Abstract
Background:
Previous studies have uncovered heightened prostatic susceptibility to hormone-induced neoplasia from early-life exposure to low-dose bisphenol A (BPA). However, significant data gaps remain that are essential to address for biological relevance and necessary risk assessment.
Objectives:
A complete BPA dose–response analysis of prostate lesions across multiple prostatic lobes was conducted that included internal BPA dosimetry, progression to adenocarcinoma with aging and mechanistic connections to epigenetically reprogramed genes.
Methods:
Male neonatal Sprague-Dawley rats were briefly exposed to 0.1 to on postnatal days (PND) 1, 3, and 5. Individual prostate lobes plus periurethral prostatic ducts were evaluated at 7 mo or 1 y of age without or with adult testosterone plus estradiol () to promote carcinogenesis. DNA methylation of five genes was quantified by bisulfite genomic sequencing in d-200 dorsal prostates across BPA doses. Serum free-BPA and BPA-glucuronide were quantitated in sera of individual PND 3 pups collected 1 hr postexposure utilizing ultra-high-pressure tandem mass spectrometry (UHPLC-MS-MS).
Results:
The lowest BPA dose initiated maximal hormonal carcinogenesis in lateral prostates despite undetectable free BPA 1 hr postexposure. Further, prostatic intraepithelial neoplasia (PIN) progressed to carcinoma in rats given neonatal low-dose BPA with adult but not in rats given adult alone. The dorsal and ventral lobes and periurethral prostatic ducts exhibited a nonmonotonic dose response with peak PIN, proliferation and apoptotic values at BW. This was paralleled by nonmonotonic and dose-specific DNA hypomethylation of genes that confer carcinogenic risk, with greatest hypomethylation at the lowest BPA doses.
Conclusions:
Developmental BPA exposures heighten prostate cancer susceptibility in a complex dose- and lobe-specific manner. Importantly, elevated carcinogenic risk is found at doses that yield undetectable serum free BPA. Dose-specific epigenetic modifications of selected genes provide a mechanistic framework that may connect early-life BPA to later-life predisposition to prostate carcinogenesis. https://doi.org/10.1289/EHP1050
Introduction
Bisphenol A (BPA), a high-volume chemical and widely used synthetic plasticizer, has known estrogenic activity and is a recognized endocrine-disrupting chemical (EDC). Extensive studies over the past two decades have evaluated its potential effects in multiple organs and biological systems including its capacity for augmenting carcinogenesis (Chapin et al. 2008; Gore et al. 2015; Rochester 2013; Seachrist et al. 2016). Although research consensus has not been reached on adverse outcomes from BPA doses relevant to human exposures, it is widely appreciated that the developmental period is particularly sensitive to BPA exposures that may lead to long-lasting effects over the life span.
The prostate gland is a hormone-dependent reproductive organ that possesses a high rate of disease with aging. Currently, prostate cancer is the most common noncutaneous cancer and the second leading cause of cancer-related deaths in U.S. men (Siegel et al. 2016). Whereas androgens are essential for prostate growth and function, substantial evidence indicates that estrogens play key roles in prostate homeostasis and disease (Nelles et al. 2011). Importantly, inappropriate estrogen exposures during prostate development, in terms of timing, type and dose, can reprogram the gland, drive differentiation defects and predispose to an increased risk of prostate cancer (Prins et al. 2001; Prins and Ho 2010). Work from our laboratory (Ho et al. 2006; Prins et al. 2011), recently confirmed in an independent study (Wong et al. 2015), determined that brief early-life exposure to low-dose BPA (), although not sufficient to induce prostate lesions on its own, increased susceptibility to estrogen-driven prostatic intraepithelial neoplasia (PIN) in adulthood. This is germane because relative estradiol levels rise in the aging male (Vermeulen et al. 2002), estrogens can transform adult prostate epithelium (Bosland et al. 1995; Hu et al. 2011), accelerate cancer progression (Chakravarty et al. 2014; Setlur et al. 2008; Takizawa et al. 2015) and estrogen activity is amplified in advanced disease (Montgomery et al. 2008). Thus we posit that BPA reprograms prostate cells early in life resulting in a cellular memory that augments adult hormonal sensitivity. The molecular underpinnings of reprogramed prostatic memory appear to lie in epigenetic modifications that have been identified in prostates exposed perinatally to low-dose BPA that poise the cells for amplified responses to later estrogenic exposures. These include hypo- or hypermethylation of DNA that directly modifies gene expression (Cheong et al. 2016; Ho et al. 2006; Tang et al. 2012), alterations in histone methylation marks that directly change gene transcription or prime the gene for elevated response to transcriptional signals in later life (Wang et al. 2016), as well as changes in the expression of noncoding RNAs (Ho et al. 2015). These epigenetic modifications initiated by BPA are mediated, in part, by changes in the activity of DNA methyltransferases (DMNTs), methyl-CpG binding domain proteins (Mbd2/4) and histone methyltransferases (HMTs) (Tang et al. 2012; Wang et al. 2016). Recent work from our laboratory has further identified that human prostate stem and progenitor cells are direct targets of BPA exposures leading to epigenomic modifications and, due to their long-lived nature, increased carcinogenic susceptibility (Calderon-Gierszal and Prins 2015; Ho et al. 2015; Prins et al. 2014; Prins et al. 2015).
Despite inroads that have been made in identifying BPA’s potential role in prostate carcinogenesis, significant knowledge gaps remain that have hindered full utilization of this work in risk assessment analysis (Chapin et al. 2008). First, a dose–response study of the prostatic carcinogenic response to BPA has not been undertaken and is necessary across a range of exposures that include average human exposure levels and occupational risks. Another critical factor is accurate determination of internal free-BPA levels soon after exposure, irrespective of exposure route, to provide environmental applicability of the responses noted as well as knowledge of the precise BPA quantities to which animals and tissues are exposed. An essential element that remains unresolved is whether the precancerous PIN lesions found in prior studies that implicate BPA-driven carcinogenic susceptibility can in fact progress to prostate cancer. Finally, connection of epigenetic reprogramed genes to carcinogenesis and documentation of altered DNA methylation in a dose-responsive manner is necessary to determine the pathological relevance of these molecular modifications.
The present study sought to address these critical elements by a multi-pronged approach. We first undertook a large dose–response analysis of rat prostatic lesions at 7 mo of age as a function of early-life BPA exposures, both without and with a 2-fold increase in adult estradiol levels to promote carcinogenesis. Endpoint analysis included detailed histopathology, apoptosis/proliferation assessments and separate examination of the lateral, dorsal, ventral prostate lobes and periurethral prostatic ducts—all with known differential sensitivities to hormonal carcinogenesis. This is especially important because some past BPA evaluations have only examined the larger ventral lobe, which has no homology in the human prostate gland (Price 1963). Periurethral prostatic ducts are particularly susceptible to estrogen-driven cancers (Bosland et al. 1995) and provide added value to the present dataset. Next, the progression of high-grade PIN lesions to microinvasion and prostate adenocarcinoma was evaluated by undertaking a properly powered study to 1 y of age. Internal dosimetry for all BPA doses in individual rat pups was addressed through development of low volume quantitative capacity for both free-BPA and BPA-glucuronide (BPA-G) using UHPLC-MS-MS. Finally, we extended our ongoing DNA methylation analysis of identified reprogramed genes (Cheong et al. 2016) across a dose-range and found that permanent methylation changes were often most robust at the lowest BPA exposure levels. Together, these results firmly document that developmental exposures to low, environmentally relevant levels of BPA modify the prostatic DNA methylome in a dose-responsive manner and drive a significant increase in rat prostate cancer incidence that is lobe-specific and BPA dose-dependent.
Materials and Methods
Animal Housing and Treatments
All animals were treated humanely and with regard for alleviation of suffering, using protocols approved by the Animal Use Committee at UIC.Timed pregnant Sprague-Dawley rats between 3 and 6 mo of age (Zivic-Miller Laboratories, Pittsburgh, PA) were shipped on gestation d 12 and housed under strict conditions as described (Prins et al. 2011). Rooms were maintained at with 50% relative humidity and a 14-hr:10-hr light:dark schedule. All rats were housed in polysulfone solid-bottom cages with steel covers and double deionized water was supplied from glass bottles. Animals were fed ad libitum a soy-free, phytoestrogen-reduced diet (Zeigler Reduced Rodent Diet 2, Ziegler Bros, Inc., Gardners, PA). Pregnant dams were monitored and the day of birth was designated postnatal d 0 (PND 0). Litter size was culled to 10 pups on PND 0 by removing or adding female pups.
