Abstract
Drug delivery in the CNS is limited by endothelial tight junctions forming the impermeable blood-brain barrier. The development of new treatment paradigms has previously been hampered by the restrictiveness of the blood-brain barrier to systemically administered therapeutics. With recent advances in stereotactic localization and noninvasive imaging, we have honed the ability to modulate, ablate, and rewire millimetric brain structures to precisely permeate the impregnable barrier. The wide range of focused radiations offers endless possibilities to disrupt endothelial permeability with different patterns and intensity following 3-dimensional coordinates offering a new world of possibilities to access the CNS, as well as to target therapies. We propose a review of the current state of knowledge in targeted drug delivery using noninvasive image-guided approaches. To this end, we focus on strategies currently used in clinics or in clinical trials such as targeted radiotherapy and magnetic resonance guided focused ultrasound, but also on more experimental approaches such as magnetically heated nanoparticles, electric fields, and lasers, techniques which demonstrated remarkable results both in vitro and in vivo. We envision that biodistribution and efficacy of systemically administered drugs will be enhanced with further developments of these promising strategies. Besides therapeutic applications, stereotactic platforms can be highly valuable in clinical applications for interventional strategies that can improve the targetability and efficacy of drugs and macromolecules. It is our hope that by showcasing and reviewing the current state of this field, we can lay the groundwork to guide future research in this realm.
Keywords: electroporation, focused ultrasound, microbubbles, photodynamic therapy, vascular permeability
Advanced stereotactic systems, particularly focused ultrasound and proton therapy, have received tremendous attention recently. Current developments in noninvasive stereotactic techniques can now effectively target diseased areas all over the CNS with extreme precision in order to eradicate tumors, ablate diseased circuits in the brain, and, with recent progress in neuroimaging, potentially improve drug delivery.1,2 The key to understanding these new breakthroughs in stereotactic radiosurgery is understanding the influence these modalities have on the blood-brain barrier (BBB) and their ability to disrupt its permeability.
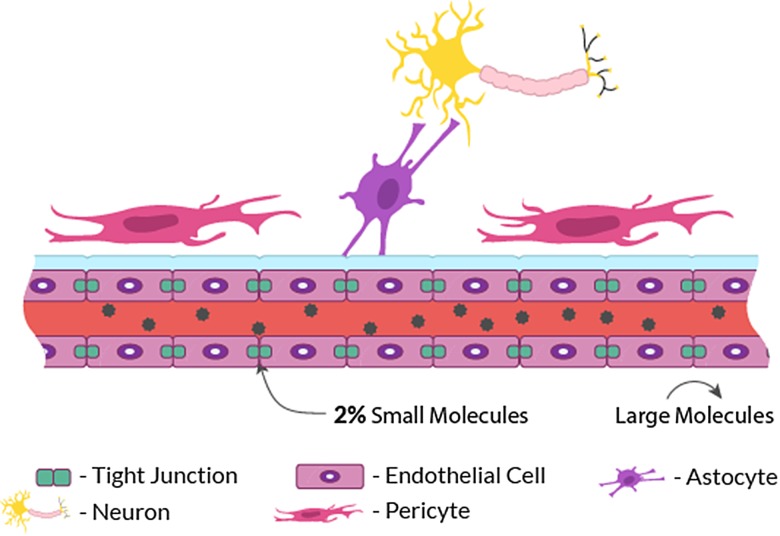
The BBB is a consistent barrier system that protects the healthy brain from harmful substances. Endothelial cells lining the blood vessels constitute the main component of the BBB. They are surrounded by extracellular matrix, astrocytes, pericytes, vascular smooth muscle cells, and microglial cells (Fig. 1). The close association of endothelial cells with astrocyte foot processes and the basement membrane of capillaries is important for the development and maintenance of the BBB properties. Furthermore, the proximity of tight junctions between brain endothelial cells limits intercellular translaminar flow to small hydrophilic molecules permitted through tight control of blood-brain exchange. In fact, compared with capillaries in other vital organs, the BBB is extremely influential at regulating molecular flow across its borders. It prevents up to 98% of all small-molecule therapeutics and essentially 100% of all unmodified large-molecule therapeutics from entering the brain due to the closely sealed tight junctions.3 Since peptide and protein therapeutics are generally excluded from the blood-brain transport, owing to the negligible permeability of the brain–capillary endothelial wall to these drugs, endothelial cells represent the major obstacle to the use of many potential therapeutics against the majority of CNS disorders.1 While the BBB essentially prevents the nonselective accumulation of potentially harmful neurotoxins, it also hinders the transport and efficacy of chemotherapeutics against tumor proliferation and invasion.
Fig. 1.
Schematic illustration of BBB permeability.
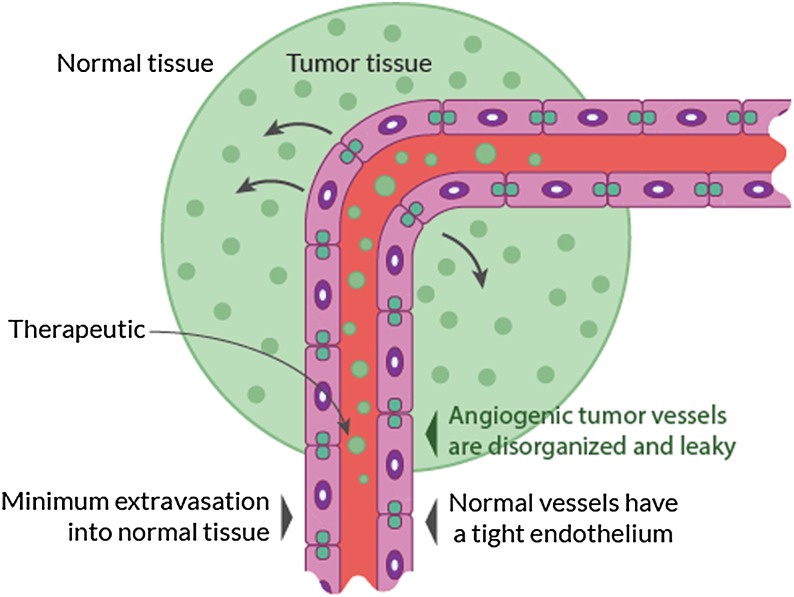
To a certain extent, brain tumors innately disrupt the BBB via local invasion of soluble secretion factors that actively degrade tight junctions, as well as the formation of abnormal blood vessels through the defective expression of tight junction proteins, namely occludin and claudin (Fig. 2). In the example of gliomas, the vasculature is immature, variably permeable, and inhomogeneously distributed4; these properties help brain tumors infiltrate intact brain parenchyma, leading to invasion and growth.
