ABSTRACT
The Accelerate Pheno system uses automated fluorescence in situ hybridization technology with morphokinetic cellular analysis to provide rapid species identification (ID) and antimicrobial susceptibility testing (AST) results for the most commonly identified organisms in bloodstream infections. The objective was to evaluate the accuracy and workflow of bacterial and yeast ID and bacterial AST using the Accelerate Pheno system in the clinical microbiology laboratory. The consecutive fresh blood cultures received in the laboratory were analyzed by the Accelerate Pheno system within 0 to 8 h of growth detection. ID/AST performance, the average times to results, and workflow were compared to those of the routine standard of care. Of the 232 blood cultures evaluated (223 monomicrobial and 9 polymicrobial) comprising 241 organisms, the overall sensitivity and specificity for the identification of organisms were 95.6% and 99.5%, respectively. For antimicrobial susceptibility, the overall essential agreement was 95.1% and categorical agreement was 95.5% compared to routine methods. There was one very major error and 3 major errors. The time to identification and the time to susceptibility using the Accelerate Pheno system were decreased by 23.47 and 41.86 h, respectively, compared to those for the standard of care. The reduction in hands on time was 25.5 min per culture. The Accelerate Pheno system provides rapid and accurate ID/AST results for most of the organisms found routinely in blood cultures. It is easy to use, reduces hands on time for ID/AST of common blood pathogens, and enables clinically actionable results to be released much earlier than with the current standard of care.
KEYWORDS: Accelerate, blood culture, identification, susceptibility, turnaround time, workflow
INTRODUCTION
Up to one in four patients with sepsis die from organ failure caused by the hosts' immune responses to infection. Blood cultures need to be collected before antimicrobials are administered, and rapid identification and susceptibility of causative organisms can aid in the lifesaving administration of targeted antimicrobial therapy (1, 2). Routine methods of microbial identification and susceptibility testing might require 24 to 48 h, delaying the use of targeted therapy and timely de-escalation (3, 4). Studies have shown that a delayed administration of antibiotics to septic patients is associated with increased mortality among adults and neonates (5, 6). Also, the initial empirical therapy can be inappropriate in up to 50% of cases (7, 8). This delay leads to increased patient morbidity, mortality, length of stay (LOS), and cost of care (9, 10).
The current standard of care (SOC) for the diagnosis of bloodstream infections (whether or not the patient presents with sepsis) includes blood cultures. Advances are needed to decrease the turnaround time (TAT) for the diagnosis of bloodstream infections. After the organisms multiply and the blood culture is flagged positive, many different platforms are available for the rapid identification of pathogens. Methods such as matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (11), fluorescence in situ hybridization with peptide nucleic acid probes (PNA-FISH) (12), multiplex PCR (13), and multiplex PCR coupled to electrospray ionization mass spectrometry (PCR/ESI-MS) (14) are rapid, and some have the ability to identify genetic antimicrobial resistance determinants, but none produce a full phenotypic susceptibility result. These methods have a clinical impact if the reporting is coupled with antimicrobial stewardship intervention (3, 15). A recent assessment of physicians' interpretations of rapid blood culture identification and antibiotic prescribing practices in a single medical center concluded that the use is suboptimal and the misinterpretation of results can be at rates of up to 50% (16). Some of the PCR-based methods can detect pathogens directly from the blood sample rather than from a positive culture, providing faster identification (17); an accompanying blood culture and isolate are necessary for susceptibility results.
We sought to evaluate the ability of the Accelerate Pheno system (AXDX) to identify bloodstream isolates and perform susceptibility testing. The system uses a fully automated fluorescence in situ hybridization (FISH) technology for a rapid genomic identification of the most common pathogens causing bloodstream infections (BSI). Each of the 16 FISH identification (ID) probe cocktails employs a proprietary combination of universal and species-specific probes for species- or in some cases genus-level identification and also supports a unique “monomicrobial call,” indicating that only one pathogen is present (18). Following an identification, the system uses morphokinetic cellular analysis for phenotypic antimicrobial susceptibility testing (AST). For each drug-specific AST, a single concentration of antibiotic is used to provide MICs and categorical interpretations (susceptible [S], intermediate [I], or resistant [R]) based on FDA and/or CLSI breakpoints (19). The Accelerate Pheno system combines ID and AST in one instrument producing an ID within 90 min and AST in approximately 7 h from a positive blood culture specimen, giving the physician timely guidance to escalate or de-escalate empirical therapy and optimize definitive therapy.
MATERIALS AND METHODS
Blood cultures collected from March 2016 to October 2016 at the University of Chicago Medicine were included in the study. The cultures were collected in Bactec Plus aerobic/F medium, Bactec Plus anaerobic/F medium or Bactec Peds Plus/F medium bottles (Becton, Dickinson, Baltimore, MD) and incubated in a Bactec FX continuous blood culture monitoring instrument (Becton, Dickinson, Baltimore, MD) until growth was detected. The time of growth detection was recorded. Positive blood culture specimens (5 ml) were tested fresh within 8 h of growth detection on an early version (pre-FDA clearance) of the Accelerate Pheno system (v1.0 software) with the Accelerate PhenoTest BC kit as per the manufacturer's instructions. Only the first positive blood culture (aerobic or anaerobic) per patient within 28 days was included in the study. Known off-panel organisms such as Gram-positive rods were excluded from the study. The start times of the runs and times when identification (ID) and AST results became available on the Accelerate Pheno system were recorded. At the same time, positive blood culture samples were processed using routine methods as per the SOC, using the Vitek MS system (bioMérieux, Durham, NC), Vitek 2 system (bioMérieux, Durham, NC), or biochemical assays (including catalase, pyrrolidonyl arylamidase [PYR], Remel; Thermo Fisher Scientific, Lenexa, KS, USA) and BactiStaph (Remel; Thermo Fisher Scientific, Lenexa, KS, USA) for identification. For antimicrobial susceptibility testing (AST), the comparator for most combinations was the Vitek 2 system using the AST-GN75 card for Gram-negative organisms and AST-GP67 for staphylococci and enterococci (bioMérieux, Durham, NC). However, the Etest (bioMérieux, Durham, NC) was the comparator for colistin, and disk diffusion (Becton Dickinson Microbiology Systems, Sparks, MD) was the comparator for testing Acinetobacter baumannii with amikacin, ampicillin-sulbactam, cefepime, ciprofloxacin, meropenem, and piperacillin-tazobactam. Moreover, for the Enterobacteriaceae, colistin was only tested against meropenem-resistant organisms.
Frozen blood aliquots were also prepared at this time to accurately capture the organisms present at the time the positive bottle was loaded on the Accelerate Pheno system. In addition, isolates were frozen at −70°C for discrepancy analysis.
