In this study, we describe the course of LOC eating in adolescents undergoing bariatric surgery and its association with long-term weight outcomes.
Abstract
BACKGROUND:
Loss-of-control (LOC) eating is common in adults undergoing bariatric surgery and is associated with poorer weight outcomes. Its long-term course in adolescent bariatric surgery patients and associations with weight outcomes are unclear.
METHODS:
Adolescents (n = 234; age range = 13–19 years) undergoing bariatric surgery across 5 US sites were assessed for postsurgery follow-up at 6 months and 1, 2, 3, and 4 years. Descriptive statistics and generalized linear mixed models were used to describe the prevalence of LOC eating episodes involving objectively large amounts of food and continuous eating, respectively. Generalized linear mixed models investigated the association of any LOC eating with short- and long-term BMI changes.
RESULTS:
At baseline, objectively large LOC eating was reported by 15.4% of adolescents, and continuous LOC eating by 27.8% of adolescents. Both forms of LOC eating were significantly lower at all postsurgical time points relative to presurgery (range = 0.5%–14.5%; Ps < .05). However, both behaviors gradually increased from 6-month to 4-year follow-up (Ps < .05). Presurgical LOC eating was not related to percent BMI change over follow-up (P = .79). However, LOC eating at 1-, 2-, and 3-year follow-up was associated with lower percent BMI change from baseline at the next consecutive assessment (Ps < .05).
CONCLUSIONS:
Although presurgical LOC eating was not related to relative weight loss after surgery, postoperative LOC eating may adversely affect long-term weight outcomes. Rates of LOC eating decreased from presurgery to 6-months postsurgery but increased thereafter. Therefore, this behavior may warrant additional empirical and clinical attention.
What’s Known on This Subject:
Loss-of-control (LOC) eating after (but not before) bariatric surgery in adults is associated with poorer weight loss outcomes. The course and outcome of LOC eating in surgery-seeking adolescents are unclear.
What This Study Adds:
LOC eating within the year immediately after surgery was unrelated to weight outcomes, but at 1-, 2-, and 3-years postsurgery, was associated with a reduced percentage of body weight lost. LOC eating sharply declined then gradually increased throughout 4-year follow-up.
Severe obesity is a major public health concern, affecting nearly 8% of adolescents in the United States.1 Multicomponent lifestyle interventions are currently the first-line treatment approaches for adolescent obesity,2 but nonresponse and relapse are significant challenges.3 Bariatric surgery is increasingly being recommended as an effective long-term treatment for severe adolescent obesity.4 However, several behaviors that are common in adolescents with obesity5 may have the potential to undermine bariatric surgery outcomes. These include loss-of-control (LOC) eating,6 which is characterized by a sense that one cannot control what or how much one is eating.7 LOC eating is reported by up to 30% of adolescents with obesity,8 including those seeking surgical treatment options.9 In addition to strong associations between LOC eating and excess weight gain and related metabolic dysfunction in naturalistic studies of youth,10–12 LOC eating is a marker for poorer health-related quality of life,13 interpersonal distress,14 and elevated eating-related and general psychopathology (eg, weight and shape concerns,15,16 symptoms of depression and anxiety,17,18 risk-taking behaviors19,20), suggesting that it is a problem of considerable clinical significance.
LOC eating may involve the consumption of objectively or subjectively large amounts of food.21 The frequency of LOC eating episodes, particularly those involving objectively large amounts of food, decreases dramatically in the immediate postoperative period among most adults,22,23 likely because of a multitude of surgery-related factors affecting the amount and types of food that can be consumed (eg, mechanical restriction, taste sensitivity and preferences, food hedonics or liking, and food reward or wanting).24 However, a minority of adult patients continue or begin to engage in subjectively large LOC episodes over 2 to 3 years after surgery,22,23 with the rates gradually increasing over time postoperatively. Although the literature regarding the impact of presurgical LOC eating on postsurgical weight outcomes in adults has been mixed,25 research consistently suggests that postsurgical LOC eating is associated with poorer weight outcomes in both the short- and long-term.26
Although our group previously reported that nearly 30% of adolescents seeking bariatric surgery endorsed LOC eating before surgery,9 the prevalence and presentation of LOC eating (eg, from objectively to subjectively large episodes) and impacts on weight outcomes in the short- and long-term postoperatively are unclear in this age group. Recent research in adolescents found presurgical LOC eating to predict a reduced rate of relative weight loss in the first year after surgery6; however, the sample was limited to adolescents undergoing laparoscopic adjustable gastric banding, and longer-term data were not reported. It is essential to characterize the course and impact of LOC eating on adolescent weight outcomes over a longer duration of follow-up and for those undergoing commonly used surgical procedures to inform screening and intervention recommendations.
