ABSTRACT
Tularemia is a zoonosis caused by the bacterium Francisella tularensis. Its specific diagnosis remains based on serological methods, while F. tularensis is rarely detected in clinical samples by culture or PCR. The aim of the present study was to evaluate the performance of the Serion enzyme-linked immunosorbent assay (ELISA) classic Francisella tularensis IgG and IgM tests (Virion/Serion GmbH Institute, Würzburg, Germany) and the VIRapid tularemia immunochromatographic test (ICT) (Vircell, Granada, Spain) compared to that of the in-house microagglutination test (MAT) and indirect immunofluorescence assay (IFA) currently used at the French National Reference Center for Francisella. We evaluated 256 consecutive sera from 208 patients, including 51 confirmed and 23 probable tularemia cases, and 134 control patients not infected with F. tularensis. The IFA tests displayed 72.5% sensitivity for IgM (cutoff titer ≥80) and 74.5% for IgG (cutoff titer ≥160), and 99.3% specificity for both IgM and IgG. Using cutoffs advocated by the manufacturer, the Serion ELISAs displayed 88.2% sensitivity for IgM and 86.3% for IgG antibodies; specificity was 94.8% for IgM and 95.5% for IgG. Compared to MAT and IFA tests, the Serion ELISAs allowed earlier detection of specific antibodies (1 to 2 weeks versus 2 to 3 weeks after the onset of symptoms). The ICT sensitivity and specificity were 90% and 83.6%, respectively, when considering the cutoff advocated by the manufacturer. In conclusion, the Serion ELISAs are useful as screening tests for tularemia diagnosis, but additional confirmatory tests (such as MAT and IFA) are needed, especially in areas of low endemicity.
KEYWORDS: zoonosis, Francisella tularensis, tularemia, serology, immunofluorescence assay, ELISA, immunochromatography
INTRODUCTION
Tularemia is a zoonosis caused by Francisella tularensis, a Gram-negative intracellular bacterium, categorized as a class A bioterrorism agent by the CDC (1, 2). Classically, two subspecies of F. tularensis cause human infections: subsp. tularensis (type A, in North America) and subsp. holarctica (type B, in the whole Northern hemisphere) (3). However, the latter subspecies has recently been detected in Australia as well (4, 5). Type B strains are usually associated with less severe symptoms and lower mortality rates compared to type A strains (3). F. tularensis can infect or colonize a wide range of animal species (especially small rodents and lagomorphs) and arthropods (especially ticks and mosquitoes). It may also survive for prolonged periods in the environment. Humans are contaminated with F. tularensis directly from infected animals (through animal bites or scratches, handling, ingestion of contaminated meat, etc.), through arthropod bites (mainly Ixodidae ticks and mosquitoes in restricted areas), or from F. tularensis-contaminated environments (e.g., contact with contaminated soils or vegetables, contact with or ingestion of contaminated water).
After a short incubation period (usually 3 to 5 days), tularemia manifests as flu-like symptoms. Then, depending on the bacteria's portal of entry, six clinical forms are classically recognized (3, 6). The ulceroglandular form corresponds to a cutaneous lesion at the site of F. tularensis inoculation, with subsequent development of regional lymphadenopathy; the glandular form also corresponds to regional lymphadenopathy, but the skin inoculation lesion is not detected; the oropharyngeal form corresponds to pharyngitis with cervical lymphadenopathy after infection via the oral route; the oculoglandular form is conjunctivitis with a periauricular or cervical lymphadenopathy after conjunctival inoculation of F. tularensis; the pneumonic form results from inhalation of a contaminated aerosol or the hematogenous spread of bacteria from other infectious loci; and the typhoidal form is a severe systemic infection usually with high fever and confusion but with no inoculation lesion or regional lymphadenopathy. Complications may occur, including lymph node suppuration, severe skin and soft tissue infections, keratitis, meningitis, encephalitis, pericarditis, endocarditis, peritonitis, hepatitis, splenitis, osteoarticular infections, septic shock with rhabdomyolysis, and acute renal failure.
Confirmation of tularemia diagnosis can be obtained by direct detection of F. tularensis in various clinical samples, either by culture or PCR assays, or by serological techniques showing the presence of specific antibodies in patients' sera (6, 7). Blood cultures are useful in patients with F. tularensis bacteremia (8). However, isolation of F. tularensis is obtained in less than 10% of patients, because clinical samples for culture are either not available or collected after an effective antibiotic therapy has been administered and because of the fastidious nature of this bacterium. Also, type A strains of F. tularensis are biosafety level 3 pathogens, and their isolation remains hazardous for laboratory personnel (9). PCR-based assays are useful for early confirmation of tularemia, by detecting F. tularensis DNA from skin ulcers or from conjunctival or pharyngeal exudates (6, 7). These assays may also confirm the diagnosis retrospectively by testing resected tissues, such as suppurated lymph nodes. Due to the limitations of F. tularensis culturing and PCR testing, diagnosis of tularemia remains most frequently based on serological tests (6, 7). However, specific antibody titers are usually detected at significant levels only 2 to 3 weeks following symptom onset (10). Also, residual antibody titers may persist for years, leading to false-positive results (11). Cross-reacting antibodies have been reported mainly between Francisella, Brucella, and Yersinia enterocolitica species (12–14) and more recently, mimivirus capsid antigens (15), but titers are usually low and not confounding. A major limitation of serological diagnosis of tularemia worldwide is the lack of standardization for F. tularensis antigen preparation and for the serological methods used.
