Abstract
Background:
Agricultural use of antimicrobials in subtherapeutic concentrations is increasing in response to the rising demand for food animal products worldwide. In India, the use of antimicrobials in food animal production is unregulated. Research suggests that many clinically important antimicrobials are used indiscriminately. This is the largest study to date in India that surveys poultry production to test for antimicrobial resistance and the occurrence of extended-spectrum (ESBLs) modulated by farming and managerial practices.
Objectives:
Our goal was to survey poultry production for resistance to eleven clinically relevant antimicrobials and phenotypic occurrence of ESBLs as modulated by farming and managerial practices.
Methods:
Eighteen poultry farms from Punjab were surveyed, and 1,556 Escherichia coli isolates from 530 birds were tested for susceptibility to 11 antimicrobials using the disk diffusion method and validated using VITEK 2 (bioMérieux, Marcy-L’Étoile, France). Samples from 510 of these birds were phenotypically tested for ESBL production using the combination disk method and confirmed using VITEK 2. Generalized linear mixed models were used to infer differences in resistance profiles associated with different farming practices and facility types.
Results:
Resistance profiles were significantly different between broiler and layer farms. Broiler farms were 2.2 [ampicillin (AMP), ] to 23 [nalidixic acid (NX), ] times more likely to harbor resistant E. coli strains than layer farms. Adjusting for farm type (broiler vs. layer), the odds of resistance (although not statistically significant) to all antimicrobials except nitrofurantoin (NIT) were higher in independent facilities (IUs) as compared to contracted facilities (CFs). Increased prevalence of multidrug resistance (MDR; 94% compared to 60% in layers), including prevalence of ESBL-producing strains (87% compared to 42% in layers), was observed in broiler farms.
Conclusions:
Our findings suggest that unregulated use of clinically relevant antimicrobials in Indian broiler and layer farms may contribute to the emergence of resistance and support the need to curb the nontherapeutic use of medically important antimicrobials in food animal production. https://doi.org/10.1289/EHP292
Introduction
Medically important antimicrobials are used extensively in food animal production for disease prevention (e.g., prophylaxis and metaphylaxis), treatment, and growth promotion. It is estimated that two-thirds of antimicrobials produced globally are consumed in the livestock sector (CDDEP 2015). Numerous studies suggest that the widespread use of agricultural antimicrobials contributes to increased clinical resistance to antimicrobials (Chang et al. 2015; Marshall and Levy 2011; Silbergeld et al. 2008). Since antimicrobials are routinely added to animal feeds, bacterial populations are repeatedly exposed to subtherapeutic doses ideal for the emergence and spread of antimicrobial resistance (Chang et al. 2015; Marshall and Levy 2011; Silbergeld et al. 2008; You and Silbergeld 2014). Nearly every class of antimicrobial is used in agriculture, including many closely related to clinically relevant antimicrobials, such as penicillins, cephalosporins, fluoroquinolones, tetracyclines, sulfonamides, and aminoglycosides (Marshall and Levy 2011; Schwarz et al. 2001; Silbergeld et al. 2008). The extensive use of antimicrobials in agriculture results in human exposure to antimicrobial-resistant bacteria via direct and indirect pathways. These include exposure via direct contact with livestock or contaminated food products, indirect gene transfer across bacterial species, and the widespread release of antimicrobial-resistant pathogens into the environment (Silbergeld et al. 2008). This raises serious human health concerns, since the occurrence of cross-resistance between antimicrobials of the same class is highly likely.
The use of antimicrobials in subtherapeutic concentrations is increasing in response to heightened demand for food animal products worldwide, particularly in South and Southeast Asia, due to rising incomes (Teillant et al. 2015; Van Boeckel et al. 2015). The global average annual consumption of antimicrobials in food animals was conservatively estimated in 2010 to be 63,151 () tons. In chickens, this corresponds to a consumption of antimicrobials per kilogram of animal produced of approximately (Van Boeckel et al. 2015). Global antimicrobial consumption in food animal production is expected to rise by 67% by 2030, driven primarily by BRICS (Brazil, Russia, India, China, and South Africa) countries shifting to large-scale, intensive farming operations where antimicrobials are used routinely in subtherapeutic doses. Antimicrobial consumption in food animal production in India is projected to grow by 312%, a concerning development further compounded by India’s status as the largest consumer of antimicrobials of humans (Van Boeckel et al. 2014, 2015).
A recent study of antimicrobial residues in chicken meat sold for human consumption in New Delhi, India, found that of the 70 chicken meat samples tested, 40% contained antimicrobial residues (Sahu and Saxena 2014). The most common antimicrobials detected were enrofloxacin (20%), ciprofloxacin (14.3%), doxycycline (14.3%), oxytetracycline (11.4%), and chlortetracycline (1.4%). The high use of fluoroquinolones detected is particularly concerning, given the importance of this broad-spectrum agent in human clinical medicine. Fluoroquinolones were banned for veterinary use in poultry in the United States in 2005 in response to numerous studies linking its use in treating respiratory diseases in poultry to the emergence and spread of fluoroquinolone resistance in humans and animals (Endtz et al. 1991; Nelson et al. 2007). In 2006, the European Union banned the use of antimicrobials for growth promotion in food animals in response to evidence suggesting growth promoters drove the emergence and spread of resistance (European Commission 2005).
In light of evidence of antimicrobial use in Indian poultry production and the projected 312% increase in agricultural antimicrobial consumption over the next 15 years, this study seeks to understand the prevalence of resistance [and, in particular, extended-spectrum (ESBL) production] in Escherichia coli and other Enterobacteriaceae in poultry and how farm operational and managerial practices in poultry production influence resistance emergence. Previous studies reported a high prevalence of ESBL-producing Enterobacteriaceae in poultry production (Dierikx et al. 2013b, 2013a; Kar et al. 2015; Laube et al. 2013). Moreover, ESBL-producing pathogens are resistant to numerous antimicrobials and are associated with longer hospital stays and other negative clinical outcomes in humans (Lautenbach et al. 2001). Specifically, this study investigates how resistance profiles vary between broiler and layer farms, between independent (IUs) and contracted facilities (CFs), and among farms reporting antimicrobial use (including for growth promotion) and those that do not.
This study compares various farm (i.e., broiler vs. layer) and facility types (i.e., contracted vs. independent), as these are the primary types of poultry operations in Punjab. Additionally, past research has reported that broiler farms tend to use more antimicrobials and harbor a higher level of resistance than layer farms (van den Bogaard et al. 2002). Higher antimicrobial usage in broilers is reasonable, considering the need to sustain rapid growth of chickens over short periods of time (), whereas layer farms typically use fewer antimicrobials to sustain consistent egg production over longer periods of time (). Moreover, unlike independent facilities, contracted farms are owned by large-scale poultry producers and follow a strict production process established by the producer, wherein all input materials (e.g., day-old chicks, feed, antimicrobials, etc.) are supplied by the contracting firm. Contracted farms are obligated to adhere to all instructions and protocols from the contracting firm. Given the differences in farming practices among these types of operations, it is necessary to understand how resistance prevalence varies to appropriately target policy interventions where they would have the greatest impact.