In the current study, we aimed to extend our previous research on the prevalence of presurgical LOC eating among adolescents9 by investigating the course and outcome of LOC eating from presurgery to 4-year follow-up among adolescents with severe obesity predominantly undergoing Roux-en-Y gastric bypass and vertical sleeve gastrectomy at 5 sites across the United States. We hypothesized that, similar to the adult literature,25 prevalence of LOC eating episodes would significantly decrease in the immediate postoperative period but would gradually increase after 1 to 4 years of follow-up, with slower increases in objectively large versus nonlarge LOC episodes. We further hypothesized that postsurgical LOC eating would be associated with poorer weight loss and maintenance across all 4 years of follow-up.26
Methods
Participants
Teen-Longitudinal Assessment of Bariatric Surgery is an ongoing, prospective observational cohort study of 242 adolescents, aged 13 to 19 years at baseline before surgery, who underwent bariatric surgery at 5 centers across the United States from 2007 to 2012. Participants completed baseline assessments within 30 days of their scheduled surgery date, and follow-up data were collected at 6-month, 1-year, 2-year, 3-year, and 4-year postoperative research visits. The original study was approved by the institutional review board at each of the 5 study sites, and written informed consent and assent were obtained from all participants. Additional information about the study protocol is provided in the original report (clinicaltrials.gov identifier: NCT00474318).4
Measures
Height and weight were measured by trained study staff to determine BMI. Age, race (“white or Caucasian,” “black or African-American,” “Asian,” “American Indian or Alaska Native,” "Native Hawaiian or other Pacific Islander,” “other,” or “unknown”), and ethnicity (“Hispanic,” “non-Hispanic,” or “unknown”) were based on self-report, whereas socioeconomic status was based on caregiver report of annual household income (ranging from <$5000 to ≥$200 000). LOC eating was assessed via items adapted from the Questionnaire on Eating and Weight Patterns-Revised (QEWP-R), which has good psychometric properties.27,28 In the current study, we examined 2 types of LOC eating, 1 characterized by LOC while consuming an unusually large amount of food, or loss-of-control objective binge eating (LOC-OBE), and the other by LOC while continuously eating, or continuous loss-of-control eating (LOC-C). LOC-OBE, which approximates the traditional classification of binge eating employed by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition,7 was defined by positive responses to both of the following QEWP-R questions: “In the past 6 months, did you ever eat within any 2-hour period what most people would regard as an unusually large amount of food?” and, “During these episodes, how often did you feel that your eating was out of control?” On the contrary, LOC-C allowed for examination of LOC eating episodes that were independent of the amount of food eaten, consistent with evidence that LOC (rather than episode size) is the core construct accounting for distress and impairment related to binge eating.29 LOC-C was defined by positive responses to both of the following questions adapted from QEWP-R: “During the past 6 months, have you had times when you eat continuously during the day or parts of the day without planning what and how much you would eat?” and, “Did you experience a loss of control, that is, you felt like you could not control your eating?” This item was included to approximate grazing, a behavior of clinical relevance in the bariatric surgery population that is characterized by eating small or modest amounts of food continuously in an unplanned and repetitious manner.30 In light of previous data demonstrating that LOC eating, regardless of frequency, is associated with adverse health-related correlates,31 any reported instances of LOC eating in the past 6 months categorized a participant as engaging in LOC eating at a given time point.
