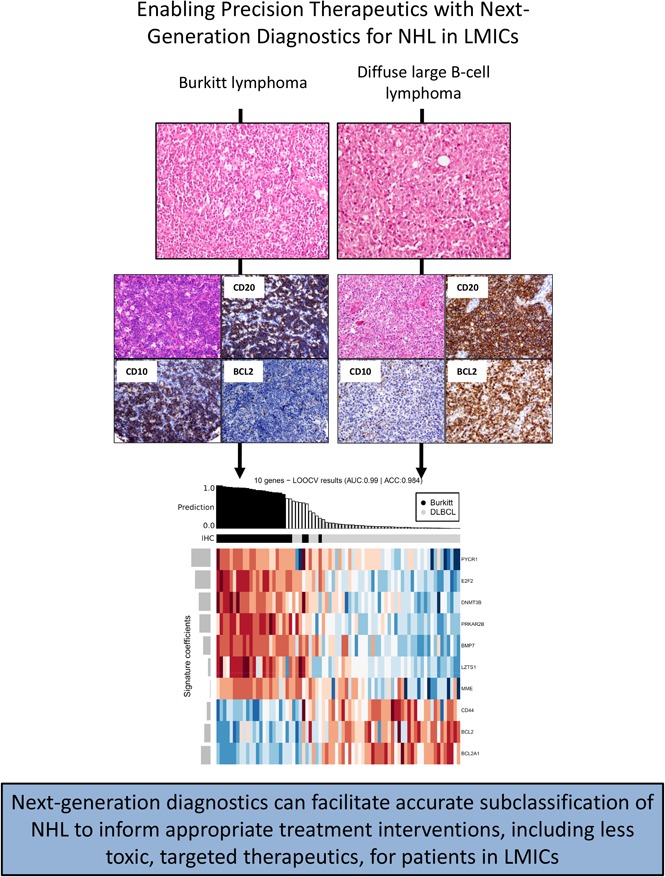
Key Points
NHL subclassification is lacking in Malawi due to resource constraints yet is critical for directing therapy.
Targeted gene expression profiling facilitates objective assessment and segregation of biologically defined subsets of NHL from Malawi.
Abstract
Diagnostics and supportive care for patients with non-Hodgkin lymphoma (NHL) in lower- and middle-income countries (LMICs) are lacking. We hypothesized that high-throughput transcription-based diagnostics could classify NHL specimens from Malawi amenable to targeted therapeutics. We established tissue microarrays and classified 328 cases diagnosed by hematoxylin and eosin as NHL at University of Malawi College of Medicine using immunohistochemistry (IHC) for conventional markers and therapeutic targets. A subset was analyzed using NanoString-based expression profiling with parsimonious transcriptional classifiers. Overall, 72% of lymphomas were high-grade B-cell tumors, subsets of which were enriched for expression of MYC, BCL2, and/or PD-L1. A 21-gene transcriptional classifier, previously validated in Western cohorts, divided 96% of diffuse large B-cell lymphomas (DLBCLs) with 100% of B-cell lymphomas, unclassifiable, into 1 cluster and 88% of Burkitt lymphomas into a separate cluster. Cell-of-origin categorization of 36 DLBCLs by NanoString lymphoma subtyping test (LST) revealed 69% concordance with IHC. All discordant cases were classified as germinal center B cell–like (GCB) by LST but non-GCB by IHC. In summary, utilization of advanced diagnostics facilitates objective assessment and segregation of biologically defined subsets of NHL from an LMIC without expert review, thereby establishing a basis for the implementation of effective and less toxic targeted agents.
Visual Abstract
Introduction
In high-income countries (HICs), diagnosis and classification of non-Hodgkin lymphoma (NHL) incorporates detailed phenotypic and genetic data with morphologic evaluation. This precise classification guides both prognosis and the selection of therapy. In contrast, detailed subtyping in sub-Saharan Africa (SSA), where NHL is the sixth-most common malignancy,1,2 is largely lacking.3 Immunohistochemistry, fluorescence in situ hybridization (FISH), and molecular assay capabilities are almost completely absent from SSA.4 Additionally, most SSA countries employ <1 pathologist per 500 000 people, which is approximately one-tenth the rate in HICs.5 This lack of comprehensive NHL subtyping derives partially from the fact that NHL-related therapeutic interventions are scarce in many SSA countries and treatment with high-dose chemotherapy is impractical due to significant limitations in supportive care; thus, the clinical pressure to fully classify NHLs is greatly diminished compared with HICs.6,7
The nation of Malawi in southeast Africa has a population of ∼16 million and is one of 31 countries worldwide classified as a low-income economy by the World Bank. A recent registry survey of 18 946 new cancer diagnoses among Malawians from 2007 to 2010 revealed that NHL accounted for 5.7% of all new cancer diagnoses and was the most common malignancy among children under age 15 years (56.4% of all pediatric cancer).6 Notably, only 17.9% of all of the registry’s cases were confirmed by pathology data.6
The Department of Histopathology at the University of Malawi College of Medicine (UOMCOM) in Blantyre, Malawi processes ∼3000 specimens per year, and pathologic diagnoses are based solely on interpretation of hematoxylin and eosin (H&E)–stained sections.8,9 Most cases diagnosed as lymphoma at UOMCOM are categorized as Burkitt lymphoma (BL) or non-BL NHL. The majority of treated persons in SSA with non-BL NHL receive CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy.10 High-intensity regimens for BL provided in HICs cannot be given in Malawi because of both the high cost and unacceptable treatment-associated mortality.11 Targeted therapies for NHL that are standard of care in HICs, most notably the anti-CD20 antibody rituximab, have historically been unavailable in countries like Malawi.12 In contrast, several targeted agents with potent activity against specific subsets of NHL and reduced toxicity compared with CHOP are either in clinical trials or recently approved in HICs. We hypothesized that next-generation approaches based on parsimonious transcriptional signatures could facilitate comprehensive characterization of NHL specimens from SSA. This improvement in pathologic classification would not only ensure appropriate application of currently available therapies but also identify patients suitable for treatment with less toxic, targeted therapies.
