Abstract
Background
The aim of this study was to evaluate the acute and short-term outcomes of transcatheter closure of secundum atrial septal defect (ASD) in children and adolescents in the first 4-year experience in two institutional centers in Upper Egypt.
Methods
This was a retrospective cohort study including 135 children and adolescents who underwent ASD closure between April 2012 and May 2016. A review of the acute and short-term outcomes and adverse events was performed.
Results
The patients had a median age of 5 years (interquartile range: 3–9 years), 71% of patients were ≤5 years, and median weight was 17 kg (interquartile range: 13–30 kg). Single defects were observed in 113 patients (84%). The remainder had multiple or multifenestrated defects that were closed by a single device. The mean defect size of single defects and the mean interatrial septum length were 15.24 ± 5.16 mm and 38.13 ± 6.3 mm, respectively. The ratio of device to TEE (Transoesophageal echocardiography) size of ASD was 1.19 ± 0.12. The devices were implanted successfully in 98.5% of patients. Six cases had concordant PS (Pulmonary stenosis), patent ductus arteriosus or perimembranous ventricular septal defect and were treated with balloon dilation, or closure. No residual flow was seen after device placement except in one patient with multiple fenestrations. There were five high-severity adverse events (3.7%) with no mortality. Device erosion was confirmed in one of two patients with massive haemopericardium; embolization of the device with retrieval in one patient; and heart block was detected in two cases. No cardiac perforation, device erosion, embolization, thrombus formation, or clinical evidence of bacterial endocarditis was observed during follow-up.
Conclusions
Transcatheter closure of ASDs in children and adolescents was feasible and safe in the first 4 years experience in our centers, with good short-term outcome. Balloon sizing is not necessary for transcatheter closure of secundum ASD. Multiple defects can be safety closed by a single device.
Keywords: Adverse events, Atrial septal defect closure, Heart block, Short-term outcomes
Abbreviations
- ASD
Atrial Septal Defect
- IAS
Interatrial Septum
- PDA
Patent Ductus Arteriosus
- PM VSD
Perimemberanous Ventricular Septal Defect
- HB
Heart Block
- ASO
Atrial Septal Occluder
- AE
Adverse Event
- CP
Cardiac Perforation
Introduction
Atrial septal defects (ASDs) have a reported prevalence of 10% among congenital heart defects and if left untreated, although recognized as a benign disease, can contribute to significant morbidity and mortality [1], [2].
For many decades, surgical intervention for ASD has been accepted as the standard treatment with excellent outcomes. However, although surgical treatment is safe, it is associated with morbidity and thoracotomy scars [3]. Successful nonsurgical closure of ASD was first described in 1974 by King and Mills [4]. Many studies have demonstrated that individual devices provide safe and efficacious alternatives to surgical closure of secundum ASD [5], [6], [7]. In developing countries, obstacles to surgical procedures include the limited number of intensive care unit beds, cardiac surgeons, intensivists, and other resources. Therefore, current advances in nonsurgical treatment of ASD with devices have become the treatment of choice in countries with limited resources [8]. In this study, we evaluated the acute and short-term outcomes of transcatheter closure of ASD in children and adolescents during the first 4-years experience in two centers in Egypt at Sohag and Assiut University Hospitals.
Methods
Study design
The present study was designed as a retrospective cohort study that included 135 patients in two referral centers in Egypt. Informed consent was obtained from parents of children and adolescents. The local ethics committees approved this study.
Inclusion criteria included children and adolescents with a clinical diagnosis of single or multiple ASDs with left-to-right shunt and right ventricular volume overload on echocardiography.
Occluders
The amplatzer septal occluder (ASO), cribriform ASD occluder and delivery system (AGA Medical, Golden Valley, MN, USA) have been used in most patients. The Figulla–Occlutech Device (FOD; Occlutech, Jena, Germany) was implanted only in three patients. The reason for the choice of ASO in most of the cases of this series in the intial experience of these centers is due to its ease of use and relatively short learning curve for practitioners' training.
