Abstract
Background
Hormonally-sensitive organs in the neonate can change size within days of birth as circulating maternal estrogen wanes. Although several reports document the size of these organs through infancy, few focus on the near-birth period. Clinical and research evaluation of hormonal and genitourinary disorders would benefit from reference size standards.
Objective
We describe the size of the uterus, ovaries, testes, and breast buds in healthy term neonates.
Materials and Methods
As part of the Infant Feeding and Early Development (IFED) study, we sonographically measured the largest diameter of these organs in sagittal, transverse, and anterior-posterior planes for 194 female and 204 male newborns up to 3 days old. We calculated mean, median, and percentiles for longest axis length and for volume calculated from measured diameters. We evaluated size differences by laterality, sex, and race and compared our observations to published values.
Results
Mean length and volume, respectively, were: uterus, 4.2 cm and 10.0 cm3; ovary, 1.0 cm and 0.2 cm3; testis, 1.1 cm and 0.3 cm3 (0.4 cm3 Lambert volume); female breast bud, 1.2 cm and 0.7 cm3; male breast bud, 1.1 cm and 0.6 cm3. Breast buds were larger in females than males. Laterality differences were typically below the precision of clinical measurement. No significant race differences were detected.
Conclusion
Using data from our large cohort together with published values, we provide guidelines for evaluating the size of reproductive organs within the first 3 days of life. Discrepancies between our results and published values are likely attributable to technique.
Keywords: Ultrasound measurements, neonate, ovary, testis, breast, uterus
Introduction
Sonographic evaluation of the uterus, ovaries, testes, or breast buds in a neonate may be prompted by concerning clinical signs, such as ambiguous genitalia, hypospadias, non-palpable testes, atypical vaginal bleeding or neonatal lactation. Renal anomalies detected in utero may initiate evaluation of the gonads. Assessment of these structures may also occur incidentally during examination of adjacent anatomy. In resource limited areas, ultrasound may be more readily available than laboratory testing for confirming suspected reproductive abnormalities [1]. In these circumstances, having available size standards for these organs in neonates would be clinically useful.
Keats and Sistrom [2], a commonly used reference book for normal organ sizes, has limited data on neonatal ovaries and contains no reference to neonatal uterus, testes, or breast buds. Although studies of infants 6 months old or younger [3–6] exist, averaging estrogen-sensitive organ sizes across a several-month age range may not accurately reflect organ size near birth because of the potential for rapid change with diminishing maternal estrogen [7]. In addition, most previous reports of neonatal organ sizes reflect small numbers of children [1,6,8–19], generally fewer than 50, with exceptions [9,13–15]. Advantages of the present study include evaluation of the size of uterus, ovaries, testes, and breast buds of 398 healthy term newborns within 3 days of birth utilizing up-to-date ultrasound technology and standardized sonographic techniques. Our goals were to produce reference size standards for these organs early in life and to compare our findings to others in the literature.
Materials and Methods
Study Recruitment
The Infant Feeding and Early Development (IFED) Study, conducted at the Children’s Hospital of Philadelphia (CHOP) under contract from the National Institute of Environmental Health Sciences (NIEHS), recruited healthy mother and infant pairs from the postpartum and well-baby nurseries at three main study sites and five additional hospitals in the Philadelphia region (see Acknowledgements). Healthy singleton infants born between 37 and 42 weeks gestation and weighing between 2,500 – 4,500 grams were eligible if their mothers were at least 18 years of age at enrollment and intended to feed the child exclusively on one of three regimens: breast milk, cow-milk formula, or soy formula. Exclusion criteria for mothers included endocrine disorders, oral or intravenous steroid use, or immunosuppressant use during the pregnancy. Exclusion criteria for infants included congenital malformations, chromosomal anomalies, significant illness affecting feeding, growth or development, a sibling already enrolled in IFED, and males with one or both non-palpable testes. There were no restrictions with respect to ethnicity or race. Enrolled mother/infant pairs completed a study visit that included the ultrasound examination within 3 days (72 hours) of delivery. Using the infants’ weights taken at that visit (termed ‘neonatal weights’), we computed corresponding Z-scores based on World Health Organization (WHO) Child Growth Standards [20]. The institutional review boards at CHOP, Virtua Hospitals, Abington Memorial Hospital, and at the NIEHS approved the protocol, and each mother provided written informed consent for her and her child’s participation in IFED. All procedures were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments.
Ultrasound Examinations
The ultrasound team included three board-certified pediatric radiologists, one lead sonographer, and six sonographers. We imaged the uterus and ovaries in females, the testes in males, and the breast buds in males and females following a standardized procedures described below for the overall exam and for each organ. All sonographers received research-protocol-specific training, certification and oversight in these ultrasound procedures. Standard sonographic landmarks were used to identify organs: nipple for breast buds, bladder for uterus, psoas for ovaries, and scrotum for testes. The two main study sites used a Philips iU22 ultrasound scanner (Philips Healthcare; Bothell, WA), whereas other study sites used a portable Philips CX50 CompactXtreme scanner and GE LOGIQ e BT11 (GE Ultrasound; Wauwatosa, WI). We employed Philips C8-5 curved array and L15-7 linear array transducers with Philips scanners and GE 8C-RS curved array and i12L-RS linear array transducers with the GE scanner.
Infants were placed in a supine position on an examination table. Sonographers scanned each organ and obtained 3 or 4 images in the sagittal (SAG) view and in the transverse (TRV) view and used the images to record measurements of the largest diameter in each of three dimensions: SAG, TRV and anterior-posterior (AP). The AP measurement was obtained in either the SAG or TRV view, specific for each organ as described below. Completion of the ultrasound procedure required approximately 15 minutes for males and up to 40 minutes for females.
Sonographers used both the curved array and linear transducers for uterus and ovaries. Imaging began with the uterus in girls. Uterine length in centimeters (cm) was measured in the midline from the external cervical os to fundus (Figure 1). Uterine AP measurements were from the SAG images at the largest diameter along the uterine length. The ovaries were scanned after the uterus, right ovary first. Ovarian AP measurements were from the TRV images (Figure 2). Due to difficulties visualizing the ovaries, our procedures directed sonographers to make a minimum of three attempts during the visit before noting an ovary as “not visualized”.
