Abstract
Background.
Patients with malignant gliomas present with variation in neurocognitive function (NCF) not attributable to lesion size or location alone. A potential contributor is the rate at which tumors grow, or “lesion momentum.” Isocitrate dehydrogenase 1 wild type (IDH1-WT) are more proliferative and aggressive than IDH1-mutant (IDH1-M) tumors. We hypothesized that patients with IDH1-WT would exhibit worse NCF than patients with IDH1-M tumors.
Methods.
Comprehensive NCF testing was completed in 119 patients with malignant glioma prior to surgical resection. IDH1 status was determined with immunohistochemistry and sequencing. Rates of impairment and mean test performances were compared by IDH1.
Results.
NCF impairment was significantly more frequent in patients with IDH1-WT tumors in memory, processing speed, visuoconstruction, language, executive functioning, and manual dexterity. Mean performances of patients with IDH1-WT were also significantly lower than those with IDH1-M tumors on measures of learning and memory, processing speed, language, executive functioning, and dexterity. Lesion volume was not statistically different between IDH1-WT and IDH1-M tumors. Tumor and lesion volume on T1-weighted and fluid attenuated inversion recovery MRI were significantly associated with most NCF tests in patients with IDH1-WT, but only significantly associated with a single measure in patients with IDH1-M tumors.
Conclusion.
Patients with IDH1-WT show reduced NCF compared with those with IDH1-M malignant gliomas. Lesion volume is inversely associated with NCF for patients with IDH1-WT, but not IDH1-M tumors. These findings are consistent with the hypothesis that patients with IDH1-WT tumors present with more severe NCF impairment due to greater lesion momentum, which may impede compensatory neuroplasticity and cerebral reorganization.
Keywords: brain tumor, cognition, glioma, genetic marker, neuropsychology.
Malignant gliomas represent a group of aggressive brain tumors, including World Health Organization (WHO) grade III anaplastic astrocytoma (AA), anaplastic oligodendroglioma, and WHO grade IV glioblastoma (GBM).1 Traditional grading is based upon the histopathological characteristics of the tumors, with GBM exhibiting both necrosis and microvascular proliferation, and AA and anaplastic oligodendroglioma characterized predominantly by microvascular proliferation. While tumor grade is predictive of survival, proposed classification schemes based upon recently discovered tumor molecular markers are more highly prognostic and better predictors of growth kinetics, regardless of grade and histology.4,5 Accordingly, numerous proposals call for a move away from traditional histological grading and toward genetic tumor profiling to further refine tumor classification.6–9 Additionally, immunohistochemical scoring of genetic markers in gliomas is less susceptible to interrater disagreement and less dependent upon volume of tissue available than histological grading.10,11 A very promising genetic marker in malignant gliomas is mutation of the isocitrate dehydrogenase 1 (IDH1) gene, first identified in gliomas about a decade ago.4,12
IDH1 is an enzyme that catalyzes the oxidative decarboxylation of isocitrate to α-ketoglutarate, which is critical to cellular protection from oxidative stress.12–14 Up to 70% of AA and 5% to 10% of primary GBM have mutant variants of the gene (IDH1-M), and over 80% of secondary GBM are also IDH1-M.15–17 IDH2, a related homolog, is mutated in rare cases, but over 90% of mutations in gliomas are in IDH1.17 Since its discovery, numerous studies have reported that patients with IDH1-M gliomas exhibit a significant survival benefit over patients with the wild-type tumor gene (IDH1-WT).13,18,19 Importantly, while younger patients with malignant glioma are more likely to have IDH1-M tumors, the survival benefit of the mutation appears to be independent of age and histological grading.19 The IDH1-M gene also predicts concurrent genetic alterations, such as 1p/19q codeletion and O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation.20–22 IDH1 status is also homogeneous within the tumor and stable throughout the disease course, unlike histological tumor grade, which can evolve over time and may vary regionally within a tumor specimen.10,23 In light of these findings, it has been suggested that IDH1 status be utilized as a classifier of etiological subtypes of gliomas.13,19,24–28
Variation in growth characteristics between IDH1 subtypes may contribute to survival differences and the spectrum of a patient’s presenting symptoms. Proliferation and dispersion characteristics, including the rate of tumor cell doubling and extent of invasion, contribute to overall “lesion momentum,” which broadly refers to the growth kinetics and aggressiveness of a tumor’s evolution. Importantly, lesion momentum varies by IDH1 subtype with implications for outcome. IDH1-M gliomas have a more diffuse pattern of growth, which has been associated with better prognosis.4 Additionally, the proliferation rate is approximately 2.5 times greater in IDH1-WT tumors compared with IDH1-M tumors. Thus, the broader dispersion and slower proliferation characteristic of IDH1-M gliomas (i.e., lesser lesion momentum) may prolong time to fatal disease burden, helping explain the observed survival benefit.
