Abstract
Purpose:
Radiation causes damage to irradiated tissues and also tissues that do not receive direct irradiation through a phenomenon called out-of-field effects. This damage through signals such as inflammatory responses can be transmitted to unirradiated cells/tissues and causes many effects such as oxidative damage. The radioprotective and anti-inflammatory effects of melatonin have been demonstrated in various studies. The aim of this study was to evaluate the effect of pretreatment with melatonin on oxidative damage caused by direct irradiation and out-of-field effects on the lung tissue after pelvic irradiation in rats.
Materials and Methods:
In this experimental study, 42 adult male Wistar albino rats were divided into seven groups (six rats per group) including control, melatonin treatment, localized irradiation to the pelvis (out-of-field group), whole-body scatter group (which gave radiation dose equal to the amount of radiation that the lung had received from the localized pelvic irradiation), direct irradiation to lung, melatonin administration before localized radiation to the pelvis, and melatonin administration before localized radiation to the lung. A 100 mg/kg of melatonin 30 min before irradiation with 5 Gy γ-rays in a local (3.75 cm × 3.75 cm) field to the lower abdomen was administered to the rats, and after 24 h, all rats were sacrificed and their lungs were excised to measure the biochemical parameters including malondialdehyde (MDA), glutathione peroxidase (GPx), and superoxide dismutase (SOD).
Results:
The results showed that localized irradiation to the lung or pelvis caused an increase in the MDA level. Moreover, pelvis and lung irradiation increased the GPx and SOD activity in the lungs. Pretreatment with melatonin before irradiation reduced the GPx and MDA levels in both targeted and nontargeted lung tissues and reduced the SOD activity after lung irradiation.
Conclusion:
Although pretreatment with melatonin did not increase the activity of SOD and GPx in comparison to the radiation groups, this study showed that preadministration of melatonin can ameliorate the oxidative damage induced by ionizing radiation.
Keywords: Glutathione peroxidase, malondialdehyde, melatonin, nontargeted effect, radiation, superoxide dismutase
INTRODUCTION
Several studies have suggested that ionizing radiation can affect not only the irradiated cells and tissues but also those outside the radiation field.[1] Studies have shown that released signals from irradiated cells/tissues affect the adjacent or distance tissues.[2] This effect is associated with DNA double-strand break, chromosomal aberrations, changes in cell proliferation processes, inflammatory response, apoptosis, cell death, mutagenesis, and increased risk of carcinogenesis.[2,3] Studies have proposed that increased level of inflammatory cytokines, mitochondrial malfunction, and upregulation of some reactive oxygen species (ROS) producing enzymes such as nicotinamide adenine dinucleotide phosphate (NADPH) Oxidase, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) are the factors involved in mediating the oxidative damage in nonirradiated tissues.[4,5]
In normal conditions at the cellular level, there is a balance between the production of free radicals and antioxidant activities.[6] The antioxidant system consists of both enzymatic and nonenzymatic parts such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), glutathione reductase (GSH-Rd), and glucose-6-phosphate dehydrogenase (G6PD or G6PDH).[7,8] The SOD enzyme is in the first line of defense against oxidative damage which converts superoxide to H2O2 and O2.[9] Similarly, GPx is also a natural enzyme, which reduces hydrogen peroxide and catalyzes a wide range of organic peroxides to the relevant alcohol and water.[10] Suppression of these enzymes is one of the main reasons for massive oxidative damage following exposure to ionizing radiation.[11] Several studies have introduced melatonin as a proper immunostimulator and radioprotector for the amelioration of oxidative damage at the directly irradiated site.[12,13] Melatonin is able to scavenge free radicals directly or through its stimulatory actions on antioxidant enzymes activity, inhibitions on pro-oxidative enzyme activity, and protective effects on macromolecules.[14] Lipid peroxidation has an important role in destruction and damage to the cells. The process of lipid peroxidation involves oxidative conversion of polyunsaturated fatty acids to several products such as malondialdehyde (MDA). MDA, being an end product of lipid peroxidation, serves as an index of oxidative damage, and because of its high cytotoxicity and inhibitory actions on protective enzymes, it acts as a tumor promoter and a cocarcinogenic agent.[15] In this study, the effect of pretreatment with melatonin on the MDA level (as index of lipid peroxidation) and SOD and GPx enzyme activities was evaluated in targeted and nontargeted lung tissues exposed to γ-rays.