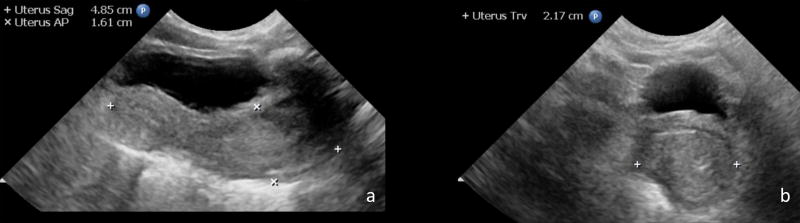
Fig. 1.
Sagittal and AP measurements of the uterus in this 1-day-old female were obtained in the sagittal plane (a). AP measurement was taken at the widest point along the length. Transverse diameter was measured at the widest point in the transverse plane (b).
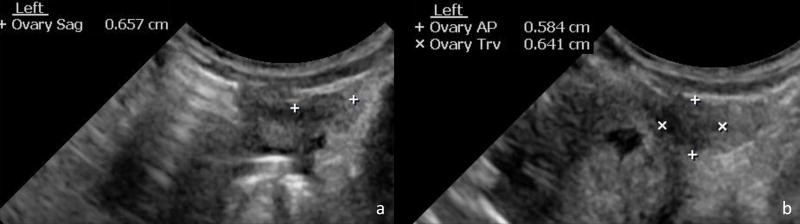
Fig. 2.
Sagittal measurement of the ovaries in this 1-day-old female was obtained in the sagittal plane (a). AP and transverse measurements of the ovaries were obtained in the transverse plane (b).
For males, imaging began with the right testis using the linear array transducer. Testicular AP measurements came from the TRV images (Figure 3).
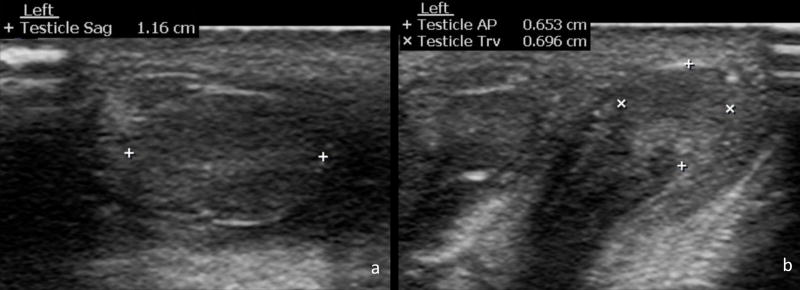
Fig. 3.
Sagittal measurement of the testes in this 1-day-old male was obtained in the sagittal plane (a). AP and transverse measurements of the testes were obtained in the transverse plane (b).
Breast buds were the last organs scanned, beginning directly over the nipple of the right breast. The sonographer used a hand-warmed standoff pad (Cone Instruments, Salon, OH) placed over the nipple for scanning with the linear array transducer. Breast bud AP measurements were from TRV images (Figure 4) and included the nipple.
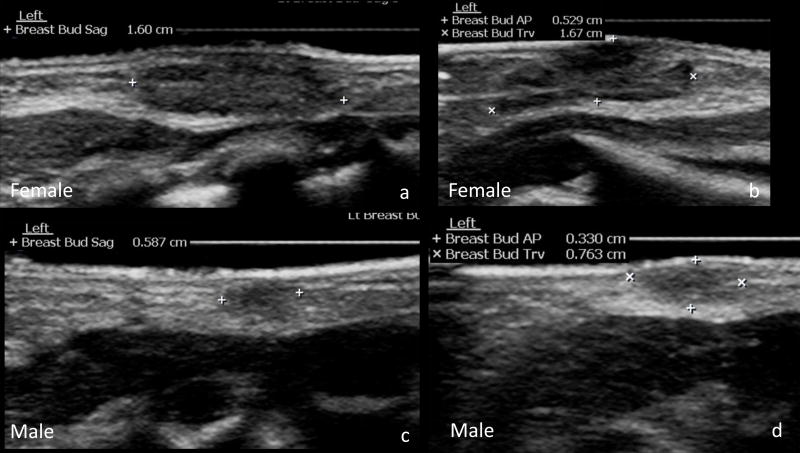
Fig. 4.
Sagittal measurements of the breast buds were obtained in the sagittal plane (a,c). AP and transverse measurements of the breast buds were obtained in the transverse plane (b,d). Female breast buds (a,b) were larger and more well defined than male breast buds (c,d). Images are from the same 1-day-old female and 1-day-old male described in Figures 1 – 3.
Longest Axis Length and Volume
The value assigned to a subject for each dimension was the geometric mean of the 3 or 4 dimension-specific diameters from the separate images. For each subject and each organ, we defined the longest axis length to be the largest diameter among the SAG, TRV, or AP geometric mean diameters, regardless of the plane. This approach simplifies clinical application when the assignment of planes may not be standard among sonographers, especially for organs like the ovaries that are typically positioned obliquely. We approximated the volumes of organs as simple geometric solids. We modeled the breast buds and uterus as cylinders with an elliptical base using the volume formula, , where L, W, and D represent the geometric mean diameters in the SAG, TRV, and AP dimensions, respectively. We modeled ovaries and testes as ellipsoids using the volume formula, , equivalently , or 0.523 × L × W × D. Because some centers use Lambert volumes (calculated as 0.71 × L × W × D), for the testes, we also computed these [21].
Statistics
We sought to summarize the distributions of longest axis length and volume for each organ using descriptive statistics. We summarized the diameter distributions for each organ in each plane. We used mean and median to assess central tendency, standard deviation and coefficient of variation to assess variability, and selected quantiles, focusing on the 5th and 95th percentiles to provide a range where most observations fall. For each paired organ (breast buds, ovaries, testes), we evaluated the right and left sides separately; but we focused on a summary applicable to either side through a combined data set that incorporated all data from both sides. Interpretation of summary statistics from this combined data set should recognize that the observations are correlated within subjects.
