Abstract
Importance
Cytotoxic CD8+ T lymphocytes (TILs) participate in immune control of ovarian cancer; however, little is known about prognostic patterns of CD8+ TILs by histotype and in relation to other clinical factors.
Objective
To define the prognostic role of CD8+ TILs in epithelial ovarian cancer.
Design
Prospective survival cohort.
Setting
Multi-center observational.
Participants
Over 5,500 patients, including 3,196 high-grade serous ovarian carcinomas (HGSOCs), followed prospectively for over 24,650 person-years.
Exposure(s)
Following immunohistochemistry, CD8+ TILs were identified within the epithelial components of tumor islets. Patients were grouped based on the estimated number of CD8+ TILs per high-powered field: negative (none), low (1–2), moderate (3–19), and high (≥20). CD8+ TILs in a subset of patients were also assessed in a quantitative, uncategorized manner, and the functional form of associations with survival was assessed using penalized B-splines.
Main Outcome Measure(s)
Overall survival time.
Results
Among the five major invasive histotypes, HGSOCs showed the most infiltration. CD8+ TILs in HGSOCs were significantly associated with longer overall survival; median survival was 2.8 years for patients with no CD8+ TILs and 3.0 years, 3.8 years, and 5.1 years for patients with low, moderate, or high levels of CD8+ TILs, respectively (p-trend=4.2 × 10−16). A survival benefit was also observed among women with endometrioid and mucinous carcinomas, but not the other histotypes. Among HGSOCs, CD8+ TILs were favorable regardless of extent of residual disease following cytoreduction, known standard treatment, and germline BRCA1 pathogenic mutation, but were not prognostic for BRCA2 mutation carriers. Evaluation of uncategorized CD8+ TIL counts showed a near linear functional form.
Conclusions and Relevance
This study demonstrates the histotype-specific nature of immune infiltration and provides definitive evidence for a dose-response relationship between CD8+ TILs and HGSOC survival. That the extent of infiltration is prognostic, not merely its presence or absence, suggests that understanding factors which drive infiltration will be key to unravelling outcome heterogeneity in this cancer.
INTRODUCTION
Epithelial ovarian cancer (OC) is the most lethal gynecologic cancer and is responsible for approximately 14,000 deaths annually in the United States.1 While initial remission is often achieved, most patients relapse and die from their disease. Immune checkpoint inhibitors have demonstrated clinical activity in a small subset of OC patients.2,3 Understanding the endogenous immune response to OC—including the frequency of CD8+ tumor infiltrating lymphocytes (TILs) and their impact on prognosis—has biological and clinical relevance.
Earlier studies demonstrated that OC prognosis is associated with TILs at the time of primary cytoreductive surgery.4–8 CD8+ T cells are stimulated by peptides from degraded proteins bound to human leukocyte antigen class I molecules.9 This can trigger CD8+ T cells to kill tumor cells and secrete proinflammatory cytokines. While the presence of CD8+ TILs within the epithelial component of OCs has been associated with favorable prognosis,2,6–8,10–12 most prior analyses used simple dichotomous classification of CD8+ TILs and neglected to specify the inclusion/exclusion of stromal tissue. Prior analyses have been inadequately powered to evaluate histotype-specific survival associations. This is critical, as the invasive histotypes (high-grade serous, HGSOC, the most common and most lethal;13 endometrioid, ENOC;14 clear cell, CCOC;14,15 mucinous, MOC;16 and low-grade serous, LGSOC17–20) represent distinct biological processes, with distinct proposed cells of origin, clinical courses, and responses to chemotherapy.21–23
We conducted a large-scale assessment of intra-epithelial CD8+ TILs in over 5,000 prospectively followed OC patients. Our goals were to clarify the associations and evaluate the functional form of CD8+ TILs with overall survival in HGSOCs, and to explore association of CD8+ TIL levels with overall survival in other histotypes.
METHODS
Study Design and Participants
We assembled a prospective cohort of 7,377 women with a primary diagnosis of epithelial ovarian, peritoneal, or fallopian tube cancer. Patients were followed from enrollment in an IRB-approved protocol until death from any cause (eTable 1).24–26 Tumors were obtained at initial debulking surgery, formalin-fixed, paraffin embedded, and arrayed on tissue microarrays (TMAs). Clinical covariates and vital status underwent standardized quality control measures. We excluded 288 patients due to loss to follow-up, 11 with missing age at diagnosis, 65 with non-epithelial disease, and 1,436 due to inadequate quality or amount of arrayed tumor tissue, resulting in a final sample size of 5,577, including 5,078 women with tumors of the five major invasive histotypes (HGSOC, ENOC, CCOC, MOC, and LGSOC) (eTable 2). Median time from diagnosis to enrollment was zero days (interquartile range, 0 – 63); however, 38% of patients were enrolled > one month from diagnosis. As some HGSOC may be mistaken as ENOC,27 we utilized WT1 and TP53 immunohistochemical staining from 17 studies to re-classify 82 ENOC cases as HGSOC; overall survival of these reclassified cases was consistent with HGSOC (eFigure 1).
