Abstract
Background
The World Health Organization recommended increased dosages of the first-line antituberculosis (anti-TB) drugs for children in 2010. We examined the frequency of and factors associated with low plasma maximum concentration (Cmax) of each drug in children treated with the revised dosages.
Methods
Children on anti-TB therapy for at least 4 weeks underwent pharmacokinetic testing. Plasma Cmax below the lower limit of proposed reference range was considered low. Bivariate and multivariate analyses were used to examine the factors associated with low Cmax of each drug.
Results
Of the 100 children, 58% were male, 50% HIV-infected and 49% younger than 5 years old. The median (IQR) Cmax was 5.9 (4.5 – 7.7) μg/mL for isoniazid, 6.5 (4.9 – 8.8) μg/mL for rifampin, 26.0 (21.2 – 33.4) μg/mL for pyrazinamide and 1.7 (0.9 – 2.7) μg/mL for ethambutol. There was a strong correlation between Cmax and AUC0–8h for all drugs. Low Cmax occurred in 9/100 (9.0%), 61/100 (61.0%), 17/97 (17.5%), and 60/97 (61.9%) for isoniazid, rifampin, pyrazinamide, and ethambutol, respectively. In addition, 75/97 (77.3%) children had pyrazinamide Cmax < 35 μg/mL. Factors associated with low Cmax were NAT2 metabolizer phenotype status for isoniazid; height, dosage, and HIV coinfection status for rifampin; height for pyrazinamide; and age, dosage and HIV coinfection status for ethambutol.
Conclusions
The high frequency of low rifampin and ethambutol Cmax in our study is consistent with emerging pharmacokinetic data in children treated according to the new WHO recommendations. Higher dosages than currently recommended especially for rifampin may be necessary in children.
Keywords: First-line antituberculosis drugs, maximum concentration, children, WHO revised dosages, Ghana
Introduction
Appropriate dosing of the antituberculosis (anti-TB) drugs in children is a significant problem in optimizing tuberculosis (TB) treatment outcomes. In the absence of direct pediatric pharmacokinetic-pharmacodynamic data, it is expected that effective anti-TB drugs in adults should be effective in children provided the recommended dosages lead to pharmacokinetic profile associated with efficacy and safety (1, 2). In vitro and animal studies suggest that antimycobacterial killing and prevention of acquired drug resistance of the first-line drugs is dependent on the area under the time curve to minimal inhibitory concentration (AUC/MIC) ratio or maximum concentration to MIC (Cmax/MIC) ratio (3–6). A number of clinical studies in adults have also shown that early bactericidal activity (EBA) is dependent on drug dosage or Cmax for isoniazid (7, 8) and dosage of rifampin or rifapentine (9). In addition, two recent studies showed that, in humans, higher rifampicin dosage or exposure leads to better outcomes (10, 11).
In practice, normal ranges of 2-hour post-dose serum concentration (C 2-h) of the first-line anti-TB drugs (an approximate surrogate for Cmax) in adults have been widely used for therapeutic drug monitoring (TDM), with C 2-h below the lower limit of the reference range of each drug considered to be low (12). Previous dosing of the first-line anti-TB drugs in children using similar dosages in mg/kg as in adults led to a large proportion of children with low Cmax (13–18). With the concern that low C 2-h or Cmax of the anti-TB drugs was associated with poor treatment outcomes, the World Health Organization (WHO) recommended increased dosages of all first-line anti-TB drugs in children in 2010 (19). As the WHO recommendations were not based on direct pharmacokinetic data, it is essential to examine the adequacy of the revised dosages in pharmacokinetic and safety studies in children.
Pharmacokinetic studies that have examined the adequacy of the revised dosages in children to date have reported conflicting findings. One study found that the revised dosages achieved target plasma Cmax of isoniazid, rifampin and pyrazinamide in a vast majority of South African children aged < 2 years old (20). However, subsequent studies, one among 31 children aged ≤ 10 years old found that 94%, 85% and 45% of the children had sub-therapeutic C 2-h of rifampin, ethambutol and pyrazinamide respectively (21), while the other in infants younger than 12 months old reported that none of the participants achieved target rifampin Cmax, and only one of the 16 infants (6%) achieved target ethambutol Cmax (22). In a preliminary study of the revised dosages in Ghanaian children, we found that most children achieved the target plasma Cmax of isoniazid and pyrazinamide but 52% and 59% of participants who received revised dosages had low Cmax for ethambutol and rifampin, respectively (23). The current analysis, which included patients in our previous publication is the largest study to date (to the best of our knowledge) to examine the frequency low Cmax of the four first-line anti-TB drugs in children treated according the current WHO guidelines. In addition, we investigated the factors associated with low Cmax of each of drug.
Materials and Methods
Study population and design
Children aged 3 months to 14 years old with clinical diagnosis of active TB were enrolled at Komfo Anokye Teaching Hospital (KATH), Kumasi, Ghana between October 2012 and August 2015. Active TB was defined as the presence of clinical symptoms, signs, radiographic findings that are consistent with active TB, with or without positive acid-fast bacilli (AFB) in sputum or tissue samples. In addition, lymph node biopsy sample showing granuloma with or without a positive AFB was considered as active TB. No Mycobacterial cultures were available at the study site or were done in this study. Eligible children were excluded if their parents refused to participate or could not return for scheduled study visits or did not receive the revised weight-band dosages. The Institutional Review Board (IRB) of KATH, Ghana and Lifespan Hospitals, Providence, Rhode Island reviewed and approved the study. All parents or guardians of the study participants provided signed informed consent. The study was registered with ClinicalTrials.gov, number NCT01687504.
