Abstract
The significance of upper gastrointestinal tract (UGI) acute graft versus host disease (aGVHD) compared to other grade II aGVHD is not clearly defined. We compared the outcomes of patients with grade II aGVHD with or without biopsy-proven UGI involvement in three groups: Grade II aGVHD without UGI (n=178), grade II aGVHD with UGI and other sites (n=102) and isolated UGI aGVHD (n=32). The overall response (ORR) to steroids at day 28 differed among the 3 groups (76%, 67% and 91%, respectively, p=0.01), but was only marginally different in direct comparison of those without or with UGI aGVHD (p=0.07) or with isolated UGI aGVHD (p=0.06). In multivariate analysis, as compared to grade II aGVHD patients without UGI involvement, those with UGI involvement and those with isolated UGI aGVHD had similar risks of chronic GVHD, relapse and non-relapse mortality and similar disease-free survival and overall survival. Our data suggest that patients with UGI aGVHD have similar outcomes as those without UGI involvement, supporting the practice that UGI aGVHD should still be included as a grade II defining event.
Keywords: GVHD, gastrointestinal, GI GVHD, upper GI GVHD, lower GI GVHD, grade II GVHD
INTRODUCTION
The extent and severity of gastrointestinal (GI) graft-versus-host disease (GVHD) is clinically assessed by the volume of diarrhea and is pathologically confirmed by the biopsy of lower GI (LGI) tract. In 1990, we described the syndrome of upper GI (UGI) acute GVHD (aGVHD) presenting as anorexia, dyspepsia, nausea or vomiting without diarrhea in a series of 469 patients who underwent bone marrow (BM) hematopoietic cell transplantation (HCT) from human leukocyte antigen (HLA)-matched siblings.1 In that study, 13–22% of patients had pathologically confirmed UGI aGVHD, more than 75% of whom developed chronic GVHD. Patients with UGI aGVHD responded well to steroids and their overall survival (OS) was similar to patients with LGI aGVHD.1 Based on these and other findings, the 1994 consensus conference on aGVHD grading added “persistent nausea with histologic evidence of GVHD but no diarrhea” as stage 1 GI aGVHD 2 – consistent with Glucksberg grade II GVHD.3
A recent preliminary study from the Center for International Blood and Marrow Transplant Research (CIBMTR) registry demonstrated that patients with isolated UGI aGVHD had similar outcomes to those without aGVHD, thus arguing against the inclusion of UGI GVHD as grade II GVHD defining entity.4 However, histological confirmation of UGI aGVHD was not required in that registry analysis, thus questioning the reliability of diagnosis because similar UGI symptoms could be attributable to a multitude of factors in the early post-HCT period. In the present study, we evaluated the outcomes of biopsy-proven grade II aGVHD in patients with or without UGI involvement. We show that patients with isolated UGI aGVHD had similar overall response to steroids by day 28 as those without UGI involvement and their longer term risks of chronic GVHD and mortality were similar to patients with grade II aGVHD without UGI involvement. UGI aGVHD should remain as a component of clinical grade II aGVHD.
METHODS
We studied patients aged 18 years or older who underwent an allogeneic HCT for malignant or non-malignant diseases at the University of Minnesota. Patients with UGI symptoms were evaluated with esophagogastroduodenoscopy (EGD) and biopsy. Out of 840 consecutive adult allogeneic transplant recipients, 77 patients were diagnosed with maximum clinical grade I Glucksberg scale aGVHD, 312 were diagnosed with maximum clinical grade II aGVHD and 36 were diagnosed with maximum clinical grade III aGVHD within 100 days of HCT. Patients with grade II aGVHD were further categorized into three groups – (1) grade II aGVHD without UGI involvement (n= 178), (2) grade II aGVHD with biopsy proven UGI aGVHD along with other grade II aGVHD-defining organ involvement (n= 102) and (3) isolated UGI aGVHD (n=32). Patients with prior allogeneic HCT and those who received both BM and peripheral blood progenitor cell (PBPC) grafts were excluded from the analysis (n=4). All other graft sources and donor types were included.
Endpoints
As overall response rate (ORR) to GVHD treatment at day 28 is correlated with non-relapse mortality (NRM),5, 6 our primary objective was to compare ORR (complete response (CR) or partial response (PR)) in the three defined groups. Additionally, we evaluated the proportion of patients with GVHD flare after initial response to treatment by day 28, and overall response, CR and PR rates at days 14 and 56. Secondary objectives were to assess long term outcomes, including the incidences of chronic GVHD, NRM, relapse/progression, disease-free survival (DFS) and OS at 1 year.