Statistical Analysis
All data analyses were conducted in SAS, version 9.4 (SAS Institute, Inc, Cary, NC). A total of 234 of the 242 participants enrolled in the Teen-Longitudinal Assessment of Bariatric Surgery study had complete BMI and QEWP-R data at the presurgical assessment and hence, were included in the current analyses. All data were reported for these 234 participants. Descriptive characteristics were used to characterize the percentage of participants engaging in LOC-OBE and LOC-C (dichotomized as yes or no) at the pre- and postsurgical assessments. Two separate generalized linear mixed models (GLMMs) were used to assess the rates of change in LOC-OBE and LOC-C from baseline to 4-year follow-up and include all participants. A GLMM was also used to examine the association of any presurgical LOC eating (main effect of reporting LOC-OBE and/or LOC-C) with percent change in BMI over the 4-year follow-up, including examination of the presurgical LOC eating by time interaction, with results reflecting least square means and associated 95% confidence intervals (CIs). Additional GLMMs were used to examine those reporting no LOC eating at any postsurgical time points, LOC eating at 1 to 2 postsurgical time points, and LOC eating at 3 or more postsurgical time points, again, with the LOC eating group by time interaction being of particular interest. Cross-lagged GLMMs were then used to further investigate the association of any LOC eating with percent change in BMI at the subsequent time point. All models included age, sex, race, ethnicity, surgery type, visit (as appropriate), and study site; for the GLMMs, study site was entered as a random effect as well as the intercept. Least square means and associated SEs are reported from the GLMMs. A Tukey-Kramer adjustment was used to adjust for multiple comparisons for the visits in the GLMMs, where appropriate. A P value, or adjusted P value (as appropriate), of <.05 was considered statistically significant.
Results
Descriptive Characteristics
The sample included 234 adolescents with a mean age of 17.1 ± 1.6 years at the presurgical assessment. They were mostly female (n = 177; 75.6%) and white (n = 170; 72.6%), with the remainder self-identifying as African American (n = 52; 22.2%), Asian (n = 1; 0.4%), or multiracial (n = 11; 4.7%). The sample was 92.7% (n = 217) non-Hispanic. The median presurgery BMI was 51 (range = 39–88). A total of 67.9% (n = 159) of participants underwent Roux-en-Y gastric bypass, 26.9% (n = 63) underwent vertical sleeve gastrectomy, and 5.1% (n = 12) underwent laparoscopic adjustable gastric banding. The 8 participants with missing baseline QEWP-R information had a lower median BMI than those with complete data (P = .03), but the 2 groups did not significantly differ with respect to age, sex, race, ethnicity, or socioeconomic status (all P > .05). Retention was high, with complete QEWP-R data ranging from 79.5% to 87.2% across the 4 years of data collection.
Prevalence of LOC Eating
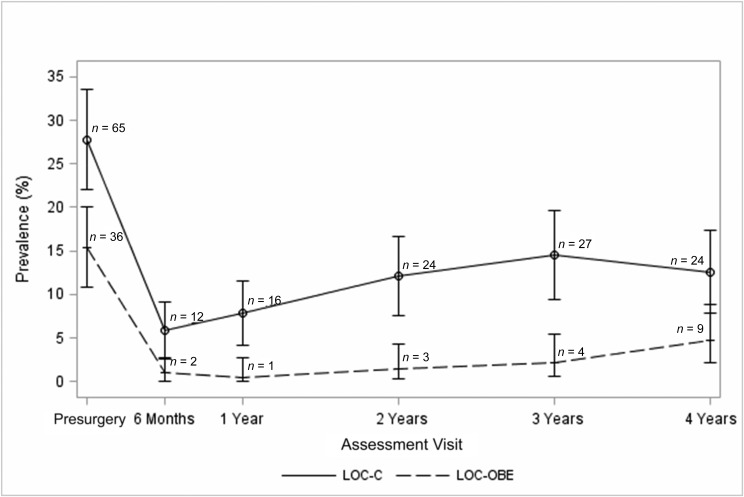
Prevalence rates of LOC-OBE and LOC-C eating at each time point are depicted in Fig 1. A total of 25 participants (10.7%) reported both LOC-OBE and LOC-C at the presurgical assessment. Thereafter, the percentage of participants who endorsed both types of LOC eating ranged from 0.5% at 6-month follow-up, to 2.1% at 4-year follow-up.
