Abstract
Human Pegivirus (HPgV, formally GB virus C) infects lymphocytes and NK cells in vivo, and infection is associated with reduced T cell and NK cell activation in HIV-infected individuals. The mechanism by which HPgV inhibits NK cell activation has not been assessed. Following IL-12 stimulation, IFNγ expression was lower in HIV-HPgV co-infected subjects compared to HIV mono-infected subjects (p = 0.02). In addition, HPgV positive human sera, extracellular vesicles containing E2 protein, recombinant E2 protein and synthetic E2 peptides containing a predicted Tyk2 interacting motif inhibited NK cell IL-12-mediated IFNγ release. E2 protein also inhibited Tyk2 activation following IL-12 stimulation. In contrast, cytolytic NK cell function was not altered by HPgV. Inhibition of NK cell-induced proinflammatory/antiviral cytokines may contribute to both HPgV persistence and reduced immune activation during HIV-coinfection. Understanding mechanisms by which HPgV alters immune activation may contribute towards novel immunomodulatory therapies to treat HIV and inflammatory diseases.
Keywords: HPgV, GBV-C, NK cells, Tyk2, Immune modulation
Introduction
Human Pegivirus (HPgV), formerly called hepatitis G virus/GB virus C (GBV-C), is an RNA virus classified within the newly assigned Pegivirus genus of the Flaviviridae (Stapleton et al., 2012; Adams et al., 2013). Both HPgV and the phylogenetically related hepatitis C virus (HCV) can establish persistent human infection through complex mechanisms that are not completely characterized (Gutierrez et al., 1997; Tanaka et al., 1998; Williams et al., 2004; Burke and Cox, 2010; Lemon, 2010). In HIV-infected humans, persistent HPgV coinfection is associated with reduced T cell activation, proliferation and function (Maidana-Giret et al., 2009; Schwarze-Zander et al., 2010; Stapleton et al., 2012, 2009) suggesting that HPgV-mediated immune modulation may contribute to viral persistence. In vitro, HPgV envelope glycoprotein (E2) inhibits T cell activation by reducing signaling through the IL-2 receptor and the T cell receptor (TCR), with resultant reduction in activation of the lymphocyte specific tyrosine kinase (Lck) (Bhattarai et al., 2013). The effects of HPgV on T cell activation may contribute to the observed protective effect of HPgV co-infection on survival of HIV- and Ebola-infected individuals (Nunnari et al., 2003; Tillmann et al., 2001; Vahidnia et al., 2012; Xiang et al., 2001; Lauck et al., 2015).
HPgV replicates in peripheral blood mononuclear cells (PBMC) ex vivo, but the primary type of cell(s) that support HPgV replication in vivo are not well characterized (Chivero and Stapleton, 2015). Among chronically infected individuals, HPgV RNA is found in multiple blood cell types including T and B lymphocytes, monocytes and natural killer (NK) cells (Chivero et al., 2014; George et al., 2006). The proportion of cells infected with HPgV is low (approximately 1–10 genome copies per 100 NK cells)(Chivero et al., 2014). The majority of serum-derived HPgV RNA is present in gradient fractions containing extracellular vesicles (EV) that have properties of exosomes (Bhattarai et al., 2013; Chivero et al., 2014). It is difficult if not impossible to exclude the presence of virions from EV preparations; however, HPgV RNA-containing particles prepared from gradients enriched for EVs deliver viral RNA to peripheral blood mononuclear cells, including NK cells (Bhattarai et al., 2013; Chivero et al., 2014).
Natural killer cells serve as rheostats modulating antiviral T cells (Waggoner et al., 2012; Welsh and Waggoner, 2013). NK cells eliminate activated CD4+ T cells that normally help CD8+ T-cell function. In the absence of CD4+ T cell help and an abundance of viral antigen, T cell exhaustion may occur. During high titer lymphocytic choriomeningitis virus (LCMV) infection, NK cells prevent fatal pathology while enabling T-cell exhaustion and viral persistence; however, at lower titer LCMV infection, NK cells paradoxically facilitate lethal T-cell-mediated pathology. Thus, NK cells regulate T-cell-mediated responses required for viral control, pathogenesis and persistence (Waggoner et al., 2012; Welsh and Waggoner, 2013). HPgV infection frequently persists in humans at high viral concentrations, yet the cellular activation marker CD69 is significantly lower on CD56+ bright NK cells in HPgV-HIV co-infected people compared to those with HIV mono-infection (Stapleton et al., 2013). Thus, HPgV infection may modulate NK cell activation. In a recent study, HPgV infection acquired by blood transfusion reduced the plasma concentration of 27 cytokines and chemokines over a 300 days period of observation. Among those down-modulated, 12 were pro-inflammatory cytokines (GM-CSF, interferon γ (IFN-γ), IFN-α2, interleukin 12p40, interleukin 12p70 (IL-12p70), interleukin 15, interleukin 1α (IL-1α), interleukin 2 (IL-2), IL-7, IL-8, TNF-α, and IL-6, suggesting an overall antiinflammatory effect of HPgV in HIV-positive subjects (Lanteri et al., 2014). The specific cell types producing these cytokines were not identified. Since HPgV RNA is found in NK cells and is associated with reduced NK cell activation in HIV-infected individuals, and because NK cells regulate antiviral T cells through both direct cytotoxicity and production of cytokines and chemokines, we hypothesized that HPgV infection may modulate NK cell function. Consequently, we investigated the effect of HPgV infection and HPgV particles on NK cell function.
Results
Patient demographics
PBMCs obtained from 26 HIV-infected subjects (5 female, 21 male) were studied. Eleven were HPgV viremic and 15 did not have HPgV viremia. All subjects were receiving cART and had undetectable HIV viral load (< 48 copies/ml plasma) for ≥ 6 months. In the viremic subjects the average HPgV plasma VL was 4.7 × 107 genome equivalent (GE) per ml of serum. The age, race, gender and mode of HIV transmission are shown in Table 1.
Table 1.
Characteristics of subjects studied.
| ID | Age yrs | Gender | Race/Ethicity | HIV transmission | CD4 | HPgV VL | HIV VL |
|---|---|---|---|---|---|---|---|
| 1 | 33 | F | AA | Het | 1034 | ND | < 48 |
| 2 | 29 | F | C/His | Het | 692 | ND | < 48 |
| 3 | 59 | M | C | MSM | 415 | ND | < 48 |
| 4 | 56 | M | C | Het | 871 | ND | < 48 |
| 5 | 30 | F | C | Het | 819 | ND | < 48 |
| 6 | 42 | M | C | MSM | 748 | ND | < 48 |
| 7 | 47 | M | C/H | MSM | 819 | ND | < 48 |
| 8 | 49 | M | C | MSM | 549 | ND | < 48 |
| 9 | 58 | M | C | MSM | 733 | ND | < 48 |
| 10 | 55 | M | C | MSM | 441 | ND | < 48 |
| 11 | 52 | M | C | MSM | 980 | ND | < 48 |
| 12 | 54 | M | C | MSM | 683 | ND | < 48 |
| 13 | 56 | M | C | MSM | 650 | ND | < 48 |
| 14 | 44 | F | AA | Het | 646 | ND | < 48 |
| 15 | 58 | M | C | MSM | 436 | ND | < 48 |
| 16 | 37 | M | C | MSM | 437 | 7.19E + 07 | < 48 |
| 17 | 53 | M | C | MSM | 1257 | 9.28E + 07 | < 48 |
| 18 | 28 | F | C | HET | 791 | 5.68E + 07 | < 48 |
| 19 | 50 | M | C | MSM | 778 | 2.01E + 08 | < 48 |
| 20 | 62 | M | C | MSM | 134 | 5.82E + 07 | < 48 |
| 21 | 40 | M | C | IDU | 307 | 1.27E + 07 | < 48 |
| 22 | 47 | M | C | MSM | 793 | 3.46E + 06 | < 48 |
| 23 | 54 | M | C | MSM | 1404 | 2.54E + 04 | < 48 |
| 24 | 54 | M | C | MSM | 508 | 7.41E + 06 | < 48 |
| 25 | 50 | M | C | MSM | 504 | 1.21E + 07 | < 48 |
| 26 | 63 | M | C | MSM | 217 | 1.79E + 06 | < 48 |
ID=subject identification code; CD4=absolute CD4+ cells/mm3; VL=viral load in RNA copies/ml; transmission category=het: heterosexual transmission, MSM: men having sex with men; IDU: intravenous drug use.
