Abstract
Metastasis involves the migration of cancer cells from a primary tumor to invade and establish secondary tumors in distant organs, and it is the main cause for cancer-related deaths. Currently, the conventional cytostatic drugs target the proliferation of malignant cells, being ineffective in metastatic disease. This highlights the need to find new anti-metastatic drugs. Toxins isolated from snake venoms are a natural source of potentially useful molecular scaffolds to obtain agents with anti-migratory and anti-invasive effects in cancer cells. While there is greater evidence concerning the mechanisms of cell death induction of several snake toxin classes on cancer cells; only a reduced number of toxin classes have been reported (i.e., disintegrins/disintegrin-like proteins, C-type lectin-like proteins, C-type lectins, serinproteases, cardiotoxins, snake venom cystatins) as inhibitors of adhesion, migration, and invasion of cancer cells. Here, we discuss the anti-metastatic mechanisms of snake toxins, distinguishing three targets, which involve (1) inhibition of extracellular matrix components-dependent adhesion and migration, (2) inhibition of epithelial-mesenchymal transition, and (3) inhibition of migration by alterations in the actin/cytoskeleton network.
Keywords: anti-cancer agents, cancer cells, invasion, migrastatic drugs, snake venom
1. Introduction
Currently, anticancer therapies target the uncontrolled clonal proliferation of cancer cells with cytostatic drugs, which are an effective therapeutic strategy for certain cancer types such as hematological malignancies. However, in solid cancers, the proliferation is accompanied by the ability to invade and execute metastasis, involving different molecular mechanisms that are not inhibited or affected by conventional anti-cancer drugs. Therefore, to search for and design specific drugs to inhibit invasion and metastasis for treatment of solid cancers is a highly relevant issue [1].
The composition of solid tumors is heterogeneous, having several cancer cell subpopulations with different tumorigenic properties [2]. In a tumor, cancer cells acquire mutations that confer them with different proliferative capacities and survival advantages. A subpopulation, named metastasis-initiating cells (MICs), exhibits high plasticity to adapt their metabolic and proliferative requirements, ability to enter and exit dormancy state, and resistance to apoptosis and immune evasion, which is responsible for metastatic growth [3]. For example, during the initial steps of tumor growth of cancer cells confined to epithelium, certain colonies of malignant cells can form a carcinoma in situ separated from the stroma. In some cells, mutations provide the ability to establish a physical relationship with stroma and changes in extracellular signals from the microenvironment, triggering the secretion of soluble factors by stromal and hematopoietic cells [4,5] and inducing phenotypic changes in cancer cells known as epithelial–mesenchymal transition (EMT). This process recapitulates properties displayed by tissues during the embrionary development [6] that facilitate the dissociation of cancer cell from the tumor bulk and dissemination to distant organs, being considered a prerequisite for invasion and metastasis [2,7].
Metastasis is a complex process in which cancer cells disseminate from a primary tumor to invade a distant organ, this ability characterizes the tumor malignancy [6]. It has been described that about 90% of cancer-related deaths are caused by a metastatic disease [8]. It is clear that dissemination to specific organs depends upon blood flow patterns and of the relationship of the migrating cells with distant organ microenvironments, the stromal cell content, vascular architecture, presence of growth factors, metabolic substrates, and signaling molecules. These characteristics can be permissive or antagonistic to metastatic colonization, determining whether these cells grow to form secondary tumors [9].
The detailed mechanistic insight of the metastatic process contrast with the minimal progress in the identification of effective therapeutic targets and in the design of new anti-metastatic drugs [1]. Based on structural characteristics and their known interactions with macromolecules, toxins isolated from snake venoms may represent a natural source of molecular scaffolds to obtain agents with anti-migratory and anti-invasive effects in cancer cells. In this review, we summarize recent evidence on the inhibitory effect of snake toxins on adhesion, migration, and invasion of cancer cells.
2. Snake Toxins as Inhibitors of Cancer Metastasis
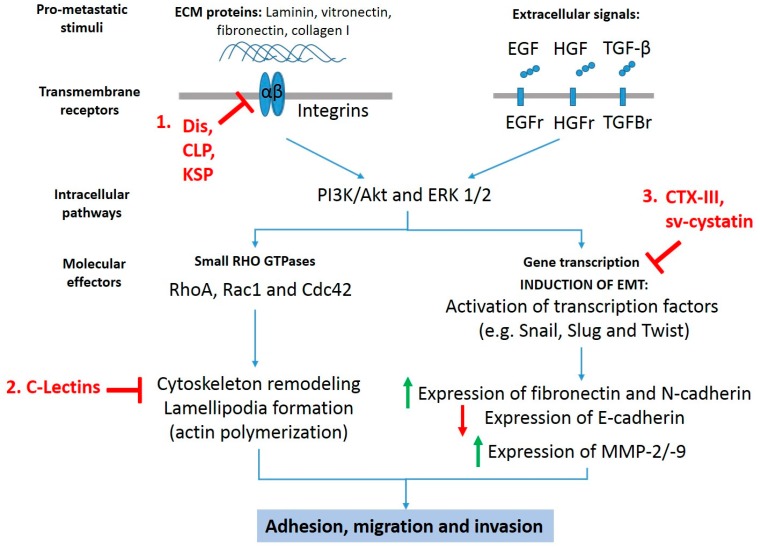
There is ample literature showing that several isolated or recombinant snake venom toxins exhibit anti-cancer effects in vitro and in vivo preclinical models, inducing cell death via mitochondrial apoptotic pathway (intrinsic pathway) or necrosis [10,11,12]. In addition, certain toxins such as snake venom metalloproteases (SVMPs), disintegrins, phospholipases A2, C-type lectins (CLP), vascular apoptosis inducing proteins, and L-amino acid oxidases are able to inhibit angiogenesis [13,14,15] and activate the immune response during tumorigenesis [16]. While greater evidence on mechanisms of death induction of snake toxins on cancer cells have been reported, reduced information on the inhibitory mechanisms of adhesion, migration, and invasion of metastatic cancer cells is available. Despite the aforementioned information, it is possible distinguish three anti-metastatic mechanisms exhibited by at least six different snake toxin classes (Figure 1): involving (1) inhibition of extracellular matrix components (ECM)-dependent adhesion and migration, (2) inhibition of epithelial-mesenchymal transition, and (3) inhibition of migration by alterations in the actin/cytoskeleton network.
