Abstract
Background
In a phase 1 dose-escalation study, combined inhibition of T-cell checkpoint pathways by nivolumab and ipilimumab demonstrated a high objective response rate, including complete responses in patients with advanced melanoma.
Methods
In this double-blind study, 142 treatment-naïve patients with metastatic melanoma were randomized 2:1 to receive ipilimumab 3 mg/kg combined with either nivolumab 1 mg/kg or placebo every 3 weeks for 4 doses, followed by nivolumab 3 mg/kg or placebo every 2 weeks until disease progression. The primary endpoint was investigator-assessed objective response in BRAF wild-type patients.
Results
Among BRAF wild-type patients, the confirmed objective response rate was 61.1% (44/72) in the nivolumab and ipilimumab combination group versus 10.8% (4/37) in the ipilimumab monotherapy group (P<0.001), with complete responses reported in 16 (22.2%) patients in the combination group; none in the ipilimumab group. Median duration of response was not reached with either treatment. Median progression-free survival was not reached for the combination versus 4.4 months for ipilimumab monotherapy (hazard ratio 0.40, 95% CI 0.23 to 0.68; P<0.001). Similar results for response and progression-free survival were also observed in 33 BRAF mutation-positive patients. Grade 3–4 drug-related adverse events were reported in 54.3% of patients receiving the combination compared with 23.9% with ipilimumab monotherapy. Select adverse events of immunological etiology were consistent with phase 1 reports, and most resolved with immune-modulating medication.
Conclusion
Nivolumab combined with ipilimumab significantly improved objective response rate and progression-free survival compared with ipilimumab monotherapy in treatment-naïve patients with advanced melanoma, and had a manageable safety profile. (ClinicalTrials.gov number, NCT01927419)
Recent approaches in the treatment of melanoma enhance antitumor immunity by blocking negative regulatory pathways, such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and the programmed death-1 (PD-1) receptor. Ipilimumab (anti-CTLA-4) is approved by the United States Food and Drug Administration (FDA) based on improvement in overall survival in patients with advanced melanoma, with objective responses in approximately 11% of patients.1, 2 Nivolumab, an anti-PD-1 monoclonal antibody, has recently been shown to improve overall survival compared with dacarbazine, with a 40% objective response rate versus 14%, respectively, in patients with advanced BRAF wild-type previously untreated melanoma.3
Targeted therapies, such as BRAF and MEK inhibitors which are approved for treatment of patients with advanced melanoma harboring BRAF V600 mutation-positive tumors, result in a high rate of initial tumor responses with a significant survival advantage over dacarbazine, although the median duration of response is less than one year.4–11 Therefore, there remains a substantial unmet need for new treatment options, particularly for the 50 to 60% of patients with BRAF wild-type melanoma.
CTLA-4 and PD-1 inhibit antitumor immunity through complementary and non-redundant mechanisms.12 Preclinical models have shown that dual blockade synergistically improves antitumor responses compared with blocking either pathway alone.13, 14 High response rates, prolonged duration of response, and favorable overall survival of 79% at 2 years were seen in a phase 1 study in patients receiving the combination regimen of nivolumab and ipilimumab.15, 16 Here, we report the results of a randomized, double-blind trial comparing the objective response rate of nivolumab in combination with ipilimumab to standard of care ipilimumab monotherapy as a first line treatment in patients with advanced melanoma.
METHODS
Patients
Eligible patients had histologically confirmed unresectable, previously-untreated, stage III or IV melanoma with measurable disease. Other inclusion criteria included a known BRAF V600 mutation status, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and availability of tumor tissue from a metastatic or unresectable site for PD-L1 immunohistochemistry. Key exclusion criteria included active brain metastases, uveal melanoma, and serious autoimmune disease.
Study Design and Treatment
Patients were randomized 2:1 in a double-blinded manner to nivolumab and ipilimumab combination or ipilimumab monotherapy. Randomization was stratified by BRAF mutation status (V600 wild-type versus mutation-positive). For the first 4 doses, nivolumab was administered intravenously at 1mg per kilogram body weight over 60 minutes, once every 3 weeks. Following a 30 minute interval, patients randomized to the combination group, received 3mg of ipilimumab per kilogram bodyweight over 90 minutes. Following the 4th dose of both agents, ipilimumab is discontinued and then nivolumab (maintenance phase) was administered as a single agent at 3 mg nivolumab per kilogram body weight over 60 minutes every 2 weeks.
In the ipilimumab monotherapy group, patients were treated with the same dosing schedule, except that nivolumab was replaced with matched placebo during both the combination and maintenance portions of the trial. Treatment was continued as long as clinical benefit as defined by the investigator was observed, or until treatment was no longer tolerated.
Patients who had investigator-assessed disease progression could be treated beyond progression (remained blinded) or be unblinded to the investigator. After unblinding, those in the ipilimumab monotherapy arm had the option to receive nivolumab at 3 mg/kg every 2 weeks until further disease progression. Patients on the combination arm, once unblinded, were required to discontinue treatment within this protocol (Fig. S1).
The primary endpoint was investigator-assessed confirmed objective response rate in patients with BRAF V600 wild-type tumors. The primary endpoint was restricted to this group of patients, because at the time of study enrolment, approved treatment options were limited for these patients and only ipilimumab had demonstrated overall survival benefit in a randomized controlled trial. Secondary endpoints included investigator-assessed progression-free survival in BRAF wild-type patients, objective response rate, and progression-free survival in BRAF V600 mutation-positive patients and safety.
