Abstract
Objective
Sepsis is the leading cause of lung injury in adults and can lead to Acute Respiratory Distress Syndrome (ARDS). Using a novel technique of isolated in vivo lung perfusion (IVLP), we hypothesized that normothermic IVLP will improve oxygenation and compliance in a porcine model of sepsis-induced lung injury.
Methods
Mature adult swine (n=8) were administered lipopolysaccharide (LPS, 50 μg/kg over 2 hours) via the external jugular vein followed by sternotomy and central ECMO cannulation (right atrium to ascending aorta). Left pulmonary artery (inflow) and left superior and inferior pulmonary veins (outflow) were dissected out and cannulated to deliver isolated perfusion to the left lung. After 4 hours normothermic IVLP with Steen solution, the left lung then underwent 4 hours of reperfusion after IVLP decannulation. Airway pressures and lung-specific pulmonary vein blood gases from right (LPS control) and left lungs (LPS+IVLP) of the same animal were compared.
Results
All animals demonstrated a significant reduction in PaO2/FiO2 ratio and total lung compliance 2 hours after the start of LPS infusion (469±19.7 vs. 222.2±21.4 mmHg, p<0.0001). After reperfusion, six (75%) animals had improved lung function allowing for ECMO decannulation. Lung-specific oxygenation was superior in the left lung after 4 hours of reperfusion (310.5±54.7 vs. 201.1±21.7 mmHg, p=0.01). Similarly, total lung compliance improved after IVLP of the left lung. Lung wet-to-dry weight ratio demonstrated reduced edema in rehabilitated left lungs (6.5±0.3 vs. 7.5±0.4, p=0.04).
Conclusions
IVLP successfully rehabilitated LPS-injured lungs compared to ECMO support alone in this preclinical porcine model.
Classifications: ARDS, Lung Rehabilitation, In Vivo Lung Perfusion, Steen, LPS, Isolated Lung Perfusion
Graphical Abstract
Introduction
Acute Respiratory Distress Syndrome (ARDS) represents the final devastating result of acute lung injury. This condition imposes a significant mortality and long-term effects on patient quality-of-life.1–3 Infection and circulatory shock represent the predominant etiologies for ARDS development.2, 4 Extracorporeal Membrane Oxygenation (ECMO) and protective ventilation strategies are the current standards of care; yet provide only passive support for innate mechanisms of healing to occur over an often-protracted time course.1, 2, 5 Current forms of pharmacotherapy are extremely limited. Data in support of early corticosteroid use is inconclusive, and administration beyond 14 days is contraindicated.6–8 Early treatment with a 48-hour infusion of the neuromuscular blocking agent cisatracurium has been shown to reduce mortality but has no effect on duration of mechanical ventilator support.9–11 Currently, no methods exist to treat and rapidly rehabilitate lungs affected by ARDS. It has become increasingly apparent that a breakthrough in technology will be required to reduce the significant mortality associated with this grave disease.
Recently, ex vivo lung perfusion (EVLP) has demonstrated clinical success as a platform to assess donor lungs prior to transplantation.12 Our laboratory has shown that EVLP may also succeed as a therapeutic platform for rehabilitation of injured lungs.13–15 This therapy involves placement of cannulas in the pulmonary artery (inflow) and the left atrium (outflow) for perfusion and an endotracheal tube into the trachea to ventilate the lungs after excision from a donor. The EVLP circuit design is modified from clinical cardiopulmonary bypass and is utilized for the ex vivo perfusion of lungs. The perfusion circuit includes a membrane deoxygenator that enables the evaluation of oxygenation capacity of the donor lungs.12, 16 Steen solution (XVIVO Perfusion Inc., Englewood, CO), used for EVLP, is a physiologic solution consisting of albumin, dextran, and electrolytes, supplemented with steroids, antibiotics, and heparin, that can reduce lung edema, provide free radical scavenging, and ameliorate harmful inflammation and endothelial damage. Steen solution provides an ideal platform for targeted drug, cellular, or gene therapy during EVLP for further improvement in lung recovery. Additionally, the EVLP circuit includes a leukocyte-reducing filter, which lowers the burden of circulating inflammatory cells. Finally, a critical principle of EVLP is maintenance of physiologic backpressure (+1–5 mmHg) on the pulmonary venous drainage to reduce cavitation and vascular injury.16 This technology has been taken one step further for isolated lung perfusion in vivo, however never used with a rehabilitative intent.17–19
Circulatory shock secondary to sepsis is the leading cause of ARDS. Using a porcine model of ARDS induced by endotoxin-mediated lung injury, we sought to test the rehabilitative potential of a novel strategy of isolated left lung In Vivo Lung Perfusion (IVLP) therapy. This previously validated model of sepsis-induced ARDS using systemic lipopolysaccharide (LPS) produces a dose-dependent reduction in oxygenation and lung compliance.20, 21 We have previously demonstrated a decrease in PaO2/FiO2 ratios and a reduction of dynamic compliance over four hours of EVLP.22 In the present study, we hypothesized that isolated left lung perfusion via IVLP would result in attenuation of lung injury with reduced inflammation and edema, as compared to lungs that received ECMO support only.
Materials and Methods
Animals and Study Groups
The Animal Care and Use Committee (ACUC) at the University of Virginia ensured this study complied with the 1996 Guide for the Care and Use of Laboratory Animals as recommended by the US National Institutes of Health and provided approval for this study. A total of eight farm-raised swine of both sexes (45–50kg) were used for this study. After induction of general anesthesia, animals underwent placement of right carotid artery catheter for blood pressure monitoring and left jugular vein catheter for vascular access, and placement of a Swan-Ganz catheter. Animals remained anesthetized with pentobarbital infusion for the duration of the experiment. Conventional ventilation was used with a tidal volume = 8 mL/kg, respiratory rate = 12–16 breaths/min, positive end-expiratory pressure = 5 mmHg, and FiO2 = 0.40. Systemic arterial blood gases and lung compliance were measured every 30 minutes for the duration of the experiment to assess lung function and performance.
