Abstract
Background
Atrial fibrillation (AF) after cardiac surgery is a major health problem that is associated with a significant financial burden. This paper aims to highlight this problem and review the current guidelines in the prevention and management of AF after cardiac surgery, providing our experience in the Australasian centers.
Methods
We conducted a literature review using mainly PubMed to compare the current practice with the available evidence. EMBASE and Cochrane library were also searched. We concurrently developed an online questionnaire to collect data from other Australasian centers regarding their approach to this problem.
Results
We identified 194 studies that were considered relevant to our research. We did not find any formal protocols published in the literature. From our Australasian experience; seven centers (58%) had a protocol for AF prophylaxis. The protocols included electrolytes replacement, use of amiodarone and/or β-blockers. Other strategies were occasionally used but were not part of a structured protocol.
Conclusion
The development of an integrated medical and surgical protocol for the prophylaxis of AF after cardiac surgery is an important aspect for the care of postoperative cardiac patients. Considerations of prophylactic strategies other than those routinely used should be included in the protocol. This area should receive considerable attention in order to reduce the postoperative complications and health costs.
Keywords: Atrial fibrillation, Arrhythmia, Cardiac surgery, Prevention of atrial fibrillation, Post operative atrial fibrillation
Abbreviation
- AF
Atrial fibrillation
Introduction
Cardiac surgery is associated with a high risk of postoperative atrial fibrillation (AF). The incidence is estimated to be around 30–40% with the greatest risk among patients undergoing valvular heart surgery [1].
AF causes a huge financial burden on the health budget. We could not find any recent data about the cost of postoperative AF. A few studies looked into the cost of AF in a general sense. The cost of additional days in-hospital, including intensive care unit care alone, adds a significant cost given the frequency of the problem. Two old studies in the USA found that, although the cost of postoperative AF in an individual patient is low in comparison to other postoperative complications, the frequency of the problem is high and therefore the total cost of AF is higher than any other complication [2], [3].
Methods
We performed a literature review about the current medical and surgical AF prophylactic strategies. We used Medline as the main search engine. EMBASE and Cochrane library were also searched. We used the keywords “atrial fibrillation” and “cardiac surgery.” The search was filtered to articles on humans and those published in English in the last 5 years. The search was completed in December 2016. We compared the current practice to our protocol in Auckland City Hospital, New Zealand, and 11 other cardiac intensive care units in Australia and New Zealand. We obtained the information from other Australasian centers through an online questionnaire that we developed. This included basic information about their centers, whether or not they had protocols and what these protocols include, specific questions about the use of some medications and/or surgical techniques, and any other approaches they might have.
Results
In the literature search, 1687 articles were identified in the initial search. Of those, only 194 were found to be relevant to our research. We manually went through the abstracts of all those articles and found that most of them did not address the topic of interest.
We had information about 12 out of the 36 training-accredited cardiac intensive units across Australia and New Zealand. Nine of those centers were in Australia. Six of the hospitals had 300–600 cardiac operations/year. Seven centers (58%) already had protocols for AF prophylaxis after cardiac surgery. These protocols included the supplementation of magnesium and electrolytes, routine use of β-blockers and amiodarone. Magnesium was used routinely in 66% of centers whether or not it was part of the protocol. Beta-blockers were used routinely in four centers as part of AF prophylaxis protocol. Amiodarone was used routinely in one center (Auckland) unless otherwise contraindicated, four other centers used it occasionally (Table 1).
Table 1.
Use of medications and electrolytes across different centers for the prophylaxis of AF.
| No | Rarely | Occasionally | Routinely | |
|---|---|---|---|---|
| Amiodarone | 5 (41.7%) | 2 (16.7%) | 4 (33.3%) | 1 (8.3%) |
| Magnesium | 1 (8.3%) | 1 (8.3%) | 2 (16.7%) | 8 (66.7%) |
| Steroids | 10 (83.3%) | 1 (8.3%) | 1 (8.3%) | 0 |
| Colchicine | 11 (91.7%) | 0 | 1 (8.3%) | 0 |
| Ranolazine | 12 (100%) | 0 | 0 | 0 |
| Antioxidants | 10 (83.3%) | 1 (8.3%) | 1 (8.3%) | 0 |
| Carvedilol | 10 (83.3%) | 1 (8.3%) | 1 (8.3%) | 0 |
| Other β-blockers | 3 (25%) | 2 (16.7%) | 3 (25%) | 4 (33.3%) |
| Other antiarrhythmic | 8 (66.7%) | 4 (33.3%) | 0 | 0 |
Steroids, other antiarrhythmic medications, and antioxidants were not routinely used in Australasia. A few comments were made with regards to the protocols keeping the potassium above 4.5 mM.