Differences in lesion momentum may also impact cerebral plasticity. Neuroplastic potential following brain injury appears to relate to the temporal pattern of the acquired damage. Broad topographical reorganization of cortical functions has been documented in patients with low-grade glioma, which has been associated with neurocognitive function (NCF) outcomes.29 It has been suggested that the chronic and slowly progressive nature of these tumors allows more time for neuroplastic reorganization and greater preservation of NCF than more acute neurological disease. While investigations regarding neuroplastic changes in high-grade glioma are lacking, it is plausible that differences in lesion momentum may influence neuroplastic potential and, in turn, NCF outcomes. More specifically, the lower lesion momentum of IDH1-M tumors may create an environment favorable for neuroplasticity. This is contrasted with the rapid proliferation characteristic of IDH1-WT tumors, potentially limiting time for plastic reorganization and resulting in more frequent and severe impairment of NCF.
No prior investigation has examined relationships between IDH1 subtypes of malignant gliomas and NCF. The present study characterizes NCF associated with IDH1 status in patients with malignant gliomas prior to surgical resection. The focus on malignant astrocytomas, specifically grade III AA and grade IV GBM, is deliberate, as prior studies indicate that IDH1-WT grade III and grade IV tumors represent a homogeneous subtype with nearly identical time to progression and survival.17,19 Given the characteristically less aggressive IDH1-M tumors, we hypothesized that the NCF of patients with IDH1-M malignant gliomas would be characterized by less frequent and less severe impairment than that of patients with IDH1-WT tumors. We further examined relationships among radiographic measures of lesion volume, NCF, and IDH1 status.
Methods
Participants
Patients with a centrally reviewed diagnosis of supratentorial malignant glioma (AA or GBM) whose first therapeutic intervention was an open surgical resection at The University of Texas MD Anderson Cancer Center (MDACC) between June 1993 and April 2009 were considered for inclusion. Patients must have had paraffin-embedded tissue scored for IDH1 as described in our prior publication.2 These patients were then cross-referenced with the Section of Neuropsychology database. One hundred and nineteen patients were identified who met criteria2 and completed detailed presurgical neuropsychological evaluations. The MDACC institutional review board approved the study. Methods of performing IDH1 R132H immunohistochemistry and exon 4 DNA sequencing have been previously described.2 Given the rarity of IDH2 mutations in astrocytic gliomas and sample limitations,30 IDH2 was not scored.
Lesion Characteristics
MRI volume calculations were performed using Vitrea 2 three-dimensional volumetric software (Vital Images). Personnel scoring the lesion volumes were blinded to molecular stratification. T1-weighted tumor volume was defined as the greater of the hypointense region on T1-weighted MRI or the hyperintense area on gadolinium-enhanced T1-weighted MRI. Presence of enhancement on T1-weighted images post-gadolinium contrast was recorded. Fluid attenuated inversion recovery (FLAIR) volume was defined as the area of hyperintensity identified on the T2 FLAIR MRI sequence and was considered representative of total lesion volume, including tumor and perilesional edema. Tumor location was categorized as frontal, temporal, parietal, insular, or occipital according to the primary location of the tumor. Multifocal tumors were assigned location based on the largest component of the tumor mass.
Neurocognitive Assessment
NCF testing was conducted by a neuropsychologist or a trained neuropsychology staff member (ie, psychometrist or neuropsychology fellow) under the supervision of a neuropsychologist as part of a comprehensive presurgical evaluation. Table 1 lists the neuropsychological tests by domain that were routinely included in the clinical test battery, which utilized a flexible approach for clinical purposes. As previously described,3 NCF test scores were standardized using published normative data and converted into demographically adjusted z-scores (mean = 0, standard deviation = 1). Demographic corrections included stratification of all normative data by age, in addition to gender, handedness, and level of education when appropriate. Per convention, performance on an individual NCF test that fell at or below a z-score of −1.5 was considered impaired.
Table 1.