Building upon a well-established relationship between UOMCOM and Brigham and Women’s Hospital (BWH), we evaluated all cases diagnosed as NHL at UOMCOM from 2004 to 2014 in order to establish diagnoses based on standard-of-care immunohistochemistry (IHC). We then tested whether a simple, high-throughput transcriptional assay could provide an alternative method for discriminating NHL subtype and thereby identify subsets suitable for targeted therapeutics.
Materials and methods
Case selection
With permission of the BWH Institutional Review Board and UOMCOM Research and Ethics Committee, archives of the UOMCOM Department of Histopathology were searched for cases of NHL diagnosed between 2004 and 2014. A total of 582 cases were identified; of these, 499 had formalin-fixed paraffin-embedded (FFPE) tissue blocks with adequate lesional tissue. Clinical parameters based on pathology requisition forms, including age, gender, HIV status, biopsy site, and presentation, were collected if available. In addition, classical Hodgkin lymphoma (CHL) and tonsil with reactive follicular hyperplasia were retrieved as controls.
TMA construction
Tissue microarray (TMA) recipient blocks were prepared at BWH and shipped to Malawi. All available H&E slides were reviewed on site, and multiple foci of lesional tissue per case were demarcated. Five TMAs were constructed at UOMCOM with a maximum of 124 cases and 4 controls per block. In >90% of cases, 3 cores per tumor (1.0 mm in diameter) were transferred. The remaining cases were represented by 1 or 2 cores.
Histology and IHC
At BWH, sections of each TMA were cut at 4 μm and stained for H&E, and the antibodies are listed in supplemental Table 1. In situ hybridization for Epstein-Barr virus encoded RNA (EBER ISH) was performed using the Ventana system (Ventana, Tucson, AZ).
Case evaluation
Full H&E slides, H&Es of TMAs, and all IHC were reviewed, and all tumors were diagnosed and classified according to standard 2008 World Health Organization criteria.13 Cases were not included if insufficient lesional or viable tissue was present or if extent of reactivity was insufficient to reach a diagnosis. “High” expression was defined for BCL2 as ≥70% of tumor cells and MYC as ≥40% of tumor nuclei.14,15
Targeted expression profiling
Three 1.0-mm cores of lesional tissue were punched from FFPE for RNA extraction. Total RNA was isolated using the AllPrep DNA/RNA FFPE kit (catalog number 80234; QIAGEN, Hilden, Germany), quantified using a NanoDrop spectrophotometer (NanoDrop Products; Thermo Scientific, Wilmington, DE) and stored at −80°C until use. RNA yield ranged from 0 to 20 µg (median, 0.85 µg). For each sample, RNA degradation was evaluated by determining RNA fragment length; the percentage of fragments between 50 and 300 bp in length ranged from 0% to 95% in all samples (median, 75%). Input quantity was calculated as 100 ng × (100/% of sample > 300 bp). For the multiplexed digital gene expression analysis, 100 to 500 ng RNA for each sample was hybridized with 20 μL reporter probes and reaction buffer and 5 μL capture probes at 65°C for 20 hours. The hybridized samples were processed on the NanoString nCounter Prep Station for 2.5 hours, and expression data were subsequently generated on the NanoString nCounter Digital Analyzer (NanoString Technologies, Seattle, WA) using the 600 fields of view setting over 4 hours.16 We extracted RNA from 150 aggressive B-cell lymphomas (diffuse large B-cell lymphoma [DLBCL]; BL; and B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and BL [BCL-U]); of these, 70 failed analytical quality control (47%). A total of 21 genes based on a previously published diagnostic classifier17 were selected to profile on the NanoString nCounter platform. Housekeeping genes and cell-of-origin (COO) analysis genes are as described previously.18 Analysis of raw nCounter output data with the lymphoma subtyping test (LST) algorithm for COO subtyping was performed at NanoString Technologies.
Normalization, unsupervised clustering, and statistical analysis
The data were normalized using the nSolver software (NanoString Technologies) by subtracting the geometric mean of 8 negative control probes. In addition, the geometric mean of 8 “positive spike” RNAs was used to account for variances in lane efficiencies, and the geometric mean expression of 5 housekeeping genes (ISY1, R3HDM1, TRIM56, UBXN4, WDR55)18 was used to account for sample content variations. The normalized dataset was transformed into log2 space to achieve an approximate normal distribution of gene expression values. Unsupervised clustering was performed with the hclust function of the R statistical framework using Ward’s minimum variance method. Optimal leaf ordering as implemented in the cba package on http://cran.r-project.org was used to get the final ordering of both samples and genes. Probability values and 95% confidence intervals (CIs) were calculated with the binom.test function. The data in the heatmap were transformed using row scaling to reflect relative differences within genes. Classification of samples was compared with the clustering results by proportions with exact binomial 95% CIs and contingency tables. Fisher’s exact test was used to test for independence of the rows and columns. All statistical tests were 2 sided, P values < .05 were considered statistically significant, and no adjustments for multiple comparisons were made.