Immunohistochemistry and Scoring
For most patients (84%), staining was performed at the Mayo Clinic using the Leica Bond RX stainer (Leica, Buffalo, IL); however, for patients enrolled at the SEA and MAY1 study sites (8% and 9%, respectively), previously stained slides were used. Immunohistochemical methods are provided in Online-Only Text. Scoring was conducted at the University of Calgary; each core was screened for a hotspot of CD8+ TILs using a Nikon eclipse 80i microscope at 200 × magnification. Within each hotspot, one high power field at 400 × magnification with a 0.55 mm field diameter was evaluated, ensuring comparable area despite different core sizes across studies. Only CD8+ TILs within the epithelial component of the tumor (tumor islets) were considered, disregarding CD8+ cells in the stroma or abutting tumor cells (as seen, for example, in eFigure 2 classified as negative). A four-point ordinal score was defined a priori based on CD8+ TIL counts per high-powered field: negative (none), low (1–2 TILs), moderate (3–19 TILs), and high (≥20 TILs), similar to the validated method of Zhang and colleagues,4 except that we decreased the low to moderate cut-off from 6 TILs to 3 TILs. We did this to increase ease and consistency of scoring, as the ≥3 TIL cut-off is routinely used in colorectal carcinoma reporting to assess Lynch syndrome.28 Multiple cores from 156 cases were evaluated blindly by two gynecologic pathologists (WC, MK), and a weighted kappa statistic was estimated. Differences in interpretation were discussed at a multi-headed microscope, and WC scored 24% and MK 76% of the remaining cohort. TMAs included an average of 2.4 cores per patient; for cases with more than one scored core, the maximum score was used, consistent with the scoring of hotspot regions.
Analysis
Chi-square tests compared CD8+ TIL categories across clinical factors. Kaplan-Meier curves visually compared survival across categories. Cox proportional hazards regression estimated hazard ratios (HRs) and 95% confidence intervals (CIs). Primary analyses were based on tests for trend, modelling the ordered CD8+ TIL categories as a one degree-of-freedom linear term. Regression models included age at diagnosis (continuous), stage (I/II, III/IV, unknown), and study site as covariates; we also ran sensitivity analyses adjusting for extent of residual disease and post-surgical treatment. Separate analyses were conducted by histotype and among histopathological groupings (e.g., combining LGSOC with their suspected precursor, serous borderline tumors), and by relevant clinical factors. This report meets REMARK reporting recommendations for tumor marker prognostic studies;29 additional statistical methods are provided in Online-Only Text.
CD8+ TIL Cutpoint Analysis
Because categorical CD8+ TIL cutpoints may artificially restrict variability in the data and can be somewhat arbitrary, MK rescored all cores from a subset of 2,175 patients (1,449 with HGSOC), recording CD8+ TIL count as a numeric marker. Each core was given a value between 0 and 20+, using a threshold of 20 for counts that exceeded that number. As before, the maximum score was used for cases with more than one scored core. Among HGSOC cases, we compared survival distributions of those with rescored levels to those without using Cox proportional hazards regression. Among HGSOC cases with rescored CD8+ TIL levels, we ran five additional sets of Cox regression analyses. We first categorized the levels using our original thresholds (0, 1–2, 3–19 and 20+ CD8+ TILs) to confirm that our original results using all HGSOC cases did not differ from the subset who were rescored. Second, we categorized the levels using the thresholds of Zhang and colleagues4 to determine the robustness of our original results to these cutpoints. Third, we assessed the functional form of the association between CD8+ TIL levels and survival using penalized B-splines.30 Fourth, we fit the numerically-valued CD8+ TIL levels as a one-degree-of-freedom linear term. Finally, we carried out a formal cutpoint analysis similar to that described by Budczies and colleagues.31 Briefly, this approach examines all possible contiguous dichotomizations of TIL levels (i.e., 0 vs. 1+, 0–1 vs. 2+, 0–2 vs. 3+, etc.) using Cox proportional hazards regression to identify the threshold which best discriminates survivors from non-survivors based on evidence of association.
RESULTS
Distribution of CD8+ TILs by Histotype
Epithelial CD8+ TILs were assessed using a four-tiered scoring system (inter-observer agreement 81.8%; weighted kappa 0.846, 95% CI 0.804–0.888). We observed intratumoral heterogeneity in CD8+ TILs across cores per patient (intraclass correlation coefficient = 0.56, 95% CI 0.54–0.57). CD8+ TILs varied across the major invasive OC histotypes (HGSOC, ENOC, CCOC, MOC, and LGSOC, chi-square p=2.8 × 10−103; eFigure 3). Most HGSOC cases (83%) had evidence of CD8+ TILs, with a lower proportion seen in LGSOC and ENOC cases (73%, 72% respectively) and CCOC and MOC cases (52%, 51%, respectively). Most borderline tumors showed evidence of CD8+ TILs (serous 84%, mucinous 70%; eTable 3).
Associations of CD8+ TILs with Overall Survival by Histotype
We observed a striking association for longer survival time with increasing levels of CD8+ TILs in HGSOC cases (p-trend adjusted for study, age, and stage = 4.2 × 10−16; Table; Figure). Median survival was 2.8 years for women negative for CD8+ TILs, and 3.0 years, 3.8 years, and 5.1 years for low, moderate, or high levels. At the extremes, women with high levels of CD8+ TILs (≥20 per field) had a 43% reduced risk of death compared to women with no evidence of CD8+ TILs (HR 0.57, 95% CI 0.49–0.65; Table). Associations were similar after adjustment for residual disease (eTable 4).
Table.