Surgical approaches were rarely used as a prophylactic measure for AF. The addition of surgical maze or intraoperative ablation to high-risk patients or patients with previous history of AF was done in some cases. Uniatrial epicardial pacing was used routinely in one center and biatrial pacing was used occasionally in two centers.
Discussion
There are a number of hypotheses as to why there is an increase in AF after cardiac surgery. Classic AF risk factors such as age and cardiac structural changes increase the incidence of AF. Inflammation is the most commonly postulated mechanism for an increase in AF after cardiac surgery, associated with abnormal intracardiac conduction in animal studies [4]. Inflammation is probably caused by direct heart manipulation during surgery and the use of cardiopulmonary bypass. Other potential factors that were described in previous studies include alteration in oxidative stress, electrical remodeling, atrial incision associated factors, and perioperative ischemia [5]. Understanding these factors has led to extensive research on the medications that may be used to alleviate this problem.
The use of certain medications and/or surgical techniques prophylactically remains a controversial issue. We did not find any clear guidelines published in literature in this area. About half of the Australasian centers had developed local protocols. The protocols were based mainly on some evidence associated with the use of amiodarone and magnesium supplementation.
Several other medical and surgical strategies have been tried in the prophylaxis of AF after cardiac surgery. We will discuss those strategies in further detail under separate headings.
Amiodarone
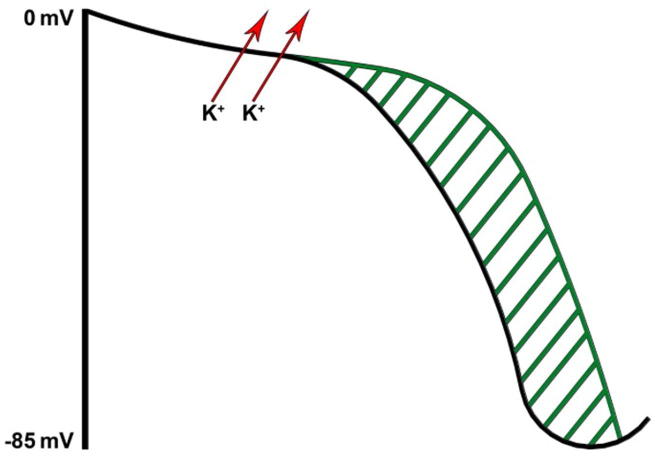
Amiodarone is a Class III antiarrhythmic that works by prolonging the action potential duration and hence refractory period of atrial, nodal, and ventricular tissues. It therefore has a broad spectrum of activity. An increase in the refractory period of the atrial cells is the major contributing action to the control of atrial tachyarrhythmias (Fig. 1).
Figure 1.
Ventricular action potential and effect of Class III antiarrhythmic prolonging refractory period.
Amiodarone has been extensively studied in the literature in its role in AF prophylaxis after cardiac surgery. Studies demonstrated a significant effect of amiodarone in reducing the rates of AF after cardiac surgery and shortening hospital stays [6]. The dose of amiodarone given varied between studies and a meta-analysis showed that high doses of amiodarone (>5-g loading dose) was associated with absolute risk reduction of 15% in comparison to low dose (<3-g loading dose) [7]. The duration of treatment is an area of uncertainty but usually ranges from a few days to a few weeks. Despite the short treatment course, concerns remain regarding amiodarone related toxicities. As the drug has a long half-life (average 40–55 days) with complex pharmacokinetics, side effects can occur at an early stage or be observed many years after drug cessation.
