Abstract
Background
Understanding the neural processes tied to the adult outcome of childhood attention-deficit/hyperactivity disorder (ADHD) could guide novel interventions to improve its clinical course. It has been argued that normalization of prefrontal cortical activity drives remission from ADHD, while anomalies in subcortical processes are ‘fixed’, present even in remission. Using multimodal neuroimaging of inhibitory processes, we test these hypotheses in adults followed since childhood, contrasting remitted against persistent ADHD.
Methods
Adult participants (35 persistent ADHD; 47 remitted ADHD; 99 never affected) were scanned with fMRI (n=85), magnetoencephalography (n=33) or both (n=63) during a response inhibition task.
Results
In fMRI analyses, during inhibition, right caudate anomalies both reflected a childhood ADHD history and were present even among those who remitted. By contrast, differences related to adult outcome emerged in cortical (right inferior frontal and inferior parietal/precuneus) and cerebellar regions. Here, the persistent ADHD group showed under-activation, whereas the remitted ADHD group did not differ significantly from the never-affected group. MEG showed that the association between adult symptom severity and prefrontal neuronal activity was confined to the time window covering the act of inhibition (300–350 ms). Group differences in cerebellar and parietal neuronal activity occurred during the time window of performance monitoring processes (500–600 ms).
Conclusions
By combining fMRI and MEG we pinpoint the location and time window of neuronal activity that underpins the adult outcome of ADHD. We thus separate the cortico-cerebellar processes tied to clinical course of ADHD from the subcortical processes that are not.
Keywords: ADHD, adults, remission, neuroimaging, fMRI, MEG
Introduction
While some children ‘grow out’ of attention-deficit/hyperactivity disorder (ADHD) by adulthood, many do not. Prospective studies find 15–45% of children with ADHD will have the full syndrome into adulthood and a further 25–48% continue to have impairing symptoms(1–5). This variable clinical outcome prompts the question: how does the adult brain differ in those whose ADHD persists after childhood compared to those who remit? Identifying the ‘plastic’ brain regions that drive remission could provide targets for interventions to improve the clinical course of the disorder.
A prominent neurodevelopmental model posits that ADHD onset is linked to early aberrations in the striato-thalamic circuitry and associated deficits in ‘bottom-up’ cognitive processes that persist into adulthood, regardless of clinical outcome(6). In support of this model, adult anomalies in subcortical, thalamic activation during response preparation (a bottom-up cognitive process) were found to reflect a childhood history of ADHD even in those who remitted (7). The other tenet of this model is that the variable adult outcome of ADHD is tied to developmental trajectories of the prefrontal cortex and associated ‘top-down’ cognitive processes(6). This is in line with behavioral observations indicating that remission from ADHD is associated with normalization in higher order cognitive functions(2). However, the delineation of the underlying prefrontal cortical circuitry and its links with adult outcome of childhood ADHD are currently not well characterized.
Here, we study adults with a childhood history of ADHD followed prospectively. Using two imaging modalities, we delineate brain activation patterns during a ‘top-down’ task of motor inhibition. We focus on motor inhibition as it has been viewed as a core deficit in ADHD, associated particularly with impulsivity(8, 9). We use the stop signal task, which—in functional magnetic resonance imaging (fMRI) studies—reliably activates both prefrontal cortical (in particular the inferior frontal cortex) and caudate regions, central to neurodevelopmental models of ADHD (10–13). However, motor inhibition unfolds rapidly, in hundreds of milliseconds. While fMRI provides excellent spatial resolution, its temporal resolution is on the orders of seconds. Therefore, to provide better temporal resolution, we incorporate magnetoencephalography (MEG), an imaging technique that maps the magnetic fields evoked by populations of activated cortical neurons. MEG thus provides a direct observation of neuronal activity during inhibitory processes which has temporal resolution in the order of milliseconds.
We test the hypothesis that the variable adult outcome of childhood ADHD is linked to differences in cortical activity. We primarily focus on associations between adult outcome of childhood ADHD and activity in the inferior frontal cortex during motor inhibition. By contrast, we predict that a childhood history of ADHD will result in fixed anomalies in striatal processing which are present even among those who remit.
Methods
Participants
In total, 181 individuals participated, 63 completing the fMRI and MEG task versions, 85 the fMRI version only, and 33 the MEG version only. Eighty-two participants (46%) had a childhood history of ADHD; the remainder had no history of ADHD (‘never affected’). Current adult ADHD symptoms were assessed using the clinician-administered ADHD Rating Scale, version IV, providing prompts appropriate for late adolescent and young adult groups (14). Seventy-three (89%) of the individuals with ADHD entered NIH research studies as children, when they received a diagnosis using the Parent Diagnostic Interview for Children and Adolescents(15). Nine participants entered the study as young adults and their childhood history of ADHD symptoms was confirmed through parental interviews. In line with DSM-5, the persistent ADHD group was defined by the presence of five or more impairing symptoms of inattention, five or more symptoms of hyperactivity-impulsivity or both (16). Remission was defined by having four or fewer symptoms in each of the two symptom dimensions. The presence of other psychiatric disorders was established through the Structured Clinical Interview for DSM Axis I Disorders(17). Two experienced clinicians (PS and WS) conducted the interviews (inter-rater reliabilities; kappa >0.9). Contrasts were made against 99 individuals never affected by ADHD, drawn from a longitudinal study of typical brain development. General exclusion criteria were a full-scale IQ of <80 (estimated from age-appropriate versions of the Wechsler intelligence scales, neurological disorders known to affect brain structure, current substance dependence or psychotic disorders. Psychostimulant medication was used among 48.6% of adults with persistent ADHD and 12.7% of those with four or fewer symptoms (remitted). To mitigate acute effects, all participants stopped psychostimulant medication the day prior to scanning. The Institutional Review Board of the National Human Genome Research Institute approved the research protocol and written informed consent was obtained.