Fig. 2.
Schematic illustration of the enhanced permeability and retention effect in and around the tumor site.
The enhanced permeability and retention (EPR) effect is attributed to the abnormal anatomy and physiology of tumors (ie, leaky vasculature, endothelial fenestrations, and poor lymphatic drainage).5–9 Many factors affect the EPR effect, including the pH, polarity, and size of the delivered substance. The tumor environment is variable and certain characteristics may hinder the EPR effect, such as hypovascularity, fibrosis, and necrosis.10 Furthermore, infiltrating cancer cells and small metastatic seeds may be protected by the BBB in surrounding intact tissue.4 Even with the breakdown of the BBB in neuro-oncological disease, the fact remains that the tissue accumulation of chemotherapeutics for CNS metastases is 85% less intracranially compared with penetration and biodistribution for extracranial neoplasms.9
The limited efficacy of chemotherapeutic drugs has been attributed to an inability to achieve effective therapeutic concentrations of these drugs in the tumor due to the presence of the BBB. With limited penetrative treatments efficacious at targeting CNS tumors and the gravity of neuro-oncological disease, it is imperative to continue exploring promising advancements in treatment options. To improve their targeting and concentration in the CNS, chemotherapeutic drugs have to respect a list of specific criteria. These include favorable properties for passive diffusion through the BBB: small molecular weight, uncharged (or only partially ionized) at physiological pH, and lipid soluble without increasing plasma protein binding to avoid uptake by the liver or reticuloendothelial system.10
Attempts to identify newer, more penetrative chemotherapeutics that can effectively infiltrate the BBB affected by the tumor are still ongoing.11 However, rather than designing therapeutic agents sufficiently small enough to penetrate the BBB, another approach is to transiently disrupt the BBB. The most recent of these great strides in neuro-oncological therapy is the modulation of both drug delivery and integrity of the BBB through functional and stereotactic mechanisms representing a promising strategy for enhancing treatment.11 Since partial permeability is insufficient to allow the accumulation of therapeutic levels of drugs,9 solutions combining stereotactic methods and systemic treatments have been shown to enhance delivery and bioavailability of therapeutics.
This field within functional neuromodulation and stereotactic radiosurgery has the potential to enhance treatment paradigms in neuroscience. Here, we discuss the different stereotactic options to enhance BBB permeability with a focus on techniques that hold the potential to be translated clinically.
Clinical Implications for Neuro-Oncology
Clinicians have long been aware of the need for systemically administered agents with excellent penetration into the CNS for the optimal treatment of brain infections, brain tumors, and other serious neurological illnesses. Brain tumors have a low incidence but high lethality compared with other cancers. Despite advanced treatment protocols, the prognosis remains poor, with an overall median survival for glioblastoma multiforme (GBM) of ∼14–15 months even after complete macroscopic surgical resection and adjuvant radiochemotherapy.12
Temozolomide is the agent of choice used in adjuvant treatment of GBM. However, the brain serum levels peak only at 17%–20% of that in the blood.13,14 Furthermore, chemotherapy drug concentrations rapidly decrease from the center of the tumor, resulting in up to 40-fold lower concentrations in the peritumoral brain zone.15,16 Up to 90% of recurrent tumors develop within the peritumoral brain zone, which is defined as the 2–3 cm margin from the primary site of surgery. This region is composed of normal BBB, decreasing the delivery of drugs to this area, leading to potential failure of water-soluble chemotherapy.17
Previous attempts to improve drug delivery to the CNS are well documented and have been pursued for over 3 decades; most of these strategies are invasive or not well localized. Intra-arterial drug administration, hyperosmolar solutions, biomolecules, high-dose chemotherapy, and direct intratumoral injection were among other solutions proposed.18 These techniques often require general anesthesia, intra-arterial catheterization, or a craniotomy, leading to many possible complications such as seizures, cerebrovascular events, and other significant toxicities.19,20 There have been innumerable efforts to attempt to minimize these risks and maximize benefits in treatment. Recent efforts have shown promise in transient disruption of the BBB in the outpatient setting with the use of pharmacological agents (bradykinin analog, verapamil, lobradimil, selective G-protein coupled receptor A2A, regadenoson).21,22 However, diffuse delivery of a high dose of chemotherapeutic agents can cause undesirable side effects in normal tissues. Direct postoperative delivery (either through slow release systems or direct infusion) into the tumor site is, in theory, an effective way to maximize the chemotherapeutic dose while limiting peripheral dose. Being able to focus drug delivery to specific cancer locations through transient and localized disruption of the BBB shows great promise for improving cancer therapy outcomes. Specifically in this review we present the salient methods of localized and noninvasive image-guided strategies to modulate the BBB. Translation and standardization of these technologies into clinical practice will potentially change the current landscape of neuro-oncological treatment paradigms.
Focused Ultrasound
A rapidly developing field of study within stereotactic modulation involves the use of high-intensity focused ultrasound, most notably magnetic resonance guided focused ultrasound (MRgFUS). This technology holds high therapeutic yields and can be modified to induce thermal ablation, sonothrombolysis, and BBB disruption, allowing targeted therapies.12 This noninvasive non-ionizing technique consists of delivering beams of focused ultrasound energy with extreme precision to heat, stimulate, and/or destroy regions of the brain while simultaneously allowing real-time imaging of the targeted brain region. These individual ultrasound beams are sent via a transducer through different parts of the skull and will sum coherently at the targeted site to modify the local environment.