Definitions
In the original Glucksberg criteria,3 grade II aGVHD was defined by the presence of stage 1 to 3 skin involvement or stage 1 liver or stage 1–2 GI involvement, while in the Consensus schema2 UGI was included as stage 1 GI aGVHD and thus clinical grade II. As previously reported,5 the diagnosis of aGVHD was made clinically and confirmed histologically by upper endoscopic biopsies of the esophagus, stomach or duodenum in all cases, as per the GVHD consensus conference criteria.2, 7 Grading of aGVHD refers to clinical (not histological) grading. Assessment of the response to steroids was determined at day 14, 28 and 56 as previously reported.8 Briefly, CR was defined as the complete resolution of aGVHD manifestations in all organs, without the need for secondary GVHD therapy. PR was defined as an improvement in aGVHD stage in all initially affected organs, without resolution or worsening in any other GVHD target organs, or need for secondary GVHD therapy. All patients with aGVHD were treated similarly with prednisone 60 mg/m2 (or 2 mg/kg body weight) by mouth or equivalent dose of intravenous methylprednisolone for two weeks followed by an eight week taper. Patients were continued on GVHD prophylaxis drugs at therapeutic concentrations as previously reported.8 GVHD diagnostic data and treatment responses are collected prospectively and confirmed by retrospective review for all patients by experienced investigators [DW and SH for adult patients; MM for pediatrics].
Statistical methods
Baseline patient and transplant characteristics and information on HCT clinical outcomes were prospectively collected and recorded in the University of Minnesota BMT Program database. Statistical comparison of categorical variables was performed by Chi-square test while Kruskal-Wallis (Wilcoxon) rank-sum test was used for comparison of continuous variables. The Kaplan-Meier method was used to estimate the probabilities of DFS and OS and the log-rank test was used for univariate comparisons.9 Cox regression analyses were used to compare survival curves adjusted by other factors. Cumulative incidence estimator was used to calculate the probabilities of relapse/progression, NRM and chronic GVHD reflecting the non-event competing risks10 with Fine and Gray regression analyses as shown.11 Multivariate models for all endpoints were created using backward selection method considering a P value of <0.20 for retention in the model. First, global analysis was performed comparing all three groups of patients with aGVHD. Then, pre-planned sub-group analysis was done to assess differences between the 3 different grade II GVHD groups for all primary and secondary outcomes. Multiple comparisons were adjusted by Bonferroni method and an adjusted significance level of 0.017 was used for all 3 group comparisons. Statistical analyses were performed with SAS 9.3 (SAS Institute, Cary, NC) system.
RESULTS
A total of 312 patients with maximum clinical grade II aGVHD were analyzed, of which 57% had no UGI involvement (n=178); approximately 33% had UGI plus another organ aGVHD (n=102) and roughly 10% had isolated UGI aGVHD (n=32) [Table 1]. Similar proportion of patients had liver involvement in grade II patients without (5.1%) and with UGI GVHD (2.9%), p=0.33. Significantly more patients without UGI aGVHD had skin involvement (96%) as compared to those with UGI aGVHD (71%), p<0.01. On the other hand, more patients with UGI aGVHD had lower GI involvement (38%) compared with only 16% in those without UGI aGVHD, p<0.01. The median age in all groups was near 40 years and 60% were males, with no differences among groups. The most common diagnoses were acute leukemia (34–40%) and chronic myeloid leukemia (30–40%) in all groups, p=0.91. More than half had standard disease risk (p=0.87), according to the American Society for Blood and Marrow Transplantation (ASBMT) 2006 risk definitions.12 In the 3 groups, most received BM grafts (50–56%), followed by PBPC (24–37%) and umbilical cord blood (UCB, 6–25%), p=0.11. More patients with isolated UGI aGVHD (78%) had sibling donors versus 45% in the other groups, p<0.01. Myeloablative conditioning was used more frequently (75–94%) in each group, p=0.08. Though correlated with conditioning intensity and graft source, a majority received methotrexate based GVHD prophylaxis, although it was used more somewhat more frequently with isolated UGI aGVHD (72%) than in other groups (56–57%), p=0.23. Only few patients (about 9% in each group) received T cell depleted grafts. The median overall follow-up was well over a year in all groups, p=0.04.
Table 1.