The aim of the present study was to evaluate commercially available serological tests for tularemia diagnosis, including the Serion enzyme-linked immunosorbent assay (ELISA) classic Francisella tularensis IgG and IgM tests (Virion/Serion GmbH Institute, Würzburg, Germany) and the VIRapid tularemia immunochromatographic test (ICT) (Vircell, Granada, Spain), and compare their performance to those of the in-house microagglutination test (MAT) and indirect immunofluorescence assay (IFA) we currently used at the French National Reference Centre for Francisella.
MATERIALS AND METHODS
Patients and clinical samples.
The present retrospective study was conducted in accordance with the Standards for Reporting of Diagnostic Accuracy (STARD) guidelines to assess the diagnostic accuracy and the clinical value of the respective assays (16, 17). As a National Reference Center for Francisella, we receive various clinical samples for tularemia diagnostic assessment, using specific culture, PCR, and serological tests (18). In this study, we evaluated 256 consecutive serum samples that were collected between 2006 and 2015 from 208 French patients. These patients included 124 men and 84 women (sex ratio, 1.47), with a mean age of 45 years (range, 5 to 95 years).
Tularemia case definition.
Tularemia cases were defined according to the WHO recommendations (19). In a patient with clinical and epidemiological findings compatible with tularemia, a confirmed case corresponded to (i) a positive F. tularensis culture; (ii) a seroconversion or a 4-fold or higher rise in specific antibody titers, as determined by the microagglutination test (MAT) and/or the indirect immunofluorescence assay (IFA), between two sera collected at least 2 weeks apart; or (iii) a positive F. tularensis PCR test. A probable case corresponded to a single positive serological titer (using MAT and/or IFA) in a patient with clinical and epidemiological findings compatible with tularemia. Patients with nonspecific clinical symptoms (usually a fever without any other symptom), absence of risk exposure for tularemia, negative tularemia diagnostic tests or a single positive MAT and/or IFA test, and resolution of symptoms without the need for administration of an appropriate antibiotic therapy were considered not infected with this pathogen, and served as non-tularemia controls.
Serological methods.
We used in-house MAT and IFA methods previously elaborated in our laboratory for detection of anti-F. tularensis antibodies in patients' sera (18). One MAT detects both specific IgM and IgG antibodies, whereas two separate IFA tests are needed, one to detect IgM (IFA-IgM) and one for IgG (IFA-IgG) antibodies. Both techniques use the F. tularensis subsp. holarctica LVS strain (NCTC 10857) as the antigen (18). Briefly, the LVS strain was grown on Polyvitex-supplemented chocolate agar plates (bioMérieux, Marcy l'Etoile, France), incubated 3 days at 37°C, in 5% CO2-enriched atmosphere, in a biosafety level 3 laboratory. Bacterial growth was harvested and homogenized in phosphate buffer saline (PBS) (Sigma-Aldrich, St. Quentin Fallavier, France), and bacteria were inactivated by adding 4% formaldehyde for 48 h at 4°C. Formaldehyde was removed by centrifugation at 16,800 relative centrifugal force (RCF) for 10 min and resuspension of the bacterial pellet in PBS, three times. The final bacterial pellet was resuspended in PBS at 1 McFarland optical density, and stored at −80°C until use. The cutoff titers are ≥80 for the MAT and IFA-IgM, and ≥160 for the IFA-IgG (18).
The Serion ELISA classic Francisella tularensis IgG and IgM tests (Virion/Serion GmbH Institute, Würzburg, Germany) are two commercial kits allowing the specific detection of either IgM or IgG antibodies predominantly directed at the bacterial lipopolysaccharide. They are referred to here as the Serion ELISA-IgM and ELISA-IgG tests. They were performed according to the manufacturer's recommendations. However, the optical densities were read using the BEP III apparatus (Siemens Healthcare, Saint-Denis, France). Briefly, serum samples were diluted in flat-bottom microtiter plates. For the IgG ELISA plate, the samples were diluted at 1:100 in two steps (1:20 then 1:5) in the buffer (DILB) provided. For the IgM ELISA plate, the samples were diluted 1:100 in rheumatoid factor (RF)-absorbent and incubated at ambient temperature for 15 min to remove any rheumatoid factor, which may give false-positive reactions. The positive controls provided for IgM and IgG were tested at each run. The manufacturer advocates using cutoff titers for optical densities (OD) ≥0.449 for IgM and ≥0.619 for IgG, which correspond to an expected 99% sensitivity for both antibody types, and >99% specificity for IgM and 96.9% for IgG.