MATERIALS AND METHODS
The experimental protocol was in line with the guidelines for care and use of laboratory animals as adopted by the Ethics Committee of the School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
Animal model and irradiation procedure
Eight- to ten-week-old male Wistar rats, each weighing 180–200 g, were obtained from Tehran University Animal Facility, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran. They were housed in the animal facility, with the room temperature maintained at 20–22°C, a relative humidity of 50%–70%, and an airflow rate of 15 exchange/h. Furthermore, a time-controlled system provided 08:00–20:00 h light and 20:00–08:00 h dark cycles. All the rats were given a standard rodent chow diet and water from sanitized bottle fitted with stopper and sipper tubes.
Experimental design and irradiation
After a 2-week acclimatization period, 1 h before the start of the experiment, all rats were transferred to a laboratory near the cobalt-60 γ-source. The experiment was performed using 42 male rats with six rats in each of the following seven groups:
Group 1 received vehicle including phosphate-buffered saline (PBS) and 5% ethanol. Group 2 received an IP injection of 100 mg/kg body weight freshly prepared melatonin (Sigma–Aldrich Co., St. Louis, MO, USA) dissolved in the same volume of vehicle. Group 3 received 18 cGy whole-body exposure as scatter group + IP injection of vehicle 30 min before the irradiation. This group was given scattering radiation dose received by the lung at the localized pelvic irradiation. The scatter group showed that the changes in the out-of-field group lung tissues were not caused by the scattered radiation. Group 4 received 5 Gy localized body irradiation at the pelvis, whereas other tissues were protected by lead shield (direct to pelvis or out-of-field group). Group 5 received an IP injection of 100 mg/kg body weight melatonin 30 min before irradiation by a dose of 5 Gy localized body irradiation at the pelvis. Group 6 rats were exposed to a dose of 5 Gy localized body gamma-irradiation at the lung plus vehicle (direct to lung group) and Group 7 received an IP injection of 100 mg/kg body weight melatonin 30 min before irradiation by a dose of 5 Gy localized body gamma-irradiation at the lung. Melatonin administered to Groups 2, 5, and 7 was first dissolved in a small amount of absolute ethanol (25 μL) and then diluted with 475 μL PBS at a final ethanol concentration of 5%. Thirty minutes after the injections, all of the rats were anesthetized with an IP injection of 60 mg/kg ketamine and 20 mg/kg xylazine, and then irradiation was performed for Groups 3, 4, 5, 6, and 7. After 24 h, all the animals were sacrificed and their lung tissues were excised to measure the biochemical parameters including SOD and GPx activity as well as MDA level. In addition, the melatonin concentrations and the dose of gamma radiation selected were based on the experience from the studies performed by other researchers.[3,16,17]
Irradiation and measurement of scattered radiation dose
Irradiation was performed using a 1.25 MeV cobalt-60 γ-radiation source at a dose rate of 101 cGy/min with a source-surface distance of 60 cm and fixed field size of 3.75 cm × 3.75 cm for localized body irradiation at room temperature (22 ± 2°C). Scattered radiation dose received by the lung at the localized pelvic irradiation was measured using rat phantom (plexiglas and cork as a lung soft-tissue equivalent) and TLD100 dosimeter. The measured scatter dose at the lung tissues equivalent after localized irradiation of the pelvis was approximately 18 cGy.