For paired organs, the utility of the combined data set requires that the distributions of sizes on either side are similar. We assessed laterality differences by applying Wilcoxon signed rank test to the right-left size differences for each subject. We also estimated the correlation between the paired sizes using Spearman’s rank correlation coefficient. When mean differences (even statistically significant ones) between sides were < 0.10 cm or 0.10 cm3, we deemed them unlikely to be clinically relevant, given the inherent operator variability and limits on sonographic precision. Consequently, we reported results to the nearest 0.01 cm or 0.01 cm3 when determining statistical significance but reported reference values only to the nearest 0.1 cm or 0.1 cm3.
Ovaries provided unique challenges as the only paired organ where we were unable to visualize both sides in every subject. We used only subjects with both sides visualized to test laterality, but we used all available measurements from both sides when summarizing size distributions. We used the Mann-Whitney U test to assess both sex differences in breast bud size and racial differences (African-American vs. European-American) in size for all organs. Because one commonly used formula for calculating testis volume assumes that the AP diameter and the TRV diameter are equivalent, we assessed this assumption by applying the Wilcoxon signed rank test to subject-specific AP-TRV diameter differences, separately for each side.
We used IBM® SPSS Statistics Version 22 for all statistical analyses.
Results
Our enrolled infants were within normal body-weight range for healthy neonates but slightly below WHO growth chart averages; they were predominantly African-American (Table 1). We provide the mean and SD of the measured diameters in each of the three planes (SAG, TRV, AP) for uterus, ovaries, testes, and male and female breast buds (Appendix A); and we provide the 10th, 25th, 50th, 75th, and 90th percentiles for longest axis length and for volume for the same organs (Appendix B). We summarize laterality differences for paired organs (Appendix C) and longest axis length and volume for all organs (Table 2 and Figure 5).
Table 1.
Infant Demographics
| Female, N = 194 | Male, N = 204 | |
|---|---|---|
| Neonatal Weighta: Mean (standard deviation) | ||
| kg | 3.15 (0.38) | 3.25 (0.41) |
| Z-scoreb | −0.15 (0.83) | −0.20 (0.85) |
| Gestational Age: N (%) | ||
| 37 weeks | 17 (9%) | 16 (8%) |
| 38 weeks | 38 (19%) | 36 (18%) |
| 39 weeks | 75 (39%) | 74 (36%) |
| 40 weeks | 43 (22%) | 52 (25%) |
| 41 weeks | 21 (11%) | 26 (13%) |
| Race: N (%) | ||
| African-American | 135 (70%) | 141 (69%) |
| European-American | 37 (19%) | 40 (20%) |
| Otherc | 22 (11%) | 23 (11%) |
Weight at the time of ultrasound exam (0–3 days after birth)
Z-scores for weight based on WHO Child Growth Standards, 2006 [20].
Includes subjects whom mothers identified as more than one race or as a race other than European-American or African-American
Table 2.
Organ Size in the Neonate
| Number | Longest Axis, cm | Volume, cm3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Subjects/Organs | Mean (SDa) | Median | 5th – 95th %-ile | CVb | Mean (SD) | Median | 5th – 95th %-ile | CV | |
| Female | |||||||||
| Uterusc | 194/194 | 4.2 (0.6) | 4.2 | 3.3 – 5.0 | 0.14 | 10.0 (3.5) | 9.8 | 5.0 – 16.2 | 0.35 |
| Ovariesd | 155/266 | 1.0 (0.3) | 0.9 | 0.6 – 1.5 | 0.30 | 0.2 (0.2) | 0.2 | 0.1 – 0.6 | 1.00 |
| Breast Budc | 194/388 | 1.2 (0.4) | 1.2 | 0.6 – 2.0 | 0.33 | 0.7 (0.5) | 0.6 | 0.1 – 1.7 | 0.71 |
| Male | |||||||||
| Testesd | 204/408 | 1.1 (0.1) | 1.1 | 0.9 – 1.3 | 0.09 | 0.3 (0.1) | 0.3 | 0.2 – 0.4 | 0.33 |
| Lamberte | _ | _ | _ | _ | _ | 0.4 (0.1) | 0.3 | 0.2 – 0.5 | 0.25 |
| Breast Budc | 204/408 | 1.1 (0.4) | 1.1 | 0.5 – 1.9 | 0.36 | 0.6 (0.5) | 0.4 | 0.1 – 1.7 | 0.83 |
SD = standard deviation
CV = coefficient of variation: SD/mean
Volume of cylinder with elliptical base: . L = length (SAG), W = width (TRV), D = depth (AP)
Volume of prolate ellipsoid: . (Note: Both ovaries visualized in 57% and at least one ovary in 80% of subjects.)
Lambert volume: 0.71 × L × W × D
Fig. 5.