Study procedures
All participants were given a regimen consisting of isoniazid (7 to 15 mg/kg), rifampin 10 – 20 mg/kg), pyrazinamide (30 – 40 mg/kg) and ethambutol (15 – 25 mg/kg) daily for 2 months then isoniazid and rifampin daily for 4 months. The medications were dosed according to WHO guidelines for using available dispersible fixed-dose combination (FDC) tablets for children in the weight bands 5 – 7 kg, 8 – 14 kg, 15 – 20 kg, 21 – 30 kg, 31 – 39 kg and 40 – 54 kg (24). The FDC tablets containing rifampin 60 mg, isoniazid 30 mg and pyrazinamide 150 mg; rifampin 60 mg and isoniazid 30 mg; and rifampin 60 mg and isoniazid 60 mg were used. Ethambutol 100 mg tablets were used. The isoniazid/rifampin/pyrazinamide dispersible FDC tablets given to children with body weight < 21 kg were manufactured by MaCleods Pharmaceuticals, Mumbai, India, and the ethambutol tablets by Riemser Pharma GmbH, Schiffweiler, Germany. Isoniazid/rifampin/pyrazinamide/ethambutol FDC tablets given to children with body weight ≥ 21 kg were manufactured by Lupin Ltd, Chkalthana, Aurangabad, India. All the medications were supplied through the WHO Global TB Drug Facility and were dosed according to WHO guidelines for using available dispersible fixed-dose combination (FDC) TB medicines for children (24). Given the ratio of pyrazinamide in the FDC tablet, it was not possible to achieve the revised dosage range in all children. The medications were either swallowed or dispersed in water in a plastic cup. Ingestion of medications after an overnight fast was observed by a healthcare worker during hospitalization and by a family member at home.
Pharmacokinetic sampling was performed after at least 4 weeks of anti-TB treatment as previously described (23). Blood samples were collected at times 0 (pre-dose), 1, 2, 4 and 8-hours after observed dosing in the hospital. The samples collected in EDTA-coated tubes were placed immediately on ice and centrifuged within 30 minutes at 3000 g for 10 minutes. Plasma was stored at − 80°C until shipment on dry ice to University of Cape Town, Cape Town, South Africa for drug concentrations assays.
Pharmacokinetic analysis
Drug concentrations were determined using validated liquid chromatography tandem with mass spectrometry (LC/MS/MS) methods as we previously described (23). The observed Cmax and time to Cmax (Tmax) were determined by inspection of the serum concentration-time graphs for each drug and AUC from time 0 to 8 hours (AUC0–8h) was calculated using noncompartmental analysis (Phoenix Software; Pharsight Corporation, Mountain View, CA). The observed Cmax of each drug below the proposed lower limit of the following references ranges in adults were considered to be low: isoniazid 3 to 6 μg/mL, rifampin 8 to 24 μg/mL and pyrazinamide 20 to 50 μg/mL and ethambutol 2 to 6 μg/mL (12). Pyrazinamide plasma Cmax < 35 μg/mL was considered as low in secondary analysis as that cut off was associated with poor treatment outcome in one study in predominantly HIV-infected adults in Botswana (25)
N-acetyltransferase-2 (NAT2) genotyping
Genotyping for NAT2 SNPs rs1801279 (191G→A), rs1801280 (341T→C), rs1799930 (590G→A) and rs1799931 (857G→A) was performed using validated TaqMan® real-time PCR assays. Samples homozygous wild-type for all SNPs were classified as rapid, those heterozygous for any one of the SNPs were classified intermediate, and those homozygous variant for one or more SNPs or heterozygous for two or more SNPs were classified slow acetylator genotypes according established criteria (26).
Sample size justification
The sample size calculation was based on a primary endpoint that compared drugs Cmax in children with TB with or without HIV coinfection as described in our previous publication (27). We assumed that a Cmax coefficient of variation of 0.35 to 0.60 based on available literature, a sample size of 23 to 64 per group (or 46 to 128) was needed to detect a 30% or greater difference in each drug Cmax between the two groups, with the minimum power of 80%. Overall, the study enrolled 113 patients, 55 with TB alone and 59 with HIV/TB coinfection (27). In the current paper, we excluded 13 patients who received drug dosages based on the old WHO recommendations, leaving 100 patients in the final analysis.
Statistical analysis
Statistical analyses were performed using Software R for Windows 3.3.1 and SAS 9.4 (SAS Institute Inc, Cary, NC). Weight-for-age Z score (WAZ), height-for-age Z score (HAZ) and body mass index (BMI)-for age were calculated based on the United States National Center for Health Statistics (NCHS) reference median values using statistical macros for children ages < 5 years old and 5 – 19 year old provided by the WHO.(28)
Bivariate analysis including Wilcoxon rank sum test and Fisher exact test was used to assess the marginal effect of demographic and clinical variables on whether Cmax falls in reference ranges. The joint effects of patient factors (HIV coinfection, sex, age, height, weight-adjusted dose and isoniazid –specific 4-SNP NAT2 non-slow metabolizing phenotype status) on probability of having low Cmax was explored by logistic regression with variable selection by the Minimax Concave Penalty (MCP) method (29, 30) to improve the efficiency of inference. Given that Cmax is the pharmacokinetic parameter used in therapeutic drug monitoring, we examined the correlation between Cmax and AUC0–8h as well with C 2-h using Pearson correlation analysis and confirmed the association by Spearman Correlation. For all analyses, a P value < 0.05 was considered significant.
Results
Study population
Of 113 children enrolled during the study period, 13 who received dosages according previous WHO TB treatment guidelines were excluded. Of the 100 study participants who were treated according to the 2010 revised dosage guidelines, 50.0% had HIV coinfection, 58.0% were male, 49.0% aged < 5 years old and 23.0% aged < 2 years old. The median (range) dosages of the drugs given were: isoniazid 11.4 (9.7 – 12.9) mg/kg, rifampin 16.5 (14.4 – 19.0) mg/kg, pyrazinamide 25.9 (22.6 – 30.4) mg/kg and ethambutol 17.2 (15.1 – 20.6) mg/kg. Overall, 86 children completed anti-TB treatment, 12 were lost to follow-up or discontinued the study and two died.