Neurocognitive tests grouped by principal domain
| Measure | Abbreviations | Norms† |
|---|---|---|
| Attention | ||
| WAIS-R/III Digit Span | Digit Span | Wechsler, 1981; Wechsler, 1997 |
| Learning and Memory | ||
| HVLT-R Total Recall | HVLT-R TR | Benedict, Schretlen, Groninger, & Brandt, 1998 |
| HVLT-R Delayed Recall | HVLT-R DR | Benedict, Schretlen, Groninger, & Brandt, 1998 |
| HVLT-R Recognition Discrimination | HVLT-R Recog | Benedict, Schretlen, Groninger, & Brandt, 1998 |
| Processing Speed | ||
| WAIS-R/III Digit Symbol | Digit Symbol | Wechsler, 1981; Wechsler, 1997 |
| Trail Making Test Part A | TMTA | Tombaugh, 2004 |
| Executive Function | ||
| Trail Making Test Part B | TMTB | Tombaugh, 2004 |
| WAIS-R/III Similarities | Similarities | Wechsler, 1981; Wechsler, 1997 |
| MAE Controlled Oral Word Association | COWA | Ruff, Light, Parker, & Levin, 1996 |
| Language | ||
| MAE Token Test | Token | Benton, Hamsher, & Sivan, 2000 |
| MAE Visual Naming Test or Boston Naming Test | Naming | Benton, Hamsher, & Sivan, 2000; Heaton, Miller, Taylor, & Grant, 2004 |
| Visuospatial Function | ||
| WAIS-R/III Block Design | Block Design | Wechsler, 1981; Wechsler, 1997 |
| Motor Function | ||
| Grooved Pegboard- Left Hand | Peg- Left | Heaton, Miller, Taylor, Grant, 2004 |
| Grooved Pegboard- Right Hand | Peg- Right | Heaton, Miller, Taylor, Grant, 2004 |
Abbreviations: WAIS-R, Wechsler Adult Intelligence Scale–Revised; WAIS-III, Wechsler Adult Intelligence Scale–Third Edition; MAE, Multilingual Aphasia Examination.
†As described and cited in Noll et al.3 Adapted from Ref.3 and used with permission.
Statistical Analysis
Descriptive statistics for demographic and clinical variables were calculated as means and standard deviations or frequencies and percentages where appropriate. Independent samples t-tests and chi-square goodness of fit tests were used to compare differences in demographic and clinical characteristics between IDH1-WT and IDH1-M groups. Rates of NCF impairment were compared between IDH1 groups with chi-square goodness of fit tests. Mean NCF test z-scores were compared across groups with independent samples t-tests. Effect sizes were measured with the Cohen’s d statistic. Using Cohen’s convention, d-values of .2, .5, and .8 correspond to small, medium, and large effect sizes, respectively. Associations between NCF, functional status, and lesion characteristics were determined with Pearson product-moment (r) or point-biserial (rpb) correlations for each IDH1 group. Histopathological grading is known to vary by IDH1 subtype15; thus, histology was not statistically controlled for, since including histology as a covariate would simultaneously suppress the effect of IDH1 status given their collinearity. All statistical analyses were performed with SPSS 21.0 (IBM). Two-sided tests were used with a significance level of P ≤ .05.
Results
Demographic and Clinical Characteristics
Sample sociodemographic and clinical characteristics are presented by IDH1 status in Table 2. Sex, ethnicity, education, and handedness did not significantly differ between groups. As expected, patients with IDH1-WT tumors were significantly older and had more frequent GBM diagnoses than those with IDH1-M tumors. Lesion location, including hemisphere and cerebral region involved, did not differ between IDH1 groups. Lesion volume on FLAIR MRI was similar across groups, though T1-weighted volume was significantly smaller in the IDH1-WT group than the IDH1-M group.
Table 2.
Demographic and clinical characteristics
| IDH1 Wild Type (N = 66) |
IDH1 Mutant (N = 53) |
P valuea | |
|---|---|---|---|
| Age, y | |||
| Mean (SD) | 54.4 (13.8) | 37.7 (10.3) | <.001* |
| Range | 22 – 84 | 19 – 66 | |
| Male (N, %) | 38 (56) | 29 (53) | .853 |
| White (N, %) | 58 (88) | 49 (92) | .410 |
| Education, years | |||
| Mean (SD) | 14.8 (2.8) | 14.4 (2.9) | |
| Range | 9–20 | 11–19 | .424 |
| Histology, (N, %) | |||
| Glioblastoma | 43 (65) | 9 (17) | <.001* |
| Anaplastic astrocytoma | 23 (35) | 44 (83) | |
| Hemisphere | |||
| Left (N, %) | 38 (58) | 38 (72) | .128 |
| Region (N, %) | |||
| Frontal | 28 (42) | 25 (47) | .532 |
| Temporal | 29 (44) | 24 (45) | |
| Parietal | 7 (11) | 3 (6) | |
| Insular | 0 (0) | 1 (2) | |
| Occipital | 2 (3) | 0 (0) | |
| Lesion volume, cm3 | |||
| T1-weighted, Mean (SD)b | 42.4 (29.0) | 63.3 (47.0) | .006* |
| FLAIR volume, Mean (SD)c | 77.5 (48.4) | 77.9 (53.6) | .964 |
| KPS (N, %) | |||
| ≥ 90 | 41 (62) | 49 (92) | <.001* |
| 70 – 80 | 24 (36) | 4 (8) | |
| 60 | 1 (2) | 0 (0) | |
aContinuous variables compared with independent samples t-tests; Categorical variables compared with chi-square goodness of fit tests.
bWild N = 64; Mutant N = 53.
cWild N = 63; Mutant N = 53.