The VIRapid tularemia test (Vircell, Granada, Spain) is an immunochromatographic (ICT) test detecting both IgM and IgG type anti-F. tularensis antibodies. This test was performed using 20 μl of pure serum sample per cassette, according to the manufacturer's recommendations. The results are determined visually and semiquantitative: 0 for negative, 0.5 for weakly positive, and 1 to 3 for positive, strong positive, and very strong positive, respectively. A result of 0.5 or higher is considered positive by the manufacturer.
The sensitivity, specificity, ROC analyses, and Cohen κ concordance between tests were performed using the R software (version 3.3.1).
RESULTS
Among the study's 208 patients, 51 were confirmed tularemia cases (37 men, 14 women; sex ratio, 2.64) based on isolation of an F. tularensis strain (26 cases), seroconversion or a 4-fold (or higher) rise in antibody titers (18 cases), or a positive F. tularensis PCR test (7 cases). Twenty-three patients were classified as probable tularemia cases. Tularemia patients corresponded to the following clinical forms: ulceroglandular (27 cases), glandular (8 cases), oculoglandular (2 cases), oropharyngeal (12 cases), pneumonic (7 cases), typhoidal (16 cases), and undetermined (2 cases). The remaining 134 patients were considered not infected by F. tularensis (Table 1).
TABLE 1.
Patients and serum samples evaluated in this study
| Patient group | No. of patients | No. of serum samples | No. of males/no. of females | Sex ratio | Median age (range [yrs]) | No. of positive serum samples/no. tested for MAT and/or IFA (%) |
|---|---|---|---|---|---|---|
| Positive F. tularensis culture | 26 | 37 | 21/5 | 4.2 | 64 (8–95) | 17/26 (65.4) |
| Seroconversion or ≥4-fold increase in antibody titers | 18 | 41 | 11/7 | 1.57 | 51 (25–76) | 18/18 (100) |
| Positive F. tularensis PCR test | 7 | 15 | 5/2 | 2 | 46 (17–87) | 7/7 (100) |
| Probable tularemia cases | 23 | 29 | 13/10 | 1.3 | 50 (17–84) | 23/23 (100) |
| Negative controls | 134 | 134 | 74/60 | 1.23 | 40 (5–82) | 5/134 (3.7) |
| Total | 208 | 256 | 124/84 | 1.47 | 45 (5–95) | 69/208 (33.2) |
The MAT displayed 75.3% sensitivity and 98.5% specificity. However, the sensitivity varied in the different groups of patients: 65.4% in patients with a positive F. tularensis culture, 72.2% for those with a seroconversion or a 4-fold (or higher) rise in antibody titers, 100% in the 7 patients with a positive F. tularensis PCR, and 83.3% in probable cases.
As for the IFAs, using previously defined cutoff titers (≥80 for IgM and ≥160 for IgG), we found a sensitivity of 72.5% for IgM and 74.5% for IgG, while the specificity was 99.3% in both cases (Table 2). Halving the cutoff titers resulted in higher sensitivity values (78.4% and 88.2% for IgM and IgG, respectively) but a lower specificity (98.5% and 97.0%, respectively). Doubling the cutoff titers resulted in a very low sensitivity of 56.8% for both IgM and IgG.
TABLE 2.
Sensitivities, specificities, and positive and negative likelihood ratios of serological methods investigated according to cutoff titers
| Method | Cutoff titer | Sensitivity (%) | Specificity (%) | LR+/LR− |
|---|---|---|---|---|
| IFA-IgM | 1:40 | 78.4 | 98.5 | 52.3/0.22 |
| 1:80 | 72.5 | 99.3 | 103.6/0.28 | |
| 1:160 | 56.8 | 99.9 | 568/0.43 | |
| IFA-IgG | 1:80 | 88.2 | 97.0 | 29.4/0.12 |
| 1:160 | 74.5 | 99.3 | 106.4/0.26 | |
| 1:320 | 56.8 | 99.3 | 81.1/0.44 | |
| ELISA-IgM | 0.449 | 88.2 | 94.8 | 17/0.12 |
| 0.9 | 86.3 | 97.8 | 39.2/0.14 | |
| 1.35 | 84.3 | 98.5 | 56.2/0.16 | |
| ELISA-IgG | 0.619 | 86.3 | 95.5 | 19.6/0.12 |
| 1.4 | 84.3 | 97.8 | 38.3/0.16 | |
| 1.8 | 70.6 | 98.5 | 47.1/0.30 |
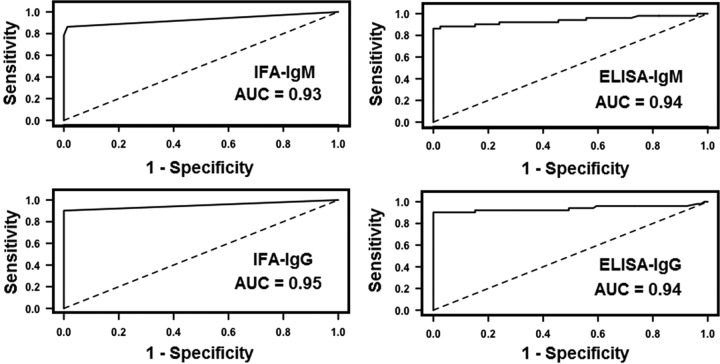
As for the Serion ELISAs, using the cutoff titers advocated by the manufacturer, we found a sensitivity of 88.2% for IgM and 86.3% for IgG, and a specificity of 94.8% for IgM and 95.5% for IgG. To obtain 97.8% specificity (close to 98%), cutoff titers had to be set at ≥0.9 for IgM and ≥1.4 for IgG with corresponding sensitivities of 86.3% for IgM and 84.3% for IgG (Table 2). Receiver operating characteristic (ROC) curves for IFA-IgM, IFA-IgG, Serion ELISA-IgM, and Serion ELISA-IgG tests are shown in Fig. 1. The negative predictive values (NPVs) and positive predictive values (PPVs) according to tularemia prevalence in the ELISA-IgM and ELISA-IgG tests, using either the lower or higher cutoff titers, are presented in Fig. 2.