Figure 1.
Anti-metastatic targets for snake toxins. Snake toxins inhibit pro-migratory and pro-invasive signals stimulated by extracellular matrix proteins and growth factors such as epidermal growth factor (EGF), hepatocyte growth factor (HGF), and transforming growth factor beta (TGF-β) though (1) inhibition of extracellular matrix (ECM) components-dependent adhesion and migration, (2) inhibition of migration by alterations in the actin/cytoskeleton network, and (3) inhibition of epithelial–mesenchymal transition (EMT). Dis: disintegrins; CLP: C-type lectin-like proteins; KSP: Kunitz-type serinprotease; C-Lectins: C-type lectins; CTX-III: cardiotoxin III; Sv-cystatin: snake venom cystatin.
3. Inhibition of Extracellular Matrix Component-Dependent Adhesion and Migration
During the initial steps of metastasis, it is required the interaction between ECM components and cancer cell, involving the ability of these cells to adhere to ECM components and migrate through them [17]. Integrins are the major receptor family present on the cell surface for adhesion to the ECM and include heterodimeric, transmembrane glycoproteins composed of α and β subunits [18], whose dimerization leads to 24 integrin pairs with distinct extracellular ligand-binding specificities [18]—such as collagen, laminin, vitronectin, and fibronectin—through the tripeptide motif Arg-Gly-Asp = RGD [19]. Abundant evidence has correlated the increased overexpression of certain integrins αvβ3, α5β1, and αvβ6 with cancer progression [20,21,22]. Integrins activate intracellular signaling that control cytoskeleton organization, cell polarity, and formation of leading edge of migrating cancer cells [22], being an attractive anti-cancer target for new antagonist molecules [23,24].
Three toxin classes (snake venom disintegrins, C-type lectin-like protein, and Kunit-like serinprotease inhibitor) have been reported with anti-migratory effect mediated by interaction with integrins in cancer cells, which are summarized in Table 1.
Table 1.
Snake toxins that inhibit the adhesion and migration of cancer cells by interaction with ECM components.
| Toxin Name | Snake Species | Adhesive Motif | Integrin Target | ECM Ligand | Effect | Ref. |
|---|---|---|---|---|---|---|
| r-Cam-dis recombinant disintegrin | Crotalus adamanteus | RGD | αvβ3 | laminin-1 | Inhibition of adhesion in pancreatic cancer cells | [41] |
| r-Colombistatins recombinant disintegrin-like domains from Class-III SVMP | Bothrops colombiensis | ECD | n.d. | collagen I | Inhibition of adhesion in SK-Mel-28 melanoma cells | [42] |
| DisBa-01, recombinant disintegrin | Bothrops alternatus | RGD | αvβ3 | fibronectin | Loss of cell directionality of migrating oral squamous carcinoma cells | [43] |
| r-mojastn-1, recombinant disintegrin | Crotalus scutulatus scutulatus | RGD | αvβ3, α3, and β1, | fibronectin and vitronectin | Inhibition of adhesion and migration of BXPC-3 pancreatic cancer cell line | [44,45] |
| r-viridistatin-2, recombinant disintigrin | Crotalus viridis viridis | RGD | αvβ3 | fibronectin and vitronectin | Inhibition of adhesion, migration and invasion of several cancer cell lines | [44,46] |
| Lebecin, C-type lectin-like protein | Macrovipera lebetina | - | αvβ3 | fibronectin and fibrinogen | Inhibition of adhesion and migration of MDA-MB-231 breast cancer cells | [47] |
| PIVL, Kunitz-type serin protease inhibitor | Macrovipera lebetina transmediterranea | RGN | αvβ3 | fibronectin and fibrinogen | Inhibition of adhesion, migration and invasion of human glioblastoma U87 cells | [48] |
n.d.: not determined.
Snake venom disintegrins are small non-enzymatic proteins mostly derived from proteolytic processing of precursors that contain a metalloprotease domain, known as snake venom metalloproteases (SVMPs), which are phylogenetically related with ADAMs (a disintegrin and metalloprotease) [25,26,27,28]. This protein family, commonly found in the venoms of the Viperidae snakes [26] and some rear-fanged snakes [28,29,30,31,32,33,34,35], is classified according to their modular architecture with multiple non-catalytic domains in SVMP P-I, P-II, and P-III classes. Disintegrins are derived from proteolytic processing of P-II SMVP class and usually exhibit the canonical “RDG” integrin-recognition motif; however, non-canonical integrin-binding motif—such as “MLD”, “KTS”, and “VGD”—are exhibited in some snake venom disintegrins [36,37]. In addition, proteolysis from P-III SVMP class originates disintegrin-like proteins, which have covalently bound the “disintegrin-like” and “cysteine (Cys)-rich” domains [27]. Comprehensive classification and structural characteristics of SVMP are found in Takeda et al., 2012 [27] and Takeda, 2016 [38].
A disintegrin isolated from the venom of the Middle American rattlesnake (Crotalus simus tzabcan) named tzabcanin [39], which has 71 amino acids and contains the canonical RGD-binding domain, exhibits a weak or null cytotoxic effect on cancer cell lines [39], but remarkable inhibitory effect of fibronectin- and vitronectin-dependent cell adhesion. This toxin binds αvβ3-integrins, which is the main receptor of the ECM protein vitronectin, inhibiting the adhesion and migration of melanoma and lung cancer cells [40].
DisBa-01, a recombinant RGD-disintegrin produced from a cDNA venom gland library of Bothrops alternatus, inhibits in vivo angiogenesis and pulmonary metastasis [49]. In oral squamous carcinoma cells, DisBa-01 selectively decreases the migration speed and directionality of fibronectin-stimulated migration, increasing the adhesion area and rate of adhesion maturation. It lacks effects on migration of non-malignant cells such as fibroblasts. DisBa-01 exhibits a high affinity on fibronectin binding receptor αvβ3 integrin [43]. Other recombinant disintegrins from Viperidae species have been reported such as αvβ3 integrin antagonists, inhibiting the migration of cancer cells (Table 1). Additional disintegrins and disintegrin-like proteins from snake venoms reported with anti-cancer effect can be found in Selistre-de-Araujo et al., 2010 [50].