Preimplantation protocol
All patients were evaluated with transthoracic two-dimensional and color Doppler echocardiography with multiple subxyphoid and precordial windows. Each of the following criteria had to be fulfilled prior to inclusion: (1) the presence of a single or multiple ostium secundum ASDs with left-to-right shunt and right ventricular volume overload on echocardiography; (2) a distance of > 5 mm from the margins of the defect to the mitral and tricuspid valves, superior vena cava, right upper pulmonary vein, and coronary sinus; (3) ASD size > 8 mm; and (4) adequate interatrial septal length, measured mainly by multiplane transthoracic echocardiography (TTE).
Exclusion criteria included other types of ASDs (sinus venosus or ostium primum), associated cardiac diseases that required surgical repair, pulmonary vascular resistance >8 Woods Units , and active infectious diseases. The patients underwent ASD closure but the procedure was aborted due to secundum ASDs that were considered to be unsuitable for transcatheter closure: too large in relation to the size of the patient, especially in small (10–15 kg) children in whom the ratio of device/body weight was >1.5; ASDs with defecient contralateral rims.
Routine examination before catheterization included standard 12-lead electrocardiography, chest X-ray, and TTE. Complete blood counts, prothrombin time, prothrombin concentration, partial thromboplastin time, and international normalized ratio were performed to exclude bleeding disorders.
Procedure
All patients received intravenous claforan injection (50 mg/kg) and unysun (ampicillin/sulbactam) (100 mg/kg) 30 min before the procedure. Intravenous heparin was injected (100 mg/kg) to achieve a therapeutic level of anticoagulation (activated clotting time >250 s).
The procedure was performed under general anesthesia. The device was deployed under fluoroscopy; LAO (left anterior oblique) cranial (35°, 30°) and TEE (transoesophageal echocardiography) guidance (Fig. 1). Multiplane TEE using Vivid S5 ultrasound systems (GE Medical Systems, Horton, Norway) was performed in all patients except two small children with good rims, in whom the procedure was done under TTE guidance. The dimensions of the defect were measured in various imaging planes of TEE. The maximal diameter of the defect was measured using atrial end-diastolic frames in 0°, 45°, 90°, and 135°. In the presence of a floppy and mobile rim, measurement of defect diameter was made between steadier and firm rims and the color flow jet width across the defect was also measured to provide supplementary information. The largest measurment was used to select device size. A device was choosed that was 20–25% larger than the largest diameter. Twenty percent was chosen if all rims of secundum ASD were preserved, except the retroaortic rim. Twenty-five percent was chosen in case of presence of two defecient rims. In small children aged <5 years, the device was used that equal or maximum 2mm larger than the largest measurment of secundum ASD. The balloon sizing was not used in assessment of defect size. Device implantation was performed using several established techniques according to previously published protocols [9].
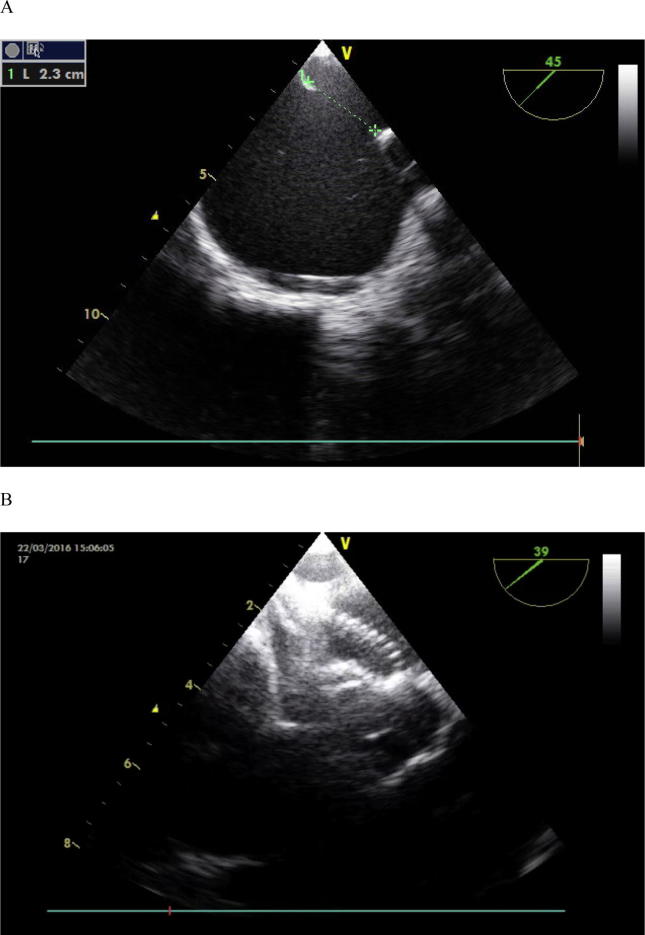
Figure 1.
