Abstract
In this review, we focus on an important aspect of ion mobility (IM) research, namely the reporting of quantitative ion mobility measurements in the form of the gas-phase collision cross section (CCS), which has provided a common basis for comparison across different instrument platforms and offers a unique form of structural information, namely size and shape preferences of analytes in the absence of bulk solvent. This review surveys the over 24,000 CCS values reported from IM methods spanning the era between 1975 to 2015, which provides both a historical and analytical context for the contributions made thus far, as well as insight into the future directions that quantitative ion mobility measurements will have in the analytical sciences. The analysis was conducted in 2016, so CCS values reported in that year are purposely omitted. In another few years, a review of this scope will be intractable, as the number of CCS values which will be reported in the next three to five years is expected to exceed the total amount currently published in the literature.
Graphical Abstract

Quantitative ion mobility methods have seen a resurgence of recent and significant interest due to the fact that in the past three years, a number of new and updated ion mobility technologies combined with mass spectrometry (IM-MS) have emerged as commercially-available instrumentation for routine chemical analysis. These have included updates to traveling wave instrumentation (TWIMS), new uniform field drift tubes (DTIMS) operated at both elevated 1 and reduced pressures (less than 10 Torr),2–4 and a newly-developed ion trapping device operated in a mobility-selective mode (trapped ion mobility spectrometry, TIMS) 5–7. Other ion mobility techniques including cyclic and extended path length traveling wave devices are currently in development.8,9 This recent and unprecedented commercial accessibility of IM-MS in combination with existing liquid chromatography and tandem MS functionality has provided powerful multidimensional separation capabilities to the greater research community,10–13 which in turn has broadened the scope of applications and fields in which IM-MS is now making a significant impact.14–19 Many of the contemporary challenges being addressed by IM-MS are grand challenges of our era of humanity.11
Ion mobility is generally utilized in one of three ways by researchers (Table 1): (1) as an added dimension of separation for increasing the peak capacity and partitioning the chemical noise from analyte signals of interest, (2) as an additional measurement for analyte identification and characterization, and/or (3) as a structural measurement technique, where the ion mobility information is used to infer some details regarding the structure (either primary or higher-order) of the analyte. The latter two strategies, analyte identification and structural measurement, are achieved by converting the ion mobility measurement (typically drift time), to an ion-neutral collision cross section value, which represents a fundamental property of the analyte comparable across different laboratories. Analyte identification and correlation can also proceed using the standardized mobility value, as has been achieved in the field of stand-alone ion mobility spectrometers utilized for chemical detection and screening,20,21 although the fundamental meaning of the mobility measurement is more accessible when discussed in the context of the analyte CCS.
TABLE 1.
Three key analytical uses of ion mobility
| Analytical Use of Ion Mobility | Description | Additional Requirements | Example Application Areas |
|---|---|---|---|
| 1. Chemical Separation | Partition signal from chemical noise and increase peak capacity of the analysis | None | Detection of Illicit compounds (e.g., drugs and explosives) and screening of exogenous metabolites (e.g., pesticides and industrial chemicals) |
| 2. Analyte Identification and Characterization | Use CCS measurement to characterize unknowns by correlation | Reference values from databases and libraries incorporating normalized drift times, reduced mobilities, and/or CCS | Emerging omic and small molecule discovery initiatives |
| 3. Structural Analysis | Utilize the experimental CCS to infer structural information | Computational methods to link theoretical structure(s) to the experimental CCS | Insights into protein complex arrangements and structure |
The Collision Cross Section
One of the contemporary challenges with interpreting the meaning of the CCS lies in the fact that it is not a true molecular cross section, but rather represents an observational property that averages all geometric orientations and interaction types (head-on, “glancing”, and “orbiting” collisions, multiple collisions within cavities of the analyte, etc.) across the experimental measurement time.22–25 These effects include both contributions from the drift gas itself (momentum transfer and gas polarization effects) and contributions arising from the ion mobility experiment (temperature and magnitude of the electric field). Classically, the CCS determined from ion-gas collision measurements is referred to as the momentum transfer or diffusion CCS to specify the importance and dependence that the drift gas has on the resulting quantity being obtained.26,27
As a result of these contributions, the empirical CCS is a macroscopic quantity which is specific to the identity of the drift gas as well as the temperature and electric field used during the measurement,28,29 and so by the strictest definition, CCS is not an intrinsic property of the analyte, although it is very closely linked to one (namely the microscopic cross section of the analyte). Mathematically, the CCS represents the area of a circle, and thus the structural information is “coarse-grained” in nature. While significant for small molecule studies, this level of granularity is less of an issue when probing coarse structural features such as domain-level information for protein assemblies. 30,31
The CCS is a quantity that is now routinely obtainable from a variety of ion mobility experiments, and, although less frequently discussed, the CCS can also be obtained from mass spectrometry experiments where gas collisions are present. Mass spectrometric methods utilized for measuring CCS have included pressure correlated ion loss studies in magnetic sector,32–34 triple quadrupole,35,36 and time-of-flight instruments;37 ion relaxation times in an ion trap;38 and peak width analysis from ion cyclotron resonance measurements.39,40 Ion mobility methods currently provide the most precise measurements of the CCS, with precision being linked to the experimental certainty in all of the parameters which govern the IM separation, such as the gas temperature, electric field, gas number density (via pressure and temperature) and the geometric distances within the instrumentation. As such, uniform field drift tubes (DTIMS) and differential mobility analyzers (DMA) afford the highest CCS precision since experimental quantities in these techniques can be well-characterized. It should be noted here that precision and accuracy are important distinctions, as very reproducible CCS values can now be obtained (better than 2%),2,41 but their accuracy cannot be validated without comparing the ion mobility results to CCS measurements obtained from other techniques, which at this time are still in development.42–44 Despite these standing questions regarding the accuracy and meaning of the CCS, it is clear that there is immense value in reporting a standardized fundamental property of an analyte in the form of a CCS which is both highly-reproducible and now readily-accessible by a large number of researchers.
Recent publications have utilized an elegant nomenclature for CCS reporting whereby the measurement technique is denoted as a superscripted prefix, while the drift gas is specified as a subscripted suffix, for example, DTCCSN2 to denote a nitrogen CCS value measured from a drift tube instrument.45–48 This nomenclature is summarized in Table 2 along with specific recommendations for the instrumentation shorthand. Given the oftentimes ambiguous nature of the experimental context in which CCS values are reported, the nomenclature formalized in Table 2 is recommended for future use in the field.
TABLE 2.
Formalized nomenclature for reporting CCS measurements in the context of the technique and drift gas utilized
| CCS Measurement Technique | Technique Shorthand a. | Nomenclature for CCS Reporting b. |
|---|---|---|
| Drift Tube Ion Mobility Spectrometry (DTIMS) | DT | DTCCSX |
| Traveling Wave Ion Mobility Spectrometry (TWIMS) | TW | TWCCSX |
| Trapped Ion Mobility Spectrometry (TIMS) | TIMS | TIMSCCSX |
| Differential Mobility Analyzer (DMA) | DMA | DMACCSX |
Only the four major ion mobility techniques which report CCS are listed.
X denotes the drift gas or drift gas equivalent for calibrated values (X = He, N2, Ar, CO2, etc.)
Significant CCS Contributions
The emerging importance of CCS to support contemporary analytical trends is evidenced by the fact that over half of the over 24,000 canonical CCS values reported between 1975 and 2015 has been published within the last five years (Figure 1A). Examining the histogram in Figure 1A indicates there was an initial surge of CCS values reported between 1995 and 1999 which was largely in response to the introduction of ESI and MALDI ionization techniques, followed by a decade of relatively few new CCS values being reported (2000–2009). Starting in 2010, the number of CCS values reported increased drastically, which is interpreted as being a direct response of the introduction of new ion mobility techniques, including commercial TWIMS technology in 2006,49 confining RF DTIMS in 2010,50 and DTIMS integrated with ion funnels, initially reported in 2005 and commercialized in 2014.2,51
Figure 1.
(A) The number of CCS values published over the 40-year span between 1975 and 2015. The drift gas used in the measurement or calibration is specified for each year bin. (B) The laboratories and studies which have made significant contributions in terms of number of values reported.
Major contributions from specific laboratories are noted in Figure 1B and include several large-scale studies from Clemmer and coworkers examining electrosprayed peptides and proteins in helium (ca. 4200 values),52–55 contributions from Bowers and coworkers on hydrocarbons and carbon clusters (ca. 400 values),56–59 studies from Jarrold and coworkers investigating carbon, silicon, and palladium clusters (ca. 550 values),60–63 contributions from Russell and coworkers reporting singly-charged CCS values of MALDI generated peptides and proteins (ca. 650 values);64,65 TWIMS and DTIMS studies from Pagel and coworkers investigating both helium and nitrogen CCS for carbohydrates (ca. 1300 values),45,66,67 work from McLean and coworkers which include a number of lipid, peptide, and carbohydrate CCS values in both helium and nitrogen (ca. 1000 values),2,68,69 and recent TWIMS work from Astarita and coworkers reporting nitrogen CCS values for both lipids and metabolites (ca. 450 values).70,71 The largest single quantitative ion mobility survey to date represents the ca. 8,700 nitrogen CCS values published by Smith and coworkers for tryptic peptides in support of proteomics studies.72 While the early studies have focused on obtaining structural information through the measurement of the CCS, several of the recent contributions have been purposed as cross sectional databases in support of analyte characterization. The motivation for utilizing CCS as a molecular descriptor (c.f., Table 1) is an emerging application area in the field of analytical chemistry. Additionally, the high quality CCS data from the Clemmer73 and Bush laboratories41,50,74 are routinely used for calibrating ion mobility instrumentation.
While only major studies are highlighted here, the majority of contributions to the CCS canon (75%) have come from smaller studies which report 50 or fewer CCS values (Figure 2). In fact, there are only three individual studies which have reported over 1,000 CCS values and thus would be considered large-scale surveys,54,55,72 underscoring the fact that the reporting of quantitative ion mobility measurements is predominantly an interlaboratory initiative.
Figure 2.

