Abstract
Background: To prevent postoperative infection the use of systemic antibiotic prophylaxis is common ground. Type of antibiotic used and duration of prophylaxis are subject to debate. In case of suspected early periprosthetic infection a debridement, antibiotics and implant retention (DAIR) procedure is treatment of first choice. This study evaluated the antibiotic prophylaxis and DAIR treatment protocols nationwide as well as reporting of these DAIR procedures to the national joint registry.
Methods: All institutions that performed total hip or knee arthroplasty were contacted to complete a 16-question online survey. Questions included availability of a protocol, type and duration of antibiotic prophylaxis used and tendency to register infectious complications in the Dutch Arthroplasty Register.
Results: All ninety-nine consulted institutions responded to this survey. All but one institutions have a standardized hospital based protocol for antibiotic prophylaxis in primary total hip or knee arthroplasty. Cefazolin was antibiotic prophylaxis of choice in ninety-four institutions for both primary hip and knee arthroplasty. In ten institutions one preoperative gift of antibiotic prophylaxis was administered. A protocol describing treatment when suspecting early periprosthetic joint infection was present in seventy-one institutions. When performing a DAIR procedure modular parts were exchanged in seventy institutions in case of a hip prosthesis and in eighty-one institutions in case of a knee prosthesis. Sixty-three institutions register DAIR procedures in the Dutch Arthroplasty Register.
Interpretation: In contradiction to the results of a recent study in Great Britain, we have found only little variety in availability of protocols and in the type of antibiotic used as prophylaxis in primary total hip and knee arthroplasty in The Netherlands. Not every institution has a protocol for treatment in suspicion of early infection. Although mobile parts are exchanged in the majority of cases, there appears to be an underreporting of DAIR procedures in the Dutch Arthroplasty Register.
Keywords: antibiotic prophylaxis, national joint registry, total hip arthroplasty, total knee arthroplasty, DAIR procedure, periprosthetic joint infection.
Introduction
Total hip and knee arthroplasty (THA and TKA) are well-proven solutions in case of end-stage osteoarthritis of hip and knee 1-5. Although, presence of complications can be devastating for the patient, especially periprosthetic joint infection (PJI) 6-8. To prevent PJI, antibiotic prophylaxis regimens are regularly used 7, 9-11. Since the introduction of systemic and local antibiotic prophylaxis in primary THA and TKA the percentage of infectious complications has decreased to 1-2% of these arthroplasty patients 7. A major part of PJI are caused by Staphylococcus species, particularly Staphylococcus (S.) aureus and coagulase negative staphylococci (CoNS) 6, 12. Generally these bacteria are susceptible to cephalosporins such as cefazolin or cefuroxime 13.
The numbers of yearly performed THA and TKA are expected to increase.14 Therefore the absolute number of infectious complications will likely increase as well, even when the percentage of infections can be limited further. Evidence based guidelines for the treatment of PJI are needed to face this challenge 15.
A worldwide consensus meeting concerning prevention, diagnosis and treatment of periprosthetic joint infections held in 2013 suggested antibiotic prophylaxis to be discontinued within 48 hours postoperatively.10, 16, 17 A recently updated guideline by the Netherlands Orthopaedic Association (NOV) advises an antibiotic prophylaxis to be discontinued within 24 hours after surgery.18 Continuation of antibiotic prophylaxis for more than 24 hours postoperatively does not provide lower infection rates.19
The duration of systemic antibiotic prophylaxis and the type of antibiotic used remain subject of discussion. The aforementioned consensus meeting suggests a first- or second-generation cephalosporin as antibiotic of first choice.16 The same was recommended in the recently updated guideline of the NOV.18 A recent study performed in Great Britain revealed a wide variety of types of antibiotics used, without region-specific bacterial occurrence to account for differences in treatment.20 This variety in treatment protocols may be caused by the absence of a national antibiotic prophylaxis guideline for all National Health Service Trusts in the UK.20 The optimal duration of antibiotic prophylaxis remains undetermined.
In case of early infection after total hip or knee arthroplasty management with a debridement, antibiotics and implant retention (DAIR) procedure is the first treatment of choice 21. According to the Dutch Arthroplasty Register (LROI) these procedures should be registered in the database as a revision procedure. Several studies of the National Joint Registries in Sweden, Denmark and Norway suggest about 30-40% of PJI and DAIR-procedures are not reported in national databases 22-25.