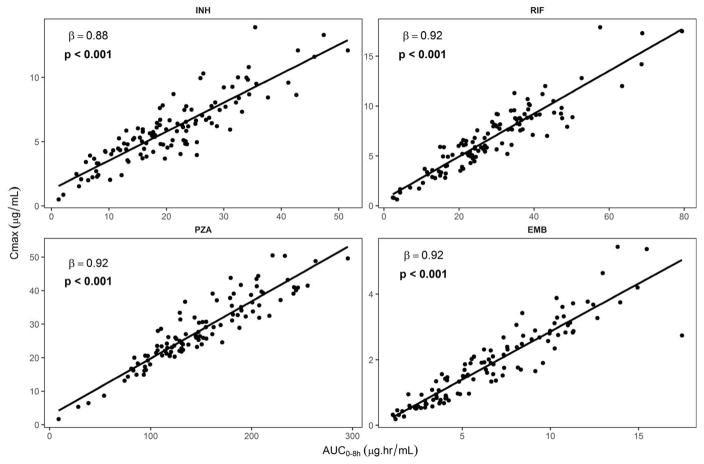
The median (IQR) plasma Cmax for isoniazid was 5.9 (4.5 – 7.7) μg/mL, for rifampin 6.5 (4.9 – 8.8) μg/mL, for pyrazinamide 26.0 (21.2 – 33.4) μg/mL and for ethambutol 1.7 (0.9 – 2.7) μg/mL. The median (IQR) AUC0–8hr for isoniazid was 21.2 (15.2 – 27.9) μg.hr/mL, for rifampin 27.5 (21.0 – 37.4) μg.hr/mL, for pyrazinamide 134.9 (110.8 – 179.4) μg.hr/mL, and for ethambutol 6.1 (3.8 – 9.6) μg.hr/mL. As shown in Fig. 1, there was strong linear correlation between plasma Cmax and AUC0–8hr for all the drugs studied. There was also a strong linear correlation between plasma Cmax and C 2-h for all the drugs (SDC Fig. 1), as well as between AUC0–8h and C 2-h (SDC Fig. 2). Among the children that we could establish Cmax and C 2-h, Cmax was either equal to or greater than but never less than C 2-h (SDC Table 1). Overall, Cmax was equal to C 2-h in 67% and 52%, respectively for ethambutol and rifampin (SDC Table 1).
Fig. 1.
Relationship between plasma Cmax and AUC0–8h of isoniazid (INH), rifampin (RIF), pyrazinamide (PZA) and ethambutol (EMB) in children with tuberculosis.
Proportion of children with low anti-TB drug Cmax
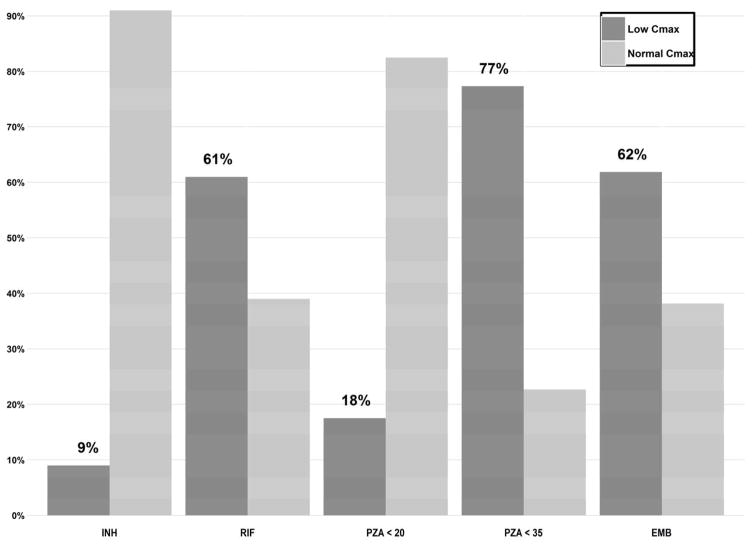
Of the 100 children, three each had undetectable pyrazinamide and ethambutol concentrations at all the sampling points. Overall, 9/100 (9.0%) had low isoniazid Cmax, 61/100 (61%) had low rifampin Cmax, 17/97 (17.5%) had low pyrazinamide Cmax, and 60/97 (61.8%) had low ethambutol Cmax (Fig. 2). Of the 97 children who had detectable concentrations of all the four first-line drugs, 42 (43.3%) had low Cmax of rifampin and ethambutol and 3 (3.1%) had low Cmax of all the drugs. If C 2-h is used as the pharmacokinetic parameter, 20/93 (21.5%) had low isoniazid C 2-h, 65/93 (69.9%) had low rifampin C 2-h, 27/91 (29.7%) had low pyrazinamide C 2-h, and 59/90 (65.6%) had low ethambutol C 2-h.
Fig. 2.
Proportion of children with plasma Cmax of isoniazid (INH) < 3 μg/mL, rifampin (RIF) < 8 μg/mL and pyrazinamide (PZA) < 20 μg/mL, PZA < 35 μg/mL and ethambutol (EMB) < 2 μg/mL.
Using other published thresholds, 75/97 (77.3%) of the children had pyrazinamide Cmax < 35 μg/mL (Fig. 2), a cut off that was associated with poor treatment outcome in one study in predominantly HIV-infected adults in Botswana (25). Also 81/97 (83.5%) of the children had pyrazinamide Cmax < 38.1 μg/mL, a recently proposed statistically derived threshold of pyrazinamide associated with treatment failure and death in Indian children with TB (31). Of the 81 children with pyrazinamide Cmax < 38.1 μg/mL, 43 (53.1%) had rifampin Cmax < 6.20 μg/mL, a rifampin threshold considered to be associated with poor outcome in the study in Indian children (31).