Lung Injury Protocol
Baseline arterial blood gas and lung compliance were collected demonstrating no intrinsic lung injury. The animals then received 50 μg/kg of LPS (from Escherichia coli 0127: B8, Sigma-Aldrich, St. Louis, MO) over a two-hour infusion to induce lung injury. Lidocaine (50 mg) was administered, followed by midline sternotomy and incision of the pericardium. After arterial blood gas confirmed lung injury, interlocking ascending aortic and running right atrial purse-string sutures were placed with 5-0 Prolene (Ethicon, Somerville, NJ). Animals then received 100 U/kg of intravenous heparin (Hospira Inc., Lake Forest, IL) and an 18F Bio-Medicus arterial cannula and 28F Bio-Medicus duel-stage venous cannula (Medtronic Inc., Minneapolis, MN) were placed for central Veno-Arterial ECMO. After de-airing, the circuit was completed with an Affinity Membrane Oxygenator (Medtronic Inc., Minneapolis, MN) and centrifugal pump (Medtronic Inc., Minneapolis, MN). ECMO was initiated with a goal mean arterial pressure (MAP) of 65 mmHg. Ventilation was continued with lung protective settings (tidal volume = 6 mL/kg, respiratory rate = 8 breaths/min, positive end-expiratory pressure = 5 mmHg, FiO2 = 0.21). The circuit was managed by a certified perfusion specialist with specific criteria for goal-directed resuscitation to maintain MAP >65 mmHg, including fluid bolus with normal saline and the addition of epinephrine and norepinephrine. Systemic arterial blood gases were collected every 30 minutes to titrate oxygen delivery and sodium bicarbonate was given for metabolic acidosis with pH<7.30. Hourly Activated Clotting Time (ACT) was measured, and heparin was administered to maintain ACT between 250–350 seconds.
In Vivo Lung Perfusion
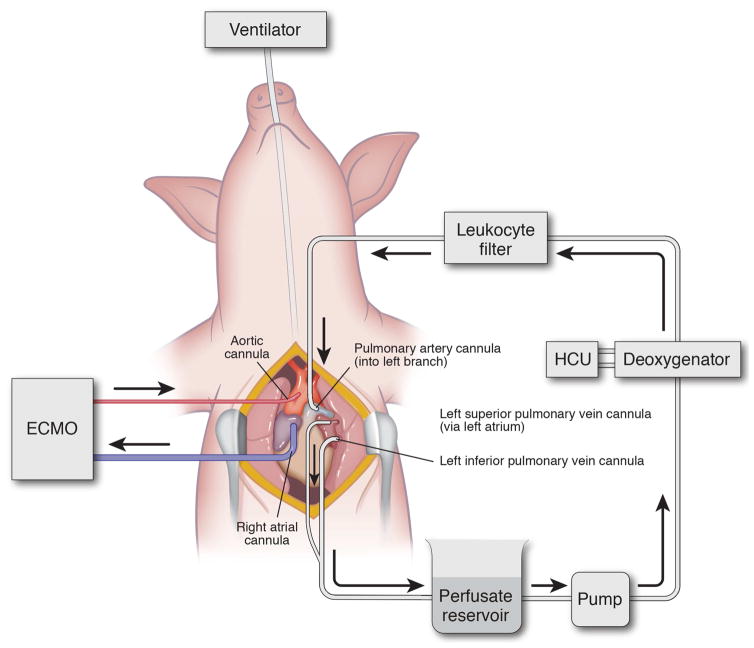
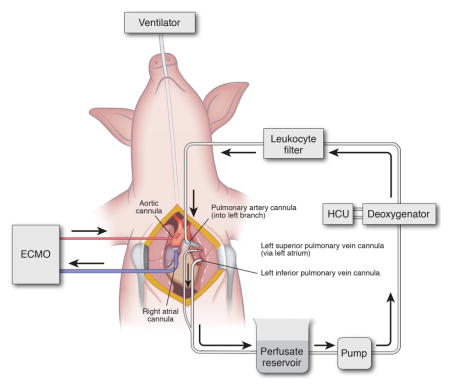
After ECMO support was initiated, perfusion to the left lung was isolated (Figure 1). The left superior and inferior pulmonary veins and left pulmonary artery (PA) were dissected circumferentially and snared with 0-Silk sutures and Rommel tourniquets. A purse string was placed in the left PA, left inferior pulmonary vein and the left atrium (for superior vein) with a 5-0 Prolene to allow for cannulation. Arterial catheters (14F) were used for the PA and superior vein while a 14F right angle cannula was used for the inferior vein. After placement of each cannula, a Rommel tourniquet was used to tighten the purse string and the snare suture was then cinched tight around the distal tip of the catheter to isolate each vessel. To prevent lung injury from outflow obstruction, the PA was cannulated and isolated first, followed by the inferior then superior veins. After de-airing the system, the circuit was completed with an Affinity Pixie Oxygenation System (Medtronic Inc., Minneapolis, MN) (to deoxygenate the perfusate) and centrifugal pump (Medtronic Inc., Minneapolis, MN). Outflow was opened, followed immediately by gentle inflow gradually increasing up to 8% of cardiac output (CO) with Steen solution (XVIVO Perfusion Inc., Englewood, CO) supplemented with cefazolin (500 mg, APP Pharmaceuticals, Schaumburg, IL), methylprednisolone (500 mg, Pfizer Inc., New York, NY), and heparin (5,000 IU) at 37.5°C. Isolated left lung perfusion (IVLP) was maintained for 4 hours while the animal was simultaneously on central ECMO support. Inflow (Left PA) and outflow (Left Pulmonary Veins) pressures were measured and outflow pressures maintained between 0–5 mmHg. Every hour, lung oxygenation capacity was calculated for each lung. For the right lung, the difference between the right pulmonary vein PaO2 and right pulmonary artery sample from the Swan-Ganz catheter was used. The oxygenation capacity of the left lung was calculated as the difference between IVLP circuit outflow (left pulmonary veins) and IVLP circuit inflow (left pulmonary artery). Serum albumin levels were measured from the animal, the perfusate prior to initiation of IVLP, and the animal after IVLP to determine extent of mixing between the circuits through the left bronchial arteries and left