Behavioral task
The stop signal task was a rapid, mixed-trial, event-related paradigm, used in previous studies (18, 19). On all trials, a fixation cross appeared centrally against a black background for 500 ms, followed by a white “X” or “O” go signal for 1000 ms. Participants were instructed to press the right button on a button box for an “X” and the left button for an “O”. During the unexpected, infrequent stop trials (25% of trials, randomly dispersed), after the “X” or “O” appeared the background changed from black to red (stop signal), and subjects were instructed to inhibit their motor response. The first stop signal appeared 250 ms after the go signal. In subsequent stop trials, the time interval between the go and stop signals (i.e. inhibit delay) adapted to individual performance. It became 50 ms longer after a successful trial, making it harder to inhibit, and 50 ms shorter after an unsuccessful trial, making it easier to inhibit. This algorithm ensured that the task was equally challenging for all individuals, resulting in approximately 50% successful and 50% failed stop trials. Trials were separated by 750 ms, with 86 trials in each run. Subjects completed 4 runs (344 trials) in the fMRI and 8 runs (688 trials) in the MEG experiment.
fMRI acquisition
Gradient echo planar images were acquired on a 3T GE Signa scanner (General Electric, Milwaukee: 26 contiguous 4 mm axial slices obtained with single shot gradient echo T2*-weighting, aligned to the anterior-posterior commissure). Images were aligned to a T1-weighted anatomical scan – see Supplementary Material.
MEG acquisition
MEG data was acquired using a 275-gradiometer whole-helmet MEG system (the former CTF Systems, Coquitlam BC, Canada) at 600Hz. The locations of 3 fiducial points (nasial, and left/ right auricular) were determined for localization of MEG signal sources.
Data analysis
Behavioral data
Performance was measured by (1) go trial accuracy and reaction time, (2) stop trial accuracy, (3) “inhibit delay” or the time interval between the onset of the go and stop signals, (4) the stop signal reaction time (SSRT) calculated as the mean go reaction time minus the mean inhibit delay in subjects who inhibited successfully on 50% of the stop trials (8, 19). If a subject’s stop trial accuracy deviated from 50%, an interpolation algorithm was used to compute SSRT: the mean inhibit delay was subtracted from the go reaction time at the Xth percentile of the go reaction times, where X is the subject’s percent accuracy on stop trials.
fMRI data
fMRI data was analyzed with Analysis of Functional Neuroimages ([AFNI] Cox, 1996). Preprocessing included slice timing correction, motion correction, spatial normalization to Talairach space, and smoothing (kernel FWHM=8mm)- see Supplementary Material
In the event-related fMRI analysis, brain activation was considered for three contrasts (1) successful inhibition versus successful go trials: this contrast holds constant (successful) performance but differs on task demand and motor response; (2) failed inhibition versus successful go: these two conditions have the same motor response but differ on task demand and performance; (3) failed versus successful inhibition: this contrast holds the task demands constant, but the conditions differ on motor response and performance. These contrasts parse out neural activation pertaining to task demands, performance success, and motor response and identify circuitry mediating successful and failed inhibition. Incorrect go trials were very infrequent and not included in the analyses.
We focused on two ROIs implicated in motor inhibition by meta-analyses of fMRI and human lesion studies, namely the inferior frontal cortex (IFC) and the caudate (10, 11, 20). These were defined using the Talairach-Tournoux atlas, applied to each subject’s normalized brain.
Differences between the persistent, remitted and never-affected adults were calculated for each of the three contrasts using factorial ANOVAs. Given the 4 ROIs, significance was set at p<0.05/4=0.01; significant group differences were examined in pair-wise, post-hoc analyses using Fisher’s least significant difference. Direct comparisons between adults with and without a childhood history of ADHD were made by categorizing individuals according to the presence or absence of a childhood diagnosis for ADHD using t-tests. We additionally explored associations with the number of adult inattentive and hyperactive-impulsive symptoms using correlation analysis.
Finally, we conducted exploratory whole-brain analyses. Results were corrected for multiple testing using the most current version of 3dClustSim (https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html), which corrects for issues with parametric approaches for preventing type 1 errors (21). Significance was declared at a voxel-wise p<0.05 and a cluster-corrected alpha level<0.05 (k=1, minimum cluster size=512 voxels).
We repeated all analyses comparing those with childhood ADHD (i.e., combining persistent and remitted subjects into one group) against the group of never-affected adults.
MEG dat
Using MNE-Python(22) and AFNI, eye blinks and other subject-related artifacts in the raw signal were removed and data visually inspected to rule out other anomalies. As we were interested in the neural processes underlying inhibition, only inhibit trials were analyzed further. Each trial was parsed into individual epochs such that time point 0 marked the onset of the stop signal. Data were averaged across trials within condition (successful inhibition, unsuccessful inhibition), and then source-localized using DICS(23) for the frequency bands: delta (1–4Hz), theta (4–8Hz), alpha (8–13Hz), beta (13–30Hz), low gamma (30–55Hz), and high gamma (65–100Hz). Further details are in the supplementary material. ANOVAs compared neural responses between the outcome groups and correlation analyses tested for associations with ADHD symptoms. We applied a False Discovery Rate (q=.05) to correct for multiple comparisons over each brain image (24). Robustness analysis tested whether results held when we considered possible effects of psychostimulant medication.
Results
Do cortical rather than subcortical anomalies reflect the adult outcome of childhood ADHD?
The three outcome groups did not differ behaviorally in the fMRI task version, but did in the MEG version which had twice as many trials (n=688 trials). In the MEG version, the persistent ADHD group was less accurate on go trials and had a longer stop signal response time compared to the never-affected group.