HPgV infection prolongs NK cell survival and inhibits IL-12 signaling ex vivo
HPgV infection reduces T cell activation and protects T cells from activation induced death following stimulation with phytohemagglutinin (PHA) and IL-2 (Rydze et al., 2012; Bhattarai et al., 2012). Since HPgV also infects NK cells (Chivero et al., 2014), the effect of HPgV infection on primary human NK cell survival following stimulation was studied. PBMCs from HPgV positive and negative subjects, both groups with undetectable HIV VL, were stimulated with PHA/IL-2 and maintained in culture for eight weeks. Essentially no NK cells obtained from HPgV RNA negative subjects were viable after 21 days in culture; while all NK cells obtained from HPgV viremic individuals were viable in culture on day 42 (Fig. 1A). Thus, primary human NK cell survival was significantly prolonged when maintained in PHA/IL-2, suggesting reduced responsiveness to activation stimulus. Since IL-12 is required to stimulate NK cells to produce interferon gamma (IFNγ) (Biron and Gazzinelli, 1995; Romani et al., 1997) and acquisition of HPgV reduces production of several cytokines including IFNγ (Lanteri et al., 2014), we hypothesized that NK cells from HPgV infected subjects have suppressed responses to cytokine stimuli such as IL-12, although reduced IL-12 receptors on the NK cells could contribute to these findings.
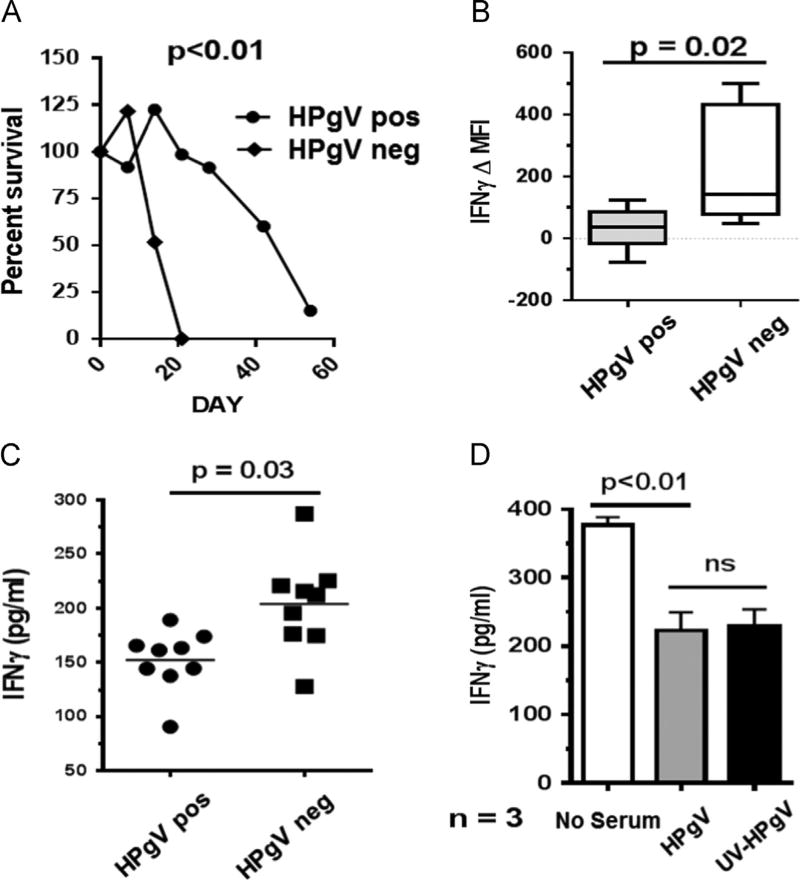
Fig. 1.
HPgV infection prolongs NK cell survival and inhibits IL-12-induced interferon gamma expression by NK cells. Peripheral blood mononuclear cells (PBMCs) from HPgV positive subjects (n = 11) and HPgV negative subjects (n = 6) were stimulated with PHA/IL-2 and maintained in culture for eight weeks NK cells obtained from HPgV viremic subjects survived significantly longer than HPgV RNA negative subjects ex vivo (p < 0.01, chi square) (A). PBMCs from HPgV positive subjects (n = 9) and HPgV negative subjects (n = 9) were studied for induction of IL12-induced interferon gamma. NK cells obtained from HPgV positive subjects had significantly less intracellular IFNγ expression following IL-12 and IL-15 stimulation (B). Similarly, IFNγ release by the human NK cell line NK92MI following stimulation with IL-12 for 18 h was significantly lower when incubated with HPgV positive human sera (n = 9) compared to HPgV negative sera (n = 9) (C). Ultraviolet inactivation of serum HPgV particles did not alter the effect of HPgV serum on IFNγ release (D). Data in panel C represent two independent experiments each using three different donors per experiment. P values represent T test results between groups.
To determine if HPgV altered NK cell function, IL-12 induced IFNγ expression was studied. Several pathogens induce IL-12 which in turn elicits IFNγ induction by NK cells (Biron and Gazzinelli, 1995; Romani et al., 1997). IFNγ has antimicrobial and immunoregularory functions critical to host protection and viral clearance (Gattoni et al., 2006; Boehm et al., 1997). PBMCs from HIV infected subjects with > 6 months suppressed HIV VL were stimulated with IL-12 and IL-15, and IFNγ was assessed by flow cytometry. NK cells obtained from HPgV infected subjects had significantly reduced IFNγ expression compared to the HPgV negative subjects (Fig. 1B). Repeat analysis using a 1%, 2% and 5% positive gating strategy confirmed that there were significant differences between the HPgV viremic and nonviremic subjects regardless of initial gating (data not shown). Furthermore, primary NK cells incubated with HPgV positive sera had significantly lower IL-12 mediated IFNγ compared to cells incubated in HPgV negative sera (Fig. 1C). Sera used in both treatments and controls were from HIV-infected subjects receiving cART. All subjects had undetectable HIV VL for > 6 months, thus making serum factors or antiretroviral drug effects unlikely to influence the results. HPgV inhibition of IL-2-mediated IFN-γ production by NK cells did not require HPgV replication, as UV-treated, replication deficient HPgV particles also inhibited IL-12 induced IFN-γ release by human PBMCs (Fig. 1D). This inhibition of IFNγ secretion was specific to the IL-12 pathway, as stimulation of PBMCs with PMA and ionomycin (bypassing the IL-12 receptor) rescued IFNγ secretion by HPgV infected primary NK cells. Representative subject data are shown in Fig. 2). Together, these data suggest that serum-derived HPgV particles can suppress IL-12 receptor (IL-12 R)-mediated NK cell function.
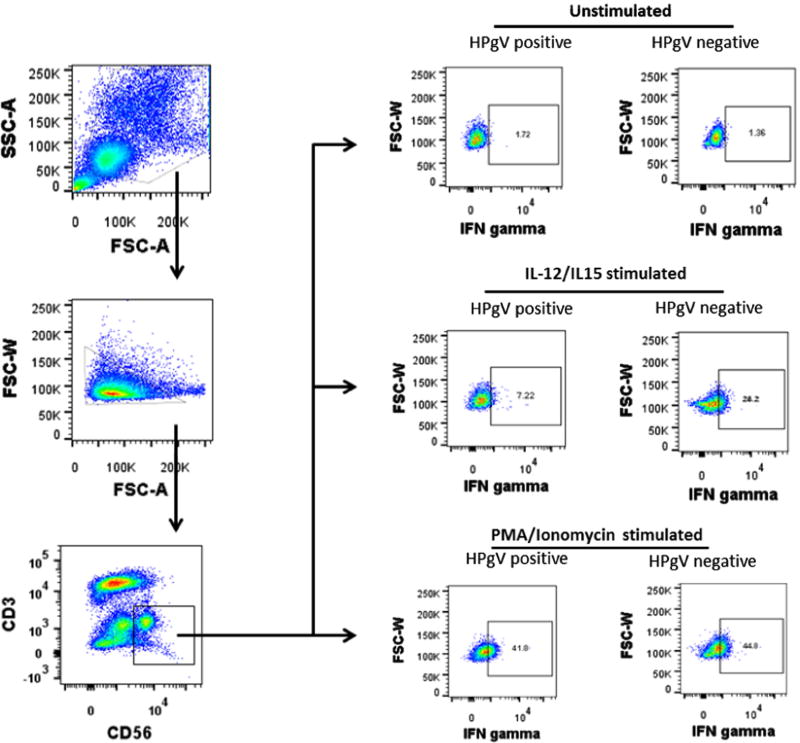
Fig. 2.
Gating strategy for intracellular staining of interferon gamma on NK cells. After gating on lymphocytes and single cells, CD3 negative and CD56 positive NK cells were gated and intracellular interferon gamma analyzed following stimulation with either IL12 plus IL15 or PMA plus ionomycin or unstimulated. Representative plots of responses to stimuli are shown.