Baseline patient and treatment characteristics
| Grade II; no UGI | Grade II; with UGI | Isolated UGI | P-value* | |
|---|---|---|---|---|
| N=178 | N=102 | N=32 | ||
| Age, Median (range), in years | 42.4(18.2–67.4) | 41.7(18.8–68.0) | 38.7(20.8–57.4) | 0.34 |
| Gender | 0.18 | |||
| Male | 113(63.5%) | 54(52.9%) | 21(65.6%) | |
| Disease | 0.91 | |||
| ALL | 22(12.4%) | 14(13.7%) | 3(9.4%) | |
| AML | 47(26.4%) | 27(26.5%) | 8(25%) | |
| CML | 56(31.5%) | 30(29.4%) | 13(40.6%) | |
| MDS/MPN | 13 (7.3%) | 11 (10.8%) | 3 (9.4%) | |
| NHL/HL | 23 (12.9%) | 12 (11.8%) | 3 (9.4%) | |
| Others | 17 (9.6%) | 8 (7.8%) | 2 (6.3%) | |
| Disease status at HCT | 0.83 | |||
| CR/CP | 121 (67.9%) | 58 (56.9%) | 20 (62.5%) | |
| PR | 2 (1.1%) | 1 (1%) | 0 | |
| Relapse/PIF/Progressive diseases | 41 (23%) | 21 (20.6%) | 9 (28.1%) | |
| Non-malignant disorders | 10 (5.6%) | 9 (8.8%) | 1 (3.1%) | |
| Missing/Others | 20 (11.3%) | 13 (12.8%) | 2 (6.3%) | |
| Disease Risk | 0.87 | |||
| Standard | 105(59.0%) | 57(55.9%) | 18(56.3%) | |
| High | 73(41.0%) | 45(44.1%) | 14(43.8%) | |
| Recipient CMV | 0.44 | |||
| Negative | 93 (52%) | 48 (47%) | 19 (59%) | |
| Positive | 85 (48%) | 54 (53%) | 13 (41%) | |
| Graft | 0.11 | |||
| BM | 90(50.6%) | 56(54.9%) | 18(56.3%) | |
| PBSC | 43(24.2%) | 21(20.6%) | 12(37.5%) | |
| UCB | 45(25.3%) | 25(24.5%) | 2(6.3%) | |
| Donor | <0.01 | |||
| Sibling | 81(45.5%) | 46(45.1%) | 25(78.1%) | |
| Unrelated | 97(54.5%) | 56(54.9%) | 7(21.9%) | |
| Conditioning | 0.08 | |||
| Myeloablative | 138(77.5%) | 77(75.5%) | 30(93.8%) | |
| RIC | 40(22.5%) | 25(24.5%) | 2(6.3%) | |
| GVHD Prophylaxis | 0.23 | |||
| ATG | 9 (5%) | 3 (3%) | 2 (6%) | |
| TCD/CD34+ | 16 (9%) | 9 (9%) | 3 (9%) | |
| CSA or Tac/MTX | 99 (56%) | 58 (57%) | 23 (72%) | |
| CSA/MMF | 53 (30%) | 32 (31%) | 3 (9%) | |
| Others | 1 (1%) | 0 | 1 (3%) | |
| Gender mismatch | ||||
| Female donor to male recipient | 56 (31.5%) | 30 (29.4%) | 15 (46.9%) | 0.13 |
| HLA ** | 0.10 | |||
| 4/6 | 31(17.4%) | 14(13.7%) | 2(6.3%) | |
| 5/6 | 28(15.7%) | 24(23.5%) | 3(9.4%) | |
| 6/6 | 119(66.9%) | 64(62.7%) | 27(84.4%) | |
| aGVHD organs involved | ||||
| Skin | 171(96.1%) | 72(70.6%) | 0 | <0.01 |
| Liver | 9(5.1%) | 3(2.9%) | 0 | 0.33 |
| LGI | 28 (15.7%) | 39 (38.2%) | 0 | <0.01 |
| aGVHD Onset, days | 0.04 | |||
| Median(Min–Max) | 29(11–76) | 28(8–73) | 34(19–72) | |
| Transplant Year | 0.14 | |||
| 1990–1995 | 45(25.3%) | 32(31.4%) | 14(43.8%) | |
| 1996–2001 | 67(37.6%) | 28(27.5%) | 11(34.4%) | |
| 2001–2005 | 52(29.2%) | 36(35.3%) | 7(21.9%) | |
| 2006–2007 | 14(7.9%) | 6(5.9%) | 0 | |
| Follow Up, months | 0.04 | |||
| Median(Min–Max) | 16.9(0.1–202) | 24.1(0.13–206) | 58.3(0.33–133.6) |
Significant cut off p-value is 0.0167, adjusted for three groups.