This technique is already used outside of the scope of CNS disease; MRgFUS is currently used for the treatment of uterine fibroids, painful osseous metastases, and breast cancer, among others.3 Yet, currently, high-intensity focused ultrasound is gaining traction to modulate central neuropathic pain, essential tremor, Parkinson's disease, and adjunctive therapy for brain tumors.19,20 The first human case of MRgFUS modulation of BBB has been reported earlier to enhance chemotherapy treatment.23
The exact mechanism of BBB modulation as a result of ultrasonic modulation is still under investigation; however, studies suggest that there is a combination of mechanical and heat stress, leading to enhanced local transport across the barrier via endocytosis, transcytosis, and tight junction disruption.4,10,24–26 Focused ultrasound (FUS) alone has been shown to thermally induce BBB disruption at the target site.27 Mild to moderate increase in temperature has the potential to modify the local environment and increase transport across the BBB. Unfortunately, high temperatures (55°–60°C) will inevitably induce cell death from thermal coagulation, even with short exposure times. It has been shown, in an MRI study assessing the effect of local hyperthermia, that the first tissue change consistently detected was disruption of the BBB, but in the setting of FUS-induced hyperthermia there is also a consistently associated damage to the healthy tissue surrounding the treatment site.14
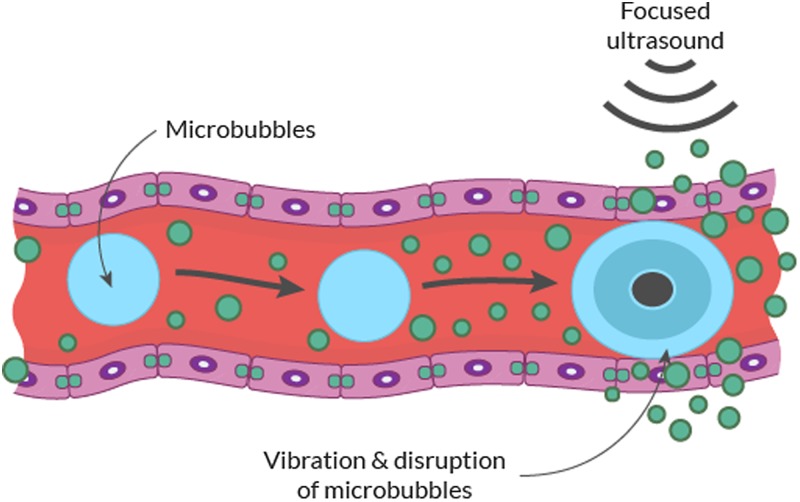
MRgFUS holds additional therapeutic yield via the use of low power FUS in combination with microbubbles (MBs). The systemic injection of MBs can mechanically disrupt the BBB within the intravascular space through the effect of FUS.28 The compressing nature of these gas bubbles will volumetrically expand and contract in response to ultrasound waves (stable cavitation), which may induce shock waves that disrupt the BBB transiently, opening it to therapeutic agents (Fig. 3). This phenomenon is also called sonoporation.29 At high pressure amplitudes these bubbles can violently collapse (inertial cavitation), causing permanent tissue damage.30 MBs increase the ultrasound signal locally disrupting the BBB and act more selectively in vascular regions, thereby allowing for tissue activation within microvessel walls.24 Immuno-electron microscopy studies in normal brains have indicated that passage through the BBB after treatment with MB-enhanced ultrasound occurs via both paracellular and transcellular routes, inducing, at endothelial cell levels, an opening of tight junctions associated with a fenestration and channelization of the cells injuring the endothelium.30,31 Furthermore, enhanced active vesicular transport has been shown in and around cell surfaces with use of MBs.31,32
Fig. 3.
Schematic illustration of FUS in the context of intravenous MB injection to transiently disrupt BBB permeability.
MRgFUS is particularly interesting, since it allows for precise thermal feedback while the patient is in the MRI machine. Promising in vitro and in vivo results have shown passage of a large range of molecular sizes through the BBB as a result. Depending on the intensity of ultrasound energy applied and the number of procedures, the BBB disruptive effect has been found to be temporary and reversible without damaging neural cells or inducing intracerebral hemorrhage. This technique demonstrated capability to deliver compounds of varying sizes, including antibodies, nanoparticles (NPs), liposomally encapsulated drugs,4,24,25,33 short interfering RNA, and viral vectors.26,34–37 In other words, MRgFUS can be used to induce reproducible BBB disruption without any neuronal damage at targeted locations, increasing the potential for safe drug delivery methods to previously inaccessible and eloquent locations.28,38,39
While MRgFUS holds much promise, several unresolved issues evident from previous clinical trials have to be addressed. The lack of standardized protocols to determine where MRgFUS yields the most efficacious results is apparent. Many factors, such as individual ultrasound parameters, MB doses, attenuation factors (eg, skull thickness and density), technical factors (eg, accuracy of the phase correction), and physiological factors (eg, tissue perfusion), remain to be determined.3 The intensity required for BBB opening may differ among patients. Similarly, the location of MRgFUS will vary based on the disease, requiring further study into particular MRgFUS protocols and outcomes based on location. Additionally, there is a variability in timing and efficiency of BBB opening, ranging in studies from 30 min post-sonication up to 72 h depending on acoustic parameters (frequency, pulse repetition frequency sonication duration, MB size, and pulse length).26,29,40–42 Translation of these results to human trials will require further studies to define the right parameters.43
The transition of preclinical studies to the development of protocols for BBB opening in large non-primate animals has highlighted several challenges to clinical translation, one of which is to maximize targeting accuracy while minimizing the time and effort necessary for accurate targeting. While MRI targeting can provide a higher level of accuracy, the presence of the magnetic field limits the accessibility of this procedure to highly specialized clinical settings. There have been several recent studies investigating MRI-independent targeted BBB opening by monitoring the acoustic emissions from MBs through the use of a passive cavitation detector for real-time monitoring and treatment efficiency verification.44,45 The protocol uses a stereotactic targeting procedure, which has initially been shown to be accurate and reliable, with observed targeting error relatively small in rhesus monkeys (2.5 ± 1.2 mm laterally, 1.5 ± 1.3 mm along depth-axis, 3.1 ± 1.3 mm total).45,46
Another challenge to translating this technology into clinical use is the real-time safety monitoring. Sonication with MBs will cause vascular damage when exposed to ultrasound levels that exceed MB thresholds, causing inertial cavitation. Specifically, the vascular damage may result in devastating and potentially fatal intracranial hemorrhage.43 In human skulls, there are particular regions that may be at risk due to high MB concentrations found in large blood vessels and highly vascularized structures.45,46 Cavitation activity can be monitored by analyzing the differences between wave reflections with and without MBs. However, further studies will need to be conducted to quantify the threshold ranges for stable or inertial cavitation.46 Unfortunately, much of the current data specifically focus on animal studies conducted in healthy brains, and there are several key differences between the BBB and environment within cancerous tissue, as well as key differences between animal tissue and human tissue, that must be accounted for.