Factors associated with low Cmax of the four first-line drugs
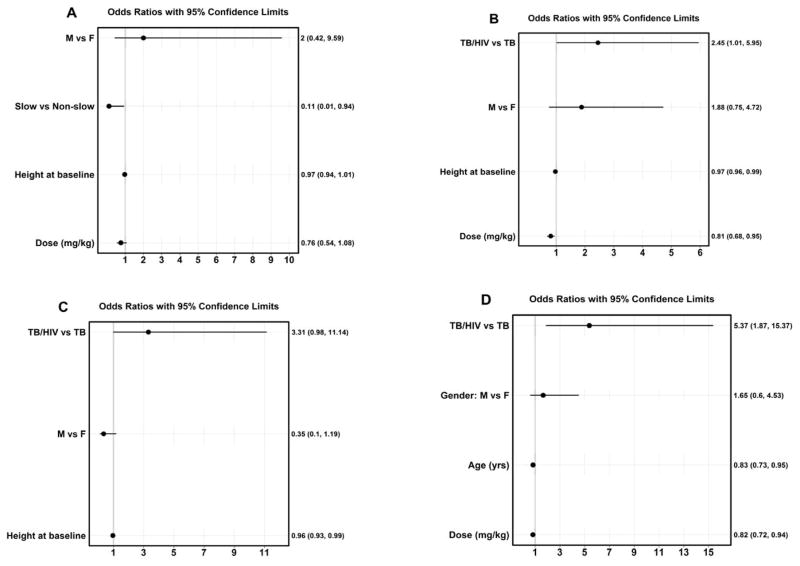
The factors associated with low Cmax of each drug were examined using bivariate (Tables 1 – 4) and multivariate analysis (Fig. 3). For isoniazid, children with 4-SNP NAT2 non-slow (intermediate/fast) compared to those with slow metabolizing phenotype were more likely to have low isoniazid Cmax in the bivariate analysis (Table 1) as well as in the multivariate analysis (odds ratio (OR), 0.11; 95% confidence interval (CI), 0.01 – 0.94) (Table 1, Fig 3A).
Table 1.
Factors associated with isoniazid plasma concentrations below target maximum concentration
| Characteristic | Cmax ≥ 3 μg/mL (N = 91) | Cmax < 3 μg/mL (N = 9) | |
|---|---|---|---|
| Median (IQR) age (years) | 5.0 (2.2 – 8.7) | 2.2 (1.0 – 7.9) | 0.224 |
| Median (IQR) body weight (Kg) | 14.0 (9.0 – 19.2) | 9.1 (5.5 – 17.5) | 0.300 |
| Median (IQR) height (Cm) | 99.0 (84.0 – 120.0) | 84.0 (63.0 – 111.0) | 0.211 |
| Nutritional status | |||
| Median (IQR) weight-for-age Z score* | −2.5 ( −3.8 – −1.3) | −2.9 ( −4.1 – −2.8) | 0.122 |
| Median (IQR) height-for-age Z score | −2.0 ( −3.1 – −1.0) | −2.2 ( −4.2 – −1.5) | 0.601 |
| Median BMI-for-age Z score | −2.0 (−3.1 – −1.0) | −2.2 ( −4.2 – −1.5) | 0.981 |
| Median (IQR) mid-arm circumference | 13.6 (12.3 – 16.0) | 12.3 (10.1 – 17.3) | 0.448 |
| Median (IQR) drug dose (mg/kg) | 11.5 (9.8 – 12.9) | 11.2 (9.4 – 11.8) | 0.674 |
| Age (years) n (%) | 0.205 | ||
| < 2 | 19 (82.6%) | 4 (17.4%) | |
| ≥ 2 | 72 (93.5%) | 5 (6.5%) | |
| Age (years) n (%) | 0.313 | ||
| < 5 | 43 (87.8%) | 6 (12.2%) | |
| ≥ 5 | 48 (94.1%) | 3 (5.9%) | |
| Sex | 0.730 | ||
| Female | 39 (92.9%) | 3 (7.1%) | |
| Male | 52 (89.7%) | 6 (10.3%) | |
| HIV positive | 1.000 | ||
| No | 45 (90.0%) | 5 (10.0%) | |
| Yes | 46 (92.0%) | 4 (8.0%) | |
| NAT2 4-SNP phenotype status | 0.052 | ||
| Fast | 10 (90.9%) | 1 (9.1%) | |
| Intermediate | 37 (84.1%) | 7 (15.9%) | |
| Slow | 44 (97.8%) | 1 (2.2%) | |
| NAT2 4-SNP phenotype status | 0.039 | ||
| Fast/intermediate | 47 (85.5%) | 8 (14.5%) | |
| Slow | 44 (97.8%) | 1 (2.2%) | |
Table 6.
Factors associated with ethambutol plasma concentrations below target maximum concentration
| Characteristic | Cmax ≥ 2 μg/mL (N = 37) | Cmax < 2 μg/mL (N = 60) | P-value |
|---|---|---|---|
| Median (IQR) age (years) | 7.3 (4.0 – 9.8) | 4.1 (1.6 – 7.0) | 0.006 |
| Median (IQR) body weight (Kg) | 16.3 (11.9 – 21.8) | 12.8 (7.2 – 18.0) | 0.003 |
| Median (IQR) height (Cm) | 107.0 (93.0 – 129.0) | 94.0 (75.0 – 114.0) | 0.007 |
| Nutritional status | |||
| Median (IQR) weight-for-age Z score* | −2.0 (−2.8 – −1.0) | −2.7 (−4.0 – −1.6) | 0.067 |
| Median (IQR) height-for-age Z score | −1.9 (−3.0 – −1.5) | −2.2 (−3.1 – −0.9) | 0.865 |
| Median (IQR) BMI-for-age Z score | −1.9 (−3.0 – −1.5) | −2.2 (−3.1 – −0.9) | 0.136 |
| Median (IQR) mid-arm circumference | 14.3 (13.0 – 16.8) | 13.0 (11.0 – 15.0) | 0.037 |
| Median (IQR) drug dose (mg/kg) | 18.8 (16.9 – 22.9) | 15.9 (14.7 – 17.5) | < 0.001 |
| Age (years) n (%) | 0.046 | ||
| < 2 | 4 (19.0%) | 17 (81.0%) | |
| ≥ 2 | 33 (43.4%) | 43 (56.6%) | |
| Age (years) n (%) | 0.313 | ||
| < 5 | 13 (27.7%) | 34 (72.3%) | |
| ≥ 5 | 24 (48.0%) | 26 (52.0%) | |
| Sex | 0.527 | ||
| Female | 18 (42.9%) | 24 (57.1%) | |
| Male | 19 (34.5%) | 36 (65.5%) | |
| HIV positive | 0.003 | ||
| No | 26 (53.1%) | 23 (46.9%) | |
| Yes | 11 (22.9%) | 37 (77.1%) | |
Fig. 3.