Interestingly, Lebecin, and PIVL isolated from Macrovipera lebetina venom, which belong two different toxin classes C-type lectin-like protein and Kunitz-type serin protease inhibitor, respectively, exhibit inhibitory effect on fibrinogen- and fibronectin-stimulated adhesion and migration.
Lebecin is a C-type lectin-like protein with α and β subunits of 129 and 131 amino acids, respectively [47]. In triple-negative breast cancer MDA-MB-231 cells, lebecin does not affect the viability. However, it inhibits the fibrinogen- and fibronectin-dependent adhesion and migration in a dose-dependent manner [47]. It has been described that lebecin interacts with αvβ3 integrin; but based on the high identity of its amino acid sequence with other C-type lectin-like protein previously reported from Macrovipera lebetina venom with inhibitory effect on adhesion, migration, and invasion of cancer cells [51,52], it has been suggested that lebecin can block other integrins such as α5β1 [47].
PIVL is a monomeric polypeptide chain bound by three disulfide linkages, which inhibits trypsin activity and lacks effects on the viability but blocks αvβ3 integrin-dependent migration, affecting the motility and cell directionality persistence of cancer cells [48]. PIVL also exhibits in vitro and in vivo anti-angiogenic effects [53].
4. Inhibition of Epithelial–Mesenchymal Transition
Epithelial–mesenchymal transition (EMT) is a process in which epithelial cells transdifferentiate into mesenchymal cells, losing their morphoinmunophenotypic characteristics. Interestingly, EMT occurs in normal and healthy tissues during angiogenesis and lymphangiogenesis; but in certain pathological conditions such as chronic inflammation, fibrosis and cancer is reactivated [6]. In tumors, EMT-like transitions involve the loss of components related with cell-cell interactions, apico-basal cell polarity and reorganization of cytoskeleton. Cancer cells with EMT have tumorigenic properties that non-EMT cells do not exhibit, such as a high migratory state that promote invasion and metastasis [4,5], lacking response to signals of oncogene-induced senescence [54] and resistance to anti-cancer drugs [55,56,57].
EMT can be induced by growth factors such as transforming growth factor beta (TGF-β), epidermal growth factor (EGF), hepatocyte growth factor (HGF), insulin-like growth factors 1 and 2 [40], activating RAS, Notch, and Wnt signalings which have been associated with poor prognosis and cancer progression [58,59]. During EMT, there is a reduction of the epithelial marker E-cadherin and an increase of the expression of mesenchymal markers vimentin, N-cadherin [60], as well as activation of transcription factors Snail, Slug, Twist, which act as repressor of E-cadherin [5,61].
Cardiotoxin III (CTX-III), a membrane toxin from Taiwan cobra (Naja naja) venom [62], inhibits the migration of cancer cells by reversion of EGF- and HGF-induced EMT. Previously, CTX-III has been described as a potent inductor of cell death in several human cancer cell lines [63,64,65] and a migration inhibitor of oral and breast cancer cells through activation of JNK and p38, without effect on ERK signaling, producing decreased metalloproteases-2 and -9 (MMP-2/-9) levels [66,67].
In breast cancer cells, the paracrine role of epidermal growth factor (EGF) and its receptor EGFR (ErbB-1) contribute to invasion, intravasation, and metastasis [68] through activation of extracellular signal-regulated kinase 1/2 (ERK1/2), STAT3, or PI3K/Akt signaling, promoting the EMT [69,70,71]. CTX-III inhibits the EGF-induced EMT in breast cancer cells, reducing EGFR phosphorylation and activation PI3K/Akt and ERK1/2. It reduces the MMP-9 levels [72] and the mesenchymal markers vimentin and N-cadherin and increases E-cadherin levels, inhibiting EGF-induced invasion and migration [72,73]. A similar effect of CTX-III on hepatocyte growth factor (HGF)-stimulated migration and invasion in breast cancer cells has been described [73,74,75].
Cancer cells can excrete cysteine-cathepsins, which are endopeptidases located intracellularly in endolysosomal vesicles [76] that are essential during the breakdown the ECM to promote the invasion and metastasis [77]. During EMT, cancer cells exhibit an increased extra- and intra-cellular proteolysis mediated by cathepsins, matrix metalloproteinases, urokinase-type plasminogen activator (uPA), and serinproteases such as kallikreins [78]. This proteolytic activity removes surface molecules involved in cell adhesion such as E-cadherin [79,80], limiting the cell–cell interaction and remodeling the extracellular matrix to uncover binding epitopes recognized by integrins and to form trials for cell migration [81]. Cysteine-cathepsins are regulated by natural inhibitors such as cystatins [82], which represent a group constituted by three types (type 1-stenfins, type-2 cystatins, type 3-kininogens) of cystatin domain containing proteins [83]. From Naja naja atra venom, it has been isolated a snake venom cystatin (Sv-cystatin) that exhibits a shorter sequence than other type-2 cystatins, such as cystatin M and cystatin C [84]. For this snake toxin, inhibitory effects on invasion and metastasis mediated by reduction of EMT markers has been described in MHCC97H liver cancer cells [85]. Sv-cystatin decreases the cathepsin B activity, MMP-2, and MMP-9 levels, increasing E-cadherin and decreasing EMT proteins N-cadherin and twist [85].
5. Alterations in the Actin/Cytoskeleton Network
During migration and invasion of cancer cells, the actin cytoskeleton is remodeled under extracellular stimuli, which is mediated by several receptors, including integrins [19]. Small GTPases Rho, Rac, and Cdc42 participate in the intracellular signaling involved in the control of the actin cytoskeleton architecture required for cell motility in individual and collective migration [86], which is a common signaling for normal and cancer cells [2]. The cell protrusion of a leading edge relies on Cdc42 and Rac activities, which are coupled to Rho activity-dependent contractility, supporting the movement of the cell body forward [87]. Consistent with the essential role of the cytoskeleton in promoting cancer migration, its deregulation may cause anti-adhesive and anti-migratory effects. Two snake venom calcium-dependent (C-type) lectins alter the actin/cytoskeleton network in cancer cells. C-type lectins identified from snake venoms are classified in two groups: C-type glycan-binding lectins; and C-type lectin-like proteins, which do not interact with sugars. The C-type glycan-binding lectins are homodimeric non-enzymatic proteins that contain a carbohydrate recognition domain (CRD), binding mainly with galactose [88].