HLA locus match. For double UCB grafts, the greatest mismatch is shown.
Abbreviations: BM, bone marrow; PBPC, peripheral blood progenitor cells; UCB, umbilical cord blood; RIC, reduced intensity conditioning; HLA, human leucocyte antigen; TCD, T cell deplete; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; NHL, Non-Hodgkin lymphoma; HL, Hodgkin lymphoma; HCT, hematopoietic cell transplantation; CR, complete remission; CP, chronic phase; PR, partial remission; PIF, primary induction failure; aGVHD, acute graft versus host disease; ATG, antithymocyte globulin; CD34+, CD34+ selected; MTX, methotrexate; CSA, cyclosporine; Tac, tacrolimus; MMF, mycophenolic acid; CMV, Cytomegalovirus; UGI, upper gastrointestinal tract; LGI, lower gastrointestinal tract.
Overall response to GVHD treatment at day 28
We found that 76% of grade II aGVHD patients without UGI involvement had an overall response (CR + PR) to steroids at day 28 as compared to 67% in those with grade II with UGI aGVHD and 91% with isolated UGI aGVHD, p=0.01. [Table 2]. However, in the pre-planned sub-group analysis, there was no statistically significant difference in the ORR between groups. The ORR at day 56 was similar between groups (69%, 67% and 88%, respectively), p=0.07.
Table 2.
Response of aGVHD to steroids
| GROUP 1 | GROUP 2 | GROUP 3 | |||||
|---|---|---|---|---|---|---|---|
| Response | Grade II; no UGI | Grade II; with UGI | Isolated UGI | Overall P-value * | Group 2 vs 3 P-value * | Group 1 vs 3 P-value * | |
| N=178 | N=102 | N=32 | |||||
| Day 14 | ORR | 128 (72%) | 51 (50%) | 23 (72%) | <0.01 | <0.01 | 1.00 |
| CR | 62 (35%) | 35 (34%) | 21 (66%) | <0.01 | <0.01 | <0.01 | |
| PR | 66 (37%) | 16 (16%) | 2 (6%) | ||||
| NR/progression | 50 (28%) | 51 (50%) | 9 (28%) | ||||
| Day28 | ORR | 135 (76%) | 67 (67%) | 29 (91%) | 0.01 | 0.07 | 0.06 |
| CR | 95 (53%) | 59 (58%) | 29 (91%) | <0.01 | <0.01 | <0.01 | |
| PR | 40 (22%) | 8 (8%) | 0 | ||||
| NR/progression | 43 (24%) | 35 (34%) | 3 (9%) | ||||
| Day 56 | ORR | 122 (69%) | 68 (67%) | 28 (88%) | 0.07 | 0.75 | 0.03 |
| CR | 108 (61%) | 59 (58%) | 28 (89%) | 0.04 | 0.89 | 0.01 | |
| PR | 14 (8%) | 9 (9%) | 0 | ||||
| NR/progression | 56 (31%) | 34 (33%) | 4 (13%) | ||||
Significant cut off p-value is p≤0.02, adjusted for three groups.
Abbreviations: CR, complete response; PR, partial response; ORR, overall response rate; UGI, upper gastrointestinal tract; NR, no response
Patients with isolated UGI involvement had significantly higher CR rates at days 14, 28 and 56 (66%, 91% and 89%) as compared to grade II without UGI involvement (35%, 53% and 61%), p<0.01. Patients with UGI and other organ grade II aGVHD had substantially lower CR rates at all 3 time points (34%, 58% and 58%).
There were no differences in the proportion of patients with GVHD flare after achieving initial response after either day 14 (9%, 4%, 0% in the 3 groups, p=0.21), day 28 (5%, 9% and 3%, p=0.47) or day 56 (2%, 0% and 0%, p=0.45).