HPgV envelope protein (E2) inhibits IL-12 signaling pathways in NK cells
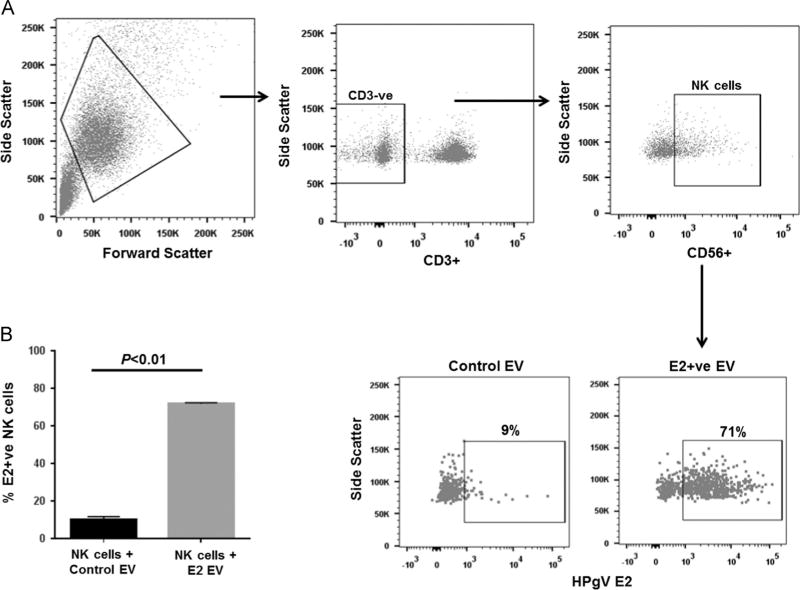
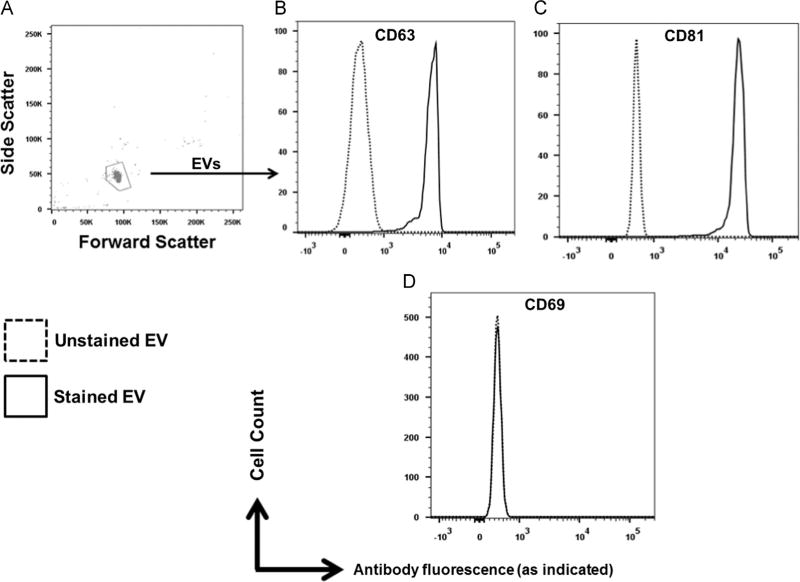
HPgV envelope protein (E2) modulates TCR and IL-2 receptor signaling pathways (Bhattarai et al., 2013, 2012). Since HPgV-mediated inhibition of IL-12 signaling in NK cells did not require viral replication (Fig. 1D) and HPgV E2 protein is predicted to be the major surface protein on virions (Mohr and Stapleton, 2009), the effect of HPgV envelope protein E2 on IL-12 pathway signaling was assessed. Recombinant Fc-fusion HPgV E2 protein inhibited IL-12 mediated IFNγ release from NK92 cells compared to cells incubated with the Fc control protein (Fig. 3A). Thus, incubation of HPgV E2 protein was sufficient to modulate IL-12 signaling by NK 92 cells. Extracellular vesicles (EVs) were purified from cell culture supernatant fluids of CHO cells as described in the methods section. EVs expressing E2-Fc contained HPgV E2 protein in immunoblots, while EVs purified from CHO cell supernatants expressing Fc without E2 protein did not (Fig. 3B). Incubation of NK92 cells with the E2-Fc EVs resulted in the uptake of HPgV E2 protein (Fig. 3C). Fig. 4 shows the gating strategy used to quantify E2 in NK92 cells. Similarly, incubation of primary human NK cells with E2-Fc-containing EVs demonstrated uptake of E2 (Fig. 5). In addition to containing HPgV E2 protein (Fig. 3B), the purified EVs contained CD63 and CD81 (Fig. 6A and B) but not CD69 (Fig. 6). Since exosomes have not been reported to contain CD69, but commonly contain CD63 and CD81, these findings are consistent with the EVs being exosomes (http://exocarta.org/query.html).
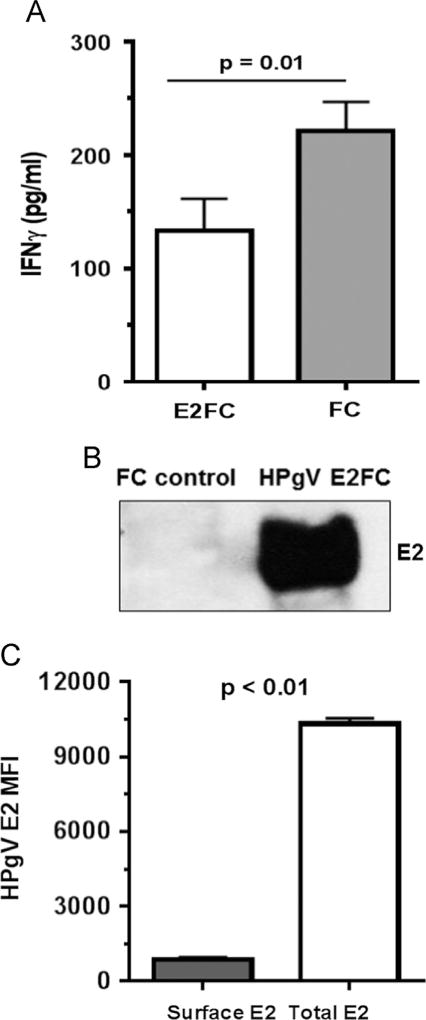
Fig. 3.
Extracellular vesicles (EVs) containing recombinant HPgV E2 protein inhibit IL-12-mediated signaling and deliver E2 to NK cells. IFNγ release by the NK cell line (NK92MI) following incubation with CHO cell culture supernatant EVs containing recombinant HPgV E2-human Fc fusion protein or human Fc protein (A). Cells were incubated 18 h prior to stimulation with IL-12 (p value for T test shown). E2-Fc and Fc concentration used was 50 µg/ml. EVs from cell culture supernatant fluids of cells expressing HPgV E2-Fc contained E2, while EVs from CHO cells expressing just Fc protein did not (B). NK92MI cells were incubated overnight with HPgV E2 positive EVs and the cells examined for surface or total cellular E2 protein following permeabilization using flow cytometry (C). The mean fluorescence intensity (MFI) of surface and total HPgV E2 in the cells is summarized in the graph (C).
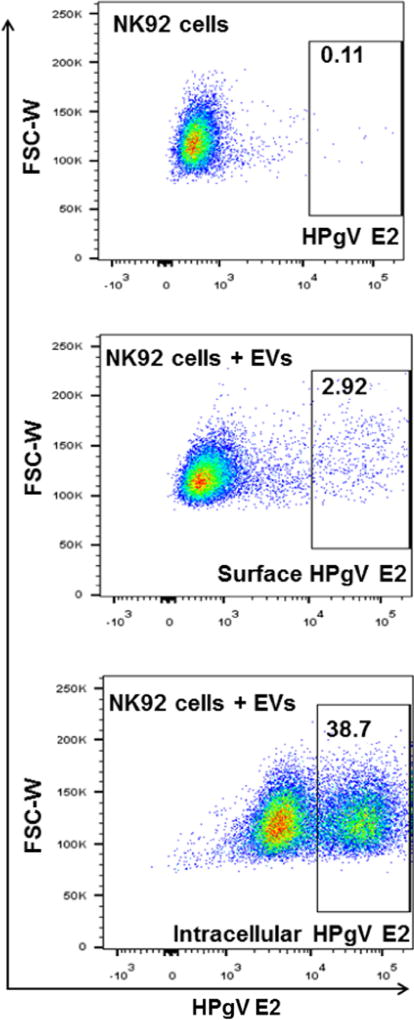
Fig. 4.
HPgV E2 positive extracellular vesicles (EVs) deliver E2 to NK cells. EVs purified from cell culture supernatant fluids of cells expressing HPgV E2-Fc protein or the Fc-vector control were analyzed delivery of E2 to NK92MI cells. NK92MI cells were incubated overnight with HPgV E2 positive EVs and the cells examined for surface or total cellular E2 protein by flow cytometry using antiE2 antibodies. Representative data are shown.
Fig. 5.