Daboialectin, a low molecular weight C-type lectin isolated from Daboia russelii venom, produces morphological changes, including spindle-like shape with loss of cell–cell contacts in lung cancer cells A549 [89]. This snake toxin decreases the mRNA and protein levels of small GTPases Rho and Rac and increases the Cdc42 expression, which is in accordance with remarkable decrease of F-actin content, inhibition of migration and invasion observed in lung cancer cells treated with it [89].
BJcuL is a C-type lectin from Bothrops jararacussu venom composed by a disulfide-linked dimer with high affinity for glycoproteins containing β-d-galactosides [90]. BJcuL binds to cancer cells without affecting the adhesion of these cells to fibronectin, laminin, and type I collagen; however, it produces complete actin filament disorganization and disassembly in malignant cells [91]. This toxin does not block the integrin signaling [92], but it binds to cell surface with ECM glycoproteins, such as its substrate d-galactose, promoting the actin disassembles, an event that could accelerate cancer cell detachment from ECM, producing cell death [91].
6. Concluding Remarks
Given that malignant cells during metastasis exhibit molecular mechanisms different from those shown by non-metastatic and highly proliferative cancer cells, the conventional cytostatic drugs, which mainly target the cell proliferation, lack effects on the capacity to disseminate and grow in distant sites of metastatic cancer cells. This review highlights the need to search new anti-metastatic drugs. We identified three anti-metastatic mechanisms of action for at least six classes of toxins from snake venoms: (1) inhibition of ECM components-dependent adhesion and migration, (2) inhibition of EMT, and (3) inhibition of migration by alterations in the actin/cytoskeleton network.
These toxins may represent a natural source of molecular scaffolds to design new anti-migratory and anti-invasive agents by obtaining recombinant proteins or small molecules that act as antagonists of integrin signaling or inductors of actin disassembling by binding of cell surface glycoproteins. A selective inhibition of the signaling machinery involved in the cancer cell migration without affect those of migrating non-malignant cells is an important challenge for the new anti-metastatic drugs.
Interestingly, all anti-cancer evaluations on tumorigenic properties—such as proliferation, angiogenesis, invasion, and metastasis of malignant cells—have been performed with toxins isolated from front-fanged snake species, especially from Viperidae species; however, the potential therapeutic applications of toxins described from rear-fanged snake species—e.g., [28,30,31,32,93,94,95]—remain unexplored.
An extensive development and conjugation of drug delivery systems with some snake toxins, which has reduced the toxicity and improved the selectivity toward cancer cells [96,97], highlight their promising applications as direct anti-cancer agents or potential tools for the development of novel therapeutic strategies [16]. Finally, the in vivo validation of anti-metastatic effect described on in vitro cancer cell lines is a pending issue for drug discovery from snake toxins.
Acknowledgments
This work was supported by FONDECYT grant #1140753 (R.A.-M.), Programa de Investigación Asociativa en Cáncer Gástrico (PIA-CG, RU2107) (R.A.-M.) and FONDECYT postdoctoral fellowship #3170813 (F.A.U.).
Author Contributions
F.A.U. and R.A.-M. designed and contributed to the literature review, discussion, and writing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gandalovičová A., Rosel D., Fernandes M., Veselý P., Heneberg P., Čermák V., Petruželka L., Kumar S., Sanz-Moreno V., Brábek J. Migrastatics-Anti-metastatic and anti-invasion drugs: Promises and challenges. Trends Cancer. 2017;3:391–406. doi: 10.1016/j.trecan.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riggi N., Aguet M., Stamenkovic I. Cancer metastasis: A reappraisal of its underlying mechanisms and their relevance to treatment. Annu. Rev. Pathol. 2017 doi: 10.1146/annurev-pathol-020117-044127. [DOI] [PubMed] [Google Scholar]
- 3.Celià-Terrassa T., Kang Y. Distinctive properties of metastasis-initiating cells. Genes Dev. 2016;30:892–908. doi: 10.1101/gad.277681.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomaskovic-Crook E., Thompson E., Thiery J. Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res. 2009;11:213. doi: 10.1186/bcr2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlsson M., Gonzalez S., Welin J., Fuxe J. Epithelial-mesenchymal transition in cancer metastasis through the lymphatic system. Mol. Oncol. 2017;11:781–791. doi: 10.1002/1878-0261.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambert A., Pattabiraman D., Weinberg R. Emerging biological principles of metastasis. Cell. 2017;168:670–691. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sleeman J., Steeg P. Cancer metastasis as a therapeutic target. Eur. J. Cancer. 2010;46:1177–1180. doi: 10.1016/j.ejca.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heath A.S., Yibin K. Determinants of organotropic metastasis. Annu. Rev. Cancer Biol. 2017;1:403–423. [Google Scholar]