FIGURE 1.
Prevalence of LOC eating during the 4 years after surgery. Note: bars represent 95% CIs.
Of 36 participants reporting LOC-OBE at the presurgical assessment (15.4% of the presurgical sample), 4 (11.1%) reported LOC-OBE at 1 or more of the follow-up assessments. Of the 198 participants without LOC-OBE at baseline, 9 (4.5%) reported LOC-OBE at 1 or more of the follow-up assessments.
Of 65 participants reporting LOC-C at the presurgical assessment (27.8% of the presurgical sample), 25 (38.5%) reported LOC-C at 1 or more of the follow-up assessments. Of the 169 participants without LOC-C at baseline, 37 (21.9%) reported LOC-C at 1 or more of the follow-up assessments.
Rates of both LOC-OBE and LOC-C were significantly lower at all 5 follow-up time points relative to baseline (all P < .001). However, rates of both LOC eating behaviors gradually increased from the 6-month to 4-year follow-up, with slightly steeper increases in LOC-C (β = .0016, SE = 0.0006; P = .01) compared with those observed for LOC-OBE (β = .0007, SE = 0.0004; P = .048).
Prospective Association of LOC Eating With Weight Change
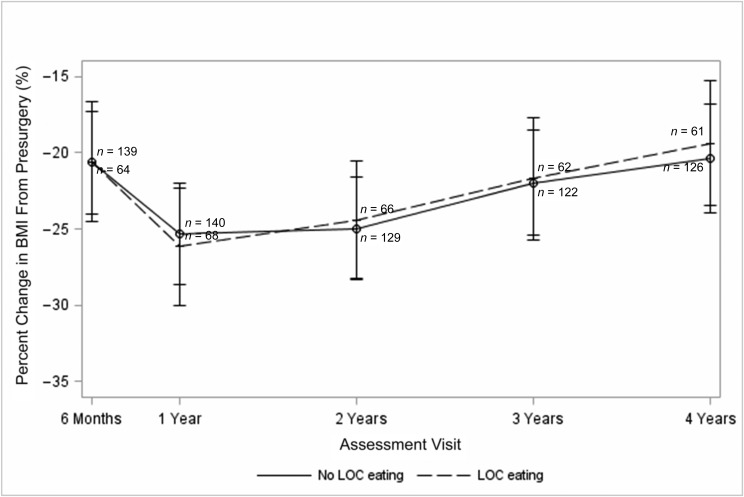
The 234 participants in this analysis reduced their BMI by an average of 25.3% ± 14.8% from baseline to 4-year follow-up. As shown in Fig 2, the GLMM revealed the absence of a significant interactive effect of presurgical LOC eating (defined as LOC-OBE or LOC-C) by time on BMI change through 4-year follow-up (P = .95). There was also no evidence of a significant main effect of presurgical LOC eating on BMI change through 4 years (P = .79).
FIGURE 2.
Prospective association between presurgical LOC eating and percent change in BMI over 4 years of follow-up. Note: bars represent 95% CIs.