Factors associated with plasma Cmax of the first-line anti-TB drugs in children identified in multivariate analysis.
Children with low rifampin Cmax compared to those with Cmax within the reference range were more likely to have received a lower median rifampin dose (15.5 mg/kg vs. 17.6 mg/kg) or have a lower HAZ (−2.6 vs. −1.6) (Table 2). In the multivariate analysis, HIV coinfection status (OR, 2.45; 95% CI 1.01 – 5.95), height (OR, 0.97; 95% CI, 0.96 – 0.99) and rifampin dose (OR, 0.81; 95% CI, 0.68 – 0.95) were associated with low rifampin Cmax (Fig 3B).
Table 4.
Factors associated with rifampin plasma concentrations below target maximum concentration
| Characteristic | Cmax ≥ 8 μg/mL (N = 39) | Cmax < 8 μg/mL (N = 61) | P-value |
|---|---|---|---|
| Median (IQR) age (years) | 6.9 (2.4 – 8.9) | 4.6 (2.0 – 7.0) | 0.264 |
| Median (IQR) body weight (Kg) | 16.3 (8.8 – 21.0) | 13.0 (8.5 – 17.4) | 0.101 |
| Median (IQR) height (Cm) | 106.0 (84.0 – 123.0) | 94.0 (82.0 – 111.0) | 0.070 |
| Nutritional status | |||
| Median (IQR) weight-for-age Z score* | −2.3 (−3.7 – −1.0) | −2.8 (−4.0 – −1.6) | 0.280 |
| Median (IQR) height-for-age Z score | −1.6 (−2.8 – −0.5) | −2.6 (−3.4 – −1.7) | 0.004 |
| Median (IQR) BMI-for-age Z score | −1.6 (−2.8 – −0.5) | −2.6 (−3.4 – −1.7) | 0.538 |
| Median (IQR) mid-arm circumference | 13.8 (12.9 – 16.5) | 13.3 (11.2 – 15.0) | 0.369 |
| Median (IQR) drug dose (mg/kg) | 17.6 (15.8 – 19.0) | 15.5 (14.1 – 18.8) | 0.043 |
| Age (years) n (%) | 0.808 | ||
| < 2 | 8 (34.8%) | 15 (65.2%) | |
| ≥ 2 | 31 (40.3%) | 46 (59.7%) | |
| Age (years) n (%) | 0.224 | ||
| < 5 | 16 (32.7%) | 33 (67.3%) | |
| ≥ 5 | 23 ((45.1%) | 28 (54.9%) | |
| Sex | 0.305 | ||
| Female | 19 (45.2%) | 23 (54.8%) | |
| Male | 20 (34.5%) | 38 (65.5%) | |
| HIV positive | 0.100 | ||
| No | 24 (48.0%) | 26 (52.0%) | |
| Yes | 15 (30.0%) | 35 (70.0%) | |
For pyrazinamide, children with low Cmax were more likely than those with Cmax within the reference range to have a lower median age (1.7 vs. 5.0 years old), body weight (7.5 vs. 14.1 kg) or shorter height (77.0 vs. 99.5 cm) (Table 3). In the multivariate model, only height (OR, 0.96; 95% CI, 0.93 – 0.99) was significantly associated with low pyrazinamide Cmax (Fig. 3C).
Table 5.
Factors associated with pyrazinamide plasma concentrations below target maximum concentration
| Characteristic | Cmax ≥ 20 μg/mL (N = 80) | Cmax < 20 μg/mL (N = 17) | P-value |
|---|---|---|---|
| Median (IQR) age (years) | 5.0 (2.6 – 9.0) | 1.7 (1.0 – 4.8) | 0.006 |
| Median (IQR) body weight (Kg) | 14.1 (9.6 – 19.7) | 7.5 (6.3 – 16.0) | 0.028 |
| Median (IQR) height (Cm) | 99.5 (84.0 – 121.5) | 77.0 (72.0 – 104.0) | 0.006 |
| Nutritional status | |||
| Median (IQR) weight-for-age Z score* | −2.6 (−3.8 – −1.5) | −2.6 (−4.0 – −1.3) | 0.927 |
| Median (IQR) height-for-age Z score | −1.9 (−3.0 – −1.0) | −2.9 (−3.3 – −1.5) | 0.319 |
| Median (IQR) BMI-for-age Z score | −1.9 (−3.0 – −1.0) | −2.9 (−3.3 – −1.5) | 0.473 |
| Median (IQR) mid-arm circumference | 13.5 (12.3 – 16.0) | 13.0 (10.6 – 14.3) | 0.232 |
| Median (IQR) drug dose (mg/kg) | 25.9 (22.8 – 31.6) | 24.6 (22.4 – 28.8) | 0.317 |
| Age (years) n (%) | < 0.001 | ||
| < 2 | 13 (56.5%) | 10 (43.5%) | |
| ≥ 2 | 67 (90.5%) | 7 (9.5%) | |
| Age (years) n (%) | 0.017 | ||
| < 5 | 35 (72.9%) | 13 (27.1%) | |
| ≥ 5 | 45 (91.8%) | 4 (8.2%) | |
| Sex | 0.419 | ||
| Female | 32 (78.0%) | 9 (22.0%) | |
| Male | 48 (85.7%) | 8 (14.3%) | |
| HIV positive | 0.108 | ||
| No | 43 (89.6%) | 5 (10.4%) | |
| Yes | 37 (75.5%0 | 12 (24.5%) | |
For ethambutol, children with low Cmax compared to those with Cmax in the proposed reference range were more likely to have a lower median age (4.1 vs. 7.3 years old), lower body weight (12.8 vs. 16.3 kg), shorter height (94.0 vs. 107 cm), shorter mid-arm circumference (13.0 vs. 14.3 cm) or received a lower median dosage (15.9 mg/kg vs. 18.8 mg/kg) (Table 4). In the multivariate analysis, HIV coinfection status (OR, 5.37; 95% CI, 1.87 – 15.37), age (OR, 0.83; 95% CI, 0.73 – 0.95) and ethambutol dose (OR, 0.82; 95% CI, 0.72 – 0.92) were associated with low Cmax (Fig. 3D).