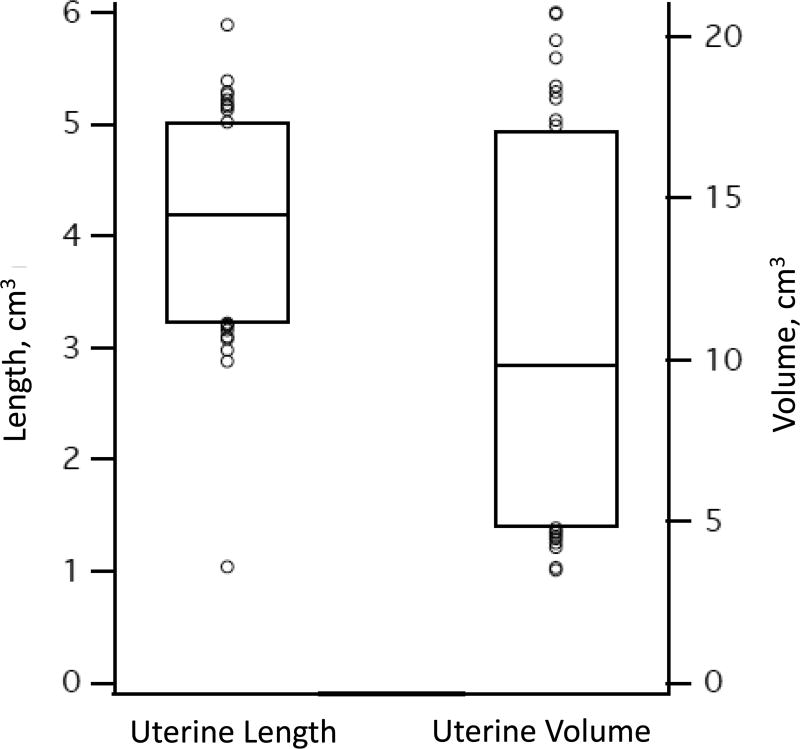
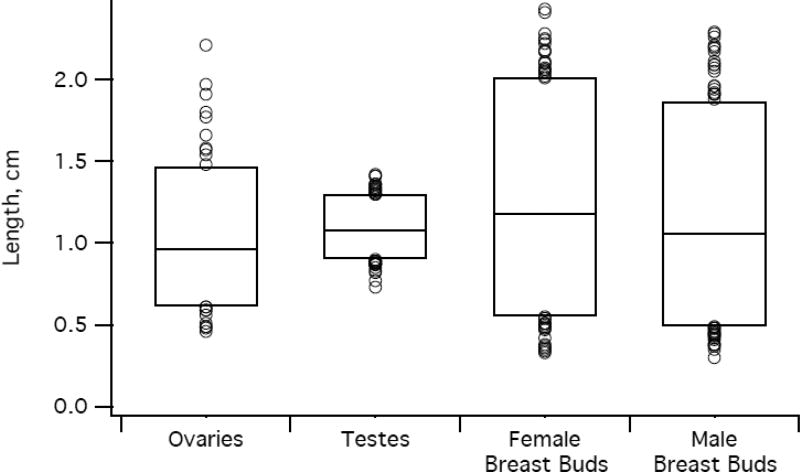
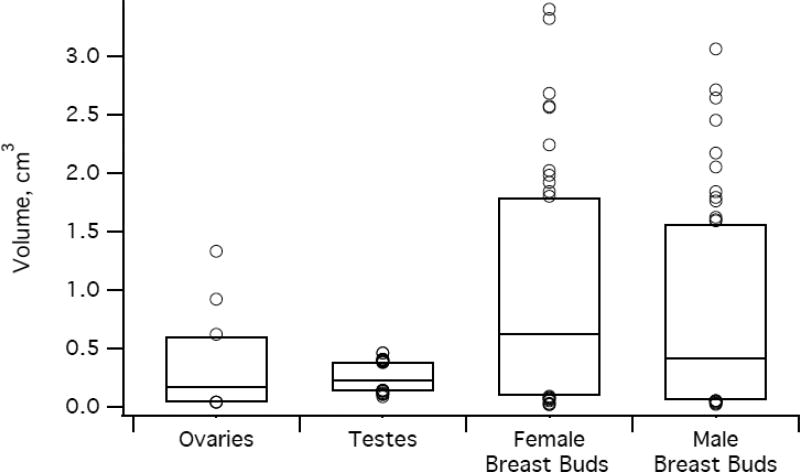
Box plots of neonatal organ length and volume. Lower and upper edges of the box correspond to 5th and 95th percentiles, respectively, while circles represent outliers < 5th percentile or > 95th percentile. Panels: a) uterine length and volume (right axis shows length, left shows volume); b) longest axis diameter of the ovaries, testes, and breast buds; c) volume of the ovaries, testes, and breast buds.
Uterus
The uterus was identified and measured in all 194 girls. The longest axis of the uterus was in the SAG plane.
Ovaries
Among 194 girls in our study population, we identified at least one ovary in 155 girls (80%) and both ovaries in 111 girls (57%). Of the 44 girls with only one identified ovary, it was the right in 23 (52%). The plane containing the longest axis of the ovary varied: SAG in 85%, TRV in 13%, and AP in 2%. The mean longest axis length did not differ by side (p > 0.05), but the mean volume did (p < 0.01)—though the difference was unlikely to be clinically relevant. The right ovarian volume exceeded the left in 64/111 girls (58%).
Testes
We obtained paired testicular measurements on all 204 boys. The longest axis was always SAG. The right testis was larger than the left by 0.02 cm in mean longest axis (p < 0.01) and 0.02 cm3 in mean volume (p < 0.001), but both differences were unlikely to be clinically relevant. The right testicular volume exceeded the left in 131/204 boys (64%).
We saw no significant left-right difference in the AP diameter of the testis (p > 0.05), but the mean TRV diameter on the right was 0.02 cm larger than on the left (p < 0.001), a difference unlikely to be clinically detectable. Based on pooled data from both testes, the mean TRV diameter was 0.06 cm larger than the AP diameter (p < 0.001) (Appendix A). This difference approached a clinically detectable 0.1 cm, drawing into question the assumption that the TRV and AP diameters of the testis can be regarded as equal.
Breast Buds
We obtained measurements from both right and left breast buds in all 194 girls and 204 boys.
In girls, the longest axis lay in SAG or TRV planes, and mean diameters in these planes were not significantly different (p = 0.4). The longest axis was SAG in 173/388 girls (45%). The left breast bud exceeded the right by 0.06 cm in mean longest axis length (p < 0.001) and by 0.05 cm3 in mean volume (p < 0.001), differences that approach clinical detection. Left breast bud volume exceeded the right in 114/194 girls (59%).
In boys, the mean SAG and TRV diameters did not differ (p = 0.7). The longest axis was SAG in 224/408 boys (55%). The mean longest axis of the breast bud was 0.04 cm greater on the right than the left (p < 0.001), a difference that unlikely to be clinically relevant. The mean volume of the right breast bud did not differ from the left (p > 0.05). The right breast bud volume exceeded the left in 105/204 boys (52%). The 0.13 cm3 greater volume of female breast bud compared with male (p < 0.05) was likely to be clinically detectable.
Race
Among males, we found weak evidence for larger breast buds in African-American than European-American neonates (p = 0.08 for volume, p = 0.10 for length). Otherwise, we saw no evidence of differences in organ volume or longest axis length by race (p values from 0.36–0.91 for testes, uterus, ovaries, and female breast buds).
Discussion
Technology
Many landmark studies on the size of hormone-sensitive organs in the neonate are decades old, dating from a time when ultrasound equipment did not have the spatial resolution available today [6,10,16]. In 1986, the uterus was identified in only 89% of subjects using ultrasound [16], whereas we were able to image the uterus in every subject without difficulty. The ovaries remain challenging to visualize, especially in neonates, in whom a full bladder is often not achieved, resulting in a limited sonographic window.