Magnesium
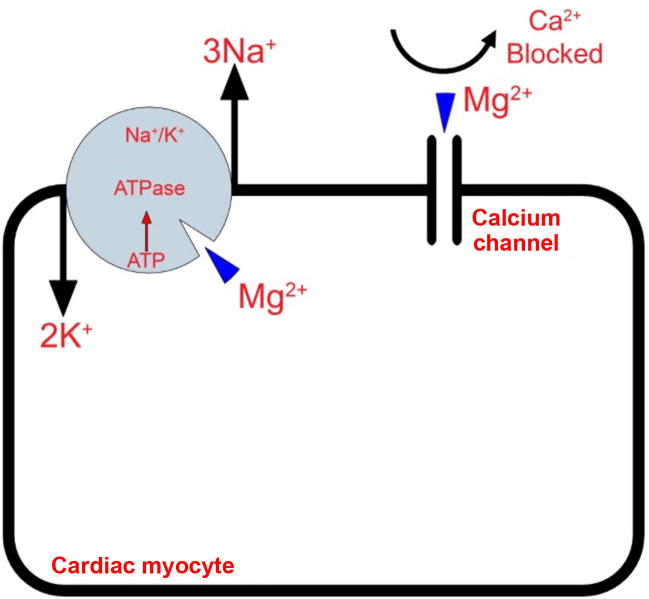
Intravenous magnesium has been shown to be effective in the prevention of AF after cardiac surgery [8]. The magnesium serum levels after cardiopulmonary bypass is often low [9] and therefore we think that routine magnesium supplementation is justifiable. There are several mechanisms by which magnesium is believed to exert its effects as antiarrhythmic. An important mechanism is that magnesium plays a role in Ca2+ transport by antagonizing its channels. This results in a reduction in Ca2+ intracellular load and therefore reduction in energy demands [10] (Fig. 2). Another important mechanism is maintaining electrical stability of the myocytes and Purkinje fibers as a cofactor in Na-K-ATPase pump. A strong relationship was found between low magnesium levels after cardiac surgery and Q-wave myocardial infarction and all-cause mortality at 1 year [11].
Figure 2.
Magnesium (Mg2+) as a cofactor has many roles including ATPase activity, regulation of contractile proteins and sarcoplasmic reticular membrane transport of calcium (Ca2+). ATP = adenosine triphosphate.
Beta-blockers
Withdrawal from β-blockers was proposed a risk factor for developing AF after cardiac surgery [12]. The same study showed that those who are not on β-blockers preoperatively had a longer hospital stay when metoprolol was used. Overall, β-blockers are effective in the prophylaxis of AF after cardiac surgery, with carvedilol being more effective than metoprolol [13] which is probably due to its Class III antiarrhythmic effect. The negative hemodynamic effects of β-blockers in unstable postsurgical makes it somewhat less favorable. Landilol, a new ultrashort acting β-blocker that can be titrated against the blood pressure shows promising results [14]. The routine use of β-blockers is usually limited by the hemodynamic status and cardiac conductive issues in the postoperative period.
Anti-inflammatory medications
The use of cardiopulmonary bypass with its associated ischemia and reperfusion leads to a systemic inflammatory response [15], which is a factor to the development of AF as discussed earlier. Steroids have been used for a long time to dampen that response. There are conflicting data in literature about the utility of postoperative steroids. A recent large study did not support the use the steroids in that situation [16].
Colchicine was also used due to its anti-inflammatory properties with conflicting evidence as well. A recent randomized controlled trial suggested that there is no significant benefit of colchince in the prevention of postoperative AF [17].
Antioxidants
Multiple antioxidant medications were trialed under the hypothesis that AF after cardiac surgery is partially due to increased oxidative stress [18]. There are a few studies on N-acetylcystine, omega-3 fatty acids, and vitamin C. There is some conflicting evidence in their use but overall these medications have a low-risk profile and low cost.
Statins
Statins were also used due to their anti-inflammatory and antioxidant effect. There is some evidence to justify their use [19], [20]. A recent study [21], however, showed that perioperative statins for elective cardiac surgery did not reduce postoperative AF and those who had statins were at higher risk of postoperative acute kidney injury.
We think that at least for patients undergoing coronary artery bypass grafting with lower risk of acute kidney injury, prescribing these medications perioperatively should be considered. Most of this cohort will be on statins already and the optimum time to restart the statin therapy is uncertain.