Large atrial septal defect using TEE monitoring: (A) Short axis view showing the diameter of defect was 2.3 mm. (B) Short axis view showing good position of device after its deployment. TEE = transesophageal echocardiography.
Patients were monitored for 24 hours and TTE with color Doppler was performed after 24 hours to assess the device position, presence of residual shunting, and the relation of the device to adjacent structures. Patients were discharged 48 hours after the procedure. Low-dose aspirin (3–5 mg/kg/day) was given for 6 months. Clopidogrel (2 mg/kg) was administered for 2 months in adolescent patients. Infective endocarditis prophylaxis was advised for 6 months after device implantation.
Follow-up clinical visits were carried out at 1 week, 1 month, 3 months, 6 months and 12 months, and annually thereafter.
We used previously established and tested definitions for adverse events severity for this study that ranged from levels 1 to 5. For this analysis, clinically important higher severity adverse events were defined as level 4 or 5 [10], [11].
Results
Between April 2012 and May 2016, transcatheter closure of secundum ASD was attempted in 135 patients. The patient demographic data are summarized in Table 1. Seventy-one patients were older than 5 years of age and 63% weighed ≤15 kg. Nine patients had noncardiac comorbidity; four had Down syndrome, one had Noonan syndrome, one had kyphoscoliosis, one had thalassemia, one had renal failure on hemodialysis, and one had cerebral palsy.
Table 1.
Demographic data of patients.
| Variable | Median (IQR)/no. (%) |
|---|---|
| Sex (F/M) | (74/61) |
| Age (y) | 5 (3–9) |
| ≤5 | 71 (53%) |
| 5–10 | 33 (24%) |
| 10–20 | 31 (23%) |
| Weight (kg) | 17 (13–30) |
| <15 | 63 (47%) |
| >15 | 72 (53%) |
| Noncardiac comorbidity | 9 (6.7%) |
| Down syndrome | 4 |
| Noonan syndrome | 1 |
| Kyphoscoliosis | 1 |
| Thalassemia | 1 |
| Renal failure and haemodiaylsis | 1 |
| Cerebral palsy | 1 |
F = female; IQR = interquartile range; M = male.
Table 2 summarizes the ASD characteristics of the 135 cases with a single treated defect. The mean ASD size by TTE was 12.83 ± 4.48 mm, the mean ASD size by TEE was 15.24 ± 5.16 mm, and the mean device/defect ratio was 1.19 ± 0.12. Successful closure of secundum ASD was achieved in 98.5% of patients. Thirteen patients had fenestrated ASD, which was closed by Amplatzer cribriform device (Fig. 2). Nine patients had two defects with a distance < than 7 mm. Four of them were closed by Amplatzer Cribriform device, and another five patients were closed by one device. Seventy-one of these patients (71%) had a deficient aortic rim. Two patients required a change to a larger device from their initial device. ASD in one of these patients was closed by a 28-mm device, which was replaced by a 34-mm device, and ASD in the other patient was closed first by a 20-mm device, which was replaced by a 26-mm device. The wide discrepancy between the initial selected size of the first device and the replacement device was due to underestimation of ASD size and sometimes enlargement of the defect occurred due to tearing of the septum by repeated closure attempts.
Table 2.