Materials and Methods
Sampling Protocol
Eighteen poultry farms (nine layers and nine broilers) were randomly selected from a list of farms provided by Guru Angad Dev Veterinary and Animal Sciences University (GADVASU), Ludhiana, Punjab, India. Layer and broiler farming operations were categorized as CFs or IUs, based on the nature of their contractual agreements with large-scale poultry producers. In this cross-sectional design, all samples were collected over a 5-month period from July to November 2014. All farms were located within six districts across the state of Punjab, India.
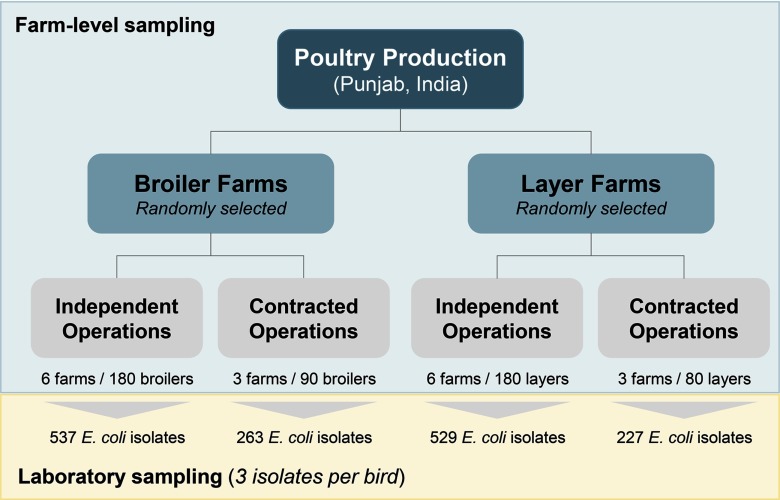
Sixty cloacal swabs were collected from 30 birds selected at random (two swabs per bird) from each farm and transported to the laboratory for isolation of E. coli (three isolates per bird) and other Enterobacteriaceae (one isolate per bird) for phenotypic detection of ESBLs. Only 20 birds were sampled from the first layer farm visited due to extreme heat causing danger to the health of the birds and only a single swab could be collected from each bird. Putative ESBL-producing Enterobacteriaceae were not isolated from these samples. Thus, 530 birds () were sampled for general susceptibility testing of E. coli, and 510 birds () were sampled for ESBL-producing Enterobacteriaceae. Since three E. coli isolates were sampled per bird, a total of 1,556 viable E. coli isolates () were tested for susceptibility against 11 antimicrobials covering a range of clinically relevant antimicrobial classes (tetracyclines, cephalosporins, quinolones, fluoroquinolones, penicillins, carbapenems, aminoglycosides, sulfonamides, and so on) (Figure 1).
Figure 1.
Sampling framework depicting differences between on-farm and in-laboratory sampling protocols.
After sampling was complete, a 30-min structured interview covering sanitation, disease control, and antimicrobial use practices was conducted with each farm manager (see Supplemental Material, Survey). All protocols used to sample isolates from animals in this study were humane and approved by the Institutional Animal Care and Use Committee at Princeton University. Informed consent was obtained from all farmers participating in interviews, and the survey protocol was approved by the Institutional Ethics Committee at the Public Health Foundation of India.
Microbiological Methodology
Cloacal swabs were transported in a Cary-Blair transport medium (HiMedia, Mumbai, India) on ice to the laboratory for bacterial isolation. One swab from each bird was cultured on selective MacConkey Lactose agar (HiMedia, Mumbai, India), while the other was inoculated in a selective pre-enrichment broth [trypticase soy broth supplemented with cefotaxime (CTX)] (HiMedia, Mumbai, India) [Diederen et al. 2012; Dierikx et al. 2013b, 2013a; EFSA Panel on Biological Hazards (BIOHAZ) 2011]. Both were incubated at under aerobic conditions for 20–24 hours.
Isolation of Escherichia coli for susceptibility testing.
Three well-isolated E. coli-like colonies (pink, doughnut-shaped) were selected at random from the MacConkey Lactose agar plate and subcultured on three separate Eosin Methylene Blue (HiMedia, Mumbai, India) agar plates at under aerobic conditions for 20–24 hours (Corry et al. 2011). Selecting bacterial isolates without selection for a specific resistance profile will detect only the most prevalent flora; thus, resistance at low levels may not be detected (Aarestrup et al. 2001). Subsequently, a single, well-isolated colony with the metallic-green sheen characteristic of E. coli on Eosin Methylene Blue from each plate was subcultured onto three separate trypticase soy agar (HiMedia, Mumbai, India) plates and incubated for 20–24 hours at under aerobic conditions for the isolation of pure cultures.
A single, well-isolated colony from each trypticase soy agar plate was then suspended in 5 mL of trypticase soy broth and incubated at until the inoculum achieved 0.5 McFarland standard ( at 620 nm). Isolates were confirmed as E. coli by using HiIMViC biochemical test kits (HiMedia, Mumbai, India). Susceptibility to 11 drugs was tested using the Kirby-Bauer disk diffusion methodology (Clinical and Laboratory Standards Institute 2013a, 2013b) (see Table S1 for zone diameter breakpoints). All 11 antimicrobial disks used for susceptibility testing: ampicillin (AMP), gentamicin (GEN), ciprofloxacin (CIP), nitrofurantoin (NIT), co-trimoxazole (COT), tetracycline (TE), cefuroxime (CXM), imipenem (IPM), nalidixic acid (NX), chloramphenicol (C), and ceftriaxone (CTR) (HiMedia, Mumbai, India), were subjected to weekly quality control tests using the standard strain E. coli ATCC 25922 (HiMedia, Mumbai, India).
Isolation of ESBL-producing Enterobacteriaceae for phenotypic confirmation.
Following incubation, the second cloacal sample, which was inoculated in trypticase soy broth supplemented with CTX (HiMedia, Mumbai, India), was vortexed and subcultured onto MacConkey agar supplemented with CTX [Diederen et al. 2012; Dierikx et al. 2013b, 2013a; EFSA Panel on Biological Hazards (BIOHAZ) 2011]. After incubation for 20–24 hours at under aerobic conditions, a single, lactose-fermenting (pink-red) colony was then subcultured onto trypticase soy agar and incubated for 20–24 hours at under aerobic conditions for the isolation of pure colonies.
A single, well-isolated colony was then suspended in 5 mL of trypticase soy broth and incubated at until the inoculum achieved 0.5 McFarland standard ( at 620 nm). Species identity was confirmed using HiIMViC biochemical test kits. Phenotypic ESBL production was tested using the combination disk method using CTX, cefotaxime–clavulanic acid (CEC), ceftazidime (CAZ), and ceftazidime–clavulanic acid (CAC) (Clinical and Laboratory Standards Institute 2013b; Dierikx et al. 2013b, 2013a). All antimicrobial disks were subjected to weekly quality control tests using the standard strain E. coli ATCC 25922, as well as an ESBL-positive Klebsiella pneumoniae isolate.