Multivariate-adjusted association of CD8+ tumor-infiltrating lymphocytes (TIL) and overall survival among cases with the five most common invasive epithelial ovarian cancer histotype (N=5,078)
| Histotype | CD8+ TILs | N | Person-Years | % events | HR (95% CI) | P value trend | P value 3 d.f. |
|---|---|---|---|---|---|---|---|
| High-grade serous | Negative | 546 | 1,709.75 | 76.2% | ref | 4.2 × 10−16 | 7.2 × 10−15 |
| Low | 546 | 1,908.39 | 72.3% | 0.86 (0.75–0.99) | |||
| Moderate | 1,394 | 5,264.82 | 69.0% | 0.77 (0.69–0.87) | |||
| High | 710 | 3,110.87 | 56.5% | 0.57 (0.49–0.65) | |||
|
| |||||||
| Endometrioid | Negative | 206 | 1,118.53 | 33.5% | ref | 0.0084 | 0.006 |
| Low | 130 | 675.44 | 34.6% | 0.80 (0.54–1.18) | |||
| Moderate | 283 | 1,844.53 | 18.0% | 0.50 (0.34–0.74) | |||
| High | 110 | 657.59 | 22.7% | 0.76 (0.47–1.23) | |||
|
| |||||||
| Clear cell | Negative | 309 | 1,640.28 | 41.1% | ref | 0.50 | 0.52 |
| Low | 141 | 658.25 | 45.4% | 1.16 (0.84–1.60) | |||
| Moderate | 118 | 618.79 | 41.5% | 0.88 (0.62–1.24) | |||
| High | 80 | 412.44 | 40.0% | 0.92 (0.61–1.39) | |||
|
| |||||||
| Mucinous | Negative | 168 | 750.75 | 44.0% | ref | 0.037 | 0.16 |
| Low | 77 | 375.26 | 31.2% | 0.91 (0.55–1.51) | |||
| Moderate | 85 | 470.62 | 27.1% | 0.56 (0.34–0.93) | |||
| High | 13 | 72.35 | 23.1% | 0.79 (0.23–2.68) | |||
|
| |||||||
| Low-grade serous | Negative | 43 | 198.06 | 48.8% | ref | 0.91 | 0.996 |
| Low | 44 | 184.89 | 65.9% | 0.94 (0.50–1.74) | |||
| Moderate | 63 | 272.13 | 47.6% | 0.98 (0.52–1.83) | |||
| High | 12 | 49.53 | 41.7% | 0.92 (0.33–2.59) | |||
Adjusted for study, age (continuous), and stage (I/II, III/IV, unknown); levels based on counts of TIL per high-powered field: negative, none; low, 1–2; moderate, 3–19; high, 20+; HR, hazard ratio; CI, confidence interval; P value trend, from a one degree-of-freedom trend test; P value 3 d.f., from an unordered three degree-of-freedom test.
Figure. Kaplan-Meier Overall Survival Plots by CD8+ Tumor-Infiltrating Lymphocyte (TIL) Levels for the High-Grade Serous, Endometrioid, and Mucinous Ovarian Cancer.
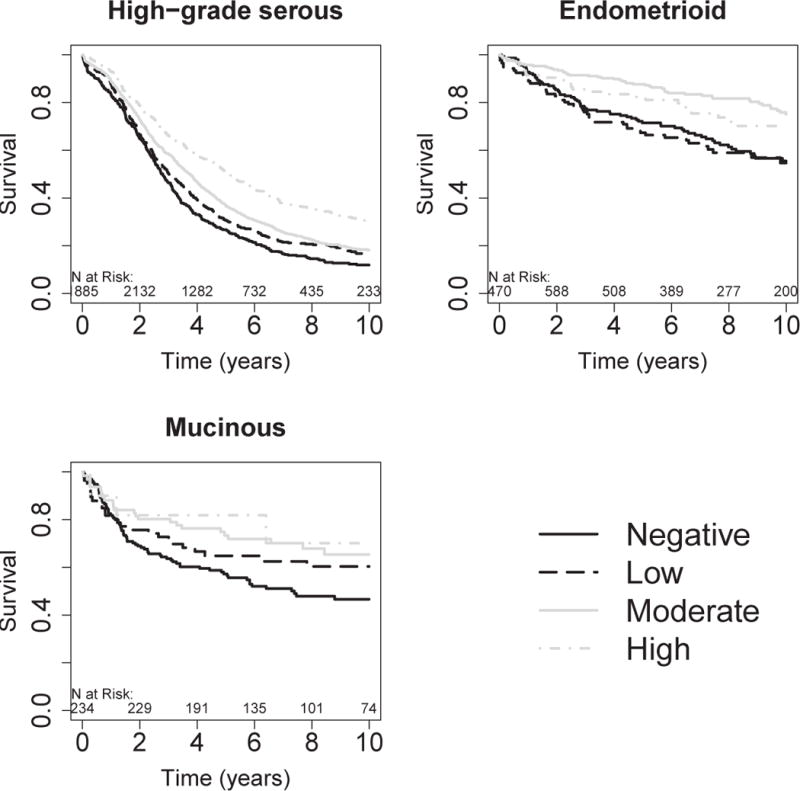
Negative, no CD8+ TILs; low, 1–2 CD8+ TILs; moderate, 3–19 CD8+ TILs; high, 20 or more CD8+ TILs per high-powered field. The numbers just above the x-axis represent the number of women at risk in two year time intervals. Number at risk on date of diagnosis may be smaller than number at risk later due to left truncation of follow-up resulting from delayed study enrollment.