Newborn male pups were assigned to one of eight neonatal treatment groups with (see Figure S1A). To control for litter effects, male pups in each litter were randomly assigned to different treatment groups and tattooed for permanent identification. The 8 neonatal groups were a) tocopherol stripped corn oil vehicle as controls, b) high dose 3-benzoate (), , c) low-dose , , or 4–8) 0.1, 1.0, 10, 100, or . The highest BPA dose is the current LOAEL for BPA established by the U.S. National Toxicology Program (Chapin et al. 2008). All dosing was administered by subcutaneous (s.c.) depot injection in the nape of the neck on PND 1, 3 and 5 as previously described (Ho et al. 2006; Prins et al. 2011) thus allowing direct comparison of findings with our prior results. The timing of exposures coincides with the d 1–6 critical window characterized for the rodent prostate gland (Pylkkänen et al. 1991). On PND 3, tail vein blood was collected at 60 min postinjection from male rats in control and BPA dose groups () using Microvette® CB300 capillary tubes (Sarstedt, Newton, NC). Sera was separated and frozen for internal dosimetry measurement of free BPA and BPA-G. All products used for collection and storage of samples were confirmed to be free of BPA contamination. The pups were weaned at PND 21 and housed two/cage.
At PND 90, approximately half of the rats from each neonatal treatment group were given implants of Silastic capsules (Dow Corning, Midland, MI; i.d. 1.02 mm, o.d. 2.16 mm) packed with estradiol (one 1-cm tube) and testosterone (two 2-cm tubes) () to drive prostate carcinogenesis as described (Ho et al. 2006). The T capsules maintain physiologic testosterone levels and are needed to maintain prostate homeostasis because estrogen treatment alone results in feedback inhibition of endogenous testosterone secretion with resultant prostatic involution. The estrogen capsules double the circulating estradiol levels, which is sufficient to promote prostate cancer in a rat model (Bosland et al. 1995). Fresh tubes were replaced every 8 wk to ensure consistent steroid levels over time. At 7 mo of age (d 200), the animals were sacrificed by decapitation, blood collected and the prostatic-urethral complex quickly removed. This included the bilateral ventral, lateral and dorsal prostate lobes and the periurethral collecting ducts from each lobe that drain into the urethra (see Figure S1B). Using a dissection microscope, a single ventral, lateral and dorsal lobe from one side of each complex was removed, snap frozen, and stored in liquid nitrogen for molecular analysis. The remaining prostate lobes and full urethral complex were fixed en masse in methacarn (BBC Biochemical, Mt Vernon, WA) for 48–72 hr, rinsed and stored in 70% EtOH until histological processing. Serum was separated and frozen for hormone assays.
To examine potential progression of prostatic lesions to carcinoma, 60 control or treated rats were sacrificed at 1 y of age (D365) with a noted loss of of rats due to bladder outlet obstruction prior to 1 y. Five treatment groups () included a) neonatal vehicle controls with empty implants at PND90, b) neonatal vehicle controls with implants at PND 90, c) neonatal low-dose with implants at PND90, d) neonatal high-dose with implants at PND90, and e) neonatal with implants at PND 90. The implants were replaced every 8 wk. At 1 y, the rats were killed by decapitation, blood was collected, and the prostatic complex was dissected and processed for histopathologic diagnosis to assess carcinoma rates. This initial analysis of cancer rates was used for power analysis that determined that a doubling of the animal number would be required for proper calculation of carcinoma incidence. The entire study was then duplicated using identical vendors, diets, conditions, and rat strains for an additional 60 rats. When the histology data was decoded for treatment group, the results were similar to the original cohort and use of Bartlett’s test for homogeneity of variance permitted the pooling of data between the two cohorts.
Histopathology
The fixed prostatic tissues were dehydrated and paraffin embedded as described (Prins et al. 2011) with the ventral, lateral, and dorsal prostate lobes mounted along one plane surrounding the urethral region. Coronal sections of this complex permitted viewing of prostate structures en masse (see Figure S1). Three to four serial sections () were made at four levels of the block apart to permit pathologic analysis along the tissue depth and 12–16 sections were analyzed for each tissue. The sections were coded to prevent reader bias and stained with hematoxylin and eosin. Each prostatic region was read in a blinded fashion and scored for presence, severity and extent of lesions that include prostatic epithelial hyperplasia, inflammatory cell infiltration, and PIN. The PIN lesions were characterized by the presence of nuclear atypia (enlarged and elongated nuclei, hyperchromasia, prominent nucleoli) with or without aberrant cellular piling. Regions of cribiform pattern and aberrant cell piling without nuclear atypia were scored as atypical hyperplasia. PIN lesions were graded on a 0–3 scale with , , and . Other pathology included adenoma and squamous metaplasia and, at 1 y, basement membrane breakdown with local epithelial microinvasion and adenocarcinoma (Shappell et al. 2004). In instances with uncertain diagnosis, a second pathology opinion was obtained and final classification reached by consensus. Once all histopathology diagnosis were completed, data were entered into a secured Excel database by a third party followed by decoding and data analysis. Incidences of the separate prostate lesions in each lobe were analyzed by chi-square and scores were analyzed by ANOVA followed by Fischer’s exact test with significance accepted at .
Immunohistochemistry and in Situ Apoptosis Labeling
To assess epithelial proliferation rates, d-200 specimens () were immunostained for using a polyclonal primary antibody (1:2,500, Novacastra, Newcastle, UK) with adjacent sections incubated in normal rabbit IgG () as negative controls. To determine apoptosis rates by TUNEL staining, d-200 sections were reacted with an ApopTag Peroxidase In Situ Apoptosis Detection Kit according to manufacturer’s instructions (Chemicon International, Temecula, CA). To calculate proliferation and apoptotic indices, multiple areas of each lobe were captured with a color digital AxioCam camera on an Axioskop microscope (Carl Zeiss, Inc., Thornwood, NY). Positive and negative -stained or TUNEL-labeled epithelial cells were counted using Zeiss Image Version 3.0 (Carl Zeiss) with cells counted per slide. Data was analyzed by ANOVA and Bonferroni post-tests with considered significant.
Bisphenol A Quantitation
Serum BPA was quantitated using a previously described HPLC-MS-MS methodology that permitted direct and simultaneous measures of free BPA and BPA-G in sera (Prins et al. 2014). As a participant in the NIEHS-coordinated round-robin BPA analysis that rigorously examined criteria required for accurate blood BPA measurements (Vandenberg et al. 2014), quality control procedures were incorporated that included procedural blanks (LC-MS grade water; Burdick & Jackson, Honeywell, Muskegon, MI) and experimental blanks (charcoal-dextran stripped serum) analyzed with each experimental run. All equipment and supplies used in sera collection and storage and BPA measurements were confirmed free of BPA contamination. (Cambridge Isotope Laboratories, Andover, MA) , BPA (Sigma-Aldrich) and bisphenol A (BPA-G; Midwest Research Institute, Kansas City, MO) were used as standards. Spiked BPA samples were analyzed for measurement accuracy.
Stock and working solutions of BPA and BPA-G were prepared in HPLC-grade methanol. Calibration standards were prepared by mixing of each working solution with blank rat serum. Sera from the rats were initially run undiluted and repeated with 1:50 dilutions to ensure accuracy. All other samples were measured undiluted and deidentified for treatment groups. Each serum sample () or calibration standard was mixed with HPLC-grade acetonitrile containing the surrogate standards and , centrifuged at at 4°C for 15 min and the supernatant was removed and evaporated to dryness. The residue was reconstituted in of 50% aqueous methanol and a aliquot was injected onto the UHPLC-MS-MS system for analysis.
Chromatographic separations were carried out using a Shimadzu (Kyoto, Japan) LCMS-8050 triple quadrupole mass spectrometer equipped with a Shimadzu Nexera UHPLC system. Free BPA and BPA-G were separated on a Waters (Milford, MA) Acquity UPLC BEH (, ) column. A 1.5-min linear gradient was used from 10% to 100% acetonitrile in water followed by a hold at 100% for 0.3 min at a flow rate of . Negative ion electrospray mass spectrometry with selected reaction monitoring (SRM) was used for measurement of each analyte using previously detailed SRM transitions (Prins et al. 2014). Data acquisition was carried out using Shimadzu Labsolution software for external calibration curve construction from standards run in each assay. The lower limits of detection (LOD) for free BPA and BPA-G in rat sera were 0.02 and , respectively, whereas the lower limits of quantitation (LLOQ) for free BPA and BPA-G were 0.2 and , respectively.
Steroid radioimmunoassays (RIA).
Frozen serum samples for testosterone (T) and () analysis were shipped to the Ligand Assay and Analysis Core Laboratory (University of Virginia, Charlottesville, VA). Hormone levels were measured using murine RIA kits (TKTT2 for T and TKE21 for ; Siemens Medical Solutions Diagnostics). Sensitivity for T was and the intra- and interassay coefficients of variance were 4.0% and 7.1%, respectively. For , sensitivity was 10 pg/mL and the intra- and interassay coefficients of variance were 7.1% and 11.6%, respectively.
Bisulfite PCR sequencing analysis.