The times when identification and AST results were available were recorded.
Research use only (RUO) organism-antimicrobial combinations were evaluated for research purposes only and are not for use in diagnostic procedures. The exclusion criteria included the following: indeterminate results, samples run >8 h after growth detection, samples for which the Accelerate Pheno system detected 4 or more organisms or AST results that were reported for 2 organisms in a sample.
This study was approved by institutional review board (IRB) under study number IRB14-1358.
Data analysis. (i) Identification and antimicrobial susceptibility.
The following Gram-positive and Gram-negative organisms and yeasts were included in the ID comparative analysis: Staphylococcus aureus, Staphylococcus lugdunensis, coagulase-negative staphylococci (i.e., Staphylococcus epidermidis, Staphylococcus haemolyticus, Staphylococcus hominis, Staphylococcus capitis, Staphylococcus lugdunensis, and Staphylococcus warneri [not differentiated]), Enterococcus faecium, Enterococcus faecalis, Streptococcus spp. (i.e., Streptococcus mitis, Streptococcus oralis, Streptococcus gallolyticus, Streptococcus agalactiae, and Streptococcus pneumoniae [not differentiated]), Escherichia coli, Klebsiella spp. (i.e., Klebsiella pneumoniae and Klebsiella oxytoca [not differentiated]), Enterobacter spp. (i.e., Enterobacter cloacae and Enterobacter aerogenes [not differentiated]), Proteus spp. (i.e., Proteus mirabilis and Proteus vulgaris [not differentiated]), Citrobacter spp. (i.e., Citrobacter freundii and Citrobacter koseri [not differentiated]), Serratia marcescens, Acinetobacter baumannii, Pseudomonas aeruginosa, Candida albicans, and Candida glabrata.
AST was performed using the following antimicrobials for the Gram-positive organisms: ampicillin, erythromycin, linezolid, trimethoprim-sulfamethoxazole, and vancomycin. Cefoxitin was also tested against S. aureus and the coagulase-negative staphylococci to detect methicillin resistance. For the Gram-negative organisms, the following were tested: amikacin, ampicillin-sulbactam, cefazolin, cefepime, ceftazidime, ceftriaxone, ciprofloxacin, colistin, ertapenem, gentamicin, meropenem, piperacillin-tazobactam, and tobramycin. For any sample with >1 strain of the same species, a single composite SOC AST profile was created using the most resistant MIC result for each drug tested and was used as the reference for analysis.
The SOC results were used as the reference comparator for bacterial identification and AST. Results were interpreted according to the CLSI 2016 M100-S26 for all antimicrobials with the exception of colistin, which was interpreted according to EUCAST 2016 standards for the Enterobacteriaceae.
To determine the identification accuracy, the sensitivity and specificity were calculated by using the FISH ID probe for the AXDX and the results were compared to those of the SOC: sensitivity = (100 × TP)/(TP + FN); specificity = (100 × TN)/(TN + FP), where TP is the true positive, FN is the false negative, TN is the true negative, and FP is the false positive.
For AST accuracy, essential agreement (EA), categorical agreement (CA), very major error (VME), major error (ME), and minor error (MiE) rates were calculated as applicable using AXDX results compared to those of the SOC for each antimicrobial tested. EA is the percentage of the total test results within one doubling dilution of the reference result. CA is the percentage of the total test results with the same categorical interpretation result as the reference result. VME is the percentage of the resistant isolates by the SOC that tested susceptible by the AXDX. ME is the percentage of the susceptible isolates by the SOC that tested resistant by the AXDX. MiE is the percentage of the total test results in which one result (from the AXDX or the SOC) is intermediate and the other is not. For resistance phenotype tests, only categorical agreement and VME and ME rates were calculated.
(ii) Discrepancy analysis.
Discrepancy testing was performed for isolates that did not show agreement between the AXDX and the SOC. Samples were plated on tryptic soy agar plates containing 5% sheep's blood and incubated at 35°C for 18 to 24 h. For frozen blood aliquots, MacConkey agar and Columbia colistin-nalidixic acid (CNA) agar containing 5% sheep's blood were also used to differentiate possible mixed sample types. Observations were made after the initial 18 to 24 h of growth to ensure purity. For mixed cultures, each morphology was isolated on blood agar plates and incubated at 35°C for 18 to 24 h. Original plates and isolated morphologies were stored at 4°C, and fresh subcultures were made from these master plates for either identification or antimicrobial susceptibility testing needs.
Frozen blood aliquots previously prepared were used for discrepant ID testing. This ensured that organisms were captured and preserved in their present state at the time the blood aliquots were originally tested. Observations of colony morphologies and Gram stains were recorded for each sample. For organisms identified by the AXDX but not by the SOC, if the organism was not detected by Gram stain and/or culture, the isolate was considered a false positive. For organisms identified by the SOC but not by the AXDX, if the organism was detected by Gram stain or culture, the isolate was considered a false negative. The organisms present in the culture were identified using a third-party comparator automated Vitek 2 system for Gram-positive, Gram-negative, and yeast identification (ID cards no. GP 21342, GN 21341, and YST 21343) according to the procedures recommended for the Vitek 2 system. If the original AXDX result matched the third-party result, the result was resolved to be a true positive. If the third-party result matched the SOC result, the error was adjudicated toward the SOC. Vitek 2 was considered a third-party comparator, because Vitek 2 was not used to obtain the original SOC ID result for any of the samples that underwent ID discrepancy testing.
Any samples with a VME/ME or select MiE or EA errors (only for drugs with overall EA <90%) were included in AST discrepancy testing. For AST discrepancy testing, previously frozen isolates from pure culture were used. The growth characteristics and purity were recorded after 24 h of incubation. Cultures were passaged twice on medium before being tested in triplicates via the CLSI standardized broth microdilution method (BMD) (20), herein referred to as the “reference method.” The modal BMD result was used as the reference. If there was no mode, the BMD was repeated and the modal BMD from the resulting 6 tests was used as the reference. For resistance screening, the isolates were subcultured twice before Kirby-Bauer disk diffusion (cefoxitin) or triplicate BMD for inducible clindamycin resistance was performed. In cases where the reference method disagreed with both the AXDX and the SOC, the reference method was used as the true result. SOC testing was not repeated for discrepancy analysis.
(iii) Workflow.
A workflow analysis was performed by Lynnova Consulting. The workflows for the Accelerate Pheno system and for SOC identification, antimicrobial susceptibility testing, and quality control methods were analyzed and compared on the basis of the average time to process samples observed during a 30-h period.
RESULTS
Identification.