Glutathione peroxidase, superoxide dismutase, and malondialdehyde assays
Supernatant preparation and ELISA
The lung tissues were perfused with a PBS solution (pH = 7.4), containing 0.16 mg/ml heparin to remove the red blood cells and clots. Then, the tissues were weighted (100 mg tissue per 1 ml PBS buffer) and homogenized (thoroughly by homogenizer at 5000 rpm for 20 min). The supernatant was carefully collected and used to quantify GPx, SOD, and MDA with a kit (Zellbio, Biocore, Germany). The GPx and SOD activity and the MDA level were measured with ELISA reader (calorimeter) at 412, 420, and 535 nm according to the manufacturer's instruction.
Statistical analysis
Each data point represents the mean ± standard error of the mean of at least five animals per group. A one-way analysis of variance was performed to compare different groups, followed by Tukey's method. P < 0.05 was considered as statistically significant difference.
RESULTS
Glutathione peroxidase activity
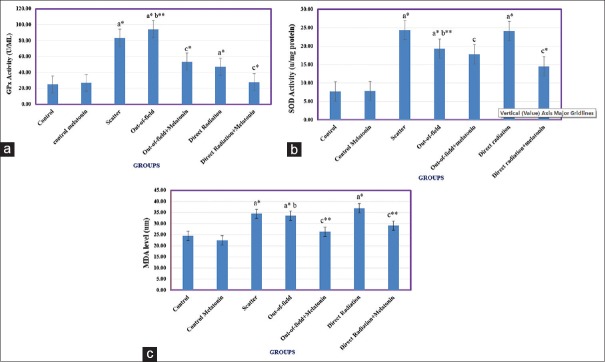
GPx activity in nontargeted, whole-body scatter and localized direct irradiation to lung group increased significantly (P < 0.001) in comparison to the control group, although increased GPx activity for localized direct irradiation to lung was lower than the other two groups (P < 0.001). GPx activity in the nontargeted irradiation group showed a significant increase (P < 0.05) compared with the scatter group. Treatment with melatonin 30 min before irradiation decreased the GPx activity in targeted and nontargeted lung tissues (P < 0.001) [Figure 1a].
Figure 1.
Changes in oxidative stress marker levels include glutathione peroxidase activity, superoxide dismutase activity and malondialdehyde level in lung tissues. (a) Glutathione peroxidase activity, (b) superoxide dismutase activity, and (c) malondialdehyde level in the lung tissues. Data analysis was based on the Tukey–Kramer method. (a) Values are expressed as a comparison between irradiated groups and control groups, (b) nontargeted group was compared to the scatter group, (c) melatonin treatment groups were compared to the corresponding irradiated groups. *P<0.001 and **P<0.05
Superoxide dismutase activity
SOD activity in X-irradiated (targeted and nontargeted) lung tissues and also the scatter group increased significantly (P < 0.001) compared with the control group, although SOD activity in nontargeted group was lower than the scatter group (P < 0.05). Treatment with melatonin 30 min before irradiation decreased the SOD activity in targeted but not for nontargeted lung groups (P < 0.001) [Figure 1b].
Malondialdehyde
MDA level in X-irradiated (targeted, nontargeted, and scatter groups) lung tissues increased significantly (P < 0.001) compared with the control group. Results showed that there is no significant difference in the MDA level between these groups. Treatment with melatonin 30 min before irradiation reduced the MDA level for both targeted and nontargeted groups (P < 0.05) [Figure 1c].