DISCUSSION
At least one million children develop active TB each year, with an estimated 210,000 deaths in 2015 (32). Optimizing the dosages of anti-TB drugs in children is challenging given the lack of reliable pediatric pharmacokinetic-pharmacodynamic data to inform dosing decisions. With the implementation of WHO revised dosages of the first-line anti-TB drugs for children, pharmacokinetic studies that sought to determine whether most children achieve plasma Cmax within proposed reference ranges have reported conflicting findings (20–23). In the current study, we found that a majority of children in the study achieved Cmax of isoniazid and pyrazinamide within proposed reference ranges (12), but at least 60% of the children had low rifampin or ethambutol Cmax. Our finding of a high frequency of low rifampin and ethambutol Cmax despite using currently recommended dosages is consistent with those of two other published studies from South Africa (21, 22), but differs from findings of one study which found the revised dosages of isoniazid, rifampin and pyrazinamide to be adequate (20). Overall, the emerging data (SDC Table 2) suggest that the revised dosages of the first-line drugs for children failed to achieve desired plasma Cmax or C 2-h except for isoniazid.
In assessing the adequacy of the current WHO recommended dosages of the first-line drugs, we used proposed reference C 2-h ranges to define low drug concentrations (12, 33) as optimal pharmacokinetic parameters associated with unfavorable clinical outcomes (especially in children) are not well established. We did not have adequate variability in clinical outcomes in our study population to evaluate the relationship between the administered dosages and clinical effect. Some clinical studies have reported a significant relationship between pyrazinamide Cmax or C 2-h and TB treatment outcome but the thresholds have not been validated (25, 34, 35). For isoniazid, rifampin and ethambutol, there is very limited direct data on the relationship between plasma Cmax and clinical outcome. For example, one retrospective study among adults with TB in Canada found that 2-month sputum conversion rate was associated with low isoniazid Cmax and a trend toward low rifampin Cmax (36) but prospective data are lacking.
Rifampin and pyrazinamide are critical components of current short-course chemotherapy that allowed for anti-TB treatment to be shortened from 24 to 6 months (37). For rifampin, our finding of at least 60% with low plasma Cmax is concerning as low exposure to the rifamycins in particular have been associated with TB treatment failure and emergence of rifamycin resistance (38–40). A comprehensive review of published data on the pharmacokinetics and pharmacodynamics of rifampin in adults and children that applied straight line regression of Cmax on dose reported that children established on rifampin require approximately twice the mg/kg body weight dosage of rifampin as in adults to reach serum concentrations equivalent to adults (41). Thus, if adults need higher rifampin dosages to improve anti-TB treatment outcomes or shorten treatment duration (10, 11, 42), then children will likely need higher than currently recommended dosages too. For pyrazinamide, the inability to achieve Cmax > 35 μg/mL in most children in our study was likely due to under dosing, as it is not possible to give the recommended dosage range using the available rifampin/isoniazid/pyrazinamide FDC tablets. Even with the newer formulation of rifampin/isoniazid/pyrazinamide 75/50/150 FDC tablet and new revised weight-band dosing guidelines recommended by WHO (43), children at the upper end of the weight-bands (except for the 12 – 15 kg) will be under dosed. Thus, newer FDC tablets with appropriate proportion of pyrazinamide are needed.
Low ethambutol Cmax was common in our study population based on the proposed reference range but whether an increase in dosage is necessary is unclear as there are no direct pharmacokinetic-pharmacodynamic data. Although ethambutol optic toxicity in adults is dose related, for severe disease in children, the upper end of the dosages range of 15 to 25 mg/kg could be used as toxicity in children given a dose of 15 – 30 mg/kg was rare (13). Also, if ethambutol is used in the setting of high prevalence of isoniazid resistance, in the absence of other alternatives, the higher recommended dosage of 25 mg/kg can be used with careful age-appropriate monitoring for optic toxicity as ethambutol efficacy is dosage related (44).
We investigated the factors associated low Cmax of each drug. In the multivariate analysis, only NAT2 metabolizing phenotype status was associated low isoniazid Cmax and height was associated with low pyrazinamide Cmax. HIV coinfection status and drug dosage were associated with low Cmax of rifampin and ethambutol. In addition, height was associated with rifampin Cmax and age with low ethambutol Cmax. While we do not propose individualized dosing of the anti-TB drugs based on the above factors as it is not practical, the significant factors should be considered when pharmacologic modeling is used to determine the appropriate dosages of the drugs in children at the population level. The finding that lower dosages were associated with low Cmax of rifampin and ethambutol suggest that a dose increase for these drugs could improve pharmacokinetic profiles in children if deemed necessary from based on clinical efficacy studies.
Another important finding of our study is that plasma Cmax or C 2-h was reflective of AUC0–8h for all the four drugs. Thus, when AUC cannot be performed, especially in large clinical studies, Cmax or C 2-h could be used as a surrogate pharmacokinetic parameter for drug exposure. Overall, Cmax tended to be higher than C 2-h in some patients especially for especially for isoniazid, pyrazinamide and about half of the time for rifampin. Thus, pharmacokinetic-pharmacodynamic studies designed to identify the best parameter most closely associated with efficacy should include both parameters.