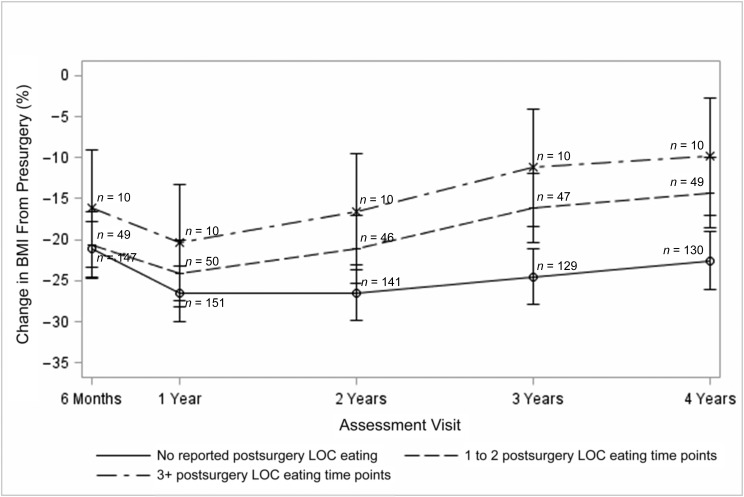
However, as illustrated in Fig 3, when the 3 postsurgical LOC eating groups (ie, no postsurgical LOC eating, LOC eating at 1–2 postsurgical time points, or LOC eating at 3 or more postsurgical time points) were compared, there was a significant group by time interaction (P = .015). Examination of the individual postsurgical time points revealed significant differences between the group reporting no postsurgical LOC eating and both of the groups reporting postsurgical LOC eating in terms of percent change in BMI from presurgery to 2-, 3- and 4-year follow-up. There were no statistically significant differences between the 2 groups that reported postsurgical LOC eating in terms of percent change in BMI from presurgery; however, the group reporting LOC during at least 3 postsurgical time points consistently showed the smallest percent change in BMI from presurgery.
FIGURE 3.
Prospective association between LOC eating and percent change in BMI at the next consecutive follow-up assessment. Note: bars represent 95% CIs.
To further observe this phenomenon, we examined a cross-lagged GLMMs at each visit from 6 months to 4 years with percent change in BMI from presurgery as the dependent variable. The presence of any LOC eating at presurgery compared with no LOC eating at presurgery (−20.3% ± 1.0% vs −19.4% ± 0.7%, respectively; P = .72) was not related to percent BMI change by 6-month follow-up, and any LOC eating at 6 months compared with no LOC eating at 6 months was not related to percent BMI change by 1 year (−22.6% ± 2.5% vs −24.8% ± 1.2%; P = .39). However, comparing those with LOC eating to those who did not endorse LOC eating at the 1-year (−18.3% ± 3.6% vs −25.1% ± 2.2%; P = .03), 2-year (−13.5% ± 4.3% vs −23.0% ± 2.9%; P = .009), and 3-year assessments (−12.3% ± 4.1% vs −22.4% ± 3.0%; P = .002) revealed that LOC eating at each assessment was associated with a significantly smaller reduction in percent BMI from baseline at the next consecutive assessment (ie, 2-, 3-, and 4-year assessments, respectively).
Discussion
In the current study, we investigated the course and outcome of LOC eating among adolescents with severe obesity who underwent bariatric surgery. As previously reported by our group,9 rates of any LOC eating (ie, LOC-OBE or LOC-C) before surgery were consistent with those reported in other weight-loss treatment–seeking samples of youth.8 In the current study, similar to findings in the adult literature,25 we observed dramatic decreases in the prevalence of LOC eating in the period immediately after surgery followed by gradual increases in this behavior over 4 years of postoperative follow-up. Although not statistically significant, these increases appeared to be steeper for eating behavior characterized by LOC-C relative to LOC-OBE. Finally, although reporting LOC eating at presurgery and 6-month follow-up was not associated with later weight change, engaging in LOC eating at 1-, 2-, or 3-year follow-up was prospectively associated with greater subsequent weight regain. Taken together, results reveal that assessing for the presence of LOC eating in the postsurgical period may be warranted because this phenotype is associated with poorer weight outcomes.
With our findings, we indicate that LOC-C is more common after surgery than LOC-OBE. This may be related to biological and/or psychological factors limiting the amount or types of food one can consume postsurgery (eg, pouch size, drive to eat) and suggests that LOC may be more likely to manifest in grazing-type behaviors than objectively large binges after surgery (the former of which nevertheless has an adverse impact on postsurgical outcomes30). Because of the low rates of LOC eating in the current sample, we were unable to examine transitions between different types of LOC eating behaviors during the postsurgical period. Therefore, it is unclear if the relatively lower rates of LOC-OBE compared with LOC-C reflected participants “migrating” from engaging in objectively large episodes to smaller, continuous episodes, or if LOC-OBE is more likely to remit after surgery. This should be investigated in future, adequately powered studies.