Underreporting of infections in implant registries is likely to be caused by the design of these databases which is not adequate for registry of infections, as the reason for revision is registered preoperatively while the diagnosis of infection can only be made after results of preoperatively taken tissue cultures are complete 2-7 days later.22 Chronic infections which are treated with antibiotic suppression therapy are also not registered in implant registries.
This study was performed to evaluate the use of standardized protocols on systemic antibiotic prophylaxis for primary THA and TKA in The Netherlands. Second, this study evaluated protocols concerning DAIR procedures and the tendency to register DAIR-procedures in the database by Dutch orthopaedic institutions. We hypothesized that, in contrast with British practice, little variety in type of antibiotics and variation in duration of antibiotic prophylaxis would exist in The Netherlands. Secondly, we hypothesized that not all DAIR treatments are performed according to a set protocol and that DAIR procedures are under-reported in the LROI.
Methods
A list of institutions performing THA and/or TKA was retrieved from the LROI annual report 2014 26. In each institution an orthopaedic surgeon was selected, who was specialised in either knee or hip arthroplasty. An electronic 16-question survey (Supplementary Material) concerning the perioperative protocol for THA and TKA was constructed and sent to the selected orthopaedic surgeons. Non-responding institutions were contacted by telephone and the survey was taken from the attending orthopaedic surgeon to assure an optimal response rate. During the period of May through July 2016 a total of ninety-nine university hospitals, teaching and regional hospitals and private orthopaedic clinics were included.
Data management and analysis were performed with SPSS 2016 software.
Results
All ninety-nine contacted institutions completed the questionnaire. All responders were orthopaedic surgeons, practicing in eight university hospitals, eighty general hospitals and eleven private orthopaedic clinics.
Systemic antibiotic prophylaxis
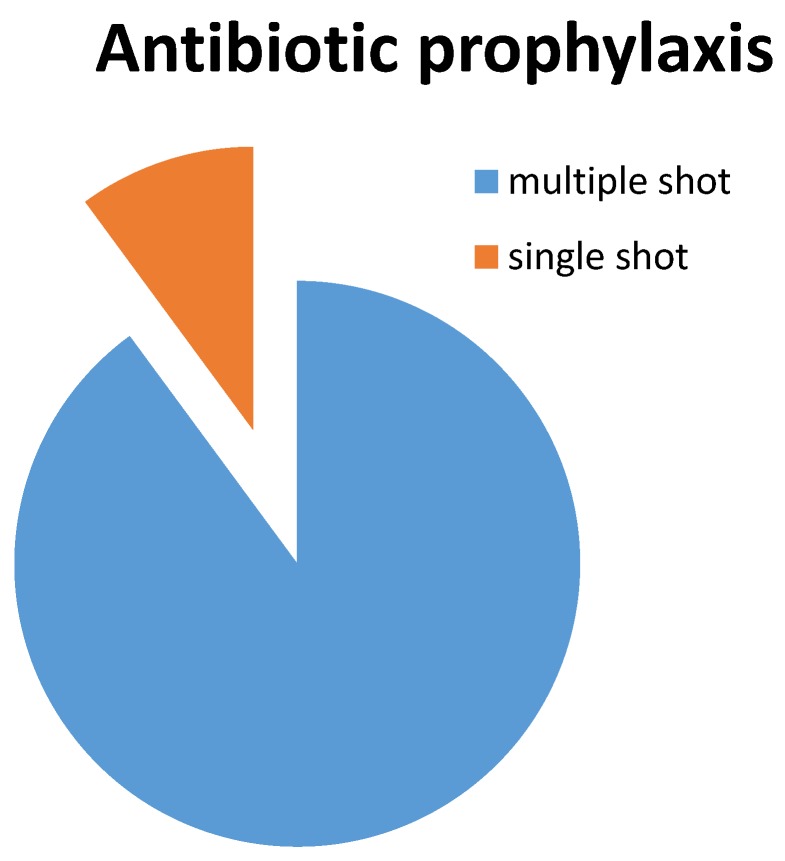
A protocol describing perioperative care including systemic antibiotic prophylaxis was present in all but one institution. In eighty-nine institutions, multiple doses of antibiotic prophylaxis were administered (three or four doses in case of cefazolin, three doses in case of cefuroxime) within twenty-four hours postoperatively. Antibiotic prophylaxis was limited to a single preoperative administration in ten institutions (Figure 1). Antibiotic of choice was cefazolin in ninety-four institutions. Four institutions administered cefuroxime as antibiotic prophylaxis, one institution administered one shot of cefazolin preoperatively and two shots of cefuroxime postoperatively. Allergy for cephalosporins or proven colonization with multi-resistant micro-organisms were reported as reason for alternative prophylaxis, in which the recommendations of the international consensus meeting were followed and either clindamycin or vancomycin were administered as second-choice antibiotic.