For testicular measurements, earlier papers relied more heavily on orchidometers consisting of standard sized beads [14,15]. Such palpation-based methods overestimate size because the epididymis is included in the palpable volume [4,22]. This overestimate is especially pronounced in neonates, where the epididymis is larger relative to the testis than in older boys or men [23]. In addition, the recorded size is limited to the available bead sizes. In one study, the smallest bead was 0.5 cm3, double our measured volumes [4].
Breast buds have also typically been measured with palpation and comparison to rulers or discs of standard sizes. Though readily accessible, these methods are prone to observer error, and recorded sizes are restricted to available disc sizes. Use of ultrasound minimizes some errors inherent to palpation-based methods.
Variability Among Organs
We found that certain organs were more variable in size than others, as assessed by the coefficient of variation. Our coefficient of variation accounts for two sources of variation: variability of actual organ size across subjects and variability associated with measurement reproducibility (e.g., across operators, instruments, and replicate measurements). A careful study of the reproducibility of ultrasound measurements in infants would be valuable for evaluating the relative contributions of these two sources, but was not feasible with our data set.
Seeing large coefficients of variation for the ovaries was unsurprising, as they have wide range in size at any age due to presence of varied numbers of follicles [8,9]. This variability may be more pronounced in the neonate still influenced by hormones in the intrauterine environment. The deep position of the ovaries and their heterogeneity also makes them exceptionally difficult to distinguish from surrounding bowel and to measure reproducibly. Moreover, their location, visibility, and appearance may change with bladder volume [24]. Like the ovaries, the uterus is located deep in the pelvis; yet the coefficients of variation for the uterus were much lower than for ovaries. The larger size of the uterus may enhance reproducibility of measurements. In addition, compared to possibly follicle-laden ovaries, the uterus has a fairly homogeneous sonographic appearance, with only a stripe of increased echogenicity of the endometrium.
Both breast buds and testes are positioned superficially, but breast buds showed larger coefficients of variation. In the neonate, breast buds may have more variation in actual size than testes -- possibly due to intrauterine exposure to maternal hormones. In addition, breast buds are more compressible than a more solid organ such as the testis, and some extra variability in breast bud measurement may be attributed to inadvertent or inconsistent compression of the tissue during scanning.
Comparisons Across Studies
Although testicular measurements in our study had the lowest coefficient of variation, they are paradoxically the least consistent across studies. Mean testicular volumes in the first week of life, as reported in the literature, range from 0.1 – 1.1 cm3 (Table 3); whereas our mean volumes are near the midpoint of that range. Some study-to-study variability may come from volume formulas. We used the prolate ellipsoid volume, , but also reported the empirically derived Lambert volume (0.71 × L × W × D) [21]. By comparing the formulae, one sees that the Lambert volume is 1.36 times the prolate ellipsoid volume, in accord with larger mean Lambert volume that we report. Alternatively, other authors use a modified ellipsoid formula, [13,22]. This formula assumes the difference between the TRV width and the AP depth is negligible; however, our data contradicted that assumption. Therefore, using this formula may lead to systematic errors. Orchidometers are also likely to introduce systematic error by inadvertently including epididymal volume or hydrocele. Review of published values shows that orchidometry tends to yield higher testicular volumes than sonography (Table 3) [4,11].
Table 3.
Summary of Literature Reports on Male Neonatal Organ Sizea
| Breast Buds | Testes | ||||||
|---|---|---|---|---|---|---|---|
| Reference | Population | Age, DOLb |
Number of Subjects |
Longest Axis, cm |
Instrument | Volume, cm3 | Instrument |
| this publication | USA | 1 – 3 | 204 | 1.1 (0.4) | Ultrasound | 0.3 (0.1) | Ultrasoundc |
| Nguyen et al. [8] | USA | 1 – 2 | 6 | 0.9d | Ultrasound | 0.2d | Ultrasoundc |
| Bernbaum et al. [11] | USA | 1 – 2 | 6 | 0.8 | Discs | 0.7 | Orchidometer |
| Kuiri-Hanninen [18] | Finland | 7 | 29 | 1.0 | Slide gauge | _ | _ |
| Francis et al. [14] | USA | 1 – 2 | 77 | 0.8 (0.2) | Ruler | _ | _ |
| McKeirnan et al. [15] | UK | 1 – 2 | 145 | 0.9 | Discs | _ | _ |
| Kuiri-Hanninen [19] | Finland | 7 | 25 | _ | _ | 0.2f | Ultrasoundg |
| Main et al. [13] | Scandinavia | 1 | 1657 | _ | _ | 0.1 | Ultrasoundg |
| Cassorla et al. [4] | USA | 1 | 10 | _ | _ | 1.1 (0.1) | Orchidometer |
Longest axis and volume entries are mean (standard deviation) unless otherwise noted.
DOL = day of life
Ellipsoid formula: . L = length (SAG diameter), W = width (TRV diameter), D = depth (AP diameter)
Geometric mean calculated for DOL = 1 from published regression equation.
Value approximated from graph; numerical values for mean and SD were not reported; median reported as 0.2 cm3.
Used ellipsoid formula: . L = length (SAG diameter), W = width (TRV diameter)
Though ovarian volume showed the largest coefficient of variation in our study, ovarian volumes are more consistent than testicular volumes across studies. Mean ovarian volumes ranged from 0.1 to 0.8 cm3 (Table 4) with our calculated volume at the lower end. All authors used the same volumetric model, the prolate ellipsoid.
Table 4.
Summary of Literature Reports on Female Neonatal Organ Sizea
| Reference | Population | Age, DOLc |
Number of Subjects (ovariesd) |
Breast Buds | Ovariesb | Uterusb | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Longest Axis, cm |
Instrument | Volume, cm3 | Volume, cm3 | Longest Axis, cm |
||||
| this publication | USA | 1 – 3 | 194 (155) | 1.2 (0.4) | Ultrasound | 0.2 (0.2)e | 10.0 (3.5)f | 4.2 (0.6) |
| Nguyen et al. [8] | USA | 1 – 2 | 6 | 0.9g | Ultrasound | 0.1e, g | 5.9f, g | _ |
| Kuiri-Hanninen [18] | Finland | 7f | 29 | 1.1 | Slide gauge | _ | _ | 3.5 |
| Bernbaum et al. [11] | USA | 1 – 2 | 6 | 1.0f | Discs | _ | _ | _ |
| Francis et al. [14] | USA | 1 – 2 | 67 | 0.9 (0.2) | Ruler | _ | _ | _ |
| McKeirnan et al. [15] | UK | 1 – 2 | 137 | 1.0 | Discs | _ | _ | _ |
| Orbak et al. [9] | Turkey | 1 – 4 | 50 | _ | _ | 0.8 (0.4)h | _ | 3.2 (0.3) |
| Griffin et al. [6] | UK | 3 | _ | _ | _ | 0.3 (left), 0.4 (right)g, h | _ | 3.1 (1.2e) |
| Hata et al. [17] | Japan | 1 – 7 | 41 | _ | _ | _ | 4.8e, i | 4.0 |
| Nussbaum et al. [16] | USA | 1 – 7 | 31 | _ | _ | _ | _ | 3.4 (0.7) |
Longest axis and volume entries are mean (standard deviation) unless otherwise noted.