- 10.Aranda-Souza M., Rossato F., Costa R., Figueira T., Castilho R., Guarniere M., Nunes E., Coelho L., Correia M., Vercesi A. A lectin from Bothrops leucurus snake venom raises cytosolic calcium levels and promotes B16-F10 melanoma necrotic cell death via mitochondrial permeability transition. Toxicon. 2014;82:97–103. doi: 10.1016/j.toxicon.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Ebrahim K., Shirazi F., Mirakabadi A., Vatanpour H. Cobra venom cytotoxins; apoptotic or necrotic agents? Toxicon. 2015;108:134–140. doi: 10.1016/j.toxicon.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Prinholato da Silva C., Costa T., Paiva R., Cintra A., Menaldo D., Antunes L., Sampaio S. Antitumor potential of the myotoxin BthTX-I from Bothrops jararacussu snake venom: Evaluation of cell cycle alterations and death mechanisms induced in tumor cell lines. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015;21 doi: 10.1186/s40409-015-0044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guimarães D., Lopes D., Azevedo F., Gimenes S., Silva M., Achê D., Gomes M., Vecchi L., Goulart L., Yoneyama K., et al. In vitro antitumor and antiangiogenic effects of Bothropoidin, a metalloproteinase from Bothrops pauloensis snake venom. Int. J. Biol. Macromol. 2017;97:770–777. doi: 10.1016/j.ijbiomac.2017.01.064. [DOI] [PubMed] [Google Scholar]
- 14.Dhananjaya B., Sivashankari P. Snake venom derived molecules in tumor angiogenesis and its application in cancer therapy; an overview. Curr. Top. Med. Chem. 2015;15:649–657. doi: 10.2174/1568026615666150225113402. [DOI] [PubMed] [Google Scholar]
- 15.Azevedo F., Lopes D., Cirilo-Gimenes S., Achê D., Vecchi L., Alves P., de Oliveira Guimarães D., Rodrigues R., Goulart L., de Melo Rodrigues V., et al. Human breast cancer cell death induced by BnSP-6, a Lys-49 PLA homologue from Bothrops pauloensis venom. Int. J. Biol. Macromol. 2016;82:671–677. doi: 10.1016/j.ijbiomac.2015.10.080. [DOI] [PubMed] [Google Scholar]
- 16.Costa T., Menaldo D., Zoccal K., Burin S., Aissa A., Castro F., Faccioli L., Greggi-Antunes L., Sampaio S. CR-LAAO, an l-amino acid oxidase from Calloselasma rhodostoma venom, as a potential tool for developing novel immunotherapeutic strategies against cancer. Sci. Rep. 2017;7:42673. doi: 10.1038/srep42673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartsch J., Staren E., Appert H. Adhesion and migration of extracellular matrix-stimulated breast cancer. J. Surg. Res. 2003;110:287–294. doi: 10.1016/S0022-4804(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 18.Anderson L., Owens T., Naylor M. Structural and mechanical functions of integrins. Biophys. Rev. 2014;6:203–213. doi: 10.1007/s12551-013-0124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longmate W., DiPersio C. Beyond adhesion: Emerging roles for integrins in control of the tumor microenvironment. F1000Research. 2017;6 doi: 10.12688/f1000research.11877.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwakwa K., Sterling J. Integrin αvβ3 signaling in tumor-induced bone disease. Cancers (Basel) 2017;9:84. doi: 10.3390/cancers9070084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niu J., Li Z. The roles of integrin αvβ6 in cancer. Cancer Lett. 2017;403:128–137. doi: 10.1016/j.canlet.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Rathinam R., Alahari S. Important role of integrins in the cancer biology. Cancer Metastasis Rev. 2010;29:223–237. doi: 10.1007/s10555-010-9211-x. [DOI] [PubMed] [Google Scholar]
- 23.Marelli U., Rechenmacher F., Sobahi T., Mas-Moruno C., Kessler H. Tumor Targeting via Integrin Ligands. Front. Oncol. 2013;3:222. doi: 10.3389/fonc.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapp T., Rechenmacher F., Neubauer S., Maltsev O., Cavalcanti-Adam E., Zarka R., Reuning U., Notni J., Wester H., Mas-Moruno C., et al. A Comprehensive Evaluation of the Activity and Selectivity Profile of Ligands for RGD-binding Integrins. Sci. Rep. 2017;7:39805. doi: 10.1038/srep39805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moura-da-Silva A., Theakston R., Crampton J. Evolution of disintegrin cysteine-rich and mammalian matrix-degrading metalloproteinases: Gene duplication and divergence of a common ancestor rather than convergent evolution. J. Mol. Evol. 1996;43:263–269. doi: 10.1007/BF02338834. [DOI] [PubMed] [Google Scholar]
- 26.Calvete J., Marcinkiewicz C., Monleón D., Esteve V., Celda B., Juárez P., Sanz L. Snake venom disintegrins: Evolution of structure and function. Toxicon. 2005;45:1063–1074. doi: 10.1016/j.toxicon.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 27.Takeda S., Takeya H., Iwanaga S. Snake venom metalloproteinases: Structure, function and relevance to the mammalian ADAM/ADAMTS family proteins. Biochim. Biophys. Acta. 2012;1824:164–176. doi: 10.1016/j.bbapap.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Weldon C., Mackessy S. Alsophinase, a new P-III metalloproteinase with α-fibrinogenolytic and hemorrhagic activity from the venom of the rear-fanged Puerto Rican Racer Alsophis portoricensis (Serpentes: Dipsadidae) Biochimie. 2012;94:1189–1198. doi: 10.1016/j.biochi.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Peichoto M.E., Paes Leme A.F., Pauletti B.A., Batista I.C., Mackessy S.P., Acosta O., Santoro M.L. Autolysis at the disintegrin domain of patagonfibrase, a metalloproteinase from Philodryas patagoniensis (Patagonia Green Racer; Dipsadidae) venom. BBA Proteins Proteom. 2010;1804:1937–1942. doi: 10.1016/j.bbapap.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Ching A.T., Paes Leme A.F., Zelanis A., Rocha M.M., Furtado M.D.F., Silva D.A., Trugilho M.R., da Rocha S.L., Perales J., Ho P.L., et al. Venomics profiling of Thamnodynastes strigatus unveils matrix metalloproteinases and other novel proteins recruited to the toxin arsenal of rear-fanged snakes. J. Proteome Res. 2012;11:1152–1162. doi: 10.1021/pr200876c. [DOI] [PubMed] [Google Scholar]