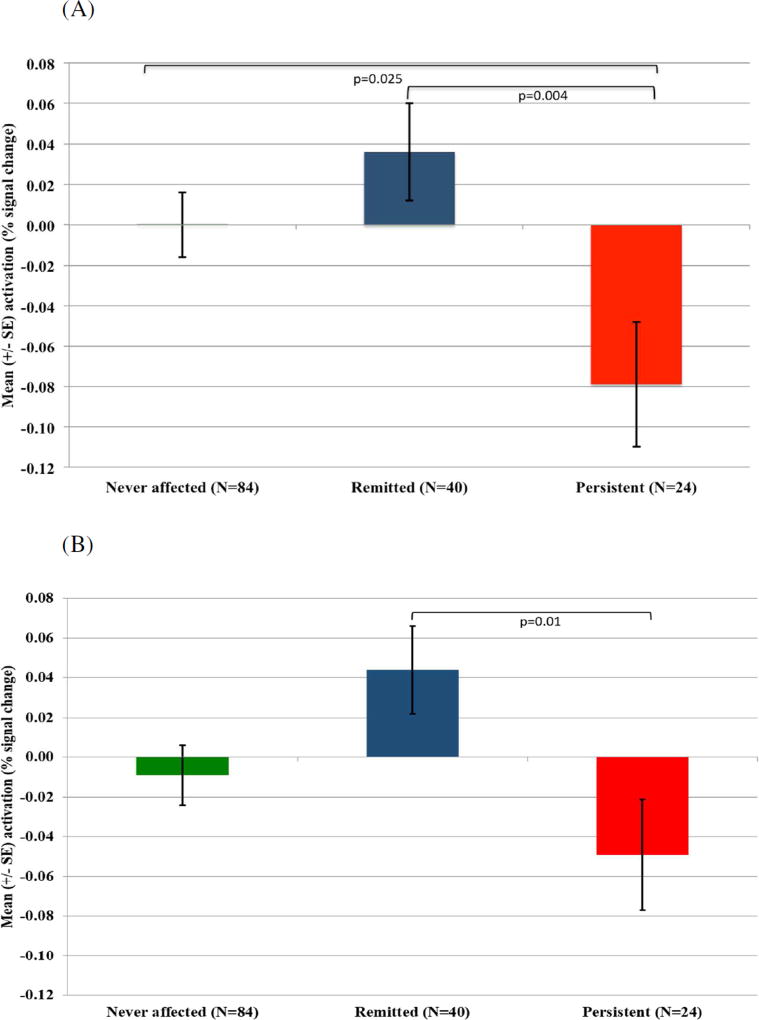
We tested the hypothesis that activation in the cortical (inferior frontal cortex) but not subcortical ROI (caudate) was tied to the degree of persistence of childhood ADHD into adulthood. First, categorical contrasts were drawn between persistent ADHD, remitted ADHD and never-affected groups. In the fMRI data, group differences emerged in inferior frontal cortex activation during failed inhibition when contrasted against either successful inhibition (left: F(2,145)=4.37, p=0.01; trend level on right: F(2,145)=3.67, p=0.03), or go trials (left: F(2,145)=5.07, p=0.007). The group effects were due to reduced inferior frontal cortex activity during failed inhibition in the persistent group compared to both the remitted group (right: p=0.01; left: p=0.004) and never-affected individuals (left: p=0.009) – Figure 1. The remitted and never-affected groups did not differ significantly (p>0.05). As predicted, no outcome group differences emerged for the subcortical ROIs, the left and right caudate.
Figure 1.
Mean activation differences for the failed versus successful inhibition contrast between the persistent ADHD, remitted ADHD and never affected groups in the left inferior frontal cortex (A). A similar pattern emerged in the right inferior frontal cortex (B).
We followed up this finding with symptom-level analyses. Higher levels of adult hyperactivity-impulsivity were associated with decreased activation in the bilateral inferior frontal cortex during failed inhibition contrasted against either successful inhibition (right: r= −0.27, p=0.03; left: r= −0.28, p=0.03) or go trials (left: r= −0.33, p=0.009) – Supplemental Figure S1. There were no significant associations with symptoms of inattention.
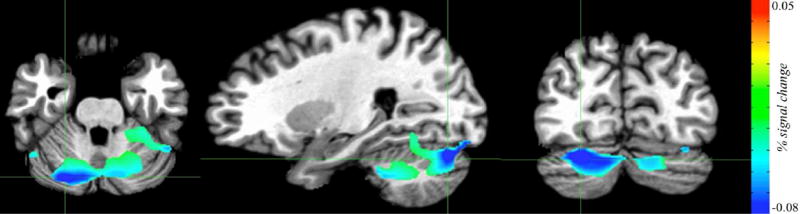
In the whole-brain fMRI analysis, no clusters showed significant differences between the three groups. However, we identified a bilateral cerebellar cluster (799 voxels) where increased adult hyperactive-impulsive symptoms were associated with decreased activation during successful inhibition (contrasted against go trials) – Figure 2. An inferior parietal cluster (492 voxels) showed a trend-level association (cluster-corrected alpha<0.06) with adult hyperactive-impulsive symptoms.
Figure 2.
Regions in the fMRI task where activation was associated with the severity of adult hyperactive-impulsive symptoms in the successful inhibition vs go contrast (clinical group, n=64). The cerebellar cluster shown (799 voxels) has peak activation in the right cerebellum (MNI coordinates x= −26.2, y=78, z= −28.5). Increased hyperactive-impulsive symptoms in adulthood were associated with decreased activation during successful inhibition.
In summary, as hypothesized, anomalous activity in the inferior frontal cortex, but not the caudate, was associated with the adult outcome of childhood ADHD during failed inhibition. Links also emerged between the degree of adult persistence of childhood hyperactive-impulsive symptoms and activation during inhibitory processes in both the right inferior frontal cortex and cerebellum.
Do subcortical rather than cortical anomalies reflect childhood ADHD?
We next categorized individuals according to the presence or absence of a childhood diagnosis for ADHD, predicting differential activation in the subcortical but not cortical ROIs. The group with a history of childhood ADHD showed reduced right caudate activity when successfully inhibiting (t(1,146)=2.28, p=0.02) and increased activity in the right caudate when failing to inhibit (t(1,146)= −2.18, p=0.03). As hypothesized, no differences were found for the cortical ROI (inferior frontal cortex) (all ps>0.1). Further, we did not detect any differences that reflected a childhood history of ADHD in whole brain analyses.
The patterns of neuronal activation tied to the adult outcome of childhood ADHD
Given our fMRI findings, we used MEG to characterize further the cortical inhibitory processes that reflect adult outcome groups, with a further focus on associations with the degree of adult persistence of childhood hyperactivity-impulsivity. We examined the neural processes relating to successful and failed inhibitory events separately, using exploratory whole brain analysis.