FIG 1.
ROC curves for detection of IgM or IgG anti-F. tularensis antibodies using immunofluorescence assays (IFAs) or ELISAs. AUC, area under the curve.
FIG 2.
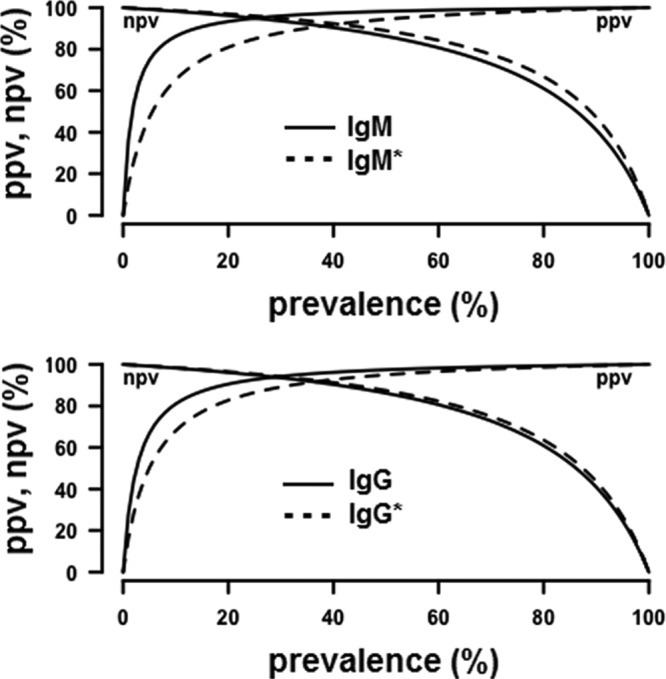
Negative predictive values (NPVs) and positive predictive values (PPVs) according to tularemia prevalence of ELISA-IgM (top)and ELISA-IgG (bottom) tests, using either the lower cutoff titers recommended by the manufacturer (dashed line) or the higher cutoff titers of 0.9 for IgM and 1.4 for IgG (solid line) defined herein to increase specificity.
The sensitivity and specificity of the VIRapid tularemia test were 90% and 83.6%, respectively, when a color intensity of 0.5 (as shown in the manufacturer's user guide) was used as a cutoff titer. Sensitivity and specificity were 90% and 92.5%, respectively, when using a cutoff at a color intensity of 1.
The kinetics of anti-F. tularensis antibodies during infection were evaluated for 20 tularemia cases (28 serum samples) for which the date of symptom onset could be determined. For this subgroup of patients, specific antibodies were detected earlier in the course of the disease with the Serion ELISA and VIRapid tularemia tests compared to the MAT and IFA tests (Table 3). During the first 2 weeks following symptom onset, the sensitivities were 15.4% (2/13 positive-tested samples) for the MAT and IFA-IgM tests and 0% (0/13) for the IFA-IgG test. The sensitivity of the VIRapid tularemia test was 20% (2/10). For the Serion ELISA-IgM and ELISA-IgG tests, the sensitivities were 38.5% (5/13) and 30.8% (4/13), respectively, when the cutoff titers recommended by the manufacturer were used. Sensitivities were 38.5% (5/13) and 15.4% (2/13) with the use of the cutoff titers determined in this study to obtain a specificity close to 98%.
TABLE 3.