Risks of chronic GVHD
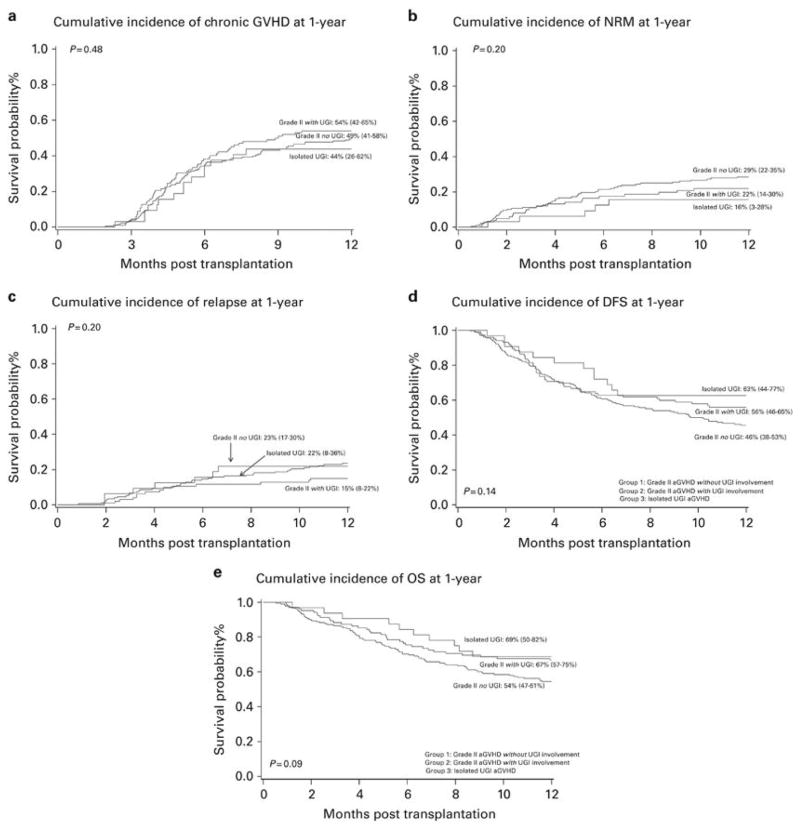
The univariate estimates of chronic GVHD at 1-year were: No UGI aGVHD, 49% (95% confidence interval (CI) 41–58%); with UGI aGVHD, 54% (95% CI 42–65%); and isolated UGI aGVHD, 44% (95% CI 26–62%), p=0.48 [Figure 1; Supplemental table 1]. After adjusting for gender, conditioning regimen, disease risk, donor, graft source, recipient CMV serostatus and year of transplantation in multivariate analysis, the risk of chronic GVHD at 1-year was similar in all groups p=0.46. As compared with BM or PBPC, UCB transplantation was associated with 46% lower risk of chronic GVHD, p<0.01 [Table 3A].
Figure 1.
Table 3A.
Chronic GVHD at 1-year: Multiple regression analysis
| Relative Risk (95% CI) | * P-value | Overall P-value | ||
|---|---|---|---|---|
| Group | Grade II aGVHD without UGI involvement | 1.00 | 0.46 | |
| Grade II aGVHD with UGI involvement | 1.13 (0.80–1.58) | 0.49 | ||
| Isolated UGI GVHD | 0.77 (0.43–1.39) | 0.39 | ||
| Graft | BM | 1.00 | 0.02 | |
| PBPC | 0.93 (0.65–1.34) | 0.71 | ||
| UCB | 0.54 (0.34–0.83) | 0.01 |
Pairwise comparison with reference group.
Covariates with p-value >0.1 now shown.
Abbreviations: BM, bone marrow; CI, confidence interval; PBPC, peripheral blood progenitor cells; UCB, umbilical cord blood; UGI, upper gastrointestinal tract;
Non-relapse mortality
The univariate estimates of NRM at 1-year were: No UGI aGVHD, 29% (95% CI 22–35%); with UGI aGVHD, 22% (95% CI 14–30%) and isolated UGI aGVHD, 16% (95% CI 3–28%), p=0.20 [Figure 1b; Supplemental table 1]. After adjusting for covariates in the multivariate analysis, there were no differences in NRM among different groups of patients with aGVHD, p=0.18. In the subgroup analysis, there was a trend towards lower risk of NRM in those with isolated UGI aGVHD compared to those without UGI involvement (HR 0.46, 95% CI 0.18–1.14, p=0.09). The risk of NRM was 60% lower in those who received RIC regimens compared with myeloablative regimens, p=0.02 [Table 3B].
Table 3B.
Non-relapse mortality at 1-year: Multiple regression analysis
| Relative Risk (95% CI) | * P-value | Overall P-value | ||
|---|---|---|---|---|
| Group | Grade II aGVHD without UGI involvement | 1.00 | 0.18 | |
| Grade II aGVHD with UGI involvement | 0.76 (0.46–1.27) | 0.30 | ||
| Isolated UGI GVHD | 0.46 (0.18–1.14) | 0.09 | ||
| Conditioning | Myeloablative | 1.00 | 0.02 | |
| RIC | 0.40 (0.19–0.84) |
Pairwise comparison with reference group
Covariates with p-value >0.1 now shown.