Increasing levels of CD8+ TILs were also associated with longer survival time among women with ENOC (p-trend=0.0084; Table; Figure). This association was also apparent in separate analyses of grade 1 ENOC and grades 2 and 3 ENOC, although these were limited in sample size (eTable 5). While there was a statistically significant dose-response similar to HGSOC, it is noteworthy that ENOCs with moderate levels (3–19 per field) showed the greatest improvement in survival time compared to women with ENOC and no detectable CD8+ TILs (HR 0.50, 95% CI 0.34–0.74).
A similar association was observed for women with MOC (p=0.037; Table; Figure), although, as the histotype with the lowest overall levels of CD8+ TILs, only 13 women (4%) had high TIL levels. Kaplan-Meier plots indicate a dose-response relationship, at least for negative to moderate levels (Figure). In contrast, CCOCs and LGSOCs showed no apparent association between CD8+ TILs and survival time (Table, eFigure 4). Because LGSOC is the rarest of the invasive histotypes, the null association in this group should be interpreted with caution. As some prior studies combined LGSOC and HGSOC, we also analyzed invasive serous cases as a group, including those with missing grade. We found that the striking HGSOC results were attenuated (eTable 5), suggesting that the relevance of CD8+ TILs among serous cases may be limited to HGSOC and confirming that immunohistochemistry-aided histotype classification is a critical first step to improving the classification of OC cases.27,32 No other patterns were observed in analyses of histopathological groups (eTable 5).
Among the five major invasive histotypes, time to disease progression was known for 52% of cases (N=2,681). Progression-free survival results were remarkably similar to overall survival results (eTable 6).
Associations of CD8+ TILs with Clinical Features in HGSOC
The extent of residual disease following primary cytoreductive surgery was available for 2,173 HGSOC cases. Our results showed a greater proportion of tumors without macroscopic residual disease had high CD8+ TIL levels than those with macroscopic disease (26% v 20%; p=0.0064; eFigure 3). Increasing CD8+ TILs were associated with improved survival in a dose-response manner in both surgical outcome groups, indicating that immune response improves prognosis regardless of the remaining residual disease after surgery (eTable 7).
Our study included 133 BRCA1 and 66 BRCA2 mutation carriers and 844 tested non-carriers. The extent of CD8+ TILs differed by mutation status (p=0.024), as 29% of BRCA1 mutation carriers had high TIL counts, yet only 18% of non-carriers and 15% BRCA2 mutation carriers did (eFigure 3). The survival benefit associated with CD8+ TILs was also found to differ by mutation status (p-interaction=0.0055). Increased CD8+ TILs were associated with favorable survival among cases without mutations (p=5.1 × 10−7) and among cases with a BRCA1 mutation (p=0.0025, eTable 7). Among BRCA2 mutation carriers, there was no evidence of association between CD8+ TILs and survival (p=0.62).
Treatment details were documented for 501 HGSOC cases who received standard first line chemotherapy, including 295 who received the standard dose (carboplatin AUC 5 or 6 and paclitaxel 135 mg/m2 or 175 mg/m²). Association with CD8+ TIL level and overall survival was also observed within this group (p trend=0.003, eTable 7).
Among HGSOC cases, CD8+ TIL level was associated with earlier stage (p=4.3 × 10−4) and younger age at diagnosis (p=1.6 × 10−4). In stratified analyses CD8+ TIL level was consistently prognostic in stage and age subgroups (eTable 5). We also observed that cases born more recently showed higher levels (n=2,734, p=0.001); we adjusted all analyses additionally for year of birth, and results were similar. CD8+ TIL level was not associated with year of diagnosis (p=0.71), self-reported racial group (p=0.74), or pre- or post-treatment CA125 (p=0.42 and 0.89; respectively).
Analysis of CD8+ TIL Cutpoints in HGSOC
Of the 3,196 HGSOC cases, 1,449 (45%) were rescored using a numeric count. There were no differences in survival between women who were rescored and those who were not (p=0.12; kappas comparing original values to rescored values 0.91 (95% CI 0.89–0.92). eTable 8 displays associations of categorized CD8+ TIL levels and survival in women with rescored tumors. After adjustment for age and stage, strong dose-response associations were observed using both the original threshold values (0, 1–2, 3–19 and 20+ CD8+ TILs) and those used by Zhang and colleagues (0, 1–5, 6–19, 20+ CD8+ TILs) (p<10−5 for each). As before, associations were slightly attenuated but remained significant after adjustment for extent of residual disease and post-surgical treatment (p<10−4 for each).
Assessment of the functional form of the association between numeric CD8+ TIL levels and survival using penalized B-splines, after adjustment for age and stage, is shown in eFigure 5. We observed a strong negative association with survival, indicating that increasing CD8+ TIL levels are progressively protective across this spectrum of values. The results of fitting CD8+ TIL levels as a one-degree-of-freedom linear term are also shown in eFigure 5 and track very closely to those using penalized B-splines, indicating that the association between CD8+ TIL levels and survival in women with HGSOC is virtually log-linear in nature.
Results of a formal cutpoint analysis examining all possible sets of contiguous dichotomizations of TIL levels can be found in eTable 9. The best discrimination of survivors from non-survivors occurred when comparing those with 0–13 TILs to those with 14 or more (HR 0.75, 95% CI 0.65–0.86, p=1.5 × 10−5). However, each of the 19 dichotomizations yielded highly significant results (all p<=1.1 × 10−3), with HRs consistently ranging from 0.75 to 0.83, again indicating that greater TIL levels are protective across the entire spectrum of values examined.