ASD and procedure characteristics.
| Variable | Mean ± SD/no. (%) |
|---|---|
| ASD diameter by TTE, mm | 12.83 ± 4.48 |
| ASD diameter by TEE, mm | 15.24 ± 5.16 |
| IAS length, mm | 38.13 ± 6.3 |
| Device diameter, mm | 20 ± 7.16 |
| Device/defect diameter | 1.19 ± 0.12 |
| Single defect | 110 (84%) |
| Multiple defects | 10 (7%) |
| Multifenestrated IAS | 13 (10%) |
| Aneurysmal floppy septum | 14 (10.5%) |
| Defecient retroaortic rim | 71 (53%) |
| Combined procedure | 6 (4.4%) |
| PDA closure | 2 |
| VSD closure | 1 |
| Pulmonary valvuloplasty | 3 |
| Qp/Qs | 1.7 ± 0.8 |
| Procedure time | 40 ± 15 |
| Fluroscopy time | 7 ± 4.3 |
| High-severity adverse events | 5 (3.7%) |
ASD = atrial septal defect; IAS = interatrial septum; PDA = patent ductus arteriosus; Qp/Qs = pulmonary to systematic flow ratio; SD = standard deviation; TEE = transoesophageal echocardiography; TTE = transthoracic echocardiography; VSD = ventricular septal defect.
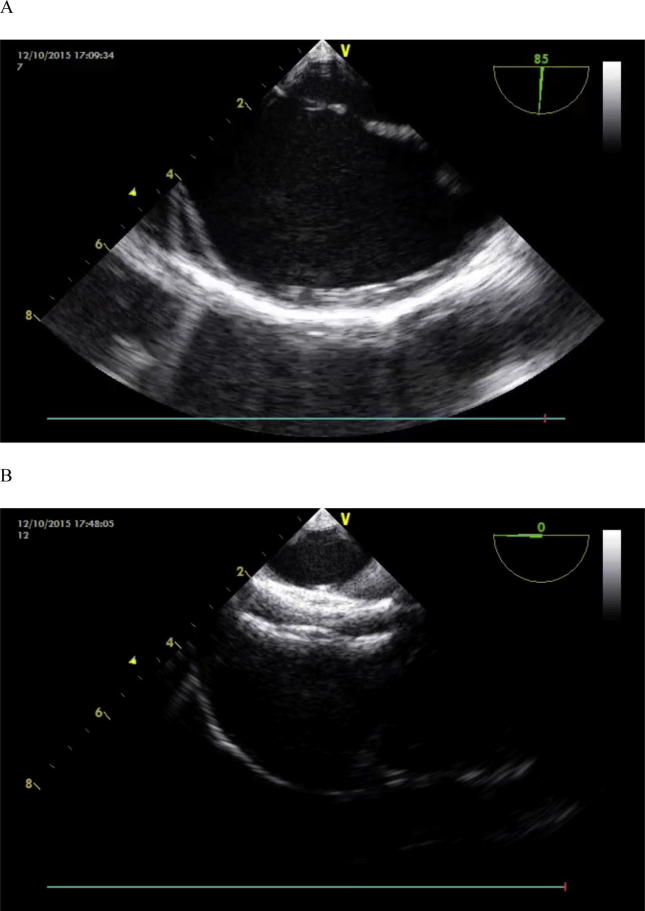
Figure 2.
Multiple atrial septal defect closure using TEE monitoring. (A) In short axis view both defects were separated by a rim of tissue <6 mm. (B) In 0 view good position of cribriform ASO after release of device. ASO = amplatzer septal occluder ; TEE = transesophageal echocardiography.
Nine patients required a right pulmonary vein technique for closing secundum ASD. ASDs in 11 patients were closed using the left pulmonary vein. ASDs in two patients were closed by a balloon-assisted technique.
In a 2-year-old boy weighing 12 kg with an aneurysm of the interatrial septum (IAS) with multiple fenestrations, a 25-mm cribriform device was used for initial closure, with repeated prolapse of the device. The device was replaced by an 18-mm device after TEE measurement revealed a larger defect due to aneurysm avulsion, thus, the procedure was abandoned and a recommendation for elective surgical repair was made.