Figure 1.
Antibiotic prophylaxis
Infection treatment: Hip
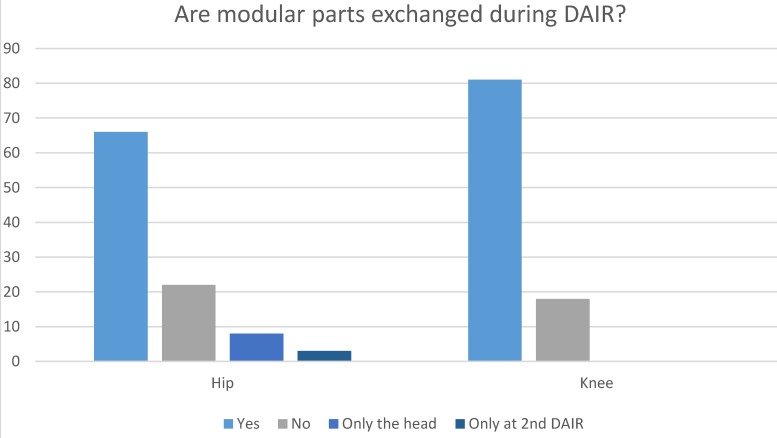
Seventy-one institutions have a protocol describing the treatment for patients with suspected early periprosthetic joint infection of the hip. When a debridement, antibiotics and implant retention (DAIR) procedure is performed, modular parts are exchanged in sixty-six institutions (Figure 2). Eight institutions reported only to exchange the femoral head, but not the acetabular liner. In four of these institutions, this was motivated by the use of a monoblock acetabular component. Three institutions only exchanged modular parts in case a second DAIR procedure was performed. In twenty-two institutions, modular parts are not exchanged during DAIR. Although many institutions exchange modular parts, DAIR procedures are registered in the LROI by only sixty-three institutions.
Figure 2.
Exchange of modular parts
Infection treatment: Knee
Seventy-three institutions have a protocol describing the treatment for patients with suspected early periprosthetic joint infection of the knee. When a DAIR procedure is performed, the insert is exchanged in eighty-one institutions, the insert is not exchanged in the remaining eighteen institutions (Figure 2). DAIR procedures are registered in the LROI by sixty-three institutions.
Discussion
Duration of systemic antibiotic prophylaxis remains a topic of discussion. First, we hypothesized that little variety in antibiotic prophylaxis protocols concerning primary total hip and knee arthroplasty would exist in The Netherlands. This hypothesis was correct. With all but one institutions stating the presence of a protocol for antibiotic prophylaxis in primary total hip and knee arthroplasty the authors believe this is an excellent basis for optimal prophylactic treatment in total hip and knee joint arthroplasty surgery. National and worldwide guidelines for administration of cephalosporin as antibiotic prophylaxis were followed by all Dutch institutions. The incidence of MRSA in the Netherlands is relatively low comparing to other European countries 12, 12, 27, 28. Therefore a prophylaxis regimen with only a cephalosporin is sufficient 12, 27.
Duration of antibiotic prophylaxis primary total joint arthroplasty surgery in The Netherlands is twenty-four hours in 89 out of 99 institutions. In 10 out of 99 institutions antibiotic prophylaxis consists of a single preoperative shot. Engesaeter and colleagues have found less infection and aseptic loosening after multiple shot antibiotic prophylaxis compared to single shot antibiotic prophylaxis in their observational study 19. Up to date insufficient evidence is available to favour either one of these protocols.