- 31.Ching A.T., Rocha M.M., Paes Leme A.F., Pimenta D.C., de Fatima D.F.M., Serrano S.M., Ho P.L., Junqueira-de-Azevedo I.L. Some aspects of the venom proteome of the Colubridae snake Philodryas olfersii revealed from a Duvernoy’s (venom) gland transcriptome. FEBS Lett. 2006;580:4417–4422. doi: 10.1016/j.febslet.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Urra F., Pulgar R., Gutiérrez R., Hodar C., Cambiazo V., Labra A. Identification and molecular characterization of five putative toxins from the venom gland of the snake Philodryas chamissonis (Serpentes: Dipsadidae) Toxicon. 2015;108:19–31. doi: 10.1016/j.toxicon.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 33.Kamiguti A., Theakston R., Sherman N., Fox J. Mass spectrophotometric evidence for P-III/P-IV metalloproteinases in the venom of the Boomslang (Dispholidus typus) Toxicon. 2000;38:1613–1620. doi: 10.1016/S0041-0101(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z., Zhang X., Hu T., Zhou W., Cui Q., Tian J., Zheng Y., Fan Q. Discovery of toxin-encoding genes from the false viper Macropisthodon rudis, a rear-fanged snake, by transcriptome analysis of venom gland. Toxicon. 2015;106:72–78. doi: 10.1016/j.toxicon.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 35.McGivern J., Wray K., Margres M., Couch M., Mackessy S., Rokyta D. RNA-seq and high-definition mass spectrometry reveal the complex and divergent venoms of two rear-fanged colubrid snakes. BMC Genom. 2014;15:1061. doi: 10.1186/1471-2164-15-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walsh E., Marcinkiewicz C. Non-RGD-containing snake venom disintegrins, functional and structural relations. Toxicon. 2011;58:355–362. doi: 10.1016/j.toxicon.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Calvete J. The continuing saga of snake venom disintegrins. Toxicon. 2013;62:40–49. doi: 10.1016/j.toxicon.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Takeda S. ADAM and ADAMTS family proteins and snake venom metalloproteinases: A structural overview. Toxins (Basel) 2016;8:E155. doi: 10.3390/toxins8050155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saviola A., Modahl C., Mackessy S. Disintegrins of Crotalus simus tzabcan venom: Isolation, characterization and evaluation of the cytotoxic and anti-adhesion activities of tzabcanin, a new RGD disintegrin. Biochimie. 2015;116:92–102. doi: 10.1016/j.biochi.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Saviola A., Burns P., Mukherjee A., Mackessy S. The disintegrin tzabcanin inhibits adhesion and migration in melanoma and lung cancer cells. Int. J. Biol. Macromol. 2016;88:457–464. doi: 10.1016/j.ijbiomac.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Suntravat M., Barret H., Jurica C., Lucena S., Perez J., Sánchez E. Recombinant disintegrin (r-Cam-dis) from Crotalus adamanteus inhibits adhesion of human pancreatic cancer cell lines to laminin-1 and vitronectin. J. Venom Res. 2015;6:1–10. [PMC free article] [PubMed] [Google Scholar]
- 42.Suntravat M., Helmke T., Atphaisit C., Cuevas E., Lucena S., Uzcátegui N., Sánchez E., Rodriguez-Acosta A. Expression, purification, and analysis of three recombinant ECD disintegrins (r-colombistatins) from P-III class snake venom metalloproteinases affecting platelet aggregation and SK-MEL-28 cell adhesion. Toxicon. 2016;122:43–49. doi: 10.1016/j.toxicon.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montenegro C., Casali B., Lino R., Pachane B., Santos P., Horwitz A., Selistre-de-Araujo H., Lamers M. Inhibition of αvβ3 integrin induces loss of cell directionality of oral squamous carcinoma cells (OSCC) PLoS ONE. 2017;12:e0176226. doi: 10.1371/journal.pone.0176226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lucena S., Castro R., Lundin C., Hofstetter A., Alaniz A., Suntravat M., Sánchez E. Inhibition of pancreatic tumoral cells by snake venom disintegrins. Toxicon. 2015;93:136–143. doi: 10.1016/j.toxicon.2014.11.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucena S., Sanchez E., Perez J. Anti-metastatic activity of the recombinant disintegrin, r-mojastin 1, from the Mohave rattlesnake. Toxicon. 2011;57:794–802. doi: 10.1016/j.toxicon.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lucena S., Jia Y., Soto J., Parral J., Cantu E., Brannon J., Lardner K., Ramos C., Seoane A., Sánchez E. Anti-invasive and anti-adhesive activities of a recombinant disintegrin, r-viridistatin 2, derived from the Prairie rattlesnake (Crotalus viridis viridis) Toxicon. 2012;60:31–39. doi: 10.1016/j.toxicon.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jebali J., Fakhfekh E., Morgen M., Srairi-Abid N., Majdoub H., Gargouri A., El Ayeb M., Luis J., Marrakchi N., Sarray S. Lebecin, a new C-type lectin like protein from Macrovipera lebetina venom with anti-tumor activity against the breast cancer cell line MDA-MB231. Toxicon. 2014;86:16–27. doi: 10.1016/j.toxicon.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 48.Morjen M., Kallech-Ziri O., Bazaa A., Othman H., Mabrouk K., Zouari-Kessentini R., Sanz L., Calvete J., Srairi-Abid N., El Ayeb M., et al. PIVL, a new serine protease inhibitor from Macrovipera lebetina transmediterranea venom, impairs motility of human glioblastoma cells. Matrix Biol. 2013;32:52–62. doi: 10.1016/j.matbio.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 49.Ramos O., Kauskot A., Cominetti M., Bechyne I., Salla-Pontes C., Chareyre F., Manent J., Vassy R., Giovannini M., Legrand C., et al. A novel alpha(v)beta (3)-blocking disintegrin containing the RGD motive, DisBa-01, inhibits bFGF-induced angiogenesis and melanoma metastasis. Clin. Exp. Metastasis. 2008;25:53–64. doi: 10.1007/s10585-007-9101-y. [DOI] [PubMed] [Google Scholar]
- 50.Selistre-de-Araujo H., Pontes C., Montenegro C., Martin A. Snake venom disintegrins and cell migration. Toxins (Basel) 2010;2:2606–2621. doi: 10.3390/toxins2112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarray S., Berthet V., Calvete J., Secchi J., Marvaldi J., El-Ayeb M., Marrakchi N., Luis J. Lebectin, a novel C-type lectin from Macrovipera lebetina venom, inhibits integrin-mediated adhesion, migration and invasion of human tumour cells. Lab. Investig. 2004;84:573–581. doi: 10.1038/labinvest.3700088. [DOI] [PubMed] [Google Scholar]