Isolate storage and quality assurance testing.
All isolates were inoculated in trypticase soy broth supplemented with 30% glycerol (v/v) (HiMedia, Mumbai, India) in cryovials and stored at for further analysis and quality assurance testing. All 347 putative ESBL-producing Enterobacteriaceae isolates (i.e., isolates that exhibited growth on MacConkey agar supplemented with CTX) and 540 E. coli isolates for general susceptibility testing were sent to SRL Diagnostics at Fortis Hospital, Noida, Uttar Pradesh, India, for quality assurance by a separate team of researchers at an independent laboratory accredited by the National Accreditation Board for Testing and Calibration Laboratories, Government of India.
Of these isolates, all putative ESBL-producing and 395 ( of total sample size, selected at random) E. coli isolates underwent species identification and susceptibility testing using VITEK 2 (bioMérieux, Marcy-L’Étoile, France). Species identification was confirmed using GN ID cards (bioMérieux, Marcy-L’Étoile, France) capable of identifying more than 150 fermentative and nonfermentative gram-negative bacilli. Antimicrobial susceptibility and phenotypic detection of ESBL production was confirmed using AST N280 cards (bioMérieux, Marcy-L’Étoile, France), which test susceptibility to the following drugs: amikacin, amoxicillin–clavulanic acid, ampicillin, cefepime, cefoperazone, sulbactam, ceftriaxone, cefuroxime, ciprofloxacin, colistin, ertapenem, gentamicin, imipenem, meropenem, nalidix acid, nitrofurantoin, piperacillin tazobactam, tigecycline, and trimethoprim/sulfamethoxazole (i.e., COT). Thus, this procedure provided quality assurance for 9 of the 11 drugs tested in this study (excluding chloramphenicol and tetracycline) as well as phenotypic detection of ESBL activity.
Statistical Analysis
Generalized linear mixed models with a logit link function were used to model the resistance profiles against farm and facility type for each antimicrobial, where the outcome for each isolate was classified as either resistant or susceptible. Isolates classified as intermediate were considered resistant for these analyses. Logistic regression models using the state of each isolate as a binary outcome (combining intermediate and resistant as nonsusceptible) with farm and facility type as the explanatory variables were used to model resistance profiles modulated by farming practices (i.e., farm or facility type). For each antimicrobial, the odds of an isolate being nonsusceptible were computed against farm and facility type. Since birds within a farm were likely to be treated similarly, random effects were incorporated in each model to account for similar resistance profiles within farms. Using the model parameters estimated from the logistic regression models, we derived prediction estimates (along with 95% prediction intervals using diagonal elements of the variance–covariance matrix of the predicted means and the estimated variance of the random intercept) for the population-based probability of resistant strains in isolates from randomly sampled broiler or layer farms, given the type of facility (contracted or independent).
Chi-square tests of independence were employed to test the difference in prevalence of ESBL-producing Enterobacteriaceae strains among farm types. Of the 11 antimicrobials tested in the study, the number of antimicrobials to which an isolate was classified as resistant was computed for every isolate. Since the number of antimicrobials did not follow a normal distribution, a categorical variable describing multidrug resistance was constructed with four levels [, , , ]. This multidrug resistance categorical variable was analyzed against farm (broiler vs. layer) and facility (independent vs. contracted) types using a proportional odds logistic regression model in order to account for the severity of resistance. Predicted probabilities for multidrug resistance were computed using the estimated parameters from the ordinal logistic regression model.
The resistance prevalence on farms that reported use of antimicrobials for growth promotion compared to those that did not was analyzed using logistic regression models with a random intercept for each farm. Adjusting for farm type (broiler vs. layer), ordinal logistic regressions were employed to understand the association of antimicrobial growth promoter (AGP) usage with multidrug resistance. Finally, a random effects logistic regression, adjusted for farm type, was used to analyze the association of AGP usage with the presence of ESBL-positive strains.
Sensitivity analyses were implemented to understand the effects of treating intermediate isolates as susceptible instead of resistant. Logistic regression models were employed to estimate the odds of resistance prevalence in broiler farms as compared to layer farms for each antimicrobial. Additionally, logistic regression models, stratified by farm type, were used to estimate the effect of farm size (in terms of number of birds) on resistance prevalence to each antimicrobial. All statistical analyses were carried out in R (version 3.2.1; R Foundation for Statistical Computing).
Results
Summary of Survey Results
Table S2 summarizes survey responses from 16 of the 18 farms sampled; two farms did not provide responses to detailed survey questions. In total, we sampled nine broiler farms (three contracted, six independent) and nine layer farms (three contracted, six independent). The average number of birds on each farm was 57,324 (). Of the farms that elected to participate in the survey, all 16 reported using antimicrobials for disease treatment and prevention, while 12 (67%) reported using antimicrobials for growth promotion. Table S3 reports purpose of antimicrobial use disaggregated by farm and facility type. Tetracyclines and fluoroquinolones were the most commonly reported antimicrobials used, with nine (56%) farms reporting their use.
Overall prevalence of resistant Escherichia coli in Poultry Farms
The overall prevalence of resistance in the 1,556 E. coli isolates was analyzed across all farm (broiler vs. layer) and facility (contracted vs. independent) types. Percentages of isolates classified as susceptible, intermediate, or resistant were used to summarize resistance prevalence overall and disaggregated by farm and facility type (Table S4). A high prevalence of E. coli resistant to NX (86.1%), TE (47.0%), AMP (43.8%), COT (42.2%), and CIP (39.4%) was observed. The degree of resistance to TE, NX, AMP, and COT was consistently high across all farm and facility types. However, resistance to CIP was detected at high levels only in broiler farms. No resistance to IPM was detected among any of the farms.
Quality Assurance Validation Results
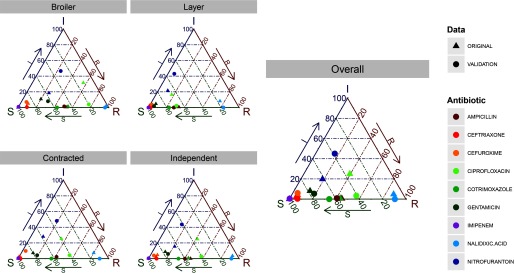
Resistance profiles of isolates against antimicrobials common to both datasets were compared. Validation analysis reveals that the original analysis conservatively underestimated the prevalence of CIP resistance by across various farm and facility types (Figure 2). For all other antimicrobials, the validation analysis reveals either no differences or differences only in the classification of isolates being susceptible vs. intermediate.
Figure 2.
Ternary diagrams showing differences in resistance prevalence between original and validation data for 395 isolates against 9 common antimicrobials. Prevalence in the original data is shown as solid triangles, and those in the validation data are shown as solid dots. Each point represents a three-component vector showing the prevalence of susceptible, intermediate, and resistant isolates that sum to 100%. A point closer to a vertex (for instance, R) represents a high prevalence of the “resistant” state, also indicated by the arrows along each edge.