DISCUSSION
Our study is by far the largest report on intra-epithelial CD8+ TILs in OC to date and shows a robust dose-dependent increase in survival for increasing TIL levels in women with HGSOC. Analyses on a subset of individuals using numeric TIL counts confirmed a progressively protective, nearly log-linear survival effect as CD8+ TILs counts increased from 0 to 20 or more per high-powered field, suggesting that the quantity of CD8+ TILs, not merely their presence, is informative and that the most immune-rich HGSOCs are the most likely to have improved clinical outcome. This effect was not modified or confounded by the extent of residual disease after cytoreductive surgery. As there are fewer than a handful of other validated prognostic biomarkers for HGSOC, e.g., BRCA1 and BRCA2 status33 and PR expression,26 these results may provide increased prognostic prediction.
This is the first CD8+ TIL study in histotypes other than HGSOC; we revealed a significant reduction in risk of death for patients diagnosed with ENOC and MOC. In ENOC, cases with moderate CD8+ TIL levels had the most favorable survival, with no additional benefit observed beyond this threshold. As prior reports suggest that ENOCs with high CD8+ TIL are more commonly mismatch repair deficient,34 we speculate that, similar to endometrial cancers,35,36 ENOC with high CD8+ TIL levels may be associated with an intermediate outcome due to the association with mismatch repair deficiency. No survival associations were seen in CCOC.
Other investigations have noted higher response rates to immune checkpoint blockade among patients with a higher burden of neoantigens,37,38 suggesting that increased neoantigens increases likelihood that T lymphocytes recognize tumor as foreign and mount an immune response. It has also been demonstrated that BRCA1-mutated HGSOC tumors have a higher average neoantigen number than non-mutated tumors.39 Here, HGSOC patients with germline BRCA1 mutations demonstrated higher CD8+ TILs than patients with BRCA2 mutations or those tested mutation negative. While neoantigen load may explain higher CD8+ TILs in BRCA1-mutated tumors, and their association with better outcome, it does not explain the better outcome of BRCA2-mutated tumors.40,41
Given its robust prognostic ability, relative ease of testing, and low inter-observer variability (percent agreement=81.8%, weighted kappa=0.846), quantitation of CD8+ TILs should be considered for clinical evaluation as suggested for other cancers.42–44 Unfortunately, as expected, we found intratumoral heterogeneity in CD8+ TILs across cores per patient (intraclass correlation coefficient = 0.56, 95% CI 0.54–0.57). To account for this, we utilize the maximum score, which is akin to the hotspot assessment of proliferation in other cancers, and is more feasible for surgical specimens with many tumor-containing slides. We also propose that, similar to the breast cancer community, a practical and robust scoring system should be developed.43 Additional issues requiring large-scale study which were not evaluated here include: utility of image analysis; evaluation of stromal CD8+ TILs; consistency across multiple tumor sites per patient; impact of neoadjuvant chemotherapy;45–47 relationships between CD8+ TIL levels, HGSOC molecular subtypes,48–50 common genetic variation,51 and epidemiologic risk factors;52 and evaluation of other lymphocyte subsets, such as CD4+ TILs, CD20+ TILs (B cells), tertiary lymphoid structures, and plasma cells.10,12–14,53 Clinically, it will be important to test whether CD8+ TILs predict response to certain therapies including standard chemo- and immune therapy, as, for example, CD8+ TILs predict chemo-response in subtypes of breast cancer.54 It will also be critical to study whether the immune response of CD8+ TILs can be activated by checkpoint blockade.
In summary, these large-scale analyses show that CD8+ TILs vary by histotype with HGSOC tumors having the highest levels and a strong association with survival, regardless of extent of residual disease or first line chemotherapy treatment. Penalized B-splines revealed that this association was nearly log-linear in nature, indicating that progressively greater TIL counts yield progressively better prognoses for HGSOC tumors. We showed for the first time that CD8+ TILs in HGSOC cases with germline BRCA2 mutations may not associate with survival. Finally, we find that ENOC and MOC tumors show trends associating CD8+ TILs with survival time and that CCOC do not. A clinically applicable scoring system for CD8+ TILs should be developed in order to incorporate into clinical trials.
Supplementary Material
KEY POINTS.
Question
To what extent are CD8+ tumor infiltrating lymphocytes (TILs) prognostic in epithelial ovarian cancer?
Findings
Analysis of over 24,000 person-years of follow-up on over 5,500 cases shows improved survival with increasing CD8+ TIL counts in high-grade serous, endometrioid, and mucinous ovarian cancers (p-trends 4.2 × 10−16; 0.0084, and 0.037, respectively). Among high-grade serous ovarian cancers, this nearly log-linear relationship was present regardless of extent of residual disease following cytoreduction, receipt of standard treatment, and germline BRCA1 mutation.
Meaning
CD8+ TILs are a key prognostic factor in certain ovarian cancer histotypes and warrant additional study in the context of immunotherapy.