- 52.Sarray S., Delamarre E., Marvaldi J., El Ayeb M., Marrakchi N., Luis J. Lebectin and lebecetin, two C-type lectins from snake venom, inhibit alpha5beta1 and alphaV-containing integrins. Matrix Biol. 2007;26:306–313. doi: 10.1016/j.matbio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Morjen M., Honoré S., Bazaa A., Abdelkafi-Koubaa Z., Ellafi A., Mabrouk K., Kovacic H., El Ayeb M., Marrakchi N., Luis J. PIVL, a snake venom Kunitz-type serine protease inhibitor, inhibits in vitro and in vivo angiogenesis. Microvasc. Res. 2014;95:149–156. doi: 10.1016/j.mvr.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 54.Ansieau S., Bastid J., Doreau A., Morel A., Bouchet B., Thomas C., Fauvet F., Puisieux I., Doglioni C., Piccinin S., et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Fischer K., Durrans A., Lee S., Sheng J., Li F., Wong S., Choi H., El Rayes T., Ryu S., Troeger J., et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kajiyama H., Shibata K., Terauchi M., Yamashita M., Ino K., Nawa A., Kikkawa F. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int. J. Oncol. 2007;31:277–283. doi: 10.3892/ijo.31.2.277. [DOI] [PubMed] [Google Scholar]
- 57.Shibue T., Weinberg R. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017;14:611–629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mulholland D., Kobayashi N., Ruscetti M., Zhi A., Tran L., Huang J., Gleave M., Wu H. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 2012;72:1878–1889. doi: 10.1158/0008-5472.CAN-11-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bo H., Zhang S., Gao L., Chen Y., Zhang J., Chang X., Zhu M. Upregulation of Wnt5a promotes epithelial-to-mesenchymal transition and metastasis of pancreatic cancer cells. BMC Cancer. 2013;13:496. doi: 10.1186/1471-2407-13-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foroni C., Broggini M., Generali D., Damia G. Epithelial-mesenchymal transition and breast cancer: Role, molecular mechanisms and clinical impact. Cancer Treat. Rev. 2012;38:689–697. doi: 10.1016/j.ctrv.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Nieto M., Cano A. The epithelial-mesenchymal transition under control: Global programs to regulate epithelial plasticity. Semin. Cancer Biol. 2012;22:361–368. doi: 10.1016/j.semcancer.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 62.Bhaskaran R., Huang C., Chang D., Yu C. Cardiotoxin III from the Taiwan cobra (Naja naja atra). Determination of structure in solution and comparison with short neurotoxins. J. Mol. Biol. 1994;235:1291–1301. doi: 10.1006/jmbi.1994.1082. [DOI] [PubMed] [Google Scholar]
- 63.Chien C., Chang S., Lin K., Chiu C., Chang L., Lin S. Taiwan cobra cardiotoxin III inhibits Src kinase leading to apoptosis and cell cycle arrest of oral squamous cell carcinoma Ca9-22 cells. Toxicon. 2010;56:508–520. doi: 10.1016/j.toxicon.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Lin K., Su J., Chien C., Chuang P., Chang L., Lin S. Down-regulation of the JAK2/PI3K-mediated signaling activation is involved in Taiwan cobra cardiotoxin III-induced apoptosis of human breast MDA-MB-231 cancer cells. Toxicon. 2010;55:1263–1273. doi: 10.1016/j.toxicon.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 65.Chen K., Lin S., Chang L. Involvement of mitochondrial alteration and reactive oxygen species generation in Taiwan cobra cardiotoxin-induced apoptotic death of human neuroblastoma SK-N-SH cells. Toxicon. 2008;52:361–368. doi: 10.1016/j.toxicon.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 66.Yen C., Liang S., Han L., Chou H., Chou C., Lin S., Chiu C. Cardiotoxin III inhibits proliferation and migration of oral cancer cells through MAPK and MMP signaling. Sci. World J. 2013;2013:650946. doi: 10.1155/2013/650946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin K., Chien C., Hsieh C., Tsai P., Chang L., Lin S. Antimetastatic potential of cardiotoxin III involves inactivation of PI3K/Akt and p38 MAPK signaling pathways in human breast cancer MDA-MB-231 cells. Life Sci. 2012;90:54–65. doi: 10.1016/j.lfs.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 68.Chiang S., Cabrera R., Segall J. Tumor cell intravasation. Am. J. Physiol. Cell Physiol. 2016;311:C1–C14. doi: 10.1152/ajpcell.00238.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lo H., Hsu S., Xia W., Cao X., Shih J., Wei Y., Abbruzzese J., Hortobagyi G., Hung M. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67:9066–9076. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Balanis N., Carlin C. Stress-induced EGF receptor signaling through STAT3 and tumor progression in triple-negative breast cancer. Mol. Cell. Endocrinol. 2017;451:24–30. doi: 10.1016/j.mce.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J., Kong J., Chang H., Kim H., Kim A. EGF induces epithelial-mesenchymal transition through phospho-Smad2/3-Snail signaling pathway in breast cancer cells. Oncotarget. 2016;7:85021–85032. doi: 10.18632/oncotarget.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsai P., Hsieh C., Chiu C., Wang C., Chang L., Lin S. Cardiotoxin III suppresses MDA-MB-231 cell metastasis through the inhibition of EGF/EGFR-mediated signaling pathway. Toxicon. 2012;60:734–743. doi: 10.1016/j.toxicon.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 73.Tsai P., Fu Y., Chang L., Lin S. Taiwan cobra cardiotoxin III suppresses EGF/EGFR-mediated epithelial-to-mesenchymal transition and invasion of human breast cancer MDA-MB-231 cells. Toxicon. 2016;111:108–120. doi: 10.1016/j.toxicon.2016.01.051. [DOI] [PubMed] [Google Scholar]
- 74.Tsai P., Chu C., Chiu C., Chang L., Lin S. Cardiotoxin III suppresses hepatocyte growth factor-stimulated migration and invasion of MDA-MB-231 cells. Cell Biochem. Funct. 2014;32:485–495. doi: 10.1002/cbf.3041. [DOI] [PubMed] [Google Scholar]
- 75.Tsai P., Fu Y., Chang L., Lin S. Cardiotoxin III Inhibits Hepatocyte Growth Factor-Induced Epithelial-Mesenchymal Transition and Suppresses Invasion of MDA-MB-231 Cells. J. Biochem. Mol. Toxicol. 2016;30:12–21. doi: 10.1002/jbt.21735. [DOI] [PubMed] [Google Scholar]