Magnetic Heating of Nanoparticles
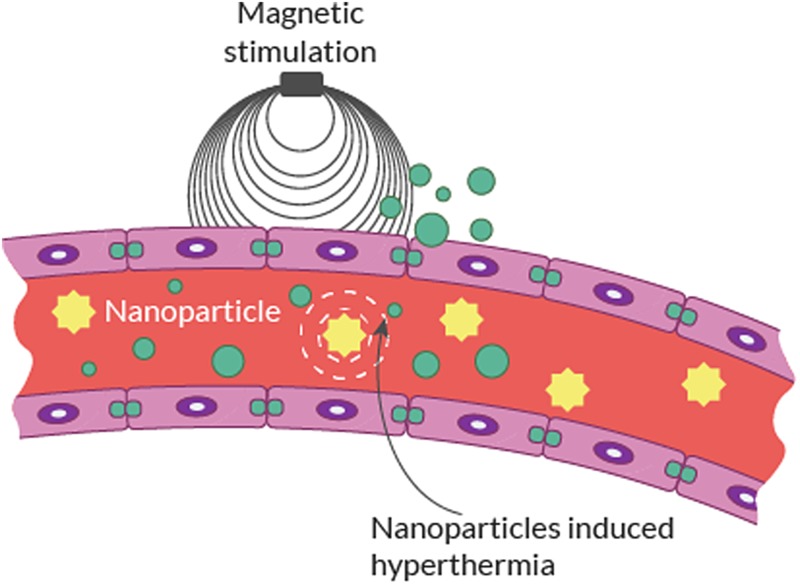
It is well known that even mild focal hyperthermia (38°–39°C) in and/or around the brain will lead to an increase in BBB permeability.6–8,27,47 Focal hyperthermia (42°C) has been shown to significantly increase flux of chemotherapy delivered to the brain in vitro and in vivo.48 In a recent study centered on this principle, Tabatabaei et al8 proposed a novel method to deliver therapeutics across the BBB. They provided preliminary evidence that magnetic heating of magnetic nanoparticles (MNPs) with a low radiofrequency (RF) source can transiently increase BBB permeability (Fig. 4). The mechanism, “Néel relaxation,” is described as a low RF field magnetically exciting MNPs to release energy in the form of heat to their surroundings.49,50 Other research groups have also applied the Néel relaxation principle to superparamagnetic iron oxide NPs with alternating magnetic field to generate local hyperthermia for BBB modulation.51
Fig. 4.
Schematic illustration of NP activation using magnetic fields to induce targeted hyperthermia.
Interestingly, no significant difference is observed when the temperature is increased, suggesting that the increased BBB permeability is not solely explained by the increase in temperature within these experiments, but could also be the result of MNP vibration or the difference of surface temperature against the surrounding temperature mechanically activating cell surface receptors.51,52 Since most of these experiments were conducted in vitro, we must remain cautious of the potential translation to in vivo studies. Furthermore, in case of imperfectly controlled environments, hyperthermia may generate a drastic increase in temperature, leading to cerebral damage.
Acoustic energy can be used to both vibrate intravenously administered MBs and magnetically heat MNPs, which allows both techniques to target brain regions and witness procedural outcomes in real time. Tabatabaei et al claim that MBs activated by MRgFUS have a shorter half-life in the vasculature and are quickly taken up by the reticuloendothelial system, in contrast to MNPs, which distribute and deposit within the surface of the target endothelium for a longer time.8 These modalities are not entirely benign. Mechanical stress associated with the MNPs can lead to high pressure shock waves that have the potential to damage the cellular membrane near a rigid surface of the brain.8 In addition, the sound waves may propagate nonlinearly over a large region of biological structures before converging into a focal point, leading to undesirable side effects in those regions.8 The affinity of the MNPs for the surface of the targeted endothelium ensures that the thermal energy is exclusively dissipated to the BBB. Therefore, this cell-specific approach ultimately minimizes potential side effects and the overheating of surrounding structures such as astrocytes and neurons.
Stereotactic Radiation Therapy
Radiation therapy plays a critical role in cancer treatment paradigms, with diagnosed tumors in nearly 50% of patients receiving ionizing radiation during the course of treatment.53 There are many types of radiation treatments, with selection based on the cancer type, disease location, and radiosensitivity. Ionizing radiation is defined as any radiant source with enough energy to generate a biologic response. Since Lars Leksell treated his first patient in 1906 with an X-ray tube attached to a stereotactic centered frame, many developments in precise stereotactic techniques have been performed. Recently, we have honed the ability to precisely focus radiation beams onto microscopic structures with a new generation of machines.54,55 Radiation therapy has the advantage to be very effective in killing cancer cells by depositing its energy in the tumor site and damaging its DNA. Ionizing radiation has the potential to not only alter tumor tissue, but also damage the glial, neuronal, and vasculature compartments of the brain. While the damage to normal brain tissue is often considered an adverse consequence of ionizing radiation, the targeted and controlled application of radiation to purposefully damage brain tissue may be key in the use of ionizing radiation to increase BBB permeability, as both endothelial cells and oligodendrocytes are radiation responsive.
There is a difference between enhancing the already permeable BBB around tumor sites and creating de novo openings of the BBB in otherwise healthy regions of the brain. Most current investigations assess the methods that modulate targeted regions around brain tumors to either limit the neo-angiogenesis or increase vessel permeability around the tumor site.9 In healthy regions of the brain, high doses of radiation have been shown to induce BBB permeability elevation, tight junction morphology changes, reductions in cell density, and the formation of actin stress fibers in cerebral endothelial cells.56 Radiation effects on the brain vasculature are of crucial importance in the progression of radiation-induced CNS toxicity but can also be used to modulate permeability. A study examining large single doses of irradiation on the cerebral microvasculature showed that ionizing radiation increases the BBB permeability to fluorescein isothiocyanate-dextran molecules of various sizes.57 Additionally, apoptosis of endothelial, neural, and glial cells, oxidative stress, and neuroinflammation mediate radiation-induced secondary cell damage that leads to further endothelial dysfunction, disruption of BBB, inhibition of cell regeneration, demyelination, and tissue necrosis.58
What remains apparent is that the therapeutic window has yet to be determined, especially with radiation-induced injury of cerebrovascular vessels as a real collateral effect. Large single doses of radiation disrupt the BBB and cause considerable edema, detectable with MRI for weeks to months post-irradiation.59 The risk of disrupting the BBB appears to be low with small doses of radiation (<100 mGy), with only a few reports describing functional or morphological changes,29 thereby establishing the accepted paradigm. Sándor et al showed recently that not only moderate and high doses of irradiation with 2 and 10 Gy, but also a single low dose of cranial irradiation with 0.1 Gy can induce BBB injury in adult mice.29
It is evident that radiation can change BBB permeability; however, it is unclear what dose is best to achieve the desired results with the least negative outcomes, and at what time post-irradiation does the permeability reach a maximum and later reverse. It has been shown that a single high dose (20 Gy) leads to an increase in permeability as early as 24 hours but can be delayed up to 90 days post-irradiation.58 Previous studies have concluded that the molecular response to single-dose irradiation is rapid, whereas the response to fractionated irradiation is slow.60 While many studies aim to characterize the best timing of single-dose radiation to achieve the desired effects, a recent study examined the duration of these effects, observing acute and early delayed effects, with no BBB impairment at 6 months follow-up.29
The potential to combine the use of radiation therapy to infiltrate the BBB with intravenous injection of NPs is an interesting new concept. NPs have recently received much attention as a potential tool in cancer treatment and diagnosis due to their low toxicity and ability to increase tissue sensitivity to radiation.61 NPs significantly increase the cellular DNA damage inflicted by ionizing radiation as well as markedly increasing DNA damage to blood-brain vessels. One can argue that low dose irradiation in conjunction with circulating NPs can increase the endothelial local dose response and therefore local BBB permeability with limited toxicity.62 Furthermore, with increased precision in both imaging and radiation treatments, we could in theory disrupt the BBB in strategic places in the brain or tumor bed before the administration of chemotherapeutics to increase their distribution and efficacy.