*Significant, P ≤ .05.
Neurocognitive Performances
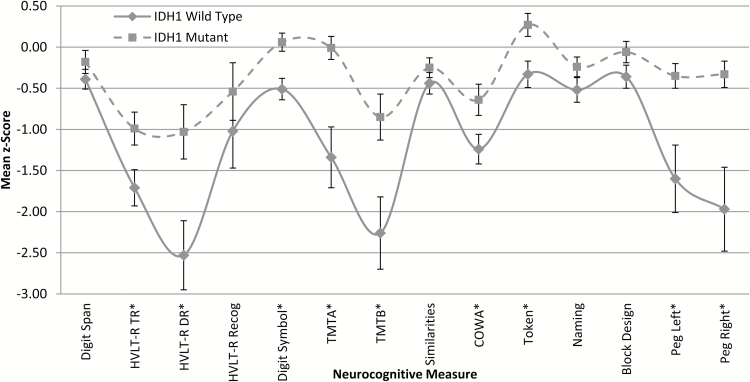
NCF test performances by IDH1 status are presented in Fig. 2, with effect sizes shown in Table 3. Mean performances significantly differed across groups on 12 of 14 measures with generally medium to large effect sizes (d = −0.43 to −1.04). Patients with IDH1-WT gliomas performed significantly worse than patients with IDH1-M tumors on measures of verbal learning and memory (HVLT–R TR, DR), processing speed (Digit Symbol; TMTA), executive function (TMTB; COWA), auditory comprehension (Token), and manual dexterity (Peg- Left; Peg- Right).
Fig. 2.
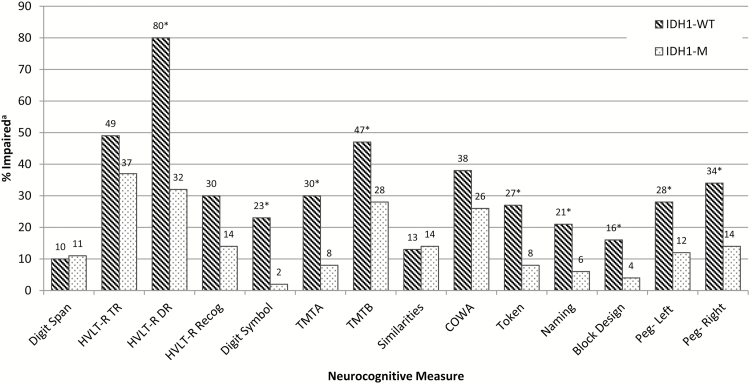
Neurocognitive impairment by IDH1 status. aImpairment defined as a z-score ≤ −1.5 for all individual measure. *Significant difference between groups, P ≤ .05; chi-square goodness of fit tests. See Table 3 for sample sizes across measure.
Table 3.
Effect sizes for differences in neurocognitive performances by IDH1 status
| Domain and Test | IDH1 Wild Type | IDH1 Mutant | Effect Sizea | P valueb | ||
|---|---|---|---|---|---|---|
| N | z-score M (SD) | N | z-score M (SD) | |||
| Attention | ||||||
| Digit Span | 63 | –0.39 (0.93) | 53 | –0.18 (1.03) | –0.29 | .250 |
| Learning and Memory | ||||||
| HVLT-R TR | 63 | –1.71 (1.73) | 51 | –0.99 (1.42) | –0.45 | .018* |
| HVLT-R DR | 10 | –2.53 (1.32) | 22 | –1.03 (1.55) | –1.04 | .013* |
| HVLT-R Recog | 10 | –1.02 (1.43) | 22 | –0.54 (1.66) | –0.30 | .434 |
| Processing Speed | ||||||
| Digit Symbol | 62 | –0.51 (1.05) | 53 | 0.56 (0.81) | –0.61 | .002* |
| TMTA | 63 | –1.34 (2.93) | 53 | –0.01 (1.00) | –0.63 | .001* |
| Executive Function | ||||||
| TMTB | 55 | –2.26 (3.23) | 53 | –0.85 (2.06) | –0.52 | .008* |
| Similarities | 62 | –0.42 (1.04) | 52 | –0.25 (0.84) | –0.18 | .331 |
| COWA | 66 | –1.23 (1.47) | 50 | –0.64 (1.32) | –0.43 | .025* |
| Language | ||||||
| Token | 64 | –0.33 (1.32) | 49 | 0.27 (0.97) | –0.53 | .006* |
| Naming | 61 | –0.52 (1.18) | 50 | –0.24 (0.87) | –0.28 | .147 |
| Visuospatial Function | ||||||
| Block Design | 63 | –0.36 (1.11) | 52 | –0.06 (0.91) | –0.30 | .118 |
| Motor Function | ||||||
| Peg- Left | 60 | –1.60 (3.19) | 51 | –0.35 (1.07) | –0.54 | .006* |
| Peg- Right | 62 | –1.97 (4.03) | 52 | –0.33 (1.18) | –0.57 | .003* |
aEffect sizes represent Cohen’s d.