Histogram illustrating the number of CCS values which are reported per publication. The bracketed bins draw attention to the fact that most of the CCS measurements have come from smaller studies reporting 50 or fewer cross section values.
Drift Gases Represented
While measurements obtained in helium and nitrogen represent the vast majority of the CCS values reported (95%, c.f., Figure 1), there have been a few quantitative studies conducted in alternative drift gases, most representing the classic atomic and small molecule studies compiled by Mason and coworkers during the early developments of analytical ion mobility,75–78 but also early work from Hill and coworkers exploring CCS differences of small peptides and drug molecules in helium, nitrogen, argon, and carbon dioxide.79 Recent studies which explicitly report CCS values in alternative drift gases include measurements of ammonium in helium, nitrogen, argon and carbon dioxide from Viehland and coworkers,80 the combined DTIMS and TWIMS study from Barran and coworkers investigating myoglobin in helium, nitrogen, argon, and neon,81 DMA measurements of CCS in air from both de la Mora and coworkers82 and Hogan and coworkers,83 and DTIMS work from Fjeldsted and coworkers exploring the CCS differences of pesticides in a variety of drift gases including helium, nitrogen, carbon dioxide, nitrous oxide, argon, and sulfur hexafluoride.84
The sparse amount of CCS data reported for gases other than helium and nitrogen is largely a combined result of both technical challenges with operating under different drift gas conditions (instrument tuning, pressure gauge calibration issues, and uncertainty with calculating the CCS from measured drift times), as well as fundamental difficulties with interpreting the structural meaning of CCS values obtained using gases other than helium. The typically better correlation of helium CCS values to theoretical results is primarily a consequence of the lower contribution of ion-neutral polarization effects in atomic helium (α=0.21 Å3) as compared to diatomic nitrogen (α=1.74 Å3) and other neutral gases (e.g., argon, α=1.64 Å3; carbon dioxide, α=2.91 Å3),79,85–87 although it should be noted that significant and recent efforts have been made in improving the fundamental theories used in predicting nitrogen-based CCS values from candidate structures.25,26,88,89 In addition to the better theoretical correlation of helium CCS, there is also some evidence that helium offers analytical benefits in reducing mass-mobility discrimination and improving ion transmission in dispersive (DTIMS and TWIMS) ion mobility instrumentation.90,91 The choice of nitrogen as a drift gas stems from practical considerations of cost and availability, fundamental considerations regarding nitrogen’s resistance to electrical discharge (dielectric breakdown) and analytical improvements in resolving power due to the longer residence time of ions (i.e., lower reduced mobility values) within the ion mobility experiment.92 While these attributes are shared by other drift gases such as argon and carbon dioxide, their use in quantitative IM research has not yet been significantly explored. It is anticipated that the meager quantitative IM data currently available for alternative gases represents only a temporary deficiency as the instrumentation and CCS measurement capabilities to support different drift gases are now becoming widely available, and evidence is mounting in support of the analytical benefits of conducting IM separations in other drift gases such as argon and carbon dioxide.93–97
Composition of Measurements
An analysis of the composition the CCS values published from 1975 to 2015 is presented in Figure 3 for a few select categories. With regards to instrumentation (Figure 3A), most (87%) of the CCS values represent measurements conducted in DTIMS instruments, which include both elevated98–102 and reduced pressure DTIMS instrumentation,103–110 as well as instrumentation utilizing electric field-mediated ion focusing strategies such as periodic DC,111,112 confining RF,4,50 and electrodynamic ion funnels.2,51,113 A cursory comparison of the measurements themselves (not shown) indicates there is no significant differences between the CCS values obtained using these different modes of DTIMS operation, suggesting these focusing strategies do not perturb the resulting CCS. Because DTIMS still exhibits the highest precision when measuring the CCS and the direct relationship between drift time and cross section allows broad scale CCS determination of mixtures, it is no surprise that DTIMS has contributed to the majority of values published to date. TWIMS values obtained from calibration represent 9% of the CCS values,114–117 while the remaining values are from other IM techniques such as DMA82,83 and TIMS.118–121
Figure 3.
Composition of CCS values with respect to (A) the ion mobility instrumentation used, (B) the drift gas, (C) specific charge state reported, and (D) the chemical classes investigated.
Regarding the selection of drift gas (Figure 3B), there are slightly more CCS values being obtained in nitrogen (49%) as compared to helium (46%), with reporting of nitrogen-based CCS values being a recent analytical trend in the field (c.f., Figure 1A). Measurements in ambient air comprise 3% of the CCS values, which are from elevated pressure DTIMS and DMA studies. The remaining 2% of values are for measurements conducted in argon (0.5%), carbon dioxide (0.3%), oxygen (0.3%), neon (0.2%), nitrous oxide (0.2%), and others (0.5%). Specific motivations for drift gas selection are discussed in the previous section.
Figure 3C indicates that the majority of CCS values are for low charge-state cations (+1, +2, and +3 ions, collectively representing 78% of all values reported), and thus anion CCS values are currently underrepresented, comprising only 8% of the total body of work. This predominance of positive ion data is expected given that MS-based studies are preferentially conducted in positive ion mode. Most of the anions CCS values reported are from two recent carbohydrate studies, one on chemically-released glycans and corresponding ion fragments generated in source,67 and another reporting negative ion CCS values on dextran and pullulan oligosaccharides.122 Remaining anion contributions represent the classic DTIMS studies on atomic and molecular clusters,57,63,123 and recent negative ion measurements for proteins,124 lipids,1,71 and metabolites.70,125 The primary ionization method used in the quantitative measurement of the CCS is ESI (87%, not shown) which tends to produce primarily +2 ions for tryptic peptides.126 As tryptic peptides represent the majority of CCS measurements reported in the literature (vide infra), it is no surprise that there are more +2 ions than any other charge state. Laser-based ionization (MALDI and LDI) which produce mainly +1 ions in positive ion mode comprise only 11% of the CCS values (not shown). Higher charge state cations (+4 or greater) comprise 14% of the CCS values reported, which is in line with the number of protein ion CCS values represented in the analysis (9% of the total, not shown).
Finally, in Figure 3D, an analysis of the contributions made within specific chemical classes reveal the majority of CCS values reported in the literature are for peptides and proteins (70%), with carbohydrates (8%), inorganics (e.g., clusters, nanomaterials, and salts; 8%), and other small molecules (e.g., hydrocarbons and metabolites; 6%) representing the remainder of values. The focus on peptide and protein work can be rationalized as being a result of continued efforts for adapting ion mobility technologies to proteomics workflows,127–129 but also a practical consequence of both the ease of generating large pools of peptides derived from enzymatic digestion130 and the fact that the structural and charge-state heterogeneity of proteins necessitates the reporting of many CCS values for a single protein. 131–134
To summarize the observations in Figure 3, most quantitative ion mobility studies to date have used DTIMS for peptide and protein analysis, with an approximate equal number of measurements represented in both helium and nitrogen drift gases.
Chemical Space Represented by IM-MS Analysis
Figures 4 and 5 projects all of the canonical CCS values as a function of the ion mass, for helium and nitrogen-based ion mobility measurements, respectively. The scattering of measurements (lower panels) are noticeably different in both gases, underscoring the fact that different analytes and charge states are represented in each type of gas. For example, a larger percentage of helium CCS values are singly-charged (37%) compared to a smaller percentage of singly-charged values in nitrogen (13%). Nitrogen CCS values also contain a significant number of triply-charged measurements (34%), in contrast to helium CCS values, which are comprised of only 14% triply-charged CCS values. This is one reason for the more prominent clustering of higher charge-state measurements in nitrogen (Figure 5, lower panel). There are also a significant number of CCS values for atomic and molecular clusters (carbon, silicon, and inorganic salts) which are unique to the helium CCS measurements, resulting in the trends prominently observed at low CCS (Figure 4, lower panel). Nitrogen CCS values are larger in magnitude than helium values due to the higher momentum contribution of the nitrogen molecule as well as the stronger polarization which in turn leads to temporally-extended ion-neutral interactions in the IM experiment.
Figure 4.
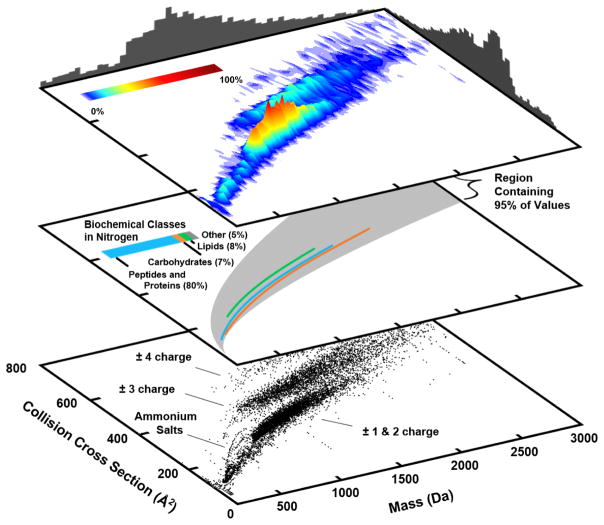
(lower panel) Helium-specific conformational space plot which projects helium-based CCS values as a function of the analyte mass. (middle panel) The composition and chemical space occupancy of specific biomolecules. (top panel) A 3-dimensional surface plot illustrating the regions of highest density in terms of the numbers of CCS values.
Figure 5.
Nitrogen-specific conformational space plot which projects nitrogen-based CCS values as a function of the analyte mass, along with (middle panel) the biomolecular composition and occupancy and (top panel) the 3-dimensional surface density plot of CCS values reported.
The central panels in both Figures 4 and 5 project the average mathematical fits to specific biochemical classes based on a power-law relationship.2 Only the fits to singly-charged analyte is shown, and fits are not extrapolated beyond the range of measurements. The total chemical occupancy of all measurements is illustrated by a 95% data inclusion area (grey shaded region). The general conformational ordering of biomolecules observed here qualitatively correlates to the gas-phase structural trends noted from previously studies, that is, lipids adopt more extended structures in the gas-phase than peptides and carbohydrates.2,135,136 The quantitative differences observed between helium and nitrogen are a consequence of evaluating the CCS values corresponding to different analytes in each figure. This can be seen by examining the biochemical class compositions which are noted in the central panel of each figure, where for example, significantly more peptides and proteins are represented in nitrogen (80%) than helium (67%).