Of the 296 blood cultures, 64 were excluded from further analysis due to technical failures (n = 4), invalid (non-report of) ID results (n = 30), a time lapse of more than 8 h after positivity of the blood culture (n = 13), or the failure to meet study inclusion criteria (i.e., a duplicate patient sample or a sample that was not a blood culture) (n = 17). Final results were obtained for the remaining 232 (221 monomicrobial and 11 polymicrobial) blood cultures comprising 244 (214 on-panel and 30 off-panel) organisms. After adjudication, there were 223 monomicrobial and 9 polymicrobial blood cultures with 241 (212 on-panel and 29 off-panel) organisms detected; of the 212 on-panel organisms, 8 indeterminate results were excluded from the analysis. Of the remaining 204 on-panel results, 195 (95.6%) were identified correctly by the AXDX.
Table 1 shows the discrepant results in organism identification. There were a total of 28 cultures with 29 discrepancies in identification that required further evaluation: 17 initial false positives (FPs) and 12 initial false negatives (FNs) (Table 1). Six of these involved polymicrobial cultures for which the two systems disagreed on one of the organism identifications in the group. Four (14%) of the discrepancies were resolved to agree with results of the AXDX, leaving 16 FPs and 9 FNs in the postadjudicated data set.
TABLE 1.
Discrepant results in organism identification
| Organism | No. of false positives |
No. of false negatives |
||||||
|---|---|---|---|---|---|---|---|---|
| Total | Resolved to AXDX | Resolved to SOC | Unresolved | Total | Resolved to AXDX | Resolved to SOC | Unresolved | |
| Gram positives | ||||||||
| Coagulase-negative Staphylococcus spp. | 3 | 0 | 3 | 0 | 1 | 1b | 0 | 0 |
| E. faecalis | —a | — | — | — | 2 | 0 | 2c | 0 |
| E. faecium | — | — | — | — | 2 | 0 | 2 | 0 |
| S. aureus | 2 | 0 | 2 | 0 | 1 | 0 | 1 | 0 |
| S. lugdunensis | — | — | — | — | — | — | — | — |
| Streptococcus spp. | 6 | 1 | 4d | 1 | — | — | — | — |
| Gram negatives | ||||||||
| A. baumannii | — | — | — | — | — | — | — | — |
| Citrobacter spp. | — | — | — | — | — | — | — | — |
| Enterobacter spp. | 1 | 0 | 1 | 0 | 3 | 1e | 2 | 0 |
| E. coli | — | — | — | — | 1 | 0 | 1f | 0 |
| Klebsiella spp. | — | — | — | — | 2 | 1g | 1 | 0 |
| Proteus spp. | — | — | — | — | — | — | — | — |
| P. aeruginosa | — | — | — | — | — | — | — | — |
| S. marcescens | — | — | — | — | — | — | — | — |
| Yeast | ||||||||
| C. albicans | — | — | — | — | — | — | — | — |
| C. glabrata | 5 | 0 | 5 | 0 | — | — | — | — |
| Total | 17 | 1 | 15 | 1 | 12 | 3 | 9 | 0 |
—, no discrepant results occurred.
False-negative coagulase-negative Staphylococcus spp. occurred in a polymicrobial sample also containing Acinetobacter haemolyticus according to the SOC.
One false-negative E. faecalis occurred in a polymicrobial sample also containing E. coli according to the SOC.
False-positive Streptococcus spp. (S. salivarius) occurred in a polymicrobial sample also containing E. coli according to the SOC.
False-negative Enterobacter spp. (E. cloacae) occurred in a polymicrobial sample also containing Acinetobacter jejuni and A. baumannii according to the SOC.
False-negative E. coli occurred in a polymicrobial sample also containing K. pneumoniae according to the SOC.
False-negative Klebsiella spp. (K. pneumoniae) occurred in a polymicrobial sample also containing E. cloacae according to the SOC.
Notably, all 5 FP Streptococcus species had genus-level agreements with the reference method. Of the 16 remaining FP results, 5 involved Streptococcus species (S. constellatus, S. dysgalactiae, S. anginosus, S. pyogenes, and S. salivarius) that had genus-level agreement but are not claimed at the species level on the Accelerate PhenoTest BC kit; the remainder included coagulase-negative staphylococci (3), S. aureus (2), Enterobacter spp. (1), and C. glabrata (5). Eight of these were mitigated by comparing to the Gram stain result, which did not indicate the presence of the false-positive organism. All 5 C. glabrata isolates tested were determined to be FPs. Per specimen (n = 232), the overall FP rate was 6.9%; the FP rates for Gram-positive, Gram-negative, and yeast organisms were 4.3%, 0.4%, and 2.2%, respectively. However, there were 16 FISH ID probe results per specimen. Per FISH ID probe result (n = 3,712), the overall FP rate was 0.4%; the FP rates for Gram-positive, Gram-negative, and yeast organisms were 0.3%, 0.03%, and 0.1%, respectively.
The 9 remaining FN results included E. faecalis (2), E. faecium (2), S. aureus (1), Enterobacter spp. (2), E. coli (1), and Klebsiella spp. (1). Eight of the 9 FNs were mitigated; one of these was a polymicrobial by the SOC method that was mitigated by comparing to the Gram stain result, which indicated an additional organism was present, and five more would likely be mitigated through additional testing as they were negative for the monomicrobial call. As a result, an expert rule would print the following note on the lab report. “Recommend culture due to possibility of another organism being present.” In other words, the system is indicating an organism is present that was not identified by the on-panel ID probes. The two FN Enterobacter spp. tested positive on the retests with updated software v1.2, which includes improved algorithms for Enterobacter spp. detection not available in software v1.0. Per specimen (n = 232), the overall FN rate was 3.9%; the FN rates for Gram-positive and Gram-negative organisms were 2.2% and 1.7%, respectively. Per FISH ID probe result (n = 3,712), the overall FN rate was 0.2%; the FN rates for Gram-positive and Gram-negative organisms were both 0.1%. There were no FNs for yeast.
Table 2 denotes the ID performance characteristics of the Accelerate Pheno system for both on-panel and off-panel isolates following adjudication. The sensitivity and specificity for bacterial identification were 95.6% and 99.1% (Gram positives), 95.3% and 99.9% (Gram negatives), and 100% and 98.9% (yeast), respectively. The overall sensitivity and specificity were 95.6% and 99.5%, respectively. The total overall sensitivity and specificity calculations considering on-panel organisms only were 95.6% and 99.7%, respectively.
TABLE 2.