Proportion of positive tests for 28 serum samples collected from 20 tularemia patients at different time intervals from the onset of clinical symptoms
| Method (cutoff titer) | % positive tests (no. positive serum samples/no. tested) at: |
||||
|---|---|---|---|---|---|
| 1st wk | 2nd wk | 3rd and 4th wks | 2nd mo | Overall | |
| MAT (≥80) | 0 (0/7) | 33.3 (2/6) | 75 (6/8) | 85.7 (6/7) | 50 (14/28) |
| IFA-IgM (≥80) | 0 (0/7) | 33.3 (2/6) | 62.5 (5/8) | 85.7 (6/7) | 46.4 (13/28) |
| IFA-IgG (≥160) | 0 (0/7) | 0 (0/6) | 87.5 (7/8) | 100 (7/7) | 50 (14/28) |
| ELISA-IgM (OD ≥ 0.45) | 14.3 (1/7) | 66.7 (4/6) | 100 (8/8) | 100 (7/7) | 71.4 (20/28) |
| ELISA-IgM (OD ≥ 0.9) | 14.3 (1/7) | 66.7 (4/6) | 100 (8/8) | 100 (7/7) | 71.4 (20/28) |
| ELISA-IgG (OD ≥ 0.62) | 0 (0/7) | 66.7 (4/6) | 100 (8/8) | 100 (7/7) | 67.8 (19/28) |
| ELISA-IgG (OD ≥ 1.4) | 0 (0/7) | 33.3 (2/6) | 100 (8/8) | 100 (7/7) | 60.7 (17/28) |
| VIRapid (band intensity ≥ 0.5) | 14.3 (1/7) | 33.3 (1/3) | 100 (4/4) | 100 (7/7) | 61.9 (13/21) |
We compared the concordance between the MATs, IFAs, and Serion ELISAs. There was a very good concordance between the MAT and the IFA-IgM (κ = 0.91) and the IFA-IgG (κ = 0.80) tests. We also found good concordance between the MATs, the IFAs, and the Serion ELISAs (using the manufacturer's cutoff titers): κ = 0.77 between the MAT and ELISA-IgM; κ = 0.79 between the MAT and ELISA-IgG; κ = 0.75 between the IFA-IgM and ELISA-IgM; and κ = 0.78 between the IFA-IgG and the ELISA-IgG. The concordance coefficients did not change significantly when higher cutoff titers were used for the Serion ELISAs (i.e., 0.9 for IgM and 1.4 for IgG). Likelihood ratios (LRs) were also highly favorable for MATs (LR+ = 50.2, LR− = 0.25), IFAs, and ELISAs (Table 2).
DISCUSSION
The aim of this study was to evaluate commercial assays for serological diagnosis of tularemia in humans. These tests were evaluated in a cohort of 208 patients whose clinical samples were sent to our national reference laboratory for tularemia diagnostic testing. All serum samples were tested using our in-house MAT and IFA tests, and tularemia diagnosis also relied upon F. tularensis culture and PCR testing of various clinical samples when available. According to the WHO definition (19), 51 patients were proven cases of tularemia and 23 patients were probable cases. Tularemia diagnosis was rejected in the remaining 134 patients, who served as negative controls in this study. Most of the confirmed and probable tularemia cases were males (50/74, 67.6%), with a median age of 55 years (range, 8 to 95 years), who suffered from the ulceroglandular or glandular form (34/74, 45.9%) of tularemia. This patient population was representative of the usual epidemiological and clinical aspects of tularemia in France (18). Interestingly, however, severe diseases with F. tularensis bacteremia were observed only in men (16 cases), with a median age of 67 years, compared to a male/female sex ratio of 1.29 and a median age of 50 years for the remaining 58 patients.
In the population studied, our MATs displayed 75.3% sensitivity and 98.5% specificity. The IFAs displayed sensitivities of 72.5% for IgM (cutoff titer ≥80) and 74.5% for IgG (cutoff titer ≥160), and a very high specificity of 99.3% for both types of antibodies. The low sensitivities found for the MAT and IFA tests were related to the fact that only acute-phase sera were available for patients with culture-proven tularemia. These serological tests are used in our laboratory for confirmation of tularemia cases, especially in patients for whom F. tularensis culture and PCR tests are not feasible or are negative. Because tularemia is a rare disease in France, we have previously determined cutoff titers to obtain specificities higher than 98% (18). In contrast, significant antibody titers are usually detected only after 3 to 4 weeks following symptom onset.