bIndependent samples t-tests used for group comparisons.
*Significant, P ≤ .05.
For the overall sample, 19% were unimpaired across all measures, 39% exhibited impaired performances on 1 to 2 measures, 24% on 3 to 4 measures, and 18% on 5 or more measures. For patients with IDH1-WT tumors, 17% were unimpaired, 27% showed impairment on 1 to 2 measures, 30% on 3 to 4 measures, and 26% on 5 or more measures. For those with IDH1-M tumors, 23% were unimpaired, 53% showed impairment on 1 to 2 measures, 15% on 3 to 4 measures, and 9% on 5 or more measures. The rate of impairment on 3 or more measures was significantly greater in patients with IDH1-WT than IDH1-M tumors (56% vs 25%, P < .001). Impairment on 5 or more measures was also significantly greater in patients with IDH1-WT than IDH1-M tumors (26% vs 9%, P = .023).
Rates of impairment on individual measures are presented in Fig. 1. Patients with IDH1-WT gliomas showed most frequent impairment on measures of verbal learning and memory (HVLT-R TR; DR) and executive functioning (TMTB; COWA). Nonetheless, impairment was common across all measures, with rates greater than 20% on 11 of 14 tests. Patients with IDH1-M tumors were also most frequently impaired on verbal learning and memory (HVLT-R TR; DR) and executive functioning (TMTB; COWA), though rates exceeded 20% on only 4 of 14 measures. Significant differences in impairment rates across IDH1 groups were identified on 9 of 14 tests administered. Specifically, patients with IDH1-WT tumors showed greater impairment frequency than patients with IDH1-M tumors on measures of verbal memory (HVLT-R DR, P = .011), processing speed (Digit Symbol, P = .001; TMTA, P = .002), executive functioning (TMTB, P = .042), auditory comprehension (Token, P = .013), object naming (Naming, P = .022), visuoconstruction (Block Design, P = .036), and manual dexterity (Peg- Left, P = .032; Peg- Right, P = .012).
Fig. 1.
Mean neurocognitive performances by IDH1 status. Independent samples t-tests used for group comparisons. Error bars represent standard error of the mean. See Table 3 for sample sizes across measures. *Significant, P ≤ .05.
Functional Status
A significantly greater proportion of patients with IDH1-WT tumors had KPS scores of 80 or lower compared with patients with IDH1-M tumors. In patients with IDH1-WT tumors, KPS scores were significantly associated with manual dexterity only; Peg- Right, r(65) = 0.40, P = .001; Peg- Left, r(65) = 0.34, P = .005. KPS scores were significantly associated with a single measures of executive functioning (COWA, r(47) = 0.32, P = .024) in the IDH1-M group.
Lesion Characteristics
Associations between measures of lesion volume and NCF are presented in Table 4. For patients with IDH1-WT tumors, T1-weighted and FLAIR MRI volumes were significantly associated with most measures of NCF. Specifically, T1-weighted tumor volume showed significant inverse associations with 8 of 14 tests, with correlation coefficients (r) ranging from −0.27 (TMTA) to −0.56 (TMTB). Associations with total lesion volume on FLAIR sequences were significant across 12 of 14 tests, ranging from −0.33 (Token) to −0.55 (TMTA). Only a single measure (Naming) was associated with T1-weighted (−0.35) or FLAIR volume (−0.45) in the patients with IDH1-M gliomas. Tumors were more frequently enhancing in patients with IDH1-WT than IDH1-M lesions (72% vs 33%, P < .001), though presence of enhancement was not associated with NCF for patients with IDH1-WT or IDH1-M lesions. Overall, lesion size from both FLAIR and T1-weighted volumes was associated with NCF, but only in patients with IDH1-WT tumors, while presence of enhancement was not associated with NCF for either group.
Table 4.