- 76.Mohamed M., Sloane B. Cysteine cathepsins: Multifunctional enzymes in cancer. Nat. Rev. Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 77.Fonović M., Turk B. Cysteine cathepsins and extracellular matrix degradation. Biochim. Biophys. Acta. 2014;1840:2560–2570. doi: 10.1016/j.bbagen.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 78.Löser R., Pietzsch J. Cysteine cathepsins: Their role in tumor progression and recent trends in the development of imaging probes. Front. Chem. 2015;3:37. doi: 10.3389/fchem.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sobotič B., Vizovišek M., Vidmar R., Van Damme P., Gocheva V., Joyce J., Gevaert K., Turk V., Turk B., Fonović M. Proteomic Identification of Cysteine Cathepsin Substrates Shed from the Surface of Cancer Cells. Mol. Cell. Proteom. 2015;14:2213–2228. doi: 10.1074/mcp.M114.044628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gocheva V., Zeng W., Ke D., Klimstra D., Reinheckel T., Peters C., Hanahan D., Joyce J. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev. 2006;20:543–546. doi: 10.1101/gad.1407406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Friedl P., Alexander S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell Biochem. Funct. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 82.Jedeszko C., Sloane B. Cysteine cathepsins in human cancer. Biol. Chem. 2004;385:1017–1027. doi: 10.1515/BC.2004.132. [DOI] [PubMed] [Google Scholar]
- 83.Shamsi A., Bano B. Journey of cystatins from being mere thiol protease inhibitors to at heart of many pathological conditions. Int. J. Biol. Macromol. 2017;102:674–693. doi: 10.1016/j.ijbiomac.2017.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brillard-Bourdet M., Nguyên V., Ferrer-di Martino M., Gauthier F., Moreau T. Purification and characterization of a new cystatin inhibitor from Taiwan cobra (Naja naja atra) venom. Biochem. J. 1998;331:239–244. doi: 10.1042/bj3310239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tang N., Xie Q., Wang X., Li X., Chen Y., Lin X., Lin J. Inhibition of invasion and metastasis of MHCC97H cells by expression of snake venom cystatin through reduction of proteinases activity and epithelial-mesenchymal transition. Arch. Pharm. Res. 2011;34:781–789. doi: 10.1007/s12272-011-0512-6. [DOI] [PubMed] [Google Scholar]
- 86.Mayor R., Etienne-Manneville S. The front and rear of collective cell migration. Nat. Rev. Mol. Cell Biol. 2016;17:97–109. doi: 10.1038/nrm.2015.14. [DOI] [PubMed] [Google Scholar]
- 87.Zegers M., Friedl P. Rho GTPases in collective cell migration. Small GTPases. 2014;5:e28997. doi: 10.4161/sgtp.28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sartim M., Sampaio S. Snake venom galactoside-binding lectins: A structural and functional overview. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015;21:35. doi: 10.1186/s40409-015-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pathan J., Mondal S., Sarkar A., Chakrabarty D. Daboialectin, a C-type lectin from Russell’s viper venom induces cytoskeletal damage and apoptosis in human lung cancer cells in vitro. Toxicon. 2017;127:11–21. doi: 10.1016/j.toxicon.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 90.Carvalho D., Marangoni S., Oliveira B., Novello J. Isolation and characterization of a new lectin from the venom of the snake Bothrops jararacussu. IUBMB Life. 1998;44:933–938. doi: 10.1080/15216549800201992. [DOI] [Google Scholar]
- 91.Nolte S., de Castro Damasio D., Baréa A., Gomes J., Magalhães A., Mello Zischler L., Stuelp-Campelo P., Elífio-Esposito S., Roque-Barreira M., Reis C., et al. BJcuL, a lectin purified from Bothrops jararacussu venom, induces apoptosis in human gastric carcinoma cells accompanied by inhibition of cell adhesion and actin cytoskeleton disassembly. Toxicon. 2012;59:81–85. doi: 10.1016/j.toxicon.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 92.De Carvalho D., Schmitmeier S., Novello J., Markland F. Effect of BJcuL (a lectin from the venom of the snake Bothrops jararacussu) on adhesion and growth of tumor and endothelial cells. Toxicon. 2001;39:1471–1476. doi: 10.1016/S0041-0101(01)00106-4. [DOI] [PubMed] [Google Scholar]
- 93.Peichoto M.E., Teibler P., Mackessy S.P., Leiva L., Acosta O., Goncalves L.R., Tanaka-Azevedo A.M., Santoro M.L. Purification and characterization of patagonfibrase, a metalloproteinase showing alpha-fibrinogenolytic and hemorrhagic activities, from Philodryas patagoniensis snake venom. BBA Gen. Subj. 2007;1770:810–819. doi: 10.1016/j.bbagen.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 94.Sánchez M.N., Timoniuk A., Maruñak S., Teibler P., Acosta O., Peichoto M.E. Biochemical and biological analysis of Philodryas baroni (Baron’s Green Racer; Dipsadidae) venom: Relevance to the findings of human risk assessment. Hum. Exp. Toxicol. 2014;33:22–31. doi: 10.1177/0960327113493302. [DOI] [PubMed] [Google Scholar]
- 95.Heyborne W.H., Mackessy S.P. Identification and characterization of a taxon-specific three-finger toxin from the venom of the Green Vinesnake (Oxybelis fulgidus; family Colubridae) Biochimie. 2013;95:1923–1932. doi: 10.1016/j.biochi.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 96.Bhowmik T., Saha P., Sarkar A., Gomes A. Evaluation of cytotoxicity of a purified venom protein from Naja kaouthia (NKCT1) using gold nanoparticles for targeted delivery to cancer cell. Chem. Biol. Interact. 2017;261:35–49. doi: 10.1016/j.cbi.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 97.Badr G., Sayed D., Maximous D., Mohamed A., Gul M. Increased susceptibility to apoptosis and growth arrest of human breast cancer cells treated by a snake venom-loaded silica nanoparticles. Cell. Physiol. Biochem. 2014;34:1640–1651. doi: 10.1159/000366366. [DOI] [PubMed] [Google Scholar]