Assessment
Tumor response was assessed according to Response Evaluation Criteria In Solid Tumors (RECIST) version 1.117 12 weeks after the first treatment, every 6 weeks thereafter for the first year, then every 12 weeks until disease progression or treatment discontinuation. Safety evaluations were performed in patients who received at least one dose of study treatment and the severity of adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events 4.0.18 Adverse event management guidelines were provided by the sponsor, and are available in the Supplementary Appendix.
Study Oversight
The study protocol was approved by the institutional review boards of the participating centers, and the study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice. All patients provided written informed consent to participate. An independent radiology review committee was established to provide a sensitivity assessment of objective responses, and a data monitoring committee provided general oversight and safety considerations. Data were collected by the sponsor, Bristol-Myers Squibb, and analyzed in collaboration with the authors. The authors vouch for the accuracy and completeness of the data and the fidelity of the study to the protocol, which includes the most recent version of the statistical analysis plan, available at NEJM.org. The manuscript was prepared by the first and last authors, with all authors contributing to subsequent drafts. All authors made the decision to submit the manuscript for publication.
PD-L1 Immunohistochemistry
Tumor cell-surface expression of PD-L1 was assessed in pretreatment tumor samples by a central laboratory using an automated BMS/Dako immunohistochemistry assay described previously.16 PD-L1 positivity was defined as at least 5% of tumor cells exhibiting cell-surface PD-L1 staining of any intensity in a section containing at least 100 evaluable tumor cells.
Statistical Analysis
A sample size of approximately 100 BRAF V600 wild-type patients, randomized 2:1 to the two treatment groups (the intention-to-treat population), was planned. Patients with BRAF V600 mutation positive tumors were eligible for the study, with approximately 50 planned to be randomized. Analyses in the BRAF V600 mutation-positive population were intended to be descriptive only and were not part of the sample size consideration. Given a 2-sided alpha of 0.05, the BRAF wild-type population provided approximately 87% power to show a statistically significant difference in the objective response rate between the combination group and the ipilimumab monotherapy group, assuming an objective response rate of 40% versus 10%, respectively. In order to preserve an experimental-wide type I error rate of 5%, a hierarchical testing approach was applied to key secondary endpoints following analysis of the primary endpoint of objective response rate in all randomized BRAF wild-type patients. The hierarchical ordering of key secondary endpoints was (1) objective response rate in all randomized patients, (2) progression-free survival in all randomized BRAF wild-type patients, and (3) progression-free survival in all randomized patients.
RESULTS
Patients
Baseline characteristics were balanced between the study groups (Table 1). At trial entry, the majority of patients (86.6%) had stage IV disease per the American Joint Commission on Cancer (AJCC) staging system, and 45.8% of patients had tumors characterized as M1c disease. Elevated lactate dehydrogenase levels were seen in 35 (24.6%) patients, and 23.2% were BRAF V600 mutation positive. Among all randomized patients with evaluable PD-L1 expression, 29.7% (35/118) were PD-L1 positive using the 5% cut-off.
Table 1.
Baseline Characteristics of Patients
| BRAF Wild-Type | All randomized patients | ||||
|---|---|---|---|---|---|
| Nivolumab + Ipilimumab N=72 |
Ipilimumab N=37 |
Nivolumab + Ipilimumab N=95 |
Ipilimumab N=47 |
Total N=142 |
|
| Age | |||||
| Median (range) | 66 (27, 87) | 69 (46, 80) | 64 (27, 87) | 67 (31, 80) | 65 (27, 87) |
| Gender, n (%) | |||||
| Male | 48 (66.7) | 23 (62.2) | 63 (66.3) | 32 (68.1) | 95 (66.9) |
| Female | 24 (33.3) | 14 (37.8) | 32 (33.7) | 15 (31.9) | 47 (33.1) |
| AJCC Stage at study entry, n (%) | |||||
| Stage III | 8 (11.1) | 8 (21.6) | 10 (10.5) | 9 (19.1) | 19 (13.4) |
| Stage IV | 64 (88.9) | 29 (78.4) | 85 (89.5) | 38 (80.9) | 123 (86.6) |
| ECOG Performance Status, n (%)† | |||||
| 0 | 62 (86.1) | 30 (81.1) | 79 (83.2) | 37 (78.7) | 116 (81.7) |
| 1 | 9 (12.5) | 7 (18.9) | 14 (14.7) | 10 (21.3) | 24 (16.9) |
| ≥2 | 1 (1.4) | 0 | 2 (2.1) | 0 | 2 (1.4) |
| Metastasis stage at study entry, n (%)a | |||||
| M0 | 6 (8.3) | 5 (13.5) | 8 (8.4) | 5 (10.6) | 13 (9.2) |
| M1a | 9 (12.5) | 7 (18.9) | 15 (15.8) | 8 (17.0) | 23 (16.2) |
| M1b | 22 (30.6) | 8 (21.6) | 27 (28.4) | 12 (25.5) | 39 (27.5) |
| M1c | 34 (47.2) | 16 (43.2) | 44 (46.3) | 21 (44.7) | 65 (45.8) |
| Not reported | 1 (1.4) | 1 (2.7) | 1 (1.1) | 1 (2.1) | 2 (1.4) |
| Baseline lactate dehydrogenase, n (%) | |||||
| ≤Upper limit of normal range | 57 (79.2) | 30 (81.1) | 70 (73.7) | 36 (76.6) | 106 (74.6) |
| >Upper limit of normal range | 15 (20.8) | 7 (18.9) | 24 (25.3) | 11 (23.4) | 35 (24.6) |
| ≤2× Upper limit of normal range | 69 (95.8) | 36 (97.3) | 88 (92.6) | 46 (97.9) | 134 (94.4) |
| >2× Upper limit of normal range | 3 (4.2) | 1 (2.7) | 6 (6.3) | 1 (2.1) | 7 (4.9) |
| History of brain metastases, n (%) | |||||
| Yes | 4 (5.6) | 0 | 4 (4.2) | 0 | 4 (2.8) |
| No | 67 (93.1) | 37 (100) | 90 (94.7) | 47 (100) | 137 (96.5) |
| BRAF V600 Mutation | 0 | 0 | 23 (24.2) | 10 (21.3) | 33 (23.2) |
M-stage as defined in the TMN system by American Joint Committee on Cancer and the International Union for Cancer Control.