pulmonary veins. We demonstrate serum albumin decreases during IVLP despite very high levels of albumin in the perfusate, indicating no mixing from the IVLP circuit into systemic circulation. (Supplemental Figure 1). After 2 hours, the IVLP perfusate (Steen solution) was exchanged for fresh Steen solution. After 4 hours total IVLP perfusion, the left lung was reperfused via the systemic circulation and the IVLP cannulas were removed. Right pulmonary vein and left pulmonary vein specific arterial blood gases were collected every hour to assess performance of each lung independently. Over the final 4-hours of in vivo reperfusion, attempts were made to wean the animal from ECMO support and if successful, the animal was decannulated from central V-A ECMO. At the end of the experiment, tissue and blood samples were collected and the animal was euthanized.
Figure 1.
Surgical technique for porcine IVLP.
Histopathology
Three samples of fresh tissue (one upper lobe, two lower lobe) were obtained and weighed from left and right lungs. A vacuum oven was used to desiccate the tissues at 55°C. Wet-to-dry weight ratios were calculated using separate tissue samples and averaged per lung to assess overall pulmonary edema accumulation.
After obtaining fresh tissue samples, the lungs were fixed via intratracheal instillation of 10% buffered formalin and stored submerged overnight at 4°C. Following overnight fixation, peripheral lung tissue samples (n=4/lung) were obtained, paraffin-embedded and sectioned. One slide from each sample was stained with hematoxylin-eosin (H&E). Subsequently, a masked pathologist assessed the H&E stained slides for presence of lung injury. Each slide was scored on a standard scale, as previously described, based on infiltration of polymorphonuclear cells per 40X high-powered field (0–3), alveolar edema (0–3), and interstitial inflammation (0–3).23, 24
Expression of Biomarkers of Injury and Inflammation
Fresh tissue samples were flash frozen in liquid nitrogen and stored at −80°C. After homogenization using a FastPrep®-24 instrument with lysing matrix D tubes (MP Biomedicals, Santa Ana, CA), the total protein concentration in the supernatant was determined with a bicinchoninic acid protein assay (Pierce, Rockford, IL). Bronchoalveolar lavage (BAL) of the upper lobe of the lung was also performed, centrifuged, and stored at −80°C. Additionally, isolated left lung perfusate samples were collected 2 hours after initialization of IVLP, centrifuged and stored at −80°C. Multiplex enzyme-linked immunosorbent assay (EMD Millipore, Billerica, MA) was used to measure cytokine levels in homogenized tissue supernatant (normalized to total protein) or BAL fluid.
Expression of Vascular Cellular Adhesion Molecule 1 (VCAM-1) and Intracellular Adhesion Molecule 1 (ICAM-1) in lung tissue was assessed by Western blot analysis. In detail, 15 μg of whole cell protein extract was electrophoresed in a 12% Bis-Tris polyacrylamide gel followed by transfer to a nitrocellulose membrane. The membranes were blocked for 1 hour in Tris-buffered saline and Tween 20 with 5% nonfat milk for 1 hour. After blocking, membranes were incubated with anti-VCAM-1 monoclonal antibody (1:1000; Abcam, City and State) or anti-ICAM-1 monoclonal antibody (1:1000; Abcam) overnight, followed by 3 washes. Membranes were then incubated in horseradish peroxidase-conjugated secondary antibody, followed by another series of washes. Protein was visualized by autoradiography and then analyzed by Image Analysis software (Bio-Rad, Hercules, CA, USA). Densities were standardized against β–actin levels for each sample.
Statistical Analysis
Wilcoxon signed-rank paired tests for non-parametric data were used to determine statistical significance. To account for repeated measures, a mixed effect linear regression model with repeated measures was fit. Prism 7 (GraphPad Software Inc., La Jolla, CA) and SAS version 9.4 (SAS Institute, Cary, NC) were used to perform statistical calculations and all data were reported as mean ± standard deviation. A p value <0.05 represented statistical significance.