Resistance Profiles Modulated by Farming Practices
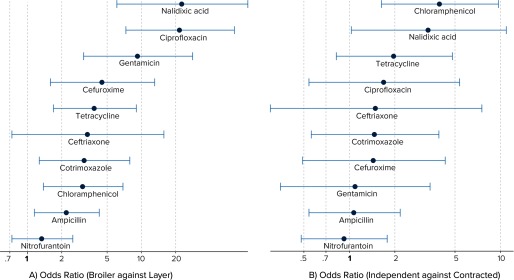
Figure 3A shows the odds ratio (OR) of resistance against each antimicrobial in broiler farms as compared to layer farms, adjusted for type of facility (contracted or independent). It is evident that for all antimicrobials, except NIT, the odds of finding a resistant isolate in broiler farms was at least two times greater than in layer farms (See also Table S6). Specifically, odds of E. coli resistant to antimicrobials, such as NX and CIP, was more than 10 times higher in broiler farms. Independent facilities had a significantly higher risk of E. coli resistant to C and NX as compared to contracted facilities, adjusted for farm type (Figure 3B). The results suggest that the odds of resistance (although not significant) to all antimicrobials tested, except NIT were higher in independent facilities.
Figure 3.
Results from logistic regression modeling the risk of resistance prevalence against farm and facility type, with random intercepts for each farm. Left panel presents the risk (in terms of odds ratios) of Escherichia coli resistance in broiler farms relative to layer farms, adjusted for facility type. Right panel presents the same risk in independent facilities as compared to contracted facilities, adjusted for farm type. For all of these analyses, intermediate isolates are treated as resistant. The x-axis represents odds ratios in a log scale. The horizontal lines represent 95% confidence intervals for the estimated odds ratios.
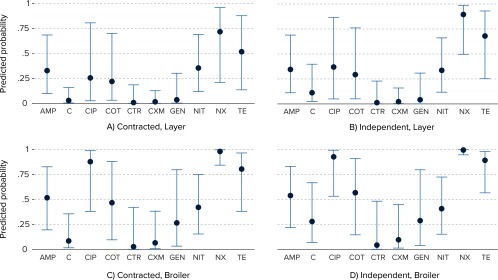
This model was further used to estimate the population average probabilities of the occurrence of E. coli strains resistant or susceptible to each antimicrobial across all farm and facility types (Figure 4). A high probability of resistance to NX, CIP, and TE was observed, with higher probabilities in broiler farms, and specifically, independent broiler facilities.
Figure 4.
Predicted probabilities of resistance (solid blue dots) and corresponding 95% confidence intervals (blue error-bars) against 10 antimicrobials [imipenem (IPM) was not included, as no resistant isolates were detected], by farm and facility type, based on a logistic regression model with random intercepts for farm. Each isolate is assumed to be either resistant or susceptible in this analysis.
Prevalence of ESBL-Positive Strains and Multidrug Resistance
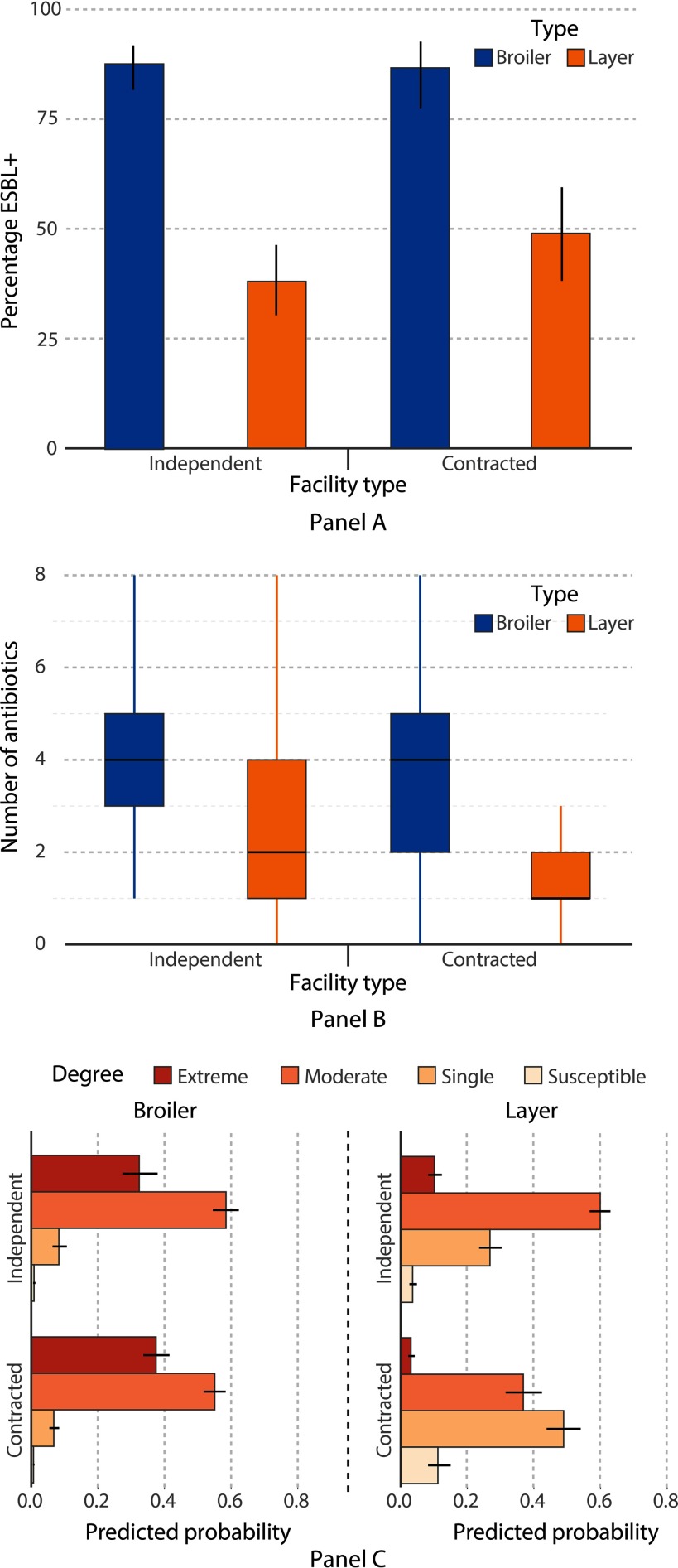
Understanding the prevalence of ESBL-positive bacteria is particularly relevant, given that infections caused by these organisms are more difficult to treat in humans. Of 510 cloacal samples collected for ESBL detection, 305 E. coli, 13 K. pneumoniae, 8 Escherichia fergusonii, 3 Proteus mirabilis, and 1 Escherichia hermannii isolate were phenotypically confirmed as ESBL-producing Enterobacteriaceae. Additionally, seven non-Enterobacteriaceae gram-negative isolates were confirmed as ESBL-positive: three Pseudomonas aeruginosa, two Bordetella trematum, and two Acinetobacter spp., and are included in our overall analysis, as they still indicate that a particular bird carried ESBL-positive flora (Table S5). The prevalence of ESBL-positive strains was significantly higher [, confidence interval (CI): 6.14, 14.85, ] in broiler farms (87%) than layer farms (42%) (Figure 5A). There was no statistically significant difference in the prevalence of ESBL-positive strains () between contracted and independent broiler farms. Contracted layer farms had a higher prevalence (49%) than independent layer farms (38%), although the difference was not statistically significant.