Electric Field Modulation
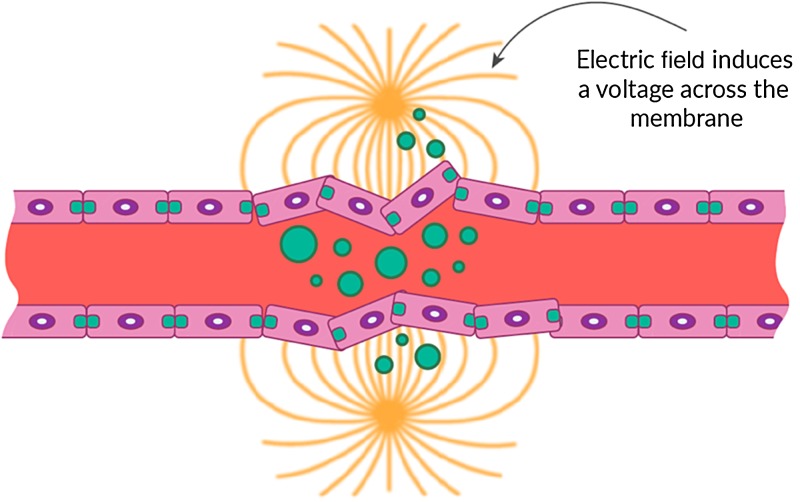
Physical modalities, other than ionizing radiation and thermal ablation, have not been systematically studied for radiomodulation of the BBB. One area of interest is the use of electric fields, which have been utilized in other fields of medicine but have remained relatively unexplored. Recently, frequency-tuned electric field therapy, also referred to as tumor-treating fields, have been studied and presented as a novel regional oncology therapy.63 One example is the transient use of an electric current to open the BBB for chemotherapeutic drugs, also known as electrochemotherapy. This technique uses sublethal pulsed electric fields to disrupt the endothelial membrane and facilitate the uptake of a chemotherapeutic agent, such as bleomycin or cisplatin, and has been tried for treatment of cutaneous and subcutaneous tumors in addition to brain metastasis (Fig. 5).64,65 Irreversible electroporation is achieved via the use of electric pulses delivered through needle electrodes inducing a nonthermal focal ablation to the target by a series of electric pulses. It induces cell death by disrupting membrane integrity and can also be used to produce nonthermal ablation of tumors.66 The mechanism of action is through both an anti-microtubule effect and a thermal ablative property of the electric field that results as the frequency increases, causing dielectric losses and developing friction between rapidly oscillating molecules.49 While there is potential for direct tumor treatment by the anti-mitotic properties of electric field therapy, what remains unclear is the potential of this modality in BBB disruption.
Fig. 5.
Schematic illustration of electric fields across the BBB membrane, regionally disrupting its permeability.
Laser Therapy
One additional neuromodulatory technology that has been shown to modulate BBB permeability within the CNS is laser therapy. For the past couple of years, MRI-guided laser interstitial thermal therapy (LITT) has emerged as an invasive ablation stereotactic technique.67,68 LITT is a minimally invasive therapy which involves inserting a thin laser probe, guided by MRI, to the core of a tumor mass, where it delivers hyperthermic ablation.69 This technique has been successfully used for treatment of primary or secondary tumors and deep seizure foci in epilepsy. In a recent study, Leuthardt et al were able to demonstrate sustained, local disruption of the peritumoral BBB using MRI-guided LITT in 14 human patients.67,69 This demonstrates an unexplored method of BBB disruption and requires further investigation and generalization.
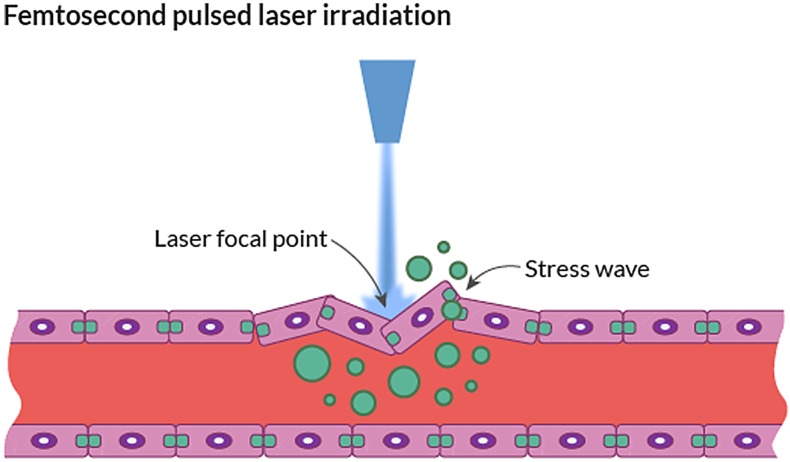
We expect other, more experimental, less invasive laser technology to be translated into the clinic. For example, laser-induced membranous defects in the capillary endothelium lead to transient disruption of the BBB and allow molecules to permeate into the brain parenchyma (Fig. 6). During the last decade, laser-induced hyperthermia has been used as a component of photodynamic therapy, which consists of treatment with a tumor-localizing photosensitizer and subsequent laser light activation.50
Fig. 6.
Schematic illustration of laser disruption through focal lesions in the BBB.