Associations between neurocognitive performances and lesion volume by IDH1 status†
| Domain and Test | IDH1-WT MRI Lesion Volume | IDH1-M MRI Lesion Volume | ||
|---|---|---|---|---|
| T1-Weighted | FLAIR | T1-Weighted | FLAIR | |
| Attention | ||||
| Digit Span | –0.12 | –0.36*** | 0.15 | 0.11 |
| Learning and Memory | ||||
| HVLT-R TR | –0.34** | –0.41*** | –0.06 | –0.02 |
| HVLT-R DR | –0.14 | 0.09 | 0.00 | –0.04 |
| HVLT-R Recog | –0.33 | –0.06 | –0.20 | –0.20 |
| Processing Speed | ||||
| Digit Symbol | –0.35** | –0.46*** | –0.01 | 0.00 |
| TMTA | –0.27* | –0.55*** | 0.08 | 0.01 |
| Executive Function | ||||
| TMTB | –0.56*** | –0.40*** | –0.08 | –0.14 |
| Similarities | –0.23 | –0.45*** | –0.17 | –0.20 |
| COWA | –0.23 | –0.45*** | –0.09 | –0.09 |
| Language | ||||
| Token | –0.19 | –0.33** | –0.04 | –0.14 |
| Naming | –0.35*** | –0.43*** | –0.35* | –0.45*** |
| Visuospatial Function | ||||
| Block Design | –0.37** | –0.48*** | –0.19 | –0.21 |
| Motor Function | ||||
| Peg- Left | –0.47*** | –0.39*** | –0.07 | –0.04 |
| Peg- Right | –0.40*** | –0.51*** | –0.07 | –0.03 |
†Correlations reflect Pearson product-moment correlations (r).
Note. Significant: *P ≤ .05, **P ≤ .01, ***P ≤ .005.
Discussion
This study represents the first characterization of relationships between NCF and IDH1 genetic mutation status in patients with newly diagnosed malignant gliomas. In line with prior studies of NCF and glioma,31 impairment was common in the overall sample, with 81% of all patients exhibiting impairment on at least one measure. While rates of impairment on one or more NCF tests were similar between patients with IDH1-WT (83%) and IDH1-M (77%) glioma, patients with IDH1-WT tumors were significantly more likely to be impaired on 3 or more (56% vs 25%) and 5 or more tests (26% vs 9%). Patients with IDH1-WT tumors were significantly more frequently impaired on individual measures of verbal memory, processing speed, executive function, language, visuoconstruction, and manual dexterity. Similarly, patients with IDH1-WT malignant gliomas exhibited more severe NCF deficits than those with IDH1-M tumors across most NCF domains with medium to large effect sizes. Accordingly, the NCF of IDH1-WT gliomas is characterized by more frequent and severe NCF impairment than patients with IDH1-M tumors. The results also demonstrate that patients with IDH1-WT tumors have lower physician-rated performance status than those with IDH1-M tumors, indicating greater impairment in both NCF and daily activities than their IDH1-M counterparts. Functional status also showed moderate associations with manual dexterity in patients with IDH1-WT tumors, consistent with the importance of motor functioning to patient daily functioning.
Importantly, FLAIR volume was similar between IDH1 groups, suggesting that worse NCF in the IDH1-WT group cannot be attributed to greater overall lesion size. Indeed, tumor volume on T1-weighted MRI was actually significantly smaller in patients with IDH1-WT gliomas, despite the fact that these patients exhibited worse NCF. Tumors were distributed throughout similar brain regions across IDH1 groups, most commonly in the frontal and temporal lobes, consistent with prior observations in malignant gliomas.32 Taken together, these findings suggest that factors other than lesion size and location, such as lesion momentum, play an important role in the etiology of NCF impairment in patients with malignant gliomas. That is, the slower proliferation rate of IDH1-M tumors may allow for greater neuroplasticity by offering the brain more time for reorganization in response to invading tumor.33
Lending additional support to the tumor momentum hypothesis is the finding of differences in associations between NCF and radiographically determined lesion size by IDH1 mutation status. T1-weighted and FLAIR MRI lesion size exhibited strong inverse associations with most NCF measures in patients with IDH1-WT tumors, but a similar pattern was not evident in patients with IDH1-M tumors. Additionally, tumors were more frequently enhancing in the IDH1-WT group, suggesting a more nodular lesion, though presence of enhancement was not associated with NCF for either group. Accordingly, rapidity of growth is likely to play a more important role in development of NCF impairment than lesion size and nodularity.