Performance characteristics of the Accelerate Pheno system for organism identification (after adjudication of discrepant results)
| Organism | Sensitivitya |
Specificityb |
||
|---|---|---|---|---|
| No. detected/no. tested | % | No. detected/no. tested | % | |
| Gram positives | ||||
| Coagulase-negative Staphylococcus spp. | 52/52 | 100 | 169/172 | 98.3 |
| Enterococcus faecalis | 15/17 | 88.2 | 215/215 | 100 |
| Enterococcus faecium | 3/5 | 60 | 227/227 | 100 |
| Staphylococcus aureus | 18/19 | 94.7 | 200/202 | 99 |
| Staphylococcus lugdunensis | 0/0 | NAc | 228/228 | 100 |
| Streptococcus spp. | 21/21 | 100 | 205/210 | 97.6 |
| Total | 109/114 | 95.6 | 1,244/1,254 | 99.1 |
| Gram negatives | ||||
| Acinetobacter baumannii | 3/3 | 100 | 229/229 | 100 |
| Citrobacter spp. | 2/2 | 100 | 230/230 | 100 |
| Enterobacter spp. | 11/13 | 84.6 | 215/216 | 99.5 |
| Escherichia coli | 30/31 | 96.8 | 201/201 | 100 |
| Klebsiella spp. | 20/21 | 95.2 | 211/211 | 100 |
| Proteus spp. | 3/3 | 100 | 229/229 | 100 |
| Pseudomonas aeruginosa | 9/9 | 100 | 223/223 | 100 |
| Serratia marcescens | 3/3 | 100 | 229/229 | 100 |
| Total | 81/85 | 95.3 | 1,767/1,768 | 99.9 |
| Yeast | ||||
| Candida albicans | 2/2 | 100 | 229/229 | 100 |
| Candida glabrata | 3/3 | 100 | 224/229 | 97.8 |
| Total | 5/5 | 100 | 453/458 | 98.9 |
| Overalld | 195/204 | 95.6 | 3,464/3,480 | 99.5 |
Eight indeterminate results were excluded from sensitivity calculations. Sensitivity calculations considering on-panel organisms only were identical.
Twenty indeterminate results were excluded from specificity calculations. The total specificity calculations considering on-panel organisms only were as follows: Gram positives, 1,090/1,095 (99.5%); Gram negatives, 1,552/1,552 (100%); yeast, 399/404 (98.8%); and overall, 3,041/3,051 (99.7%).
NA, not applicable.
Total overall genus-level agreement for all probes was 91.4%.
The Streptococcus genus probe is claimed to detect Streptococcus mitis, Streptococcus oralis, Streptococcus gallolyticus, Streptococcus agalactiae, and Streptococcus pneumoniae (not differentiated). However, the probe also detected other Streptococcus species that are not claimed for the Accelerate Pheno system. The agreement on a genus level was 100% for the streptococci. The other genus probes showed high performance except for Enterobacter; genus-level performance for this probe was 78.6%.
Antimicrobial susceptibility.
Antimicrobial susceptibility results were produced for 151 isolates with a total of 944 (236 Gram-positive and 708 Gram-negative) organism-antimicrobial test results. Thirty-seven (15.7%) of the Gram-positive and 75 (10.6%) of the Gram-negative results were resistant to one or more antimicrobials.
There were 33 samples with 33 discrepancies requiring discrepancy resolution for AST and/or resistance phenotype detection. There were initially 8 VMEs, 4 MEs, and 21 MiEs. Table 3 lists the summary of VME and ME discrepancy testing results, and Tables 4, 5, and 6 provide the AST performance results obtained by antibiotic and organism groups. Three of the VMEs involved Gram-positive organisms tested against erythromycin (2) and linezolid (1); four involved Gram-negative organisms tested against ampicillin-sulbactam (1), ceftazidime (1), ciprofloxacin (1), and ertapenem (1); one involved cefoxitin resistance testing for methicillin-resistant coagulase-negative Staphylococcus (MRS) spp. Four of the VMEs were resolved to agree with the AXDX results, and one was resolved to agree with the SOC result. The remaining 3 were not resolved with either system (i.e., they repeatedly tested as “intermediate” by the reference method), resulting in those VMEs being reclassified as MiEs Therefore, in the final analysis, there was one VME for cefoxitin and coagulase-negative Staphylococcus spp., resulting in an overall VME rate of 0.7%.
TABLE 3.
Summary of very major and major errors in antimicrobial susceptibility
| Antibiotic | No. of VMEsa |
No. of MEsb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Resolved to AXDX | Resolved to SOC | Resolved to neither | Unresolved | Total | Resolved to AXDX | Resolved to SOC | Resolved to neither | Unresolved | |
| Gram-positive organisms | ||||||||||
| Erythromycin | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Linezolid | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gram-negative organisms | ||||||||||
| Ampicillin-sulbactam | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefepime | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Ceftazidime | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 |
| Ciprofloxacin | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ertapenem | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 |
| Resistance phenotype tests | ||||||||||
| Cefoxitin (methicillin resistance) | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Total | 8 | 4 | 1 | 3 | 0 | 4 | 1 | 2 | 1 | 0 |
VME, very major error.
ME, major error.
TABLE 4.
Performance characteristics of the Accelerate Pheno system for Gram-positive AST performance by antibiotic and organism groupinga
| Antibiotic | EA |
CA |
No. of results |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. results/totalb | % | No. results/totalc | % | VME | ME | MiE | S | I | R | |
| Staphylococcus aureus | ||||||||||
| Erythromycin | 16/17 | 94.1 | 16/17 | 94.1 | 0 | 0 | 1 | 9 | 1 | 7 |
| Linezolid | 17/17 | 100 | 17/17 | 100 | 0 | 0 | 0 | 17 | 0 | 0 |
| Trimethoprim-sulfamethoxazoled | 17/17 | 100 | 17/17 | 100 | 0 | 0 | 0 | 16 | 0 | 0 |
| Vancomycin | 16/17 | 94.1 | 17/17 | 100 | 0 | 0 | 0 | 17 | 0 | 0 |
| Total | 66/68 | 97.1 | 67/68 | 98.5 | 0 | 0 | 1 | 59 | 1 | 7 |
| Coagulase-negative Staphylococcus spp. | ||||||||||
| Erythromycind | 44/45 | 97.8 | 44/45 | 97.8 | 0 | 0 | 1 | 18 | 0 | 27 |
| Linezolidd | 46/47 | 97.9 | 47/47 | 100 | 0 | 0 | 0 | 47 | 0 | 0 |
| Vancomycin | 44/46 | 95.7 | 46/46 | 100 | 0 | 0 | 0 | 46 | 0 | 0 |
| Total | 134/138 | 97.1 | 137/138 | 99.3 | 0 | 0 | 1 | 111 | 0 | 27 |
| Enterococcus spp. | ||||||||||
| Ampicillin | 8/8 | 100 | 8/8 | 100 | 0 | 0 | 0 | 7 | 0 | 1 |
| Linezolid | 8/8 | 100 | 8/8 | 100 | 0 | 0 | 0 | 8 | 0 | 0 |
| Vancomycin | 13/14 | 93 | 13/14 | 93 | 0 | 0 | 1 | 12 | 0 | 2 |
| Total | 29/30 | 96.7 | 29/30 | 96.7 | 0 | 0 | 1 | 27 | 0 | 3 |
| Overall | 229/236 | 97 | 233/236 | 98.7 | 0 | 0 | 3 | 197 | 1 | 37 |
Results based on CLSI 2016 breakpoints.