Figure 5.
(A) Distribution of ESBL-producing status of the 510 cloacal samples, disaggregated by farm and facility type. ESBL status was tested for all Enterobacteriaceae isolated, including seven non-Enterobacteriaceae, gram-negative isolates. The figure shows proportions and associated 95% confidence intervals of ESBL-positive strains within each group. (B) Distribution of 1,556 multidrug-resistant Escherichia coli isolates by farm type and facility type. The vertical axis shows the number of antimicrobials (maximum 10) to which an isolate was resistant. The horizontal lines within the boxes indicate the median number of antibiotics to which an isolate was resistant, while the length of the box represents the interquartile range (IQR). The lower and upper limits of the whiskers represent and , where Q1 and Q3 are the first and third quartiles, respectively. (C) Predicted probabilities and associated 95% confidence intervals for multidrug resistance according to a proportional odds logistic regression of categories of multidrug resistance against farm and facility type.
Given that all isolates were tested against multiple antimicrobials, the prevalence of MDR E. coli strains was analyzed against farming practices (Table S6). A higher prevalence of MDR E. coli was detected on broiler farms (94%) as compared to layer farms (60%) (Figure 5B). Isolates on independent broiler farms were 15 times more likely (CI: 10.3, 21.9, ) to be multidrug resistant than those from an independent layer farm. The odds were significant yet lower in the case of contracted farming facilities (, CI: 4.1, 7.0, ). Predicted probabilities (Figure 5C) show that 90% of isolates were likely to be moderately or extremely resistant in broiler farms, while contracted layer farms had a lower probability (36%) of being moderately or extremely resistant.
Impact of Antimicrobial Use for Growth Promotion on Resistance Profiles
The prevalence patterns of antimicrobial resistance within certain subgroups were analyzed to better understand the impact of farming practices on resistance. Prevalence percentages within farms that reported using antimicrobials for growth promotion in poultry chickens were analyzed compared to farms that did not. Of the 16 farms that elected to participate in the survey, 75% reported using antimicrobials for growth promotion. Irrespective of the type of farming operation, our results indicate increased resistance prevalence in farms using AGPs. AGP use is correlated with significantly increased odds of resistance for all antimicrobials except CTR and NIT. Within broiler farms that reported use of AGPs, a significantly higher prevalence of resistance to CIP, C, NIT, and COT was detected. Within layer farms, significantly higher odds of resistance were observed for all antimicrobials except C, NIT, CXM, and CTR (Table 1).
Table 1.
Odds ratios of increased resistance prevalence to all antibiotics for farms reporting antimicrobial use for growth promotion as compared to farms that did not report antimicrobial use for growth promotion.
| Antimicrobial | Odds ratios stratified by farm type | ||
|---|---|---|---|
| Overall | Broilers | Layers | |
| Ampicillin (AMP) | 1.413* (1.088, 1.839) | 0.784* (0.485, 1.256) | 1.460* (1.031, 2.079) |
| Chloramphenicol (C) | 1.698* (1.179, 2.489) | 2.721* (1.361, 6.040) | 0.916 (0.562, 1.513) |
| Ciprofloxacin (CIP) | 4.980** (3.792, 6.567) | 2.258* (1.168, 4.189) | 4.297** (2.899, 6.473) |
| Cotrimoxazole (COT) | 4.242** (3.131, 5.809) | 1.669* (1.042, 2.693) | 6.638** (4.221, 10.764) |
| Ceftriaxone (CTR) | 1.680 (0.885, 3.464) | 1.284 (0.529, 3.769) | 1.061 (0.363, 3.492) |
| Cefuroxime (CXM) | 2.032* (1.130, 3.923) | 1.888 (0.792, 5.479) | 0.993 (0.388, 2.747) |
| Gentamicin (GEN) | 2.559** (1.734, 3.875) | 1.151 (0.694, 1.954) | 3.279* (1.498, 8.190) |
| Nalidixic Acid (NX) | 5.572** (3.761, 8.298) | N/A | 3.977** (2.590, 6.158) |
| Nitrofurantoin (NIT) | 1.290 (0.984, 1.698) | 2.942** (1.669, 5.457) | 0.879 (0.624, 1.240) |
| Tetracycline (TE) | 1.969** (1.490, 2.598) | 1.101 (0.563, 2.036) | 1.633* (1.162, 2.295) |
Note: Fisher’s exact test was employed to compute the odds ratios and associated 95% confidence intervals (given in parentheses below) with the outcome as the presence or absence of resistant isolates. Odds ratios are presented for all farms (“overall”) and disaggregated by farm type (“broilers” vs. “layers”). Note that an OR could not be calculated for NX in broiler farms, as the cell frequency was zero. *; **.
After accounting for farm type (broiler vs. layer), our results indicate that reported AGP use had no significant association with the prevalence of ESBL-positive strains (, CI: 0.01, 30.58, ). In contrast, isolates from farms reporting AGP use were 2.92 times more likely (CI: 2.24, 3.81, ) to be multidrug resistant than those from farms not reporting AGP use. Moreover, in this analysis, the effect of farm type on the odds of multidrug resistance was maintained as broiler farms were still 6.17 times (CI: 4.80, 8.00, ) more likely to harbor MDR strains compared to layer farms.
Sensitivity Analysis
We analyzed the impact of treating intermediate isolates as susceptible instead of resistant on the associations described earlier, and we provide these results in the Supplemental Material (Figure S1). We did not observe any significant changes in the direction and statistical significance of associations between farming practices and patterns of antimicrobial resistance. However, in some cases (e.g., GEN, CXM, C), the associations were stronger, indicating a higher prevalence of intermediate isolates in layer farms.
We also conducted a sensitivity analysis to examine associations between farm size (number of birds) and the prevalence of resistance among different types of farms (broilers and layers). The median farm size in terms of number of birds was 15,000 in broiler farms compared to 40,000 in layer farms. With an increase in farm size by 10,000 birds, we observed that changes in the odds of resistance to all antimicrobials were not statistically significant within broiler farms. In the case of layer farms, we observed marginally lower odds of resistance to NX, CIP, and NIT with increases in farm size. These results are summarized in the Supplemental Materials (Figure S2).