All studies of ovaries and uterus used ultrasound.
DOL = days of life
Number of subjects with one or both ovaries detected.
Used formula for volume of ellipsoid: . L = length (SAG diameter), W = width (TRV diameter), D = depth (AP diameter)
Used formula for volume of cylinder with elliptical base: . (This volume is 3/2 times the ellipsoid volume for the same L, W, D)
Geometric mean calculated for DOL = 1 from published regression equation.
Used approximate formula for volume of ellipsoid: 0.5 × L × W × D. Note: 4π/(3·2·2·2), so the approximation is slightly low.
Used ellipsoid volume formula separately for uterine cervix and body, then summed. Translated to the cylinder volume using the relationship mentioned in f, this volume becomes 4.8×(3/2) = 7.2 cm3.
Breast buds showed the most consistency across studies of all organs, with reported mean longest axis ranging from 0.8 to 1.1 cm in males (Table 3) and from 0.9 to 1.2 cm in females (Table 4); our measurements were at the top of both ranges.
The range of reported mean uterine longest axis lengths, 3.1 to 4.2 cm, is narrower than that of volumes, 4.8 to 6.7 cm3, as might be expected for volumes calculated from three individual measurements, each with its own variability. Uterine sizes in our study were at the top of the range for both length and volume. Uterine volume measurement at the top of the range may be secondary to our method of using the largest diameter in the AP and TRV planes, which we selected for its consistency. Though most studies used the ellipsoid model for uterine volume; we used a cylinder with an ellipse as its base -- but transformed our cylindrical volume into the corresponding ellipsoid volume for comparison with other published data. The uterus is especially difficult to model accurately, as its shape varies widely among different neonates and, even for one neonate, throughout the day as bladder volume changes. The uterine shape has been described as 58% tubular (cervical width = fundal), 32% spade-shaped (cervical width > fundal), and 10% heart-shaped (cervical width < fundal) [16]. We believe that our cylindrical model combined with AP measurements taken at the widest point along the uterine length will provide more consistent volume estimates in the face of shape heterogeneity than would a prolate ellipsoid model with AP measurements anchored at either cervix or fundus; our approach may also contribute to our mean uterus volume being greater than other published volumes.
Laterality
Other authors have found, as we did, that the right ovary was larger than the left on average [6,9]. We also found the right testis to be larger than that of the left, a finding that corroborates some authors’ work [21], while others detected no difference [12]. Gonads are bilaterally symmetric in their anatomy, with the exception of venous drainage, so we see no compelling etiology for laterality in size – though too few studies have assessed it in gonads to allow a meaningful conclusion. In our study, the statistically evident difference was below the level of uncertainty inherent to single-subject clinical ultrasound measurements. Though we found some evidence that the left breast was larger on average than the right in both sexes, differences were small and likely without clinical import. Other studies of neonatal breast buds have not examined laterality.
Population
Our study population was not a random sample, but rather a sample composed of families who elected to commit to a specific infant feeding regimen. Any bias arising from self-selection into the IFED study has not been assessed. Because we acquired measurements within 72 hours of birth, we considered any contribution of feeding regimen negligible due to the extremely short duration of feeding.
Neonatal organ sizes vary depending on birth weight [9,13,14]. We selected infants of normal birth weight, as did other authors; however, the negative average Z-score for neonatal weight indicated that our enrolled subjects were smaller than average, according the multi-national, multi-ethnic 2006 WHO criteria [20]. Nevertheless, only the mean ovarian volume of our infants was below the median of values reported in the literature. Testicular volumes were at the median, while uterus and both male and female mean breast bud sizes were at the top of the literature-reported ranges for means. We found no racial difference in organ size, except weak evidence for larger breast buds in African American boys. Most studies reporting male organ size sample Northern European populations, while other studies in the United States do not always specify the racial and ethnic identity of participants (Table 3). Reports of female organ size cover a wider range of heritage, including Turkish and Japanese in addition to European and North American (Table 4). With the exception of a large study designed to compare testicular volumes in Danish and Finnish boys [13], no meaningful analysis of racial or ethnic differences in organ size is possible due to differences in technique across studies and the small numbers of subjects.
Conclusion
We offer reference ranges for sonographic size of uterus, ovaries, testes, and breast buds in the healthy term neonate 0 – 3 days old based on data from ~ 200 girls and ~ 200 boys. Our study shows that the smaller of these organs (excluding the uterus) have longest axis lengths within a small range, from 1.0 – 1.2 cm. The ovaries and breast buds show wider variability in their measured sizes compared to the testes and uterus, which may reflect a wider range of actual organ sizes or may reflect different measurement errors introduced by the depth, parenchymal heterogeneity, or compressibility of these organs. Mean longest axis diameter is more consistent than mean volume across multiple studies. When volume is used to compare measurements, it is important to specify the volumetric model used, especially for the testes.