Genome-wide DNA methylation analysis using Roche-NimbleGen Rat ChIP 385 Promoter methylation arrays previously identified 20 genes with significant differentially methylated promoter regions in d-90 dorsal prostates of rats neonatally exposed to high-dose or as compared to oil controls (Cheong et al. 2016). Bisulfite sequencing validated the differential methylation patterns in 15 of the 20 genes and seven were confirmed to have gene expression status inversely correlated with promoter methylation status. In the present study, the CpG methylation status of five of these seven confirmed genes—Sox2, Creb314, Paqr4, Pitx3 and Tpd52—was examined across the five neonatal BPA doses and high-dose exposure of d-200 dorsal prostates from rats without exogenous exposures to examine their persistence with aging and whether methylation status exhibited a dosage-dependent response.
Genomic DNA from d-200 dorsal prostates was extracted using DNeasy Blood & Tissue kit (Qiagen, Valencia, CA) with RNase A. Bisulfite sequencing of the gene promoters was conducted using 500-ng genomic DNA and EZ DNA Methylation kits (Zymo Research, Irvine, CA) as described (Cheong et al. 2016). Following 40 cycles of PCR amplification, the amplicons were gel-purified and TA-cloned into pGEM T Easy Vector (Promega, Madison, WI). Plasmids from a single E. coli colony were directly amplified by TempliPhi DNA amplification kits (GE Healthcare, Buckinghamshire, UK) and sequenced (Macrogen USA, Rockville, MD). The methylation status at each CpG site was analyzed using BiQ Analyzer. Five day-200 dorsal prostate samples were used in each group. Promoter methylation at each CpG site was expressed as from four to five samples/group with . The exception was Creb3/4 of which bisulfite sequencing analysis was performed on pooled samples () with six clones/sample. For statistical analysis, area under the curve for promoter CpG methylation sites at each dose was calculated and significance determined by two-way ANOVA and Tukey test when compared to vehicle control prostates, with considered significant.
Results
Quantitation of Free and Glucuronidated BPA in D-3 Rat Serum
An essential element for evaluating the biologic relevance of BPA administration across a dose–response range is internal dosimetry measurements that accurately quantitate circulating free-BPA and BPA-G levels shortly after exposure, irrespective of mode of BPA administration. Because BPA was administered on PND 1, 3, and 5, serum samples were collected from individual PND 3 rats via tail vein sampling 60 min after s.c. injection to determine the median free-BPA and BPA-G levels to which the developing tissues were exposed. As shown in Table 1, all vehicle-treated control rats had undetectable () levels of free BPA and BPA-G, documenting a contamination-free system. Total BPA rose linearly in rat sera with increasing doses of BPA up to . At the two lowest BPA doses of 0.1 and , free BPA was below the LOD and LLOQ, respectively, whereas BPA-G was and , respectively. Free BPA was first quantifiable in rats treated with , appearing at , which is similar to our previous study (Prins et al. 2011) and within the range of fetal and newborn human exposures (Gerona et al. 2013; Padmanabhan et al. 2008), thus implicating direct biologic relevance of this dosage. A nonlinear increase in free BPA was observed in the 100 and treated rats, reaching and , respectively. Direct measurement of BPA-G revealed in the treated rats, which is markedly higher than our previous measures using indirect enzymatic treatment and extrapolation for BPA-G (Prins et al. 2011), emphasizing the improved accuracy and importance of direct BPA-G measurements. With this direct quantitative method, % free BPA in PND 3 rats was 2.2%, 7.7%, and 15% of total BPA for 10, 100, and dosing levels, respectively, at 60 min postexposure.
Table 1.
Serum BPA levels in individual d 3 rats quantitated by UHPLC-MS-MS.
| Treatment () | BPA Concentration (ng/mL) | ||
|---|---|---|---|
| Free BPA | G-BPA | Total BPAa | |
| Vehicle | |||
| 0.1 | |||
| 1 | |||
| 10 | |||
| 100 | |||
| 5000 | |||
Note: BPA in oil administered by subcutaneous injection. Serum collected at 1 h via tail vein. ; ; G-BPA, glucuronidated BPA; LOD, limit of detection; LLOQ, lower limit of quantitation.
.
Neonatal BPA Exposures Increase Prostatic PIN and Hyperplasia at D 200 in a Lobe- and Dose-Specific Manner
Rats exposed to neonatal hormones without or with adult treatment were aged to d 200 and their prostates assessed in a blinded manner for the presence of PIN, atypical hyperplasia, epithelial hyperplasia and inflammatory cell infiltration. Neonatal exposure to increasing doses of BPA without adult hormone exposure produced minimal prostatic lesions at d 200 with no significant differences compared to aging controls (data not shown). The only group with significant pathologic alterations was neonatal high-dose as previously described by our laboratory (Ho et al. 2006; Prins 1992).
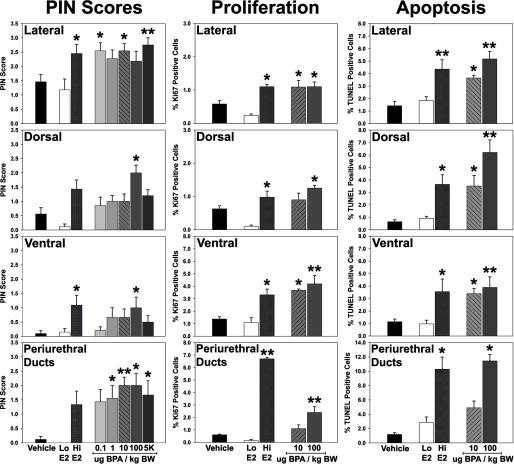
In contrast, marked prostatic pathology was noted in a lobe-specific and dose-responsive manner in all neonatal and BPA treated rats when exposed to for 4 mo during adulthood (Figure 1, Table 2; see also Table S1). It is important to note that the capsules produced identical serum testosterone levels and only 2-fold higher levels as compared to control rats given empty capsules (see Figure S2), which is sufficient to initiate moderate prostate carcinogenesis with aging in this rat model (Bosland et al. 1995). As expected, the lateral prostate lobe was most sensitive to adult hormone-induced carcinogenesis. PIN incidence (%) and severity score (scale 0–3) increased from 20% and 0.30, respectively, in the neonatal vehicle controls-empty adult capsule group to 67% and 1.46 in neonatal controls given during adulthood (Table 2). Importantly, PIN incidence and scores in the lateral lobe were further increased to near maximal values when exposed early in life to high- and all BPA doses plus adult , with an incidence and score of 100% and 2.54, respectively, at the lowest dose of (Figure 1, Table 2). It is noteworthy that serum free BPA and BPA-G were and LLOQ, respectively, at 1 hr postexposure in this dosing group, documenting that lateral prostatic effects occur at exceedingly low BPA exposures. Lateral lobe epithelial hyperplasia was prevalent in all aged prostates including neonatal controls without additional adult hormone exposures (empty) (Table 2) and this was not further exacerbated with neonatal or BPA exposures or adult . Of note, the lateral lobes contained marked inflammatory cell infiltration upon adult treatment that was significantly augmented by neonatal high-dose and BPA exposures (Table 2).
Figure 1.
Prostatic intraepithelial neoplasia (PIN) scores (0–3 scale), % proliferation () and % apoptosis (TUNEL labeling) in the lateral, dorsal and ventral prostate lobes and periurethral prostate ducts at d 200. Rats were treated neonatally with vehicle, low-dose , high-dose and increasing doses of BPA on d 1, 3, and 5 of life. All rats were given implants at d 90 to drive hormonal carcinogenesis. . and vs, neonatal vehicle within each lobe.
Table 2.
Lateral prostate (T&E) pathology in d-200 rats.
| PIN | Hyperplasia | Inflammation | |||||
|---|---|---|---|---|---|---|---|
| Score | Incidence | Score | Incidence | Score | Incidence | ||
| All | HGPIN | ||||||
| 0.3 | 20% | 0% | 0.90 | 40% | 0.00 | 0% | |
| 1.46 | 67% | 33% | 1.10 | 60% | 0.89 | 67% | |
| Low | 1.18 | 62% | 25% | 0.38 | 25% | 1.38 | 75% |
| High | 2.46a,c | 91% | 73%c | 0.73 | 64% | 2.00b | 100% |
| 0.1 BPA | 2.54a,c | 100% | 70% | 0.80 | 60% | 1.70a | 90% |
| 1.0 BPA | 2.27c | 89% | 56% | 0.56 | 67% | 1.33 | 89% |
| 10 BPA | 2.54a,c | 100% | 67% | 0.67 | 56% | 2.22b | 100% |
| 100 BPA | 2.18 | 75% | 62% | 0.62 | 38% | 1.25 | 50% |
| 5K BPA | 2.75b,c | 90% | 80%a,c | 0.80 | 80%c | 1.90a | 100% |
| (a) vs. | NS | NS | NS | NS | |||
| (b) vs. | |||||||
| (c) vs. Low | |||||||
Note: Scores were analyzed by ANOVA with post hoc Dunnett multiple comparisons.
Incidence was analyzed by chi-square and Fischer’s exact test.
All PIN includes tissues with 1, 2, or 3 PIN scores.
HGPIN includes only 2 and 3 PIN scores. ; NS, nonsignificant.