Abbreviations: aGVHD, acute graft versus host disease; CI, confidence interval; RIC, reduced intensity conditioning; UGI, upper gastrointestinal tract.
Risk of relapse/progression
In those with malignant disease, the univariate estimates of relapse/progression at 1-year were: No UGI aGVHD, 23% (95% CI 17–30%); with UGI aGVHD, 15% (95% CI 8–22%) and isolated UGI aGVHD, 22% (95% CI 8–36%), p=0.20 [Figure 1c; Supplemental table 1]. In multivariate analysis, the risk of relapse was similar across groups, p=0.13. Patients who received reduced intensity conditioning (RIC) had 2.05 fold greater relative risk (RR) of relapse as compared to those who received myeloablative regimens, p=0.03. Although year of HCT was not an overall significant predictor of relapse, there was a trend towards increased risk of relapse in recent years. [Table 3C] Higher risk of relapse in later years was correlated with significantly increasing use of RIC regimens and UCB graft and more patients over the age of 40 years who underwent HCT than in the previous years (data not shown).
Table 3C.
Relapse at 1-year: Multiple regression analysis
| Relative Risk (95% CI) | * P-value | Overall P-value | ||
|---|---|---|---|---|
| Group | Grade II aGVHD without UGI involvement | 1.00 | 0.13 | |
| Grade II aGVHD with UGI involvement | 0.59 (0.32–1.08) | 0.09 | ||
| Isolated UGI GVHD | 1.41 (0.59–3.36) | 0.44 | ||
| Conditioning | Myeloablative | 1.00 | 0.03 | |
| RIC | 2.05 (1.09–3.86) | |||
| Year of HCT | 1990–1995 | 1.00 | 0.13 | |
| 1996–2001 | 1.12 (0.51–2.47) | 0.78 | ||
| 2001–2005 | 2.05 (0.89–4.75) | 0.09 | ||
| 2006–2007 | 2.78 (1.02–7.60) | 0.05 |
Pairwise comparison with reference group
Covariates with p-value >0.1 now shown.
Abbreviations: aGVHD, acute graft versus host disease; CI, confidence interval; HCT, hematopoietic cell transplantation; RIC, reduced intensity conditioning; UGI, upper gastrointestinal tract;
Disease free survival and overall survival
The estimates of DFS at 1-year were: No UGI aGVHD, 46% (95% CI 38–53%); with UGI aGVHD, 56% (95% CI 46–65%) and isolated UGI aGVHD, 63% (95% CI 44–77%), p=0.14 [Figure 1d; Supplemental table 1]. In multivariate analysis, all groups of patients with Grade II aGVHD had similar DFS, p=0.10. Patients with high risk disease had significantly inferior DFS (HR 1.64, 95% CI 1.19–2.25, p<0.01) than those with standard risk disease [Table 3D].
Table 3D.
DFS at 1-year: Multiple regression analysis
| Hazard Ratio (95% CI) | * P-value | Overall P-value | ||
|---|---|---|---|---|
| Group | Grade II aGVHD without UGI involvement | 1.00 | 0.10 | |
| Grade II aGVHD with UGI involvement | 0.60 (0.33–1.09) | 0.10 | ||
| Isolated UGI GVHD | 0.74 (0.52–1.06) | 0.09 | ||
| Gender | Male | 1.00 | 0.08 | |
| Female | 1.33 (0.97–1.84) | |||
| Disease risk | Standard | 1.00 | <0.01 | |
| High | 1.64 (1.19–2.25) |
Pairwise comparison with reference group
Covariates with p-value >0.1 now shown.
Abbreviations: aGVHD, acute graft versus host disease; CI, confidence interval; UGI, upper gastrointestinal tract.
The estimates of OS at 1-year were: No UGI aGVHD, 54% (95% CI 47–61%); with UGI aGVHD, 67% (95% CI 57–75%) and isolated UGI aGVHD, 69% (95% CI 50–82%), p=0.09 [Figure 1e; Supplemental table 1]. In multivariate analysis, OS was similar among the aGVHD groups, p=0.12. In the subgroup analysis, there was a trend towards lower hazards of mortality in those with isolated UGI aGVHD than those without UGI involvement (HR 0.48, 95% CI 0.22–1.06, p=0.07). [Table 3E].
Table 3E.