Ranolazine
Ranolazine is a sodium channel inhibitor that has antianginal and antiarrhythmic properties [22]. In recent years, this drug gained interest in the management of AF. There are some studies showing significant effect in the reduction AF after cardiac surgery [23].
Biatrial pacing
Prophylactic pacing may reduce the incidence of AF after cardiac surgery by several mechanisms that include preventing bradycardia-induced AF and overdrive the suppression of ectopic beats [24]. Earlier studies suggested that biatrial pacing has the advantage of promoting interatrial synchrony [25] that overall decreases the chance of the AF development and therefore should be the preferred method of pacing. Although there were some negative studies this could be explained by technical faults that may lead to a proarrhythmic effect of pacing [26]. Appropriate surgical epicardial lead placement and routine sensing and pacing threshold checks postoperatively should make this technique safe and an effective way of reducing AF after cardiac surgery.
Surgical techniques
A few novel surgical interventions have been used in the last few years that are showing promising results with minimal adverse effects. These include amiodarone-eluting biological adhesive [27] and botulinum toxin injection in epicardial fat pads [28]. Older surgical approaches such as leaving the posterior pericardium open are still performed with significant effect in AF prophylaxis after cardiac surgery [29]. None of the novel techniques are used in the Australasian centers but posterior pericardiotomy is still commonly performed.
Once AF occurs after cardiac surgery then the issue of rate control versus rhythm control is raised. A recent large randomized trial showed no overall advantage of a strategy over the other [30]. At our center, we do not have a protocol for the management of AF after its occurrence and there is huge variability among clinicians in the treatment approach. We usually tend to adopt a rhythm control strategy with amiodarone or direct current cardioversion for reasons that include avoiding the use of long-term anticoagulation, which is generally preserved for cases of permanent or recurrent AF after cardiac surgery.
Conclusion
It is highly important for each cardiac center to have a well-developed protocol for AF prophylaxis after cardiac surgery that ideally should start in the perioperative period. This should improve the surgical outcome and reduce healthcare cost.
We believe that prophylactic protocols should include the routine magnesium supplementation postoperatively ± potassium if indicated. Short-term amiodarone and early reinstitution of β-blockers should be considered and given unless otherwise contraindicated. The hemodynamic and the cardiac conduction status of the patient are important factors to be considered when using these medications. Our protocols do not take into account the left ventricular function when considering prophylactic measures. Restarting statins should be considered on case by case basis as there is conflicting evidence regarding its benefit. Steps to ensure the adherence to these protocols such as the use of preprinted drug charts were used at our center and at least one other center are extremely useful.
Surgical approaches may be considered on a case by case basis and more attention should be made to the mode of pacing after cardiac surgery. There are new techniques on the horizon that will likely benefit this field and more studies are needed.
Conflicts of interest
All authors have no conflicts of interest to declare.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Mathew J.P., Fontes M.L., Tudor I.C., Ramsay J., Duke P., Mazer C.D. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–1729. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 2.Taylor G.J., Mikell F.L., Moses H.W., Dove J.T., Katholi R.E., Malik S.A. Determinants of hospital charges for coronary artery bypass surgery: the economic consequences of postoperative complications. Am J Cardiol. 1990;65:309–313. doi: 10.1016/0002-9149(90)90293-a. [DOI] [PubMed] [Google Scholar]