DISCUSSION
In this research, it has been demonstrated that administration of melatonin before irradiation caused a significant reduction in oxidative stress in both targeted and nontargeted lung tissues. It was shown that direct irradiation to the lung resulted in an increase in the MDA level and GPx activity in both targeted and nontargeted lung tissues. Direct irradiation leads to an increase in SOD activity, whereas nontargeted effect suppressed the SOD activity. Inhibition of antioxidant enzymes such as SOD in bystander cells has been demonstrated in some studies. These studies indicated that transforming growth factor beta 1 (TGF-β)–miR-21 pathway has a key role for suppression of SOD level and increased ROS.[18] The results presented in this report indicate that melatonin is able to ameliorate oxidative damage in both targeted and nontargeted lung tissues. The radioprotective effect of melatonin for targeted lung tissues was associated with reduced SOD and GPx activities. These results are in contrast to several previous studies. The altered level of antioxidant enzymes is dose dependent. Reduction in SOD activity and GSH levels has been demonstrated following exposure to high doses (18 Gy), but at a lower-dose (3 Gy), an increase in the levels of these enzymes was reported.[19] Moreover, previous studies have indicated that SOD activity and GSH level were suppressed in nontargeted cells.[20,21] These studies indicated that increased level of oxidative damage in nontargeted tissues reach the peak 24 h after exposure. These results are in line with the results of this study.
Previous studies have shown that melatonin is able to mitigate micronuclei formation in bystander cells.[22] In addition, in an in vivo study, melatonin has been shown to reduce inflammatory markers including COX-2 and iNOS in nontargeted lung tissues.[17] Given that the production of free radicals in both targeted and nontargeted tissues has a potent relation to inflammatory responses, it seems that both antioxidative and anti-inflammatory effect of melatonin are involved in this process. Moreover, inhibition of some signaling pathways in nontargeted effect such as TGF-β–mir-21 or TGF-β–COX-2 may be involved in radioprotection by melatonin. The previous studies confirmed that inhibition of these pathways result in abolished oxidative stress and DNA damage.[1,3,18] Given that melatonin has been shown to ameliorate increased TGF-β after irradiation, it is possible that suppression of TGF-β–mir-21 is involved in the upregulation of SOD activity after treatment with melatonin.[12] In addition, suppression of ROS-producing enzymes such as COX-2 by melatonin can help to reduce the oxidative stress. Radioprotection of both targeted and nontargeted tissues by melatonin may help to reduce the risk of second primary cancers in patients undergoing radiation treatment for their diseases.
The differences observed in the present study compared to those reported by others may be attributed to the differences in the experimental conditions, and hence, direct comparisons are not possible.
CONCLUSION
Exposure to gamma rays with 5 Gy causes upregulation of oxidative damage. This was associated with increased SOD and GPx activities in targeted tissues and decreased SOD and GPx activities in nontargeted lung tissues. Melatonin could ameliorate oxidative stress in both the targeted and nontargeted lung tissues.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Tehran University of medical sciences, grant number: 31495.
REFERENCES
- 1.Fardid R, Najafi M, Salajegheh A, Kazemi E, Rezaeyan A. Radiation-induced non-targeted effect in vivo: Evaluation of cyclooygenase-2 and endothelin-1 gene expression in rat heart tissues. J Cancer Res Ther. 2017;13:51–5. doi: 10.4103/0973-1482.203601. [DOI] [PubMed] [Google Scholar]