OS at 1-year: Multiple regression analysis
| Parameter | Class | Hazard Ratio (95% CI) | * P-value | Overall P-value |
|---|---|---|---|---|
| Group | Grade II aGVHD without UGI involvement | 1.00 | 0.12 | |
| Grade II aGVHD with UGI involvement | 0.76 (0.49–1.18) | 0.21 | ||
| Isolated UGI GVHD | 0.48 (0.22–1.06) | 0.07 | ||
| Gender | Male | 1.00 | 0.06 | |
| Female | 1.47 (0.99–2.18) |
Pairwise comparison with reference group
Covariates with p-value >0.1 now shown.
Abbreviations: aGVHD, acute graft versus host disease; CI, confidence interval; KPS, Karnofsky performance score; UGI, upper gastrointestinal tract.
Discussion
We found that patients with isolated UGI aGVHD had higher responses to initial steroid treatment as compared to other grade II aGVHD groups, but it was only marginally different from those without UGI aGVHD (p=0.06) or those with UGI aGVHD (p=0.07). We chose ORR at day 28 as our primary endpoint because ORR at day 28 or day 56 are reported to predict NRM.5, 6 Consistent with these findings, all 3 cohorts with grade II aGVHD had similar risks of NRM, chronic GVHD, relapse, and similar DFS and OS irrespective of UGI involvement. These results confirm that patients with UGI aGVHD have outcomes similar to those with any other grade II aGVHD, thus supporting the continuing appropriateness of including UGI aGVHD as a grade II GVHD defining entity.
About 10–30% of patients with aGVHD have biopsy proven UGI involvement1, 2 while nearly 40% of patients with unidentified causes of nausea or vomiting post allogeneic HCT may have UGI aGVHD.13 Although the involvement of UGI tract by inflammatory cells was reported in an autopsy series more than four decades ago,14, 15 it wasn’t correlated with clinical symptoms of GVHD until later.16 In a large series of patients who underwent myeloablative BM HCT, Weisdorf et al 1 described the syndrome of UGI aGVHD as an independent entity, after which it was recognized as stage 1 GI GVHD by the 1994 consensus conference on aGVHD,2 consistent with Glucksberg grade II GVHD.3 However, the clinical significance of UGI aGVHD had not been systematically re-investigated since its initial description. In a preliminary analysis of the CIBMTR database, Nikiforow et al4 included 8567 adult recipients of myeloablative allogeneic HCT, of which 229 had isolated UGI GVHD. In this study, among recipients of matched related donor (MRD) HCT, patients with clinically diagnosed (though not biopsy confirmed) isolated UGI aGVHD had similar risks of chronic GVHD, relapse, NRM, and similar DFS and OS as those with grade II aGVHD without UGI involvement and those with grades 0 or I aGVHD; while in unrelated donor (URD) HCT recipients, isolated UGI aGVHD patients had lower NRM and tended to have superior DFS and OS as compared to patients with grade II aGVHD without UGI involvement. The URD outcomes with isolated UGI GVHD were similar to those with grades 0 or I aGVHD suggesting that UGI GVHD should perhaps not be included as a grade II GVHD defining event. As similar UGI symptoms (nausea, vomiting, anorexia) can have other etiologies, such as conditioning regimen toxicity, gastritis or infection with herpes virus, cytomegalovirus or candida,13, 17 the clinical diagnosis of UGI GVHD without histological confirmation may be overestimated in the registry database and potentially confound these conclusions.
UGI aGVHD was confirmed with biopsy in all our patients. Additionally, all patients with grade II aGVHD are treated similarly at our center, which limits the bias of differential diagnosis and treatment approaches recognized in a multicenter analysis. Moreover, the response to GVHD treatment is assessed prospectively in all of our patients and confirmed retrospectively by experienced physicians, which generates consistency.
With the development of novel clinical and biomarker-based models to risk stratify aGVHD, 8, 18 risk-adapted approach to treatment should be considered rather than treatment based on individual organ involvement (reviewed by Holtan and MacMillan19). The presence of isolated UGI aGVHD is considered as standard risk according to the refined Minnesota aGVHD risk score,8 while grade II aGVHD with or without UGI involvement may be standard or high risk, depending upon the absence or presence of liver GVHD. As patients with standard risk GVHD have excellent overall responses to steroids compared to those with high risk disease,8, 18 a lower toxicity approach to treatment of standard risk disease may be preferred, such as that being tested by the Blood and Marrow Transplant Clinical Trials Network protocol 1501.