Results
Tumor classification
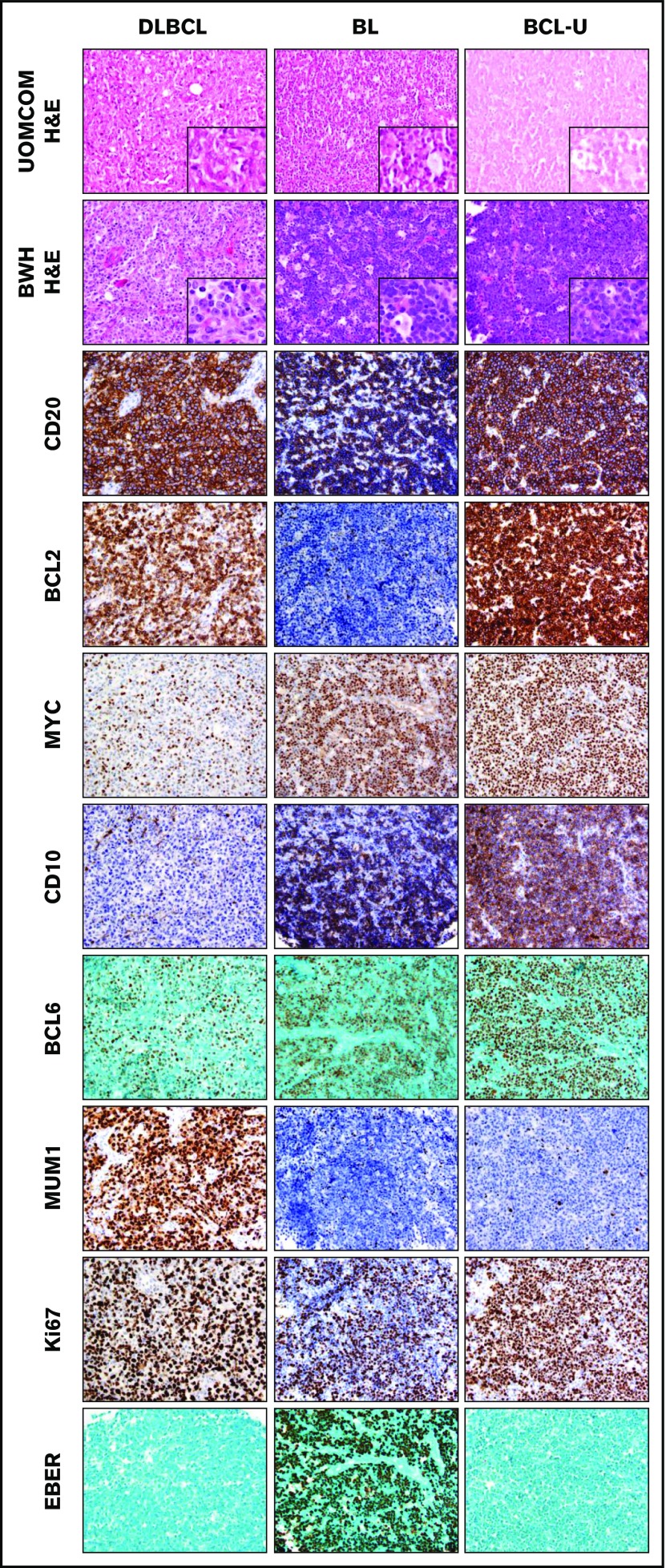
We identified 499 pediatric and adult tumors diagnosed as NHL and/or suspicious for NHL at UOMCOM from 2004 to 2014. The median patient age was 31 years (range, 1-87 years; 25% <16 years). The cohort included 274 males and 191 females (data were unavailable for 19 patients). HIV status was available for 43 patients (9%); of those, 31 were HIV positive. Specimens included lymph node (n = 219), extranodal tissue (n = 261), bone marrow (n = 2), and not specified (n = 17). We generated TMAs at UOMCOM and performed additional classification refinement using these TMAs at BWH based on review of the original H&E slide and H&E staining of a TMA section, as well as incorporation of the reactivity pattern of 30 antibodies (n = 9750 assessments; Table 1 and supplemental Table 2). Representative examples are shown in Figure 1.
Table 1.