High-severity adverse events were recorded in five of 116 cases (3.7%), with no mortality (Table 3). There was one case of device embolization of the right ventricle in a 14-year-old girl weighing 55 kg who had a large defect with a deficient inferior vena cava rim (28 mm × 26 mm), and a 32-mm device was used for closure of the defect. Successful retrieval using a snare was performed followed by elective surgical repair (Fig. 3). Massive hemopericardium and cardiac tamponade occurred in two patients at 24 hours after device deployment. The first patient was a 6-year-old boy who had a 9-mm secundum ASD that was closed by a 10-mm device. The cause of hemopericardium was not detected as no erosion was seen by TTE. The hemopericardium was treated by pericardiocentesis without device removal. The second patient was a 5-year-old girl who had a 12.5-mm secundum ASD that was closed by a 15-mm device. Pericardiocentesis was performed with accumulation that indicated surgical removal of the device. The erosion was confirmed at surgery, and its location was anteriosuperior (atrial wall–aorta).
Table 3.
Patients and procedural charecteristics with high-severity adverse events.
| Age (y) | ASD diameter (mm) | ASD device diameter (mm) | Time from implantion (h) | Presentation | Intervention and outcome |
|---|---|---|---|---|---|
| 6 | 9 | 10 | 14 | Chest pain, tachycardia diagnosed with pericardial effusion | Pericardiocentesis, no surgical device removal |
| 5 | 12.5 | 15 | 24 | Collapse CPR, convulsion diagnosed with pericardial effusion | Pericardiocentesis followed by reaccumulation, surgical device removal |
| 3 | Aneurysm with multiple fenestration, largest one = 12 mm | Cribriform, 25 | Immediately after device depolyment | Bradycardia, 2nd degree heart block | After 2 d predinsolone, normal heart rate regained |
| 11 | 18 mm | 32 | 18 | Chest pain and discomfort, diagnosed by device embolization | Transcatheter reterieval of device |
| 4 | 19 mm | 24 | Immediately after device depolyment | Bradycardia, complete heart block | Recapture of device and surgical closure of ASD |
ASD = atrial septal defect; CPR = cardiopulmonary resuscitation.
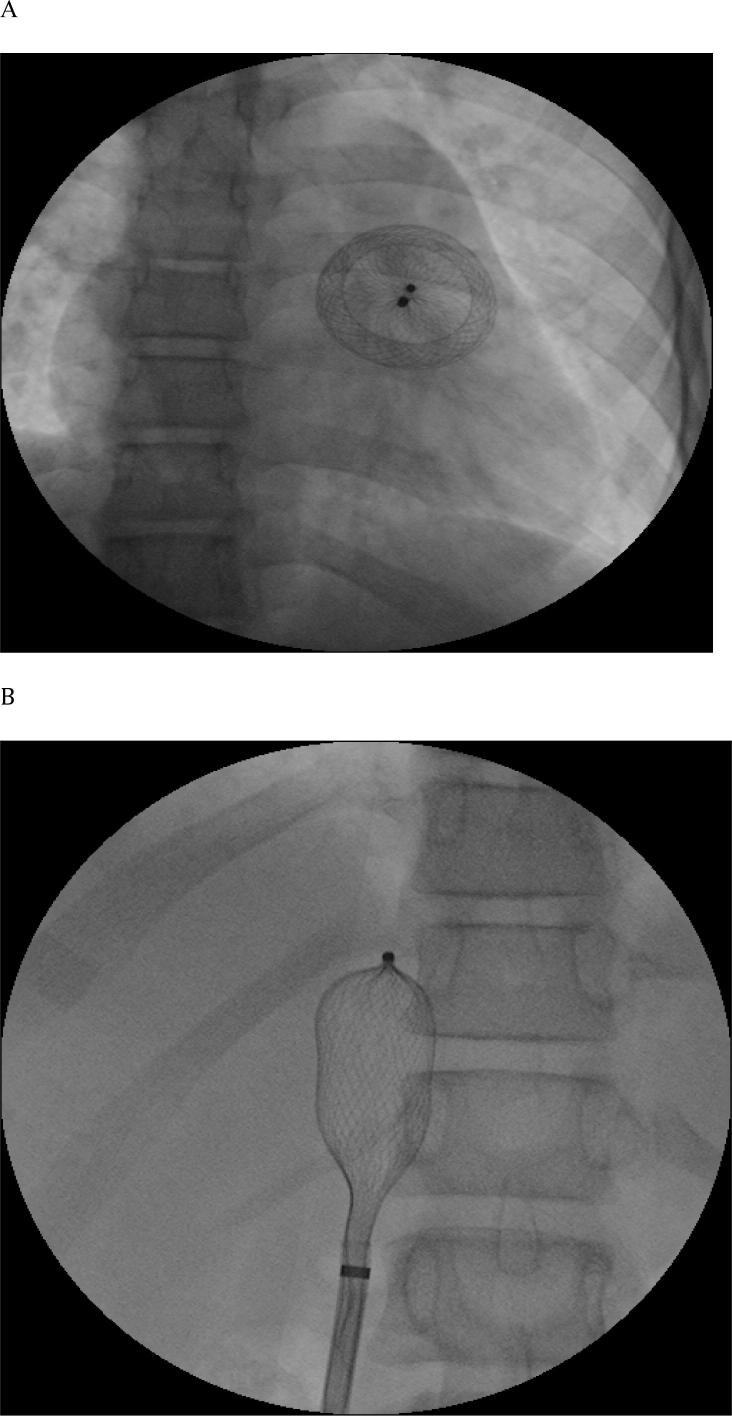
Figure 3.
Embolized device. (A) AP = anterioposterior view showed embolized ASO = amplatzer septal occluder in right ventricle. (B) AP view showed successful retrieval of embolized device into the sheath in IVC = inferior vena cava.