Our study has some limitations. The sparse pharmacokinetic sampling could have missed the true Cmax of the drugs in some patients especially between the 2 to 4-hour or 4 to 8-hour sampling windows. However, such sampling schemes have been used in the other pediatric studies that we compared our results to (21, 22, 45). As discussed above, our findings in relation to pyrazinamide could be due to under-dosing because of the use of currently available FDC tablets and weight-band dosing and does not truly assess the recommended dosage range of 30 – 40 mg/kg. Also, while drug dosages were observed by the study team on the day of pharmacokinetic sampling, poor medication adherence at home prior to admission for sampling could have also affected the drug concentrations. Finally, we were unable to examine the relationship between the drugs Cmax and clinical outcome as majority of children in the study had smear negative TB and severely ill children who were likely to die were excluded from the study. Notwithstanding the above limitation, the findings of this study showed that current WHO weight-band dosage recommendations of the first-line anti-TB drugs (except for isoniazid) did not achieve desirable Cmax or C 2-h of rifampin and ethambutol in most children. While pharmacokinetic studies have so far provided important data, clinical efficacy studies of the WHO revised pediatric dosages are essential to adequately inform dosing decisions aimed at optimizing TB treatment outcomes in children.
Acknowledgments
We thank the study participants, the supportive staff of the TB and HIV clinics and the malnutrition ward at KATH who helped with patient enrolment. The study funded by grant support from Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) at the National Institutes of Health (Award # HD071779). Laboratory research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases (NIAID) (U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) (AI068632) (University of Cape Town pharmacology laboratory). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Source of support: This work was funded by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) at the National Institutes of Health [Award # HD071779, PI, AK]. Additional support from National Institutes of Health [Award #s AI068632 LW, AI042853 FG, and AI1078498, HY].
Footnotes
Previous presentation: Part of the data was presented at the 46th Union World Conference on Lung Health, Cape Town, South Africa. December 2 – 6, 2015. [Oral presentation, # OA-424-05].
Disclosures: The authors have no conflicts of interest to disclose.
All authors report no conflicts of interest.
References
- 1.Donald PR, Ahmed A, Burman WJ, et al. Requirements for the clinical evaluation of new anti-tuberculosis agents in children. Int J Tuberc Lung Dis. 2013;17:794–799. doi: 10.5588/ijtld.12.0567. [DOI] [PubMed] [Google Scholar]
- 2.Schaaf HS, Garcia-Prats AJ, Donald PR. Antituberculosis drugs in children. Clin Pharmacol Ther. 2015;98:252–265. doi: 10.1002/cpt.164. [DOI] [PubMed] [Google Scholar]
- 3.Gumbo T, Dona CS, Meek C, Leff R. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob Agents Chemother. 2009;53:3197–3204. doi: 10.1128/AAC.01681-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gumbo T, Louie A, Deziel MR, et al. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob Agents Chemother. 2007;51:3781–3788. doi: 10.1128/AAC.01533-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gumbo T, Louie A, Liu W, et al. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob Agents Chemother. 2007;51:2329–2336. doi: 10.1128/AAC.00185-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayaram R, Gaonkar S, Kaur P, et al. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother. 2003;47:2118–2124. doi: 10.1128/AAC.47.7.2118-2124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coates AR, Hu Y, Jindani A, Mitchison DA. Contradictory results with high-dosage rifamycin in mice and humans. Antimicrob Agents Chemother. 2013;57:1103. doi: 10.1128/AAC.01705-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donald PR, Sirgel FA, Botha FJ, et al. The early bactericidal activity of isoniazid related to its dose size in pulmonary tuberculosis. Am J Respir Crit Care Med. 1997;156:895–900. doi: 10.1164/ajrccm.156.3.9609132. [DOI] [PubMed] [Google Scholar]
- 9.Sirgel FA, Fourie PB, Donald PR, et al. The early bactericidal activities of rifampin and rifapentine in pulmonary tuberculosis. Am J Respir Crit Care Med. 2005;172:128–135. doi: 10.1164/rccm.200411-1557OC. [DOI] [PubMed] [Google Scholar]
- 10.Boeree MJ, Heinrich N, Aarnoutse R, et al. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis. 2017;17:39–49. doi: 10.1016/S1473-3099(16)30274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorman SE, Savic RM, Goldberg S, et al. Daily rifapentine for treatment of pulmonary tuberculosis. A randomized, dose-ranging trial. Am J Respir Crit Care Med. 2015;191:333–343. doi: 10.1164/rccm.201410-1843OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs. 2002;62:2169–2183. doi: 10.2165/00003495-200262150-00001. [DOI] [PubMed] [Google Scholar]
- 13.Donald PR, Maher D, Maritz JS, Qazi S. Ethambutol dosage for the treatment of children: literature review and recommendations. Int J Tuberc Lung Dis. 2006;10:1318–1330. [PubMed] [Google Scholar]
- 14.Graham SM, Bell DJ, Nyirongo S, Hartkoorn R, Ward SA, Molyneux EM. Low levels of pyrazinamide and ethambutol in children with tuberculosis and impact of age, nutritional status, and human immunodeficiency virus infection. Antimicrob Agents Chemother. 2006;50:407–413. doi: 10.1128/AAC.50.2.407-413.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussels H, Kroening U, Magdorf K. Ethambutol and rifampicin serum levels in children: second report on the combined administration of ethambutol and rifampicin. Pneumonologie. 1973;149:31–38. doi: 10.1007/BF02179950. [DOI] [PubMed] [Google Scholar]
- 16.McIlleron H, Willemse M, Werely CJ, et al. Isoniazid plasma concentrations in a cohort of South African children with tuberculosis: implications for international pediatric dosing guidelines. Clin Infect Dis. 2009;48:1547–1553. doi: 10.1086/598192. [DOI] [PubMed] [Google Scholar]