The 3-dimensional surface plots and associated histograms projected on the top panels in Figures 4 and 5 illustrate the distribution of CCS values reported for both helium and nitrogen drift gas. Overall, the analytes surveyed from both gases fall within a similar mass window between 500 to 1500 Da with more values at lower mass reported for helium than nitrogen. As many of the helium measurements are from earlier work in the field and represent singly-charged analytes, it is no surprise that the overall coverage concerns lower mass analytes.
CCS Coverage over Time
Figure 6 compares the number of CCS values reported over the past 40 years as they correlate to mass. This analysis reveals that, as expected, the focus of quantitative ion mobility studies has shifted over time to higher mass due to improvements in technology and methods used to desorb, ionize, and stabilize large analytes such as biomolecules. Prior to the widespread use of soft ionization methods (ca. 1995), the average mass of ions for which CCS values were being reported was less than 100 Da,75–78,137–141 and in the decade following the adoption of MALDI and ESI (1996–2005) in research instrumentation, a broad range of ion masses up to ca. 2500 Da were investigated, though the majority of measurements were centered on low mass studies around 300 Da. In the past decade (2006–2015), the average ion mass was approximately 1000 Da and represents predominately peptide CCS values, however significant efforts were also made for reporting CCS values of lower mass ions centered around 400 Da, the latter representing analytical interests in short-chain carbohydrates,142–147 metabolites,70,125,148–151 and drug-like molecules.84,87,89,152 Figure 6B contains the distribution of CCS reporting with analyte masses extending up to the megaDalton range, which illustrates the recent analytical trend of utilizing quantitative ion mobility methods to study the structure of large protein assemblies,50,124,153–159 some of which are annotated in the figure. These studies specifically target IM-based measurements towards the interpretation of molecular structure. Note that the vertical scale in Figure 6 is the same in both panels, however, the bin size is increased in Figure 6B (from 50 Da to 10 kDa) to accommodate the broader mass range being projected. A final observation to make from Figure 6A is that the bimodal distribution observed over the past five years (2011–2015) closely mimics the analytical trend observed within the largest chemical database, PubChem,160,161 where chemical entries have, over time, shifted to focusing on lower mass analytes while the total number of entries in PubChem currently exhibits a bimodal mass distribution.11
Figure 6.
Histogram illustrating the number of CCS values reported as a function of mass with data sets delineated into specific timespans. Panel (A) contains the histogram for low mass analytes below 3000 Da, with arrows denoting the approximate mass where each distribution exhibits a maximum. Panel (B) contains the histogram for high mass analytes above 3000 Da, with labels calling out select protein assemblies which have been studied. Note that the vertical scales are the same in both panels; however, the bin size in panel B (10 kDa) is different than the bin sized used in panel A (50 Da).
The bubble plot projection in Figure 7 compares the number of CCS values reported over time with respect to specific analyte classes and types. In this projection, the bubble size correlates to the number of values reported for each corresponding year. Early quantitative IM studies focused on atomics and small molecules. A significant number of the small molecules CCS values consists of aromatic hydrocarbons.98,119,139,162–164 Starting in the 1990s, interest in inorganic compounds (metal salts, atomic and molecular clusters) began to emerge. Very few inorganic compound CCS values were reported between 2000 and 2010, with a resurgence of interest starting in 2013 which were primarily focused on gaining fundamental insights into the structures of inorganic salt and metal clusters.83,165–169 Protein CCS values were initially reported in the late 1990’s by the Jarrold, Clemmer, and Bowers groups,170–173 with sparse numbers of measurements reported thereafter for several years. From the year 2000 onward, efforts in the field were largely concentrated on biological molecules. A significant number of peptide and protein CCS values started appearing again in the literature in 2007. The large blue bubble in Figure 7 corresponds to the 8676 peptide cross sections published by Smith and coworkers in 2010 in support of developing theoretical methods for predicting the IM drift time based upon the primary amino acid sequence.72 While most of the CCS values have been for tryptic peptides, there is recent and significant efforts being made in the quantitative IM analysis of structurally-interesting peptide and protein classes, including helical peptides,174–176 metalloproteins,177–180 intrinsically-disordered proteins,181–184 metamorphic proteins,185,186 amyloids,187–194 and membrane-bound proteins and assemblies.117,195–198 The last three years has seen a balance of cross section reporting across most of the chemical classes, including lipids and carbohydrates. The exception is nucleotide CCS values, which, aside for the 2009 study from McLean and coworkers,68 have been published in small numbers spread across several studies and years, and currently comprise about 1% all CCS values reported.153,199–209 This observation is reflected in the fact that while many of these IM-based biomolecular studies have coincided strongly with developments in MS-based lipidomics, glycomics and metabolomics, the role of mass spectrometry related techniques in genomics research is, and has always been, relatively small.
Figure 7.
Bubble plot projecting the number of CCS values reported over time for the top 7 chemical classes represented. The size of each bubble encodes the relative number of CCS values for each respective year.
Not shown in Figure 7 are the large number of studies which have focused on synthetic polymers,210–220 which, like nucleotides, have seen a small but gradual number of CCS value reporting since the initial measurements by Bowers and coworkers in the late 1990s.221–225 Several recent polymer studies have focused on reporting CCS values for dendrimers.226–228, and polymeric supermolecular assemblies utilized in molecular sensing, catalysis, and advanced materials applications.229–237 Overall, synthetic polymers comprise about 1% of the total number of CCS values reported to date, virtually all of which are measured in helium drift gas or are calibrated to helium-equivalent values. Also not reflected in the analysis presented in Figure 7 are the recent interests in characterizing natural products by IM-MS based CCS measurements.69,116,238–240 Many natural products contain complex and unusual scaffolds which motivates their study by a structurally-selective technique such as IM-MS, however, natural products are conventionally classified based on bioactivity rather than structure and as such molecules which can be considered natural products are represented in virtually all of the chemical classes delineated in this review. A similar issue is seen in metabolites (not shown) which is a classification that includes small peptides, carbohydrates and lipids. Finally, there are a number of CCS measurements which cannot easily be classified into a given chemical class category, such as compounds derived from chemical synthesis.241–245 It is anticipated that additional trends in the analysis of chemical classes not described in this review will become evident as the field of quantitative IM continues to grow.
CONCLUDING REMARKS
This current analysis of all collision cross section values published into the canonical literature from 1975 to 2015 reveals both important analytical trends in the field, such as the focus on biomolecules and drift tube studies, and also avenues where future efforts will make a significant impact. These future analytical prospects include, (1) the use of emerging ion mobility methods and mass-spectrometry based techniques for validating the accuracy of CCS measurements, (2) quantitative IM experiments exploring alternative drift gases such as carbon dioxide and argon, (3) overlapping analyte studies which explicitly compare fundamental differences across different gases, charge states, and polarities (4) anion studies to test whether or not conformational ordering observed for cations is retained in negative ion mode, (5) quantitative studies of underrepresented chemical classes such as nucleotides, lipids, and synthetic polymers, and (6) comprehensive CCS mapping of suites of analytes (e.g., chemical classes, pharmacologically-active, or disease-implicated) in support of unknown identification and characterization by means of searching databases and libraries. In terms of the immediate analytical impact of this current work, the compilation of CCS measurements will provide a basis for correlating future measurements to the canonical literature, enable large-scale studies of the quantitative relationships within chemical classes and across different drift gases, and serve as a basis for developing predictive methods for CCS chemical space occupancy. Importantly, the compilation of these measurements will provide a foundation for supporting future efforts aimed at utilizing the CCS as an additional metric for analyte identification, with correspondence to other analytical measurements such as exact mass, tandem MS data, and chromatographic retention time. Given the rapid growth now being seen in the field of quantitative ion mobility, many of the analytical prospects outlined in this review will likely be realized in the next few years.
Acknowledgments
The authors thank Katrina L. Leaptrot and Stacy D. Sherrod for valuable suggestions during the preparation of this manuscript. This work was supported in part using the resources of the Center for Innovative Technology at Vanderbilt University. Financial support for this work was generously provided by the Vanderbilt Center for Quantitative Sciences under a CQS pilot project award, the National Institutes of Health (NIH R01GM092218), the U.S. Army Research Office and the Defense Advanced Research Projects Agency (DARPA) under Cooperative Agreement Number W911 NF-14-2-0022, and the U.S. Environmental Protection Agency (EPA) under Assistance Agreement No. 83573601. This work has not been formally reviewed by EPA and EPA does not endorse any products or commercial services mentioned in this publication. The views expressed in this document are solely those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the EPA, the Army Research Office, DARPA, or the U.S. Government. The U.S. Government is authorized to reproduce and distribute reprints for Government purposes notwithstanding any copyright notation herein.
Biographies
Jody C. May, Associate Director of the Center for Innovative Technology and Research Assistant Professor, completed his B.S. degree in chemistry at the University of Central Arkansas and his Ph.D. in analytical chemistry from Texas A&M University. His research interests are in the development of multidimensional analytical methods based on ion mobility-mass spectrometry and the application of quantitative ion mobility measurements for chemical characterization in untargeted workflows.
Caleb B. Morris, Ph.D. candidate at Vanderbilt University, completed his B.S. degree in chemistry at the University of Kentucky. His research focuses on broad-scale collision cross section relationships and the development of parametric descriptors for structural classes in quantitative ion mobility studies.
John A. McLean, Director of the Center for Innovative Technology and Stevenson Professor of Chemistry, completed his B.S. degree in chemistry at the University of Michigan, his Ph.D. in analytical chemistry at the George Washington University, and completed his postdoctoral training at Texas A&M University prior to joining the faculty at Vanderbilt University. His research focuses on the intersection of instrumentation and bioinformatics in support of systems, synthetic, and chemical biology initiatives.