Major conduction abnormality was recorded in two cases. CHB (Complete Heart Block) occurred in one patient immediately after device deployment and the heart rate recovered by the next morning. Second degree heart block occurred in a patient with Down syndrome who had IAS aneurysm with multiple fenestrations that was closed by a 25-mm Cribriform device. The condition was treated with oral steroids and resolved after 2 days. There were three minor adverse events, including: transient arrhythmia resolving spontaneously or with only catheter manipulation (two cases); and rebleeding from the access site (one case).
One patient who had an IAS aneurysm with multiple defects that were closed by a single 28-mm device, 3 mm residual remained at posterior–inferior margin of the defect that uncovered by device after implantation. The residual flow closed during follow up.
Seventy-six (65.5%) patients completed 2 years of follow-up. All patients had sinus rhythm. There was no late embolization, erosion, and thrombus formation on TTE, endocarditis, or atrioventricular valve dysfunction.
Discussion
ASD is the second most common cause of congenital heart disease and its prevalence is ∼1.5 per 1000 live births [12]. Transcatheter closure has become the method of choice to manage most patients with secundum ASDs [13], [14].
In this study, we evaluated procedural characteristics, and acute and mid-term outcomes encountered during percutaneous transcatheter closure of secundum ASDs in 135 children and adolescence during the first 4-years experience at two institutions in Upper Egypt. We found that high-severity AE (Advere Events) rate (levels 3–5) was 3.7% without any mortality.
In the present study, successful closure was achieved in 98.5% of cases. Small residual shunting occurred in one patient who had an IAS aneurysm with multiple defects. Balloon sizing of the defect was not performed in all cases, even in those with complex morphology of the defect, as it was recommended in previous studies [15]. However, recent studies have reported the safety and feasibility of transcatheter closure of ASD without balloon sizing [16], [17].
In our study, there were 21 cases that had multiple or multifenestrated defects that were were closed by a single device; either Cribriform Amplatzer septal occluder or a larger device. This agrees with Hu et al. [18] who reported the safety and efficacy of closure of multiple defects by a single device, with no difference from dual occluders, even though the risk of residual shunting was greater with dual occluders. However, may previous studies have recommended closure of multiple defects with a distance >7 mm with two devices [19].