Values are the numbers of results within one doubling dilution of the reference results over the total numbers of test results.
Values are the numbers of results with same categorical interpretation as the reference results over the total numbers of test results.
Includes data from one or more organism/antimicrobial combinations that are research use only (RUO) and not for use in diagnostic procedures.
TABLE 5.
Performance characteristics of the Accelerate Pheno system for Gram-negative AST performance by antibiotic and organism groupinga
| Antibiotic | EA |
CA |
No. of results |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. results/totalb | % | No. results/totalc | % | VME | ME | MiE | S | I | R | |
| Enterobacteriaceae | ||||||||||
| Amikacin | 61/61 | 100 | 61/61 | 100 | 0 | 0 | 0 | 60 | 0 | 1 |
| Ampicillin-Sulbactam | 31/37 | 83.8 | 30/37 | 81.1 | 0 | 1e | 6 | 18 | 6 | 13 |
| Cefazolind | 42/47 | 89.4 | 40/47 | 85.1 | 0 | 0 | 7 | 0 | 40 | 7 |
| Cefepime | 59/63 | 93.7 | 59/63 | 93.7 | 0 | 0 | 4 | 58 | 2 | 3 |
| Ceftazidime | 52/62 | 83.9 | 55/62 | 88.7 | 0 | 1 | 6 | 54 | 0 | 8 |
| Ceftriaxone | 62/62 | 100 | 60/62 | 96.8 | 0 | 0 | 2 | 54 | 0 | 8 |
| Ciprofloxacin | 61/62 | 98.4 | 60/62 | 96.8 | 0 | 0 | 2 | 45 | 1 | 16 |
| Colistind | NAf | NAg | 1/1 | 100 | 0 | 0 | 0 | 1 | 0 | 0 |
| Ertapenem | 62/63 | 98.4 | 59/63 | 93.7 | 0 | 1 | 3 | 60 | 2 | 1 |
| Gentamicin | 60/63 | 95.2 | 63/63 | 100 | 0 | 0 | 0 | 56 | 1 | 6 |
| Meropenem | 60/60 | 100 | 60/60 | 100 | 0 | 0 | 0 | 59 | 0 | 1 |
| Tobramycin | 58/61 | 95.1 | 58/61 | 95.1 | 0 | 0 | 3 | 50 | 6 | 5 |
| Total | 608/641 | 94.9 | 606/642 | 94.4 | 0 | 3 | 33 | 515 | 58 | 69 |
| Pseudomonas aeruginosa | ||||||||||
| Amikacin | 8/8 | 100 | 8/8 | 100 | 0 | 0 | 0 | 7 | 0 | 1 |
| Cefepime | 8/8 | 100 | 7/8 | 87.5 | 0 | 0 | 1 | 7 | 0 | 1 |
| Ceftazidime | 4/8 | 50 | 4/8 | 50 | 0 | 0 | 4 | 7 | 1 | 0 |
| Ciprofloxacin | 7/7 | 100 | 7/7 | 100 | 0 | 0 | 0 | 6 | 0 | 1 |
| Colistind | NA | NAg | 1/1 | 100 | 0 | 0 | 0 | 1 | 0 | 0 |
| Gentamicin | 6/8 | 75 | 8/8 | 100 | 0 | 0 | 0 | 7 | 0 | 1 |
| Meropenem | 8/8 | 100 | 8/8 | 100 | 0 | 0 | 0 | 7 | 0 | 1 |
| Tobramycin | 7/7 | 100 | 7/7 | 100 | 0 | 0 | 0 | 6 | 0 | 1 |
| Total | 48/54 | 88.9 | 50/55 | 90.9 | 0 | 0 | 5 | 48 | 1 | 6 |
| Acinetobacter baumannii | ||||||||||
| Amikacin | NA | NA | 2/2 | 100 | 0 | 0 | 0 | 2 | 0 | 0 |
| Ampicillin-sulbactamd | NA | NA | 2/2 | 100 | 0 | 0 | 0 | 2 | 0 | 0 |
| Cefepimed | NA | NA | 2/2 | 100 | 0 | 0 | 0 | 2 | 0 | 0 |
| Ciprofloxacind | NA | NA | 2/2 | 100 | 0 | 0 | 0 | 2 | 0 | 0 |
| Meropenemd | NA | NA | 2/2 | 100 | 0 | 0 | 0 | 2 | 0 | 0 |
| Piperacillin-tazobactam | NA | NA | 1/1 | 100 | 0 | 0 | 0 | 1 | 0 | 0 |
| Total | NA | NA | 11/11 | 100 | 0 | 0 | 0 | 11 | 0 | 0 |
| Overall | 656/695 | 94.4 | 667/708 | 94.2 | 0 | 3 | 38 | 574 | 59 | 75 |
Results based on CLSI 2016 breakpoints.
Values are the numbers of results within one doubling dilution of the reference results over the total numbers of test results.
Values are the numbers of results with same categorical interpretation as the reference results over the total numbers of test results.
Includes data from one or more organism/antimicrobial combinations that are research use only (RUO) and not for use in diagnostic procedures.
For this ME, Accelerate had an MIC of 32 μg/ml (R), Vitek 2 had an MIC of 16 μg/ml (I), and broth microdilution had an MIC of 8 μg/ml (S), such that all results were around the breakpoints.
NA, not applicable.
RUO colistin tested by disk diffusion.
TABLE 6.
Performance characteristics of the Accelerate Pheno system for resistance phenotype test performance
| Combination | Count | CA |
No. of results |
||||
|---|---|---|---|---|---|---|---|
| No. results/totala | % | VME | ME | S | R | ||
| Cefoxitin and S. aureus | 17 | 17/17 | 100 | 0 | 0 | 10 | 7 |
| Cefoxitin and CNSb | 40 | 39/40 | 97.5 | 1 | 0 | 16 | 24 |
Values are the numbers of results with same categorical interpretation as the reference results over the total numbers of test results.
CNS, coagulase-negative Staphylococcus spp.
The 4 MEs involved Gram-negative organisms tested against cefepime (1), ceftazidime (1), and ertapenem (1); one involved cefoxitin resistance testing for methicillin-resistant coagulase-negative Staphylococcus (MRS) spp. One ME was resolved to agree with the AXDX result, 2 resolved with the SOC results, and 1 was resolved with neither, yielding that ME to be reclassified as an MiE according to the reference AST method.