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Groessl M, Graf S, Knochenmuss R. Analyst. 2015;140:6904–6911. doi: 10.1039/c5an00838g. [DOI] [PubMed] [Google Scholar]
- 2.May JC, Goodwin CR, Lareau NM, Leaptrot KL, Morris CB, Kurulugama RT, Mordehai A, Klein C, Barry W, Darland E, Overney G, Imatani K, Stafford GC, Fjeldsted JC, McLean JA. Anal Chem. 2014;86:2107–2116. doi: 10.1021/ac4038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ibrahim YM, Baker ES, Danielson WF, III, Norheim RV, Prior DC, Anderson GA, Belov ME, Smith RD. Int J Mass Spectrom. 2014;377:655–662. doi: 10.1016/j.ijms.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen SJ, Giles K, Gilbert T, Bush MF. Analyst. 2016;141:884–891. doi: 10.1039/c5an02107c. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Lima FA, Kaplan DA, Park MA. Rev Sci Instrum. 2011;82:126106. doi: 10.1063/1.3665933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silveira JA, Ridgeway ME, Park MA. Anal Chem. 2014;86:5624–5627. doi: 10.1021/ac501261h. [DOI] [PubMed] [Google Scholar]
- 7.Michelmann K, Silveira JA, Ridgeway ME, Park MA. J Am Soc Mass Spectrom. 2015;26:14–24. doi: 10.1007/s13361-014-0999-4. [DOI] [PubMed] [Google Scholar]
- 8.Deng L, Ibrahim YM, Baker ES, Aly NA, Hamid AM, Zhang X, Zheng X, Garimella SV, Webb IK, Prost SA. ChemistrySelect. 2016;1:2396–2399. doi: 10.1002/slct.201600460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamid AM, Garimella SVB, Ibrahim YM, Deng L, Zheng X, Webb IK, Anderson GA, Prost SA, Norheim RV, Tolmachev AV, Baker ES, Smith RD. Anal Chem. 2016;88:8949–8956. doi: 10.1021/acs.analchem.6b01914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherrod SD, McLean JA. Clinical Chemistry. 2016;62:77–83. doi: 10.1373/clinchem.2015.238261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.May JC, McLean JA. Ann Rev Anal Chem. 2016;9 doi: 10.1146/annurev-anchem-071015-041734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortmayr K, Causon TJ, Hann S, Koellensperger G. TrAC, Trends Anal Chem. 2016;82:358–366. [Google Scholar]
- 13.Stephan S, Jakob C, Hippler J, Schmitz OJ. Anal Bioanal Chem. 2016;408:3751–3759. doi: 10.1007/s00216-016-9460-9. [DOI] [PubMed] [Google Scholar]
- 14.Chouinard CD, Wei MS, Beekman CR, Kemperman RHJ, Yost RA. Clinical Chemistry. 2016;62:124–133. doi: 10.1373/clinchem.2015.238840. [DOI] [PubMed] [Google Scholar]
- 15.Lanucara F, Holman SW, Gray CJ, Eyers CE. Nat Chem. 2014;6:281–294. doi: 10.1038/nchem.1889. [DOI] [PubMed] [Google Scholar]
- 16.Zhong Y, Hyung SJ, Ruotolo BT. Expert Rev Proteomic. 2012;9:47–58. doi: 10.1586/epr.11.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kliman M, May JC, McLean JA. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2011;1811:935–945. doi: 10.1016/j.bbalip.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray CJ, Thomas B, Upton R, Migas LG, Eyers CE, Barran PE, Flitsch SL. Biochimica et Biophysica Acta (BBA) - General Subjects. 2016;1860:1688–1709. doi: 10.1016/j.bbagen.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 19.May JC, Goodwin CR, McLean JA. In: Encyclopedia of Drug Metabolism and Interactions. Lyubimov AV, editor. John Wiley & Sons, Inc; 2011. [Google Scholar]
- 20.Kaur-Atwal G, O’Connor G, Aksenov AA, Bocos-Bintintan V, Paul Thomas CL, Creaser CS. International Journal for Ion Mobility Spectrometry. 2009;12:1–14. [Google Scholar]
- 21.Crawford CL, Hauck BC, Tufariello JA, Harden CS, McHugh V, Siems WF, Hill HH., Jr Talanta. 2012;101:161–170. doi: 10.1016/j.talanta.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Mesleh MF, Hunter JM, Shvartsburg AA, Schatz GC, Jarrold MF. J Phys Chem. 1996;100:16082–16086. [Google Scholar]
- 23.Shvartsburg AA, Jarrold MF. Chem Phys Lett. 1996;261:86–91. [Google Scholar]
- 24.Wyttenbach T, Bleiholder C, Bowers MT. Anal Chem. 2013;85:2191–2199. doi: 10.1021/ac3029008. [DOI] [PubMed] [Google Scholar]
- 25.Larriba C, Hogan CJ. Journal of Computational Physics. 2013;251:344–363. [Google Scholar]
- 26.Bleiholder C, Johnson NR, Contreras S, Wyttenbach T, Bowers MT. Anal Chem. 2015;87:7196–7203. doi: 10.1021/acs.analchem.5b01429. [DOI] [PubMed] [Google Scholar]
- 27.Mason EA, McDaniel EW. Transport Properties of Ions in Gases. John Wiley & Sons; New York: 1988. p. 560. [Google Scholar]
- 28.Siems WF, Viehland LA, Hill HH. Anal Chem. 2012;84:9782–9791. doi: 10.1021/ac301779s. [DOI] [PubMed] [Google Scholar]
- 29.McDaniel EW. Collision Phenomena in Ionized Gases. Wiley; New York: 1964. [Google Scholar]
- 30.Marklund Erik G, Degiacomi Matteo T, Robinson Carol V, Baldwin Andrew J, Benesch Justin LP. Structure. 2015;23:791–799. doi: 10.1016/j.str.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Benesch JL, Ruotolo BT. Curr Opin Struct Biol. 2011;21:641–649. doi: 10.1016/j.sbi.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon BU, Kim MS. Org Mass Spectrom. 1990;25:397–403. [Google Scholar]
- 33.van Houte JJ, de Koster CG, van Thuijl J. Int J Mass Spectrom Ion Processes. 1992;115:173–183. [Google Scholar]
- 34.Roussis SG. J Am Soc Mass Spectrom. 1995;6:803–811. doi: 10.1016/1044-0305(95)00323-6. [DOI] [PubMed] [Google Scholar]
- 35.Covey T, Douglas D. J Am Soc Mass Spectrom. 1993;4:616–623. doi: 10.1016/1044-0305(93)85025-S. [DOI] [PubMed] [Google Scholar]
- 36.Javahery G, Thomson BA. J Am Soc Mass Spectrom. 1997;8:697–702. [Google Scholar]
- 37.Ring S, Naaman R, Rudich Y. Anal Chem. 1999;71:648–651. doi: 10.1021/ac980779j. [DOI] [PubMed] [Google Scholar]
- 38.Plass WR, Gill LA, Bui HA, Cooks RG. J Phys Chem A. 2000;104:5059–5065. [Google Scholar]
- 39.Wobschall D, Graham JR, Malone DP. Phys Rev. 1963;131:1565–1571. [Google Scholar]
- 40.Wobschall DC, Fluegge RA, Graham JR. J Appl Phys. 1967;38:3761–3767. [Google Scholar]
- 41.Salbo R, Bush MF, Naver H, Campuzano I, Robinson CV, Pettersson I, Jorgensen TJ, Haselmann KF. Rapid communications in mass spectrometry: RCM. 2012;26:1181–93. doi: 10.1002/rcm.6211. [DOI] [PubMed] [Google Scholar]
- 42.Yang F, Voelkel JE, Dearden DV. Anal Chem. 2012;84:4851–4857. doi: 10.1021/ac300379a. [DOI] [PubMed] [Google Scholar]
- 43.Mao L, Chen Y, Xin Y, Chen Y, Zheng L, Kaiser NK, Marshall AG, Xu W. Anal Chem. 2015;87:4072–4075. doi: 10.1021/acs.analchem.5b00102. [DOI] [PubMed] [Google Scholar]
- 44.Jiang T, Chen Y, Mao L, Marshall AG, Xu W. Physical Chemistry Chemical Physics. 2016;18:713–717. doi: 10.1039/c5cp02987b. [DOI] [PubMed] [Google Scholar]
- 45.Hofmann J, Hahm HS, Seeberger PH, Pagel K. Nature. 2015;526:241. doi: 10.1038/nature15388. [DOI] [PubMed] [Google Scholar]
- 46.Benigni P, Bravo C, Quirke JME, DeBord JD, Mebel AM, Fernandez-Lima F. Energy & Fuels. 2016 doi: 10.1021/acs.energyfuels.5b02292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reading E, Munoz-Muriedas J, Roberts AD, Dear GJ, Robinson CV, Beaumont C. Anal Chem. 2016;88:2273–2280. doi: 10.1021/acs.analchem.5b04068. [DOI] [PubMed] [Google Scholar]
- 48.Pacholarz KJ, Barran PE. Anal Chem. 2015;87:6271–6279. doi: 10.1021/acs.analchem.5b01063. [DOI] [PubMed] [Google Scholar]
- 49.Pringle SD, Giles K, Wildgoose JL, Williams JP, Slade SE, Thalassinos K, Bateman RH, Bowers MT, Scrivens JH. Int J Mass Spectrom. 2007;261:1–12. [Google Scholar]
- 50.Bush MF, Hall Z, Giles K, Hoyes J, Robinson CV, Ruotolo BT. Anal Chem. 2010;82:9557–9565. doi: 10.1021/ac1022953. [DOI] [PubMed] [Google Scholar]