Results
In Vivo Lung Perfusion Model
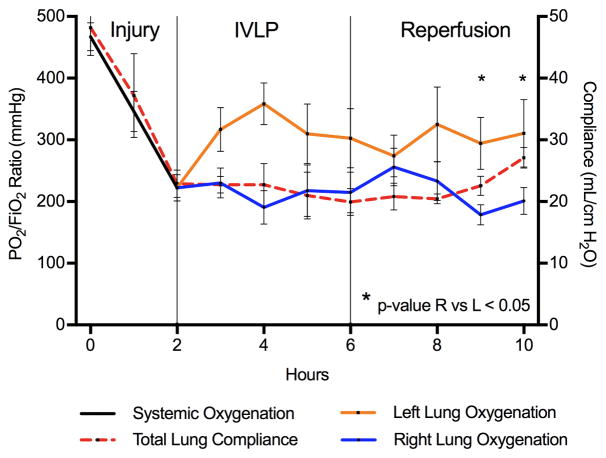
A total of 8 adult swine underwent the experimental protocol with significant acute lung injury after infusion of LPS (initial P/F ratio = 469.0±60.1vs. 2 hours after LPS = 222.2±60.6, p<0.008) (Figure 2). Of note, there was no difference oxygenation performance between right and left lungs of each animal at baseline or 2-hour injury time periods (Supplemental Figure 2A and B). All animals were placed on ECMO for respiratory and circulatory support. After isolating the left pulmonary vasculature all animals underwent successful IVLP cannulation. Subsequently, the left lung oxygenation increased significantly after 2 hours of IVLP (left P/F ratio = 358.7±127.6 vs. right = 190.8±110.6, p=0.035), which remained significantly higher after 4 hrs of IVLP (left P/F ratio = 302.7±135.2 vs. right = 214.7±93.0, p=0.048) (Figure 2). Following IVLP decannulation and three hours of reperfusion, the left lung significantly outperformed the right lung in oxygenation (right P/F ratio = 178.5±46.2 vs. left = 294.4±119.2, p=0.039) (Supplemental Figure 3C). Final lung specific oxygenation at the end of the reperfusion period demonstrated that the right lung continued to worsen while the left lung remained significantly improved (right P/F ratio = 201.1±64.4 vs. left = 310.5±154.4 mmHg, p=0.008) (Figure 2 and Supplemental Figure 3D). Furthermore, following four hours of reperfusion, 75% (6/8 animals) of animals had adequate improvement in respiratory status to successfully undergo ECMO decannulation. Additionally, the total lung compliance significantly improved from post injury to post 4 hours of reperfusion (22.9±6.3vs. 27.1±4.7 mL/cmH2O, respectively, p=0.008) (Figure 2 and Supplemental Figure 2C). Finally, mixed effects linear regression model demonstrates a parameter estimate of +73.9±18.9 mmHg (p<0.0001) for IVLP treatment.
Figure 2.
Oxygenation (P/F ratio) and total lung compliance. Both systemic oxygenation (Black) and total lung compliance (Red) drop precipitously over the two-hour lung injury with LPS. After isolation and cannulation for IVLP the Left lung oxygenation (Orange) improved significantly compared to Right (Blue). After decannulation and reperfusion the left lung continued to improve in addition to total lung compliance (Red). * = p-value<0.05 for left vs right lung oxygenation.
Histopathology
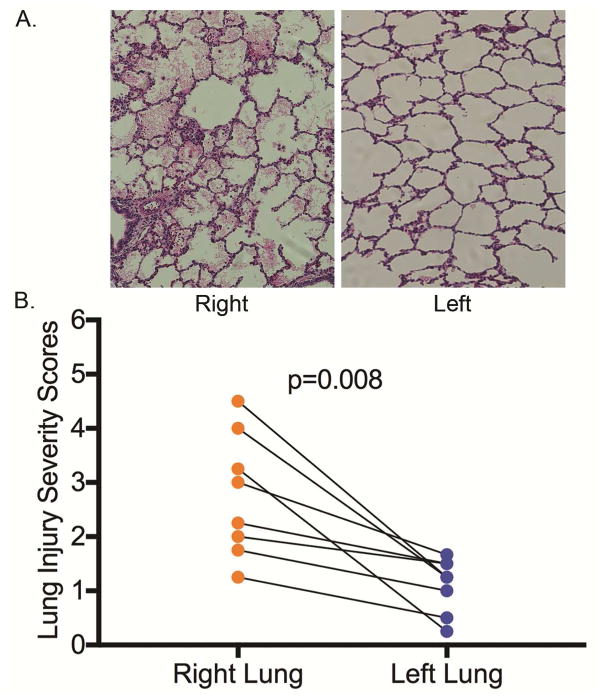
Measurement of lung wet-to-dry weight ratios after reperfusion demonstrated significantly reduced edema in the left lung compared to the right (6.5±0.8 vs. 7.5±1.1, respectively, p=0.039) (Figure 3). Furthermore, lung histology was noticeably improved and lung injury severity scores were significantly reduced in the left lungs compared to right lungs (1.1±0.5 vs. 2.8±1.1, respectively, p=0.008) (Figure 4).
Figure 3.
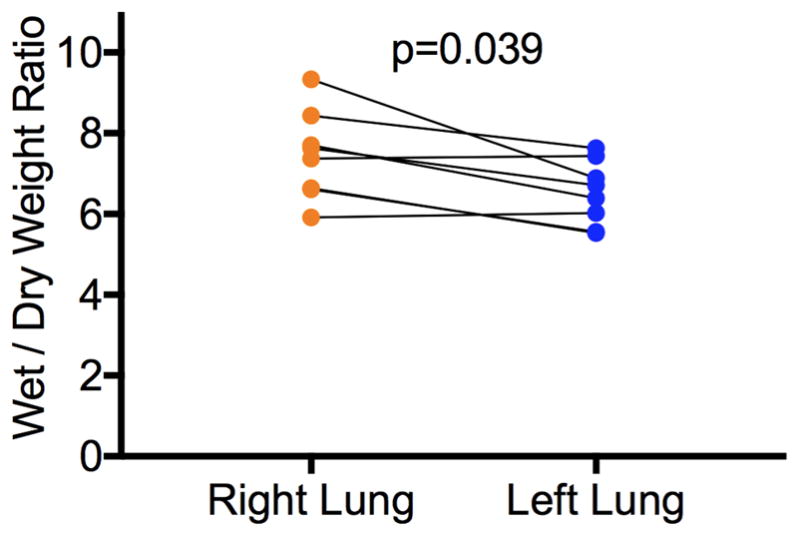
Wet/Dry weight ratio of left lung compared to right lung for each animal as a measurement of lung edema.