Contrary to previous findings in adolescents6 but consistent with most (but not all32–34) findings in adults,25 presurgical LOC eating had no discernible association with percent BMI change after surgery. On the basis of these results, presurgical LOC eating does not appear to be a contraindication for surgery in adolescents. Indeed, in the first year after surgery, LOC eating was not associated with weight reduction at subsequent time points, which could lead clinicians to relax their attentiveness to problematic eating behaviors in their adolescent patients. Because we observed that LOC eating gradual increased in the years after surgery, and that endorsing any LOC eating (regardless of size) at 1-, 2-, or 3-years postsurgery was related to greater weight regain at the following time point, we suggest that continued vigilance regarding patients’ eating behaviors is warranted. It is possible that LOC eating also is related to surgical outcomes other than weight (eg, psychosocial functioning, medical complications, cardiometabolic health), thus warranting future research to examine the more general health impact of LOC eating over time in adolescent bariatric surgery patients. In future work, researchers should also clarify whether interventions focused on reducing LOC improve weight outcomes in postsurgical patients as well as the optimal timing and content of such interventions.
This study was marked by several strengths, including the large sample, which included participants undergoing multiple different types of surgical procedures, and the inclusion of long-term, prospective, follow-up data. Furthermore, with the sophisticated cross-lagged analyses, we were able to establish precedence in the relationship between LOC eating and weight change over time, showing that the presence of LOC eating at a given time point from 1- to 3-year follow-up was associated with greater weight regain at the subsequent time point. The major limitation concerned the use of a self-report questionnaire to assess LOC eating over the 6 months before assessment. LOC is a complex construct that may be difficult for adolescents to understand; therefore, it is unclear if similar findings would emerge had LOC eating been assessed by trained raters (although the predictive validity of LOC eating in terms of objectively measured BMI provides some support for the utility of the current measure). Similarly, some participants may have incorrectly categorized the size of reported LOC episodes, especially in recalling eating episodes over a 6-month time frame. Moreover, although LOC was assessed in the context of both objectively large and continuous eating episodes (the latter of which may be particularly applicable to postsurgical populations30), the lack of assessment of other types of historically defined LOC eating episodes (ie, subjectively large binges) may limit cross-study comparisons. Other limitations included the inability to investigate whether the prevalence and/or outcome of LOC eating are related to surgical procedure because of insufficient power (although analyses did adjust for surgery type), the lack of a nonsurgical control group, and the limited information available regarding interventions received before surgery, which could have impacted LOC eating (eg, behavioral weight loss treatment, psychotherapy).
Conclusions
In adolescents, the postbariatric surgery period is marked by sharp declines in LOC eating followed by gradual increases over 4 years of follow-up that appear to differ according to the amount of food consumed. Moreover, postsurgical LOC eating, irrespective of size, may be associated with poorer weight outcomes in the 4 years after surgery. With these findings taken together, we suggest that continued monitoring of eating patterns is warranted in postoperative patients, regardless of history of LOC eating. In future work, researchers should clarify the impact of treating LOC eating on weight outcomes and assess whether LOC eating influences other postsurgical outcomes (eg, medical complications).