Prostatic lesions in the dorsal and ventral lobes and periurethral prostatic ducts presented at reduced incidence and severity upon neonatal /BPA plus adult hormone exposures when compared to the lateral prostate. This provided an opportunity to assess the dose-responsiveness of neonatal BPA exposures on altering carcinogenic susceptibility. PIN lesions in the neonatal vehicle-adult group were low in these three regions, not affected by neonatal low-dose and increased by high-dose exposure (Figure 1; see also Table S1). In contrast to the lateral lobe, PIN scores exhibited an inverted U-shaped dose response to rising neonatal BPA doses. Although nonsignificant increases were noted at 1 and as compared to neonatal vehicle controls, a significant increase in PIN scores was observed in dorsal and ventral lobes at . Notably, this dropped at the high dose of to levels seen with lower-dose BPA. In the periurethral prostatic ducts, PIN scores increased at all BPA doses compared to neonatal vehicle controls with significance at 1, 10, 100, and and peak values at the 10- and doses. The decrease PIN score noted at the highest BPA dose suggests that increased carcinogenic response in these regions may be limited to low-dose BPA exposures. Although dorsal lobe hyperplasia was not affected by neonatal /BPA (see Table S1), the ventral lobe and periurethral prostatic ducts exhibited significant augmentation in hyperplastic incidence and severity in the high and all BPA doses as compared to adult only (see Table S1). Additionally, inflammation was low in these prostatic regions with only modest and nonsignificant increases from neonatal BPA exposures.
Neonatal BPA Augments Prostatic Epithelial Proliferation and Apoptosis
Perturbations in epithelial proliferation and apoptosis are accepted biomarkers of precancerous prostatic lesions when combined with histopathology (Shappell et al. 2004). To assess the pathological significance of high-grade PIN lesions, tissues from neonatal and selected BPA dose-groups given adult through d 200 were histologically assessed for epithelial proliferation and apoptosis using Ki67 and TUNEL assays. Although not affected by neonatal low-dose , the proliferative and apoptotic indices were markedly increased in all prostate regions with neonatal high-dose exposure plus adult (Figure 1), matching the known capacity for carcinoma progression with combined hormonal exposures (Leav et al. 1988; Yuen et al. 2005). Importantly, neonatal exposures to 10 or increased proliferation in the lateral, dorsal and ventral prostate epithelium to levels observed with high- exposure. Although a marked response to high-/adult was seen in the periurethral prostatic ducts, the effect of neonatal BPA was less pronounced with a proliferative increase only noted at . Matching the proliferative changes, a significant increase in epithelial apoptosis was noted in all prostatic regions at and a further augmentation was noted at the dose (Figure 1). Together, this imbalance in proliferation/apoptosis rates indicates pre-carcinogenic activity and provides objective support for the pathologic relevance of the heightened PIN susceptibility upon neonatal BPA exposures.
Progression to Local Invasion and Adenocarcinoma upon Aging to 1 Y
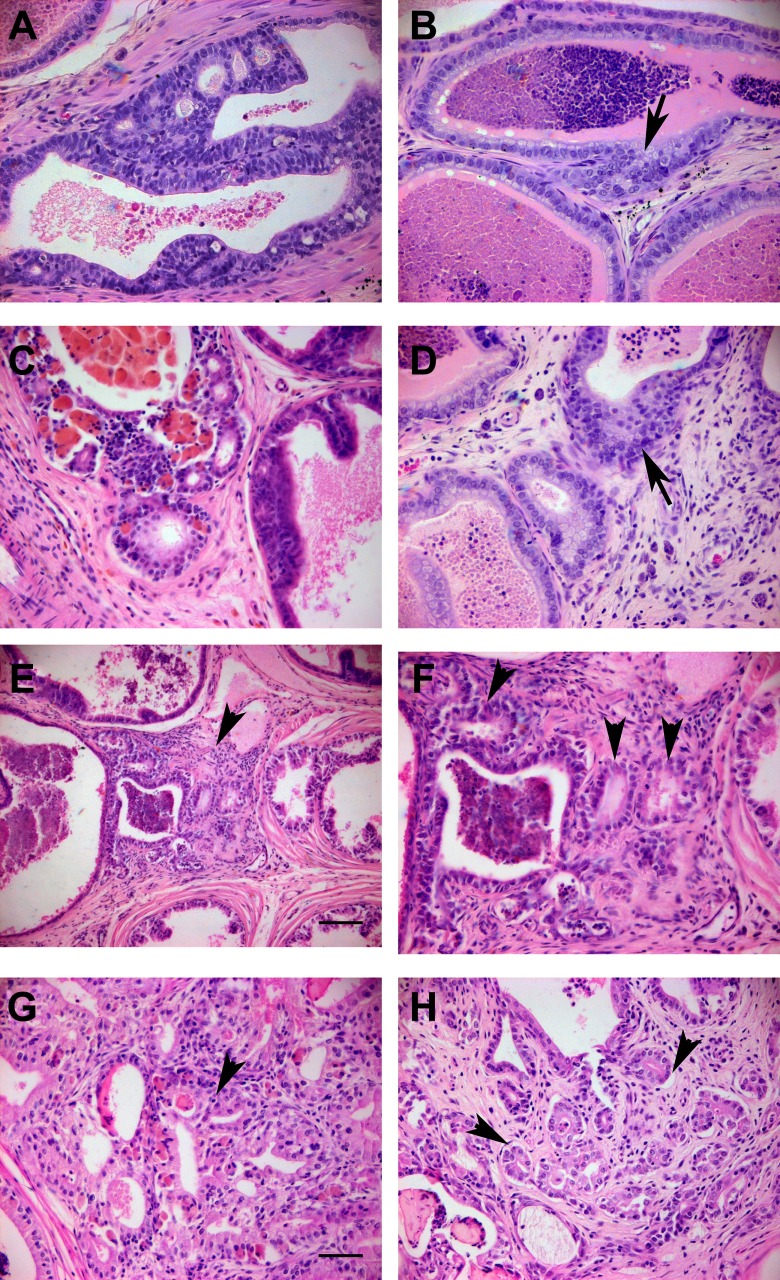
To directly determine whether the d-200 high-grade PIN lesions progressed to cancer, a cohort of control rats (neonatal vehicle without or with adult ) and rats neonatally exposed to low/high or with adult were aged to 1 y for detailed histopathologic analysis. Although serum testosterone and estradiol were lower at d-365 vs. d-200 rats given empty capsules, the capsules maintained equivalent testosterone and a 2-fold elevation in circulating in rats at 1 y (see Figure S2). Control rats without adult hormones showed no lesion progression whereas controls with exhibited limited cancerous progression with a 17% incidence of microinvasion, the earliest recognizable form of prostate cancer as evidenced by basement membrane breakdown with epithelial cells invading the stroma (Bostwick 1996; Bostwick and Cheng 2012). However, there were no newly formed malignant acini/glands, used for grading adenocarcinoma (Shappell et al. 2004), in any of the prostatic regions in the vehicle empty or control rats (Figure 2, Table 3; see also Table S2). In contrast, a progressive increase in microinvasion and formation of focal well-differentiated adenocarcinoma glands (carcinoma – glandular) were observed in prostates of rats treated neonatally with or BPA plus adult hormones. The response to low- and high-dose was dose-related in the lateral lobe with maximal incidence of microinvasion (58%; ) and newly formed adenocarcinoma (carcinoma – glandular incidence of 23%; ) seen with neonatal high-dose (Table 3). Notably, neonatal produced an equivalent incidence of microinvasive carcinoma (62%; ) as high-dose in the lateral lobe, whereas the incidence of newly formed cancerous glands was somewhat lower (carcinoma: glandular incidence of 14%; ) in the BPA-treated rats. Similar progression to microinvasive cancer without or with discreet, newly formed cancerous glands was found in the other prostatic regions for the low-dose BPA group, although this did not reach significance (see Table S2). Together, these findings demonstrate for the first time that neonatal exposure to low-dose BPA combines with rising adult estradiol levels to drive prostate cancer with aging.
Figure 2.
Prostatic histopathology lesions at d 365 in the lateral lobe (A–G) and periurethral prostate ducts (H) of rats treated neonatally with and implants at d 90. Examples of HG-PIN and in situ carcinoma (A,C) and local microinvasion of epithelium into stroma (B,D, arrows) were observed in the majority of treated lateral prostates. Focal regions of well-differentiated adenocarcinoma (arrowheads in E, 10 and F, 20 of same region and G from a different lateral lobe) were seen in several lateral prostates as evidenced by irregular small glandular structures within stroma with abortive glandular lumens, back-to-back lumens and loss of basement membranes (arrowheads). Regions of adenocarcinoma were also noted in periurethral prostatic ducts (H, arrowheads). A–D, F–H, same magnification as G with ; .
Table 3.