Refined classification by IHC of 328 cases originally diagnosed at UOMCOM by H&E only
| Original pathologic classification | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Refined diagnosis incorporating IHC | DLBCL/high-grade NHL (n = 116) | BL (n = 70) | CLL/SLL (n = 7) | MZL (n = 2) | PBL (n = 2) | LBL (n = 1) | FL (n = 3) | ALCL (n = 2) | PCN (n = 1) | NHL/low-grade NHL/lymphoma NOS/low-grade BCL (n = 101) | Suspicious for NHL (n = 23) |
| DLBCL (n = 133) | 70 | 8 | — | — | — | — | 1 | 1 | 1 | 45 | 7 |
| BL (n = 73) | 12 | 43 | — | — | — | — | — | — | — | 14 | 4 |
| BCL (n = 27) | 6 | 2 | 2 | — | — | — | 2 | — | — | 14 | 1 |
| PBL (n = 20) | 11 | 5 | — | — | — | 1 | — | — | — | 2 | 1 |
| Neuroendocrine tumor (n = 20) | 4 | 6 | — | — | — | — | — | — | — | 6 | 4 |
| BCL-U (n = 9) | 4 | 3 | 1 | — | 1 | — | — | — | — | — | — |
| T-ALL (n = 8) | 2 | 1 | — | — | — | — | — | — | — | 3 | 2 |
| Carcinoma NOS (n = 8) | 2 | — | — | — | — | — | — | — | — | 6 | — |
| CLL/SLL (n = 7) | 1 | — | 4 | — | — | — | — | — | — | 2 | — |
| MZL (n = 5) | 1 | — | — | 2 | — | — | — | — | — | — | 2 |
| CHL (n = 4) | 1 | 1 | — | — | — | — | — | 1 | — | 1 | — |
| FL (n = 3) | 2 | — | — | — | — | — | — | — | — | 1 | — |
| Myeloid sarcoma (n = 2) | — | — | — | — | — | — | — | — | — | 2 | — |
| MCL (n = 2) | — | — | — | — | — | — | — | — | — | 2 | — |
| ALK + ALCL (n = 2) | — | — | — | — | — | — | — | — | — | 1 | 1 |
| Plasmacytoma (n = 2) | — | — | — | — | 1 | — | — | — | — | 1 | — |
| PTCL NOS (n = 1) | — | 1 | — | — | — | — | — | — | — | — | — |
| Nasopharyngeal carcinoma (n = 1) | — | — | — | — | — | — | — | — | — | — | 1 |
| Reactive (n = 1) | — | — | — | — | — | — | — | — | — | 1 | — |
ALCL, anaplastic large cell lymphoma; BCL, B-cell lymphoma not further classified; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; FL, follicular lymphoma; LBL, lymphoblastic lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; NOS, not otherwise specified; PBL, plasmablastic lymphoma; PCN, plasma cell neoplasm; PTCL, peripheral T-cell lymphoma; T-ALL, T-lymphoblastic lymphoma.
Figure 1.
Classification by morphology and immunophenotype. Representative examples of H&E staining at UOMCOM and BWH, as well as immunostaining at BWH, for cases of DLBCL, BL, and BCL-U. Original magnification ×40 for all panels (original magnification ×100 for insets).
Of the 499 cases, 328 had adequate material in the TMA and the remaining 171 cases (34%) could not be further classified, largely due to poor tissue antigenicity. Diagnoses from UOMCOM based solely on H&E, and from BWH based on H&E and extensive IHC, are outlined in Table 1. Of the 328 cases with adequate material in the TMA, 326 cases were originally diagnosed as NHL or suspicious for NHL at UOMCOM based on H&E alone; the 2 remaining cases were originally diagnosed as lymphoblastic lymphoma or plasma cell neoplasm. Of those 326 cases, 280 (86%) were confirmed as NHL at BWH by immunophenotypic analysis (Table 1), a concordance rate similar to prior reports.4 The remaining 14% were neuroendocrine tumor (n = 20), T-lymphoblastic lymphoma (8), carcinoma, not otherwise specified (8), CHL (4), myeloid sarcoma (2), plasmacytoma (2), nasopharyngeal carcinoma (1), and reactive (1). As previously reported, there was a striking paucity of mature T-cell neoplasms (1 peripheral T-cell lymphoma not otherwise specified, 2 ALK-positive anaplastic large cell lymphoma)4 and very few low-grade lymphomas (Table 1).
Characterization of aggressive lymphoma subsets
DLBCL accounted for 133 (40%) of the classifiable cases. COO analysis by the Hans algorithm (which used CD10, BCL6, and MUM1 IHC) demonstrated that 50 (38%) were GCB, 79 (59%) were non-GCB, and 3% could not be characterized (Table 2).19,20 We also assessed DLBCLs to identify cases with overexpression of both MYC protein and the antiapoptotic protein BCL2 (“double expressors”). Among the 97 DLBCL cases with interpretable IHC, high MYC protein expression was detected in 39 (40%). Consistent with prior reports in Western cohorts,21 concurrent high expression of both BCL2 and MYC was enriched among non-GCB DLBCLs (31% vs 8% among GCB; P = .01 by 2-tailed Fisher’s exact test). The immunomodulatory molecule programmed cell death ligand 1 (PD-L1) was expressed on all or a subset of tumor cells in 5 (45%) of 11 EBER ISH–positive DLBCLs but only 14 (11%) of 122 EBER ISH–negative DLBCLs (P = .0094), which is similar to reports in Western cohorts.22
Table 2.
Comparison of clinical and pathologic features of 129 DLBCLs from UOMCOM divided by COO classification (Hans criteria)
| COO by Hans criteria* | P | ||
|---|---|---|---|
| GCB (n = 50) | non-GCB (n = 79) | ||
| Median age (y)† | 35, range 9-75 (5% <16) | 40, range 13-76 (6% <16) | .3165‡ |
| EBER ISH positive | 3 (6%) | 7 (9%) | .7397§ |
| BCL2/MYC high expression|| | 3 out of 37 (8%) | 18 out of 58 (31%) | .0106§ |
| Extranodal | 26 (52%) | 48 (62%)¶ | .2732§ |
| HIV positive | 2 out of 3 tested patients (67%) | 8 out of 9 tested patients (89%) | |
Four cases were excluded due to insufficient data.