- 2.Morgan WF, Sowa MB. Non-targeted bystander effects induced by ionizing radiation. Mutat Res. 2007;616:159–64. doi: 10.1016/j.mrfmmm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Chai Y, Lam RK, Calaf GM, Zhou H, Amundson S, Hei TK. Radiation-induced non-targeted response in vivo: Role of the TGFß-TGFBR1-COX-2 signalling pathway. Br J Cancer. 2013;108:1106–12. doi: 10.1038/bjc.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Najafi M, Fardid R, Hadadi G, Fardid M. The mechanisms of radiation-induced bystander effect. J Biomed Phys Eng. 2014;4:163–72. [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TJ, Wu CC, Chai Y, Lam RK, Hamada N, Kakinuma S, et al. Induction of non-targeted stress responses in mammary tissues by heavy ions. PLoS One. 2015;10:e0136307. doi: 10.1371/journal.pone.0136307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- 7.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mihandoost E, Shirazi A, Mahdavi SR, Aliasgharzadeh A. Consequences of lethal-whole-body gamma radiation and possible ameliorative role of melatonin. ScientificWorldJournal. 2014;2014:621570. doi: 10.1155/2014/621570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukai T, Ushio-Fukai M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Bhandar B, Kavdia M. Interaction of ROS and RNS with GSH and GSH/GPX Systems. FASEB J. 2015;29:636–7. [Google Scholar]
- 11.Rezaeyan A, Haddadi GH, Hosseinzadeh M, Moradi M, Najafi M. Radioprotective effects of hesperidin on oxidative damages and histopathological changes induced by X-irradiation in rats heart tissue. J Med Phys. 2016;41:182–91. doi: 10.4103/0971-6203.189482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Najafi M, Shirazi A, Motevaseli E, Geraily G, Norouzi F, Heidari M, et al. The melatonin immunomodulatory actions in radiotherapy. Biophys Rev. 2017;9:139–48. doi: 10.1007/s12551-017-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Najafi M, Shirazi A, Motevaseli E, Rezaeyan AH, Salajegheh A, Rezapoor S. Melatonin as an anti-inflammatory agent in radiotherapy. Inflammopharmacology. 2017;25:403–13. doi: 10.1007/s10787-017-0332-5. [DOI] [PubMed] [Google Scholar]
- 14.Shirazi A, Ghobadi G, Ghazi-Khansari M. A radiobiological review on melatonin: A novel radioprotector. J Radiat Res. 2007;48:263–72. doi: 10.1269/jrr.06070. [DOI] [PubMed] [Google Scholar]
- 15.Taysi S, Koc M, Büyükokuroglu ME, Altinkaynak K, Sahin YN. Melatonin reduces lipid peroxidation and nitric oxide during irradiation-induced oxidative injury in the rat liver. J Pineal Res. 2003;34:173–7. doi: 10.1034/j.1600-079x.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 16.Chai Y, Calaf GM, Zhou H, Ghandhi SA, Elliston CD, Wen G, et al. Radiation induced COX-2 expression and mutagenesis at non-targeted lung tissues of gpt delta transgenic mice. Br J Cancer. 2013;108:91–8. doi: 10.1038/bjc.2012.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fardid R, Salajegheh A, Mosleh-Shirazi MA, Sharifzadeh S, Okhovat MA, Najafi M, et al. Melatonin ameliorates the production of COX-2, iNOS, and the formation of 8-OHdG in non-targeted lung tissue after pelvic irradiation. Cell J. 2017;19:324–31. doi: 10.22074/cellj.2016.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Y, Chen X, Tian W, Yin X, Wang J, Yang H. The role of TGF-ß1-miR-21-ROS pathway in bystander responses induced by irradiated non-small-cell lung cancer cells. Br J Cancer. 2014;111:772–80. doi: 10.1038/bjc.2014.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Najafi M, Fardid R, Takhshid MA, Mosleh-Shirazi MA, Rezaeyan AH, Salajegheh A. Radiation-induced oxidative stress at out-of-field lung tissues after pelvis irradiation in rats. Cell J. 2016;18:340–5. doi: 10.22074/cellj.2016.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu S, Wang J, Ding N, Hu W, Zhang X, Wang B, et al. Exosome-mediated microRNA transfer plays a role in radiation-induced bystander effect. RNA Biol. 2015;12:1355–63. doi: 10.1080/15476286.2015.1100795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian W, Yin X, Wang L, Wang J, Zhu W, Cao J, et al. The key role of miR-21-regulated SOD2 in the medium-mediated bystander responses in human fibroblasts induced by a-irradiated keratinocytes. Mutat Res. 2015;780:77–85. doi: 10.1016/j.mrfmmm.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Marozik P, Mothersill C, Seymour CB, Mosse I, Melnov S. Bystander effects induced by serum from survivors of the Chernobyl accident. Exp Hematol. 2007;35 4 Suppl 1:55–63. doi: 10.1016/j.exphem.2007.01.029. [DOI] [PubMed] [Google Scholar]