Figure 4.
A. H&E histologic micrographs at 40x magnification of left and right lung from the same animal. B. Lung injury scores of left and right lungs from each animal.
Inflammatory Biomarker Analysis
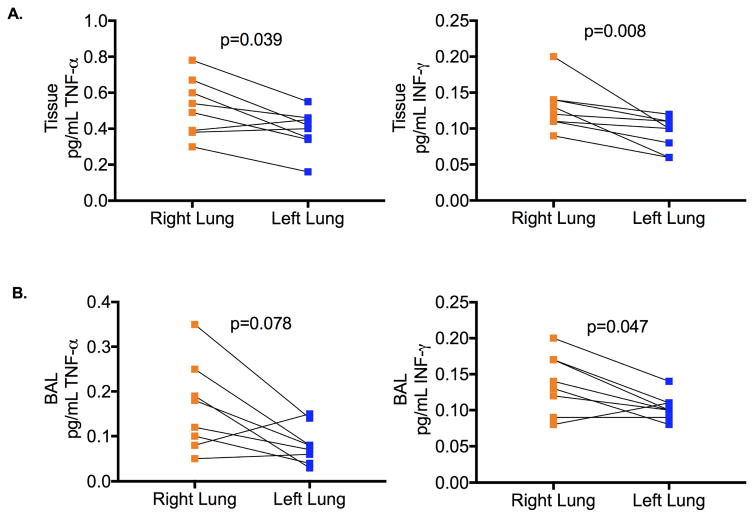
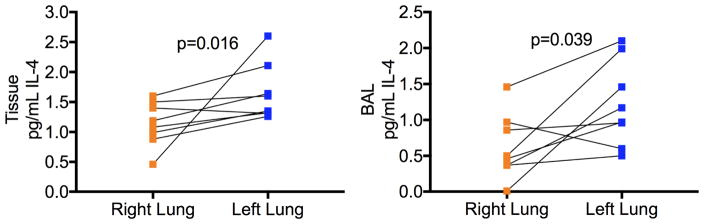
Expression of pro-inflammatory the cytokine TNF-α were significantly attenuated in homogenized lung tissue from left lungs compared to right lungs from the same animals (TNF-α left lung tissue 0.164±0.12 pg/mL vs right lung tissue 0.163±0.14 pg/mL, p=0.039, left lung BAL 0.081±0.04 pg/mL vs right lung BAL 0.165±0.10 pg/mL, p=0.078) (Figure 5A and B). Additionally, expression of the pro-inflammatory cytokine IFN-γ was significantly lower both lung tissue homogenate and BAL from the treated left lung compared to the control right lung of each animal (IFN-γ left lung tissue 0.093±0.02 pg/mL vs right lung tissue 0.130±0.03 pg/mL, p=0.046, left lung BAL 0.104±0.02 pg/mL vs right lung BAL 0.138±0.04 pg/mL, p=0.078) (Figure 5A and B). Furthermore, expression of the anti-inflammatory cytokine IL-4 was significantly elevated in the left lung compared to right lungs of the same animal (IL-4 left lung tissue 1.649±0.48 pg/mL vs right lung tissue 1.138±0.37 pg/mL, p=0.039, left lung BAL 1.219±0.59 pg/mL vs right lung BAL 0.625±0.45 pg/mL, p=0.016) (Figure 6).
Figure 5.
A. Pro-inflammatory cytokine concentrations in tissue of left compared to right lung of each animal. B. Pro-inflammatory cytokine concentrations in Bronchial Alveolar Lavage (BAL) samples of left compared to right lung of each animal.
Figure 6.
Anti-inflammatory cytokine (IL-4) concentrations in tissue and Bronchial Alveolar Lavage (BAL) samples of left compared to right lung of each animal.
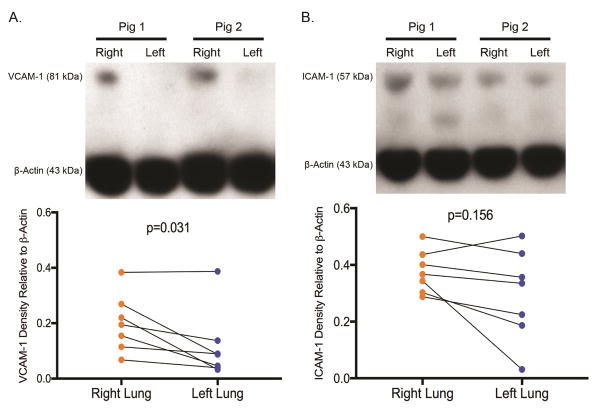
Western blot analysis demonstrated significantly decreased expression ratio of VCAM-1 in the left lungs compared to the right (VCAM-1 left lung tissue 0.201±0.10 vs right lung tissue 0.117±0.12, p=0.0391) (Figure 7A). Similarly, ICAM-1 expression ratio was lower in the left lungs, which did not reach statistical significance (ICAM-1 left lung tissue 0.377±0.08 vs right lung tissue 0.297±0.16, p=0.156) (Figure 7B).
Figure 7.