Extracellular vesicles (EVs) deliver HPgV E2 protein to primary human NK cells. EVs released into cell culture supernatant from CHO cells expressing HPgV E2 protein or control CHO cells were incubated with healthy donor peripheral blood mononuclear cells (PBMCs). PBMCs were gated as shown (A), and total cellular E2 uptake was measured (B).
Fig. 6.
Characterization of extracellular vesicles (EVs) containing HPgV E2 for exosomal markers. Gating of EVs used to deliver E2 to NK cells (A) were characterized for exosomal markers by flow cytometry. EVs were positive for cellular receptors CD69 and CD81 (B, C) but negative for CD69 (D).
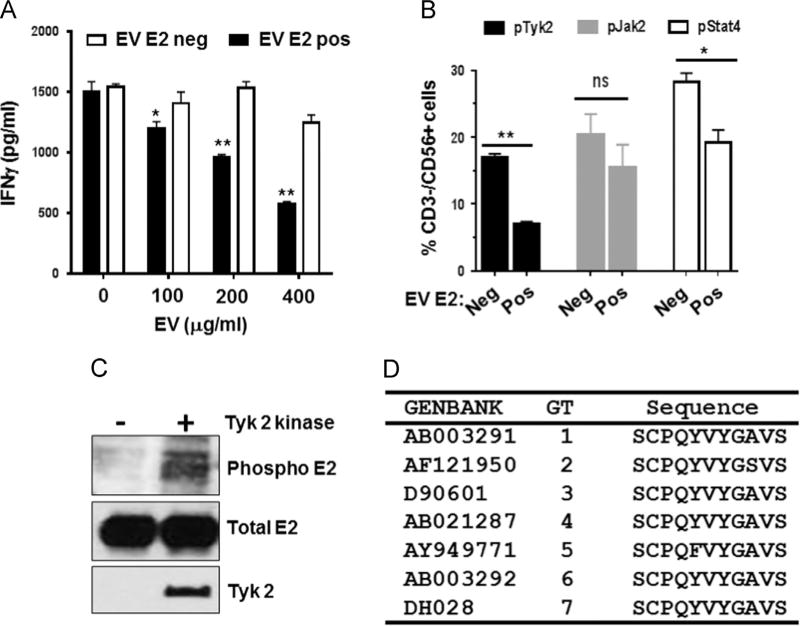
Following IL-12 stimulation, HPgV E2-positive EVs significantly inhibited IFNγ release by NK92 cells in a dose dependent manner (Fig. 7A) compared to cells incubated in HPgV E2-negative, Fc-positive control EVs. Thus E2 protein-containing EVs from cell culture supernatant (Fig. 7A) inhibited IL-12-mediated signaling in NK92 cells. Although the concentration of E2 protein used in these in vitro studies is high, IFNγ release was also reduced in cells incubated with HPgV positive serum (Fig. 1C and D), suggesting that physiologically relevant amounts of E2 protein present in serum or circulating virions suppress cytokine responses.
Fig. 7.
HPgV envelope protein (E2) in extracellular vesicles inhibits IL-12 signaling pathways in NK cells. IFNγ released into NK92MI cell culture supernatants was inhibited following incubation with a range of volumes of HPgV E2 positive or negative extracellular vesicles (EVs) prepared from CHO cell culture supernatants. EVs were quantified by protein-A280 spectrometry. (A). IL-12-mediated signaling of healthy donor peripheral blood mononuclear cells following 18 h incubation with HPgV E2 positive or negative EVs was assessed by intracellular pTyk2, pJak2 and pSTAT4 phosphorylation. HPgV E2 positive EVs inhibited Tyk2 and STAT4 but not Jak2 phosphorylation compared to HPgV E2 negative EV controls (B). Tyk2 phosphorylated HPgV E2 protein in vitro as measured by anti-phosphotyrosine antibodies (C). Two tyrosine residues within HPgV E2 protein (a.a. 85, 87) that are conserved among all 7 HPgV genotypes (representative sequences shown in D) were predicted to interact with a kinase (Tyk2) required for IL-12 induced interferon induction * = p < 0.05, ** = p < 0.01 (T test).
HPgV E2 inhibits NK cell Tyk2 activation
To understand potential mechanisms by which HPgV E2 inhibited IL-12 R-mediated NK cell function, NK92MI cells were incubated with HPgV positive or negative EVs overnight, and IL-12 mediated phosphorylation of STAT4, Janus Kinase 2 (Jak2), and tyrosine kinase 2 (Tyk2) was assessed. Following IL-12 stimulation, the membrane proximal kinases Tyk2 and Jak2 are activated (phosphorylated) resulting in recruitment, phosphorylation, and nuclear translocation of STAT4 leading to stimulation of cytokine production (Babon et al., 2014). NK92 cells incubated with HPgV E2 positive EVs demonstrated reduced phosphorylation of Tyk2 and STAT4 following IL12 stimulation compared to cells incubated in control, Fc-containing EVs (Fig. 7B). HPgV E2 mediated IL-12 signaling inhibition was specific to Tyk2 phosphorylation, as JAK2 phosphorylation was not impaired (Fig. 7B).
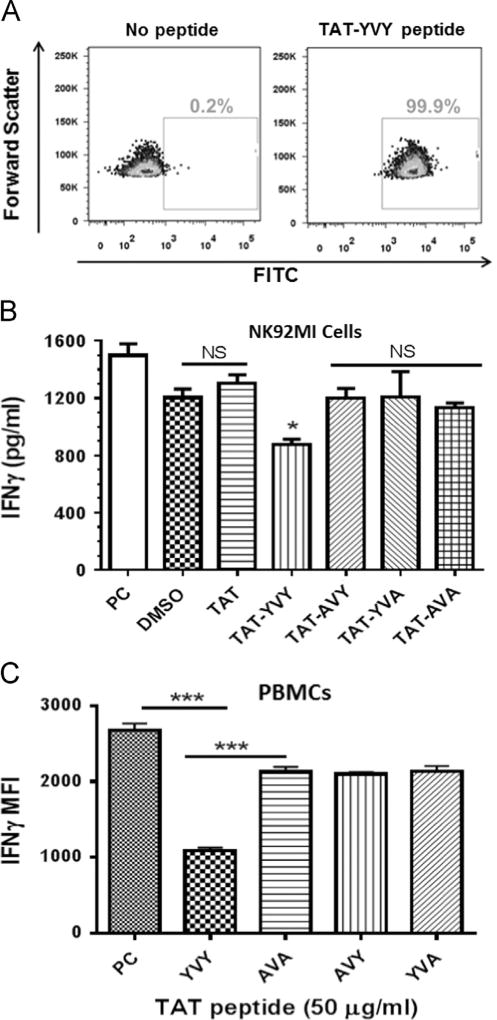
Bioinformatic analyses identified highly conserved motifs within HPgV E2 that are predicted to serve as phosphorylation substrates for Tyk2 (Xue et al., 2006). Consistent with this, incubation of recombinant HPgV E2 and Tyk2 kinase resulted in the phosphorylation of E2 in vitro (Fig. 6C), thus HPgV E2 may serve as a Tyk2 substrate. The motif on HPgV E2 predicted to serve as a Tyk2 substrate from isolates representing all 7 HPgV genotypes are shown in Fig. 7D. FITC-labeled synthetic HPgV E2 peptides were conjugated with the HIV TAT protein transduction domain (TAT) to lead to peptide internalization. Following incubation with NK92 cells, the peptides were present intracellularly as shown by flow cytometry (Fig. 8A). The native HPgV E2 peptide (81-SCPQYVYGSVSVT-93) significantly inhibited IL-12-mediated IFNγ production in the NK92 cell line, whereas substitution of an alanine for either of the conserved tyrosines (Y85 or Y87), or both restored IL-12 R signaling (Fig. 8B). The HPgV E2 peptides also inhibited IFNγ in primary human NK cells following IL-12 stimulation when compared to the Y85A or Y87A or AVA double mutant peptide (Fig. 8C). Incubation of NK92 or primary human NK cells with the native HPgV E2 peptide not conjugated to the TAT domain did not alter IL-12 signaling (data not shown), thus peptide uptake by the cells was required for the inhibition to occur.
Fig. 8.
Conserved tyrosine residues within HPgV envelope protein (E2) protein were required for inhibition of IL-12 signaling in primary NK cells and NK cell lines. Conserved tyrosine residues within HPgV E2 protein (a.a. 85, 87) predicted to modulate Tyk2 kinase function were predicted to serve as a substrate for Tyk2. NK92MI cells were incubated with no peptide or a FITC-labeled HPgV E2 peptide containing the HIV TAT protein transduction domain (TAT). Peptide uptake is shown by FITC fluorescence (A). NK92MI cells (B) or peripheral blood mononuclear cells (PBMCs; C) were incubated with the peptide vehicle (DMSO; PC), TAT peptide, native HPgV peptide with TAT (TAT-YVY) or mutant TAT-HPgV peptides as indicated for 6 h prior to IL12 stimulation (2 ng/ml). Following 18 h incubation, IFNγ was quantified in cell culture supernatant by ELISA (B) or intracellularly gating on CD3−/CD56+ NK cells by flow cytometry (C). Data represent cultures from three healthy, HPgV negative independent blood donors. * = p < 0.01 vs. DMSO and TAT only peptide (T test). *** = p < 0.01 vs. PC.