- 3.Mauldin P.D., Weintraub W.S., Becker E.R. Predicting hospital costs for first-time coronary artery bypass grafting from preoperative and postoperative variables. Am J Cardiol. 1994;74:772–775. doi: 10.1016/0002-9149(94)90432-4. [DOI] [PubMed] [Google Scholar]
- 4.Ishii Y., Schuessler R.B., Gaynor S.L., Yamada K., Fu A.S., Boineau J.P. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005;111:2881–2888. doi: 10.1161/CIRCULATIONAHA.104.475194. [DOI] [PubMed] [Google Scholar]
- 5.Loh S.H., Jin J.S., Tsai C.S., Chao C.M., Tsai Y., Chen W.H. Possible underlying mechanism for hydrogen peroxide-induced electromechanical suppression in human atrial myocardium. J Pharmacol Sci. 2003;91:53–60. doi: 10.1254/jphs.91.53. [DOI] [PubMed] [Google Scholar]
- 6.Aasbo J.D., Lawrence A.T., Krishnan K., Kim M.H., Trohman R.G. Amiodarone prophylaxis reduces major cardiovascular morbidity and length of stay after cardiac surgery: a meta-analysis. Ann Intern Med. 2005;143:327–336. doi: 10.7326/0003-4819-143-5-200509060-00008. [DOI] [PubMed] [Google Scholar]
- 7.Onalan O., Lashevsky I., Crystal E. Prophylaxis and management of postoperative atrial fibrillation. Curr Cardiol Rep. 2005;7:382–390. doi: 10.1007/s11886-005-0093-4. [DOI] [PubMed] [Google Scholar]
- 8.Gu W.J., Wu Z.J., Wang P.F., Aung L.H., Yin R.X. Intravenous magnesium prevents atrial fibrillation after coronary artery bypass grafting: a meta-analysis of 7 double-blind, placebo-controlled, randomized clinical trials. Trials. 2012;13:41. doi: 10.1186/1745-6215-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casthely P.A., Yoganathan T., Komer C., Kelly M. Magnesium and arrhythmias after coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 1994;8:188–191. doi: 10.1016/1053-0770(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 10.Caputo M., Santo K.C., Angelini G.D., Fino C., Agostini M., Grossi C. Warm-blood cardioplegia with low or high magnesium for coronary bypass surgery: a randomised controlled trial. Eur J Cardiothorac Surg. 2011;40:722–729. doi: 10.1016/j.ejcts.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Booth J.V., Phillips-Bute B., McCants C.B., Podgoreanu M.V., Smith P.K., Mathew J.P. Low serum magnesium level predicts major adverse cardiac events after coronary artery bypass graft surgery. Am Heart J. 2003;145:1108–1113. doi: 10.1016/S0002-8703(03)00077-2. [DOI] [PubMed] [Google Scholar]
- 12.Crystal E., Thorpe K.E., Connolly S.J., Lamy A., Cybulsky I., Carroll S. Metoprolol prophylaxis against postoperative atrial fibrillation increases length of hospital stay in patients not on pre-operative beta blockers: the beta blocker length of stay (BLOS) trial. Heart. 2004;90:941–942. doi: 10.1136/hrt.2003.016048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozaydin M., Icli A., Yucel H., Akcay S., Peker O., Erdogan D. Metoprolol vs. carvedilol or carvedilol plus N-acetyl cysteine on post-operative atrial fibrillation: a randomized, double-blind, placebo-controlled study. Eur Heart J. 2013;34:597–604. doi: 10.1093/eurheartj/ehs423. [DOI] [PubMed] [Google Scholar]
- 14.Osumi M., Tashiro T., Morita Y., Kamiya S., Minematsu N., Nishimi M. Preventive effect of intraoperative landiolol administration on atrial fibrillation after off-pump coronary artery bypass grafting. Adv Ther. 2014;31:1109–1117. doi: 10.1007/s12325-014-0158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halter J., Steinberg J., Fink G., Lutz C., Picone A., Maybury R. Evidence of systemic cytokine release in patients undergoing cardiopulmonary bypass. J Extra Corpor Technol. 2005;37:272–277. [PMC free article] [PubMed] [Google Scholar]
- 16.Van Osch D., Dieleman J.M., van Dijk D., Jacob K.A., Kluin J., Doevendans P.A. Dexamethasone for the prevention of postoperative atrial fibrillation. Int J Cardiol. 2015;182:431–437. doi: 10.1016/j.ijcard.2014.12.094. [DOI] [PubMed] [Google Scholar]