- 17.Schaaf HS, Willemse M, Cilliers K, et al. Rifampin pharmacokinetics in children, with and without human immunodeficiency virus infection, hospitalized for the management of severe forms of tuberculosis. BMC Med. 2009;7:19. doi: 10.1186/1741-7015-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaaf HS, Parkin DP, Seifart HI, et al. Isoniazid pharmacokinetics in children treated for respiratory tuberculosis. Arch Dis Child. 2005;90:614–618. doi: 10.1136/adc.2004.052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. [Last accessed 9/29/16];Rapid advice: treatment of tuberculosis in children. 2010 Avaialable at: http://apps.who.int/iris/bitstream/10665/44444/1/9789241500449_eng.pdf. [PubMed]
- 20.Thee S, Seddon JA, Donald PR, et al. Pharmacokinetics of isoniazid, rifampin, and pyrazinamide in children younger than two years of age with tuberculosis: evidence for implementation of revised World Health Organization recommendations. Antimicrob Agents Chemother. 2011;55:5560–5567. doi: 10.1128/AAC.05429-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiruy H, Rogers Z, Mbowane C, et al. Subtherapeutic concentrations of first-line anti-TB drugs in South African children treated according to current guidelines: the PHATISA study. J Antimicrob Chemother. 2015;70:1115–1123. doi: 10.1093/jac/dku478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bekker A, Schaaf HS, Draper HR, et al. Pharmacokinetics of Rifampin, Isoniazid, Pyrazinamide, and Ethambutol in Infants Dosed According to Revised WHO-Recommended Treatment Guidelines. Antimicrob Agents Chemother. 2016;60:2171–2179. doi: 10.1128/AAC.02600-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwara A, Enimil A, Gillani FS, et al. Pharmacokinetics of First-Line Antituberculosis Drugs Using WHO Revised Dosage in Children With Tuberculosis With and Without HIV Coinfection. J Pediatric Infect Dis Soc. 2016;5:356–365. doi: 10.1093/jpids/piv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO. Dosing instructions for the use of currently available fixed-dose combination TB medicines for children. 2009:1–12. [Google Scholar]
- 25.Chideya S, Winston CA, Peloquin CA, et al. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis. 2009;48:1685–1694. doi: 10.1086/599040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hein DW, Doll MA. Accuracy of various human NAT2 SNP genotyping panels to infer rapid, intermediate and slow acetylator phenotypes. Pharmacogenomics. 2012;13:31–41. doi: 10.2217/pgs.11.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antwi S, Yang H, Enimil A, et al. Pharmacokinetics of the First-Line Antituberculosis Drugs in Ghanaian Children with Tuberculosis with or without HIV Coinfection. Antimicrob Agents Chemother. 2017:61. doi: 10.1128/AAC.01701-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO. Technical report series no. 854. World Health Organization; Geneva, Swizerland: 2011. Physical status: the use and interpretation of anthropometry. Report of a WHO expert committee; pp. 1–463. [PubMed] [Google Scholar]
- 29.Breheny P, Huang J. Coordinate Descent Algorithms for Nonconvex Penalized Regression, with Applications to Biological Feature Selection. Ann Appl Stat. 2011;5:232–253. doi: 10.1214/10-AOAS388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C-H. Nearly unbaised variable selection under minimax concave penalty. Ann of Statist. 2010;38:894–942. [Google Scholar]
- 31.Swaminathan S, Pasipanodya JG, Ramachandran G, et al. Drug Concentration Thresholds Predictive of Therapy Failure and Death in Children With Tuberculosis: Bread Crumb Trails in Random Forests. Clin Infect Dis. 2016;63:S63–S74. doi: 10.1093/cid/ciw471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO. Global tuberculosis report 2016. 2016 WHO/HTM/TB/2016.13. Available at: http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?ua=1.
- 33.Alsultan A, Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs. 2014;74:839–854. doi: 10.1007/s40265-014-0222-8. [DOI] [PubMed] [Google Scholar]
- 34.Burhan E, Ruesen C, Ruslami R, et al. Isoniazid, rifampin, and pyrazinamide plasma concentrations in relation to treatment response in Indonesian pulmonary tuberculosis patients. Antimicrob Agents Chemother. 2013;57:3614–3619. doi: 10.1128/AAC.02468-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramachandran G, Kumar AK, Bhavani PK, et al. Pharmacokinetics of first-line antituberculosis drugs in HIV-infected children with tuberculosis treated with intermittent regimens in India. Antimicrob Agents Chemother. 2015;59:1162–1167. doi: 10.1128/AAC.04338-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mah A, Kharrat H, Ahmed R, et al. Serum drug concentrations of INH and RMP predict 2-month sputum culture results in tuberculosis patients. Int J Tuberc Lung Dis. 2015;19:210–215. doi: 10.5588/ijtld.14.0405. [DOI] [PubMed] [Google Scholar]
- 37.Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis. 1999;3:S231–279. [PubMed] [Google Scholar]
- 38.Chang KC, Leung CC, Yew WW, Chan SL, Tam CM. Dosing schedules of 6-month regimens and relapse for pulmonary tuberculosis. Am J Respir Crit Care Med. 2006;174:1153–1158. doi: 10.1164/rccm.200605-637OC. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Munsiff SS, Driver CR, Sackoff J. Relapse and acquired rifampin resistance in HIV-infected patients with tuberculosis treated with rifampin- or rifabutin-based regimens in New York City, 1997–2000. Clin Infect Dis. 2005;41:83–91. doi: 10.1086/430377. [DOI] [PubMed] [Google Scholar]
- 40.Weiner M, Benator D, Burman W, et al. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin Infect Dis. 2005;40:1481–1491. doi: 10.1086/429321. [DOI] [PubMed] [Google Scholar]
- 41.Donald PR, Maritz JS, Diacon AH. The pharmacokinetics and pharmacodynamics of rifampicin in adults and children in relation to the dosage recommended for children. Tuberculosis (Edinb) 2011 doi: 10.1016/j.tube.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Milstein M, Lecca L, Peloquin C, et al. Evaluation of high-dose rifampin in patients with new, smear-positive tuberculosis (HIRIF): study protocol for a randomized controlled trial. BMC Infect Dis. 2016;16:453. doi: 10.1186/s12879-016-1790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WHO. WHO FDC_Factsheet-final. 2016 Available at: http://www.tballiance.org/sites/default/files/child-resources/WHO%20FDC_Factsheet_Final.pdf.
- 44.Jindani A, Aber VR, Edwards EA, Mitchison DA. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1980;121:939–949. doi: 10.1164/arrd.1980.121.6.939. [DOI] [PubMed] [Google Scholar]
- 45.Thee S, Detjen A, Wahn U, Magdorf K. Rifampicin serum levels in childhood tuberculosis. Int J Tuberc Lung Dis. 2009;13:1106–1111. [PubMed] [Google Scholar]