Acknowledgments
FUNDING/SUPPORT
Funding was provided by Canadian Institutes for Health Research (MOP-86727), Brazilian National Council for Scientific and Technological Development – Grant Number 478416/2009-1, Calgary Laboratory Services Internal Research Competition RS10-533, German Federal Ministry of Education and Research of Germany (01 GB 9401), German Cancer Research Center (DKFZ), US National Cancer Institute K07-CA80668, P50-CA159981, R01CA095023; NIH/National Center for Research Resources/General Clinical Research Center grant MO1-RR000056, US Army Medical Research and Materiel Command DAMD17-02-1-0669, National Institutes of Health (R01-CA122443, P50-CA136393, P30-CA15083, U01-CA71966, U01-CA69417, R01-CA16056, K07-CA143047), Cancer Research UK (C490/A10119, C490/A10123, C490/A16561), UK National Institute for Health Research Biomedical Research Centres at the University of Cambridge and at University College Hospital “Womens Health Theme”, NIH SFB 685, the Eve Appeal, the Oak Foundation, and Deutsche Forschungsgemein-schaft. AOCS was supported by the U.S. Army Medical Research and Materiel Command under DAMD17-01-1-0729, The Cancer Council Victoria, Queensland Cancer Fund, The Cancer Council New South Wales, the Tom Baker Cancer Centre Translational Laboratories The Cancer Council South Australia, The Cancer Foundation of Western Australia, The Cancer Council Tasmania and the National Health and Medical Research Council of Australia (NHMRC; ID400413, ID400281). AOCS gratefully acknowledges additional support from the Peter MacCallum Cancer Foundation and Ovarian Cancer Australia (OCA). The Gynaecological Oncology Biobank at Westmead, a member of the Australasian Biospecimen Network-Oncology group, was funded by the National Health and Medical Research Council Enabling Grants ID 310670 & ID 628903 and the Cancer Institute NSW Grants 12/RIG/1-17 and 15/RIG/1-16. Funding for MALOVA was provided by research grant R01-CA61107 from the National Cancer Institute, Bethesda, MD; research grant 94 222 52 from the Danish Cancer Society, Copenhagen, Denmark; and the Mermaid I project. Drs DeFazio and Harnett are funded by Cancer Institute NSW Grant 15/TRC/1-01. Dr Karlan is funded by the American Cancer Society Early Detection Professorship (SIOP-06-258-01-COUN) and the National Center for Advancing Translational Sciences (NCATS), Grant UL1TR000124.
Role of the Funder/Sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
- TIL
tumor infiltrating lymphocytes
- HGSOC
high-grade serous ovarian carcinoma
- OC
ovarian cancer
- PD-1
programmed death 1
- ENOC
endometrioid ovarian cancer
- CCOC
clear cell ovarian cancer
- MOC
mucinous ovarian cancer
- LGSOC
low-grade serous ovarian cancer
- TMA
tissue microarray
Footnotes
Author Contributions: Dr Goode had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Mr Vierkant and Dr Winham conducted and were responsible for the data analysis.
Study concept and design: Goode, Block, Vierkant, Hartmann, Nation, Jimenez-Linan, Ness, Longacre, Bowtell, Moysich, Deen, Brenton, Pharoah, Köbel, Ramus.
Acquisition, analysis, or interpretation of data: Goode, Block, Kalli, Vierkant, Chen, Fogarty, Gentry-Maharaj, Tołoczko, Hein, Bouligny, Jensen, Osorio, Hartkopf, Ryan, Chudecka-Głaz, Magliocco, Hartmann, Jung, Gao, Hernandez, Fridley, McCauley, Kennedy, Wang, Karpinsky, de Sousa, Tiezzi, Wachter, Herpel, Taran, Modugno, Nelson, Lubiński, Menkiszak, Alsop, Lester, García-Donas, Hung, Palacios, Rothstein, Kelley, de Andrade, Robles-Díaz, Intermaggio, Widschwendter, Beckmann, Ruebner, Singh, Oszurek, Harnett, Rambau, Sinn, Wagner, Ghatage, Sharma, Edwards, Orsulic, Brucker, Johnatty, Eilber, McGuire, Sieh, Natanzon, Li, Whittemore, deFazio, Staebler, Karlan, Gilks, Bowtell, Høgdall, Candido dos Reis, Steed, Campbell, Gronwald, Benítez, Koziak, Chang-Claude, Moysich, Kelemen, Cook, Goodman, García, Fasching, Kommoss, Kjaer, Menon, Pharoah, Chenevix-Trench, Huntsman, Winham, Köbel, Ramus.
Drafting of the manuscript: Goode, Block, Vierkant, Fogarty, Jimenez-Linan, Rambau, Sharma, Brucker, Li, Bowtell, Köbel, Ramus.
Critical revision of the manuscript for important intellectual content: Goode, Block, Kalli, Vierkant, Chen, Gentry-Maharaj, Tołoczko, Hein, Bouligny, Jensen, Osorio, Hartkopf, Ryan, Chudecka-Głaz, Magliocco, Hartmann, Jung, Gao, Hernandez, Fridley, McCauley, Kennedy, Wang, Karpinskyj, de Sousa, Tiezzi, Wachter, Herpel, Taran, Modugno, Nelson, Lubiński, Menkiszak, Alsop, Lester, García-Donas, Nation, Hung, Palacios, Rothstein, Kelley, de Andrade, Robles-Díaz, Intermaggio, Widschwendter, Beckmann, Ruebner, Singh, Oszurek, Harnett, Sinn, Wagner, Ghatage, Sharma, Edwards, Ness, Orsulic, Brucker, Johnatty, Longacre, Eilber, McGuire, Sieh, Natanzon, Whittemore, deFazio, Staebler, Karlan, Gilks, Høgdall, Candido dos Reis, Steed, Campbell, Gronwald, Benítez, Koziak, Chang-Claude, Moysich, Kelemen, Cook, Goodman, García, Fasching, Kommoss, Deen, Kjaer, Menon, Brenton, Pharoah, Chenevix-Trench, Huntsman, Winham, Köbel, Ramus.