The rapid proliferation of IDH1-WT tumors likely limits time for functional reorganization. As such, growth of IDH1-WT gliomas is accompanied by greater NCF dysfunction, as the brain is unable to compensate in the face of rapid growth. On the other hand, IDH1-M gliomas are more diffuse and proliferate at a slower rate, allowing for greater functional reorganization. Accordingly, tumor growth does not always cause the same degree of NCF worsening in patients with IDH1-M gliomas, explaining the lack of association between lesion size and NCF in these patients. Moving forward, incorporation of functional neuroimaging techniques (eg, functional MRI) would be helpful in understanding potential differences in neuroplastic reorganization in patients with malignant gliomas of differing molecular subtypes. More precise segmentation of tumor compartments (necrosis, edema, etc) would also improve understanding of the relationships between lesion characteristics and NCF across IDH1 subtypes.
Consistent with previous reports, IDH1-M malignant gliomas were more common in younger patients and those with AA histology.12,15–18 NCF test performances were adjusted for age among other demographic variables; thus, differences in age do not account for the large differences in NCF between IDH1 groups. Group comparisons deliberately did not control for histology, as the difference in distribution of AA and GBM tumors by IDH1 mutation status is reflective of the known etiological differences in tumor genesis by IDH1 subtype. Specifically, the IDH1-M gene is found in a majority of AA and only a minority of primary GBM, despite IDH1 status representing a more homogeneous grouping of tumors with similar etiology, growth kinetics, and survival compared with traditional grading.20–22 Accordingly, statistical control of histology would, in effect, simultaneously suppress the effect of IDH1 status, the variable of primary interest.
NCF outcomes are increasingly incorporated into clinical trials to aid in establishing the clinical benefit, therapeutic efficacy, and treatment safety of brain tumor therapies. Further, trials focused on preserving or enhancing cognitive function clearly will require consideration of stratification factors related to cognition. Given the strong associations between IDH1 status and NCF, as well as the differences in the associations between NCF and lesion characteristics between the 2 molecular subtypes, it is important to consider potential impact of tumor molecular subtype (eg, stratification) in clinical trials incorporating NCF outcomes.
It should be noted that differences in sample size by measure administered presents a challenge in this sample of clinic patients. Relatively few patients had data regarding delayed memory (HVLT-R DR and Recognition). As such, the lack of difference on some test variables (eg, HVLT-R Recognition) may reflect reduced power, particularly in light of the sizable differences between IDH1 groups on other verbal memory indices. Cross-validation in an independent sample will be of great importance and likely possible in the near future given the more common practice of evaluating IDH1 genotype and incorporating NCF outcomes in modern neuro-oncological practice. As molecular neuropathology continues to identify important variants for tumor growth and survival, it is likely that additional associations between tumor biology and NCF will be identified. Indeed, recent work suggests that combined classification by IDH1 mutation and 1p/19q deletion status may further refine subgrouping, again regardless of grade and histology.34 While this additional genetic marker was unavailable for the present analyses, future work regarding NCF in gliomas should strive to evaluate these and other prognostic molecular markers.
In sum, NCF impairment is common in patients with malignant gliomas. Here we show for the first time that the frequency and severity of impairment vary according to IDH1 gene mutation status, as patients with IDH1-WT tumors exhibit greater preoperative NCF impairment than those with IDH1-M tumors. Further, NCF was inversely associated with lesion volume only in patients with IDH1-WT tumors, suggesting that the greater lesion momentum characteristic of this tumor subtype overwhelms compensatory neuroplasticity, thereby leading to greater NCF dysfunction. We speculate that this difference in NCF will persist across the postoperative adjuvant treatment and survivorship period. Longitudinal investigations are ongoing to evaluate this hypothesis.
Funding
Research reported in this publication was supported by the National Institutes of Health through the National Institute of Nursing Research award number R01NR014195 (J.S.W.), National Institutes of Neurological Disorders and Stroke award number K08NS070928 (G.R.), and National Cancer Institute SPORE award number P50CA165962 (D.P.C.). Additional support was received from the Burroughs Wellcome Fund Career Award (D.P.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Burroughs Wellcome Fund.
Acknowledgments
This work was previously presented as a rapid report at the 19th annual Scientific Meeting and Education Day of the Society for Neuro-Oncology, Miami Beach, Florida, November 16, 2014.
Conflict of interest statement. The authors report no conflicts of interest.