A. Western Blot demonstrates significantly decreased VCAM-1 expression in left compared to right lung of each animal. B. Western Blot demonstrates lower ICAM-1 expression in left compared to right lung of each animal.
Discussion
The present study demonstrates that IVLP of the left lung results in significant rehabilitation of sepsis-induced ARDS over a 10-hour study period in a preclinical porcine model. Both lung-specific oxygenation and total lung compliance improved after IVLP therapy with 75% of animals’ no longer requiring ECMO support. These improvements in lung function were likely multifactorial but can be attributed to reduction of pulmonary edema and attenuation of the inflammatory pathways in the treated lung. These data indicate that IVLP therapy with
Steen solution reduces the expression of pro-inflammatory cytokines TNF-α and INF-γ while providing an increase in the anti-inflammatory cytokine IL-4. Finally, these results correlate with reduced expression of adhesion molecules VCAM-1 and ICAM-1 after IVLP treatment, which are critical mediators of leukocyte transmigration into lung tissue. This proof-of-concept study serves as a launch pad for a novel, advanced, and rapid surgical method for the treatment of ARDS, which will require conversion into a percutaneous platform for future translation to clinical use. Steen solution was first described by Steen et al. in 2001 as a physiologic acellular perfusate consisting of albumin, dextran, and electrolytes and further modified by the Toronto group for use in EVLP and lung transplantation.12, 25, 26 Several groups have demonstrated that perfusion with Steen solution can reduce pulmonary edema in ex vivo perfused lungs.23, 27, 28 Our LPS-induced model of porcine ARDS simulates the increased vascular permeability observed during sepsis. This injury is ameliorated with 4 hours of IVLP perfusion, and these data demonstrate enduring edema reduction even after 4 hours of reperfusion. A reduction in pulmonary edema is a critical mechanism by which lung function improves after IVLP therapy in this preclinical model.
Sepsis-induced lung injury through the LPS pathway stimulates an inflammatory cascade by activation of toll-like receptor 4 (TLR-4), leading to activation of neutrophils and macrophages.29 Both TNF-α and INF-γ are pro-inflammatory cytokines within this pathway helping to amplify the inflammatory response. After 4 hours of IVLP treatment, we observed reduced expression of these cytokines in the rehabilitated left lungs compared to the right control lungs despite 4 additional hours of reperfusion. Additionally, IL-4 is a potent immunomodulatory cytokine synthesized and released by Th2 lymphocytes, which suppressed LPS-induced COX-2 expression in monocytes.30, 31 Furthermore, IL-4 is known to suppress pro-inflammatory cytokine production.32 A recent paper by Wehrmann and colleagues suggests IL-4 expression decreases TNF-α production by resident alveolar macrophages, thus reducing accumulation of M1 macrophages, inflammation, and alveolar-capillary leak.33 This anti-inflammatory pathway represents another potential mechanism by which IVLP rehabilitates sepsis induced lung injury via induction of pathways that mediate resolution of injury.
The pathophysiology of sepsis is associated with aberrant interaction between leukocytes and vascular endothelium, which involve the upregulation of the expression of cell adhesion molecules including vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1).34 Endothelial adhesion molecules play a critical roll in LPS-induced lung injury allowing transmigration of inflammatory cells.35 This up regulation of adhesion molecules attracts neutrophils and lymphocytes, further amplifying the inflammatory cascade through release of additional chemo-attractants. Thus the protein expression of VCAM-1 and ICAM-1 were measured in lung tissue as further indicators of vascular inflammation. IVLP-treated lungs in this study exhibited significantly reduced expression of VCAM-1 compared to untreated lungs, suggesting another mechanism by which IVLP attenuated acute lung injury in this porcine model of sepsis.
The limitations of this study include the complexity of this large animal model resulting in some variation between animals, however comparing results between left and right lungs of the same animal minimizes this effect. While this validated porcine model of sepsis-induced acute lung injury demonstrates a reproducible reduction in lung compliance and oxygenation, it does not directly translate to the chronic nature of sepsis in a critically ill patient suffering severe ARDS requiring ECMO. Additional mechanistic studies are needed to discover specific signaling pathways that are affected by IVLP with Steen solution providing attenuation of lung injury. Finally, prior to translation into human studies IVLP will require an entirely percutaneous approach hopefully transforming ARDS from a long-term condition with high morbidity and mortality to a treatable illness with shortened recovery, lower healthcare related cost, and increased survival.
In conclusion, 4 hours of IVLP significantly rehabilitated LPS-induced lung injury and attenuated the need for ECMO support in 75% of animals. Lung injury is ameliorated by IVLP through several mechanisms involving reduced lung edema, reduction in pro-inflammatory cytokines and adhesion molecules, and an increase of an anti-inflammatory cytokine. In the near future, when translated into a percutaneous platform, IVLP may provide a reliable means to rehabilitate various types of acute lung injury in patients on ECMO leading to reduced morbidity and mortality in patients with ARDS.
Supplementary Material
Albumin concentration in serum at Baseline and End IVLP Perfusion as well as IVLP circuit concentration. Demonstrates serum albumin decreased from beginning to end of IVLP despite IVLP circuit having high concentration of albumin.
Right vs Left lung oxygenation at A. Baseline and the end of the B. 2- hour injury period does not differ within animals. C. Dynamic lung compliance significantly decreases from baseline to 2 hour injury with a subsequent significant increase from 2 hour injury to end of reperfusion.
Right vs Left lung oxygenation after reperfusion. A. 1 Hour, B. 2 Hour C. 3 Hour, D. 4 Hours.
Video.