- 51.Tang K, Shvartsburg AA, Lee HN, Prior DC, Buschbach MA, Li F, Tolmachev AV, Anderson GA, Smith RD. Anal Chem. 2005;77:3330–3339. doi: 10.1021/ac048315a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valentine SJ, Counterman AE, Clemmer DE. J Am Soc Mass Spectrom. 1999;10:1188–1211. doi: 10.1016/S1044-0305(99)00079-3. [DOI] [PubMed] [Google Scholar]
- 53.Valentine SJ, Counterman AE, Hoaglund-Hyzer CS, Clemmer DE. J Phys Chem B. 1999;103:1203–1207. [Google Scholar]
- 54.Dilger JM, Valentine SJ, Glover MS, Ewing MA, Clemmer DE. Int J Mass Spectrom. 2012;330–332:35–45. [Google Scholar]
- 55.Dilger JM, Valentine SJ, Glover MS, Clemmer DE. J Am Soc Mass Spectrom. 2013;24:768–779. doi: 10.1007/s13361-013-0579-z. [DOI] [PubMed] [Google Scholar]
- 56.von Helden G, Hsu M, Gotts N, Kemper P, Bowers M. Chem Phys Lett. 1993;204:15–22. [Google Scholar]
- 57.Gotts NG, von Helden G, Bowers MT. Int J Mass Spectrom Ion Processes. 1995;149:217–229. [Google Scholar]
- 58.Lee S, Gotts N, von Helden G, Bowers MT. J Phys Chem A. 1997;101:2096–2102. [Google Scholar]
- 59.von Helden G, Porter E, Gotts NG, Bowers MT. J Phys Chem. 1995;99:7707–7714. [Google Scholar]
- 60.Clemmer DE, Jarrold MF. J Am Chem Soc. 1995;117:8841–8850. [Google Scholar]
- 61.Shelimov KB, Jarrold MF. J Phys Chem. 1995;99:17677–17679. [Google Scholar]
- 62.Shelimov KB, Jarrold MF. J Am Chem Soc. 1996;118:1139–1147. [Google Scholar]
- 63.Hudgins RR, Imai M, Jarrold MF, Dugourd P. J Chem Phys. 1999;111:7865–7870. [Google Scholar]
- 64.Tao L, McLean JR, McLean JA, Russell DH. J Am Soc Mass Spectrom. 2007;18:1727–1728. doi: 10.1016/j.jasms.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 65.Fernandez-Lima FA, Blase RC, Russell DH. International Journal for Mass Spectrometry. 2010;298:111–118. doi: 10.1016/j.ijms.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pagel K, Harvey DJ. Anal Chem. 2013;85:5138–5145. doi: 10.1021/ac400403d. [DOI] [PubMed] [Google Scholar]
- 67.Hofmann J, Struwe WB, Scarff CA, Scrivens JH, Harvey DJ, Pagel K. Anal Chem. 2014;86:10789–10795. doi: 10.1021/ac5028353. [DOI] [PubMed] [Google Scholar]
- 68.Fenn LS, Kliman M, Mahsut A, Zhao SR, McLean JA. Anal Bioanal Chem. 2009;394:235–244. doi: 10.1007/s00216-009-2666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goodwin CR, Fenn LS, Derewacz DK, Bachmann BO, McLean JA. Journal of Natural Products. 2012;75:48–53. doi: 10.1021/np200457r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paglia G, Williams JP, Menikarachchi L, Thompson JW, Tyldesley-Worster R, Halldórsson S, Rolfsson O, Moseley A, Grant D, Langridge J, Palsson BO, Astarita G. Anal Chem. 2014;86:3985–3993. doi: 10.1021/ac500405x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paglia G, Angel P, Williams JP, Richardson K, Olivos HJ, Thompson JW, Menikarachchi L, Lai S, Walsh C, Moseley A, Plumb RS, Grant DF, Palsson BO, Langridge J, Geromanos S, Astarita G. Anal Chem. 2015;87:1137–1144. doi: 10.1021/ac503715v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shah AR, Agarwal K, Baker ES, Singhal M, Mayampurath AM, Ibrahim YM, Kangas LJ, Monroe ME, Zhao R, Belov ME, Anderson GA, Smith RD. Bioinformatics. 2010;26:1601–1607. doi: 10.1093/bioinformatics/btq245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clemmer DE. http://www.indiana.edu/~clemmer/Research/Cross%20Section%20Database/cs_database.php.
- 74.Bush MF. http://depts.washington.edu/bushlab/ccsdatabase/
- 75.Ellis HW, Pai RY, McDaniel EW, Mason EA, Viehland LA. At Data Nucl Data Tables. 1976;17:177–210. [Google Scholar]
- 76.Ellis HW, McDaniel EW, Albritton DL, Viehland LA, Lin SL, Mason EA. At Data Nucl Data Tables. 1978;22:179–217. [Google Scholar]
- 77.Ellis HW, Thackston MG, McDaniel EW, Mason EA. At Data Nucl Data Tables. 1984;31:113–151. [Google Scholar]
- 78.Viehland LA, Mason EA. At Data Nucl Data Tables. 1995;60:37–95. [Google Scholar]
- 79.Matz LM, Hill HH, Jr, Beegle LW, Kanik I. J Am Soc Mass Spectrom. 2002;13:300–307. doi: 10.1016/S1044-0305(01)00366-X. [DOI] [PubMed] [Google Scholar]
- 80.Abedi A, Sattar L, Gharibi M, Viehland LA. Int J Mass Spectrom. 2014;370:101–106. [Google Scholar]
- 81.Jurneczko E, Kalapothakis J, Campuzano IDG, Morris M, Barran PE. Anal Chem. 2012;84:8524–8531. doi: 10.1021/ac301260d. [DOI] [PubMed] [Google Scholar]
- 82.Fernandez-Garcia J, de la Mora JF. J Am Soc Mass Spectrom. 2013;24:1872–1889. doi: 10.1007/s13361-013-0702-1. [DOI] [PubMed] [Google Scholar]
- 83.Ouyang H, Larriba-Andaluz C, Oberreit DR, Hogan CJ. J Am Soc Mass Spectrom. 2013;24:1833–1847. doi: 10.1007/s13361-013-0724-8. [DOI] [PubMed] [Google Scholar]
- 84.Kurulugama RT, Darland E, Kuhlmann F, Stafford G, Fjeldsted J. Analyst. 2015;14:6834–6844. doi: 10.1039/c5an00991j. [DOI] [PubMed] [Google Scholar]
- 85.Bush MF, Campuzano IDG, Robinson CV. Anal Chem. 2012;84:7124–7130. doi: 10.1021/ac3014498. [DOI] [PubMed] [Google Scholar]
- 86.Chudinov AV, Martynovich YG, Sulimenkov IV, Brusov VS, Filatov VV, Pikhtelev AR, Kozlovskiy VI. Journal of Analytical Chemistry. 2015;70:1647–1653. [Google Scholar]
- 87.Lapthorn C, Pullen FS, Chowdhry BZ, Wright P, Perkins GL, Heredia Y. Analyst. 2015;140:6814–6823. doi: 10.1039/c5an00411j. [DOI] [PubMed] [Google Scholar]
- 88.Larriba-Andaluz C, Hogan CJ. J Chem Phys. 2014;141:194107. doi: 10.1063/1.4901890. [DOI] [PubMed] [Google Scholar]
- 89.Campuzano I, Bush MF, Robinson CV, Beaumont C, Richardson K, Kim H, Kim HI. Anal Chem. 2011;84:1026–1033. doi: 10.1021/ac202625t. [DOI] [PubMed] [Google Scholar]
- 90.Giles K, Williams JP, Campuzano I. Rapid Commun Mass Spectrom. 2011;25:1559–1566. doi: 10.1002/rcm.5013. [DOI] [PubMed] [Google Scholar]
- 91.Ibrahim YM, Garimella SV, Tolmachev AV, Baker ES, Smith RD. Anal Chem. 2014;86:5295–5299. doi: 10.1021/ac404250z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.May JC, Dodds JN, Kurulugama RT, Stafford GC, Fjeldsted JC, McLean JA. Analyst. 2015;140:6824–6833. doi: 10.1039/c5an00923e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lalli PM, Corilo YE, Fasciotti M, Riccio MF, de Sa GF, Daroda RJ, Souza GHMF, McCullagh M, Bartberger MD, Eberlin MN, Campuzano IDG. J Mass Spectrom. 2013;48:989–997. doi: 10.1002/jms.3245. [DOI] [PubMed] [Google Scholar]
- 94.Reid Asbury G, Hill HH., Jr Anal Chem. 2000;72:580–584. doi: 10.1021/ac9908952. [DOI] [PubMed] [Google Scholar]
- 95.Fasciotti M, Sanvido GB, Santos VG, Lalli PM, McCullagh M, de Sá GF, Daroda RJ, Peter MG, Eberlin MN. J Mass Spectrom. 2012;47:1643–1647. doi: 10.1002/jms.3089. [DOI] [PubMed] [Google Scholar]
- 96.Fasciotti M, Lalli PM, Klitzke CcF, Corilo YE, Pudenzi MA, Pereira RC, Bastos W, Daroda RJ, Eberlin MN. Energy & Fuels. 2013;27:7277–7286. [Google Scholar]
- 97.Howdle MD, Eckers C, Laures AMF, Creaser CS. Int J Mass Spectrom. 2010;298:72–77. [Google Scholar]
- 98.Beitz T, Laudien R, Löhmannsröben HG, Kallies B. J Phys Chem A. 2006;110:3514–3520. doi: 10.1021/jp055335n. [DOI] [PubMed] [Google Scholar]
- 99.Bonakdarzadeh P, Topic F, Kalenius E, Bhowrnik S, Sato S, Groessl M, Knochenmuss R, Rissanen K. Inorg Chem. 2015;54:6055–6061. doi: 10.1021/acs.inorgchem.5b01082. [DOI] [PubMed] [Google Scholar]
- 100.Wu C, Siems WF, Reid Asbury G, Hill HH., Jr Anal Chem. 1998;70:4929–4938. doi: 10.1021/ac980414z. [DOI] [PubMed] [Google Scholar]
- 101.Steiner WE, Clowers BH, Fuhrer K, Gonin M, Matz LM, Siems WF, Schultz JA, Hill HH., Jr Rapid Commun Mass Spectrom. 2001;15:2221–2226. doi: 10.1002/rcm.495. [DOI] [PubMed] [Google Scholar]