Lateral prostate (T&E) pathology in aged (d 365) rats.
| PIN | Carcinoma-Microinvasion | Carcinoma- Glandular | Inflammation | Hyperplasia | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | Incidence | Incidence | Incidence | Score | Incidence | Score | Incidence | ||||
| Vehicle Empty | 0.12 | 1/17 | 6% | 0/17 | 0% | 0/17 | 0% | 0.00 | 0% | 0.76 | 47% |
| Vehicle T&E | 1.50a | 15/18 | 83%a | 3/18 | 17% | 0/18 | 0% | 1.28a | 78%a | 0.50 | 39% |
| Lo T&E | 2.00a | 12/14 | 86%a | 6/14 | 43%a | 1/14 | 7% | 1.14a | 71%a | 0.86 | 57% |
| Hi T&E | 2.50a,b | 24/26 | 92%a | 15/26 | 58%a,b | 6/26 | 23%a,b | 1.77a | 77%a | 1.81a,b | 88%a,b |
| BPA 10 T&E | 2.48a,b | 20/21 | 95%a | 13/21 | 62%a,b | 3/21 | 14% | 1.43a | 95%a | 0.67 | 52% |
| (a) vs. Veh empty | |||||||||||
| (b) vs. Veh T&E | NS | NS | NS | ||||||||
Note: Scores were analyzed by ANOVA with post hoc Dunnett multiple comparison.
Incidence was analyzed by chi-square and Fischer’s exact Test. . NS, nonsignificant.
DNA Methylome reprograming across BPA Doses at D 200
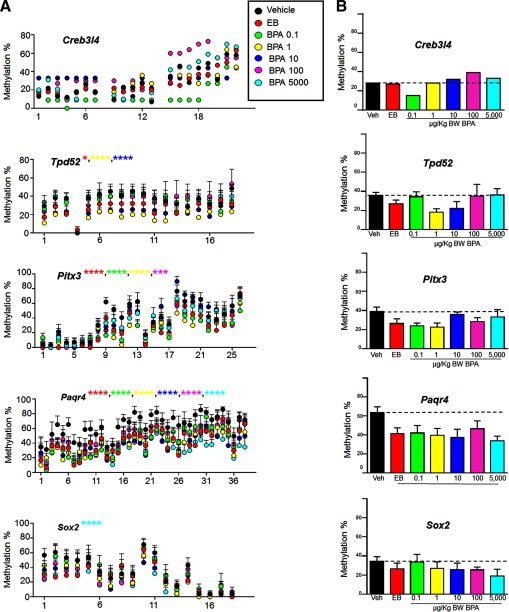
We recently identified multiple genes in d-90 rat dorsal prostates whose promoters bore reprogramed DNA methylation patterns as a result of neonatal and BPA exposures, leading to altered gene transcription (Cheong et al. 2016). In the present study, five of these genes were selected for a dose–response analysis of DNA methylation status using bisulfite PCR sequencing in d-200 dorsal prostates of rats without adult treatment. The five genes (Creb3l4, Tpd52, Pitx3, Paqr4, and Sox2) were selected because they showed the greatest changes in promoter methylation in PND90 dorsal prostates as a function of neonatal /BPA exposures as compared to vehicle-treated controls. Although the lateral lobes exhibited the highest PIN and carcinoma rates upon treatment, the dorsal lobes were selected given that a dose–response PIN phenotype incidence was observed thus pathologically validating other dose–response measures. Importantly, the dorsal lobe exhibited minimal inflammatory cells with hormone treatments whereas lateral prostates had a high incidence of inflammatory cell infiltration thus precluding a clean separation of prostatic cells from immune cells in lateral lobes. As shown in Figure 3, Creb314, Tpd52, Pitx3, Paqr4, and Sox2 were hypomethylated at promoter CpG sites by neonatal high-dose and BPA in a dose-dependent manner when compared to vehicle controls. Three dose-dependent patterns emerged. For three genes—Creb314, Tpd52 and Pitx3—significant hypomethylation was observed at the lower BPA doses of 0.1, 1.0, and/or , with a return towards vehicle control methylation levels at the higher BPA exposures. Of note, the hypomethylation changes in Creb314 and Tpd52 induced by neonatal 0.1 or , respectively, were greater than that observed with high-dose . A second methylation pattern was noted in Paqr4 in which high-dose and all BPA doses significantly reduced promoter gene methylation to the same extent as compared to the vehicle control group. The third pattern observed was seen with Sox 2 where hypomethylation first appeared at and gradually decreased further with increasing BPA doses, reaching a nadir at the highest BPA dose of ().
Figure 3.
Dose–response analysis of promoter DNA methylation patterns of five previously identified /BPA reprogramed rat prostate genes (Cheong et al. 2016). A) Promoter methylation at each CpG site is expressed as from four to five samples/group with for each BPA dose, treatment and vehicle control. The exception is Creb3/4 of which bisulfite sequencing analysis was performed on pooled samples () with six clones/sample. B) Mean % methylation of all promoter-region CpG sites combined across the separate doses. The dotted line represents the vehicle control total promoter % methylation for comparison.
Discussion
The present study fills several critical data gaps necessary to thoroughly assess the influence of developmental BPA, at levels relevant to daily human exposures, on prostate cancer risk with aging. Most notably, it provides a logarithmic dose–response analysis across separate rat prostate lobes, direct measurement of internal free BPA and BPA-G across doses in individual rat pups, documentation of PIN lesion progression to locally invasive carcinoma, and mechanistic connections to epigenetically reprogramed genes across multiple doses. At all doses tested, early-life BPA exposure alone was insufficient to drive prostate precancerous lesions with aging, which substantiates previous findings (Ho et al. 2006; Milman et al. 2002; Yoshino et al. 2002) and indicates that up to the current LOAEL (), BPA is not a complete carcinogen in the prostate. Nevertheless, we found that BPA exposures across multiple doses, prostatic lobes and endpoints act in conjunction with elevated estradiol levels, as seen with aging, to heighten prostate carcinogenesis and progression. Combined with previously published studies (Cheong et al. 2016; Ho et al. 2006; Ho et al. 2015; Seachrist et al. 2016; Tang et al. 2012; Wang et al. 2016; Wong et al. 2015), the present work further supports the postulation that early-life BPA exposure acts on estrogen-sensitive prostate cells to epigenetically reprogram and prime selective genes for enhanced responses to later-life estrogenic triggers. To that extent, BPA may be considered an epigenetic initiator of tumorigenesis during early development that results in increased cancer risk to rising estrogens that act as a promoter (Sharma et al. 2010).
Specific BPA dose–response patterns were observed across the separate prostatic regions, which aligns with known differential sensitivities to multiple hormones, including estrogens, in the prostate gland (Bosland et al. 1995; Prins 1987; Prins 1989; Prins 1992). Of particular note, the lateral prostate lobe exhibited maximal carcinogenic susceptibility to adult estradiol when neonatally exposed to , that is, at a 100-fold lower dose than previously reported (Ho et al. 2006; Prins et al. 2011), despite no detectable free BPA or BPA-G 1 hr after exposure. This indicates that markedly lower BPA doses will be required to reach a LOAEL for the rat prostate. Remarkably, this low-dose BPA response was equivalent to that found with neonatal high-dose . These results are similar to mammary gland lesions induced by perinatal exposure to the very low dose of 25 ng BPA/kg BW (Durando et al. 2007) and emphasize that BPA levels within the range of human gestational exposures can predispose to adverse effects with aging. That similar responses were observed with BPA and high-dose treatment, but not the low-dose treatment suggests that BPA may act through additional pathways beyond estrogen receptors as has been documented in many studies including our recent findings with human prostate progenitor cells (Delfosse et al. 2014b; Ho et al. 2015).
Relative to the lateral prostate, the less estrogen-sensitive dorsal and ventral lobes and periurethral prostatic ducts exhibited a nonmonotonic dose response for -induced PIN incidence and scores. That a rising carcinogenic response was observed across these three regions with rising neonatal BPA levels through the dose, supports the biologic relevance of the response. Of note, a significant increase in PIN scores was found in periurethral ducts at , when serum free BPA was , and rose to peak values at the dose when mean serum free BPA was , a value reported in some pregnant women (Gerona et al. 2013). That peak neoplastic responses were observed between BPA/kgBW and dropped to lower responses at the dose in these three prostatic regions is likely explained by the multiple receptors and signaling pathways that can be engaged by different BPA levels, leading to a complex response pattern over the wide range of exposures tested herein (Delfosse et al. 2014a; Viñas and Watson 2013). Similar shaped BPA dose responses have been reported for other organs and benign prostatic growth (Vandenberg et al. 2012; vom Saal et al. 1997) and the current data extend this to carcinogenic susceptibility.