The most frequent serious complications as reported in a recent analysis of the Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE) database are device dislodgement and cardiac perforation, erosion, or rupture [20], [21]. The rate of confirmed device erosion in the present study was 0.7%. This was higher than in a multicenter study by EL-Said et al. [22], which reported a rate of device erosion of 0.5% in patients with ASO implants.
Technique-related CP (Cardiac Perforation) during catheterization is inherent to the procedure, and typically occurs before hospital discharge and is amenable to intervention. In contrast, device-related CP occurs after a technically adequate procedure, frequently after hospital discharge and has the potential for a fatal outcome [20]. In the present study, hemopericardium occurred early before hospital discharge, but the technique was carried out smoothly without oversizing of the implants and the device splayed promptly over the aortic root. Therefore, no modification of the technique of the procedure occurred, and only a postponing of the discharge of patients 48 hours after the procedure with urgent echocardiography was required in case of device- related CP symptoms.
Other AEs reported in our study were device embolization and heart block. The rate of device embolization was 0.7% with successful retrieval. This rate is higher than the rate of ASO device embolization that has previously been estimated as 0.5–0.6% from MAUDE and survey data [21]. El-Said et al. [22] reported in a multicenter study a higher rate of device embolization of 1.5%. Heart block occurred in two patients (1.5%); one regained normal conduction with medical treatment, and normal heart rate resolved in another patient on the next morning without medical treatment. Fifteen patients (2.1%) developed heart block and 13 patients reverted to normal conduction with observation or medical treatment.
Our study had some limitations, including its retrospective nature and the small number of patients. More studies with a larger number of patients are needed to analyze the safety and efficacy of transcatheter closure of ASD in this age group.
In conclusion, transcatheter closure of secundum ASD in children and adolescents was feasible and safe in the first 4-years experience in our two centers, with good mid-term outcomes. Balloon sizing is not necessary for transcatheter closure of secundum ASD. Multiple ASDs can be closed safety by a single device.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Campbell M. Natural history of atrial septal defect. Br Heart J. 1970;32:820–826. doi: 10.1136/hrt.32.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig R.J., Selzer A. Natural history and prognosis of atrial septal defect. Circulation. 1968;37:805–815. doi: 10.1161/01.cir.37.5.805. [DOI] [PubMed] [Google Scholar]
- 3.Galal M.O., Wobst A., Halees Z., Hatle L., Schmaltz A.A., Khougeer F. Peri-operative complications following surgical closure of atrial septal defects type II in 232 patients: a baseline study. Eur Heart J. 1994;15:1381–1384. doi: 10.1093/oxfordjournals.eurheartj.a060398. [DOI] [PubMed] [Google Scholar]
- 4.Pastorek J.S., Allen H.D., Davis J.T. Current outcomes of surgical closure of secundum atrial septal defect. Am J Cardiol. 1994;74:75–77. doi: 10.1016/0002-9149(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 5.Du Z.D., Hijazi Z.M., Kleinman C.S., Silverman N.H., Larntz K. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults. J Am Coll Cardiol. 2002;39:1836–1844. doi: 10.1016/s0735-1097(02)01862-4. [DOI] [PubMed] [Google Scholar]
- 6.Nugent A., Britt A., Gauvreau K., Piercey G., Lock J., Jenkins Device closure rates of simple atrial septal defects optimized by the STARflex. J Am Coll Cardiol. 2006;48:538–544. doi: 10.1016/j.jacc.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 7.Jones T.K., Latson L.A., Zahn E., Fleishman C.E., Jacobson J., Vincent R. Results of the US multicenter pivotal study of the HELEX septal occluder for percutaneous closure of secundum atrial septal defects. J Am Coll Cardiol. 2007;49:2215–2221. doi: 10.1016/j.jacc.2006.11.053. [DOI] [PubMed] [Google Scholar]