Appendix A
Summary of Measured Neonatal Organ Diameters in Three Planes
| Number | SAGa, cm | TRVb, cm | APc, cm | |
|---|---|---|---|---|
| Subjects/Organs | Mean (SDd) | Mean (SD) | Mean (SD) | |
| Female | ||||
| Uterus | 194/194 | 4.15 (0.56) | 1.98 (0.27) | 1.51 (0.24) |
| Ovaries | 155/266 | 0.97 (0.28) | 0.74 (0.25) | 0.53 (0.17) |
| Left | 132/132 | 0.96 (0.26) | 0.71 (0.25) | 0.53 (0.15) |
| Right | 134/134 | 0.98 (0.29) | 0.76 (0.24) | 0.53 (0.18) |
| Breast Buds | 194/388 | 1.22 (0.43) | 1.24 (0.46) | 0.50 (0.11) |
| Left | 194/194 | 1.25 (0.43) | 1.27 (0.47) | 0.49 (0.11) |
| Right | 194/194 | 1.20 (0.43) | 1.21 (0.44) | 0.51 (0.11) |
| Male | ||||
| Testes | 204/408 | 1.09 (0.12) | 0.70 (0.09) | 0.64 (0.08) |
| Left | 204/204 | 1.08 (0.12) | 0.69 (0.08) | 0.63 (0.08) |
| Right | 204/204 | 1.10 (0.12) | 0.71 (0.09) | 0.64 (0.08) |
| Breast Buds | 204/408 | 1.11 (0.41) | 1.10 (0.42) | 0.49 (0.12) |
| Left | 204/204 | 1.12 (0.41) | 1.13 (0.43) | 0.48 (0.11) |
| Right | 204/204 | 1.10 (0.41) | 1.08 (0.42) | 0.51 (0.12) |
SAG = sagittal (length)
TRV = transverse (width)
AP = antero-posterior (height)
SD = standard deviation
Appendix B
Quantiles for Organ Length and Volume
| Number | Percentiles (Length, cm / Volume, cm3) | |||||
|---|---|---|---|---|---|---|
| Subjects/Organs | 10th | 25th | 50th | 75th | 90th | |
| Female | ||||||
| Uterus | 194/194 | 3.44 / 5.52 | 3.82 / 7.37 | 4.18 / 9.83 | 4.48 / 12.44 | 4.81 / 13.96 |
| Ovaries | 155/266 | 0.70 / 0.07 | 0.82 / 0.10 | 0.96 / 0.17 | 1.15 / 0.26 | 1.39 / 0.43 |
| Left | 132/132 | 0.71 / 0.07 | 0.81 / 0.11 | 0.94 / 0.16 | 1.10 / 0.25 | 1.30 / 0.41 |
| Right | 134/134 | 0.64 / 0.07 | 0.79 / 0.11 | 0.95 / 0.17 | 1.15 / 0.30 | 1.41 / 0.52 |
| Breast Buds | 194/388 | 0.65 / 0.14 | 0.91 / 0.30 | 1.18 / 0.59 | 1.55 / 0.99 | 1.80 / 1.44 |
| Left | 194/194 | 0.65 / 0.14 | 0.93 / 0.31 | 1.19 / 0.60 | 1.57 / 1.01 | 1.82 / 1.52 |
| Right | 194/194 | 0.64 / 0.14 | 0.89 / 0.29 | 1.15 / 0.59 | 1.54 / 0.94 | 1.79 / 1.39 |
| Male | ||||||
| Testes | 204/408 | 0.95 / 0.18 | 1.01 / 0.21 | 1.08 / 0.25 | 1.17 / 0.30 | 1.26 / 0.36 |
| Left | 204/204 | 0.93 / 0.17 | 1.01 / 0.20 | 1.07 / 0.25 | 1.16 / 0.29 | 1.24 / 0.35 |
| Right | 204/204 | 0.96 / 0.18 | 1.01 / 0.21 | 1.09 / 0.26 | 1.17 / 0.31 | 1.27 / 0.37 |
| Breast Buds | 204/408 | 0.56 / 0.10 | 0.82 / 0.25 | 1.06 / 0.44 | 1.34 / 0.71 | 1.67 / 1.29 |
| Left | 204/204 | 0.56 / 0.10 | 0.84 / 0.25 | 1.07 / 0.45 | 1.36 / 0.75 | 1.71 / 1.30 |
| Right | 204/204 | 0.55 / 0.09 | 0.78 / 0.23 | 1.06 / 0.43 | 1.33 / 0.69 | 1.64 / 1.24 |
Appendix C
Laterality Difference Statistics
| Number | Longest Axis Length, cm | Volume, cm3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Subjects/Organs | Mean (SDa) | p-valueb | rhoc | Mean (SDa) | p-valueb | rhoc | |||
| Right | Left | Right | Left | ||||||
| Female | |||||||||
| Ovariesd | 111/222 | 1.00 (0.29) | 0.98 (0.26) | > 0.05 | 0.74 | 0.25 (0.26) | 0.22 (0.22) | < 0.01 | 0.90 |
| Breast Buds | 194/388 | 1.20 (0.43) | 1.26 (0.44) | < 0.001 | 0.92 | 0.68 (0.51) | 0.73 (0.57) | < 0.01 | 0.95 |
| Male | |||||||||
| Testes | 204/408 | _ | _ | _ | _ | 0.27 (0.07) | 0.25 (0.07) | < 0.001 | 0.82 |
| SAG | 1.10 (0.12) | 1.08 (0.12) | < 0.01 | 0.86 | _ | _ | _ | _ | |
| TRV | 0.71 (0.09) | 0.69 (0.09) | < 0.001 | 0.71 | _ | _ | _ | _ | |
| AP | 0.64 (0.09) | 0.64 (0.09) | > 0.05 | 0.65 | _ | _ | _ | _ | |
| Breast Buds | 204/408 | 1.08 (0.41) | 1.12 (0.41) | < 0.001 | 0.94 | 0.58 (0.53) | 0.58 (0.52) | > 0.05 | 0.93 |
SD = standard deviation
Wilcoxon signed rank test for left-right differences
Spearman’s rank correlation coefficient
Restricted to girls who had both ovaries visualized
References
- 1.Khadilkar VV, Khadilkar AV, Kinare AS, Tapasvi HS, Deshpande SS, Maskati GB. Ovarian and uterine ultrasonography in healthy girls between birth to 18 years. Indian Pediatr. 2006;43(7):625–630. [PubMed] [Google Scholar]
- 2.Keats TE, Sistrom C. Atlas of radiologic measurement. 7. Mosby: St. Louis, MO; 2001. [Google Scholar]
- 3.Gilchrist JM, Moore MB, Andres A, Estroff JA, Badger TM. Ultrasonographic patterns of reproductive organs in infants fed soy formula: comparisons to infants fed breast milk and milk formula. J Pediatr. 2010;156(2):215–220. doi: 10.1016/j.jpeds.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 4.Cassorla FG, Golden SM, Johnsonbaugh RE, Heroman WM, Loriaux DL, Sherins RJ. Testicular volume during early infancy. J Pediatr. 1981;99(5):742–743. doi: 10.1016/s0022-3476(81)80398-8. [DOI] [PubMed] [Google Scholar]