On another note, near-infrared femtosecond pulsed lasers have been widely used for in vivo imaging because of their deep tissue penetration, reduced scattering, and localized nonlinear absorption, which are ideal properties for CNS applications.51 Furthermore, it has been shown that femtosecond pulsed laser irradiation induces transient and reversible permeability of the targeted blood vessel wall, enabling extravasation of plasma along with bioactive macromolecules. This technology enables noninvasive tissue modulation via multiple effects, including the generation of intracellular calcium, dissection of intracellular organelles, transient plasma membrane permeability, induction of arterial contraction, and disruption of blood flow.49 This ability to alter vascular and BBB permeability holds great promise for the role of laser therapy in BBB modulation for the administration of chemotherapeutics to treat neuro-oncological disease.
Conclusions
New strides in noninvasive stereotactic techniques effectively target diseased areas all over the CNS with extreme precision in order to eradicate tumors and improve therapeutic drug delivery. These modalities, including MRgFUS, ionizing radiation, electric field therapy, and laser therapy, exert their effects via a disruption in the BBB integrity, thereby altering its permeability and enabling the ability of locally applied or systemically infused therapeutically active agents to reach and penetrate targeted diseased areas. It is this key modulation of BBB permeability that holds the promise of these modalities in treating neuro-oncological diseases.
MRgFUS has become one of the most salient mechanisms of modulating BBB permeability in otherwise “healthy” areas of the brain, where the disruption needs to be transient to enhance drug delivery. When examining the literature, it is evident that MRgFUS holds many potential advantages, as it is characterized by a sharp thermal gradient, creating a more focal effect compared with the broader gradient of radiation dose. Theoretically, MRgFUS produces a sharply delineated lesion resulting from the homogeneous thermal dose, whereas radiofrequency heating dissipates with distance from a central ablation electrode. One imaging manifestation of this observation is that there is usually more vasogenic edema around RF lesions compared with MRgFUS.3
Compared with MRgFUS and RF ablation, stereotactic radiosurgery has the disadvantage of latent treatment effects and the possibility of more extensive tissue damage beyond the intended target.19 MRgFUS has the unique advantages of being able to track changes in BBB permeability in real time and to define the minimal dose necessary to provide drug delivery, thereby efficiently controlling the therapeutic window. This was demonstrated by tracking changes in the MRI signal intensity with the extravasation via detection of hemorrhage during FUS-induced BBB opening.70
In the population of patients with brain tumors, ionizing radiation holds many advantages as well. We have decades of clinical expertise manipulating radiation in the oncologic brain, and validated recommendations exist pertaining to the radiosensitivity of key brain structures. Moreover, radiosurgery is widely available around the world and radiation specialists could combine existing treatment protocols with BBB targeting, allowing for enhanced chemotherapeutic drug delivery. Depending on the underlying disease, a more permanent opening of the BBB may be more appropriate. We can therefore postulate that targeted low dose radiation around the tumor vasculature, before chemotherapy, could be an interesting option. Furthermore, recent research has been focusing on increasing endothelial cell sensitivity through the use of NPs. One recent study showed how combined gold NPs and radiotherapy resulted in markedly increased DNA damage to brain blood vessels, leading to a more targeted BBB disruption limiting peripheral toxicity and allowing for an intravascular radiosensitization.60
Regardless of the modality of BBB permeabilization, what remains to be characterized is the optimal timeline for maximal BBB disruption and subsequent penetration. A short window can be positive but can also limit utility in a scenario where chemotherapeutic agents need to be administered to the patient for a longer period of time. Besides the importance of the timing for systemic treatments, length of infusions, and planning of chemotherapy regimens, it is important to know the time-to-recovery window to limit any possible brain injury via undesired penetration of nontherapeutic agents.54 Therefore, while it is clear that noninvasive stereotactic neuromodulatory modalities increase BBB permeability, with various modalities having been studied to assorted degrees, much remains to be characterized to make the use of this technology a widely accepted tool for tumor treatment in the CNS.
Funding
No funding was received for this research.
Conflict of interest statement. This review is the sole work of its authors, and there are no conflicts of interest.
References
- 1. Gabrieli JDE, Ghosh SS, Whitfield-Gabrieli S. Prediction as a humanitarian and pragmatic contribution from human cognitive neuroscience. Neuron. 2015;85(1):11–26 10.1016/j.neuron.2014.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fritz JV. Neuroimaging trends and future outlook. Neurol Clin. 2014;32(1):1–29 10.1016/j.ncl.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 3. Ghanouni P, Pauly KB, Elias WJ et al. . Transcranial MRI-guided focused ultrasound: a review of the technologic and neurologic applications. AJR Am J Roentgenol. 2015;205(1):150–159 10.2214/AJR.14.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Treat LH, McDannold N, Zhang Y et al. . Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med Biol. 2012;38(10):1716–1725 10.1016/j.ultrasmedbio.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lammers T, Koczera P, Fokong S et al. . Theranostic USPIO-loaded microbubbles for mediating and monitoring blood-brain barrier permeation. Adv Funct Mater. 2014;25(1):36–43 10.1002/adfm.201401199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kiyatkin EA, Sharma HS. Permeability of the blood-brain barrier depends on brain temperature. Neuroscience. 2009;161(3):926–939 10.1016/j.neuroscience.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dokladny K, Moseley PL, Ma TY. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am J Physiol Gastrointest Liver Physiol. 2006;290(2):G204–G212 10.1152/ajpgi.00401.2005. [DOI] [PubMed] [Google Scholar]