References
- 1. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beiko J, Suki D, Hess KR, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Noll KR, Sullaway C, Ziu M, et al. Relationships between tumor grade and neurocognitive functioning in patients with glioma of the left temporal lobe prior to surgical resection. Neuro Oncol. 2015;17(4):580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baldock AL, Yagle K, Born DE, et al. Invasion and proliferation kinetics in enhancing gliomas predict IDH1 mutation status. Neuro Oncol. 2014;16(6):779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ceccarelli M, Barthel FP, Malta TM, et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nobusawa S, Watanabe T, Kleihues P, et al. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15(19):6002–6007. [DOI] [PubMed] [Google Scholar]
- 7. Weller M, Wick W, von Deimling A. Isocitrate dehydrogenase mutations: a challenge to traditional views on the genesis and malignant progression of gliomas. Glia. 2011;59(8):1200–1204. [DOI] [PubMed] [Google Scholar]
- 8. Lai A, Kharbanda S, Pope WB, et al. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol. 2011;29(3):4482–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res 2013;19(4):764–772. [DOI] [PubMed] [Google Scholar]
- 10. Glantz MJ, Burger PC, Herndon JE, et al. Influence of the type of surgery on the histologic diagnosis in patients with anaplastic gliomas. Neurology. 1991;41(11):1741–1741. [DOI] [PubMed] [Google Scholar]
- 11. Kim BY, Jiang W, Beiko J, et al. Diagnostic discrepancies in malignant astrocytoma due to limited small pathological tumor sample can be overcome by IDH1 testing. J Neurooncol. 2014;118(2):405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hodges TR, Cho BD, Bigner DD, et al. Isocitrate dehydrogenase 1 (IDH1): what it means to the neurosurgeon. J Neurosurg 2013;118(6):1176–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheng HB, Yue W, Xie C, et al. IDH1 mutation is associated with improved overall survival in patients with glioblastoma: a meta-analysis. Tumor Biol. 2013;34(6):3555–3559. [DOI] [PubMed] [Google Scholar]
- 14. Reitman ZJ, Yan H. Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism. J Natl Cancer Inst. 2010;102(13):932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kloosterhof NK, Bralten LB, Dubbink HJ, et al. Isocitrate dehydrogenase-1 mutations: a fundamentally new understanding of diffuse glioma?. Lancet Oncol. 2011;12(1):83–91. [DOI] [PubMed] [Google Scholar]
- 16. Ichimura K, Pearson DM, Kocialkowski S, et al. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol. 2009;11(4):341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27(25):4150–4154. [DOI] [PubMed] [Google Scholar]
- 19. Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120(6):707–718. [DOI] [PubMed] [Google Scholar]
- 20. Zhang C, Moore LM, Li X, et al. IDH1/2 mutations target a key hallmark of cancer by deregulating cellular metabolism in glioma. Neuro Oncol. 2013;15(9):1114–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirose Y, Sasaki H, Abe M, et al. Subgrouping of gliomas on the basis of genetic profiles. Brain Tumor Pathol. 2013;30(4):203–208. [DOI] [PubMed] [Google Scholar]
- 22. Gravendeel LA, Kouwenhoven MC, Gevaert O, et al. Intrinsic gene expression profiles of gliomas are a better predictor of survival than histology. Cancer Res. 2009;69(23):9065–9072. [DOI] [PubMed] [Google Scholar]
- 23. Prayson RA, Agamanolis DP, Cohen ML, et al. Interobserver reproducibility among neuropathologists and surgical pathologists in fibrillary astrocytoma grading. J Neurol Sci. 2000;175(1):33–39. [DOI] [PubMed] [Google Scholar]
- 24. Theeler BJ, Yung WA, Fuller GN, et al. Moving toward molecular classification of diffuse gliomas in adults. Neurology. 2012;79(18):1917–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874–5880. [DOI] [PubMed] [Google Scholar]
- 26. Weller M, Felsberg J, Hartmann C, et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol. 2009;27(34):5743–5750. [DOI] [PubMed] [Google Scholar]
- 27. Hegi ME, Janzer RC, Lambiv WL, et al. Presence of an oligodendroglioma-like component in newly diagnosed glioblastoma identifies a pathogenetically heterogeneous subgroup and lacks prognostic value: central pathology review of the EORTC_26981/NCIC_CE. 3 trial. Acta Neuropathol. 2012;123(6):841–852. [DOI] [PubMed] [Google Scholar]
- 28. Kayl AE, Meyers CA. Does brain tumor histology influence cognitive function? Neuro Oncol. 2003;5(4):255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duffau H. Diffuse low-grade gliomas and neuroplasticity. Diagn Interv imaging. 2014;95(10):945–955. [DOI] [PubMed] [Google Scholar]
- 30. Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118(4):469–474. [DOI] [PubMed] [Google Scholar]
- 31. Tucha O, Smely C, Preier M, et al. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47(2):324–333. [DOI] [PubMed] [Google Scholar]
- 32. Zada G, Bond AE, Wang YP, et al. Incidence trends in the anatomic location of primary malignant brain tumors in the United States: 1992–2006. World Neurosurg. 2012;77(3–4):518–524. [DOI] [PubMed] [Google Scholar]
- 33. Desmurget M, Bonnetblanc F, Duffau H. Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain. 2007;130(Pt 4):898–914. [DOI] [PubMed] [Google Scholar]
- 34. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. New Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]