The cornerstone in the treatment of PJI should be evidence based treatment protocols 16. Protocolled care is expected to minimise the chance of errors during treatment 16, 29. Availability of such local protocols when suspecting PJI is 71 and 73 out of 99 institutions for hip and knee arthroplasties respectively in The Netherlands. The Netherlands Orthopaedic Association (NOV) has recently updated its treatment recommendations in presence of PJI 29. The NOV recommendation suggests exchange of all modular parts of a total joint implant in case a DAIR procedure is performed. This is in concordance with the recommendations of the international consensus meeting.16 Exchange of modular parts during a DAIR procedure is performed in seventy-four of ninety-nine institutions in case of the hip and in eighty-one of ninety-nine institutions in case of the knee. Registration of DAIR procedure is mandatory according to the LROI instructions on registration of revision procedures (i.e. registration would be done in case of an exchange of any implant). However, DAIR procedures are registered in the LROI by only sixty-four percent of the institutions. This means that currently there is a significant under registration, with consequently a potential underestimation of the rate of implant-related infections in our nationwide joint registry. It should be taken into account that a DAIR is also performed for prolonged wound drainage after a primary joint arthroplasty and is thus not always identical to a PJI. The possibility to register a DAIR procedures as such instead of as a partial revision may lead to improved registration of these procedures, especially in hospitals where modular parts are not exchanged during DAIR. Nevertheless, registration of DAIR procedures require better attention. First to relate DAIR procedures to primary surgery, secondly to relate them to (suspected) early and late infections resulting in implant removal. The latter can be early or late after the initial DAIR procedure, these data can then be used as part of a quality surveillance systems to improve patient care.
Weaknesses of this study are caused by its design, a questionnaire survey. Despite presence of a protocol, still differences between orthopaedic surgeons within the same institute might occur, the latter cannot be accounted for in this study. But since prevention of a PJI by antibiotic prophylaxis is common practice, the likelihood of not conferring to the prophylaxis protocol is highly unlikely. As for DAIR procedures, larger inter-surgeon variation may exist. Due to the present form of the LROI registry it has a reporting bias for PJI, which makes it difficult to draw conclusions concerning postoperative infections.
We have managed to achieve an excellent response rate, which is a pearl of this study. This study provides a perspective on the use of current prophylactic regimes and availability of protocolled care in The Netherlands. Also, the availability and characteristics of protocols describing treatment when suspecting early infection after primary hip or knee prosthesis are evaluated.
With the number of primary prosthesis expected to increase in years to come, the orthopaedic society faces a tremendous challenge. Even if the percentage of infectious complications can be decreased, the absolute number of PJI is likely to increase drastically. To cope with this challenge, research studying the optimal prevention of infectious complications, of which is also DAIR in presence of persistent wound leakage, following total hip or knee arthroplasty is crucial. Future research should show which type and duration of antibiotic prophylaxis regime provides lowest risk for infection after primary total hip or knee arthroplasty.
Supplementary Material
Survey questionnaire.
Contribution of authors
All authors contributed to study design and draft and/or revision of the manuscript. Data collection and analysis were performed by ESV.
References
- 1.Arirachakaran A, Wande T, Pituckhanotai K, Predeeprompan P, Kongtharvonskul J. Clinical outcomes after high-flex versus conventional total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23:1610–21. doi: 10.1007/s00167-015-3557-0. [DOI] [PubMed] [Google Scholar]
- 2.Kane RL, Saleh KJ, Wilt TJ, Bershadsky B. The functional outcomes of total knee arthroplasty. J Bone Joint Surg Am. 2005;87:1719–24. doi: 10.2106/JBJS.D.02714. [DOI] [PubMed] [Google Scholar]
- 3.De Anta-Diaz B, Serralta-Gomis J, Lizaur-Utrilla A, Benavidez E, Lopez-Prats FA. No differences between direct anterior and lateral approach for primary total hip arthroplasty related to muscle damage or functional outcome. Int Orthop. 2016;40(10):2025–2030. doi: 10.1007/s00264-015-3108-9. [DOI] [PubMed] [Google Scholar]