An Eastern Cooperative Oncology Group (ECOG) performance-status score of 0 indicates no symptoms, 1 mild symptoms, and 2 moderate symptoms, with the patient being ambulatory and capable of all self-care but unable to carry out any work activities. Two patients randomly assigned to the nivolumab and ipilimumab group were inadvertently enrolled in the study, despite having an ECOG performance-status score of 2.
From September 16, 2013 to February 6, 2014, 179 patients were screened in the United States and France, and 142 patients were randomly assigned (Table S1), 109 with BRAF wild-type tumors and 33 with BRAF V600 mutation-positive tumors, to the two treatment groups. Clinical database lock for the results reported here occurred on January 30, 2015, allowing a minimum follow-up of 11 months after randomization.
Efficacy
The investigator-assessed confirmed objective response rate in the BRAF wild-type patients was 61.1% (95% confidence interval [CI], 48.9 to 72.4) in the nivolumab and ipilimumab combination group (Table 2), versus 10.8% (95% CI 3.0 to 25.4) in the ipilimumab group (odds ratio, 12.96, 95% CI, 3.91 to 54.49; P<0.001). Complete response was observed in 16 patients (22.2%) treated with the combination and none in the ipilimumab group. Figure 1A describes the distribution of tumor burden change from baseline in the BRAF wild-type population. The median reduction in investigator-assessed tumor volume was 68.1% in the nivolumab and ipilimumab group, compared with a 5.5% increase in the ipilimumab group.
Table 2.
Investigator-Assessed Confirmed Objective Response
| BRAF Wild-Type | BRAF V600 Mutant | |||
|---|---|---|---|---|
| Nivolumab + Ipilimumab | Ipilimumab | Nivolumab + Ipilimumab | Ipilimumab | |
| All randomized population, N | 72 | 37 | 23 | 10 |
| Best overall response, n (%)a | ||||
| Complete response | 16 (22.2) | 0 | 5 (21.7) | 0 |
| Partial response | 28 (38.9) | 4 (10.8) | 7 (30.4) | 1 (10.0) |
| Stable disease | 9 (12.5) | 13 (35.1) | 3 (13.0) | 1 (10.0) |
| Progressive disease | 10 (13.9) | 15 (40.5) | 5 (21.7) | 7 (70.0) |
| Unable to determine | 9 (12.5) | 5 (13.5) | 3 (13.0) | 1 (10.0) |
| Objective response rateb | ||||
| Number of responders (%) | 44 (61.1) | 4 (10.8) | 12 (52.2) | 1 (10.0) |
| 95% CI | 48.9, 72.4 | 3.0, 25.4 | 30.6, 73.2 | 0.3, 44.5 |
| Estimate odds ratio (95% CI)c | 12.96 (3.91, 54.49) | 9.82 (0.99, 465.39) | ||
| P value for comparison | <0.001 | NE | ||
Assessed by investigator using RECIST v1.1.
Complete response + partial response; CI based on the Clopper and Pearson method.
Ratio of nivolumab + ipilimumab over ipilimumab.
NE denotes not evaluated.
Fig 1. Clinical activities.