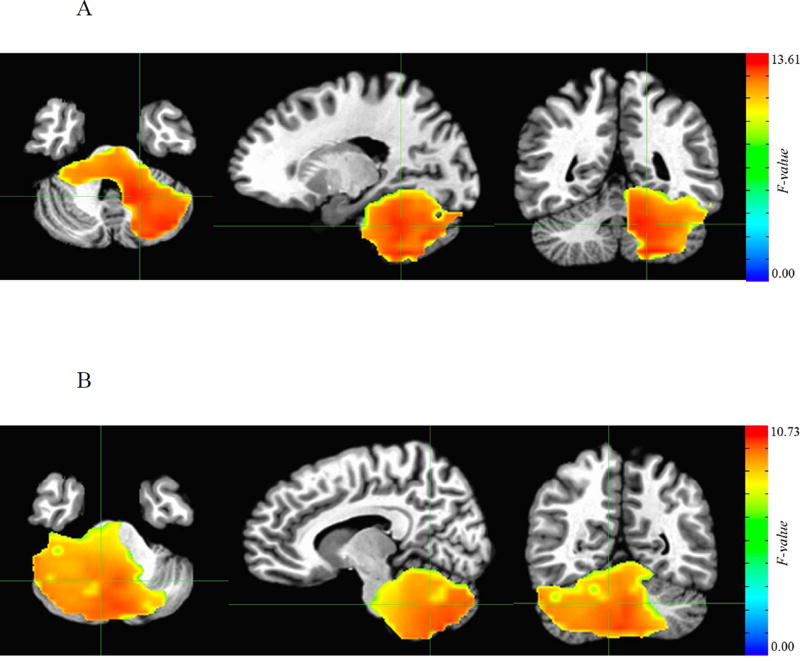
We found significant differences between the three groups during successful inhibition. These differences occurred in two frequency bands at 500–600 ms after the stop signal in cerebellar and precuneal/parietal regions; these regions overlapped with those identified in the fMRI analysis. Specifically, in the delta frequency band the three groups differed in evoked responses 550–600 ms after the stop signal (minimum F=6.05; FDR-corrected p<0.05) in two clusters. The first cluster (697 voxels) encompassed the cerebellum (Figure 3); the second cluster (69 voxels) was centered on the left precuneus – Supplemental Figure S2. Pairwise group analyses showed that neural activity was highest in never-affected individuals, and lowest in the persistent ADHD group, with the remitted group showing intermediate levels of activation.
Figure 3.
Cerebellar regions from MEG whole brain analysis showing differential neural response in relation to adult outcome during successful inhibition. A: Cerebellar cluster in the delta band where group differences emerged at 550–600 ms following the stop signal (699 voxels centered on the left cerebellum, MNI coordinates: x=16.9, y=47.1, z= −28.5). B: Cerebellar cluster in the theta band where group differences emerged at 500–550 ms following the stop signal (1102 voxels centered on the right cerebellum, MNI coordinates: x= −9.3, y=53.6, z= −31.5).
In the theta band, the three outcome groups also showed differences in neural activity at 500–550 ms after the stop signal (minimum F=5.25, FDR-corrected p<0.05) in a bilateral cerebellar cluster (1002 voxels) that overlapped with the cerebellar cluster found in the delta band, shown in Figure 3. As in the delta band, the lowest amplitude was observed in the persistent ADHD group and the highest in the never-affected group, with the remitted group showing intermediate levels of activation. Further clusters showing difference between outcome groups were noted in the right inferior parietal cluster (341 voxels); and a right cuneus/posterior cingulate cluster (172 voxels) – Supplemental Figure S3.
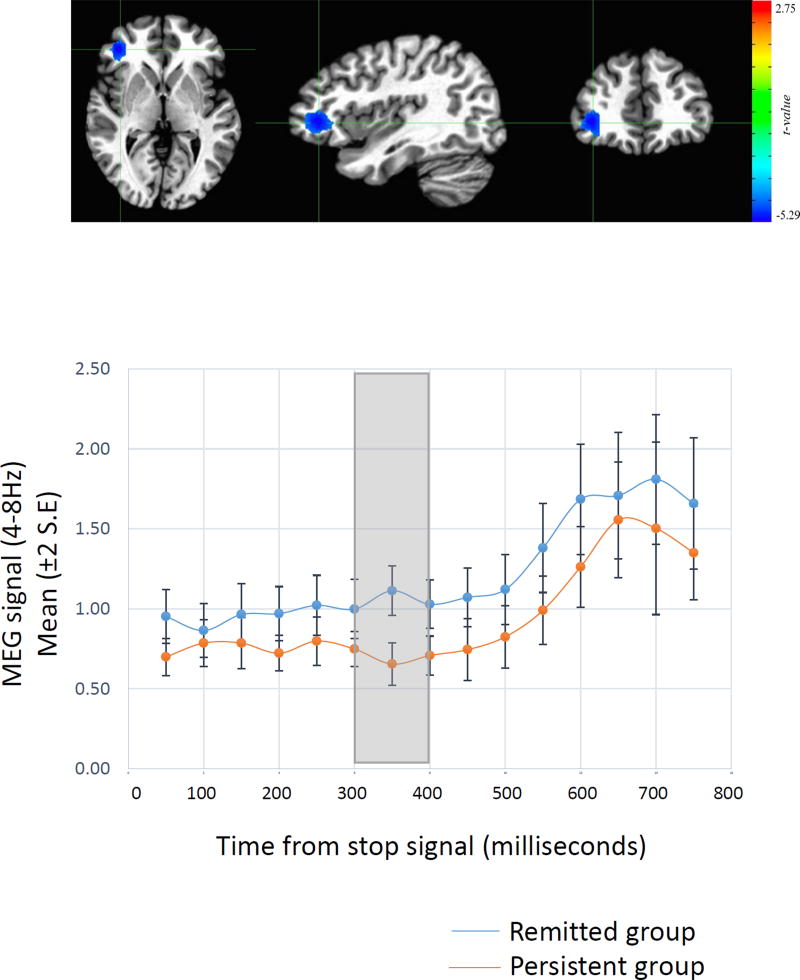
In analyses of adult symptoms of hyperactivity-impulsivity we detected associations with neural responses in the right inferior prefrontal cortex during successful inhibition; this region overlapped with the cortical ROI in the fMRI analysis. This theta band cluster (42 voxels; minimum t=4.06, FDR-corrected p<0.05) was confined to the time window during which successful inhibition typically occurs (i.e, 300–350 ms after the stop signal). During this ‘inhibitory’ time window those who had remitted exhibited decreased theta activity within the right inferior frontal cortex, compared to persistent ADHD (Figure 4). In summary, the degree of persistence into adulthood of hyperactive-impulsive symptoms was associated with neuronal activity during the time window of the act of inhibition.
Figure 4.