In each group, data were not available for 7 patients.
Unpaired Student t test.
Two-tailed P value from Fisher’s exact test.
Data were not available for 13 and 21 patients, respectively.
Data were not available for 2 patients.
Phenotypically confirmed BL accounted for 73 of the 328 classifiable cases (22%). This encompassed 43 cases originally diagnosed as BL; the remainder were originally diagnosed as NHL or suspicious for NHL. The median age was 12 years (range, 3-74 years; 62% < 16 years). EBER ISH was positive in 63 of 73 cases (86%), including 98% of cases of BL among patients aged < 16 yr. The median age was younger in EBER ISH–positive cases (9 years; range, 3-39 years) than in EBER ISH–negative cases (34 years; range, 15-74 years). Of all BLs, 70% were from extranodal sites. Among BL with interpretable MYC IHC, 93.5% (43 of 46 cases) showed high MYC protein expression in tumor nuclei. No cases of BL showed PD-L1 expression in the tumor cells, consistent with prior reports.22
Application of next-generation diagnostics to FFPE samples
We assessed the feasibility of COO classification using RNA extracted from DLBCLs with the NanoString LST algorithm (also known as Lymph2Cx).18 RNA was extracted from 81 randomly selected DLBCLs, of which 48 passed analytical quality control (supplemental Table 3). Of these 48 cases, 11 (22.9%) were unclassifiable by the LST algorithm, including 2 GCB and 9 non-GCB cases by IHC. This predominance of cases classified as non-GCB by the Hans algorithm that are unclassifiable by LST is similar to what has been observed in Western cohorts.18 One additional case had insufficient data for IHC classification. Of the remaining 36 DLBCLs classified by both methods, comparison of COO revealed concordance in 25 cases (69%) and discordance in 11 cases (31%), where concordance was defined as GCB/GCB or non-GCB/activated B cell–like for the IHC and LST algorithms, respectively. All 11 discordant cases were classified as non-GCB by IHC but GCB by LST. In 10 of 11 cases, classification as non-GCB by IHC was based on the absence of both CD10 and BCL6 staining.
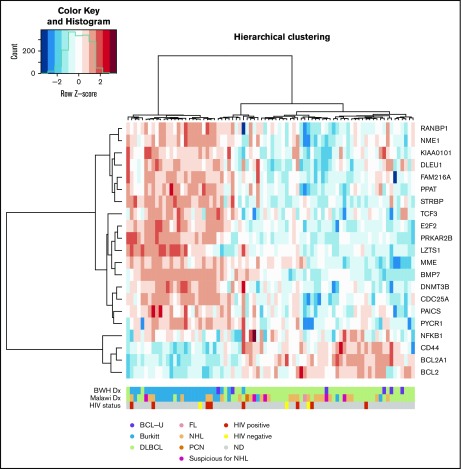
Next, we assessed 21 genes with a custom research-use NanoString gene expression panel. These 21 genes were selected from a previously published, NanoString-based classifier that distinguished ∼85% of pathologically defined BLs from DLBCLs in Western cohorts.17 We performed unsupervised hierarchical clustering with the 21-gene signature on 48 DLBCL, 26 BL, and 6 BCL-U samples (based on IHC). The cases clustered into 2 groups (n = 25, 55; Figure 2). A total of 23 out of 26 BL subjects (88%) were classified into cluster 1 (exact binomial 95% CI, 70%-98%). Two additional DLBCLs (2 of 48 [4%]; 95% CI, 0%-14%) were assigned to this group; both were BCL2 IHC negative, EBER ISH negative, MYC IHC high, and GCB-like. Cluster 2 combined all 6 BCL-U lymphomas (95% CI, 54%-100%), the remaining 46 DLBCLs (96%; 95% CI, 86%-99%), and the remaining 3 BLs (11%; 95% CI, 2%-30%). The 46 DLBCLs in cluster 2 included all 13 BCL2/MYC dual-expressor DLBCLs in the cohort. All 3 BLs classified in cluster 2 were EBER ISH positive. All 6 (100%) BCL-Us were genetically classified with DLBCLs despite appearance on H&E as BL-like. Further refinement of the classification signature resulted in a parsimonious 10-gene list that distinguished ∼85% of pathologically defined BLs from DLBCLs (supplemental Figure 1).
Figure 2.
Unsupervised hierarchical clustering of transcriptional profiles of DLBCL, BCL-U, and BL cases based on the 21-gene classifier.17 Red indicates upregulation and blue downregulation of gene expression. Each row represents a gene, and each column represents a sample.