- 102.Sysoev A, Adamov A, Viidanoja J, Ketola RA, Kostiainen R, Kotiaho T. Rapid Commun Mass Spectrom. 2004;18:3131–3139. doi: 10.1002/rcm.1738. [DOI] [PubMed] [Google Scholar]
- 103.Jarrold MF. J Phys Chem. 1995;99:11–21. [Google Scholar]
- 104.Dugourd P, Hudgins RR, Clemmer DE, Jarrold MF. Rev Sci Instrum. 1997;68:1122–1129. [Google Scholar]
- 105.Kemper PR, Bowers MT. J Am Soc Mass Spectrom. 1990;1:197–207. [Google Scholar]
- 106.Hoaglund CS, Valentine SJ, Clemmer DE. Anal Chem. 1997;69:4156–4161. [Google Scholar]
- 107.Hoaglund CS, Valentine SJ, Sporleder CR, Reilly JP, Clemmer DE. Anal Chem. 1998;70:2236–2242. doi: 10.1021/ac980059c. [DOI] [PubMed] [Google Scholar]
- 108.Gillig KJ, Ruotolo BT, Stone EG, Russell DH, Fuhrer K, Gonin M, Schultz JA. Anal Chem. 2000;72:3965–3971. doi: 10.1021/ac0005619. [DOI] [PubMed] [Google Scholar]
- 109.Sundarapandian S, May JC, McLean JA. Anal Chem. 2010;82:3247–3254. doi: 10.1021/ac902980r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McCullough BJ, Kalapothakis J, Eastwood H, Kemper P, MacMillan D, Taylor K, Dorin J, Barran PE. Anal Chem. 2008;80:6336–6344. doi: 10.1021/ac800651b. [DOI] [PubMed] [Google Scholar]
- 111.Gillig KJ, Ruotolo BT, Stone EG, Russell DH. Int J Mass Spectrom. 2004;239:43–49. [Google Scholar]
- 112.Blase RC, Silveira JA, Gillig KJ, Gamage CM, Russell DH. Int J Mass Spectrom. 2011;301:166–173. [Google Scholar]
- 113.Koeniger SL, Merenbloom SI, Valentine SJ, Jarrold MF, Udseth HR, Smith RD, Clemmer DE. Anal Chem. 2006;78:4161–4174. doi: 10.1021/ac051060w. [DOI] [PubMed] [Google Scholar]
- 114.Ridenour WB, Kliman M, McLean JA, Caprioli RM. Anal Chem. 2010;82:1881–1889. doi: 10.1021/ac9026115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hilton GR, Thalassinos K, Grabenauer M, Sanghera N, Slade SE, Wyttenbach T, Robinson PJ, Pinheiro TJ, Bowers MT, Scrivens JH. J Am Soc Mass Spectrom. 2010;21:845–854. doi: 10.1016/j.jasms.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 116.Fouque KJD, Afonso C, Zirah S, Hegemann JD, Zimmermann M, Marahiel MA, Rebuffat S, Lavanant H. Anal Chem. 2015;87:1166–1172. doi: 10.1021/ac503772n. [DOI] [PubMed] [Google Scholar]
- 117.Watkinson TG, Calabrese AN, Giusti F, Zoonens M, Radford SE, Ashcroft AE. Int J Mass Spectrom. 2015;391:54–61. doi: 10.1016/j.ijms.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schenk ER, Almeida R, Miksovska J, Ridgeway ME, Park MA, Fernandez-Lima F. J Am Soc Mass Spectrom. 2015;26:555–563. doi: 10.1007/s13361-014-1067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Castellanos A, Benigni P, Hernandez DR, DeBord JD, Ridgeway ME, Park MA, Fernandez-Lima F. Analytical Methods. 2014;6:9328–9332. doi: 10.1039/C4AY01655F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Molano-Arevalo JC, Hernandez DR, Gonzalez WG, Miksovska J, Ridgeway ME, Park MA, Fernandez-Lima F. Anal Chem. 2014;86:10223–10230. doi: 10.1021/ac5023666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Benigni P, Thompson CJ, Ridgeway ME, Park MA, Fernandez-Lima F. Anal Chem. 2015;87:4321–4325. doi: 10.1021/ac504866v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rashid AM, Saalbach G, Bornemann S. Rapid Commun Mass Spectrom. 2014;28:191–199. doi: 10.1002/rcm.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dugourd P, Hudgins RR, Jarrold MF. Chem Phys Lett. 1997;267:186–192. [Google Scholar]
- 124.Allen SJ, Schwartz AM, Bush MF. Anal Chem. 2013;85:12055–12061. doi: 10.1021/ac403139d. [DOI] [PubMed] [Google Scholar]
- 125.Zhang LW, Vertes A. Anal Chem. 2015;87:10397–10405. doi: 10.1021/acs.analchem.5b02502. [DOI] [PubMed] [Google Scholar]
- 126.Liu H, Zhang J, Sun H, Xu C, Zhu Y, Xie H. Procedia Environmental Sciences. 2011;8:483–491. [Google Scholar]
- 127.Baker ES, Livesay EA, Orton DJ, Moore RJ, Danielson WF, Prior DC, Ibrahim YM, LaMarche BL, Mayampurath AM, Schepmoes AA, Hopkins DF, Tang K, Smith RD, Belov ME. J Proteome Res. 2010;9:997–1006. doi: 10.1021/pr900888b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McLean JA, Ruotolo BT, Gillig KJ, Russell DH. Int J Mass Spectrom. 2005;240:301–315. [Google Scholar]
- 129.Shliaha PV, Bond NJ, Gatto L, Lilley KS. J Proteome Res. 2013;12:2323–2339. doi: 10.1021/pr300775k. [DOI] [PubMed] [Google Scholar]
- 130.Lietz CB, Yu Q, Li L. J Am Soc Mass Spectrom. 2014;25:2009–2019. doi: 10.1007/s13361-014-0920-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Knapman TW, Valette NM, Warriner SL, Ashcroft AE. Current Analytical Chemistry. 2013;9:181–191. doi: 10.2174/1573411011309020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vahidi S, Stocks BB, Konermann L. Anal Chem. 2013;85:10471–10478. doi: 10.1021/ac402490r. [DOI] [PubMed] [Google Scholar]
- 133.May JC, McLean JA. Proteomics. 2015;15:2862–2871. doi: 10.1002/pmic.201400551. [DOI] [PubMed] [Google Scholar]
- 134.Phillips AS, Gomes AF, Kalapothakis JMD, Gillam JE, Gasparavicius J, Gozzo FC, Kunath T, MacPhee C, Barran PE. Analyst. 2015;140:3070–3081. doi: 10.1039/c4an02306d. [DOI] [PubMed] [Google Scholar]
- 135.McLean JA. J Am Soc Mass Spectrom. 2009;20:1775–1781. doi: 10.1016/j.jasms.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 136.Wilkins CL, Trimpin S, May JC, McLean JA. Ion Mobility Spectrometry-Mass Spectrometry: Theory and Applications. CRC Press; 2010. pp. 327–343. [Google Scholar]
- 137.Karpas Z, Berant Z, Shahal O. J Am Chem Soc. 1989;111:6015–6018. [Google Scholar]
- 138.Berant Z, Karpas Z. J Am Chem Soc. 1989;111:3819–3824. [Google Scholar]
- 139.Krishnamurthy M, de Gouw JA, Bierbaum VM, Leone SR. J Phys Chem. 1996;100:14908–14913. [Google Scholar]
- 140.de Gouw JA, Krishnamurthy M, Bierbaum VM, Leone SR. Int J Mass Spectrom Ion Processes. 1997;167:281–289. [Google Scholar]
- 141.Karpas Z, Tironi C. Structural Chemistry. 1991;2:655–659. [Google Scholar]
- 142.Li H, Giles K, Bendiak B, Kaplan K, Siems WF, Hill HH., Jr Anal Chem. 2012;84:3231–3239. doi: 10.1021/ac203116a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fenn LS, McLean JA. Phys Chem Chem Phys. 2011;13:2196–2205. doi: 10.1039/c0cp01414a. [DOI] [PubMed] [Google Scholar]
- 144.Gelb AS, Jarratt RE, Huang YT, Dodds ED. Anal Chem. 2014;86:11396–11402. doi: 10.1021/ac503379e. [DOI] [PubMed] [Google Scholar]
- 145.Huang YT, Dodds ED. Anal Chem. 2015;87:5664–5668. doi: 10.1021/acs.analchem.5b00759. [DOI] [PubMed] [Google Scholar]
- 146.Gaye MM, Kurulugama R, Clemmer DE. Analyst. 2015;140:6922–6932. doi: 10.1039/c5an00840a. [DOI] [PubMed] [Google Scholar]
- 147.Williams JP, Grabenauer M, Holland RJ, Carpenter CJ, Wormald MR, Giles K, Harvey DJ, Bateman RH, Scrivens JH, Bowers MT. Int J Mass Spectrom. 2010;298:119–127. [Google Scholar]
- 148.Zhang F, Guo S, Zhang M, Zhang Z, Guo Y. J Mass Spectrom. 2015;50:906–913. doi: 10.1002/jms.3600. [DOI] [PubMed] [Google Scholar]
- 149.Pacini T, Fu WQ, Gudmundsson S, Chiaravalle AE, Brynjolfson S, Palsson BO, Astarita G, Paglia G. Anal Chem. 2015;87:2593–2599. doi: 10.1021/ac504707n. [DOI] [PubMed] [Google Scholar]
- 150.Aqai P, Blesa NG, Major H, Pedotti M, Varani L, Ferrero VE, Haasnoot W, Nielen MW. Anal Bioanal Chem. 2013;405:9427–9436. doi: 10.1007/s00216-013-7384-1. [DOI] [PubMed] [Google Scholar]
- 151.Shimizu A, Chiba M. Drug Metabolism and Disposition. 2013;41:1295–1299. doi: 10.1124/dmd.113.051953. [DOI] [PubMed] [Google Scholar]