HPgV does not inhibit NK cell degranulation and cytolytic function
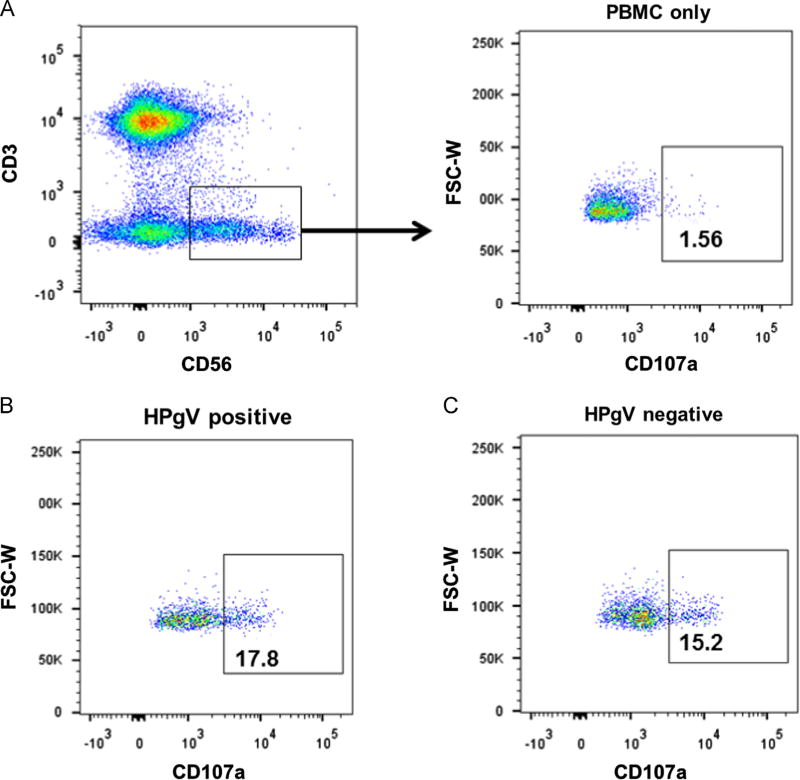
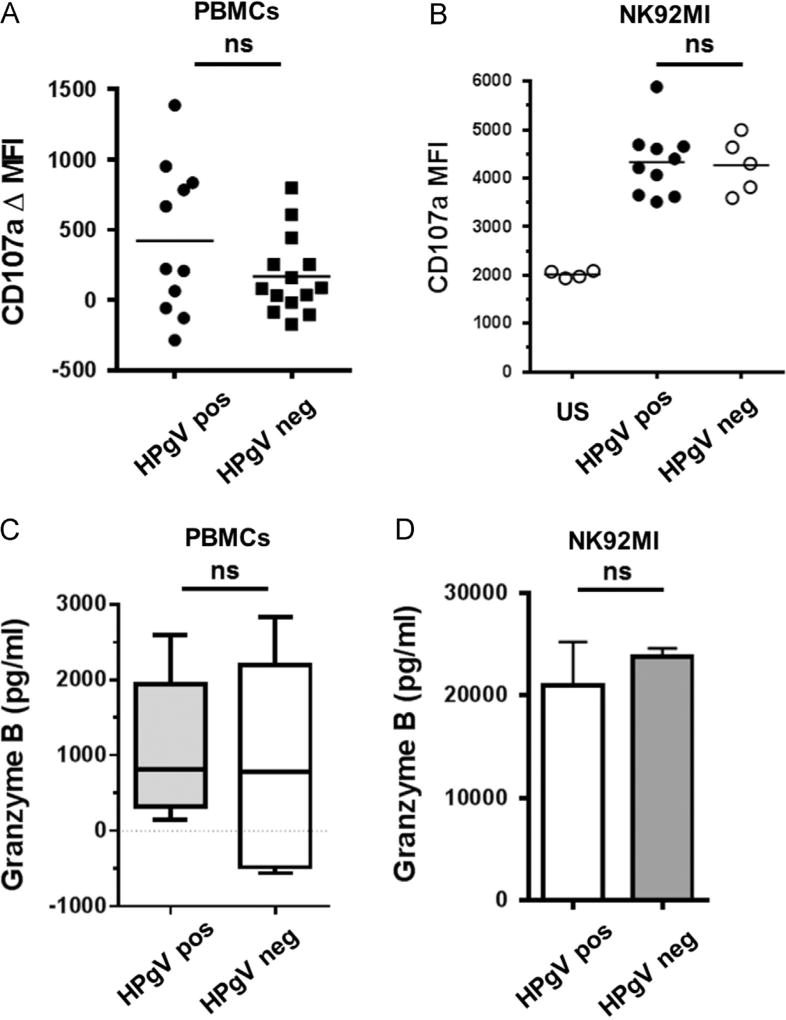
Since HPgV inhibited non-cytolytic NK cell function (IL-12 signaling), the effect of HPgV on NK cell cytolytic function was assessed. PBMCs from HPgV positive and negative subjects were incubated with K562 target cells, and cytolytic function was measured by quantifying surface expression of CD107a on CD56+/CD3− NK cells by flow cytometry or by measuring granzyme B release from PBMCs into culture supernatant fluids. Representative gating from HPgV RNA positive and negative subjects are shown in Fig. 9. There was no difference in CD107a expression on primary NK cells obtained from HPgV viremic or negative subjects (Fig. 10A). Furthermore, CD107a expression was not altered on NK92 cells incubated with serum (300 µl in 1 ml total volume) from HPgV viremic or uninfected subjects prior to incubation with K562 target cells (Fig. 10B). Similar to the CD107a results, there were no differences in the amount of granzyme B released by PBMCs obtained from HPgV viremic and uninfected subjects following incubation with K562 cells (Fig. 10C), or from NK92 cells incubated in serum from HPgV infected or uninfected prior to the addition of K562 target cells (Fig. 10D). Together these results demonstrate that HPgV serum containing viral particles did not alter primary human PBMC or NK92 cell killing (cytolytic) functions.
Fig. 9.
HPgV does not inhibit NK cell degranulation. PBMCs from HPgV positive or negative subjects were studied for K562 target cell-induced CD107a surface expression (a marker of degranulation) by flow cytometry. Representative gating for CD3−/CD56+/CD107a+ surface expression on NK cells is shown in panel A, and results from representative HPgV infected (B) and uninfected (C) subjects are shown.
Fig. 10.
HPgV does not inhibit NK cell cytolytic functions. PBMCs from HPgV positive or negative subjects or NK92MI cells were studied for K562 target cell-induced CD107a surface expression by flow cytometry and for granzyme B release by ELISA. Following incubation with K562 cells for 5 h, there were no significant differences in CD107a expression on NK cells between HPgV infected and uninfected donors in PBMCs (A) or NK92MI cells (B). Similarly, there was no difference in the release of granzyme B by PBMCs (C) or NK92MI cells (D). ns = p > 0.05.
Materials and methods
Subjects
Following written informed consent, blood was obtained from HIV infected subjects whose viral load (VL) was suppressed (< 48 copies/mL for ≥ 6 months) by combination antiretroviral therapy (cART), or from healthy blood donors. For HIV infected subjects, HIV VL was tested on the day of blood sampling using the COBAS® AmpliPrep/COBAS® TaqMan® HIV-1 Test (Roche) by the University of Iowa clinical laboratory as part of routine medical care. HPgV-HIV co-infected subjects were selected based on prior studies characterizing their HPgV status. The study was approved by the University of Iowa Institutional Review Board.
Cells and clinical samples
Serum was prepared and HPgV viral load determined as previously described (Rydze et al., 2012; George et al., 2003). PBMCs (2 × 106 cells/ml) were prepared as previously described (Lymphocyte Separation Media Mediatech, Manassas, VA) and maintained in RPMI 1640 media containing 10% fetal bovine serum, 25 U/ml recombinant human IL-2 (Zeptometrix), 10 mcg/mL phytohemagglutinin (PHA, Gibco) 100 U/ml penicillin, 100 U/ml streptomycin, and 2 mM l-glutamine. Fifty percent of the media was removed weekly and replaced with fresh media. Cells were counted using the Countess™ automated cell counter (Invitrogen Inc.). Viability was assessed using trypan blue exclusion. Serum was treated with, or without UV inactivation (T8 Germicidal UV lamp, GE) as indicated for 30 min prior to use.