University of Virginia Porcine In Vivo Lung Perfusion Cannulation Procedure: Dissecting Pulmonary Hilum (00:04), Left PA purse-string (00:22), Left Atrial Purse-string (00:35), Central ECMO Cannulation (01:00), Dissection Left Pulmonary Veins (01:15), Left PA Cannulation (01:30), Left Inferior Pulmonary Vein Cannulation (01:50), Left Superior Pulmonary Vein Cannulation (02:35), Connecting IVLP (02:55), IVLP Diagram (03:15).
Perspective Statement.
When translated into a percutaneous platform, in vivo lung perfusion may provide a reliable means to rehabilitate various types of acute lung injury in patients on ECMO to reduce the morbidity and mortality in ARDS.
Acknowledgments
The National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Numbers T32HL007849, UM1HL088925 and R01HL119218 supported research reported in this publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Special thanks to Tony Herring, Cindy Dodson, and Sheila Hammond for their technical support and continued commitment to the completion of this project.
Definitions
- ARDS
Acute Respiratory Distress Syndrome
- ECMO
Extracorporeal Membrane Oxygenation
- EVLP
Ex Vivo Lung Perfusion
- IVLP
In Vivo Lung Perfusion
- LPS
Lipopolysaccharide
- MAP
Mean Arterial Pressure
- BAL
Bronchial Alveolar Lavage
- CO
Cardiac Output
- TNF-α
Tumor Necrosis Factor Alpha
- INF-γ
Interferon Gamma
- IL-4
Interleukin 4
- VCAM-1
Vascular Cell Adhesion Molecule 1
- ICAM-1
Intercellular Adhesion Molecule 1
Footnotes
Meeting Presentation: AATS Lillehei Resident Forum, May 2017, Boston MA
There are no financial disclosures or conflicts of interest from any of the authors.
Disclosure
There are no financial disclosures or conflicts of interest from any of the authors. The National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Numbers T32HL007849 (I.L.K.), UM1 HL088925 (I.L.K.), and R01HL119218 (I.L.K. and V.E.L.) supported research reported in this publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schmidt M, Zogheib E, Roze H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013;39:1704–1713. doi: 10.1007/s00134-013-3037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonelli AR, Zein J, Adams J, Ioannidis JP. Effects of interventions on survival in acute respiratory distress syndrome: an umbrella review of 159 published randomized trials and 29 meta-analyses. Intensive Care Med. 2014;40:769–787. doi: 10.1007/s00134-014-3272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 4.Modig J. Adult respiratory distress syndrome. Pathogenesis and treatment. Acta Chir Scand. 1986;152:241–249. [PubMed] [Google Scholar]
- 5.Windsor AC, Mullen PG, Fowler AA. Acute lung injury: what have we learned from animal models? Am J Med Sci. 1993;306:111–116. doi: 10.1097/00000441-199308000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Meduri GU, Schwingshackl A, Hermans G. Prolonged Glucocorticoid Treatment in ARDS: Impact on Intensive Care Unit-Acquired Weakness. Front Pediatr. 2016;4:69. doi: 10.3389/fped.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortiz-Diaz E, Festic E, Gajic O, Levitt JE. Emerging pharmacological therapies for prevention and early treatment of acute lung injury. Semin Respir Crit Care Med. 2013;34:448–458. doi: 10.1055/s-0033-1351118. [DOI] [PubMed] [Google Scholar]
- 8.Peter JV, John P, Graham PL, Moran JL, George IA, Bersten A. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ. 2008;336:1006–1009. doi: 10.1136/bmj.39537.939039.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serpa Neto A, Simonis FD, Barbas CS, et al. Association between tidal volume size, duration of ventilation, and sedation needs in patients without acute respiratory distress syndrome: an individual patient data meta-analysis. Intensive Care Med. 2014;40:950–957. doi: 10.1007/s00134-014-3318-4. [DOI] [PubMed] [Google Scholar]
- 10.Alhazzani W, Alshahrani M, Jaeschke R, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2013;17:R43. doi: 10.1186/cc12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosma KJ, Taneja R, Lewis JF. Pharmacotherapy for prevention and treatment of acute respiratory distress syndrome: current and experimental approaches. Drugs. 2010;70:1255–1282. doi: 10.2165/10898570-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cypel M, Yeung JC, Hirayama S, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27:1319–1325. doi: 10.1016/j.healun.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Mulloy DP, Sharma AK, Fernandez LG, et al. Adenosine A3 receptor activation attenuates lung ischemia-reperfusion injury. Ann Thorac Surg. 2013;95:1762–1767. doi: 10.1016/j.athoracsur.2013.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone ML, Sharma AK, Zhao Y, et al. Sphingosine-1-phosphate receptor 1 agonism attenuates lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1245–1252. doi: 10.1152/ajplung.00302.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaPar DJ, Laubach VE, Emaminia A, et al. Pretreatment strategy with adenosine A2A receptor agonist attenuates reperfusion injury in a preclinical porcine lung transplantation model. J Thorac Cardiovasc Surg. 2011;142:887–894. doi: 10.1016/j.jtcvs.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulloy DP, Stone ML, Crosby IK, et al. Ex vivo rehabilitation of non-heart-beating donor lungs in preclinical porcine model: delayed perfusion results in superior lung function. The Journal of thoracic and cardiovascular surgery. 2012;144:1208–1215. doi: 10.1016/j.jtcvs.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cypel M, Keshavjee S. Isolated lung perfusion. Front Biosci (Elite Ed) 2012;4:2226–2232. doi: 10.2741/e538. [DOI] [PubMed] [Google Scholar]