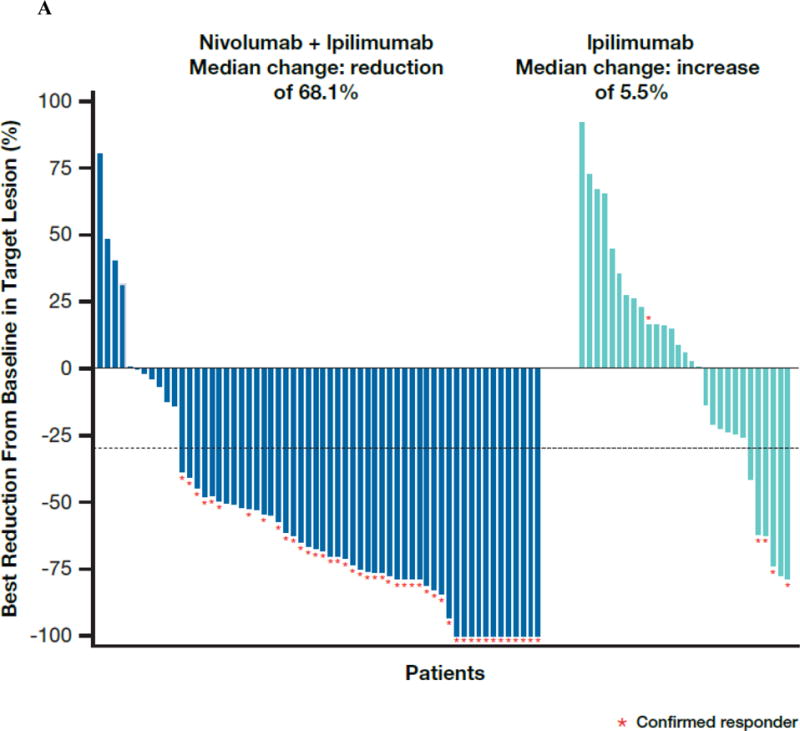
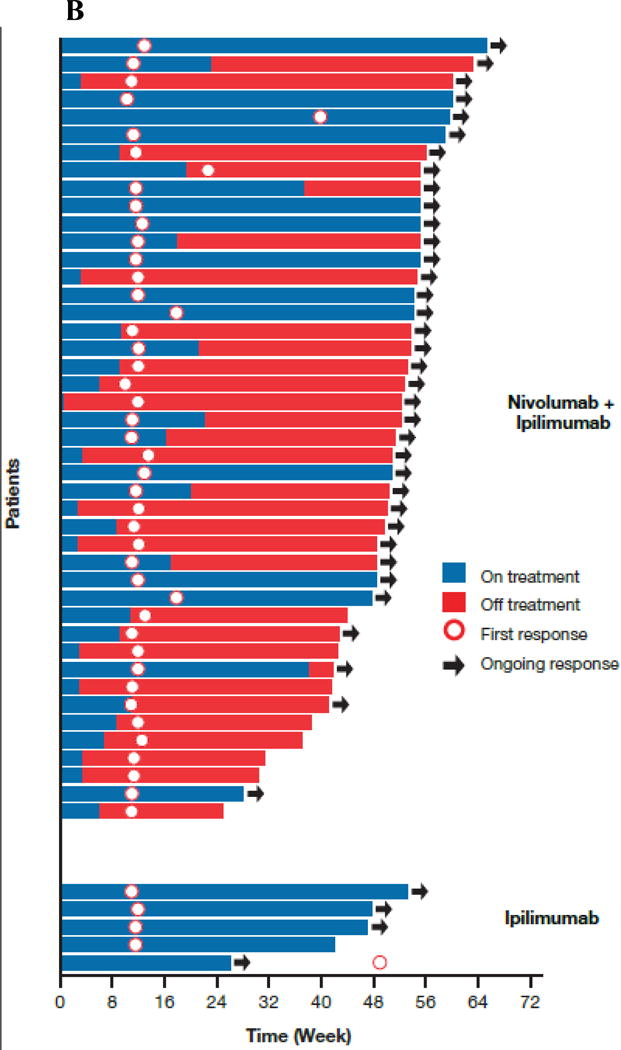
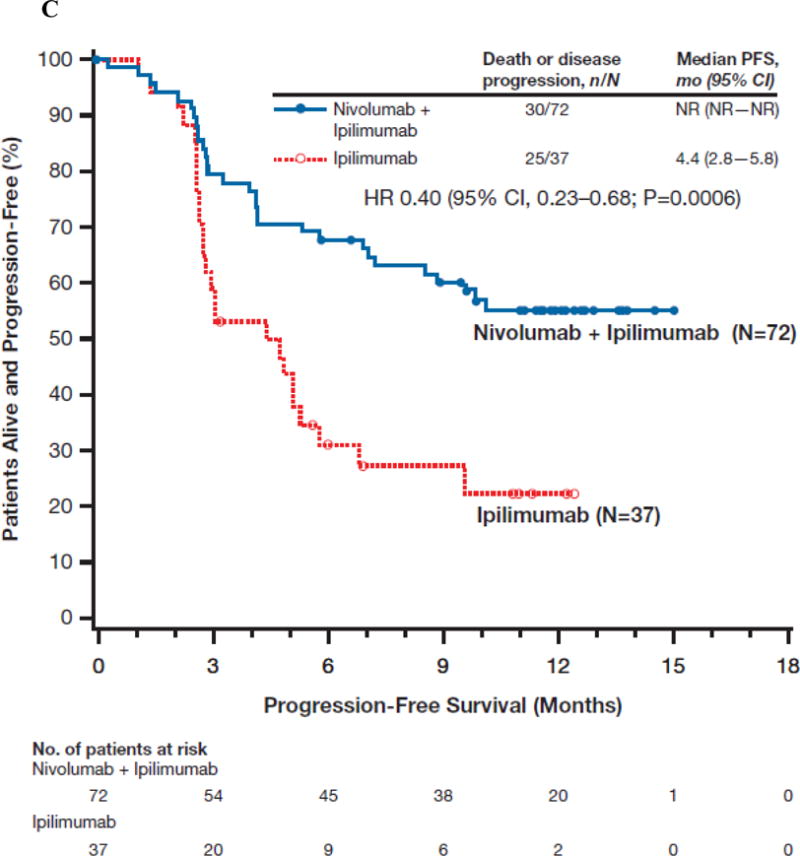
(Panel A) Tumor burden change from baseline in the sum of reference diameters of target lesion among patients receiving combination nivolumab and ipilimumab (left) or ipilimumab monotherapy (right); Horizontal reference line indicates the 30% reduction in tumor burden consistent with a RECIST 1.1 response; (Panel B) Durability of tumor regressions in patients with advanced melanoma with BRAF wild-type tumors who had objective responses to combination nivolumab and ipilimumab or ipilimumab alone according to conventional RECIST criteria. Blue bars indicate the time to and duration of response while on treatment; red bars indicate duration of response after treatment discontinuation; open circles indicate first evidence of objective response; arrows indicate ongoing response at time of analysis. (Panel C) Kaplan-Meier curves for progression-free survival of patients with BRAF wild-type tumors treated with combination nivolumab and ipilimumab or ipilimumab alone.