A complete prostate lobe-specific analysis was essential for multiple reasons: a) the rat dorsal and lateral lobes have homology in humans, both embryologically and histologically, whereas there is no human homolog for the ventral prostate (Price 1963), b) the individual lobes express specific genes, particularly the high-expression genes that encode lobe-specific secretory proteins (Gerhardt et al. 1983), c) the ventral prostate is larger than the dorsal and lateral lobes combined and analysis of genes in entire prostatic complex will mask significant changes in the smaller lateral and dorsal lobes, and d) the ventral lobe undergoes branching morphogenesis between PND 1 and 6, whereas the lateral and dorsal lobes are delayed by 4–5 d, thus studies prior to 1 wk of age will be primarily ventral in nature (Hayashi et al. 1991). This may explain why a recent study that examined global genomic DNA methylation % and gene expression changes in the d-4 and -90 full prostate complex was not able to identify a dose–response effect in the prostate (Camacho et al. 2015). This further emphasizes the necessity for lateral and dorsal prostate evaluations given that they are considered the human homolog in rodent studies and will have the greatest applicability to prostate diseases in aging men.
The pathological relevance of the PIN lesions to prostate cancer was confirmed in the present studies in two ways. First, significantly accelerated rates of epithelial proliferation and apoptosis were found in all prostate regions at the doses when elevated and maximal PIN scores were noted, respectively. These aberrant cell turn-over rates are considered key evidence of similarity to human high-grade PIN, the precursor to prostate cancer (Shappell et al. 2004). Second, and most importantly, the prostate PIN lesions progressed to microinvasion and adenocarcinoma in the lateral lobes of rats treated with neonatal BPA () or plus adult when aged to 1 y as compared to neonatal vehicle-controls. Although not significant, similar trends were noted in the dorsal lobe and periurethral ducts aged to 1 y. That adult exposures alone had limited invasion (17%) and no glandular carcinoma in the lateral prostate, whereas the addition of neonatal BPA produced a 62% incidence of microinvasive carcinoma and 14% glandular carcinoma incidence clearly confirms, for the first time, that early-life BPA exposures heighten prostate cancer risk.
The route of BPA exposure in the present study deserves discussion. A prior study from our laboratory directly compared the BPA pharmacokinetics in PND 3 rat pups after exposure to by s.c. injection or orally and further assessed prostatic lesions in adult treated rats on d 200 (Prins et al. 2011). Whereas free BPA was significantly higher at 30 min after s.c. injection compared with oral exposure, this rapidly declined by 1–2 hours to equivalent levels in the two exposure groups with serum values matching the present study. Importantly, the prostate exhibited nearly identical heightened susceptibility to PIN lesions with either exposure route suggesting that the early metabolic differences in free BPA did not influence the pathologic outcomes. A recent independent study comparing exposure routes in PND 3 rats reported equivalent serum free-BPA levels 1 hr postexposure in pups given by s.c. injection and oral administration of , the current BPA NOAEL, with similar carcinogenic risk at both doses (Wong et al. 2015). Although the present experiments utilized the s.c. injection route to permit direct comparisons with previous datasets, we directly measured the internal BPA dosimetry in the neonatal pups, and most importantly, find significant prostatic lesions when free BPA was below detection limits. It is now appreciated that humans are exposed to BPA through multiple routes including ingestion, skin absorption, inhalation, and intravenous medical devices with which newborns are in increasing contact (Duty et al. 2013; Hines et al. 2017; Vandenberg et al. 2007). As such, studies that utilize nonoral routes should not be discounted a priori and may in fact encompass the variety of human exposures to this chemical. Thus internal dosimetry measures utilizing a contamination-free system as performed herein should be the ultimate determinant of applicability of study results for risk assessment and relevance.
The measurement of free BPA and BPA-G in of sera permitted direct monitoring of BPA levels in individual exposed rats in this study, markedly increasing the robustness of the present results. Prior work on rat neonates required pooling sera from 10 pups for BPA quantitation (Prins et al. 2011; Wong et al. 2015), extrapolation from higher-dose exposures or utilization of isotopically labeled tracers (Doerge et al. 2010). Further, until recently, direct BPA-G measures were not possible due to lack of an available standard, relying instead on enzymatic digestion for indirect BPA-G calculations (Doerge et al. 2010; Prins et al. 2011). Using a direct assay, the current study found that the BPA-G levels were considerably higher than we previously observed in PND3 rats and result in a 2–15% level of free BPA that closely aligns with studies by Doerge for PND-4 rats (Doerge et al. 2010). The higher levels of free BPA with increasing BPA doses is expected due to the immaturity of UDP glucuronosyltransferase 2 family, polypeptide B1 (Ugt2b1) expression and thus limited capacity for glucuronidation in the neonatal liver (Matsumoto et al. 2002). As a result, the dose, which produced peak PIN lesions in the dorsal and ventral prostates, resulted in free BPA 1 hr postexposure that, while far higher than daily exposures reported in the adult human population, has been reported in mid-gestational sera of pregnant women using a documented contamination-free sera collection and assay (Gerona et al. 2013).
Complementing the nonmonotonic dose response in carcinogenic risk, we herein identified nonmonotonic dose responses for DNA hypomethylation in several gene promoters of d-200 dorsal prostates, with three of five examined genes (Creb3L4, Tpd52, Pitx3) showing marked changes only at the lowest BPA doses. This is supported by recent reports of nonmonotonic DNA methylation responses to low- and high-dose BPA in the mouse and human fetal liver (Faulk et al. 2015; Kim et al. 2014). In addition to the nonmonotonic low-dose methylation marks, other patterns emerged including consistent Paqr4 promoter hypomethylation at all doses and increasing Sox2 promoter hypomethylation with increasing BPA levels. The five prostate genes examined at d 200 in the present study were selected from a previous genome-wide DNA methylation analysis of the same rat cohort that identified 86 genes differentially methylated by neonatal BPA exposure in d-90 dorsal prostates as compared to neonatal vehicle controls (Cheong et al. 2016). These genes were specifically chosen as potential BPA- reprogramed gene candidates because they exhibited an inverse relationship between DNA methylation and gene expression at d 90, long after neonatal BPA exposure. That differential methylation modifications persist through d 200 indicates that these genes are permanently reprogramed throughout life by developmental BPA exposures. Our previous work found that upon neonatal low-dose BPA treatment, the prostatic de novo DNA methylation transferases (Dmnt3a/b) and methyl-CpG binding domain proteins (Mbd2/4) were elevated throughout life and modified by adult estrogens which may underpin the dynamic alterations in DNA methylation marks across genes, doses and with aging (Tang et al. 2012). It is important to note that the hypomethylation marks in the present study were quantified in the d-200 dorsal prostate in the absence of adult hormone treatment. Given that the onset of dorsal lobe PIN lesions and microinvasion was only observed with the addition of adult , the present data suggest that the altered gene methylomes may contribute to increased carcinogenic susceptibility to adult estrogen exposure. Utilizing the same rat prostate model, a parallel mechanism of neonatal BPA- reprogramed genes in the KEGG prostate cancer pathway was recently identified (Wang et al. 2016). In those studies, persistent H3K4me3 marks were elevated at several cancer-associated gene promoters, a function of increased histone methyltransferase mixed-lineage leukemia 1 (MLL1) activation by BPA, which primed them for enhanced sensitivity to induction in the adult prostate. Undoubtedly several epigenomic modifications initiated by early-life BPA, including altered noncoding RNAs such as SNORDs as recently shown in prostate progenitor cells (Cheong et al. 2016; Faulk et al. 2015), act in parallel to poise the prostate gland for increased carcinogenic risk as a function of later-life events, supporting the proposal that BPA acts as an epigenetic initiator in early life as epigenetic marks are established in developing tissues.
Given that the five BPA- reprogramed genes examined herein were hypomethylated with aging, their expression may be aberrantly elevated in the aging prostate. Paqr4 (progestin and adipoQ receptor family member 4) was hypomethylated at equivalent levels by all BPA doses and matched high-dose , suggesting maximal and sustained expression changes at low-to-high BPA exposures. Although studies on Paqr4 are limited, AdipoQ, its ligand, plays an important role in cellular metabolism and abnormal expression has been associated with prostate cancer risk (Kaklamani et al. 2011). Pitx3 is a homeobox transcription factor with established roles in development and stem cells, although its function in the prostate has not been examined. Interestingly, although Tpd52 and Creb3L4 were hypermethylated in d-90 prostates by neonatal BPA/ exposures with resultant down regulation of gene expression (Cheong et al. 2016), the profiles of these two genes was reversed with aging, becoming significantly hypomethylated by d 200. This is noteworthy because Tpd52 and Creb3L4, along with Sox 2, are upregulated in prostate cancer (Jia et al. 2011; Labrie et al. 2008; Tennstedt et al. 2014) and, along with Pitx3, have established roles in stem cells. Because epigenetic mechanisms are central to maintaining stem cell identity, disruption of their epigenome may give rise to high-risk progenitor populations that readily become neoplastic upon gain of additional insults throughout life (Sharma et al. 2010). Taken together with our recent findings that BPA targets human embryonic and adult prostate stem cells and modifies their epigenome (Calderon-Gierszal and Prins 2015; Ho et al. 2015; Prins et al. 2014; Prins et al. 2015) and that chronic low-dose BPA treatment alters rat prostate stem cell homeostasis (Hu et al. 2015), a picture emerges whereby early-life BPA exposure heightens later-life carcinogenic risk by permanently modifying prostate stem and progenitor cells for increased sensitivity to carcinogenic hits. Although stem and progenitor cells were not studied in the present experiments, the epigenetic modifications of certain prostate genes related to stemness found herein leads us to postulate that BPA exposures might epigenetically poise prostate epithelium towards a cancer stem cell phenotype following additional hormonal stimuli. Of particular note, these genes were found to be associated with recurrence-free survival in human prostate cancer when the Cancer Genome Atlas (TCGA) data set was interrogated, indicating potential clinical relevance of these genes to human prostate cancer (Cheong et al. 2016).