There were initially 43 minor errors, 40 involving Gram-negative and 3 involving Gram-positive organisms. Thirty-six (83.7% of MiEs) specifically involved the Enterobacteriaceae. Twenty-one fulfilled criteria for further discrepancy resolution. Five were resolved in favor of the AXDX and 10 in favor of the SOC. The remaining 6 MiEs either remained unresolved (n= 5) due to the lack of a BMD mode or nonviable organisms or were resolved to neither AXDX nor SOC results (n = 1), i.e., if the AXDX result was intermediate, the SOC result was susceptible and the discrepant test result was resistant.
One ampicillin-sulbactam minor error was reclassified as an ME according to the reference AST method. Therefore, in the final analysis, there were 3 MEs and 41 MiEs, resulting in an overall ME rate of 0.4% (Tables 4 to 6).
EA was obtained in 95.1% and CA in 95.5% of results. For the Gram-positive organisms, EA and CA were 97% and 98.7%, respectively (Table 4). For the Gram-negative organisms, EA and CA were 94.4% and 94.2%, respectively (Table 5).
The EAs and CAs ranged from 93% to 100% for the Gram-positive organisms; the results obtained for all antimicrobials were >90% (Table 4). For the Enterobacteriaceae, the EAs ranged from 83.8% (ampicillin-sulbactam) to 100% and CAs ranged from 81.1% (ampicillin-sulbactam) to 100% (Table 5). For P. aeruginosa (n = 8 isolates), the EAs and CAs ranged from 50% to 100%; the EA and CA were particularly low for ceftazidime (50%) and EA was low for gentamicin (75%) (Table 5). For the two A. baumannii isolates, the CAs were 100% for all the drugs tested (EA was not applicable, as testing was performed by disk diffusion) (Table 5).
Workflow.
Workflow results are presented in Table 7. When using the Accelerate Pheno system for positive blood cultures, the times to identification and susceptibility were reduced by 23.47 h and 41.86 h, respectively; the hands-on time per positive culture was reduced by 25.5 min.
TABLE 7.
Workflow summary of SOC versus AXDXa
| Category | SOC Workflow | AXDX Workflow | Difference (SOC − AXDX) |
|---|---|---|---|
| No. of intervention points for: | |||
| Gram-positive organisms | 12 | 5 | 7 |
| Gram-negative organisms | 12 | 5 | 7 |
| Yeast | 6 | 4 | 2 |
| Technician hands-on time for ID and AST (min) | 35.5 | <10 | 25.5 |
| Time to result for patient specimen from positive blood culture (min [h]) | |||
| ID | 1,494 (24.9) | 85.5 (1.43) | 1,408.5 (23.47) |
| AST | 2,937.5 (48.95) | 425.5 (7.09) | 2,512 (41.86) |
| Technician needed | Yes | For prep only | Time savings |
| Process | Manual | Automated | Technician autonomy |
| Wait time | Exceeds 24 h and covers shift changes | Within 1 shift | Wait time is minimized |
SOC, standard of care; AXDX, Accelerate Pheno system.
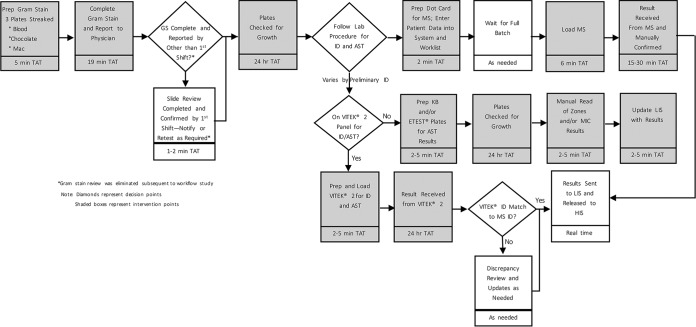
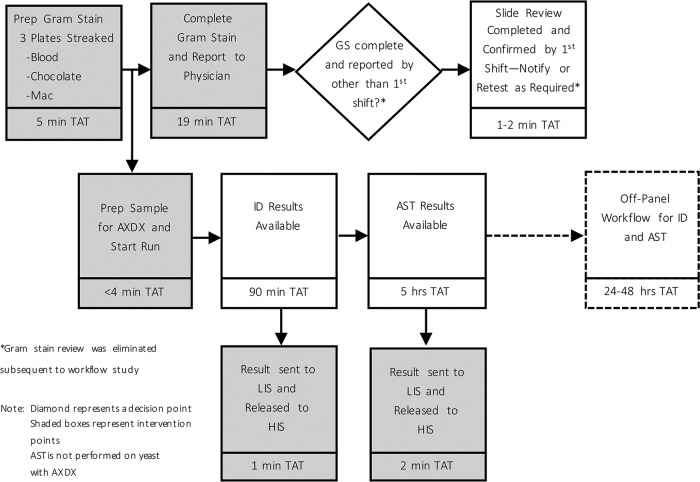
Overviews of the workflows associated with the SOC and the AXDX for Gram-positive organisms are shown in Fig. 1 and 2.
FIG 1.
Overview of workflow for the standard of care for Gram-positive blood cultures.
FIG 2.
Overview of workflow with the Accelerate Pheno system (AXDX) for positive blood cultures.
DISCUSSION
We evaluated the performance of the Accelerate Pheno system for the identification and antimicrobial susceptibility of organisms directly from positive blood culture bottles. To our knowledge, this is the first study to evaluate the workflow and real-time performance characteristics of the system for both Gram-positive and Gram-negative organisms from fresh blood cultures in a routine diagnostic setting and for a broad patient population.
The Accelerate Pheno system performed well for the identification of bloodstream pathogens, correctly identifying 95.6% of organisms. The performance was good for Gram-positive and Gram-negative organisms, with an overall sensitivity and specificity of 95.6% and 99.5%, respectively. There were 16 false-positive and 9 false-negative results, some of which were due to the presence of off-panel organisms. Moreover, this study was performed using software v1.0; updated software is now available (v1.2.1) and incorporates interpretive rules that may limit FP (specifically for C. glabrata, coagulase-negative staphylococci, and S. aureus) and FN (specifically for Enterobacter spp. and P. aeruginosa) results in addition to decreasing invalid rates.
The system also performed well for antimicrobial susceptibility, achieving an overall essential agreement of 95.1% and categorical agreement of 95.5% compared to routine methods. There was one VME, 3 MEs, and 41 MiEs.