Among the BRAF wild-type patients randomized, the median duration of response was not reached in either group with ongoing response seen in 36 of the 44 (81.8%) responders in the combination group and in 3 of the 4 (75.0%) responders receiving ipilimumab monotherapy (Fig. 1B). Time to response did not differ between groups, with the majority of all responses taking place at the time of first scan (Fig. 1B).
In BRAF mutation-positive patients (Table 2), objective response rate was 52.2% (12/23) in the combination group, with similar percentage of complete responses (n=5, 21.7%) as in BRAF wild-type patients. In the BRAF wild-type population, median progression-free survival was not reached for the combination therapy and 4.4 months (95% CI, 2.8 to 5.8) for ipilimumab monotherapy (hazard ratio [HR] 0.40, 95% CI, 0.23 to 0.68; P<0.001; Fig. 1C). Among BRAF mutant-positive patients, median progression-free survival was 8.5 (95% CI, 2.8 to not estimable) and 2.7 (95% CI, 1.0 to 5.4) months for the combination and ipilimumab monotherapy, respectively (HR 0.38, 95% CI, 0.15 to 1.00; Fig. S2). Among all randomized patients who discontinued due to study drug toxicity, objective response rate was 30/44 (68.2%, 95% CI, 52.4 to 81.4) in the combination arm, as compared with 1/10 (10%, 95% CI to 0.4, 44.5) in the ipilimumab monotherapy group.
In BRAF wild-type patients, the response benefit with the nivolumab and ipilimumab combination compared with ipilimumab alone was observed across all pre-specified patient subgroups, including those with M1c stage disease and elevated lactate dehydrogenase levels (Fig. S3). The response rate for nivolumab combined with ipilimumab was independent of patients’ tumor PD-L1 status. Objective response rate was 58.3% (95% CI, 36.6 to 77.9) among the PD-L1 positive patients receiving the combination regimen, 55.4%, (95% CI, 41.5 to 68.7) among the PD-L1 negative patients (Table S2; Fig S3). In the ipilimumab monotherapy group, numerically higher objectives response rates were observed in patients whose tumors were PD-L1 positive (18.2% (95% CI, 2.3 to 51.8) versus those with PD-L1 negative tumors (7.4%. 95% CI, 0.9 to 24.3).
Safety
In the combination group, 58.5% and 57.4% of patients received at least 4 doses of nivolumab and ipilimumab, respectively compared with 69.6% of patients in the ipilimumab plus matched placebo group (Table 4). The investigator-assessed treatment-related adverse event rate was 91.5% in the combination group and 93.5% in the ipilimumab monotherapy group (Table 3). Grade 3–4 drug-related adverse events were reported more frequently in the combination group (54.3%) than in the ipilimumab monotherapy group (23.9%), with most adverse events having first onset in the combination portion versus the nivolumab monotherapy phase. The most common grade 3–4 adverse events associated with the combination were colitis (17.0%), diarrhea (10.6%) and increased alanine aminotransferase (10.6%). Diarrhea was the most frequently reported grade 3–4 adverse event associated with ipilimumab monotherapy (10.9%), followed by colitis in 6.5% of patients.
Table 4.