In summary, the present study extends our knowledge on the effects of developmental BPA on adult prostate health, documenting a dose-dependent effect, providing internal BPA dosimetry for direct comparisons to humans and showing that low-dose exposures heighten the risk for developing lesions that progress to prostate cancer. That PIN progresses to adenocarcinoma provides the required biologic and pathologic relevance of these earlier lesions and raises the bar for adverse outcomes due to early-life BPA exposures. Further, dose-specific modifications in the DNA methylome of genes connected to human prostate cancer provide a mechanistic framework for connecting early-life exposures to later-life disease risk. Together, this data reinforces the assertion that low-dose BPA at levels comparable to human exposures negatively affects prostatic health. This may bear particular relevance to at-risk populations for developing prostate cancer due to race, metabolic polymorphisms, hormonal therapeutics and/or genetics.
Supplemental Material
Acknowledgments
This study was supported by grants from the National Institute of Environmental Health Sciences/National Institutes of Health (NIH): R01ES015584 (G.S.P., S.-M.H.), RC2ES018758 (G.S.P., S.-M.H.), P30ES006096 (S.-M.H.) and the Michael Reese Research and Education Foundation (G.S.P.). The authors gratefully thank G. Shi, D. Hu (Department of Urology, UIC) for their technical assistance; M. Bosland (Department of Pathology, UIC) for pathologic consultations; J. Ying (UC) for biostatistics consultation; M. Medvedovich (UC) for Bioinformatics assistance; and the Genomics, Epigenomics and Sequencing Core (UC) for the array service. The University of Virginia’s Center for Research in Reproduction Ligand Assay and Analysis Core performed steroid RIA services supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)/NIH grant U54-HD28934.
References
- Bosland MC, Ford H, Horton L. 1995. Induction at high incidence of ductal prostate adenocarcinomas in NBL/Cr and Sprague-Dawley Hsd:SD rats treated with a combination of testosterone and estradiol-17β or diethylstilbestrol. Carcinogenesis 16(6):1311–1317, PMID: 7788848. [DOI] [PubMed] [Google Scholar]
- Bostwick DG. 1996. Progression of prostatic intraepithelial neoplasia to early invasive adenocarcinoma. Eur Urol 30(2):145–152, PMID: 8875195. [DOI] [PubMed] [Google Scholar]
- Bostwick DG, Cheng L. 2012. Precursors of prostate cancer. Histopathology 60(1):4–27, PMID: 22212075, 10.1111/j.1365-2559.2011.04007.x. [DOI] [PubMed] [Google Scholar]
- Calderon-Gierszal E, Prins GS. 2015. Directed differentiation of human embryonic stem cells into prostate organoids in vitro and its perturbation by low-dose bisphenol A exposure. PloS One 10(7):e0133238, PMID: 26222054, 10.1371/journal.pone.0133238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho L, Basavarajappa MS, Chang CW, Han T, Kobets T, Koturbash I, et al. . 2015. Effects of oral exposure to bisphenol A on gene expression and global genomic DNA methylation in the prostate, female mammary gland, and uterus of NCTR Sprague-Dawley rats. Food Chem Toxicol 81:92–103, PMID: 25862956, 10.1016/j.fct.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty D, Sboner A, Nair SS, Giannopoulou E, Li R, Hennig S, et al. . 2014. The oestrogen receptor alpha-regulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nat Commun 5:5383, PMID: 25415230, 10.1038/ncomms6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin RE, Adams J, Boekelheide K, Gray LE Jr, Hayward SW, Lees PS, et al. . 2008. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol 83(3):157–395, PMID: 18613034, 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- Cheong A, Zhang X, Cheung YY, Tang WY, Chen J, Ye SH, et al. . 2016. DNA methylome changes by estradiol benzoate and bisphenol A links early-life environmental exposures to prostate cancer risk. Epigenetics 11(9):674–689, PMID: 27415467, 10.1080/15592294.2016.1208891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfosse V, Grimaldi M, Cavaillès V, Balaguer P, Bourguet W. 2014a. Structural and functional profiling of environmental ligands for estrogen receptors. Environ Health Perspect 122(12):1306–1313, PMID: 25260197, 10.1289/ehp.1408453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfosse V, Grimaldi M, le Maire A, Bourguet W, Balaguer P. 2014b. Nuclear receptor profiling of bisphenol-A and its halogenated analogues. Vitam Horm 94:229–251, PMID: 24388193, 10.1016/B978-0-12-800095-3.00009-2. [DOI] [PubMed] [Google Scholar]
- Doerge D, Twaddle NC, Vanlaningham M, Fischer JW. 2010. Pharmacokinetics of bisphenol-A in neonatal and adult Sprague-Dawley rats. Toxicol Appl Pharmacol 247(2):158–165, PMID: 20600215, 10.1016/j.taap.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Durando M, Kass L, Piva J, Sonnenschein C, Soto AM, Luque EH, et al. . 2007. Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ Health Perspect 115(1):80–86, PMID: 17366824, 10.1289/ehp.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, Mendonca K, Hauser R, Calafat AM, Ye X, Meeker JD, et al. . 2013. Potential sources of bisphenol A in the neonatal intensive care unit. Pediatrics 131(3):483–489, PMID: 23420909, 10.1542/peds.2012-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk C, Kim JH, Jones TR, McEachin RC, Nahar MS, Dolinoy DC, et al. . 2015. Bisphenol A-associated alterations in genome-wide DNA methylation and gene expression patterns reveal sequence-dependent and non-monotonic effects in human fetal liver. Environ Epigenet 1(1):dvv006, PMID: 27358748, 10.1093/eep/dvv00610.1093/eep/dvv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt PG, Mevåg B, Tveter KJ, Purvis K. 1983. A systematic study of biochemical differences between the lobes of the rat prostate. Int J Androl 6(6):553–562, PMID: 6668082, 10.1111/j.1365-2605.1983.tb00346.x. [DOI] [PubMed] [Google Scholar]
- Gerona RR, Woodruff TJ, Dickenson CA, Pan J, Schwartz JM, Sen S, et al. . 2013. Bisphenol-A (BPA), BPA glucuronide, and BPA sulfate in midgestation umbilical cord serum in a northern and central California population. Environ Sci Technol 47(21):12477–12485, PMID: 23941471, 10.1021/es402764d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. . 2015. EDC-2: the Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev 36:E1–E150, PMID: 26544531, 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi N, Sugimura Y, Kawamura J, Donjacour AA, Cunha GR. 1991. Morphological and functional heterogeneity in the rat prostatic gland. Biol Reprod 45(2):308–321, PMID: 1786296. [DOI] [PubMed] [Google Scholar]
- Hines CJ, Jackson MV, Deddens JA, Clark JC, Ye X, Christianson AL, et al. . 2017. Urinary bisphenol A (BPA) concentrations among workers in industries that manufacture and use BPA in the USA. Ann Work Expo Health 61(2):164–182, PMID: 28395354, 10.1093/annweh/wxw021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SM, Cheong A, Lam HM, Hu WY, Shi GB, Zhu X, et al. . 2015. Exposure of human prostaspheres to bisphenol A epigenetically regulates SNORD family non-coding RNAs via histone modification. Endocrinology 156(11): 3984–3995, PMID: 26248216, 10.1210/en.2015-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. 2006. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res 66(11):5624–5632, PMID: 16740699, 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WY, Shi GB, Lam HM, Hu DP, Ho SM, Madueke IC, et al. . 2011. Estrogen-initiated transformation of prostate epithelium derived from normal human prostate stem-progenitor cells. Endocrinology 152(6):2150–2163, PMID: 21427218, 10.1210/en.2010-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WY, Shi GB, Xie L, Birch L, Hu DP, Majumdar S, et al. . 2015. Chronic low-dose bisphenol A (BPA) exposure alters rat prostate stem cell homeostasis. In: Proceedings of the Society of Toxicology 54th Annual Meeting, 22-26 March 2015 San Diego, CA, Vol. 144 Toxicologist: Late-Breaking Supplement to Toxicological Sciences 144(1). Abstract No. 2835. [Google Scholar]
- Jia X, Li X, Xu Y, Zhang S, Mou W, Liu Y, et al. . 2011. SOX2 promotes tumorigenesis and increases the anti-apoptotic property of human prostate cancer cell. J Mol Cell Biol 3(4):230–238, PMID: 21415100, 10.1093/jmcb/mjr002. [DOI] [PubMed] [Google Scholar]