A human NK cell line (NK92MI, ATCC® CRL2408™) was cultured Alpha Minimum Essential Medium (Alpha-MEM) supplemented with 10% heat-inactivated Fetal Calf Serum, 5% horse serum, 0.02 g/0.5 L Inositol, 0.005 g/0.5 L Folic acid, 909 µl/0.5 L mercaptoethanol, 100 IU/ml Penicillin and 100 µg/ml streptomycin. K562 cells were cultured in RPMI-1640 (GIBCO) supplemented with 10% heat-inactivated Fetal Calf Serum, 2 mM l-glutamine, 100 IU/ml Penicillin and 100 µg/ml streptomycin.
NK cell functional assays
NK92MI cells were incubated with serum (300 µl) from HPgV viremic and HPgV RNA-negative subjects, or with UV-treated serum (as specified) for 6 h prior to overnight incubation with or without IL-12 stimulation with (2 ng/ml; BD) in a total volume of 1 ml. IFN-γ was quantified in cell culture supernatants obtained 16 h later using the Human IFN-γ ELISA kit (BD Biosciences) according to manufacturer's instructions. Alternatively, intracellular IFN-γ was measured in primary peripheral blood NK cells following stimulation with IL-12 (5 ng/ml; BD) and IL15 (5 ng/ml; BD) or phorbol 12-myristate 13-acetate (PMA 50 ng/ml) and Ionomycin (500 ng/ml) as specified. One hour after stimulation, Brefeldin A (BFA; 10 µg/ml; Sigma) was added and after seven hours, cells were harvested, fixed and permeabilized (BD Cytofix/Cytoperm Solution, BD Biosciences) using the manufacturer's instructions. IFN-γ in NK cells (CD3−/CD56+) was quantified by flow cytometry (BD LSR-II) and analyzed using FlowJo program software (Tree Star). Antibodies used in these studies included: CD3-V450, CD8-A700, CD56-PE, IFN-γ A647 (BD Biosciences). Data was acquired on BD LSR II flow cytometer using single stained CompBeads (BD Biosciences) for compensation. At least 10,000 total events were collected in each experiment and the FlowJo program (Tree Star Inc.) was used for data analysis.
Freshly isolated PBMCs (1 × 106/ml) were co-cultured with K562 target cells (1 × 106/ml) for 5 h (37 °C) in the presence of anti-CD107a-FITC (Southern BioTech). Degranulation was quantified by measuring CD107a surface expression on CD56+/CD3− NK cells (flow cytometry) and granzyme B release in culture supernatant (ELISA, Southern Biotech).
Phosphorylation of Tyk 2, Jak 2 or Stat 4 was measured in peripheral blood NK cells following incubation with HPgV E2-Fc positive or Fc-only extracellular vesicles (EVs) for 18 h followed by IL 12 (5 ng/ml; BD) stimulation for 15 min. Cells were fixed and permeabilized (BD Cytofix/Cytoperm) prior to staining in BD permwash buffer. Antibodies used included: pJAK2 (Y1007/1008; Cell Signaling), pTyk2 (Y1054/Y1055; BD), pSTAT4 (Y693; BD), goat anti-rabbit FITC (Invitrogen), goat anti-mouse A647 (Invitrogen).
Viral proteins and extracellular vesicles preparation
HPgV E2-Fc protein (nt 1167–2161 based on GenBank AF121950) or the vector control expressing Fc were expressed in CHO S cell lines previously described (McLinden et al., 2006). HPgV E2 was analyzed by immunoblot using polyclonal rabbit anti-E2 peptide antibody using chemiluminescence (Amersham ECL;GE Healthcare) as described (McLinden et al., 2006; Bhattarai et al., 2012). CHO cells were maintained in serum-free media in a bioreactor (CELLine CL1000; Wilson Wolf) and supernatants were clarified and exosomes purified from supernatant using ExoQuick reagent (Systems Biosciences) as recommended by the manufacturer. Isolated EVs were incubated with aldehyde/sulfate latex beads (Invitrogen) at room temperature (RT). After 1 h, 100 µL of glycine (100 mM) and 100 µL of BSA (10% w/v) was added to the reaction and incubated for additional 30 min at RT. Beads were washed in PBS (3×) and pelleted by centrifugation for 2 min at 10,000g prior to flow cytometry analysis. CD63, CD81 and CD69 monoclonal antibodies (BD Biosciences) were used to detect the cognate molecules on exosomes using flow cytometry.
FITC labeled synthetic HPgV E2 peptides with an N-terminal HIV TAT protein transduction domain (TAT) alone (GGGGGRKKRRQRRR), with wild type HPgV E2 aa 81–93 (GGGGGRKKRRQRRRSCPQYVYGSVSVT), or mutants (AVA mutant GGGGGRKKRRQRRRSCPQAVAGSVSVT; AVY mutant (GGGGGRKKRRQRRRSCPQAVYGSVSVT; or YVA mutant GGGGGRKKRRQRRRSCPQYVAGSVSVT) were purchased from EzBiolabs. PBMCs (1 × 106 cells/ml) were incubated with 50 µg of peptide at 37 °C for 3 h and subsequently stimulated with 2 ng/ml IL-12 in the presence of BFA for 8 h. Intracellular IFN-γ was quantified by flow cytometry as described above.
Recombinant E2 protein (40 µg) was incubated with or without active human Tyk2 (250 ng) using an in vitro kinase assay as recommended by the manufacturer (Signal Chem). Phosphorylation was determined by immunoblot analysis using phosphotyrosine antibodies (Invitrogen).
Statistics
Statistics and graphs were performed using GraphPad software V5.0 (GraphPad Prism Software). Student's t test was used to compare results between test and controls. P values less than 0.05 were considered statistically significant.
Conclusions
HPgV and HCV are unusual among cytoplasmic human RNA viruses in that they commonly cause persistent infection, thus they have developed effective mechanisms to evade host immune clearance. For HCV, several mechanisms are proposed to contribute to immune evasion, including the presence of two hypervariable regions on the envelope (E2) protein, rapid mutation of immunodominant T cell epitopes (Keck et al., 2014), interference with intracellular signaling pathways resulting in reduced innate and adaptive immune responses, and chronic stimulation of T cells leading to T cell exhaustion (Burke and Cox, 2010; Lemon, 2010). In contrast, HPgV envelope proteins do not have hypervariable regions, display limited sequence variability within infected hosts, and HPgV infection is associated with reduced T cell activation (Stapleton et al., 2012; Mohr and Stapleton, 2009). Although the primary permissive cell type for HPgV remains unclear, viral RNA is detected in several types of peripheral blood cells including NK cells (Chivero et al., 2014), suggesting that HPgV may modulate NK cell function.
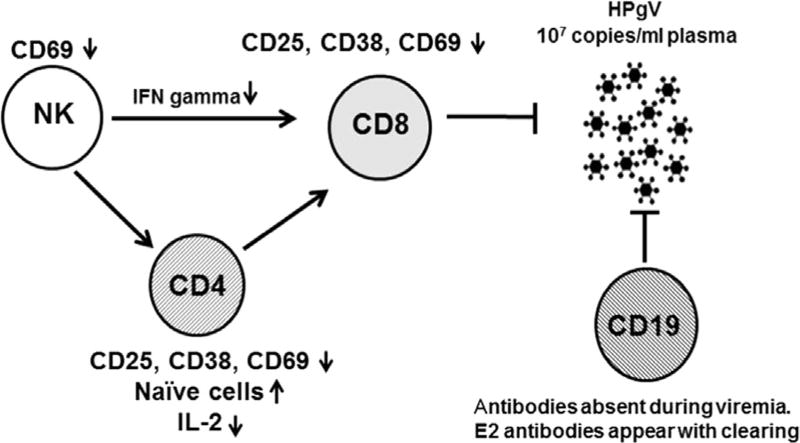
In this study, NK cells from persistently infected people had reduced IFNγ expression following IL-12 stimulation compared to those without HPgV viremia. HPgV replication was not required for inhibition, suggesting that components of HPgV particles modulate NK cell function. Indeed, HPgV E2 glycoprotein was sufficient to inhibit IFNγ expression by NK cells, and an E2 protein YVY-containing motif (amino acids 85–87) was required for inhibition, as mutation of either tyrosine to alanine in the context of these peptides restored IL-12 R-mediated induction of IFNγ. This conserved motif is predicted to be a Tyk2 substrate, and Tyk2 phosphorylated HPgV E2 in vitro (Fig. 7C). The high VL observed in HPgV infection (typically greater that 1 × 107 genome copies/ml plasma) (Tillmann et al., 2001) implies that interactions constantly occur between NK cells and HPgV particles. Tyk2 activation is required for IFNγ induction (Karaghiosoff et al., 2000; Shimoda et al., 2002; Tokumasa et al., 2007), and Tyk2 deficient mice are unable to clear vaccinia virus and have reduced T cell responses after LCMV challenge (Karaghiosoff et al., 2000). NK cell depletion also enhances T-cell immunity leading to virus control preventing chronic infection (Lang et al., 2012; Waggoner et al., 2014), confirming the important role NK cells play in chronic viral infections. Our study highlights a potential role of NK cells in contributing to chronic HPgV viremia. However, the absence of an animal model makes it impossible to demonstrate NK cell depletion or Tyk2 inhibition effects on HPgV persistence in vivo. The current understanding of HPgV persistence and clearance mechanisms are shown in Fig. 11. The failure to detect antibody responses to HPgV proteins and delayed development of E2 antibodies also suggests impaired B cell function during HPgV infection. This, plus the reduction in T cell and NK cell activation state during HIV infection (Maidana-Giret et al., 2009; Stapleton et al., 2012, 2013; Bhattarai et al., 2012) and as shown in these studies suggests a modest HPgV-mediated effect resulting in global impairment of cellular immune responses.