Treatment-related select adverse event and their management with immunomodulatory medication (IMM) by organ category
| Select Adverse Event Organ Category | Nivolumab + Ipilimumab (N=94)
|
Ipilimumab (N=46)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Patients reporting select adverse event, n (A) | Patients managed with IMM, n (% of A) (B) | Patients with resolutiona of select adverse event after treatment with IMM, n (% of B) | Median time to resolutiona, weeks (95% CI) | Patients reporting select adverse event, n (A) | Patients managed with IMM, n (% of A) (B) | Patients with resolutiona of select adverse event after treatment with IMM, n (% of B) | Median time to resolutiona, weeks (95% CI) | |
|
| ||||||||
| Skin | 67 | 41 (61.2) | 24 (68.6) | 18.6 (9.3, 35.1) | 26 | 13 (50.0) | 11 (84.6) | 8.6 (3.3, 22.0) |
| Grade 3–4 | 9 | 9 (100.0) | 8 (88.9) | 6.1 (0.9, 24.1) | 0 | 0 | 0 | NE |
|
| ||||||||
| Gastrointestinal | 48 | 31 (64.6) | 26 (92.9) | 4.7 (3.0, 6.7) | 17 | 11 (64.7) | 7 (77.8) | 5.0 (1.4, 12.1) |
| Grade 3–4 | 20 | 16 (80.0) | 15 (88.2) | 4.3 (1.4, 10.7) | 5 | 5 (100.0) | 4 (80.0) | 3.6 (0.7, 5.0) |
|
| ||||||||
| Endocrineb | 32 | 14 (43.8) | 2 (14.3) | NE (NE, NE) | 8 | 3 (37.5) | 1 (33.3) | NE (0.9, NE) |
| Grade 3–4 | 5 | 4 (80.0) | 1 (25.0) | NE (5.6, NE) | 2 | 2 (100) | 1 (50.0) | NE (0.9, NE) |
|
| ||||||||
| Hepatic | 26 | 13 (50.0) | 11 (84.6) | 14.1 (3.1, 19.6) | 2 | 0 | 0 | NE |
| Grade 3–4 | 14 | 12 (85.7) | 10 (83.3) | 8.3 (2.1, 14.1) | 0 | 0 | 0 | NE |
|
| ||||||||
| Pulmonary | 11 | 8 (72.7) | 6 (75.0) | 6.1 (0.3, 9.0) | 2 | 2 (100.0) | 2 (100.0) | 3.2 (2.9, 3.6) |
| Grade 3–4 | 3 | 3 (100.0) | 2 (66.7) | 9.0 (0.3, 9.0) | 1 | 1 (100.0) | 1 (100.0) | 3.6 (NE, NE) |
|
| ||||||||
| Renal | 3 | 2 (66.7) | 2 (100.0) | 0.4 (0.3, 0.6) | 1 | 0 | 0 | NE |
| Grade 3–4 | 1 | 1 (100.0) | 1 (100.0) | 0.6 (NE, NE) | 0 | 0 | 0 | NE |
Table includes events reported after the first dose and within 100 days of the last dose of study treatment.
Resolution of an event was defined as complete resolution or improvement to the baseline level among all clustered events in a given category experienced by the patient.
Endocrine events were managed with hormone replacement therapy. Patients requiring long term hormone replacement therapy were not counted as resolved.
NE, not estimable
Table 3.
Summary of Treatment-Related Adverse Events
| Patients Reporting Adverse Events | Nivolumab + Ipilimumab (N=94) | Ipilimumab (N=46) | |||
|---|---|---|---|---|---|
| Any Grade | Grade 3–4 | Any Grade | Grade 3–4 | ||
| Treatment-Related Adverse Events, n (%) | 86 (91.5) | 51 (54.3) | 43 (93.5) | 11 (23.9) | |
| Most frequent (>10% any grade) terms, n (%) | Rash | 39 (41.5) | 5 (5.3) | 12 (26.1) | 0 |
| Pruritus | 33 (35.1) | 1 (1.1) | 13 (28.3) | 0 | |
| Diarrheaa | 42 (44.7) | 10 (10.6) | 17 (37.0) | 5 (10.9) | |
| Fatigue | 37 (39.4) | 5 (5.3) | 20 (43.5) | 0 | |
| Colitisa | 22 (23.4) | 16 (17.0) | 6 (13.0) | 3 (6.5) | |
| Nausea | 21 (22.3) | 1 (1.1) | 11 (23.9) | 1 (2.2) | |
| AST increased | 20 (21.3) | 7 (7.4) | 2 (4.3) | 0 | |
| ALT increased | 21 (22.3) | 10 (10.6) | 2 (4.3) | 0 | |
| Pyrexia | 19 (20.2) | 3 (3.2) | 7 (15.2) | 0 | |
| Rash maculo-papular | 15 (16.0) | 3 (3.2) | 8 (17.4) | 0 | |
| Hypothyroidism | 15 (16.0) | 0 | 7 (15.2) | 0 | |
| Hypophysitis | 11 (11.7) | 2 (2.1) | 3 (6.5) | 2 (4.3) | |
| Headache | 13 (13.8) | 2 (2.1) | 5 (10.9) | 0 | |
| Pneumonitisb | 10 (10.6) | 2 (2.1) | 2 (4.3) | 1 (2.2)b | |
| Arthralgia | 10 (10.6) | 0 | 4 (8.7) | 0 | |
| Chills | 10 (10.6) | 0 | 3 (6.5) | 0 | |
| Decreased appetite | 14 (14.9) | 0 | 4 (8.7) | 0 | |
| Lipase increased | 12 (12.8) | 8 (8.5) | 2 (4.3) | 1 (2.2) | |
| Vitiligo | 10 (10.6) | 0 | 4 (8.7) | 0 | |
| Vomiting | 13 (13.8) | 1 (1.1) | 5 (10.9) | 0 | |
| Rash pruritic | 3 (3.2) | 0 | 5 (10.9) | 0 | |
| Abdominal pain | 10 (10.6) | 0 | 4 (8.7) | 1 (2.2) | |
| Myalgia | 9 (9.6) | 0 | 6 (13.0) | 0 | |
| Dyspnea | 9 (9.6) | 9 (3.2) | 5 (10.9) | 0 | |
| Constipation | 10 (10.6) | 1 (1.1) | 4 (8.7) | 0 | |
| Treatment-Related Adverse Events Leading to Discontinuation, n (%) | 44 (46.8) | 36 (38.3) | 8 (17.4) | 6 (13.0) | |
Table includes events reported after the first dose and within 100 days of the last dose of study treatment.