- 8. Tabatabaei SN, Girouard H, Carret A-S et al. . Remote control of the permeability of the blood–brain barrier by magnetic heating of nanoparticles: a proof of concept for brain drug delivery. J Control Release. 2015;206(C):49–57 10.1016/j.jconrel.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 9. Baetke SC, Lammers T, Kiessling F. Applications of nanoparticles for diagnosis and therapy of cancer. BJR. 2015;88(1054):20150207. 10.1259/bjr.20150207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu H-L, Hua M-Y, Yang H-W et al. . Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc Natl Acad Sci U S A. 2010;107(34):15205–15210 10.1073/pnas.1003388107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baumann BC, Kao GD, Mahmud A et al. . Enhancing the efficacy of drug-loaded nanocarriers against brain tumors by targeted radiation therapy. Oncotarget. 2013;4(1):64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stupp R, Hegi ME, Mason WP et al. . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 13. Portnow J, Badie B, Chen M et al. . The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin Cancer Res. 2009;15(22):7092–7098 10.1158/1078-0432.CCR-09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ostermann S, Csajka C, Buclin T et al. . Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10(11):3728–3736 10.1158/1078-0432.CCR-03-0807. [DOI] [PubMed] [Google Scholar]
- 15. Deeken JF, Löscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13(6):1663–1674 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 16. Stewart DJ, Richard MT, Hugenholtz H et al. . Penetration of VP-16 (etoposide) into human intracerebral and extracerebral tumors. J Neurooncol. 1984;2(2):133–139. [DOI] [PubMed] [Google Scholar]
- 17. Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30(9):907–911. [DOI] [PubMed] [Google Scholar]
- 18. Neuwelt EA, Maravilla KR, Frenkel EP, et al. Osmotic blood-brain barrier disruption. Computerized tomographic monitoring of chemotherapeutic agent delivery. J Clin Invest. 1979;64(2):684–688 10.1172/JCI109509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Warren K, Jakacki R, Widemann B et al. . Phase II trial of intravenous lobradimil and carboplatin in childhood brain tumors: a report from the Children's Oncology Group. Cancer Chemother Pharmacol. 2006;58(3):343–347 10.1007/s00280-005-0172-7. [DOI] [PubMed] [Google Scholar]
- 20. Valtonen S, Timonen U, Toivanen P et al. . Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery. 1997;41(1): 44–49; discussion 48–49. [DOI] [PubMed] [Google Scholar]
- 21. Jackson S, Anders NM, Mangraviti A et al. . The effect of regadenoson-induced transient disruption of the blood–brain barrier on temozolomide delivery to normal rat brain. J Neurooncol. 2015;126(3):433–439 10.1007/s11060-015-1998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fenart L, Buée-Scherrer V, Descamps L et al. . Inhibition of P-glycoprotein: rapid assessment of its implication in blood-brain barrier integrity and drug transport to the brain by an in vitro model of the blood-brain barrier. Pharm Res. 1998;15(7):993–1000. [DOI] [PubMed] [Google Scholar]
- 23. Mitrasinovic S, Appelboom G, Detappe A et al. . Focused ultrasound to transiently disrupt the blood brain barrier. J Clin Neurosci. 2016;28:187–189. 10.1016/j.jocn.2015.12.011 [DOI] [PubMed] [Google Scholar]
- 24. Liu H-L, Hua M-Y, Chen P-Y et al. . Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment 1. Radiology. 2010;255(2):415–425 10.1148/radiol.10090699. [DOI] [PubMed] [Google Scholar]
- 25. Aryal M, Vykhodtseva N, Zhang Y-Z et al. . Multiple sessions of liposomal doxorubicin delivery via focused ultrasound mediated blood-brain barrier disruption: a safety study. J Control Release. 2015;204(C):60–69 10.1016/j.jconrel.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burgess A, Huang Y, Querbes W et al. . Focused ultrasound for targeted delivery of siRNA and efficient knockdown of Htt expression. J Control Release. 2012;163(2):125–129 10.1016/j.jconrel.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McDannold N, Vykhodtseva N, Jolesz FA et al. . MRI investigation of the threshold for thermally induced blood-brain barrier disruption and brain tissue damage in the rabbit brain. Magn Reson Med. 2004;51(5):913–923 10.1002/mrm.20060. [DOI] [PubMed] [Google Scholar]
- 28. Rodriguez A, Tatter S, Debinski W. Neurosurgical techniques for disruption of the blood–brain barrier for glioblastoma treatment. Pharmaceutics. 2015;7(3):175–187 10.3390/pharmaceutics7030175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sirsi SR, Borden MA. Advances in ultrasound mediated gene therapy using microbubble contrast agents. Theranostics. 2013;2(12):1208–1222 10.7150/thno.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Behrens S, Daffertshofer M, Spiegel D et al. . Low-frequency, low-intensity ultrasound accelerates thrombolysis through the skull. Ultrasound Med Biol. 1999;25(2):269–273. [DOI] [PubMed] [Google Scholar]
- 31. Sheikov N, McDannold N, Jolesz F et al. . Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood-brain barrier. Ultrasound Med Biol. 2006;32(9):1399–1409 10.1016/j.ultrasmedbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 32. Sheikov N, McDannold N, Sharma S et al. . Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med Biol. 2008;34(7):1093–1104 10.1016/j.ultrasmedbio.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wei K-C, Chu P-C, Wang H-YJ et al. . Focused ultrasound-induced blood–brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study. PLoS One. 2013;8(3):e58995. 10.1371/journal.pone.0058995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang S, Olumolade OO, Sun T et al. . Noninvasive, neuron-specific gene therapy can be facilitated by focused ultrasound and recombinant adeno-associated virus. Gene Therapy. 2014;22(1):104–110 10.1038/gt.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hsu P-H, Wei K-C, Huang C-Y et al. . Noninvasive and targeted gene delivery into the brain using microbubble-facilitated focused ultrasound. PLoS One. 2013;8(2):e57682. 10.1371/journal.pone.0057682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin C-Y, Hsieh H-Y, Pitt WG et al. . Focused ultrasound-induced blood-brain barrier opening for non-viral, non-invasive, and targeted gene delivery. J Control Release. 2015;212(C):1–9 10.1016/j.jconrel.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 37. Downs ME, Buch A, Sierra C et al. . Long-term safety of repeated blood-brain barrier opening via focused ultrasound with microbubbles in non-human primates performing a cognitive task. PLoS One. 2015;10(5):e0125911. 10.1371/journal.pone.0125911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ali IU, Chen X. Penetrating the blood–brain barrier: promise of novel nanoplatforms and delivery vehicles. ACS Nano. 2015;910:9470–9474 10.1021/acsnano.5b05341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Watson KD, Lai C-Y, Qin S et al. . Ultrasound increases nanoparticle delivery by reducing intratumoral pressure and increasing transport in epithelial and epithelial-mesenchymal transition tumors. Cancer Res. 2012;72(6):1485–1493 10.1158/0008-5472.CAN-11-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Samiotaki G, Konofagou EE. Dependence of the reversibility of focused- ultrasound-induced blood-brain barrier opening on pressure and pulse length in vivo. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60(11):2257–2265 10.1109/TUFFC.2013.6644731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Choi JJ, Selert K, Vlachos F et al. . Noninvasive and localized neuronal delivery using short ultrasonic pulses and microbubbles. Proc Natl Acad Sci U S A. 2011;108(40):16539–16544 10.1073/pnas.1105116108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Choi JJ, Feshitan JA, Baseri B et al. . Microbubble-size dependence of focused ultrasound-induced blood-brain barrier opening in mice in vivo. IEEE Trans Biomed Eng. 2010;57(1):145–154 10.1109/TBME.2009.2034533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xu Z, Carlson C, Snell J et al. . Intracranial inertial cavitation threshold and thermal ablation lesion creation using MRI-guided 220-kHz focused ultrasound surgery: preclinical investigation. J Neurosurg. 2015;122(1):152–161 10.3171/2014.9.JNS14541. [DOI] [PubMed] [Google Scholar]