- 152.Warnke S, Seo J, Boschmans J, Sobott F, Scrivens JH, Bleiholder C, Bowers MT, Gewinner S, Schollkopf W, Pagel K, von Helden G. J Am Chem Soc. 2015;137:4236–4242. doi: 10.1021/jacs.5b01338. [DOI] [PubMed] [Google Scholar]
- 153.Ma X, Shah S, Zhou M, Park CK, Wysocki VH, Horton NC. Biochemistry. 2013;52:4373–4381. doi: 10.1021/bi3013214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Politis A, Park AY, Hall Z, Ruotolo BT, Robinson CV. Journal of Molecular Biology. 2013;425:4790–4801. doi: 10.1016/j.jmb.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 155.Ma X, Zhou MW, Wysocki VH. J Am Soc Mass Spectrom. 2014;25:368–379. doi: 10.1007/s13361-013-0790-y. [DOI] [PubMed] [Google Scholar]
- 156.Zhang Y, Ju Y, Huang CS, Wysocki VH. Anal Chem. 2014;86:1342–1346. doi: 10.1021/ac403383d. [DOI] [PubMed] [Google Scholar]
- 157.Pacholarz KJ, Porrini M, Garlish RA, Burnley RJ, Taylor RJ, Henry AJ, Barran PE. Angew Chem Int Ed. 2014;53:7765–7769. doi: 10.1002/anie.201402863. [DOI] [PubMed] [Google Scholar]
- 158.Beveridge R, Covill S, Pacholarz KJ, Kalapothakis JM, MacPhee CE, Barran PE. Anal Chem. 2014;86:10979–10991. doi: 10.1021/ac5027435. [DOI] [PubMed] [Google Scholar]
- 159.Quintyn RS, Zhou MW, Yan J, Wysocki VH. Anal Chem. 2015;87:11879–11886. doi: 10.1021/acs.analchem.5b03441. [DOI] [PubMed] [Google Scholar]
- 160.Bolton EE, Wang Y, Thiessen PA, Bryant SH. In: Annual Reports in Computational Chemistry. Ralph AW, David CS, editors. Elsevier; 2008. pp. 217–241. [Google Scholar]
- 161.Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, Han L, He J, He S, Shoemaker BA. Nucleic Acids Res. 2015:gkv951. doi: 10.1093/nar/gkv951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Creaser CS, Benyezzar M, Griffiths JR, Stygall JW. Anal Chem. 2000;72:2724–2729. doi: 10.1021/ac991409d. [DOI] [PubMed] [Google Scholar]
- 163.Momoh PO, Attah IK, El-Shall MS, Kanters RPF, Pinski JM, Abrash SA. J Phys Chem A. 2014;118:8251–8263. doi: 10.1021/jp5010488. [DOI] [PubMed] [Google Scholar]
- 164.Rusyniak M, Ibrahim YM, Alsharaeh E, Meot-Ner M, El-Shall MS. J Phys Chem A. 2003;107:7656–7666. [Google Scholar]
- 165.Ota K, Koyasu K, Ohshimo K, Misaizu F. Chem Phys Lett. 2013;588:63–67. [Google Scholar]
- 166.Ohshimo K, Komukai T, Moriyama R, Misaizu F. J Phys Chem A. 2014;118:3899–3905. doi: 10.1021/jp5015687. [DOI] [PubMed] [Google Scholar]
- 167.Ohshimo K, Takahashi T, Moriyama R, Misaizu F. J Phys Chem A. 2014;118:9970–9975. doi: 10.1021/jp5061396. [DOI] [PubMed] [Google Scholar]
- 168.Ohshimo K, Norimasa N, Moriyama R, Misaizu F. Journal of Chemical Physics. 2016;144:8. doi: 10.1063/1.4949269. [DOI] [PubMed] [Google Scholar]
- 169.Wu JWJ, Moriyama R, Tahara H, Ohshimo K, Misaizu F. J Phys Chem A. 2016;120:3788–3796. doi: 10.1021/acs.jpca.6b03403. [DOI] [PubMed] [Google Scholar]
- 170.Shelimov KB, Clemmer DE, Hudgins RR, Jarrold MF. J Am Chem Soc. 1997;119:2240–2248. [Google Scholar]
- 171.Valentine SJ, Anderson JG, Ellington AD, Clemmer DE. J Phys Chem B. 1997;101:3891–3900. [Google Scholar]
- 172.Valentine SJ, Counterman AE, Clemmer DE. J Am Soc Mass Spectrom. 1997;8:954–961. doi: 10.1016/S1044-0305(99)00079-3. [DOI] [PubMed] [Google Scholar]
- 173.Wyttenbach T, Batka JJ, Gidden J, Bowers MT. Int J Mass Spectrom. 1999;193:143–152. [Google Scholar]
- 174.Morrison LJ, Wysocki VH. J Am Chem Soc. 2014;136:14173–14183. doi: 10.1021/ja507298e. [DOI] [PubMed] [Google Scholar]
- 175.Xiao CY, Perez LM, Russell DH. Analyst. 2015;14:6933–6944. doi: 10.1039/c5an00826c. [DOI] [PubMed] [Google Scholar]
- 176.Tao L, Dahl DB, Pérez LM, Russell DH. J Am Soc Mass Spectrom. 2009;20:1593–1602. doi: 10.1016/j.jasms.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 177.Wyttenbach T, Grabenauer M, Thalassinos K, Scrivens JH, Bowers MT. J Phys Chem B. 2009;114:437–447. doi: 10.1021/jp906242m. [DOI] [PubMed] [Google Scholar]
- 178.Chen SH, Chen LX, Russell DH. J Am Chem Soc. 2014;136:9499–9508. doi: 10.1021/ja5047878. [DOI] [PubMed] [Google Scholar]
- 179.Calabrese AN, Bowie JH, Pukala TL. Biochemistry. 2015;54:567–576. doi: 10.1021/bi5004124. [DOI] [PubMed] [Google Scholar]
- 180.Berezovskaya Y, Porrini M, Nortcliffe C, Barran PE. Analyst. 2015;140:2847–2856. doi: 10.1039/c4an00427b. [DOI] [PubMed] [Google Scholar]
- 181.D’Urzo A, Konijnenberg A, Rossetti G, Habchi J, Li JY, Carloni P, Sobott F, Longhi S, Grandori R. J Am Soc Mass Spectrom. 2015;26:472–481. doi: 10.1007/s13361-014-1048-z. [DOI] [PubMed] [Google Scholar]
- 182.Dickinson ER, Jurneczko E, Pacholarz KJ, Clarke DJ, Reeves M, Ball KL, Hupp T, Campopiano D, Nikolova PV, Barran PE. Anal Chem. 2015;87:3231–3238. doi: 10.1021/ac503720v. [DOI] [PubMed] [Google Scholar]
- 183.Beveridge R, Phillips AS, Denbigh L, Saleem HM, MacPhee CE, Barran PE. Proteomics. 2015;15:2872–2883. doi: 10.1002/pmic.201400605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Saikusa K, Kuwabara N, Kokabu Y, Inoue Y, Sato M, Iwasaki H, Shimizu T, Ikeguchi M, Akashi S. Analyst. 2013;138:1441–1449. doi: 10.1039/c2an35878f. [DOI] [PubMed] [Google Scholar]
- 185.Harvey SR, Porrini M, Konijnenberg A, Clarke DJ, Tyler RC, Langridge-Smith PRR, MacPhee CE, Volkman BF, Barran PE. Journal of Physical Chemistry B. 2014;118:12348–12359. doi: 10.1021/jp504997k. [DOI] [PubMed] [Google Scholar]
- 186.Harvey SR, Porrini M, Tyler RC, MacPhee CE, Volkman BF, Barran PE. Physical Chemistry Chemical Physics. 2015;17:10538–10550. doi: 10.1039/c4cp05136j. [DOI] [PubMed] [Google Scholar]
- 187.Scarff CA, Sicorello A, Tome RJL, Macedo-Ribeiro S, Ashcroft AE, Radford SE. Int J Mass Spectrom. 2013;345:63–70. doi: 10.1016/j.ijms.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cole H, Porrini M, Morris R, Smith T, Kalapothakis J, Weidt S, Mackay CL, MacPhee CE, Barran PE. Analyst. 2015;140:7000–7011. doi: 10.1039/c5an01253h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Young LM, Cao P, Raleigh DP, Ashcroft AE, Radford SE. J Am Chem Soc. 2014;136:660–670. doi: 10.1021/ja406831n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bernstein SL, Wyttenbach T, Baumketner A, Shea JE, Bitan G, Teplow DB, Bowers MT. J Am Chem Soc. 2005;127:2075–2084. doi: 10.1021/ja044531p. [DOI] [PubMed] [Google Scholar]
- 191.Dupuis NF, Wu C, Shea JE, Bowers MT. J Am Chem Soc. 2009;131:18283–18292. doi: 10.1021/ja903814q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Murray MM, Krone MG, Bernstein SL, Baumketner A, Condron MM, Lazo ND, Teplow DB, Wyttenbach T, Shea JE, Bowers MT. J Phys Chem B. 2009;113:6041–6046. doi: 10.1021/jp808384x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Daly S, Kulesza A, Poussigue F, Simon AL, Choi CM, Knight G, Chirot F, MacAleese L, Antoine R, Dugourd P. Chemical Science. 2015;6:5040–5047. doi: 10.1039/c5sc01463h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bernstein SL, Dupuis NF, Lazo ND, Wyttenbach T, Condron MM, Bitan G, Teplow DB, Shea JE, Ruotolo BT, Robinson CV, Bowers MT. Nat Chem. 2009;1:326–331. doi: 10.1038/nchem.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Calabrese AN, Watkinson TG, Henderson PJF, Radford SE, Ashcroft AE. Anal Chem. 2015;87:1118–1126. doi: 10.1021/ac5037022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Konijnenberg A, Yilmaz D, Ingolfsson HI, Dimitrova A, Marrink SJ, Li ZL, Venien-Bryan C, Sobott F, Kocer A. Proc Nat Acad Sci USA. 2014;111:17170–17175. doi: 10.1073/pnas.1413118111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Laganowsky A, Reading E, Allison TM, Ulmschneider MB, Degiacomi MT, Baldwin AJ, Robinson CV. Nature. 2014;510:172. doi: 10.1038/nature13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kelker MS, Berry C, Evans SL, Pai R, McCaskill DG, Wang NX, Russell JC, Baker MD, Yang C, Pflugrath JW, Wade M, Wess TJ, Narva KE. Plos One. 2014;9:15. doi: 10.1371/journal.pone.0112555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hoaglund CS, Liu Y, Ellington AD, Pagel M, Clemmer DE. J Am Chem Soc. 1997;119:9051–9052. [Google Scholar]