Fig. 11.
Working model for HPgV persistence. Clinical studies show HPgV to be associated with significantly reduced expression of the activation marker CD69 on NK cells, and activation markers CD38, CD69 and CD25 on CD4+ and CD8+ T cells. Thus, HPgV infection is associated with reduced immune cell activation. In addition, B cells usually do not mount an antibody response to HPgV proteins during viremia, though they develop E2 antibodies at the time of, or after clearance of viremia. Due to the impairment of both cellular and humoral immunity, HPgV replicates to titers typically greater than 1 × 107 genome copies/ml of plasma. In this study, NK cells expressed less interferon gamma following IL-12 stimulation, potentially contributing to dysfunctional immune regulation of CD8+ T cells.
While interferonγ was decreased by HPgV in this study, direct cytotoxicity measures (CD107a degranulation and granzyme B release) were not altered, suggesting that HPgV selectively interferes with the noncytoloytic functions of NK cells. In contrast, the related virus HCV increases NK cell cytotoxicity (i.e. degranulation and TNF-related apoptosis-inducing ligand; TRAIL) (Ahlenstiel et al., 2010; Oliviero et al., 2009), potentially contributing to HCV induced hepatocyte cell death, and conversely explaining in part the lack of disease outcomes in HPgV infected hosts.
While HPgV is not associated with any acute or chronic illnesses other than non-Hodgkin's lymphoma, its immunomodulatory capacity illustrates the complex relationships between viral infection and host immune activation, particularly in HIV co-infected subjects. HIV infection is associated with chronic immune cell activation which is an important factor in HIV disease progression, and immune activation enhances HIV replication (Gonzalez et al., 2010; Sandberg et al., 2010; Nixon and Landay 2010; Giorgi et al., 1999). Immune activation is also associated with immune dysfunction (Hazenberg et al., 2003) and reduced responses to antiretroviral therapy (Hunt et al., 2003). Several clinical studies found that among HIV-infected subjects HPgV infection is associated with reduced T, B and NK cell activation (Maidana-Giret et al., 2009; Stapleton et al., 2012; Bhattarai et al., 2012), and HPgV infection acquired through blood transfusion is associated with reduced proinflammatory cytokines compared to controls in HIV co-infected subjects (Lanteri et al., 2014). The data above show that HPgV viremia inhibits IL-12-mediated IFNγ release by NK cells. Given the paracrine and pleomorphic effects of IFNγ, this inhibition likely contributes to reduced immunomodulation and recruitment of cytotoxic CD8+ T cells, contributing to a survival benefit for the host. Consistent with this, a recent study found a survival benefit in Ebola infected subjects co-infected with HPgV (Lauck et al., 2015). Ebola infection causes hemorrhagic fever by inducing the release of pro-inflammatory cytokines. It is tempting to speculate that the effect of HPgV infection on cytokine induction in HIV disease contributes to a survival benefit with Ebola infection (Lauck et al., 2015), and may alter outcomes in other immune-mediated disease states.
In summary, HPgV prolonged NK cell survival ex vivo, and impaired noncytolytic function as exemplified by IL-12-induced IFNγ without inhibiting NK cytolytic function as measured by granzyme B release and CD107a degranulation. HPgV E2 glycoprotein was sufficient to inhibit IL-12-induced IFNγ expression in NK cells through the inhibition of Tyk2 and STAT4 phosphorylation. We show that HPgV E2 can be delivered to primary NK cells and the NK92 cell line by EVs. Although beyond the scope of this investigation, further characterization of the mechanism of EV interactions with cells is important. Specifically, EVs may enter target cells by being taken up by the target cell endocytic pathway or by fusion with the cell membrane with direct release of the EV contents into the cytoplasm. This suggests that interference with NK cell function may contribute to both the chronicity and high viral loads observed in HPgV infection, and that these effects may contribute to the reduced immune activation and proinflammatory cytokines involved in the immunopathogenesis of HIV and other diseases.
Acknowledgments
We thank our patients for participating in the research, Thomas Kaufman and Donna Klinzman for assistance with cell culture and PCR studies. We also thank Justin Fishbaugh and the University of Iowa Flow cytometry core for assistance with these studies. This work was supported by Grants from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Merit Review Grants BX000207 and CX000821, JTS and BX001241, JX), and the International Fulbright Science and Technology Student Fellowship (I.D. number 15101093) (ETC).
Footnotes
Conflicts of interest
Drs. Stapleton, Bhattarai, Xiang, and McLinden have patents or provisional patents related to the use of HPgV/GBV-C for therapeutic use. The authors have no additional financial interests. This potential conflict does not interfere with requirement of free exchange of any materials used in studies published by Virology and as recommended by the National Academy of Sciences.
This work was presented in part at the Keystone Symposium on Innate immunity to viral infections at Keystone, CO on January 20, 2014 (poster 1027), and at the Mechanisms of HIV persistence, implications for a cure (E1) Keystone Symposia, Boston, MA, April 27, 2015 (poster 1025).
References
- Adams MJ, King AMQ, Carstens EB. Ratification vote on taxonomic proposals to the international committee on taxonomy of viruses (2013) Arch. Virol. 2013;158:2023–2030. doi: 10.1007/s00705-013-1688-5. [DOI] [PubMed] [Google Scholar]
- Ahlenstiel G, Titerence RH, Koh C, et al. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138(325–35):e1–e2. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babon JJ, Lucet IS, Murphy JM, Nicola NA, Varghese LN. The molecular regulation of Janus kinase (JAK) activation. Biochem. J. 2014;462:1–13. doi: 10.1042/BJ20140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai N, Rydze RT, Chivero ET, Stapleton JTGB, Virus C. Viremia is associated with higher levels of double-negative T cells and lower T-cell activation in HIV-infected individuals receiving antiretroviral therapy. J. Infect. Dis. 2012;206:1469–1472. doi: 10.1093/infdis/jis515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai N, McLinden JH, Xiang JH, Kaufman TM, Stapleton JTGB, Virus C. Envelope protein E2 inhibits TCR-induced IL-2 production and alters IL-2-signaling pathways. J. Immunol. 2012;189:2211–2216. doi: 10.4049/jimmunol.1201324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai N, McLinden JH, Xiang JH, Landay AL, Chivero ET, Stapleton JTGB, Virus C. Particles inhibit T cell activation via envelope E2 protein-mediated inhibition of TCR signaling. J. Immunol. 2013;190:6351–6359. doi: 10.4049/jimmunol.1300589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron CA, Gazzinelli RT. Effects of IL-12 on immune responses to microbial infections: a key mediator in regulating disease outcome. Curr. Opin. Immunol. 1995;7:485–496. doi: 10.1016/0952-7915(95)80093-x. [DOI] [PubMed] [Google Scholar]
- Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- Burke KP, Cox AL. Hepatitis C virus evasion of adaptive immune responses: a model for viral persistence. Immunol. Res. 2010;47:216–227. doi: 10.1007/s12026-009-8152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivero ET, Stapleton JT. Tropism of human pegivirus (formerly known as GB virus C/Hepatitis G virus) and host immunomodulation: insights into a highly successful viral infection. J. Gen. Virol. 2015;96:1531–1532. doi: 10.1099/vir.0.000086. http//dx.doi.org/10.1099/vir.0.000086, Published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivero ET, Bhattarai N, Rydze RT, Winters MA, Holodniy M, Stapleton JT. Human pegivirus RNA is found in multiple blood mononuclear cells in vivo and serum-derived viral RNA-containing particles are infectious in vitro. J. Gen. Virol. 2014;95:1307–1319. doi: 10.1099/vir.0.063016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattoni A, Parlato A, Vangieri B, Bresciani M, Derna R. Interferon-gamma: biologic functions and HCV therapy (type I/II) (1 of 2 parts) La Clin. Ter. 2006;157:377–386. [PubMed] [Google Scholar]