- 44. Tung Y-S, Vlachos F, Choi JJ et al. . In vivo transcranial cavitation threshold detection during ultrasound-induced blood-brain barrier opening in mice. Phys Med Biol. 2010;55(20):6141–6155 10.1088/0031-9155/55/20/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marquet F, Teichert T, Wu S-Y et al. . Real-time, transcranial monitoring of safe blood-brain barrier opening in non-human primates. PLoS One. 2014;9(2):e84310. 10.1371/journal.pone.0084310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McDannold N, Arvanitis CD, Vykhodtseva N et al. . Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012;72(14):3652–3663 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lai C-Y, Fite BZ, Ferrara KW. Ultrasonic enhancement of drug penetration in solid tumors. Front Oncol. 2013;3:204. 10.3389/fonc.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gong W, Wang Z, Liu N et al. . Improving efficiency of adriamycin crossing blood brain barrier by combination of thermosensitive liposomes and hyperthermia. Biol Pharm Bull. 2011;34(7):1058–1064. [DOI] [PubMed] [Google Scholar]
- 49. Hergt R, Dutz S, Zeisberger M. Validity limits of the Néel relaxation model of magnetic nanoparticles for hyperthermia. Nanotechnology. 2010;21(1):015706. 10.1088/0957-4484/21/1/015706. [DOI] [PubMed] [Google Scholar]
- 50. Shah RR, Davis TP, Glover AL et al. . Impact of magnetic field parameters and iron oxide nanoparticle properties on heat generation for use in magnetic hyperthermia. J Magn Magn Mater. 2015;387:96–106 10.1016/j.jmmm.2015.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dan M, Bae Y, Pittman TA et al. . Alternating magnetic field-induced hyperthermia increases iron oxide nanoparticle cell association/uptake and flux in blood-brain barrier models. Pharm Res. 2015;32(5):1615–1625 10.1007/s11095-014-1561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Busquets M, Espargaró A, Sabaté R et al. . Magnetic nanoparticles cross the blood-brain barrier: when physics rises to a challenge. Nanomaterials. 2015;5(4):2231–2248 10.3390/nano5042231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moding EJ, Kastan MB, Kirsch DG. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat Rev Drug Discov. 2013;12(7):526–542 10.1038/nrd4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. De Salles AA, Gorgulho AA, Pereira JL et al. . Intracranial stereotactic radiosurgery. Neurosurg Clin N Am. 2013;24(4):491–498 10.1016/j.nec.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 55. Adler JR. The future of robotics in radiosurgery. Neurosurgery. 2013;72(suppl 1):8–11 10.1227/NEU.0b013e318271ff20. [DOI] [PubMed] [Google Scholar]
- 56. Fauquette W, Amourette C, Dehouck M-P et al. . Radiation-induced blood-brain barrier damages: an in vitro study. Brain Res. 2012;1433:114–126 10.1016/j.brainres.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 57. Yuan H, Gaber MW, McColgan T et al. . Radiation-induced permeability and leukocyte adhesion in the rat blood-brain barrier: modulation with anti-ICAM-1 antibodies. Brain Res. 2003;969(1-2):59–69. [DOI] [PubMed] [Google Scholar]
- 58. Sándor N, Walter FR, Bocsik A et al. . Low dose cranial irradiation-induced cerebrovascular damage is reversible in mice. PLoS One. 2014;9(11):e112397. 10.1371/journal.pone.0112397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yuan H, Gaber MW, Boyd K et al. . Effects of fractionated radiation on the brain vasculature in a murine model: blood-brain barrier permeability, astrocyte proliferation, and ultrastructural changes. Int J Radiat Oncol Biol Phys. 2006;66(3):860–866 10.1016/j.ijrobp.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 60. Gaber MW, Yuan H, Killmar JT et al. . An intravital microscopy study of radiation-induced changes in permeability and leukocyte-endothelial cell interactions in the microvessels of the rat pia mater and cremaster muscle. Brain Res Brain Res Protoc. 2004;13(1):1–10 10.1016/j.brainresprot.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 61. Kunjachan S, Detappe A, Kumar R et al. . Nanoparticle mediated tumor vascular disruption: a novel strategy in radiation therapy. Nano Lett. 2015;15(11):7488–7496 10.1021/acs.nanolett.5b03073. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62. Joh DY, Sun L, Stangl M et al. . Selective targeting of brain tumors with gold nanoparticle-induced radiosensitization. PLoS One. 2013;8(4):e62425. 10.1371/journal.pone.0062425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Davies AM, Weinberg U, Palti Y. Tumor treating fields: a new frontier in cancer therapy. Ann N Y Acad Sci. 2013;1291(1):86–95 10.1111/nyas.12112. [DOI] [PubMed] [Google Scholar]
- 64. Miklavčič D, Mali B, Kos B et al. . Electrochemotherapy: from the drawing board into medical practice. Biomed Eng Online. 2014;13(1):29. 10.1186/1475-925X-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Linnert M, Iversen HK, Gehl J. Multiple brain metastases—current management and perspectives for treatment with electrochemotherapy. Radiol Oncol. 2012;46(4):271–278 10.2478/v10019-012-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Neal RE, Rossmeisl JH, D'Alfonso V et al. . In vitro and numerical support for combinatorial irreversible electroporation and electrochemotherapy glioma treatment. Ann Biomed Eng. 2013;42(3):475–487 10.1007/s10439-013-0923-2. [DOI] [PubMed] [Google Scholar]
- 67. Leuthardt EC, Duan C, Kim MJ et al. . Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS One. 2016;11(2):e0148613. 10.1371/journal.pone.0148613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hawasli AH, Bagade S, Shimony JS et al. . Magnetic resonance imaging-guided focused laser interstitial thermal therapy for intracranial lesions. Neurosurgery. 2013;73(6):1007–1017 10.1227/NEU.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hawasli AH, Ray WZ, Murphy RKJ et al. . Magnetic resonance imaging-guided focused laser interstitial thermal therapy for subinsular metastatic adenocarcinoma: technical case report. Neurosurgery. 2012;70:332–337; discussion 338 10.1227/NEU.0b013e318232fc90. [DOI] [PubMed] [Google Scholar]
- 70. Zhang F, Xu C-L, Liu C-M. Drug delivery strategies to enhance the permeability of the blood-brain barrier for treatment of glioma. Drug Des Devel Ther. 2015;9:2089–2100 10.2147/DDDT.S79592. [DOI] [PMC free article] [PubMed] [Google Scholar]