When the cutoff titers advocated by the manufacturer were used, for the same group of patients the Serion ELISAs displayed 88.2% sensitivity for IgM and 86.3% for IgG; specificity was 94.8% for IgM and 95.5% for IgG. These are lower sensitivity and specificity levels than those reported by the manufacturer, i.e., sensitivity of 99% for both IgM and IgG, and specificity greater than 99% for IgM and 96.9% for IgG. This difference could be partly related to the use of an automated procedure (the BEP III system) whereas these kits are intended to be used manually. However, in most diagnostic laboratories ELISAs are now performed in automated systems, which usually ensure greater reproducibility of test results. It is more likely that the observed differences in sensitivity and specificity are related to different characteristics between the patient population tested by the manufacturer and our cohort of patients. As for the VIRapid tularemia test, we found a sensitivity of 90% and a specificity of 83.6%, using the cutoff titer advocated by the manufacturer (i.e., a color intensity of the test line at 0.5 or higher). These values were much lower than those claimed in the manufacturer's user guide, i.e., 99.13% sensitivity and 98.58% specificity. Increasing the cutoff at a color intensity of the test line at 1 or higher did not change the sensitivity (90%) but increased the specificity (92.5%). In our hands, the VIRapid tularemia test was not specific enough to serve as a screening test for tularemia in a reference diagnostic laboratory.
A few studies have evaluated commercial serological tests for tularemia diagnosis. Chaignat et al. (20) evaluated the performance of three commercial assays: the Serazym anti-Francisella tularensis ELISA (Seramun Diagnostica GmbH, Heidesee OT Wolzig, Germany), the Serion ELISA classic F. tularensis IgG and IgM tests (Virion/Serion, GmbH Institute, Würzburg, Germany), and the VIRapid tularemia test (Vircell, Granada, Spain). These tests were evaluated at the same time as an in-house MAT, taken as the reference. Serum samples were collected in Serbia from a consecutive series of 110 tularemia patients. The sensitivities and specificities of the tests evaluated were, respectively, 97% and 91.5% for the Serazym ELISAs, 96.3% and 96.8% for the Serion ELISA-IgG tests, 94.8% and 96.8% for the Serion ELISA-IgM tests, and 97% and 84% for the VIRapid tularemia tests. Interestingly, for the three latter serological assays, we found lower sensitivities but similar specificities. The higher sensitivities found by Chaignat et al. (20) might be explained by the fact that only patients with typical tularemia clinical manifestations and a positive MAT were included in their study. Tularemia may correspond to various clinical manifestations, some of which are poorly specific (3, 21). Our cases had more variable clinical patterns, including nonspecific symptoms, but were defined as confirmed or probable tularemia cases according to the WHO definition (19). Performing serological tests in patients with typical symptoms of tularemia in a country of high endemicity such as Serbia may have resulted in overevaluation of their performance. Another study from Kiliç et al. (22) reported a sensitivity of 99.3% and a specificity of 94.6% for the VIRapid tularemia test. However, this study involved 106 tularemia cases (mostly oropharyngeal forms) occurring during a tularemia outbreak. This is quite different from the situation of our study where tularemia cases were independent and sporadic. Also, tularemia cases were defined as the combination of compatible clinical findings and a MAT titer of 160 or higher.
To evaluate the kinetics of anti-F. tularensis antibodies during infection, we determined the proportion of positive serological tests among 28 serum samples collected from 20 tularemia patients for which the date of symptom onset could be accurately determined. Our goal was to determine the most appropriate serological tests for early detection of specific antibodies in patients' sera. The MAT and IFA-IgM tests were negative for serum samples collected during the 1st week of symptom progression and positive for 33.3% of those collected during the 2nd week, giving an overall sensitivity of 15.4% for the first 2 weeks of the course of the disease. The Serion ELISA-IgM test was positive for 14.3% and 66.7% of serum samples collected during the 1st and 2nd weeks of symptom progression, respectively, giving an overall sensitivity of 38.5% for the first 2 weeks of the disease course. Specific IgG antibodies were also detected earlier with the ELISA-IgG test compared with the IFA-IgG test (30.8% versus 0% for the first 2 weeks of the disease course). Earlier detection of specific anti-F. tularensis antibodies using the ELISA method was previously reported (10, 23). Using an in-house ELISA and a lipopolysaccharide (LPS) extract from the F. tularensis subsp. holarctica LVS strain as the antigen, Eliasson et al. (23) reported a sensitivity of 50% for detection of IgM and/or IgG antibodies 10 days after the onset of clinical symptoms. The VIRapid tularemia test also allowed earlier detection of specific antibodies compared to the MAT and IFA-IgM tests (20% sensitivity for the first 2 weeks of disease progression compared to 15.4% for the MAT and IFA-IgM tests). Kiliç et al. (22) reported higher sensitivities for the VIRapid tularemia test, at 20% during the 1st week following symptom onset and 67.4% during the 2nd week. Altogether, the results of the present study and those of previous reports in the literature demonstrate better performance of the ELISAs (compared to the MATs and IFAs) in detecting anti-F. tularensis antibodies in serum samples collected from tularemia patients during the first 2 weeks of symptom progression. However, earlier detection of low titers of anti-F. tularensis antibodies may be associated with a higher risk of false-positive results. Thus, positive ELISA results should be confirmed by MAT or IFA on the same serum or negative ELISA results by MAT or IFA on a subsequent serum.