Glossary
- CI
confidence interval
- GLMM
generalized linear mixed model
- LOC
loss of control
- LOC-C
continuous loss-of-control eating
- LOC-OBE
loss-of-control objective binge eating
- QEWP-R
Questionnaire on Eating and Weight Patterns-Revised
Footnotes
Dr Goldschmidt conceptualized and designed the study and drafted the initial manuscript; Drs Inge, Zeller, and Mitchell conceptualized and designed the study, designed the data collection instruments, coordinated and supervised data collection, and critically reviewed the manuscript; Drs Khoury and Jenkins conducted the statistical analyses and reviewed and revised the manuscript; Drs Bond, Thomas, and Utzinger conceptualized and designed the study and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00474318).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by grants from the National Institute of Diabetes and Digestive and Kidney Disease (U01-DK072493 and K23-DK105234). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Inge has been a consultant with and owns stock in Standard Bariatrics; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Ogden CL, Carroll MD, Lawman HG, et al. . Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315(21):2292–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirschenbaum DS, Gierut K. Treatment of childhood and adolescent obesity: an integrative review of recent recommendations from five expert groups. J Consult Clin Psychol. 2013;81(2):347–360 [DOI] [PubMed] [Google Scholar]
- 3.Coppock JH, Ridolfi DR, Hayes JF, St Paul M, Wilfley DE. Current approaches to the management of pediatric overweight and obesity. Curr Treat Options Cardiovasc Med. 2014;16(11):343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inge TH, Courcoulas AP, Jenkins TM, et al. ; Teen-LABS Consortium . Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. 2016;374(2):113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldschmidt AB, Aspen VP, Sinton MM, Tanofsky-Kraff M, Wilfley DE. Disordered eating attitudes and behaviors in overweight youth. Obesity (Silver Spring). 2008;16(2):257–264 [DOI] [PubMed] [Google Scholar]
- 6.Sysko R, Devlin MJ, Hildebrandt TB, Brewer SK, Zitsman JL, Walsh BT. Psychological outcomes and predictors of initial weight loss outcomes among severely obese adolescents receiving laparoscopic adjustable gastric banding. J Clin Psychiatry. 2012;73(10):1351–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013 [Google Scholar]
- 8.He J, Cai Z, Fan X. Prevalence of binge and loss of control eating among children and adolescents with overweight and obesity: an exploratory meta-analysis. Int J Eat Disord. 2017;50(2):91– 103 [DOI] [PubMed] [Google Scholar]
- 9.Utzinger LM, Gowey MA, Zeller M, et al. ; Teen Longitudinal Assessment of Bariatric Surgery (Teen-LABS) Consortium . Loss of control eating and eating disorders in adolescents before bariatric surgery. Int J Eat Disord. 2016;49(10):947–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanofsky-Kraff M, Cohen ML, Yanovski SZ, et al. . A prospective study of psychological predictors of body fat gain among children at high risk for adult obesity. Pediatrics. 2006;117(4):1203–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanofsky-Kraff M, Yanovski SZ, Schvey NA, Olsen CH, Gustafson J, Yanovski JA. A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. Int J Eat Disord. 2009;42(1):26–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanofsky-Kraff M, Shomaker LB, Stern EA, et al. . Children’s binge eating and development of metabolic syndrome. Int J Obes (Lond). 2012;36(7):956–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranzenhofer LM, Columbo KM, Tanofsky-Kraff M, et al. . Binge eating and weight-related quality of life in obese adolescents. Nutrients. 2012;4(3):167–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott CA, Tanofsky-Kraff M, Shomaker LB, et al. . An examination of the interpersonal model of loss of control eating in children and adolescents. Behav Res Ther. 2010;48(5):424–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goossens L, Soenens B, Braet C. Prevalence and characteristics of binge eating in an adolescent community sample. J Clin Child Adolesc Psychol. 2009;38(3):342–353 [DOI] [PubMed] [Google Scholar]