- George SL, Xiang JH, Stapleton JT. Clinical isolates of GB virus type C vary in their ability to persist and replicate in peripheral blood mononuclear cell cultures. Virology. 2003;316:191–201. doi: 10.1016/s0042-6822(03)00585-3. [DOI] [PubMed] [Google Scholar]
- George SL, Varmaz D, Stapleton JT. GB virus C replicates in primary T and B lymphocytes. J. Infect. Dis. 2006;193:451–454. doi: 10.1086/499435. [DOI] [PubMed] [Google Scholar]
- Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- Gonzalez VD, Landay AL, Sandberg JK. Innate immunity and chronic immune activation in HCV/HIV-1 co-infection. Clin. Immunol. 2010;135:12–25. doi: 10.1016/j.clim.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Gutierrez RA, Dawson GJ, Knigge MF, et al. Seroprevalence of GB virus C and persistence of RNA and antibody. J. Med. Virol. 1997;53:167–173. doi: 10.1002/(sici)1096-9071(199710)53:2<167::aid-jmv10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J. Infect. Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549–560. doi: 10.1016/s1074-7613(00)00054-6. [DOI] [PubMed] [Google Scholar]
- Keck ZY, Angus AG, Wang W, et al. Non-random escape pathways from a broadly neutralizing human monoclonal antibody map to a highly conserved region on the hepatitis C virus E2 glycoprotein encompassing amino acids 412–423. Plos Pathog. 2014;10:e1004297. doi: 10.1371/journal.ppat.1004297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PA, Lang KS, Xu HC, et al. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc. Natl. Acad. Sci. USA. 2012;109:1210–1215. doi: 10.1073/pnas.1118834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanteri MC, Vahidnia F, Tan S, et al. Downregulation of Cytokines and chemokines by GB virus C after transmission via blood transfusion in HIV-positive blood recipients. J. Infect. Dis. 2014 doi: 10.1093/infdis/jiu660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauck M, Bailey AL, Andersen KG, Goldberg TL, Sabeti PC, O’Connor DHGB, Virus C. Coinfections in west African ebola patients. J. Virol. 2015;89:2425–2429. doi: 10.1128/JVI.02752-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon SM. Induction and evasion of innate antiviral responses by hepatitis C virus. J. Biol. Chem. 2010;285:22741–22747. doi: 10.1074/jbc.R109.099556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidana-Giret MT, Silva TM, Sauer MM, et al. GB virus type C infection modulates T-cell activation independently of HIV-1 viral load. AIDS. 2009;23:2277–2287. doi: 10.1097/QAD.0b013e32832d7a11. [DOI] [PubMed] [Google Scholar]
- McLinden JH, Kaufman TM, Xiang J, et al. Characterization of an immunodominant antigenic site on GB virus C glycoprotein E2 that is involved in cell binding. J. Virol. 2006;80:12131–12140. doi: 10.1128/JVI.01206-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr EL, Stapleton JT. GB virus type C interactions with HIV: the role of envelope glycoproteins. J. Viral Hepat. 2009;16:757–768. doi: 10.1111/j.1365-2893.2009.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon DE, Landay AL. Biomarkers of immune dysfunction in HIV. Curr. Opin. HIV AIDS. 2010;5:498–503. doi: 10.1097/COH.0b013e32833ed6f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnari G, Nigro L, Palermo F, et al. Slower progression of HIV-1 infection in persons with GB virus C co-infection correlates with an intact T-Helper 1 cytokine profile. Ann. Int. Med. 2003;139:26–30. doi: 10.7326/0003-4819-139-1-200307010-00009. [DOI] [PubMed] [Google Scholar]
- Oliviero B, Varchetta S, Paudice E, et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137(1151–60):60 e1–60 e7. doi: 10.1053/j.gastro.2009.05.047. [DOI] [PubMed] [Google Scholar]
- Romani L, Puccetti P, Bistoni F. Interleukin-12 in infectious diseases. Clin. Microbiol. Rev. 1997;10:611–636. doi: 10.1128/cmr.10.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydze RT, Bhattarai N, Stapleton JT. GB virus C infection is associated with a reduced rate of reactivation of latent HIV and protection against activation-induced T-cell death. Antivir. Ther. 2012;17:1271–1279. doi: 10.3851/IMP2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg JK, Falconer K, Gonzalez VD. Chronic immune activation in the T cell compartment of HCV/HIV-1 co-infected patients. Virulence. 2010;1:177–179. doi: 10.4161/viru.1.3.11206. [DOI] [PubMed] [Google Scholar]
- Schwarze-Zander C, Neibecker M, Othman S, et al. GB virus C coinfection in advanced HIV type-1 disease is associated with low CCR5 and CXCR4 surface expression on CD4(+) T-cells. Antivir. Ther. 2010;15:745–752. doi: 10.3851/IMP1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda K, Tsutsui H, Aoki K, et al. Partial impairment of interleukin-12 (IL-12) and IL-18 signaling in Tyk2-deficient mice. Blood. 2002;99:2094–2099. doi: 10.1182/blood.v99.6.2094. [DOI] [PubMed] [Google Scholar]
- Stapleton JT, Chaloner K, Zhang JY, et al. GBV-C viremia is associated with reduced CD4 expansion in HIV-infected people receiving HAART and interleukin-2 therapy. AIDS. 2009;23:605–610. doi: 10.1097/QAD.0b013e32831f1b00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton JT, Chaloner K, Martenson JA, et al. GB Virus C infection is associated with altered lymphocyte subset distribution and reduced T cell activation and proliferation in HIV-infected individuals. PLoS One. 2012;7:e50563, 1–9. doi: 10.1371/journal.pone.0050563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton JT, Martinson JA, Klinzman D, Xiang J, Desai SN, Landay A. GB virus C infection and B-cell, natural killer cell, and monocyte activation markers in HIV-infected individuals. AIDS. 2013;27:1829–1832. doi: 10.1097/QAD.0b013e328363089f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton JTBJ, Muerhoff AS, Foung S, Simmonds P. Assignment of human, simian and bat pegiviruses (previously described as GBV-A, GBV-C, and GBV-D) as members of a new Genus (Pegivirus) within the Flaviviridae. 2012 〈 http://www.ictvonline.org/proposals/2012.011a-dV.A.v2.Pegivirus.pdf〉.
- Tanaka E, Kiyosawa K, Shimoda K, et al. Evolution of hepatitis G virus infection and antibody response to envelope protein in patients with transfusion-associated non-A, non-B hepatitis. J. Vir. Hepat. 1998;5:153–159. doi: 10.1046/j.1365-2893.1998.00095.x. [DOI] [PubMed] [Google Scholar]
- Tillmann HL, Heiken H, Knapik-Botor A, et al. Infection with GB virus C and reduced mortality among HIV-infected patients. N. Engl. J. Med. 2001;345:715–724. doi: 10.1056/NEJMoa010398. [DOI] [PubMed] [Google Scholar]
- Tokumasa N, Suto A, Kagami S, et al. Expression of Tyk2 in dendritic cells is required for IL-12, IL-23, and IFN-gamma production and the induction of Th1 cell differentiation. Blood. 2007;110:553–560. doi: 10.1182/blood-2006-11-059246. [DOI] [PubMed] [Google Scholar]
- Vahidnia F, Petersen M, Stapleton JT, Rutherford GW, Busch M, Custer B. Acquisition of GB virus type C and lower mortality in patients with advanced HIV disease. Clin. Infect. Dis. 2012;55:1012–1019. doi: 10.1093/cid/cis589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481:394–U183. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner SN, Daniels KA, Welsh RM. Therapeutic depletion of natural killer cells controls persistent infection. J. Virol. 2014;88:1953–1960. doi: 10.1128/JVI.03002-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RM, Waggoner SN. NK cells controlling virus-specific T cells: rheostats for acute vs. persistent infections. Virology. 2013;435:37–45. doi: 10.1016/j.virol.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CF, Klinzman D, Yamashita TE, et al. Persistent GB virus C infection and survival in HIV-infected men. N. Engl. J. Med. 2004;350:981–990. doi: 10.1056/NEJMoa030107. [DOI] [PubMed] [Google Scholar]
- Xiang JH, Wunschmann S, Diekema DJ, et al. Effect of coinfection with GB virus C on survival among patients with HIV infection. N. Engl. J. Med. 2001;345:707–714. doi: 10.1056/NEJMoa003364. [DOI] [PubMed] [Google Scholar]
- Xue Y, Li A, Wang L, Feng H, Yao X. PPSP: prediction of PK-specific phosphorylation site with Bayesian decision theory. BMC Bioinform. 2006;7:163. doi: 10.1186/1471-2105-7-163. [DOI] [PMC free article] [PubMed] [Google Scholar]