The study was limited by the organisms isolated during the study period at a single institution. For some organisms, very few isolates were tested (e.g., only 2 A. baumannii isolates); more would be needed for an optimal determination of system performance. Moreover, there were only 2 vancomycin-resistant enterococci (VRE) and 1 carbapenem-resistant Enterobacteriaceae tested. Additional resistant organisms would be useful to further challenge the system's performance, particularly for specific antimicrobials that might only be tested on resistant organisms (i.e., RUO colistin in our institution). For polymicrobial cultures, AST results were reported only for the dominant on-panel organism and were not reported for samples where 2 or more on-panel, AST-eligible organisms were identified.
Furthermore, identification was achieved at the genus level only for some organisms, such as Streptococcus species; it would be preferred to achieve a species-level ID for such isolates, as identification to the species level can be critical and would still have to be performed using alternative methodologies. Additionally, in some cases, the AST results are limited and reporting is not always feasible when one does not have the full species identification, as MICs and interpretations may vary depending on the species identified. This was not a significant issue for the Enterobacteriaceae identified only to the genus level, as the CLSI M100-27 groups these together. For example, K. pneumoniae has the same reporting requirements as K. oxytoca; therefore, identifying the isolate as a Klebsiella spp. would not impact AST reporting. However, there may be challenges, even within this group. For example, P. mirabilis and P. vulgaris have different intrinsic resistance profiles (CLSI M100-S27, appendix B). Although the AXDX provides AST results for Proteus spp., a P. vulgaris isolate might test as susceptible to the penicillins or cephalosporins, though it should still be reported as resistant, as per its intrinsic resistance profile (CLSI M100-S27, appendix B1). This is not true for P. mirabilis. In cases like this, the laboratory may have to perform additional identification and susceptibility testing.
The greater challenge lies with the Gram-positive organisms, particularly, the Streptococcus spp., for which the antimicrobials tested and interpretations reported can vary greatly depending on the species present. In the present study, a comparison of MIC results could not be performed for the Streptococcus species isolates, as they are identified to the genus level only by the AXDX and antimicrobial testing and reporting can only follow species identification for this group.
Moreover, additional AST testing may have to be performed in a number of cases, as the antimicrobials assessed in this study were limited, particularly for the Gram-positive organisms, for which only ampicillin, erythromycin, linezolid, trimethoprim-sulfamethoxazole, vancomycin, and cefoxitin were compared between the AXDX and the SOC. While daptomycin, ceftaroline, and doxycycline were tested using the AXDX, SOC data were not available for comparative analysis. A larger selection of antimicrobials from different classes was assessed for the Gram-negative organisms.
Furthermore, an identification to the species level may be important for epidemiologic and infection control purposes, even if not critical for reporting AST results.
Finally, certain organism-antimicrobial combinations did not perform well, such as ceftazidime for P. aeruginosa; further studies are needed to determine the acceptability of using this system for testing this particular combination. A limitation of the system is that it can process only one sample at a time per module, although it can currently accommodate up to 4 modules. In addition, samples have to be processed within 8 h, and the system currently does not include ID and AST for Gram-positive rods or AST for yeast.
An adoption of lean principles in the microbiology laboratory is highly desirable to provide better operational success, to standardize processes, to reduce costs, and ultimately, to provide better patient care (21, 22). The Accelerate Pheno system is a streamlined “sample-to-result” platform that has laboratory information system (LIS) connectivity. In our study, the average hands-on time per specimen was reduced by 25.5 min from routine testing, similar to the 30-min reduction described by Romano et al. (23). The time to ID based on a single specimen was observed to be 85.5 min from the time of positive blood culture (cartridge set up, 3.5 min; run on the platform, 82 min). In the United States, FDA marketing authorization requires the results to be interpreted in conjunction with Gram stain results. The time to ID is similar to that for multiplex PCR methods (13) and compared to the SOC was reduced by 23.47 h. The Accelerate Pheno system required 7.09 h to deliver susceptibility results and, compared to the 48.95 h required for the SOC results, significantly reduces the time for AST by 41.86 h. The times to ID/AST were similar to the times noted by other authors using the Accelerate Pheno system (24, 25). The integration of the Accelerate Pheno system with LIS for automated resulting, with the implementation of an automated LIS alert (either by text paging or email), will deliver actionable results to a stewardship team or provider. This test will increase the availability of results around the clock; this might require changes in practice by stewardship teams or covering physicians so that targeted antimicrobial coverage is implemented when laboratory results are ready. The capacity on the current Accelerate Pheno system is restricted to one sample per instrument module at a time. An institution might need multiple modules, depending on the volume of blood cultures with detectable growth. The system has a small footprint and several modules could be easily placed on the benchtop, allowing easy installation even in small laboratories. On the basis of our laboratory's procedures and policies for working up unique positive blood cultures, we typically see between 3 and 4 samples per day that require full identification and susceptibility testing. These would be eligible for testing with the AXDX and would require a minimum of 2 modules.
On the basis of our workflow analysis, there are 2 to 7 fewer technician intervention points in the workflow depending on which organisms are encountered in the positive blood culture (Gram-positive versus Gram-negative versus yeast) if the Accelerate Pheno system is used. The manual steps required were significantly reduced with the system, allowing time savings, greater technologist autonomy, and decreased wait times.
As with many new laboratory instruments, any return-on-investment calculations should take factors outside the laboratory into account to the extent possible; these factors include the antimicrobial costs, the length of stay, and the impacts on patient care and outcomes.
If susceptibility result reporting is coupled with antimicrobial stewardship intervention, the impact on patient care can be greater than that reported in earlier studies, which used limited AST results based on genotypic determinants (3, 4, 9, 14, 15) rather than a fuller panel of phenotypic organism-antimicrobial susceptibility results that are available 41.86 h sooner. Additional studies are needed to show the impacts on costs, reducing the risk of adverse drug effects, reducing the pressure for resistance development, optimizing and de-escalating therapies, possibly reducing LOS, and improving patient outcomes.
In conclusion, the Accelerate Pheno system provides fast and accurate results for most of the organisms found routinely in blood cultures. A failure to identify species was due in most cases to invalids or the presence of an off-panel organism not found on the Accelerate PhenoTest BC kit panel. The Accelerate Pheno system is easy to use, reduces hands-on time for ID/AST of common blood pathogens, and allows clinically actionable results to be released much earlier than the current standard of care. For antimicrobial susceptibility, the Accelerate Pheno system performed comparably to our current AST methodologies for the majority of organism-antimicrobial combinations. However, additional testing is needed for some organisms (i.e., Acinetobacter baumannii and carbapenemase-producing Gram-negative organisms), as well as for some of the organism-antimicrobial combinations that were either not well represented or that did not perform as expected.
ACKNOWLEDGMENTS
This study was funded by Accelerate Diagnostics, Inc.
We have no conflicts of interest to declare.