Statistical analysis: Vierkant, Chen, Fogarty, Gao, Fridley, McCauley, Wang, Winham.
Obtained funding: Goode, Modugno, Edwards, Ness, Moysich, Kelemen, Cook, Goodman, Kjaer, Chenevix-Trench.
Administrative, technical, or material support: Goode, Block, Kalli, Gentry-Maharaj, Tołoczko, Hein, Bouligny, Jensen, Osorio, Hartkopf, Chudecka-Głaz, Magliocco, Hartmann, Jung, Hernandez, Kennedy, Karpinskyj, de Sousa, Tiezzi, Wachter, Herpel, Taran, Lubiński, Alsop, Lester, Nation, Rothstein, de Andrade, Intermaggio, Widschwendter, Beckmann, Ruebner, Jimenez-Linan, Singh, Harnett, Wagner, Ness, Orsulic, Johnatty, Eilber, McGuire, Sieh, Natanzon, deFazio, Staebler, Karlan, Gilks, Bowtell, Høgdall, Candido dos Reis, Campbell, Benítez, Koziak, Chang-Claude, Moysich, Kelemen, Cook, García, Fasching, Kommoss, Deen, Menon, Huntsman, Ramus.
Study supervision: Goode, Vierkant, Nelson, Menkiszak, García-Donas, Ghatage, Sharma, Edwards, Ness, Moysich, Brenton, Huntsman.
Other contributions: Whittemore, Köbel.
Other Contributors: Australian Ovarian Cancer Study Group.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Bowtell receives funding from Astra Zeneca and Genentech Roche for the conduct of research studies and clinical trials unrelated to the work described in this article. Dr Huntsman is a founder and Chief Medical Officer of Contextual Genomics, a somatic mutation testing laboratory; the company’s work and interests do not overlap with the subject of and methodologies used in this manuscript. No other disclosures were reported.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of anti-PD-1 antibody, Nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015–4022. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 3.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 5.Tomsova M, Melichar B, Sedlakova I, Steiner I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol. 2008;108(2):415–420. doi: 10.1016/j.ygyno.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Han LY, Fletcher MS, Urbauer DL, et al. HLA class I antigen processing machinery component expression and intratumoral T-cell infiltrate as independent prognostic markers in ovarian carcinoma. Clin Cancer Res. 2008;14(11):3372–3379. doi: 10.1158/1078-0432.CCR-07-4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke B, Tinker AV, Lee CH, et al. Intraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type, and BRCA1 loss. Mod Pathol. 2009;22(3):393–402. doi: 10.1038/modpathol.2008.191. [DOI] [PubMed] [Google Scholar]
- 8.Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124(2):192–198. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callahan MJ, Nagymanyoki Z, Bonome T, et al. Increased HLA-DMB expression in the tumor epithelium is associated with increased CTL infiltration and improved prognosis in advanced-stage serous ovarian cancer. Clin Cancer Res. 2008;14(23):7667–7673. doi: 10.1158/1078-0432.CCR-08-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preston CC, Maurer MJ, Oberg AL, et al. The ratios of CD8+ T cells to CD4+CD25+ FOXP3+ and FOXP3− T cells correlate with poor clinical outcome in human serous ovarian cancer. PLoS One. 2013;8(11):e80063. doi: 10.1371/journal.pone.0080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurman RJ. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Ann Oncol. 2013;24(Suppl 10):x16–21. doi: 10.1093/annonc/mdt463. [DOI] [PubMed] [Google Scholar]
- 14.Worley MJ, Welch WR, Berkowitz RS, Ng SW. Endometriosis-associated ovarian cancer: a review of pathogenesis. Int J Mol Sci. 2013;14(3):5367–5379. doi: 10.3390/ijms14035367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mabuchi S, Sugiyama T, Kimura T. Clear cell carcinoma of the ovary: molecular insights and future therapeutic perspectives. J Gynecol Oncol. 2016;27(3):e31. doi: 10.3802/jgo.2016.27.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelemen LE, Kobel M. Mucinous carcinomas of the ovary and colorectum: different organ, same dilemma. Lancet Oncol. 2011;12(11):1071–1080. doi: 10.1016/S1470-2045(11)70058-4. [DOI] [PubMed] [Google Scholar]
- 17.Emmanuel C, Chiew YE, George J, et al. Genomic classification of serous ovarian cancer with adjacent borderline differentiates RAS pathway and TP53-mutant tumors and identifies NRAS as an oncogenic driver. Clin Cancer Res. 2014;20(24):6618–6630. doi: 10.1158/1078-0432.CCR-14-1292. [DOI] [PubMed] [Google Scholar]
- 18.Oswald AJ, Gourley C. Low-grade epithelial ovarian cancer: a number of distinct clinical entities? Curr Opin Oncol. 2015;27(5):412–419. doi: 10.1097/CCO.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 19.Grabowski JP, Harter P, Heitz F, et al. Operability and chemotherapy responsiveness in advanced low-grade serous ovarian cancer. An analysis of the AGO Study Group metadatabase. Gynecol Oncol. 2016;140(3):457–462. doi: 10.1016/j.ygyno.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Fader AN, Java J, Ueda S, et al. Survival in women with grade 1 serous ovarian carcinoma. Obstet Gynecol. 2013;122(2 Pt 1):225–232. doi: 10.1097/AOG.0b013e31829ce7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobel M, Bak J, Bertelsen BI, et al. Ovarian carcinoma histotype determination is highly reproducible, and is improved through the use of immunohistochemistry. Histopathology. 2014;64(7):1004–1013. doi: 10.1111/his.12349. [DOI] [PubMed] [Google Scholar]
- 22.Ramalingam P. Morphologic, immunophenotypic, and molecular features of epithelial ovarian cancer. Oncology (Williston Park) 2016;30(2):166–176. [PubMed] [Google Scholar]