We conclude that patients with UGI grade II aGVHD have similar responses to initial therapy and similar later outcomes as others with grade II aGVHD. This clinically more modest GI involvement has the same clinical impact as other grade II aGVHD. Future studies should incorporate these findings in the context of risk-adapted approach to treatment of GVHD.
Supplementary Material
Acknowledgments
The authors thank all patients, their families, nurses who took care of them and research nurses for their support and dedication in collecting these data.
Footnotes
Conflicts of interest: Authors have no conflicts of interest to declare.
References
- 1.Weisdorf DJ, Snover DC, Haake R, Miller WJ, McGlave PB, Blazar B, et al. Acute upper gastrointestinal graft-versus-host disease: clinical significance and response to immunosuppressive therapy. Blood. 1990;76(3):624–629. [PubMed] [Google Scholar]
- 2.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 3.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Nikiforow S, Hemmer M, Spellman SR, Alousi AM, Couriel DR, Pidala JA, et al. Upper Gastrointestinal Acute Graft-Versus-Host Disease Adds Minimal Prognostic Value When Present in Isolation or in Addition to Grade I or Other Grade II-Defining GvHD Manifestations. Blood. 2015;126(857) [Google Scholar]
- 5.MacMillan ML, DeFor TE, Weisdorf DJ. The best endpoint for acute GVHD treatment trials. Blood. 2010;115(26):5412–5417. doi: 10.1182/blood-2009-12-258442. [DOI] [PubMed] [Google Scholar]
- 6.Levine JE, Logan B, Wu J, Alousi AM, Ho V, Bolaños-Meade J, et al. Graft-versus-host disease treatment: predictors of survival. Biol Blood Marrow Transplant. 2010;16(12):1693–1699. doi: 10.1016/j.bbmt.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hings IM, Filipovich AH, Miller WJ, Blazar BL, McGlave PB, Ramsay NK, et al. Prednisone therapy for acute graft-versus-host disease: short- versus long-term treatment. A prospective randomized trial. Transplantation. 1993;56(3):577–580. doi: 10.1097/00007890-199309000-00016. [DOI] [PubMed] [Google Scholar]
- 8.MacMillan ML, Robin M, Harris AC, DeFor TE, Martin PJ, Alousi A, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21(4):761–767. doi: 10.1016/j.bbmt.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53(282):457–481. [Google Scholar]
- 10.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16(8):901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 12.ASBMT. American Society for Blood and Marrow Transplantation RFI 2006. 2006 http://www.asbmt.org.
- 13.Spencer GD, Hackman RC, McDonald GB, Amos DE, Cunningham BA, Meyers JD, et al. A prospective study of unexplained nausea and vomiting after marrow transplantation. Transplantation. 1986;42(6):602–607. doi: 10.1097/00007890-198612000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Kruger GR, Berard CW, DeLellis RA, Graw RG, Jr, Yankee RA, Leventhal BG, et al. Graft-versus-host disease. Morphologic variation and differential diagnosis in 8 cases of HL-A matched bone marrow transplantation. Am J Pathol. 1971;63(2):179–202. [PMC free article] [PubMed] [Google Scholar]
- 15.Sale GE, Shulman HM, McDonald GB, Thomas ED. Gastrointestinal graft-versus-host disease in man. A clinicopathologic study of the rectal biopsy. Am J Surg Pathol. 1979;3(4):291–299. doi: 10.1097/00000478-197908000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Snover DC, Weisdorf SA, Vercellotti GM, Rank B, Hutton S, McGlave P. A histopathologic study of gastric and small intestinal graft-versus-host disease following allogeneic bone marrow transplantation. Hum Pathol. 1985;16(4):387–392. doi: 10.1016/s0046-8177(85)80232-x. [DOI] [PubMed] [Google Scholar]
- 17.Wu D, Hockenberry DM, Brentnall TA, Baehr PH, Ponec RJ, Kuver R, et al. Persistent nausea and anorexia after marrow transplantation: a prospective study of 78 patients. Transplantation. 1998;66(10):1319–1324. doi: 10.1097/00007890-199811270-00010. [DOI] [PubMed] [Google Scholar]
- 18.Levine JE, Braun TM, Harris AC, Holler E, Taylor A, Miller H, et al. A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study. Lancet Haematol. 2015;2(1):e21–29. doi: 10.1016/S2352-3026(14)00035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holtan SG, MacMillan ML. A risk-adapted approach to acute GVHD treatment: are we there yet? Bone Marrow Transplant. 2016;51(2):172–175. doi: 10.1038/bmt.2015.261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.