- 16.Schlüter N, Schmidt R, Kittel R, Tetzlaff A, Hilbert A. Loss of control eating in adolescents from the community. Int J Eat Disord. 2016;49(4):413–420 [DOI] [PubMed] [Google Scholar]
- 17.Shomaker LB, Tanofsky-Kraff M, Elliott C, et al. . Salience of loss of control for pediatric binge episodes: does size really matter? Int J Eat Disord. 2010;43(8):707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldschmidt AB, Jones M, Manwaring JL, et al. . The clinical significance of loss of control over eating in overweight adolescents. Int J Eat Disord. 2008;41(2):153–158 [DOI] [PubMed] [Google Scholar]
- 19.Goldschmidt AB, Loth KA, MacLehose RF, Pisetsky EM, Berge JM, Neumark-Sztainer D. Overeating with and without loss of control: associations with weight status, weight-related characteristics, and psychosocial health. Int J Eat Disord. 2015;48(8):1150–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonneville KR, Horton NJ, Micali N, et al. . Longitudinal associations between binge eating and overeating and adverse outcomes among adolescents and young adults: does loss of control matter? JAMA Pediatr. 2013;167(2):149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fairburn CG, Cooper Z. The eating disorder examination In: Fairburn CG, Wilson GT, eds. Binge Eating: Nature, Assessment, and Treatment. 12th ed. New York, NY: Guilford Press; 1993:317–360 [Google Scholar]
- 22.de Zwaan M, Hilbert A, Swan-Kremeier L, et al. . Comprehensive interview assessment of eating behavior 18-35 months after gastric bypass surgery for morbid obesity. Surg Obes Relat Dis. 2010;6(1):79–85 [DOI] [PubMed] [Google Scholar]
- 23.White MA, Kalarchian MA, Masheb RM, Marcus MD, Grilo CM. Loss of control over eating predicts outcomes in bariatric surgery patients: a prospective, 24-month follow-up study. J Clin Psychiatry. 2010;71(2):175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.le Roux CW, Bueter M. The physiology of altered eating behaviour after Roux-en-Y gastric bypass. Exp Physiol. 2014;99(9):1128–1132 [DOI] [PubMed] [Google Scholar]
- 25.Meany G, Conceição E, Mitchell JE. Binge eating, binge eating disorder and loss of control eating: effects on weight outcomes after bariatric surgery. Eur Eat Disord Rev. 2014;22(2):87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldschmidt AB, Conceição EM, Thomas JG, Mitchell JE, Raynor HA, Bond DS. Conceptualizing and studying binge and loss of control eating in bariatric surgery patients-time for a paradigm shift? Surg Obes Relat Dis. 2016;12(8):1622–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spitzer RL, Yanovski SZ, Marcus MD. The Questionnaire on Eating and Weight Patterns-Revised (QEWP-R). New York, NY: New York State Psychiatric Institute; 1993 [Google Scholar]
- 28.Nangle DW, Johnson WG, Carr-Nangle RE, Engler LB. Binge eating disorder and the proposed DSM-IV criteria: psychometric analysis of the Questionnaire of Eating and Weight Patterns. Int J Eat Disord. 1994;16(2):147–157 [DOI] [PubMed] [Google Scholar]
- 29.Goldschmidt AB. Are loss of control while eating and overeating valid constructs? A critical review of the literature. Obes Rev. 2017;18(4):412–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conceição EM, Mitchell JE, Engel SG, Machado PPP, Lancaster K, Wonderlich SA. What is “grazing”? Reviewing its definition, frequency, clinical characteristics, and impact on bariatric surgery outcomes, and proposing a standardized definition. Surg Obes Relat Dis. 2014;10(5):973–982 [DOI] [PubMed] [Google Scholar]
- 31.Vannucci A, Theim KR, Kass AE, et al. . What constitutes clinically significant binge eating? Association between binge features and clinical validators in college-age women. Int J Eat Disord. 2013;46(3):226–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chao AM, Wadden TA, Faulconbridge LF, et al. . Binge-eating disorder and the outcome of bariatric surgery in a prospective, observational study: two-year results. Obesity (Silver Spring). 2016;24(11):2327–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pekkarinen T, Koskela K, Huikuri K, Mustajoki P. Long-term results of gastroplasty for morbid obesity: binge-eating as a predictor of poor outcome. Obes Surg. 1994;4(3):248–255 [DOI] [PubMed] [Google Scholar]
- 34.Sallet PC, Sallet JA, Dixon JB, et al. . Eating behavior as a prognostic factor for weight loss after gastric bypass. Obes Surg. 2007;17(4):445–451 [DOI] [PubMed] [Google Scholar]