductal carcinoma in situ and margins <2 mm: contemporary outcomes with breast conservation. Ann Surg. 2017 Jul 24; doi: 10.1097/SLA.0000000000002439. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haffty BG. Molecular and genetic markers in the local-regional management of breast cancer. Semin Radiat Oncol. 2002;12:329–340. doi: 10.1053/srao.2002.35252. [DOI] [PubMed] [Google Scholar]
- 17.Kerlikowske K, Molinaro AM, Gauthier ML, Berman HK, Waldman F, Bennington J, et al. Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J Natl Cancer Inst. 2010;102:627–637. doi: 10.1093/jnci/djq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han K, Nofech-Mozes S, Narod S, Hanna W, Vesprini D, Saskin R, et al. Expression of HER2neu in ductal carcinoma in situ is associated with local recurrence. Clin Oncol (R Coll Radiol) 2012;24:183–189. doi: 10.1016/j.clon.2011.09.008. [DOI] [PubMed] [Google Scholar]