Discussion
The past few decades have witnessed an unprecedented movement to improve health care in lower- and middle-income countries (LMICs), as evidenced by a fivefold increase in global health funding between 1990 and 2013.23 Notably, the majority of resources are allocated for HIV/AIDS and maternal, newborn, and child health (45% of ∼$30 billion), whereas non-communicable diseases, including cancer, heart disease, and diabetes, received ∼1% of the funding. However, cancer is a growing burden; by 2030, ∼70% of all new cancer diagnoses will occur in LMICs.24 Thus there is an existing and rapidly increasing crisis of inadequate cancer care in LMICs.25
Accurate diagnosis will be a critical step in this effort to improve cancer care and inform drug choice.26 However, detailed pathologic diagnosis is often not available in LMICs because of a lack of both diagnostic tools and pathologists.5 Although H&E evaluation is sufficient to diagnose many high-incidence tumors in LMICs (eg, squamous cell carcinoma of the cervix8), hematologic malignancies require broad immunophenotyping and expert review for classification. We reasoned that advanced diagnostics could be applied to classify aggressive lymphomas and potentially obviate the need for expert pathologists, who are in dire shortage within the region. To this end, we manufactured TMAs of NHL at UOMCOM, analyzed them with detailed immunophenotyping, and assessed the diagnostic accuracy of targeted gene expression profiling to classify aggressive B-cell lymphomas.
High-grade B cell lymphomas (DLBCL, BL, plasmablastic lymphoma, and BCL-U) accounted for nearly three-quarters of cases, and T-cell lymphomas were extremely uncommon (<1%), similar to prior reports from SSA.4 Although chemotherapy regimens for aggressive B-cell lymphomas in Malawi do not mirror HIC protocols, in part due to a lack of sufficient supportive care, BL and non-BL aggressive NHL are nonetheless differentially treated in Malawi, and therefore, accurate segregation of these diagnoses is immediately relevant.10,11 Unsupervised hierarchical clustering using a 21-gene NanoString-based classifier previously only applied to Western cohorts17 had a diagnostic accuracy for DLBCL and BL of 96% and 88%, respectively, in our cohort. The significant advantage of this approach is the ability to perform targeted profiling on FFPE with relatively straightforward sample preparation. Given the relative simplicity of this system, which negates the need for multiple IHC studies, karyotype or FISH, and associated expert review, it is an attractive alternative for diagnosis in LMICs.
We further used our pathologically defined cases to develop a simplified and potentially less expensive 10-gene classifier that correctly classified 85% of SSA BLs and DLBCLs. Although additional studies are needed to validate this classifier, these initial results are an enticing first step toward streamlining high-grade NHL diagnostics in SSA. Of note, within the group of 150 UOMCOM specimens selected for testing, 47% failed quality control (compared with ∼5% in Western cohorts).17 The use of standardized fixation conditions to maintain RNA integrity is likely to overcome this issue. In addition, the specimens were archived (in some cases for over 10 years), which may have also reduced the quality of RNA.
Detailed information regarding COO subtype in representative SSA patients is lacking; the one published study of COO in de novo DLBCL from SSA was from an 81% Caucasian origin, South African cohort.27 We used both an IHC algorithm (Hans) and the NanoString LST targeted expression profiling algorithm to determine COO. Application of the IHC algorithm to all 133 DLBCL cases revealed a predominance of the non-GCB subtype, similar in proportion to the original report from a Western cohort.20 In contrast, analysis by the LST algorithm of 48 randomly selected DLBCL cases revealed a predominance of the GCB subtype. All “discordant” cases were classified as non-GCB by IHC and GCB by the LST algorithm, with the former almost exclusively based on the absence of CD10 and BCL6 expression. This was apparently not due to poor tissue antigenicity, as all cases showed reactivity with other antibodies (such as CD20). While the definitive reason for this discrepancy is uncertain, one interpretation is that the Hans algorithm may misclassify a subset of cases from SSA that have transcriptional signatures consistent with GCB but lack CD10 and BCL6 immunoreactivity. Unfortunately, HIV status was not available in the large majority of our cases, so it was not possible to determine whether HIV infection may have contributed to this finding.
Broad application of targeted therapeutics is the most promising strategy to address the looming burden of cancer in LMICs, where there are critical shortages of supportive care, radiotherapy, and cancer surgery. Several targeted agents with potent activity against specific subsets of NHL and reduced toxicity compared with multiagent chemotherapy are either in clinical trials or recently approved in HICs. These include small-molecule inhibitors of EZH2, BCL2, BTK, BRD4, phosphatidylinositol 3-kinase, MDM2, and PD-1/PD-L1; naked and conjugated antibodies targeting CD19, CD20, and CD22; and adoptive cellular therapies against Epstein-Barr virus and B-cell–specific antigens.28 Investigations are underway to assess the use of certain classes of drugs in lymphomas with specific molecular signatures, such as EZH2 inhibitors and BTK inhibitors in cases classified as GCB and non-GCB, respectively.29,30 While it may seem overly ambitious at the current time to envision a future that includes precision medicine for patients with NHL in SSA, the experiences with both HIV and chronic myeloid leukemia in this region serve as models for what the future could hold once agents with adequate therapeutic index against NHL are available.31,32 We believe that the willingness of pharmaceutical companies to grant such regions larger access to these types of potentially transformative medications will likely be contingent on diagnostic precision beyond what is currently available.