Top panel. Regions from whole brain MEG analyses where neuronal activity during successful inhibition was associated with the severity of hyperactive-impulsive symptoms persisting from childhood. This signal was detected in the theta band and centered on the right inferior prefrontal cortex (42 voxels, peak MNI coordinates: x= −38.0, y= −38.4, z=2.3). Lower panel. The time course is shown of the average magnetic field response in this right inferior frontal cortical region during successful inhibition. The blue line indicates adults with remittent ADHD and the red line indicates adults with persistent ADHD. The time window of the significant group difference is highlighted in the grey box and occurred at 300–350 ms following the stop signal.
Discussion
By combining fMRI and MEG we pinpoint the location and time window of neuronal activity that is tied to the adult outcome of childhood ADHD. This detailed spatiotemporal mapping of the neural substrate tied to remission could guide more targeted interventions for improving the clinical course of ADHD. We separate the cortico-cerebellar processes that underpin adult outcome from the striatal processes that are not. Specifically, we find inhibition-related anomalies in the inferior frontal cortex to be associated with the adult outcome of childhood ADHD, particularly the persistence of hyperactive-impulsive, rather than inattentive symptoms. Leveraging the exquisite temporal resolution of MEG, we pinpointed these prefrontal anomalies to the time window covering the act of inhibition. Additional activations in cerebellar and precuneal/inferior parietal regions also reflected adult outcome. Here, MEG indicated that neuronal activation differences occurred relatively late after the onset of the stop sign, compatible with disruption of cerebellar performance monitoring processes. Not all neural differences were tied to adult outcome. Subcortical, right caudate anomalies reflected a childhood history of ADHD and were present even in those who had remitted as adults.
The neurobiology of the variable adult outcome of childhood ADHD
What do these findings tell us about the processes underpinning adult remission versus persistence of childhood ADHD? Our results support a model in which prefrontally-mediated cognitive processes are tied to the adult outcome of ADHD(6, 10). In general, the remitted ADHD group did not differ from the never-affected group, compatible with the concept that remission is underpinned by a normalization of inferior frontal cortex activity. Although not suggested by the original model (6), we note that cerebellar and parietal regions also showed partial normalization with remission from ADHD. It is noteworthy that adult severity of hyperactive-impulsive rather than inattentive symptoms accounted for these findings. This is in keeping with an influential model of ADHD, which links inhibitory deficits with hyperactive-impulsive symptoms While there is empirical support from neuropsychological studies for this model(25–28), there are also some conflicting reports(29–31). Prior neuroimaging studies have primarily examined ADHD-related inhibitory deficits using diagnostic categories and not included separate analyses of hyperactive-impulsive and inattentive symptom dimensions. Mapping the neural substrate of inhibition onto symptom dimensions, as we attempted in this study, is an area of active interest. Additionally, more recent models have linked inattention, particularly deficient sustained attention and increased distractibility, with intrusions of the default mode network (prominent during introspective processing) into neural activity tied to task performance (32–34) A future goal is to examine whether such dysregulated interactions between brain networks account for the persistence of inattention into adulthood.
As expected, only caudate anomalies were related to childhood symptoms of ADHD irrespective of later outcome. This is consonant with a recent, independent finding of atypical subcortical—specifically thalamic—activity among adults with a childhood diagnosis of ADHD, regardless of adult status(7). This supports the concept that fixed subcortical abnormalities drive the onset of ADHD and remain relatively unchanged throughout development and recovery(6). We further demonstrate that these subcortical anomalies are not only present during bottom-up cognitive processes but also in higher-order processes of cognitive control.
Mapping the patterns of neuronal activation sensitive to the adult outcome of childhood ADHD
Using MEG we showed that the link between adult hyperactive-impulsive symptoms and right inferior prefrontal neuronal activation was confined to 300–350 ms following the stop signal. This time window overlaps with the time it takes a subject to successfully stop an already initiated response (35–37). This confirms prior Event Related Potential (ERP) studies in healthy subjects showing that that right inferior frontal cortical activation during successful inhibition occurs during this time window (38). Here, we further demonstrate that this epoch of prefrontal inhibitory processing is sensitive to the adult outcome of childhood ADHD.
Activation differences associated with adult ADHD symptoms extended to the cerebellum, in both fMRI and MEG. This finding is congruent with the role of the cerebellum in response inhibition. Prior PET studies find cerebellar activation during the inhibition of prepotent responses in Stroop tasks, while deficits in the Stroop task are seen in patients with cerebellar lesions(39, 40). Similarly, slower go signal reaction times in the go/no-go task have been linked to the extent of cerebellar lesions (41). Such human lesion studies implicate the cerebellum in response inhibition, and align with previous reports of subtle cerebellar anatomic anomalies in adults with persistent ADHD(42). Using MEG, we additionally find that ADHD symptoms were associated with cerebellar activity in a time window 500–600 ms after the stop signal. This time window points to anomalies in the processing of feedback around successful inhibition, which is reliant on prefrontal-cerebellar interactions(43, 44). It suggests that adults with persistent symptoms show impairment in such processing, whereas those who have remitted may have more typical performance monitoring.
Electrophysiological differences that tracked with adult outcome lay in the theta and delta bands. Interestingly, ERP studies also show augmented power in both theta and delta frequencies during inhibition (45). Specifically, frontal theta activation has been observed during response inhibition (46, 47), while delta oscillations are observed during the evaluation of motor inhibition (46). Indeed, frontal-midline theta is associated with a wide range of cognitive processes including working memory, spatial navigation and episodic memory, thus indicating a general role in action monitoring(46). Our findings support the emerging picture that both theta and delta bands are sensitive both to increased cognitive demands during response inhibition and to the severity of adult symptoms of ADHD (48).
Limitations
This study has its limitations. First, MEG is best suited to detect cortical activation, and could not specify the characteristics of the caudate anomalies we found using fMRI. Second, nine participants were not followed from childhood, but recruited in adulthood. Consequently, we had to establish the presence of a childhood history of ADHD retrospectively. Nevertheless, whenever possible, we also collected collateral information about childhood history and the results held when we reanalyzed the sample excluding the individuals who entered the study as adults (Supplemental Information). Third, follow-up assessments in adulthood were not blind. Consequently, there may have been a bias toward underestimating ADHD symptoms in adults who entered as typically developing children, and overestimating adult ADHD symptoms in those who entered the study as children with the diagnosis. This is of relevance given the recent interest in the possibility of adult-onset ADHD (49–51). Finally, regional neural activation is partly task-dependent (52) and it is interesting to consider whether the same general finding of cortical anomalies in persisters, but typical activity in remitters, would emerge using different cognitive probes. One pertinent study found greater integration of thalamo-cortical activity in remitters compared to persisters during a task of response preparation, while childhood history was characterized by a general hypoactivation(7). This adult imaging study, embedded within a longitudinal study starting in childhood provides hypotheses about mechanisms of recovery. Ideally, these should be tested and refined though the future collection of longitudinal imaging data.