Tularemia is a rare zoonotic disease in France, where the annual incidence of human infections is close to 0.13 cases per 105 inhabitants (18, 24). The low specificities of the Serion ELISA and VIRapid tularemia tests we obtained in our study population would certainly correspond to low positive predictive values (PPVs), although the PPV could not be calculated because the true prevalence in the population tested remains unknown. The same reasoning applies to other countries that have tularemia endemicity with low prevalence of the disease, especially in most European countries (25). As a national reference center, we need high-PPV diagnostic tests to accurately confirm suspected tularemia cases. We therefore determined ROC curves from the results obtained with the Serion ELISAs and new cutoff titers allowing approximately 98% specificities in our patient cohort. Using cutoff titers of optical density (OD) ≥0.9 for the Serion ELISA-IgM and OD ≥1.4 for the Serion ELISA-IgG, we obtained 97.8% specificity for both ELISAs, while the sensitivities were 86.3% and 84.3% for the ELISA-IgM and ELISA-IgG, respectively. Thus, even using these higher cutoffs, the ELISAs remained more sensitive than the IFAs. In areas where tularemia has a low prevalence, an approximately 95% specificity of the ELISAs would result in a significant number of false-positive results and a low PPV. As an example, when using the Serion ELISA-IgG test in a population with a 10% tularemia prevalence, the negative predictive value (NPV) and PPV would be 98.6% and 66.2%, respectively, for the cutoff advocated by the manufacturer (OD ≥0.619), but 98.3% and 80.9% for an OD ≥1.4 as the cutoff (Fig. 2). The IFA-IgG test would give a NPV of 97.2% and a PPV of 92.5% in the same situation. Altogether, there was a very good correlation between the results obtained with the MAT, IFA, and Serion ELISAs, and highly favorable likelihood ratios.
In our routine practice, serum samples from patients with suspected tularemia are still tested using the combination of microagglutination and IFA methods (18). The microagglutination test is still considered a reference test for serological diagnosis of tularemia (19), although it does not allow separate quantification of IgM and IgG titers. The IFA method allows separate detection and quantification of specific IgM and IgG antibodies. However, it is time-consuming and difficult to standardize, especially because the antigen tested often varies from one laboratory to another, and fluorescence reading and quantification remain subjective. Commercialized ELISAs for detection and titration of anti-F. tularensis IgM or IgG antibodies are easier to automate and standardize. The results obtained in the present study and previously published studies show that, compared to the MAT and IFA techniques, the ELISAs allow earlier detection of specific antibodies but display lower specificities. Because all these serological tests detect predominantly anti-lipopolysaccharide antibodies, serological cross-reactions with Brucella spp. and Y. enterocolitica should be considered. The ELISAs are useful for screening a large number of serum samples, while the MAT and IFA tests could serve as confirmatory tests for samples with positive ELISAs. A similar proposal was previously made by Porsch-Özcürümez et al. (26) but using an in-house Western blot as the confirmatory test. In our hands, the VIRapid tularemia test displayed much lower specificity than the MAT, IFA, and ELISA tests, and its use in hospital laboratories should be discouraged. However, this test is a valuable tool for field studies, allowing easy and rapid screening of people and animals with potential F. tularensis infection.
ACKNOWLEDGMENTS
We acknowledge Santé Publique France for funding. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We also thank Linda Northrup for editing the manuscript.
We have no conflict of interest to declare.
REFERENCES
- 1.Oyston PCF, Sjostedt A, Titball RW. 2004. Tularaemia: bioterrorism defense renews interest in Francisella tularensis. Nat Rev Microbiol 2:967–978. doi: 10.1038/nrmicro1045. [DOI] [PubMed] [Google Scholar]
- 2.Maurin M. 2015. Francisella tularensis as a potential agent of bioterrorism? Expert Rev Anti Infect Ther 13:141–144. doi: 10.1586/14787210.2015.986463. [DOI] [PubMed] [Google Scholar]
- 3.Sjöstedt A. 2007. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann N Y Acad Sci 1105:1–29. doi: 10.1196/annals.1409.009. [DOI] [PubMed] [Google Scholar]
- 4.Eden J-S, Rose K, Ng J, Shi M, Wang Q, Sintchenko V, Holmes EC. 2017. Francisella tularensis ssp. holarctica in ringtail possums, Australia. Emerg Infect Dis 23:1198–1201. doi: 10.3201/eid2307.161863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson J, McGregor A, Cooley L, Ng J, Brown M, Ong CW, Darcy C, Sintchenko V. 2012. Francisella tularensis subspecies holarctica, Tasmania, Australia, 2011. Emerg Infect Dis 18:1484–1486. doi: 10.3201/eid1809.111856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hepburn MJ, Simpson AJH. 2008. Tularemia: current diagnosis and treatment options. Expert Rev Anti Infect Ther 6:231–240. doi: 10.1586/14787210.6.2.231. [DOI] [PubMed] [Google Scholar]