- 23.Kobel M, Kalloger SE, Boyd N, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 2008;5(12):e232. doi: 10.1371/journal.pmed.0050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobel M, Madore J, Ramus SJ, et al. Evidence for a time-dependent association between FOLR1 expression and survival from ovarian carcinoma: implications for clinical testing. An Ovarian Tumour Tissue Analysis consortium study. Br J Cancer. 2014;111(12):2297–2307. doi: 10.1038/bjc.2014.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobel M, Kalloger SE, Lee S, et al. Biomarker-based ovarian carcinoma typing: a histologic investigation in the Ovarian Tumor Tissue Analysis consortium. Cancer Epidemiol Biomarkers Prev. 2013;22(10):1677–1686. doi: 10.1158/1055-9965.EPI-13-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieh W, Kobel M, Longacre TA, et al. Hormone-receptor expression and ovarian cancer survival: an Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013;14(9):853–862. doi: 10.1016/S1470-2045(13)70253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobel M, Rahimi K, Rambau PF, et al. An immunohistochemical algorithm for ovarian carcinoma typing. Int J Gynecol Pathol. 2016;35(5):430–441. doi: 10.1097/PGP.0000000000000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.College of American Pathologists. Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Colon and Rectum. 2016 http://www.cap.org/ShowProperty?nodePath=/UCMCon/ContributionFolders/WebContent/pdf/cp-colon-16protocol-3400.pdf.
- 29.McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK) Br J Cancer. 2005;93(4):387–391. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eilers PHC, Marx BD. Flexible smoothing with B-splines and penalties. Statistical Science. 1996;11(2):89–102. [Google Scholar]
- 31.Budczies J, Klauschen F, Sinn BV, et al. Cutoff finder: a comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7(12):e51862. doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kar SP, Berchuck A, Gayther SA, et al. Common genetic variation and susceptibility to ovarian cancer: current insights and future directions. Cancer Epidemiol Biomarkers Prev. 2017 doi: 10.1158/1055-9965.EPI-17-0315. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Candido-dos-Reis FJ, Song H, Goode EL, et al. Germline mutation in BRCA1 or BRCA2 and ten-year survival for women diagnosed with epithelial ovarian cancer. Clin Cancer Res. 2015;21(3):652–657. doi: 10.1158/1078-0432.CCR-14-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rambau PF, Duggan MA, Ghatage P, et al. Significant frequency of MSH2/MSH6 abnormality in ovarian endometrioid carcinoma supports histotype-specific Lynch syndrome screening in ovarian carcinomas. Histopathology. 2016;69(2):288–297. doi: 10.1111/his.12934. [DOI] [PubMed] [Google Scholar]
- 35.McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol. 2016;34(25):3062–3068. doi: 10.1200/JCO.2016.67.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strickland KC, Howitt BE, Shukla SA, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7(12):13587–13598. doi: 10.18632/oncotarget.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolton KL, Chenevix-Trench G, Goh C, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307(4):382–389. doi: 10.1001/jama.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9(5):e95285. doi: 10.1371/journal.pone.0095285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galon J, Pages F, Marincola FM, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denkert C, Wienert S, Poterie A, et al. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol. 2016;29(10):1155–1164. doi: 10.1038/modpathol.2016.109. [DOI] [PubMed] [Google Scholar]
- 44.Hida AI, Ohi Y. Evaluation of tumor-infiltrating lymphocytes in breast cancer; proposal of a simpler method. Ann Oncol. 2015;26(11):2351. doi: 10.1093/annonc/mdv363. [DOI] [PubMed] [Google Scholar]
- 45.Mesnage SJ, Auguste A, Genestie C, et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC) Ann Oncol. 2017;28(3):651–657. doi: 10.1093/annonc/mdw625. [DOI] [PubMed] [Google Scholar]
- 46.Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 47.Melamed A, Hinchcliff EM, Clemmer JT, et al. Trends in the use of neoadjuvant chemotherapy for advanced ovarian cancer in the United States. Gynecol Oncol. 2016;143(2):236–240. doi: 10.1016/j.ygyno.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Verhaak RG, Tamayo P, Yang JY, et al. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J Clin Invest. 2013;123(1):517–525. doi: 10.1172/JCI65833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tothill RW, Tinker AV, George J, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14(16):5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 50.Konecny GE, Wang C, Hamidi H, et al. Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. J Natl Cancer Inst. 2014;106(10):dju249. doi: 10.1093/jnci/dju249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phelan CM, Kuchenbaecker KB, Tyrer JP, et al. Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nat Genet. 2017;49(5):680–691. doi: 10.1038/ng.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cannioto RA, Trabert B, Poole EM, Schildkraut JM. Ovarian cancer epidemiology in the era of collaborative team science. Cancer Causes Control. 2017;28(5):487–495. doi: 10.1007/s10552-017-0862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kroeger DR, Milne K, Nelson BH. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian cancer. Clin Cancer Res. 2016;22(12):3005–3015. doi: 10.1158/1078-0432.CCR-15-2762. [DOI] [PubMed] [Google Scholar]
- 54.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