REFERENCES
- 1.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, et al. 2017. Surviving Sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 2.Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, Hsueh PR, Viale P, Pano-Pardo JR, Venditti M, Tumbarello M, Daikos G, Canton R, Doi Y, Tuon FF, Karaiskos I, Perez-Nadales E, Schwaber MJ, Azap OK, Souli M, Roilides E, Pournaras S, Akova M, Perez F, Bermejo J, Oliver A, Almela M, Lowman W, Almirante B, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodriguez-Bano J. 2017. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis 17:726–734. doi: 10.1016/S1473-3099(17)30228-1. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee R, Teng CB, Cunningham SA, Ihde SM, Steckelberg JM, Moriarty JP, Shah ND, Mandrekar JN, Patel R. 2015. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis 61:1071–1080. doi: 10.1093/cid/civ447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buehler SS, Madison B, Snyder SR, Derzon JH, Cornish NE, Saubolle MA, Weissfeld AS, Weinstein MP, Liebow EB, Wolk DM. 2016. Effectiveness of practices to increase timeliness of providing targeted therapy for inpatients with bloodstream infections: a laboratory medicine best practices systematic review and meta-analysis. Clin Microbiol Rev 29:59–103. doi: 10.1128/CMR.00053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Cheang M. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 6.Apisarnthanarak A, Holzmann-Pazgal G, Hamvas A, Olsen MA, Fraser VJ. 2004. Antimicrobial use and the influence of inadequate empiric antimicrobial therapy on the outcomes of nosocomial bloodstream infections in a neonatal intensive care unit. Infect Control Hosp Epidemiol 25:735–741. doi: 10.1086/502469. [DOI] [PubMed] [Google Scholar]
- 7.Herzke CA, Chen LF, Anderson DJ, Choi Y, Sexton DJ, Kaye KS. 2009. Empirical antimicrobial therapy for bloodstream infection due to methicillin-resistant Staphylococcus aureus: no better than a coin toss. Infect Control Hosp Epidemiol 30:1057–1061. doi: 10.1086/606163. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146–155. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 9.Perez KK, Olsen RJ, Musick WL, Cernoch PL, Davis JR, Land GA, Peterson LE, Musser JM. 2013. Integrating rapid pathogen identification and antimicrobial stewardship significantly decreases hospital costs. Arch Pathol Lab Med 137:1247–1254. doi: 10.5858/arpa.2012-0651-OA. [DOI] [PubMed] [Google Scholar]
- 10.Kaye KS, Marchaim D, Chen TY, Baures T, Anderson DJ, Choi Y, Sloane R, Schmader KE. 2014. Effect of nosocomial bloodstream infections on mortality, length of stay, and hospital costs in older adults. J Am Geriatr Soc 62:306–311. doi: 10.1111/jgs.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatti MM, Boonlayangoor S, Beavis KG, Tesic V. 2014. Rapid identification of positive blood cultures by matrix-assisted laser desorption ionization-time of flight mass spectrometry using prewarmed agar plates. J Clin Microbiol 52:4334–4338. doi: 10.1128/JCM.01788-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaleta EJ, Clark AE, Johnson DR, Gamage DC, Wysocki VH, Cherkaoui A, Schrenzel J, Wolk DM. 2011. Use of PCR coupled with electrospray ionization mass spectrometry for rapid identification of bacterial and yeast bloodstream pathogens from blood culture bottles. J Clin Microbiol 49:345–353. doi: 10.1128/JCM.00936-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatti MM, Boonlayangoor S, Beavis KG, Tesic V. 2014. Evaluation of FilmArray and Verigene systems for rapid identification of positive blood cultures. J Clin Microbiol 52:3433–3436. doi: 10.1128/JCM.01417-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosgrove SE, Li DX, Tamma PD, Avdic E, Hadhazy E, Wakefield T, Gherna M, Carroll KC. 2016. Use of PNA FISH for blood cultures growing Gram-positive cocci in chains without a concomitant antibiotic stewardship intervention does not improve time to appropriate antibiotic therapy. Diagn Microbiol Infect Dis 86:86–92. doi: 10.1016/j.diagmicrobio.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Huang AM, Newton D, Kunapuli A, Gandhi TN, Washer LL, Isip J, Collins CD, Nagel JL. 2013. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis 57:1237–1245. doi: 10.1093/cid/cit498. [DOI] [PubMed] [Google Scholar]
- 16.Donner LM, Campbell WS, Lyden E, Van Schooneveld TC. 2017. Assessment of rapid-blood-culture-identification result interpretation and antibiotic prescribing practices. J Clin Microbiol 55:1496–1507. doi: 10.1128/JCM.02395-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Opota O, Jaton K, Greub G. 2015. Microbial diagnosis of bloodstream infection: towards molecular diagnosis directly from blood. Clin Microbiol Infect 21:323–331. doi: 10.1016/j.cmi.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Chantell C. 2015. Multiplexed automated digital microscopy for rapid identification and antimicrobial susceptibility testing of bacteria and yeast from cultured samples. Clin Microbiol Newsl 37:161–167. doi: 10.1016/j.clinmicnews.2015.10.001. [DOI] [Google Scholar]
- 19.Accelerate Diagnostics, Inc. 2017. Accelerate PhenoTest BC kit instructions for use. Diagnostics, Inc; Tucson, AZ. [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 10th ed CLSI document M07-A10 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 21.Mitchell PS, Mandrekar JN, Yao JD. 2014. Adoption of lean principles in a high-volume molecular diagnostic microbiology laboratory. J Clin Microbiol 52:2689–2693. doi: 10.1128/JCM.00430-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samuel L, Novak-Weekley S. 2014. The role of the clinical laboratory in the future of health care: lean microbiology. J Clin Microbiol 52:1812–1817. doi: 10.1128/JCM.00099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romano F, De Angelis G, Menchinelli G, Fiori B, Liotti FM, Spanu T, Posteraro B, Cicchetti A, Sanguinetti M. 2017. Organizational impact of a rapid automated microbiological diagnostic testing: a preliminary study, abstr P0998. Abstr 27th ECCMID, Vienna, Austria. [Google Scholar]
- 24.Marschal M, Bachmaier J, Autenrieth I, Oberhettinger P, Willmann M, Peter S. 2017. Evaluation of the Accelerate Pheno system for fast identification and antimicrobial susceptibility testing from positive blood culture in Gram-negative bloodstream infection. J Clin Microbiol 55:2116–2126. doi: 10.1128/JCM.00181-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brazelton de Cárdena JN, Su Y, Rodriguez A, Hewitt C, Tang L, Garner CD, Hayden RT. 2017. Evaluation of rapid phenotypic identification and antimicrobial susceptibility testing in a pediatric oncology center. Diagn Microbiol Infect Dis 89:52–57. doi: 10.1016/j.diagmicrobio.2017.06.014. [DOI] [PubMed] [Google Scholar]