- 4.Graves SC, Dropkin BM, Keeney BJ, Lurie JD, Tomek IM. Does Surgical Approach Affect Patient-reported Function After Primary THA? Clin Orthop Relat Res. 2016;474:971–81. doi: 10.1007/s11999-015-4639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins BT, Barlow DR, Heagerty NE, Lin TJ. Anterior vs. posterior approach for total hip arthroplasty, a systematic review and meta-analysis. J Arthroplasty. 2015;30:419–34. doi: 10.1016/j.arth.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–54. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 7.Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387:386–94. doi: 10.1016/S0140-6736(14)61798-0. [DOI] [PubMed] [Google Scholar]
- 8.Bosco JA, Bookman J, Slover J, Edusei E, Levine B. Principles of Antibiotic Prophylaxis in Total Joint Arthroplasty: Current Concepts. J Am Acad Orthop Surg. 2015;23:e27–e35. doi: 10.5435/JAAOS-D-15-00017. [DOI] [PubMed] [Google Scholar]
- 9.Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94:e104. doi: 10.2106/JBJS.K.01417. [DOI] [PubMed] [Google Scholar]
- 10.AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br. 2008;90:915–9. doi: 10.1302/0301-620X.90B7.20498. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organisation. Global Guidelines for the Prevention of Surgical Site Infection. 2016. Ref Type: Online Source. [PubMed]
- 12.Wertheim HF, Vos MC, Boelens HA, Voss A, Vandenbroucke-Grauls CM, Meester MH. et al. Low prevalence of methicillin-resistant Staphylococcus aureus (MRSA) at hospital admission in the Netherlands: the value of search and destroy and restrictive antibiotic use. J Hosp Infect. 2004;56:321–5. doi: 10.1016/j.jhin.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg; 2017. pp. 395–404. [DOI] [PubMed] [Google Scholar]
- 14.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 15.Simpson AH, Dave J, Ghert M. Prophylactic antibiotics in total joint arthroplasty: evolution or devolution? Bone Joint Res. 2015;4:195–7. doi: 10.1302/2046-3758.412.BJR-2015-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parvizi J, Gehrke T. International consensus on periprosthetic joint infection: let cumulative wisdom be a guide. J Bone Joint Surg Am. 2014;96:441. doi: 10.2106/JBJS.N.00023. [DOI] [PubMed] [Google Scholar]
- 17.Gillespie WJ, Walenkamp GH. Antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures. Cochrane Database Syst Rev. 2010:CD000244. doi: 10.1002/14651858.CD000244.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nederlandse Orthopaedische Vereniging. Preventie van postoperatieve wondinfectie bij een totale heupprothese. orthopeden.org. 2016. Ref Type: Online Source.
- 19.Engesaeter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74:644–51. doi: 10.1080/00016470310018135. [DOI] [PubMed] [Google Scholar]
- 20.Hickson CJ, Metcalfe D, Elgohari S, Oswald T, Masters JP, Rymaszewska M. et al. Prophylactic antibiotics in elective hip and knee arthroplasty: an analysis of organisms reported to cause infections and National survey of clinical practice. Bone Joint Res. 2015;4:181–9. doi: 10.1302/2046-3758.411.2000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuiper JW, Willink RT, Moojen DJ, van den Bekerom MP, Colen S. Treatment of acute periprosthetic infections with prosthesis retention: Review of current concepts. World J Orthop. 2014;5:667–76. doi: 10.5312/wjo.v5.i5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witso E. The rate of prosthetic joint infection is underestimated in the arthroplasty registers. Acta Orthop. 2015;86:277–8. doi: 10.3109/17453674.2015.1042320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gundtoft PH, Overgaard S, Schonheyder HC, Moller JK, Kjaersgaard-Andersen P, Pedersen AB. The "true" incidence of surgically treated deep prosthetic joint infection after 32,896 primary total hip arthroplasties: a prospective cohort study. Acta Orthop. 2015;86:326–34. doi: 10.3109/17453674.2015.1011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gundtoft PH, Pedersen AB, Schonheyder HC, Overgaard S. Validation of the diagnosis 'prosthetic joint infection' in the Danish Hip Arthroplasty Register. Bone Joint J. 2016;98B:320–5. doi: 10.1302/0301-620X.98B3.36705. [DOI] [PubMed] [Google Scholar]
- 25.Lindgren JV, Gordon M, Wretenberg P, Karrholm J, Garellick G. Validation of reoperations due to infection in the Swedish Hip Arthroplasty Register. BMC Musculoskelet Disord. 2014;15:384. doi: 10.1186/1471-2474-15-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nederlandse Orthopaedische Vereniging, Stichting LROI. LROI jaarrapportage 2014 - Orthopedische Implantaten in Beeld. 2014. Ref Type: Report.
- 27.RIVM. European Antimicrobial Resistance Surveillance System (EARSS) 2017. Ref Type: Online Source.
- 28.Wannet WJB, Huijsdens XW, Heck MEOC, Pluister GN, Santen-Verheuvel MG van, Spalburg E. MRSA in Nederlandse ziekenhuizen: surveillanceresultaten 2005-2006 en recente ontwikkelingen. Infectieziekten Bulletin. 2017;2007:347–51. [Google Scholar]
- 29.Nederlandse Orthopaedische Vereniging. Aanbeveling Werkwijze Behandeling Prothese Infecties Orthopedie. orthopeden.org. 1-9-2015. Ref Type: Online Source.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survey questionnaire.