Diarrhea is defined as a disorder characterized by frequent and watery bowel movements; colitis is defined as a disorder characterized by inflammation of the colon. Grade 3–4 drug-related adverse events were reported more frequently in the combination group than in the ipilimumab monotherapy group with most adverse events appearing to occur in the combination portion compared with nivolumab monotherapy.
An ipilimumab patient had progressive disease on April 28, 2014 and was unblinded before starting nivolumab monotherapy a day later. This patient received 10 cycles of nivolumab monotheapy before the onset of pneumonitis after the last dose on September 25, 2014
Select adverse events of potentially immune-mediated etiology occurred most frequently in skin, gastrointestinal, and hepatic organ categories (Table 4, and S5), and were observed more frequently with the combination than with ipilimumab monotherapy. Immunosuppressive medications for management of adverse events, including topical agents for dermatological adverse events were used in a higher percentage of patients in the combination group compared with the ipilimumab group (89.4 and 58.7%, respectively). The most common systemic immunosuppressive agents across both treatment arms were glucocorticoids (81.9% and 50.0% in the combination and ipilimumab monotherapy groups, respectively). Infliximab was administered to 12.8% and 8.7% of patients, respectively, for adverse event management. Hormone replacement was used to manage endocrine adverse events. The majority (37/46, 80.4%) of grade 3–4 drug-related select adverse events resolved either completely or to baseline in the combination group, and there was a similar resolution rate across organ categories in both arms (Table 4).
The most common reason for discontinuing study treatment was drug-related toxicity in the combination group (52.1%) and disease progression in the ipilimumab monotherapy group (32.4%; Table S5). After the initial 4 doses, 40.4% of patients in the combination arm continued to receive nivolumab monotherapy (Table S3).
A total of 25 (26.6%) and 17 (37.0%) deaths were reported in the combination and the ipilimumab monotherapy groups, respectively, mostly due to progressive disease. Three deaths were related to the combination therapy by investigator assessment—one patient with a history of cardiac disease died due to ventricular arrhythmia 29 days after the last dose of study treatment; the second died suddenly while clinically improving from pneumonitis and undergoing an iatrogenic pneumothorax 69 days after the last dose. A third patient sustained sudden death in the combination group, 86 days after the last dose of study treatment (3 days after resolution of grade 3 pneumonia and grade 4 hypercalcemia). None of the deaths in the ipilimumab monotherapy group was deemed study drug-related.
DISCUSSION
In this double-blind, randomized study, the combination of nivolumab and ipilimumab resulted in a significantly higher objective response rate, more frequent complete responses, and significantly improved progression-free survival compared with ipilimumab alone in untreated patients with advanced melanoma. The confirmed response rate of the combination therapy in this trial (61%) is numerically higher than the 40% response rate of nivolumab monotherapy recently reported in the first-line setting for patients with advanced melanoma who have tumors that are BRAF wild-type, and also in trials of pembrolizumab monotherapy, another anti-PD-1 agent.3, 24 However, it is inherently difficult to compare efficacy of the combination therapy to anti-PD-1 monotherapy as patient demographics differ among trials.
Based upon the high degree of tumor reduction in this current study with frequent complete responses (22.2%), favorable clinical benefit can be anticipated with longer follow-up. Overall, the characteristics of response observed with nivolumab plus ipilimumab in the current study are consistent with previously reported results,15, 16 with most responses occurring by time of first tumor assessment, and in many patients, responses continuing despite discontinuation of therapy. The response rate of the combination regimen in this current phase 2 study was even higher than response rates previously reported, which may be explained by the patient population being treatment naïve in this study. A prior phase 1 trial of the combination regimen at varying doses showed high 1- (85%) and 2-year (79%) overall survival.15, 16
The primary endpoint of this study specifically addressed patients with BRAF wild-type melanoma because at the time of study enrollment, for this group of patients, ipilimumab was the only approved therapy that had demonstrated overall survival benefit in a randomized phase 3 trial. While BRAF inhibitors as single agents and BRAF/MEK inhibitor combinations can result in high response rates in patients with BRAF mutant melanoma,13, 14, 19 no single agent or combination of agents has similarly been shown to result in a high response rate in patients with BRAF wild-type melanoma. Nevertheless, for patients with BRAF mutant melanoma, the overall response rate and progression-free survival of the combination therapy regimen was also substantially higher than with ipilimumab alone. This was consistent with prior phase 1 experience and suggests that the presence of the BRAF V600 mutation does not influence the efficacy of checkpoint blockade.15, 16, 21
In general, the spectrum of select adverse events seen in this study was consistent with the prior experience with the combination.15 Three deaths related to the combination were reported in this study and could be linked to pre-existing condition related to the cause of death or required medical procedures that might have contributed to the death. The proportion of patients experiencing a drug-related adverse event with the nivolumab and ipilimumab combination was higher than ipilimumab monotherapy, with approximately half of patients (54%) experiencing a grade 3–4 treatment-related adverse event compared with 24% in the ipilimumab monotherapy group. Select grade 3–4 adverse events generally manifested within the first 15 weeks of treatment with the combination and typically required less than 9 weeks to resolve dependent on the specific adverse event. Aside from endocrinopathies, which typically require continued hormone replacement, the majority of patients eventually had complete resolution of their grade 3–4 adverse event. It is noteworthy that among the patients who discontinued combination treatment due to toxicity, 68.2% experienced an objective response and most continue to be in a response.