IHC alone did reveal several potential therapeutic targets in the aggressive B-cell NHL cohorts. PD-L1 is an immunomodulatory molecule expressed by selected tumors that inhibits antitumoral T-cell immunity by engaging specific receptors on T cells, most notably PD-1. PD-L1 expression is common in certain subtypes of lymphoma such as CHL, primary mediastinal large B-cell lymphoma, and Epstein-Barr virus–associated DLBCL but uncommon in the remaining DLBCLs, BL, and low-grade lymphomas.22 PD-1 blockade in patients with relapsed or refractory CHL is associated with durable remissions in up to 85% of patients.33 Our cases exhibited rates of PD-L1 expression similar to Western cohorts, suggesting that specific populations in SSA may be responsive to therapeutic and less toxic PD-1/PD-L1 blockade.
Similarly, overexpression of MYC by IHC may indicate responsiveness to BRD4 inhibition, which can target MYC transcription in DLBCL.34 In addition, MYC/BCL2 double-expressor DLBCLs appear to have a worse prognosis than non–double-expressor DLBCLs and are enriched among the non-GCB subset in both HIC cohorts15,35-38 and our cohort. Notably, MYC expression could not be interpreted in 69 of the 235 evaluated cases (30%) of high-grade B-cell lymphomas (BL, DLBCL, BCL-U, and plasmablastic lymphoma) by IHC. This may be attributable to variability in fixation conditions between different samples, which can likely be resolved with implementation of standardized fixation protocols.39
There are a number of limitations to this study. Our cohort is lacking in staging, treatment, and outcomes data. Most notably, HIV status was available in <10% of samples. Without FISH data, we do not have a comprehensive understanding of the prevalence of “double-hit” or “triple-hit” lymphomas in this patient population and we do not have genetic confirmation of MYC translocation in cases of BL. Additionally, we detected very low rates of low-grade B-cell NHL and T-cell NHL. Although similar to prior reports,4 we are uncertain if this reflects the true epidemiology of this patient population, as we simply characterized available cases rather than prospectively screening patients who presented with possible NHL.
In summary, we performed comprehensive immunophenotypic analysis and targeted expression profiling on a large cohort of tumors diagnosed as NHL in Malawi. We demonstrate that both IHC and NanoString-based classification can identify targetable subsets of NHL in this population that match signatures established using lymphomas from HICs. The next step is to prospectively evaluate diagnostics like NanoString within LMICs to assess the feasibility and accuracy of performance on-site. If those studies are confirming, the application of less toxic and more effective therapeutics for patients with NHL in LMICs will depend on the availability of these diagnostics.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Roland Zunda for his invaluable assistance in organizing pathology cases at UOMCOM and Leslie Grimmett and Zach Herbert at the DFCI Genomics Core for their technical expertise.
This work was supported in part by the Harvard Medical School Eleanor and Miles Shore Fellowship (E.A.M.) Dana-Farber/Harvard Cancer Center (R.A.R. and D.S.N.) is supported in part by National Institutes of Health National Cancer Institute Cancer Center Support Grant no. 5 P30 CA006516. D.M.W. is a Leukemia and Lymphoma Society Scholar.
Authorship
Contribution: E.A.M., C.D.C., S.J.R., D.A.M., and D.M.W. were responsible for conception and design of the study. M.P.S., T.T., L.M., S.K., N.K., and G.S.P. were responsible for acquisition of data. E.A.M., D.G., R.A.R., and D.S.N. interpreted and analyzed data. E.A.M. and D.M.W. wrote the manuscript. All authors critically revised the article and approved the final version.
Conflict-of-interest disclosure: D.G., C.D.C, and S.J.R. have a pending patent application (WO/2015/157291) for MYCKEY NanoString-based molecular signature. The remaining authors declare no competing financial interests.
Correspondence: Elizabeth A. Morgan, Department of Pathology, Brigham and Women’s Hospital, 75 Francis St, Amory 3, Boston, MA 02115; e-mail: eamorgan@partners.org.
References
- 1.Gopal S, Wood WA, Lee SJ, et al. Meeting the challenge of hematologic malignancies in sub-Saharan Africa. Blood. 2012;119(22):5078-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. [DOI] [PubMed] [Google Scholar]
- 3.Perry AM, Diebold J, Nathwani BN, et al. Non-Hodgkin lymphoma in the developing world: review of 4539 cases from the International Non-Hodgkin Lymphoma Classification Project. Haematologica. 2016;101(10):1244-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naresh KN, Raphael M, Ayers L, et al. Lymphomas in sub-Saharan Africa—what can we learn and how can we help in improving diagnosis, managing patients and fostering translational research? Br J Haematol. 2011;154(6):696-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adesina A, Chumba D, Nelson AM, et al. Improvement of pathology in sub-Saharan Africa. Lancet Oncol. 2013;14(4):e152-e157. [DOI] [PubMed] [Google Scholar]
- 6.Msyamboza KP, Dzamalala C, Mdokwe C, et al. Burden of cancer in Malawi; common types, incidence and trends: national population-based cancer registry. BMC Res Notes. 2012;5:149-0500-5-149. [DOI] [PMC free article] [PubMed]