Clinical implications
We show that the ‘plasticity’ underpinning remission from ADHD lies in inferior prefrontal/cerebellar rather than striatal regions. This implies that cognitive or pharmacological interventions targeting prefrontal or cerebellar functions (such as executive or temporal information processes) may prove more successful than interventions that ‘train’ more automatic processes, supported by the striatum. In devising novel interventions is noteworthy that both fMRI and MEG have been successfully used as real-time neurofeedback devices, allowing subjects to modulate task-related brain activation and oscillatory rhythms associated with awareness and attention (53, 54). For example, it is possible to provide real-time MEG feedback on right inferior frontal gyrus during critical time windows for response inhibition (55, 56). Thus the tools we use to map the cognitive deficits underlying persistent symptoms of ADHD could feasibly also be used for their remediation. Finally, the findings are consonant with studies that tie recovery from ADHD to a convergence towards typical brain structure and function (5).
Supplementary Material
Table 1.
Behavioral performance and descriptives of adults with persistent ADHD, remitted ADHD, and never-affected individuals on the fMRI and MEG versions of the stop signal task.
|
|
||||||
|---|---|---|---|---|---|---|
| fMRI analysis | ||||||
|
|
||||||
| Never affected (n=84) |
Remitted (n=40) | Persistent (n=24) | ||||
|
|
|
|
||||
| Characteristic | Mean (SD) | Mean (SD) | Mean (SD) |
Test statistic |
p | |
|
|
||||||
| Percent accuracy go trials | 89.43 (10.17) | 89.49 (10.98) | 91.78 (8.36) | 0.53 | 0.59 | |
| Percent accuracy stop trials | 50.37 (8.65) | 53.37 (12.11) | 50.94 (7.37) | 1.36 | 0.26 | |
| Mean go response time (ms) | 714.84 (107.98) | 708.62 (102.11) | 663.31 (120.99) | 1.66 | 0.19 | |
| Stop signal response time (ms)a | 271.87 (95.53) | 241.10 (65.32) | 243.00 (64.24) | 2.34 | 0.1 | |
| Inhibit delay (ms) | 450.64 (114.73) | 489.43 (121.43) | 433.56 (144.98) | 1.97 | 0.14 | |
|
|
||||||
| Age (yrs) | 24.46 (4.09) | 24.33 (3.85) | 23.34 (3.95) | 0.74 | 0.48 | |
| Sex (male), N (%) | 48 (57.1) | 25 (62.5) | 11 (45.8) | 1.71 | 0.42 | |
| Inattentive symptoms | NA | 2.03 (1.31) | 5.92 (1.74) | −10.15 | <0.001 | |
| Hyperactive-impulsive symptoms | NA | 1.43 (1.45) | 4.08 (2.76) | −4.36 | <0.001 | |
| Psychostimulant use, N (%) | NA | 4 (10) | 15 (62.5) | 19.81 | <0.001 | |
| Presence of comorbidity, N (%) | NA | 22 (55) | 12 (50) | 0.15 | 0.7 | |
|
|
||||||
| MEG analysis | ||||||
|
|
||||||
| Never affected (n=46) | Remitted (n=26) | Persistent (n=25) | ||||
|
|
|
|
||||
| Characteristic | Mean (SD) | Mean (SD) | Mean (SD) | Test statistic | p | |
|
|
||||||
| Percent accuracy go trials | 86.65 (10.50) | 79.81 (11.98) | 80.67 (8.56) | 4.54 | 0.01 | NV>remitted, persistent |
| Percent accuracy stop trials | 47.39 (7.12) | 46.81 (12.14) | 49.02 (6.44) | 0.46 | 0.63 | |
| Mean go response time (ms) | 735.41 (92.80) | 749.17 (94.31) | 765.74 (47.78) | 1.06 | 0.35 | |
| Stop signal response time (ms)a | 296.96 (79.57) | 305.64 (98.67) | 353.23 (90.27) | 3.46 | 0.04 | persistent>NV |
| Inhibit delay (ms) | 437.42 (127.40) | 442.74 (178.39) | 414.93 (111.54) | 0.3 | 0.74 | |
|
|
||||||
| Age (yrs) | 23.31 (2.96) | 24.79 (3.86) | 23.73 (4.18) | 1.46 | 0.24 | |
| Sex (male), N (%) | 22 (47.8) | 16 (61.5) | 13 (52.0) | 1.26 | 0.53 | |
| Inattentive symptoms | NA | 1.88 (1.39) | 6.40 (1.68) | −10.45 | <0.001 | |
| Hyperactive-impulsive symptoms | NA | 1.15 (1.35) | 4.04 (2.91) | −4.52 | <0.001 | |
| Psychostimulant use, N (%) | NA | 3 (11.5) | 13 (52) | 9.69 | 0.002 | |
| Presence of comorbidity, N (%) | NA | 15 (57.7) | 12 (48) | 0.48 | 0.49 | |
|
|
||||||
Calculated as the mean go reaction time at the Xth percentile minus the mean inhibit delay. X is the subject’s percent accuracy on stop trials.