- 5.Cohen HL, Shapiro MA, Mandel FS, Shapiro ML. Normal ovaries in neonates and infants: a sonographic study of 77 patients 1 day to 24 months old. AJR Am J Roentgenol. 1993;160(3):583–586. doi: 10.2214/ajr.160.3.8430559. [DOI] [PubMed] [Google Scholar]
- 6.Griffin IJ, Cole TJ, Duncan KA, Hollman AS, Donaldson MD. Pelvic ultrasound measurements in normal girls. Acta Paediatr. 1995;84(5):536–543. doi: 10.1111/j.1651-2227.1995.tb13689.x. [DOI] [PubMed] [Google Scholar]
- 7.Forest MG, De Peretti E, Bertrand J. Hypothalamic-pituitary-gonadal relationships in man from birth to puberty. Clin Endocrinol (Oxf) 1976;5(5):551–569. doi: 10.1111/j.1365-2265.1976.tb01985.x. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen RH, Umbach DM, Parad RB, Stroehla B, Rogan WJ, Estroff JA. US assessment of estrogen-responsive organ growth among healthy term infants: piloting methods for assessing estrogenic activity. Pediatr Radiol. 2011;41(5):633–642. doi: 10.1007/s00247-010-1895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orbak Z, Kantarci M, Yildirim ZK, Karaca L, Doneray H. Ovarian volume and uterine length in neonatal girls. J Pediatr Endocrinol Metab. 2007;20(3):397–403. doi: 10.1515/jpem.2007.20.3.397. [DOI] [PubMed] [Google Scholar]
- 10.Haber HP, Mayer EI. Ultrasound evaluation of uterine and ovarian size from birth to puberty. Pediatr Radiol. 1994;24(1):11–13. doi: 10.1007/BF02017650. [DOI] [PubMed] [Google Scholar]
- 11.Bernbaum JC, Umbach DM, Ragan NB, Ballard JL, Archer JI, Schmidt-Davis H, Rogan WJ. Pilot studies of estrogen-related physical findings in infants. Environ Health Perspect. 2008;116(3):416–420. doi: 10.1289/ehp.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuijper EA, van Kooten J, Verbeke JI, van Rooijen M, Lambalk CB. Ultrasonographically measured testicular volumes in 0- to 6-year-old boys. Hum Reprod. 2008;23(4):792–796. doi: 10.1093/humrep/den021. [DOI] [PubMed] [Google Scholar]
- 13.Main KM, Toppari J, Suomi AM, Kaleva M, Chellakooty M, Schmidt IM, Virtanen HE, Boisen KA, Kai CM, Damgaard IN, Skakkebaek NE. Larger testes and higher inhibin B levels in Finnish than in Danish newborn boys. J Clin Endocrinol Metab. 2006;91(7):2732–2737. doi: 10.1210/jc.2005-2443. [DOI] [PubMed] [Google Scholar]
- 14.Francis GL, Hoffman WH, Gala RR, McPherson JC, Zadinsky J. A relationship between neonatal breast size and cord blood testosterone level. Ann Clin Lab Sci. 1990;20(4):239–244. [PubMed] [Google Scholar]
- 15.McKiernan JF, Hull D. Breast development in the newborn. Arch Dis Child. 1981;56(7):525–529. doi: 10.1136/adc.56.7.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nussbaum AR, Sanders RC, Jones MD. Neonatal uterine morphology as seen on real-time US. Radiology. 1986;160(3):641–643. doi: 10.1148/radiology.160.3.3526401. [DOI] [PubMed] [Google Scholar]
- 17.Hata K, Nishigaki A, Makihara K, Takamiya O, Hata T, Kitao M. Ultrasonic evaluation of the normal uterus in the neonate. J Perinat Med. 1989;17(4):313–317. doi: 10.1515/jpme.1989.17.4.313. [DOI] [PubMed] [Google Scholar]
- 18.Kuiri-Hänninen T, Haanpää M, Turpeinen U, Hämäläinen E, Seuri R, Tyrväinen E, Sankilampi U, Dunkel L. Postnatal ovarian activation has effects in estrogen target tissues in infant girls. J Clin Endocrinol Metab. 2013;98(12):4709–4716. doi: 10.1210/jc.2013-1677. [DOI] [PubMed] [Google Scholar]
- 19.Kuiri-Hänninen T, Seuri R, Tyrväinen E, Turpeinen U, Hämäläinen E, Stenman UH, Dunkel L, Sankilampi U. Increased activity of the hypothalamic-pituitary-testicular axis in infancy results in increased androgen action in premature boys. J Clin Endocrinol Metab. 2011;96(1):98–105. doi: 10.1210/jc.2010-1359. [DOI] [PubMed] [Google Scholar]
- 20.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 21.Lambert B. The frequency of mumps and of mumps orchitis and the consequences for sexuality and fertility. Acta Genet Stat Med. 1951;2(Suppl 1):1–166. [PubMed] [Google Scholar]
- 22.Rivkees SA, Hall DA, Boepple PA, Crawford JD. Accuracy and reproducibility of clinical measures of testicular volume. J Pediatr. 1987;110(6):914–917. doi: 10.1016/s0022-3476(87)80412-2. [DOI] [PubMed] [Google Scholar]
- 23.Lin CC, Huang WJ, Chen KK. Measurement of testicular volume in smaller testes: how accurate is the conventional orchidometer? J Androl. 2009;30(6):685–689. doi: 10.2164/jandrol.108.006460. [DOI] [PubMed] [Google Scholar]
- 24.Fawcett SL, Gomez AC, Barter SJ, Ditchfield M, Set P. More harm than good? The anatomy of misguided shielding of the ovaries. Br J Radiol. 2012;85(1016):e442–447. doi: 10.1259/bjr/25742247. [DOI] [PMC free article] [PubMed] [Google Scholar]