Discussion
Research on antimicrobial use and resistance in food animal production in India remains a relatively new field. However, research is urgently needed given the projected large-scale increase in poultry production and antimicrobial use in the poultry sector (Brahmachari et al. 2013; Van Boeckel et al. 2015). Sarma et al. (1981) isolated E. coli from healthy and diseased fowl in Ludhiana, Punjab, India, and discovered that approximately 80% of isolates were resistant to chlortetracycline, tetracycline, oxytetracycline, and triple sulfas. Shivachandra et al. (2004) found 100% resistance to sulfadiazine and widespread resistance to amikacin, carbenicillin, erythromycin, and penicillin in Pasteurella multocida isolates from chickens and other birds from 11 separate states in India. More recently, Dhanarani et al. (2009) found extensive resistance to streptomycin (75%), erythromycin (57%), tobramycin (54%), ampicillin (50%), rifampicin (46%), and kanamycin (40%) in Staphylococcus and other bacterial isolates from poultry litter in Tamil Nadu, India.
Recent surveys suggest that 70–90% of Enterobacteriaceae in India are ESBL producers and that colonization of humans with such bacteria is widespread(Hawkey 2008; Kumarasamy et al. 2010; Mathai et al. 2002). However, researchers in India have only recently begun to investigate ESBL-producing bacteria of food animal origin. Kar et al. (2015) conducted the first systematic study on multidrug resistant ESBL-producing E. coli in food producing animals from India in which 316 E. coli isolates were collected from poultry and dairy cattle in Odisha with 18 (6%) isolates confirmed as ESBL-positive by combination disc method and ESBL E-test. A study in Hyderabad, India, isolated E. coli from 150 food samples (vegetable salad, raw egg surface, raw chicken, unpasteurized milk, and raw meat) and detected 6 (4%) ESBL producers, two of which were isolated from raw chicken samples (Rasheed et al. 2014). Another study in West Bengal, India, of 360 healthy layers and their environment did not detect ESBL production by PCR, but did record high levels of phenotypic resistance to several drugs: erythromycin (95.83%), chloramphenicol (87.52%), and cotrimoxazole (78.%) (Samanta et al. 2014).
We found a high degree of antimicrobial resistance and ESBL production in poultry facilities, which varied according to farming practices (i.e., farm and facility type). Moreover, we found a high prevalence of E. coli resistant to antimicrobials, such as CIP (fluoroquinolone), AMP (), and tetracycline, which are commonly used in clinical settings. Broiler farms, and especially independent broiler farms, were associated with a higher prevalence of resistant E. coli strains than layer farms, corroborating past research (van den Bogaard et al. 2002). Additionally, the prevalence of ESBL-producing Entero-bacteriaceae was higher for broiler farms (87%) than layer farms (42%), and for contracted layer farms (49%) than independent layer farms (38%). These results may indicate that independent layer farms are using more drugs such as tetracyclines and fluoroquinolones, while contracted layer and broiler farms are beginning to shift to more recently developed drugs, such as third-generation cephalosporins, accounting for the higher prevalence of ESBLs. Additionally, higher odds of resistance among independent facilities may suggest that contracted facilities are employing better hygiene practices and veterinary care, which seems reasonable, considering these protocols and services are supplied by the contracting firm. In contrast, independent farmers do not have a comparable support system and could be misusing antimicrobials to a greater degree. However, it is critical that future studies identify the specific farming practices that are driving increased prevalence of resistant strains in order to mitigate the risk of spreading antimicrobial resistance.
Most of the farms surveyed in this study were large (average number of birds greater than 50,000), and all farms (that participated in the survey) reported using antimicrobials. Large flocks in small, confined areas, a lack of proper sanitation measures, and the unregulated application of broad-spectrum antimicrobials drive the spread of resistance at the farm level. The questionnaire-based surveys employed in this study revealed disturbing trends regarding the indiscriminate use of antimicrobials; all poultry farms included in this study used antimicrobials, and over half of them used antimicrobials for growth promotion rather than solely for disease prevention or treatment. Antimicrobials are often employed when broilers are being transported or held prior to slaughter to help them tolerate stress. Anecdotally, one farmer noted that antimicrobials were more effective than hygiene or sanitation measures because labor on poultry farms is unskilled, making it difficult to ensure that all hygiene procedures are followed. A majority of the farmers surveyed reported being unaware of the presence of AGPs premixed in chicken feed purchased from feed mills. Given the size and reach of these poultry farms in the retail market, the risk of exposure to resistant bacteria and antimicrobial compounds to humans is a significant concern.
This study reports a high prevalence of ESBL-producing Enterobacteriaceae in poultry farms (87% and 42% in broilers and layers, respectively). These results corroborate similar studies in broiler production facilities, but the prevalence reported here is similar or higher (Dierikx et al. 2013b, 2013a; Kar et al. 2015; Laube et al. 2013). ESBL-producing Enterobacteriaceae are highly resistant to multiple drugs, can contribute to acquired resistance through horizontal gene transfer across a wide range of bacterial species, and are associated with longer hospital stays and negative clinical outcomes in humans (Lautenbach et al. 2001; Marshall and Levy 2011; Rawat and Nair 2010; Silbergeld et al. 2008). Emerging resistance to widely used antimicrobials, such as fluoroquinolones and cephalosporins, reduces the efficacy of treating enteric, urinary tract, and skin infections, resulting in prolonged and more serious courses of illness. Enterobacteriaceae resistant to third-generation cephalosporins as a result of cephalosporin overuse in poultry production have been associated with increased human deaths in Europe (Collignon et al. 2013). In the present study, the prevalence of ESBL-producing E. coli and other Enterobacteriaceae was higher in broiler facilities, where antimicrobials are more commonly administered for growth promotion and disease prevention. This highlights the need to regulate the use of antimicrobials in these intensive farming operations, especially since researchers predict that this region will experience a major shift towards this type of farming operation over the next 15 years (Van Boeckel et al. 2015).
Direct contact with livestock colonized with resistant bacteria is the most documented route of resistance transmission from the agricultural reservoir into human populations (Bergeron et al. 2012; Jakobsen et al. 2010a, 2010b; Marshall and Levy 2011; Schmithausen et al. 2015; van den Bogaard et al. 2002; Zhang et al. 2009). These high-risk individuals provide a conduit of entry for resistant bacteria and resistance genes into the community and hospitals, where further person-to-person transmission is possible (Marshall and Levy 2011; Silbergeld et al. 2008). Of particular concern in this study was the lack of sanitation measures to prevent the transfer of resistant bacteria from animals to farm workers. Among survey respondents, 67% indicated they take no precautions when entering poultry sheds (and farm workers often do not wear closed-toe shoes); thus, the risk of colonization of farm workers is likely much higher than in other countries where strict disease control practices are implemented.