- 8.Putra S.T., Djer M.M., Idris N.S., Samion H., Sastroasmoro S. Transcatheter closure of atrial septal defects in a center with limited resources: outcomes and short term follow-up. Iran J Pediatr. 2015;25:e3906. doi: 10.5812/ijp.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedra C.A., Pedra S.R., Esteves C.A., Cassar R., Pontes S.C., Jr, Braga S.L. Transcatheter closure of secundum atrial septal defects with complex anatomy. J Invasive Cardiol. 2004;16:117–122. [PubMed] [Google Scholar]
- 10.Bergersen L., Marshall A., Gauvreau K., Beekman R., Hirsch R., Foerster S. Adverse event rates in congenital cardiac catheterization – a multi-center experience. Catheter Cardiovasc Interv. 2010;75:389–400. doi: 10.1002/ccd.22266. [DOI] [PubMed] [Google Scholar]
- 11.Bergersen L., Gauvreau K., Foerster S.R., Marshall A.C., McElhinney D.B., Beekman R.H. Catheterization for Congenital Heart Disease Adjustment for Risk Method (CHARM) JACC Cardiovasc Interv. 2011;4:1037–1046. doi: 10.1016/j.jcin.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Van der Linde D., Konings E.E., Slager M.A., Witsenburg M., Helbing W.A., Takkenberg J.J. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–2247. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 13.Feltes T.F., Bacha E., Beekmand R.H., 3rd, Cheatham J.P., Feinstein J.A., Gomes A.S. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2607–2652. doi: 10.1161/CIR.0b013e31821b1f10. [DOI] [PubMed] [Google Scholar]
- 14.Baumgartner H., Bonhoeffer P., De Groot N.M., de Haan F., Deanfield J.E., Galie N. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010) Eur Heart J. 2010;31:2915–2957. doi: 10.1093/eurheartj/ehq249. [DOI] [PubMed] [Google Scholar]
- 15.Vijarnsorn C., Durongpisitkul K., Chanthong P., Chungsomprasong P., Soongswang J., Loahaprasitiporn D. Transcatheter closure of atrial septal defects in children, middle-aged adults, and older adults: failure rates, early complications; and balloon sizing effects. Cardiol Res Pract. 2012;2012:584236. doi: 10.1155/2012/584236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amin Z., Daufors D.A. Balloon sizing is not necessary for closure of secundum atrial septal defects. J Am Coll Cardiol. 2005;45(Suppl 1):317. [Google Scholar]
- 17.Wang J.K., Tsai S.K., Lin S.M., Chiu S.N., Lin M.T., Wu M.H. Catheter Cardiovasc Interv. 2008;71:214–221. doi: 10.1002/ccd.21308. [DOI] [PubMed] [Google Scholar]
- 18.Hu Z., Zhang Y., Zhang T., Cheng G., Xie X., He X. Comparison of the effectiveness and safety of single versus dual occluders for the closure of multiple atrial septal defects. J Invasive Cardiol. 2015;27:E90–E97. [PubMed] [Google Scholar]
- 19.Bramlet M.T., Hoyer M.H. Single pediatric center experience with multiple device implantation for complex secundum atrial septal defects. Catheter Cardiovasc Interv. 2008;72:531–537. doi: 10.1002/ccd.21668. [DOI] [PubMed] [Google Scholar]
- 20.Amin Z., Hijazi Z.M., Bass J.L., Cheatham J.P., Hellenbrand W.E., Kleinman C.S. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: review of registry of complications and recommendations to minimize future risk. Catheter Cardiovasc Interv. 2004;63:496–502. doi: 10.1002/ccd.20211. [DOI] [PubMed] [Google Scholar]
- 21.DiBardino D.J., McElhinney D.B., Kaza A.K., Mayer J.E., Jr. Analysis of the US food and drug administration manufacturer and user facility device experience database for adverse events involving Amplatzer septal occluder devices and comparison with the Society of Thoracic Surgery Congenital Cardiac Surgery database. J Thorac Cardiovasc Surg. 2009;137:1334–1341. doi: 10.1016/j.jtcvs.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Said H., Hegde S., Foerster S., Hellenbrand W., Kreutzer J., Trucco S.M. Device therapy for atrial septal defects in a multicenter cohort: acute outcomes and adverse events. Catheter Cardiovasc Interv. 2015;85:227–233. doi: 10.1002/ccd.25684. [DOI] [PubMed] [Google Scholar]