- 18.dos Santos PR, Iskender I, Machuca T, et al. Modified in vivo lung perfusion allows for prolonged perfusion without acute lung injury. J Thorac Cardiovasc Surg. 2014;147:774–781. doi: 10.1016/j.jtcvs.2013.10.009. discussion 781–772. [DOI] [PubMed] [Google Scholar]
- 19.Reck Dos Santos P, Sakamoto J, Chen M, et al. Modified In Vivo Lung Perfusion for Local Chemotherapy: A Preclinical Study With Doxorubicin. Ann Thorac Surg. 2016;101:2132–2140. doi: 10.1016/j.athoracsur.2015.12.043. [DOI] [PubMed] [Google Scholar]
- 20.Modig J, Borg T. High-dose methylprednisolone in a porcine model of ARDS induced by endotoxemia. Acta Chir Scand Suppl. 1985;526:94–103. [PubMed] [Google Scholar]
- 21.Wang HM, Bodenstein M, Markstaller K. Overview of the pathology of three widely used animal models of acute lung injury. Eur Surg Res. 2008;40:305–316. doi: 10.1159/000121471. [DOI] [PubMed] [Google Scholar]
- 22.Mehaffey JH, Charles EJ, Sharma AK, et al. Ex Vivo Lung Perfusion Rehabilitates Sepsis-Induced Lung Injury. Ann Thorac Surg. 2017;103:1723–1729. doi: 10.1016/j.athoracsur.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehaffey JH, Charles EJ, Sharma AK, et al. Airway pressure release ventilation during ex vivo lung perfusion attenuates injury. J Thorac Cardiovasc Surg. 2017;153:197–204. doi: 10.1016/j.jtcvs.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charles EJ, Huerter ME, Wagner CE, et al. Donation After Circulatory Death Lungs Transplantable Up to Six Hours After Ex Vivo Lung Perfusion. Ann Thorac Surg. 2016;102:1845–1853. doi: 10.1016/j.athoracsur.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steen S, Liao Q, Wierup PN, Bolys R, Pierre L, Sjoberg T. Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann Thorac Surg. 2003;76:244–252. doi: 10.1016/s0003-4975(03)00191-7. discussion 252. [DOI] [PubMed] [Google Scholar]
- 26.Cypel M, Rubacha M, Yeung J, et al. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant. 2009;9:2262–2269. doi: 10.1111/j.1600-6143.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes LM, Mariani AW, Medeiros IL, et al. Alternative solution for ex vivo lung perfusion, experimental study on donated human lungs non-accepted for transplantation. Acta Cir Bras. 2015;30:359–365. doi: 10.1590/S0102-865020150050000008. [DOI] [PubMed] [Google Scholar]
- 28.Yeung JC, Cypel M, Waddell TK, van Raemdonck D, Keshavjee S. Update on donor assessment, resuscitation, and acceptance criteria, including novel techniques--non-heart-beating donor lung retrieval and ex vivo donor lung perfusion. Thorac Surg Clin. 2009;19:261–274. doi: 10.1016/j.thorsurg.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz DA. TLR4 and LPS hyporesponsiveness in humans. Int J Hyg Environ Health. 2002;205:221–227. doi: 10.1078/1438-4639-00117. [DOI] [PubMed] [Google Scholar]
- 30.Endo T, Ogushi F, Kawano T, Sone S. Comparison of the regulations by Th2-type cytokines of the arachidonic-acid metabolic pathway in human alveolar macrophages and monocytes. Am J Respir Cell Mol Biol. 1998;19:300–307. doi: 10.1165/ajrcmb.19.2.2915. [DOI] [PubMed] [Google Scholar]
- 31.Maloney CG, Kutchera WA, Albertine KH, McIntyre TM, Prescott SM, Zimmerman GA. Inflammatory agonists induce cyclooxygenase type 2 expression by human neutrophils. J Immunol. 1998;160:1402–1410. [PubMed] [Google Scholar]
- 32.Bingisser R, Stey C, Weller M, Groscurth P, Russi E, Frei K. Apoptosis in human alveolar macrophages is induced by endotoxin and is modulated by cytokines. Am J Respir Cell Mol Biol. 1996;15:64–70. doi: 10.1165/ajrcmb.15.1.8679223. [DOI] [PubMed] [Google Scholar]
- 33.Wehrmann F, Lavelle JC, Collins CB, et al. gammadelta T cells protect against LPS-induced lung injury. J Leukoc Biol. 2016;99:373–386. doi: 10.1189/jlb.4A0115-017RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCulloh RJ, Spertus JA. Separating signal from noise: the challenge of identifying useful biomarkers in sepsis. Crit Care. 2014;18:121. doi: 10.1186/cc13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo YL, Huang H, Zeng DX, Zhao JP, Fang HJ, Lavoie JP. Interleukin (IL)-4 induces production of cytokine-induced neutrophil chemoattractants (CINCs) and intercellular adhesion molecule (ICAM)-1 in lungs of asthmatic rats. J Huazhong Univ Sci Technolog Med Sci. 2013;33:470–478. doi: 10.1007/s11596-013-1144-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Albumin concentration in serum at Baseline and End IVLP Perfusion as well as IVLP circuit concentration. Demonstrates serum albumin decreased from beginning to end of IVLP despite IVLP circuit having high concentration of albumin.
Right vs Left lung oxygenation at A. Baseline and the end of the B. 2- hour injury period does not differ within animals. C. Dynamic lung compliance significantly decreases from baseline to 2 hour injury with a subsequent significant increase from 2 hour injury to end of reperfusion.
Right vs Left lung oxygenation after reperfusion. A. 1 Hour, B. 2 Hour C. 3 Hour, D. 4 Hours.