Expression of PD-L1, one of the ligands for PD-1, has been associated with higher response rates in patients treated with nivolumab when administered as a single agent.22, 23 For patients treated with the combination regimen in this study; however, there was no difference in response rates between patients whose pretreatment tumor cells were defined as PD-L1-positive vs. PD-L1 negative. These data suggest that PD-L1 should not be used to select patients to receive combination treatment. The mechanism for response independent of baseline PD-L1 status remains unclear. It is possible that since ipilimumab drives T cells into the tumor, this on-treatment T-cell infiltration leads to a more favorable microenvironment for anti-PD-1 efficacy.25 It is also possible that assessing PD-L1 status on tumor infiltrating macrophages or T cells may be most relevant as opposed to the tumor cells as performed in this study, but this requires additional investigation.26
In summary, the combination of ipilimumab plus nivolumab, as compared with ipilimumab monotherapy, resulted in a substantially higher objective response rate, durable responses, improved progression-free survival, and higher complete response rates in patients with both BRAF wild-type and BRAF mutant advanced melanoma. The incidence of adverse events was higher with combination therapy, but remained generally manageable when established safety guidelines were utilized. The risk-and-benefit profile of combined PD-1 and CTLA-4 blockade compared with monotherapy will ultimately be further clarified by data from ongoing phase 3 double-blind randomized trials, such as the CheckMate 067/NCT01844505 study.
Supplementary Material
Acknowledgments
The authors would like to thank the patients who participated in this study; clinical faculty and personnel, including Elizabeth Buchbinder formerly of the Beth-Israel Deaconess Medical Center, currently of Dana Farber Cancer Institute; Ryan Sullivan of Massachusetts General Hospital; Jason Luke, formerly of the Dana Farber Cancer Institute currently at the University of Chicago; Marta Colgan, Jenessa Holder, Yelena Shames, Vanessa Reed, and Alyona Weinstein of Memorial Sloan Kettering Cancer Center; Rajni Kannan, Kathleen Madden, Ethel Yepes, Crystal Escano, Caroline Muren, Claire Stein, and Martina Romain of the New York University Medical Center; Miriam Faruqi and Jaclyn Neely of Bristol-Myers Squibb. Editorial and writing assistance was provided by Wayne Peng and Karin McGlynn of StemScientific, funded by Bristol-Myers Squibb.
Footnotes
Disclosure:
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2014 doi: 10.1056/NEJMoa1412082. Epub ahead of print September 29. [DOI] [PubMed] [Google Scholar]
- 4.Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 5.Long GV, Stroyakovsky D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 6.Robert C, Karaszewska B, Schachter J, et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. N Engl J Med. 2014 doi: 10.105/NEJMoa1412690. Epub ahead of print September 29. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DB, Flaherty KT, Weber JS, et al. Combined BRAF (dabrafenib) and MEK inhibition (trametinib) in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor. J Clin Oncol. 2014;32:3697–3704. doi: 10.1200/JCO.2014.57.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323–32. doi: 10.1016/S1470-2045(14)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ascierto PA, Schadendorf D, Berkng C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol. 2013;14:249–256. doi: 10.1016/S1470-2045(13)70024-X. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki T, Chikuma S, Iwai Y, et al. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14:1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 13.Curran MA, Montalvo W, Yagita H, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selby M, Englehardt J, Lu L-S, et al. Antitumor activity of concurrent blockade of immune checkpoint molecules CTLA-4 and PD-1 in preclinical models. J Clin Oncol. 2013;31(suppl) abstr 3061. [Google Scholar]
- 15.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sznol M, Kluger HM, Callahan MK, et al. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL) J Clin Oncol. 2014;32(suppl) abstr LBA9003^. [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaert J, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.NCI. NCI Common Terminology Criteria for Adverse Events (CTCAE) v.4. Accessed October 23, 2014 at http://evs.nci.nih.gov/ftp1/CTCAE/About.html.
- 19.Shahabi V, Whitney G, Hamid O, et al. Assessment of association between BRAF-V600E mutation status in melanomas and clinical response to ipilimumab. Can Immunol Immunother. 2012;61:733–737. doi: 10.1007/s00262-012-1227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber J, Minor D, D’Angelo S, et al. A phase 3 randomized, open-label study of nivolumab (anti-PD-1; BMS-936558; ONO-4538) versus investigator’s choice chemotherapy (ICC) in patients with advanced melanoma after prior anti-CTLA-4 therapy. ESMO. 2014 Abstract #7218;Presentation #LBA3. [Google Scholar]
- 21.Kluger H, Sznol M, Callahan M, et al. Survival, response duration, and activity by BRAF mutation (MT) status in a phase 1 trial of nivolumab (anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL) Ann Oncol. 2014;25(suppl_4):iv374–iv393. [Google Scholar]
- 22.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Can Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribas A, et al. A randomized controlled comparison of pembrolizumab and chemotherapy in patients with ipilimumab-refractory melanoma. Society for Melanoma Research 2014 Congress. J Pigment Cell Mel Res. 2014 doi: 10.1111/pcmr.12317. Epub ahead of print Oct. 23. [DOI] [Google Scholar]
- 25.Hamid O, Schmidt H, Nissan A, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011 doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces response by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