- 17.Imazio M., Brucato A., Ferrazzi P., Pullara A., Adler Y., Barosi A. Colchicine for prevention of postpericardiotomy syndrome and postoperative atrial fibrillation: the COPPS-2 randomized clinical trial. JAMA. 2014;312:1016–1023. doi: 10.1001/jama.2014.11026. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigo R., Korantzopoulos P., Cereceda M., Asenjo R., Zamorano J., Villalabeitia E. A randomized controlled trial to prevent post-operative atrial fibrillation by antioxidant reinforcement. J Am Coll Cardiol. 2013;62:1457–1465. doi: 10.1016/j.jacc.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Dehghani M.R., Kasianzadeh M., Rezaei Y., Sepehrvand N. Atorvastatin reduces the incidence of postoperative atrial fibrillation in statin-naive patients undergoing isolated heart valve surgery: a double-blind, placebo-controlled randomized trial. J Cardiovasc Pharmacol Ther. 2015;20:465–472. doi: 10.1177/1074248414564869. [DOI] [PubMed] [Google Scholar]
- 20.Chen W.T., Krishnan G.M., Sood N., Kluger J., Coleman C.I. Effect of statins on atrial fibrillation after cardiac surgery: a duration- and dose-response meta-analysis. J Thorac Cardiovasc Surg. 2010;140:364–372. doi: 10.1016/j.jtcvs.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Z., Jayaram R., Jiang L., Emberson J., Zhao Y., Li Q. Perioperative rosuvastatin in cardiac surgery. N Engl J Med. 2016;374:1744–1753. doi: 10.1056/NEJMoa1507750. [DOI] [PubMed] [Google Scholar]
- 22.Burashnikov A., Di Diego J.M., Zygmunt A.C., Belardinelli L., Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–1457. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammond D.A., Smotherman C., Jankowski C.A., Tan S., Osian O., Kraemer D. Short-course of ranolazine prevents postoperative atrial fibrillation following coronary artery bypass grafting and valve surgeries. Clin Res Cardiol. 2015;104:410–417. doi: 10.1007/s00392-014-0796-x. [DOI] [PubMed] [Google Scholar]
- 24.Murgatroyd F.D., Nitzsché R., Slade A.K., Limousin M., Rosset N., Camm A.J. A new pacing algorithm for overdrive suppression of atrial fibrillation. Chorus Multicentre Study Group. Pacing Clin Electrophysiol. 1994;17:1966–1973. doi: 10.1111/j.1540-8159.1994.tb03782.x. [DOI] [PubMed] [Google Scholar]
- 25.Saksena S., Prakash A., Hill M., Krol R.B., Munsif A.N., Mathew P.P. Prevention of recurrent atrial fibrillation with chronic dual-site right atrial pacing. J Am Coll Cardiol. 1996;28:687–694. doi: 10.1016/0735-1097(96)00232-x. [DOI] [PubMed] [Google Scholar]
- 26.Kurz D.J., Naegeli B., Kunz M., Genoni M., Niederhäuser U., Bertel O. Epicardial, biatrial synchronous pacing for prevention of atrial fibrillation after cardiac surgery. Pacing Clin Electrophysiol. 1999;22:721–726. doi: 10.1111/j.1540-8159.1999.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 27.Feng X.D., Wang X.N., Yuan X.H., Wang W. Effectiveness of biatrial epicardial application of amiodarone-releasing adhesive hydrogel to prevent postoperative atrial fibrillation. J Thorac Cardiovasc Surg. 2014;148:939–943. doi: 10.1016/j.jtcvs.2014.05.049. [DOI] [PubMed] [Google Scholar]
- 28.Pokushalov E., Kozlov B., Romanov A., Strelnikov A., Bayramova S., Sergeevichev D. Botulinum toxin injection in epicardial fat pads can prevent recurrences of atrial fibrillation after cardiac surgery: results of a randomized pilot study. J Am Coll Cardiol. 2014;64:628–629. doi: 10.1016/j.jacc.2014.04.062. [DOI] [PubMed] [Google Scholar]
- 29.Biancari F., Mahar M.A. Meta-analysis of randomized trials on the efficacy of posterior pericardiotomy in preventing atrial fibrillation after coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2010;139:1158–1161. doi: 10.1016/j.jtcvs.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Gillinov A.M., Bagiella E., Moskowitz A.J., Raiten J.M., Goh M.A., Bowdish M.E. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N Engl J Med. 2016;374:1911–1921. doi: 10.1056/NEJMoa1602002. [DOI] [PMC free article] [PubMed] [Google Scholar]