Acknowledgments
All authors are funded by the Intramural Programs of the National Human Genome Research Institute and the National Institute of Mental Health. This work utilized the computational resources of the NIH HPC Biowulf cluster. (http://hpc.nih.gov)
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Biederman J, Petty CR, Evans M, Small J, Faraone SV. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. PSY Psychiatry Research. 2010;177:299–304. doi: 10.1016/j.psychres.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halperin JM, Trampush JW, Miller CJ, Marks DJ, Newcorn JH. Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. Journal of Child Psychology and Psychiatry. 2008;49:958–966. doi: 10.1111/j.1469-7610.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein RG, Mannuzza S, Olazagasti MA, Roizen E, Hutchison JA, Lashua EC, Castellanos FX. Clinical and functional outcome of childhood attention-deficit/hyperactivity disorder 33 years later. Archives of general psychiatry. 2012;69:1295–1303. doi: 10.1001/archgenpsychiatry.2012.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, Epstein JN, Hoza B, Hechtman L, Abikoff HB, Elliott GR, Greenhill LL, Newcorn JH, Wells KC, Wigal T, Gibbons RD, Hur K, Houck PR Group MTAC. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw P, Malek M, Watson B, Greenstein D, de Rossi P, Sharp W. Trajectories of Cerebral Cortical Development in Childhood and Adolescence and Adult Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2013;74:599–606. doi: 10.1016/j.biopsych.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychological bulletin. 2006;132:560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- 7.Clerkin SM, Schulz KP, Berwid OG, Fan J, Newcorn JH, Tang CY, Halperin JM. Thalamo-cortical activation and connectivity during response preparation in adults with persistent and remitted ADHD. The American journal of psychiatry. 2013;170:1011–1019. doi: 10.1176/appi.ajp.2013.12070880. [DOI] [PubMed] [Google Scholar]
- 8.Logan GD, Schachar RJ, Tannock R. Impulsivity and Inhibitory Control. Psychological Science. 1997;8:60–64. [Google Scholar]
- 9.Rubia K. The dynamic approach to neurodevelopmental psychiatric disorders: use of fMRI combined with neuropsychology to elucidate the dynamics of psychiatric disorders, exemplified in ADHD and schizophrenia. BBR</cja:jid> Behavioural Brain Research. 2002;130:47–56. doi: 10.1016/s0166-4328(01)00437-5. [DOI] [PubMed] [Google Scholar]
- 10.Aron AR, Poldrack RA. The Cognitive Neuroscience of Response Inhibition: Relevance for Genetic Research in Attention-Deficit/Hyperactivity Disorder. BPS Biological Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in cognitive sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Band GP, van Boxtel GJ. Inhibitory motor control in stop paradigms: review and reinterpretation of neural mechanisms. Acta psychologica. 1999;101:2–3. doi: 10.1016/s0001-6918(99)00005-0. [DOI] [PubMed] [Google Scholar]
- 13.Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SCR, Giampietro V, Andrew CM, Taylor E. Mapping Motor Inhibition: Conjunctive Brain Activations across Different Versions of Go/No-Go and Stop Tasks. YNIMG</cja:jid>. NeuroImage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- 14.DuPaul GJ, Power JD, Anastopouls AA, Reid R. ADHD Rating Scale-IV: Checklists, Norms and Clinical Interpretation. New York: The Guilford Press; 1998. [Google Scholar]
- 15.Reich W. Diagnostic Interview for Children and Adolescents (DICA) Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 16.APA. DSM 5. American Psychiatric Association; 2013. [Google Scholar]
- 17.First MB, Spitzer RL, Gibbon M, Williams JB. User’s guide for the Structured clinical interview for DSM-IV axis I disorders SCID-I: clinician version. American Psychiatric Pub; 1997. [Google Scholar]
- 18.Deveney CM, Connolly ME, Jenkins SE, Kim P, Fromm SJ, Brotman MA, Pine DS, Leibenluft E. Striatal dysfunction during failed motor inhibition in children at risk for bipolar disorder. PNP Progress in Neuropsychopharmacology & Biological Psychiatry. 2012;38:127–133. doi: 10.1016/j.pnpbp.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, Joshi P, Robb A, Schachar RJ, Dickstein DP, McClure EB, Pine DS. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. The American journal of psychiatry. 2007;164:52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- 20.Hart H, Rubia K, Radua J, Nakao T, Mataix-Cols D. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: Exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry JAMA Psychiatry. 2013;70:185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- 21.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gramfort A, Gramfort A, Luessi M, Hamalainen M, Gramfort A, Larson E, Engemann DA, Engemann DA, Strohmeier D, Brodbeck C, Brooks T, Goj R, Jas M, Parkkonen L, Jas M, Parkkonen L, Hamalainen M. MEG and EEG data analysis with MNE-Python. Front Neurosci Frontiers in Neuroscience. 2013 doi: 10.3389/fnins.2013.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laaksonen H, Kujala J, Salmelin R. A method for spatiotemporal mapping of event-related modulation of cortical rhythmic activity. NeuroImage. 2008;42:207–217. doi: 10.1016/j.neuroimage.2008.04.175. [DOI] [PubMed] [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Multiple Hypotheses Testing with Weights. Scandinavian Journal of Statistics. 1997;24:407–418. [Google Scholar]
- 25.Houghton S, Douglas G, West J, Whiting K, Wall M, Langsford S, Powell L, Carroll A. Differential patterns of executive function in children with attention-deficit hyperactivity disorder according to gender and subtype. Journal of child neurology. 1999;14:801–805. doi: 10.1177/088307389901401206. [DOI] [PubMed] [Google Scholar]
- 26.Lockwood KA, Marcotte AC, Stern C. Differentiation of attention-deficit/hyperactivity disorder subtypes: application of a neuropsychological model of attention. Journal of clinical and experimental neuropsychology. 2001;23:317–330. doi: 10.1076/jcen.23.3.317.1179. [DOI] [PubMed] [Google Scholar]
- 27.Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD. Neuropsychological executive functions and DSM-IV ADHD subtypes. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:59–66. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Solanto MV, Gilbert SN, Raj A, Zhu J, Pope-Boyd S, Stepak B, Vail L, Newcorn JH. Neurocognitive functioning in AD/HD, predominantly inattentive and combined subtypes. Journal of Abnormal Child Psychology. 2007;35:729–744. doi: 10.1007/s10802-007-9123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chhabildas N, Pennington BF, Willcutt EG. A comparison of the neuropsychological profiles of the DSM-IV subtypes of ADHD. Journal of abnormal child psychology. 2001;29:529–540. doi: 10.1023/a:1012281226028. [DOI] [PubMed] [Google Scholar]