- 7.Tärnvik A, Chu MC. 2007. New approaches to diagnosis and therapy of tularemia. Ann N Y Acad Sci 1105:378–404. doi: 10.1196/annals.1409.017. [DOI] [PubMed] [Google Scholar]
- 8.Karagöz S, Kiliç S, Berk E, Uzel A, Celebi B, Çomoğlu Ş, Karagöz A, Akyar I, Can S. 2013. Francisella tularensis bacteremia: report of two cases and review of the literature. New Microbiol 36:315–323. [PubMed] [Google Scholar]
- 9.Shapiro DS, Schwartz DR. 2002. Exposure of laboratory workers to Francisella tularensis despite a bioterrorism procedure. J Clin Microbiol 40:2278–2281. doi: 10.1128/JCM.40.6.2278-2281.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koskela P, Salminen A. 1985. Humoral immunity against Francisella tularensis after natural infection. J Clin Microbiol 22:973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bevanger L, Maeland JA, Kvan AI. 1994. Comparative analysis of antibodies to Francisella tularensis antigens during the acute phase of tularemia and eight years later. Clin Diagn Lab Immunol 1:238–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behan KA, Klein GC. 1982. Reduction of Brucella species and Francisella tularensis cross-reacting agglutinins by dithiothreitol. J Clin Microbiol 16:756–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koskela P, Herva E. 1982. Immunity against Francisella tularensis in northern Finland. Scand J Infect Dis 14:195–199. doi: 10.3109/inf.1982.14.issue-3.07. [DOI] [PubMed] [Google Scholar]
- 14.Sato T, Fujita H, Ohara Y, Homma M. 1990. Microagglutination test for early and specific serodiagnosis of tularemia. J Clin Microbiol 28:2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelletier N, Raoult D, La Scola B. 2009. Specific recognition of the major capsid protein of Acanthamoeba polyphaga mimivirus by sera of patients infected by Francisella tularensis. FEMS Microbiol Lett 297:117–123. doi: 10.1111/j.1574-6968.2009.01675.x. [DOI] [PubMed] [Google Scholar]
- 16.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, Lijmer JG, Moher D, Rennie D, de Vet HC, Kressel HY, Rifai N, Golub RM, Altman DG, Hooft L, Korevaar DA, Cohen JF, STARD Group. 2015. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 351:h5527. doi: 10.1136/bmj.h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, Irwig L, Levine D, Reitsma JB, de Vet HCW, Bossuyt PMM. 2016. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 6:e012799. doi: 10.1136/bmjopen-2016-012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maurin M, Pelloux I, Brion JP, Del Banõ J-N, Picard A. 2011. Human tularemia in France, 2006-2010. Clin Infect Dis 53:e133–141. doi: 10.1093/cid/cir612. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. 2007. WHO guidelines on tularaemia. WHO Press, Geneva, Switzerland. [Google Scholar]
- 20.Chaignat V, Djordjevic-Spasic M, Ruettger A, Otto P, Klimpel D, Müller W, Sachse K, Araj G, Diller R, Tomaso H. 2014. Performance of seven serological assays for diagnosing tularemia. BMC Infect Dis 14:234. doi: 10.1186/1471-2334-14-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maurin M, Gyuranecz M. 2016. Tularaemia: clinical aspects in Europe. Lancet Infect Dis 16:113–124. doi: 10.1016/S1473-3099(15)00355-2. [DOI] [PubMed] [Google Scholar]
- 22.Kiliç S, Celebi B, Yeşilyurt M. 2012. Evaluation of a commercial immunochromatographic assay for the serologic diagnosis of tularemia. Diagn Microbiol Infect Dis 74:1–5. doi: 10.1016/j.diagmicrobio.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 23.Eliasson H, Olcén P, Sjöstedt A, Jurstrand M, Bäck E, Andersson S. 2008. Kinetics of the immune response associated with tularemia: comparison of an enzyme-linked immunosorbent assay, a tube agglutination test, and a novel whole-blood lymphocyte stimulation test. Clin Vaccine Immunol 15:1238–1243. doi: 10.1128/CVI.00434-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mailles A, Vaillant V. 2014. 10 years of surveillance of human tularaemia in France. Euro Surveill 19:20956. doi: 10.2807/1560-7917.ES2014.19.45.20956. [DOI] [PubMed] [Google Scholar]
- 25.Hestvik G, Warns-Petit E, Smith LA, Fox NJ, Uhlhorn H, Artois M, Hannant D, Hutchings MR, Mattsson R, Yon L, Gavier-Widen D. 2015. The status of tularemia in Europe in a one-health context: a review. Epidemiol Infect 143:2137–2160. doi: 10.1017/S0950268814002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porsch-Özcürümez M, Kischel N, Priebe H, Splettstösser W, Finke E-J, Grunow R. 2004. Comparison of enzyme-linked immunosorbent assay, Western blotting, microagglutination, indirect immunofluorescence assay, and flow cytometry for serological diagnosis of tularemia. Clin Diagn Lab Immunol 11:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]