- 200.Gidden J, Bowers M. The European Physical Journal D-Atomic, Molecular, Optical and Plasma Physics. 2002;20:409–419. [Google Scholar]
- 201.Gidden J, Bowers MT. J Phys Chem B. 2003;107:12829–12837. [Google Scholar]
- 202.Gidden J, Bowers MT. J Am Soc Mass Spectrom. 2003;14:161–170. doi: 10.1016/S1044-0305(02)00866-8. [DOI] [PubMed] [Google Scholar]
- 203.Gidden J, Ferzoco A, Baker ES, Bowers MT. J Am Chem Soc. 2004;126:15132–15140. doi: 10.1021/ja046433+. [DOI] [PubMed] [Google Scholar]
- 204.Baker ES, Bernstein SL, Bowers MT. J Am Soc Mass Spectrom. 2005;16:989–997. doi: 10.1016/j.jasms.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 205.Baker ES, Manard MJ, Gidden J, Bowers MT. J Phys Chem B. 2005;109:4808–4810. doi: 10.1021/jp0501190. [DOI] [PubMed] [Google Scholar]
- 206.Baker ES, Hong JW, Gaylord BS, Bazan GC, Bowers MT. J Am Chem Soc. 2006;128:8484–8492. doi: 10.1021/ja060069s. [DOI] [PubMed] [Google Scholar]
- 207.Baker ES, Bowers MT. J Am Soc Mass Spectrom. 2007;18:1188–1195. doi: 10.1016/j.jasms.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 208.Baker ES, Dupuis NF, Bowers MT. J Phys Chem B. 2009;113:1722–1727. doi: 10.1021/jp807529m. [DOI] [PubMed] [Google Scholar]
- 209.Burmistrova A, Gabelica V, Duwez AS, De Pauw E. J Am Soc Mass Spectrom. 2013;24:1777–1786. doi: 10.1007/s13361-013-0721-y. [DOI] [PubMed] [Google Scholar]
- 210.Gidden J, Bowers MT, Jackson AT, Scrivens JH. J Am Soc Mass Spectrom. 2002;13:499–505. doi: 10.1016/S1044-0305(02)00367-7. [DOI] [PubMed] [Google Scholar]
- 211.Baker ES, Gidden J, Simonsick WJ, Grady MC, Bowers MT. Int J Mass Spectrom. 2004;238:279–286. [Google Scholar]
- 212.Jackson AT, Scrivens JH, Williams JP, Baker ES, Gidden J, Bowers MT. Int J Mass Spectrom. 2004;238:287–297. [Google Scholar]
- 213.Forsythe JG, Stow SM, Nefzger H, Kwiecien NW, May JC, McLean JA, Hercules DM. Anal Chem. 2014;86:4362–4370. doi: 10.1021/ac5001652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Stow SM, Onifer TM, Forsythe JG, Nefzger H, Kwiecien NW, May JC, McLean JA, Hercules DM. Anal Chem. 2015;87:6288–6296. doi: 10.1021/acs.analchem.5b01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kim K, Lee JW, Chang T, Kim HI. J Am Soc Mass Spectrom. 2014;25:1771–1779. doi: 10.1007/s13361-014-0949-1. [DOI] [PubMed] [Google Scholar]
- 216.Guo K, Guo ZH, Ludlow JM, Xie TZ, Liao SY, Newkome GR, Wesdemiotis C. Macromolecular Rapid Communications. 2015;36:1539–1552. doi: 10.1002/marc.201500084. [DOI] [PubMed] [Google Scholar]
- 217.Alalwiat A, Grieshaber SE, Paik BA, Kiick KL, Jia XQ, Wesdemiotis C. Analyst. 2015;140:7550–7564. doi: 10.1039/c5an01600b. [DOI] [PubMed] [Google Scholar]
- 218.Katzenmeyer BC, Cool LR, Williams JP, Craven K, Brown JM, Wesdemiotis C. Int J Mass Spectrom. 2015;378:303–311. [Google Scholar]
- 219.Liu XM, Cool LR, Lin K, Kasko AM, Wesdemiotis C. Analyst. 2015;140:1182–1191. doi: 10.1039/c4an01599a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Alsharaeh EH, El-Shall MS. Polymer. 2011;52:5551–5559. [Google Scholar]
- 221.Wyttenbach T, von Helden G, Bowers MT. Int J Mass Spectrom Ion Processes. 1997;165:377–390. [Google Scholar]
- 222.Gidden J, Wyttenbach T, Batka JJ, Weis P, Bowers MT, Jackson AT, Scrivens JH. J Am Soc Mass Spectrom. 1999;10:883–895. [Google Scholar]
- 223.Gidden J, Wyttenbach T, Jackson AT, Scrivens JH, Bowers MT. J Am Chem Soc. 2000;122:4692–4699. [Google Scholar]
- 224.Gidden J, Jackson AT, Scrivens JH, Bowers MT. Int J Mass Spectrom. 1999;188:121–130. [Google Scholar]
- 225.von Helden G, Wyttenbach T, Bowers MT. Int J Mass Spectrom Ion Processes. 1995;146–147:349–364. [Google Scholar]
- 226.Maire F, Coadou G, Cravello L, Lange CM. J Am Soc Mass Spectrom. 2013;24:238–248. doi: 10.1007/s13361-012-0527-3. [DOI] [PubMed] [Google Scholar]
- 227.Tintaru A, Pricl S, Denbigh L, Liu XX, Peng L, Charles L. Int J Mass Spectrom. 2013;354:235–241. [Google Scholar]
- 228.Leriche ED, Afonso C, Lange CM, Grossel MC, Truong L, Coadou G, Oulyadi H, Loutelier-Bourhis C. Rsc Advances. 2014;4:1744–1753. [Google Scholar]
- 229.Baker ES, Gidden J, Fee DP, Kemper PR, Anderson SE, Bowers MT. Int J Mass Spectrom. 2003;227:205–216. [Google Scholar]
- 230.Anderson SE, Baker ES, Mitchell C, Haddad TS, Bowers MT. Chemistry of Materials. 2005;17:2537–2545. [Google Scholar]
- 231.Anderson SE, Mitchell C, Haddad TS, Vij A, Schwab JJ, Bowers MT. Chemistry of Materials. 2006;18:1490–1497. [Google Scholar]
- 232.Brocker ER, Anderson SE, Northrop BH, Stang PJ, Bowers MT. J Am Chem Soc. 2010;132:13486–13494. doi: 10.1021/ja105702y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Liang YP, He YJ, Lee YH, Chan YT. Dalton Transactions. 2015;44:5139–5145. doi: 10.1039/c4dt03055a. [DOI] [PubMed] [Google Scholar]
- 234.Lee JW, Shin MH, Mobley W, Urbach AR, Kim HI. J Am Chem Soc. 2015;137:15322–15329. doi: 10.1021/jacs.5b10648. [DOI] [PubMed] [Google Scholar]
- 235.Xie TZ, Guo K, Guo ZH, Gao WY, Wojtas L, Ning GH, Huang MJ, Lu XC, Li JY, Liao SY, Chen YS, Moorefield CN, Saunders MJ, Cheng SZD, Wesdemiotis C, Newkome GR. Angewandte Chemie-International Edition. 2015;54:9224–9229. doi: 10.1002/anie.201503609. [DOI] [PubMed] [Google Scholar]
- 236.Zhang H, Grabenauer M, Bowers MT, Dearden DV. J Phys Chem A. 2009;113:1508–1517. doi: 10.1021/jp808625v. [DOI] [PubMed] [Google Scholar]
- 237.Chan YT, Li X, Yu J, Carri GA, Moorefield CN, Newkome GR, Wesdemiotis C. J Am Chem Soc. 2011;133:11967–11976. doi: 10.1021/ja107307u. [DOI] [PubMed] [Google Scholar]
- 238.Poyer S, Loutelier-Bourhis C, Coadou G, Mondeguer F, Enche J, Bossee A, Hess P, Afonso C. J Mass Spectrom. 2015;50:175–181. doi: 10.1002/jms.3515. [DOI] [PubMed] [Google Scholar]
- 239.Baker ES, Dupuis NF, Bowers MT. Int J Mass Spectrom. 2009;283:105–111. [Google Scholar]
- 240.Poyer S, Loutelier-Bourhis C, Tognetti V, Joubert L, Enche J, Bossée A, Mondeguer F, Hess P, Afonso C. Int J Mass Spectrom. 2016;402:20–28. doi: 10.1002/jms.3515. [DOI] [PubMed] [Google Scholar]
- 241.Nortcliffe C, Migas LG, Liu XJ, Ngo HT, Jolliffe KA, Barran PE. Int J Mass Spectrom. 2015;391:62–70. [Google Scholar]
- 242.Domalain V, Tognetti V, Hubert-Roux M, Lange CM, Joubert L, Baudoux J, Rouden J, Afonso C. J Am Soc Mass Spectrom. 2013;24:1437–1445. doi: 10.1007/s13361-013-0690-1. [DOI] [PubMed] [Google Scholar]
- 243.Stojko J, Fieulaine S, Petiot-Becard S, Van Dorsselaer A, Meinnel T, Giglione C, Cianferani S. Analyst. 2015;140:7234–7245. doi: 10.1039/c5an01311a. [DOI] [PubMed] [Google Scholar]
- 244.Coughlan NJA, Catani KJ, Adamson BD, Wille U, Bieske EJ. Journal of Chemical Physics. 2014;140:10. doi: 10.1063/1.4871883. [DOI] [PubMed] [Google Scholar]
- 245.Baker ES, Bushnell JE, Wecksler SR, Lim MD, Manard MJ, Dupuis NF, Ford PC, Bowers MT. J Am Chem Soc. 2005;127:18222–18228. doi: 10.1021/ja0553699. [DOI] [PubMed] [Google Scholar]