- 7.Gopal S. Moonshot to Malawi. N Engl J Med. 2016;374(17):1604-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berezowska S, Tomoka T, Kamiza S, Milner DA Jr, Langer R. Surgical pathology in sub-Saharan Africa--volunteering in Malawi. Virchows Arch. 2012;460(4):363-370. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery ND, Liomba NG, Kampani C, et al. Accurate real-time diagnosis of lymphoproliferative disorders in Malawi through clinicopathologic teleconferences: a model for pathology services in Sub-Saharan Africa. Am J Clin Pathol. 2016;146(4):423-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanley CC, Westmoreland KD, Heimlich BJ, et al. Outcomes for paediatric Burkitt lymphoma treated with anthracycline-based therapy in Malawi. Br J Haematol. 2016;173(5):705-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hesseling P, Molyneux E, Kamiza S, Israels T, Broadhead R. Endemic Burkitt lymphoma: a 28-day treatment schedule with cyclophosphamide and intrathecal methotrexate. Ann Trop Paediatr. 2009;29(1):29-34. [DOI] [PubMed] [Google Scholar]
- 12.Kingham TP, Alatise OI, Vanderpuye V, et al. Treatment of cancer in sub-Saharan Africa. Lancet Oncol. 2013;14(4):e158-e167. [DOI] [PubMed] [Google Scholar]
- 13.Swerdlow S, Campo E, Harris NL, et al., eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues Lyon: IARC; 2008 [Google Scholar]
- 14.Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3460-3467. [DOI] [PubMed] [Google Scholar]
- 15.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geiss GK, Bumgarner RE, Birditt B, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26(3):317-325. [DOI] [PubMed] [Google Scholar]
- 17.Carey CD, Gusenleitner D, Chapuy B, et al. Molecular classification of MYC-driven B-cell lymphomas by targeted gene expression profiling of fixed biopsy specimens. J Mol Diagn. 2015;17(1):19-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott DW, Wright GW, Williams PM, et al. Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood. 2014;123(8):1214-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503-511. [DOI] [PubMed] [Google Scholar]
- 20.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275-282. [DOI] [PubMed] [Google Scholar]
- 21.Nowakowski GS, Czuczman MS. ABC, GCB, and double-hit diffuse large B-cell lymphoma: does subtype make a difference in therapy selection? Am Soc Clin Oncol Educ Book. 2015;35:e449-e457. [DOI] [PubMed] [Google Scholar]
- 22.Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19(13):3462-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Institute for Health Metrics and Evaluation. Global health funding reaches new high as funding priorities shift. Available at: http://www.healthdata.org/news-release/global-health-funding-reaches-new-high-funding-priorities-shift. Accessed 23 November, 2015.
- 24.Farmer P, Frenk J, Knaul FM, et al. Expansion of cancer care and control in countries of low and middle income: a call to action. Lancet. 2010;376(9747):1186-1193. [DOI] [PubMed] [Google Scholar]
- 25.Knaul FM, Atun R, Farmer P, Frenk J. Seizing the opportunity to close the cancer divide. Lancet. 2013;381(9885):2238-2239. [DOI] [PubMed] [Google Scholar]
- 26.Roberts DJ. Pathology: functionality in resource-poor settings. Arch Pathol Lab Med. 2013;137(6):748-751. [DOI] [PubMed] [Google Scholar]
- 27.Sissolak G, Wood L, Smith L, Chan JW, Armitage J, Jacobs P. Tissue microarray in a subset of South African patients with DLBCL. Transfus Apheresis Sci. 2013;49(2):120-132. [DOI] [PubMed] [Google Scholar]
- 28.Camicia R, Winkler HC, Hassa PO. Novel drug targets for personalized precision medicine in relapsed/refractory diffuse large B-cell lymphoma: a comprehensive review. Mol Cancer. 2015;14(207):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Shaffer AL III, Emre NC, et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell. 2012;21(6):723-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Béguelin W, Popovic R, Teater M, et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell. 2013;23(5):677-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Global Health Sector Response to HIV, 2000-2015: Focus on Innovations in Africa: Progress Report. Geneva, Switzerland: World Health Organization; 2015.
- 32.Garcia-Gonzalez P, Boultbee P, Epstein D. Novel Humanitarian Aid Program: The Glivec International Patient Assistance Program—lessons learned from providing access to breakthrough targeted oncology treatment in low- and middle-income countries. J Glob Oncol. 2015;1:37-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapuy B, McKeown MR, Lin CY, et al. Discovery and characterization of super-enhancer-associated dependencies in diffuse large B cell lymphoma [published correction appears in Cancer Cell. 2014;25(4):545-546]. Cancer Cell. 2013;24(6):777-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green TM, Nielsen O, de Stricker K, Xu-Monette ZY, Young KH, Møller MB. High levels of nuclear MYC protein predict the presence of MYC rearrangement in diffuse large B-cell lymphoma. Am J Surg Pathol. 2012;36(4):612-619. [DOI] [PubMed] [Google Scholar]
- 36.Hu S, Xu-Monette ZY, Tzankov A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121(20):4021-4031, quiz 4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott DW, Mottok A, Ennishi D, et al. Prognostic significance of diffuse large B-cell lymphoma cell of origin determined by digital gene expression in formalin-fixed paraffin-embedded tissue biopsies. J Clin Oncol. 2015;33(26):2848-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kendrick S, Tus K, Wright G, et al. Diffuse large B-cell lymphoma cell-of-origin classification using the Lymph2Cx assay in the context of BCL2 and MYC expression status. Leuk Lymphoma. 2016;57(3):717-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kluk MJ, Ho C, Yu H, et al. MYC Immunohistochemistry to Identify MYC-Driven B-Cell Lymphomas in Clinical Practice. Am J Clin Pathol. 2016;145(2):166-179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.