Despite extensive evidence linking the use of antimicrobials in food animal production to resistance in human populations, little has been done to address the problem in the majority of developing and developed countries worldwide. Recent publications have highlighted the rise of antimicrobial resistance and the emergence of new mechanisms of resistance in the Indian subcontinent (Kumarasamy et al. 2010). Policy makers and researchers have focused their attention on the clinical overuse and misuse of antimicrobials (to treat colds and other viral infections, for example) as drivers of resistance emergence. Furthermore, a recent review of the effectiveness of AGPs in food animal production suggests that the effects of AGPs on improving production and decreasing mortality in the poultry industry are minimal and do not offset the costs of the AGPs themselves (Cogliani et al. 2011; Engster et al. 2002; Graham et al. 2007; Sneeringer et al. 2015; Teillant et al. 2015). Moreover, it is possible to reduce the prevalence of antimicrobial resistance by placing restrictions on the use of antimicrobials in food animal production without negative impacts on productivity, as evidenced by the experience of both the poultry and pork industries in Denmark (Aarestrup et al. 2001; Levy 2014).
Notwithstanding the growing body of evidence, India has no regulatory provisions for the use of antimicrobials in cattle, chicken, or pigs raised for domestic consumption (Ganguly et al. 2011; Van Boeckel et al. 2015). The only laws on antimicrobial use in food animal production for domestic consumption mandate withdrawal of antimicrobials before processing of food animal products (Brahmachari et al. 2013). The lack of uniform regulations by the various agencies involved in poultry farming (and other food animal production industries) hinders enforcement of the appropriate use of antimicrobials. Policy actions should be implemented immediately in order to safeguard the effectiveness of antimicrobials, since antimicrobial effectiveness is a globally shared resource and responsibility (Ganguly et al. 2011).
This study analyzes a large sample size of isolates for resistance patterns and ESBL production, utilizing samples from farms following a variety of different operational and managerial practices, but only covers 18 farms located in one particular region of India. Future surveys of farms across multiple locations will be needed to obtain estimates at the district, state, and national levels. Furthermore, resistance prevalence at the farm level should be tracked longitudinally over longer periods of time to account for seasonality and to ascertain how resistance profiles are fluctuating over time. Supply of, and demand for, poultry meat and the economics of poultry farming are other critical factors that determine the proliferation of broiler farms and associated farming practices. Augmenting this study with antimicrobial sales and consumption data would help in formulating strategies to curb the rise of resistance in this growing reservoir.
This study only examines the prevalence of resistance on farms that reported using antimicrobials. Future studies should attempt to include antimicrobial-free or organic farms in their sampling frame. However, this may be difficult to accomplish in this region, as we did not encounter any such farms. Additionally, this study relies on survey data to create analytical variables used in our statistical models; all variables used were selected for analysis based on their likelihood of impacting resistance profiles and whether survey responses were reliable across all farms. For instance, we were unable to obtain detailed information on the types and amounts of antimicrobials used for all farms, since some farmers were unaware of this information or unwilling to report it. In light of these limitations, our study primarily compares resistance profiles among different farm (broiler vs. layer) and facility (independent vs. contracted) types instead of investigating specific practices influencing resistance development. Future studies should augment this survey data with observational periods on farms to better understand specific managerial and antimicrobial use practices.
Finally, given the high rates of background resistance in both human populations and the environment in India, this study cannot definitively link increased farm use of antimicrobials to increased resistance. A more detailed genetic investigation of the isolates to identify specific resistance genes could shed light on the mechanisms of resistance propagation within and among high-risk farms. Moreover, genetic analysis also would enable tracking of resistance genes in poultry birds to farmers and laborers in order to better quantify the risk of transmission from animals to humans, as well as help us understand the sources of resistance (e.g., humans, animals, environment, etc.).
Conclusion
This study presents evidence that antimicrobial resistance in E. coli and other pathogenic bacteria is correlated with particular farming practices. In particular, the results of this study revealed that broiler farms were associated with a higher prevalence of resistance, including ESBL-producing Enterobacteriaceae and multidrug resistance, than layer farms. Moreover, our findings suggest that antimicrobial use for growth promotion promoted the development of reservoirs of highly resistant bacteria on the studied farms, with potentially serious implications for human health. The prevalence of resistance to multiple antimicrobials was higher in both broiler and layer farms that used antimicrobials for growth promotion. ESBL-positive and multidrug-resistant strains are equipped with an arsenal of mechanisms that enable them to survive against last-resort treatments in human clinical settings. Furthermore, these highly resistant strains contribute to acquired resistance through horizontal gene transfer of resistance determinants to other microbial strains and species (including commensal microbes) further propagating antimicrobial resistance across various reservoirs of resistance, a threat that is both real and imminent (Marshall and Levy 2011; Silbergeld et al. 2008).
Until recently, resistance to polymyxin (a drug of last resort when other modern antimicrobials are ineffective) had only been reported to evolve via chromosomal mutations. A recent study of commensal E. coli in Chinese food animal production has identified a plasmid-mediated polymyxin resistance mechanism, MCR-1, in Enterobacteriaceae that has spread from animals to humans (detected in 1% of inpatients with infection) (Liu et al. 2015). Although we did not test for MCR-1 in this study, the emergence of such a highly mobile resistance determinant to such an important class of antimicrobials, with risk of global dissemination similar to NDM-1 (New Delhi metallo-β-lactamase), further emphasizes the need to regulate and curb antimicrobial overuse in food animal production (Liu et al. 2015). We conclude that withdrawal of nontherapeutic use of agricultural antimicrobials in India would be prudent to protect public health.
Supplemental Material
Acknowledgments
C.H.B. and R.L.’s time, as well as the cost of the study, was funded by the Global Antibiotic Resistance Partnership (funded by the Bill and Melinda Gates Foundation). C.H.B.’s time was also partially funded by the Fulbright-Nehru Fellowship. S.P. was funded by Princeton University’s Grand Challenges in Health program. We thank the poultry farmers for providing access to their farms to sample bacteria from their birds, SRL and Guru Angad Dev Veterinary and Animal Sciences University (GADVASU) for providing laboratory facilities, bioMérieux for providing us VITEK supplies, Suraj Pant for helping us create the figures presented in this article, and Manish Kakkar and Elizabeth Rogawski for helping develop the survey we administered.