- 30.Pasini A, Paloscia C, Alessandrelli R, Porfirio MC, Curatolo P. Attention and executive functions profile in drug naive ADHD subtypes. Brain and Development. 2007;29:400–408. doi: 10.1016/j.braindev.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Thorell LB. Do delay aversion and executive function deficits make distinct contributions to the functional impact of ADHD symptoms? A study of early academic skill deficits. Journal of Child Psychology and Psychiatry. 2007;48:1061–1070. doi: 10.1111/j.1469-7610.2007.01777.x. [DOI] [PubMed] [Google Scholar]
- 32.Sonuga-Barke EJS, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neuroscience & Biobehavioral Reviews. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB. A lack of default network suppression is linked to increased distractibility in ADHD. Brain research. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenberg MD, Finn ES, Scheinost D, Papademetris X, Shen X, Constable RT, Chun MM. A neuromarker of sustained attention from whole-brain functional connectivity. Nature neuroscience. 2015 doi: 10.1038/nn.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boehler CN, Münte TF, Krebs RM, Heinze H-J, Schoenfeld MA, Hopf J-M. Sensory MEG responses predict successful and failed inhibition in a stop-signal task. Cerebral Cortex. 2009;19:134–145. doi: 10.1093/cercor/bhn063. [DOI] [PubMed] [Google Scholar]
- 36.Curtis CE, Cole MW, Rao VY, D’Esposito M. Canceling planned action: an fMRI study of countermanding saccades. Cerebral Cortex. 2005;15:1281–1289. doi: 10.1093/cercor/bhi011. [DOI] [PubMed] [Google Scholar]
- 37.Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychological review. 1984;91:295. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- 38.Schmajuk M, Liotti M, Busse L, Woldorff MG. Electrophysiological activity underlying inhibitory control processes in normal adults. Neuropsychologia. 2006;44:384–395. doi: 10.1016/j.neuropsychologia.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Neau JP, Anllo E, Bonnaud V, Ingrand P, Gil R. Neuropsychological disturbances in cerebellar infarcts. Acta Neurologica Scandinavica. 2000;102:363–370. doi: 10.1034/j.1600-0404.2000.102006363.x. [DOI] [PubMed] [Google Scholar]
- 40.Taylor SF, Kornblum S, Lauber EJ, Minoshima S, Koeppe RA. Isolation of Specific Interference Processing in the Stroop Task: PET Activation Studies. NeuroImage. 1997;6:81–92. doi: 10.1006/nimg.1997.0285. [DOI] [PubMed] [Google Scholar]
- 41.Brunamonti E, Chiricozzi FR, Clausi S, Olivito G, Giusti MA, Molinari M, Ferraina S, Leggio M. Cerebellar damage impairs executive control and monitoring of movement generation. PloS one. 2014;9:e85997. doi: 10.1371/journal.pone.0085997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF, 3rd, Sharp WS, Giedd JN, Rapoport JL. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. The American journal of psychiatry. 2007;164:647–655. doi: 10.1176/ajp.2007.164.4.647. [DOI] [PubMed] [Google Scholar]
- 43.Picazio S, Ponzo V, Koch G. Cerebellar Control on Prefrontal-Motor Connectivity During Movement Inhibition. The Cerebellum. 2015:1–8. doi: 10.1007/s12311-015-0731-3. [DOI] [PubMed] [Google Scholar]
- 44.Rustemeier M, Koch B, Schwarz M, Bellebaum C. Processing of Positive and Negative Feedback in Patients with Cerebellar Lesions. The Cerebellum. 2015:1–14. doi: 10.1007/s12311-015-0702-8. [DOI] [PubMed] [Google Scholar]
- 45.De Jong R, Coles MG, Logan GD, Gratton G. In search of the point of no return: the control of response processes. Journal of Experimental Psychology: Human Perception and Performance. 1990;16:164. doi: 10.1037/0096-1523.16.1.164. [DOI] [PubMed] [Google Scholar]
- 46.Huster RJ, Enriquez-Geppert S, Lavallee CF, Falkenstein M, Herrmann CS. Electroencephalography of response inhibition tasks: functional networks and cognitive contributions. International Journal of Psychophysiology. 2013;87:217–233. doi: 10.1016/j.ijpsycho.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Isabella S, Ferrari P, Jobst C, Cheyne JA, Cheyne D. Complementary roles of cortical oscillations in automatic and controlled processing during rapid serial tasks. NeuroImage. 2015;118:268–281. doi: 10.1016/j.neuroimage.2015.05.081. [DOI] [PubMed] [Google Scholar]
- 48.Harper J, Malone SM, Bernat EM. Theta and delta band activity explain N2 and P3 ERP component activity in a go/no-go task. Clinical Neurophysiology. 2014;125:124–132. doi: 10.1016/j.clinph.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agnew-Blais JC, Polanczyk GV, Danese A, Wertz J, Moffitt TE, Arseneault L. Evaluation of the persistence, remission, and emergence of attention-deficit/hyperactivity disorder in young adulthood. JAMA Psychiatry. 2016;73:713–720. doi: 10.1001/jamapsychiatry.2016.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caye A, Rocha T, Anselmi L, et al. Attention-deficit/hyperactivity disorder trajectories from childhood to young adulthood: Evidence from a birth cohort supporting a late-onset syndrome. JAMA Psychiatry. 2016;73:705–712. doi: 10.1001/jamapsychiatry.2016.0383. [DOI] [PubMed] [Google Scholar]
- 51.Moffitt TE, Houts R, Asherson P, Belsky DW, Corcoran DL, Hammerle M, Harrington H, Hogan S, Meier MH, Polanczyk GV. Is adult ADHD a childhood-onset neurodevelopmental disorder? Evidence from a four-decade longitudinal cohort study. American Journal of Psychiatry. 2015;172:967–977. doi: 10.1176/appi.ajp.2015.14101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith AB, Taylor E, Brammer M, Toone B, Rubia K. Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. American Journal of Psychiatry. 2006;163:1044–1051. doi: 10.1176/ajp.2006.163.6.1044. [DOI] [PubMed] [Google Scholar]
- 53.Okazaki YO, Horschig JM, Luther L, Oostenveld R, Murakami I, Jensen O. Realtime MEG neurofeedback training of posterior alpha activity modulates subsequent visual detection performance. NeuroImage. 2015;107:323–332. doi: 10.1016/j.neuroimage.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 54.Sacchet MD, Mellinger J, Sitaram R, Braun C, Birbaumer N, Fetz E. Volitional control of neuromagnetic coherence. Frontiers in neuroscience. 2012;6:189. doi: 10.3389/fnins.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sudre G, Parkkonen L, Bock E, Baillet S, Wang W, Weber DJ. rtMEG: a real-time software interface for magnetoencephalography. Computational intelligence and neuroscience. 2011;2011:11. doi: 10.1155/2011/327953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Florin E, Bock E, Baillet S. Targeted reinforcement of neural oscillatory activity with real-time neuroimaging feedback. Neuroimage. 2014;88:54–60. doi: 10.1016/j.neuroimage.2013.10.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.