- Kaklamani V, Yi N, Zhang K, Sadim M, Offit K, Oddoux C, et al. . 2011. Polymorphisms of ADIPOQ and ADIPOR1 and prostate cancer risk. Metab Clin Exp 60(9):1234–1243, PMID: 21397927, 10.1016/j.metabol.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Sartor MA, Rozek LS, Faulk C, Anderson OS, Jones TR, et al. . 2014. Perinatal bisphenol A exposure promotes dose-dependent alterations of the mouse methylome. BMC genomics 15:30, PMID: 24433282, 10.1186/1471-2164-15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie C, Lessard J, Ben Aicha S, Savard MP, Pelletier M, Fournier A, et al. . 2008. Androgen-regulated transcription factor AibZIP in prostate cancer. J Steroid Biochem Mol Biol 108(3–5):237–244, PMID: 17933519, 10.1016/j.jsbmb.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Leav I, Ho S, Ofner P, Merk F, Kwan P, Damassa D. 1988. Biochemical alterations in sex hormone-induced hyperplasia and dysplasia of the dorsolateral prostates of Noble rats. J Natl Cancer Inst 80(13):1045–1053, PMID: 2457709. [DOI] [PubMed] [Google Scholar]
- Matsumoto J, Yokota H, Yuasa A. 2002. Developmental increases in rat hepatic microsomal UDP-glucuronyltransferase activities toward xenoestrogens and decreases during pregnancy. Environ Health Perspect 110(2):193–196, PMID: 11836149, 10.1289/ehp.02110193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman HA, Bosland MC, Walden PD, Heinze JE. 2002. Evaluation of the adequacy of published studies of low-dose bisphenol A on rodent prostate for use in human risk assessment. Regul Toxicol Pharmacol 35(3):338–346, PMID: 12202049. [DOI] [PubMed] [Google Scholar]
- Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. . 2008. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 68(11):4447–4454, PMID: 18519708, 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles JL, Hu WY, Prins GS. 2011. Estrogen action and prostate cancer. Expert Rev Endocrinol Metab 6(3):437–451, PMID: 21765856, 10.1586/eem.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Siefert K, Ransom S, Johnson T, Pinkerton J, Anderson L, et al. . 2008. Maternal bisphenol-A levels at delivery: a looming problem? J Perinatol 28(4):258–263, PMID: 18273031, 10.1038/sj.jp.7211913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. 1963. Comparative aspects of development and structure in the prostate. In: Biology of the Prostate and Related Tissues, Vol. 12 Vollmer EP, ed. Washington, DC:National Cancer Institute, 1–27. [PubMed] [Google Scholar]
- Prins GS. 1987. Prolactin influence on cytosol and nuclear androgen receptors in the ventral, dorsal, and lateral lobes of the rat prostate. Endocrinology 120(4):1457–1464, PMID: 3493896, 10.1210/endo-120-4-1457. [DOI] [PubMed] [Google Scholar]
- Prins GS. 1989. Differential regulation of androgen receptors in the separate rat prostate lobes: androgen independent expression in the lateral lobe. J Steroid Biochem 33(3):319–326, PMID: 2779222. [DOI] [PubMed] [Google Scholar]
- Prins GS. 1992. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology 130(6):3703–3714, PMID: 1597166, 10.1210/endo.130.6.1597166. [DOI] [PubMed] [Google Scholar]
- Prins GS. 1992. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology 130(4):2401–2412, PMID: 1547747, 10.1210/endo.130.4.1547747. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L, Habermann H, Chang WY, Tebeau C, Putz O, et al. . 2001. Influence of neonatal estrogens on rat prostate development. Reprod Fertil Dev. 13(4):241–252, 10.1071/RD00107. [DOI] [PubMed] [Google Scholar]
- Prins GS, Calderon-Gierszal EL, Hu WY. 2015. Stem cells as hormone targets that lead to increased cancer susceptibility. Endocrinology 156(10):3451–3457, PMID: 26241068, 10.1210/en.2015-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Ho SM. 2010. Early life estrogens and prostate cancer in an animal model. J Dev Orig Health Dis 1(6):365–370, PMID: 24795802, 10.1017/S2040174410000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Hu WY, Shi GB, Hu DP, Majumdar S, Li G, et al. . 2014. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology 155(3):805–817, PMID: 24424067, 10.1210/en.2013-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Ye SH, Birch L, Ho SM, Kannan K. 2011. Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague-Dawley rats. Repro Toxicology 31(1):1–9, PMID: 20887781, 10.1016/j.reprotox.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylkkänen L, Santti R, Newbold R, McLachlan J. 1991. Regional differences in the prostate of the neonatally estrogenized mouse. Prostate 18(2):117–129, PMID: 2006118. [DOI] [PubMed] [Google Scholar]
- Rochester JR. 2013. Bisphenol A and human health: a review of the literature. Reprod Toxicol 42:132–155, PMID: 23994667, 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Seachrist DD, Bonk KW, Ho SM, Prins GS, Soto AM, Keri RA. 2016. A review of the carcinogenic potential of bisphenol A. Reprod Toxicol 59:167–182, PMID: 26493093, 10.1016/j.reprotox.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlur SR, Mertz KD, Hoshida Y, Demichelis F, Lupien M, Perner S, et al. . 2008. Estrogen-dependent signaling in a molecularly distinct subclass of aggressive prostate cancer. J Natl Cancer Inst 100(11):815–825, 10.1093/jnci/djn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shappell S, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, et al. . 2004. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Biology Committee. Cancer Res 64(6):2270–2305, PMID: 15026373, 10.1158/0008-5472.CAN-03-0946. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kelly TK, Jones PA. 2010. Epigenetics in cancer. Carcinogenesis 31(1):27–36, PMID: 19752007, 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. 2016. Cancer statistics, 2016. CA: A Cancer Journal for Clinicians 66(1):7–30, 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Takizawa I, Lawrence MG, Balanathan P, Rebello R, Pearson HB, Garg E, et al. . 2015. Estrogen receptor alpha drives proliferation in PTEN-deficient prostate carcinoma by stimulating survival signaling, MYC expression and altering glucose sensitivity. Oncotarget 6(2):604–616, PMID: 25436982, 10.18632/oncotarget.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WY, Morey LM, Cheung YY, Birch L, Prins GS, Ho SM. 2012. Neonatal exposure to estradiol/bisphenol A alters promoter methylation and expression of Nsbp1 and Hpcal1 genes and transcriptional programs of Dnmt3a/b and Mbd2/4 in the rat prostate gland throughout life. Endocrinology 153(1):42–55, PMID: 22109888, 10.1210/en.2011-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennstedt P, Bölch C, Strobel G, Minner S, Burkhardt L, Grob T, et al. . 2014. Patterns of TPD52 overexpression in multiple human solid tumor types analyzed by quantitative PCR. Int J Oncol 44(2):609–615, PMID: 24317684, 10.3892/ijo.2013.2200. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, et al. . 2012. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33(3):378–455, PMID: 22419778, 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Gerona RR, Kannan K, Taylor JA, van Breemen RB, Dickenson CA, et al. . 2014. A round robin approach to the analysis of bisphenol A (BPA) in human blood samples. Environ Health 13:25, 10.1186/1476-069X-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. 2007. Human exposure to bisphenol A (BPA). Repro Toxicology 24(2):139–177, 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Kaufman JM, Goemaere S, van Pottelberg I. 2002. Estradiol in elderly men. Aging Male 5(2):98–102, PMID: 12198740. [PubMed] [Google Scholar]
- Viñas R, Watson CS. 2013. Mixtures of xenoestrogens disrupt estradiol-induced non-genomic signaling and downstream functions in pituitary cells. Environ Health 12:26, 10.1186/1476-069X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, et al. . 1997. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci U S A 94(5):2056–2061, PMID: 9050904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Trevino LS, Wong RL, Medvedovic M, Chen J, Ho SM, et al. . 2016. Reprogramming of the epigenome by MLL1 links early-life environmental exposures to prostate cancer risk. Mol Endocrinol 30(8):856–871, 10.1210/me.2015-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RLY, Wang Q, Treviño LS, Bosland MC, Chen J, Medvedovic M, et al. . 2015. Identification of secretaglobin Scgb2a1 as a target for developmental reprogramming by BPA in the rat prostate. Epigenetics 10(2):127–134, PMID: 25612011, 10.1080/15592294.2015.1009768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino H, Ichihara T, Kawabe M, Imai N, Hagiwara A, Asamoto M, et al. . 2002. Lack of significant alteration in the prostate or testis of F344 rat offspring after transplacental and lactational exposure to bisphenol A. J Toxicol Sci 27(5):433–439, PMID: 12533913. [DOI] [PubMed] [Google Scholar]
- Yuen M, Leung L, Wang J, Wong YC, Chan FL. 2005. Enhanced induction of prostatic dysplasia and carcinoma in the Noble rat model by combination of neonatal estrogen exposure and hormonal treatments in adulthood. Int J Oncology 27(6):1685–1695, PMID: 16273225. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.