References
- Aarestrup FM, Seyfarth AM, Emborg HD, Pedersen K, Hendriksen RS, Bager F. 2001. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal Enterococci from food animals in Denmark. Antimicrob Agents Chemother 45(7):2054–2059, PMID: 11408222, 10.1128/AAC.45.7.2054-2059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron CR, Prussing C, Boerlin P, Daignault D, Dutil L, Reid-Smith RJ, et al. 2012. Chicken as reservoir for extraintestinal pathogenic Escherichia coli in humans, Canada. Emerging Infect Dis 18(3):415–421, PMID: 22377351, 10.3201/eid1803.111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmachari SK, Misri J, Vijayakumar K, George VI, Chakraborty P, Bhalla S, et al. 2013. Report of the Committee on Antibiotic Use and Resistance in Food Animals. New Delhi, India: The Public Health Foundation of India. [Google Scholar]
- Center for Disease Dynamics, Economics & Policy (CDDEP). 2015. State of the World’s Antibiotics, 2015. Washington, DC:CDDEP; http://www.cddep.org/publications/state_worlds_antibiotics_2015#sthash.XhiMCKG6.dpbs [accessed 14 July 2016]. [Google Scholar]
- Chang Q, Wang W, Regev-Yochay G, Lipsitch M, Hanage WP. 2015. Antibiotics in agriculture and the risk to human health: how worried should we be?. Evol Appl 8(3):240–247, PMID: 25861382, 10.1111/eva.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). 2013a. “Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard––Fourth Edition; CLSI Document VET01-A4.” Wayne, PA:Clinical and Laboratory Standards Institute. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). 2013b. “Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement; CLSI Document M100-S23.” Wayne, PA:Clinical and Laboratory Standards Institute. [Google Scholar]
- Cogliani C, Goossens H, Greko C. 2011. Restricting antimicrobial use in food animals: lessons from Europe. Microbe 6(6):274–279, 10.1128/microbe.6.274.1. [DOI] [Google Scholar]
- Collignon P, Aarestrup FM, Irwin R, McEwen S. 2013. Human deaths and third-generation cephalosporin use in poultry, Europe. Emerging Infect Dis 19(8):1339–1340, PMID: 23876416, 10.3201/eid.1908.120681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corry JEL, Curtis GDW, Baird RM, eds 2011. Culture media for detection and enumeration of “total” Enterobacteriaceae, coliforms and Escherichia coli from foods. In: Handbook of Culture Media for Food and Water Microbiology. Cambridge, UK:Royal Society of Chemistry, 233–260. [Google Scholar]
- Dhanarani TS, Shankar C, Park J, Dexilin M, Kumar RR, Thamaraiselvi K. 2009. Study on acquisition of bacterial antibiotic resistance determinants in poultry litter. Poult Sci 88(7):1381–1387, PMID: 19531707, 10.3382/ps.2008-00327. [DOI] [PubMed] [Google Scholar]
- Diederen B, Chang C, Euser S, Cohen Stuart J. 2012. Evaluation of four screening protocols for detection of extended-spectrum β-lactamase-producing members of the Enterobacteriaceae. J. Med Microbiol 61:452–453, 10.1099/jmm.0.036467-0. [DOI] [PubMed] [Google Scholar]
- Dierikx CM, van der Goot JA, Smith HE, Kant A, Mevius DJ. 2013a. Presence of ESBL/AmpC -producing Escherichia coli in the broiler production pyramid: A descriptive study. A. Cloeckaerted. PLoS ONE 8:e79005, 10.1371/journal.pone.0079005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierikx C, van der Goot J, Fabri T, van Essen-Zandbergen A, Smith H, Mevius D. 2013b. Extended-spectrum-β-lactamase- and AmpC-β-lactamase-producing Escherichia coli in Dutch broilers and broiler farmers. J. Antimicrob Chemother 68(1):60–67, 10.1093/jac/dks349. [DOI] [PubMed] [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ). 2011. Scientific Opinion on the public health risks of bacterial strains producing extended-spectrum β-lactamases and/or AmpC β-lactamases in food and food-producing animals. EFSA J 9(8):1–95, 10.2903/j.efsa.2011.2322. [DOI] [Google Scholar]
- Endtz HP, Ruijs GJ, van Klingeren B, Jansen WH, van der Reyden T, Mouton RP. 1991. Quinolone resistance in campylobacter isolated from man and poultry following the introduction of fluoroquinolones in veterinary medicine. J Antimicrob Chemother 27(2):199–208, PMID: 2055811. [DOI] [PubMed] [Google Scholar]
- Engster HM, Marvil D, Stewart-Brown B. 2002. The effect of withdrawing growth promoting antibiotics from broiler chickens: a long-term commercial industry study. J Appl Poult Res 11(4):431–436, 10.1093/japr/11.4.431. [DOI] [Google Scholar]
- European Commission. 2005. Ban on Antibiotics as Growth Promoters in Animal Feed Enters into Effect. http://europa.eu/rapid/press-release_IP-05-1687_en.htm [accessed 4 March 2016].
- Ganguly NK, Arora NK, Chandy SJ, Fairoze MN, Gill JP, Gupta U, et al. 2011. Rationalizing antibiotic use to limit antibiotic resistance in India. Indian J Med Res 134:281–294, PMID: 21985810. [PMC free article] [PubMed] [Google Scholar]
- Graham JP, Boland JJ, Silbergeld E. 2007. Growth promoting antibiotics in food animal production: an economic analysis. Public Health Rep 122(1):79–87, PMID: 17236612, 10.1177/003335490712200111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkey PM. 2008. Prevalence and clonality of extended-spectrum beta-lactamases in Asia. Clin. Microbiol. Infect 14(suppl 1):159–165, PMID: 18154540, 10.1111/j.1469-0691.2007.01855.x. [DOI] [PubMed] [Google Scholar]
- Jakobsen L, Kurbasic A, Skjøt-Rasmussen L, Ejrnaes K, Porsbo LJ, Pedersen K, et al. 2010a. Escherichia coli isolates from broiler chicken meat, broiler chickens, pork, and pigs share phylogroups and antimicrobial resistance with community-dwelling humans and patients with urinary tract infection. Foodborne Pathog. Dis 7(5):537–547, 10.1089/fpd.2009.0409. [DOI] [PubMed] [Google Scholar]
- Jakobsen L, Spangholm DJ, Pedersen K, Jensen LB, Emborg HD, Agersø Y, et al. 2010b. Broiler chickens, broiler chicken meat, pigs and pork as sources of ExPEC related virulence genes and resistance in Escherichia coli isolates from community-dwelling humans and UTI patients. Int J Food Microbiol 142(1-2):264–272, 10.1016/j.ijfoodmicro.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Kar D, Bandyopadhyay S, Bhattacharyya D, Samanta I, Mahanti A, Nanda PK, et al. 2015. Molecular and phylogenetic characterization of multidrug resistant extended spectrum beta-lactamase producing Escherichia coli isolated from poultry and cattle in Odisha, India. Infect Genet Evol 29:82–90, 10.1016/j.meegid.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, et al. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 10(9):597–602, PMID: 20705517, 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube H, Friese A, von Salviati C, Guerra B, Käsbohrer A, Kreienbrock L, et al. 2013. Longitudinal monitoring of extended-spectrum-beta-lactamase/AmpC-producing Escherichia coli at German broiler chicken fattening farms. Appl Environ Microbiol 79:4815–4820, PMID: 23747697, 10.1128/AEM.00856-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenbach E, Patel JB, Bilker WB, Edelstein PH, Fishman NO. 2001. Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis 32(8):1162–1171, 10.1086/319757. [DOI] [PubMed] [Google Scholar]
- Levy S. 2014. Reduced antibiotic use in livestock: How Denmark tackled resistance. Environ Health Perspect 122(6):A160–A165, PMID: 24892505, 10.1289/ehp.122-A160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, et al. 2015. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis 16(2):161–168. [DOI] [PubMed] [Google Scholar]
- Marshall BM, Levy SB. 2011. Food animals and antimicrobials: Impacts on human health. Clin Microbiol Rev 24(4):718–733, PMID: 21976606, 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai D, Rhomberg PR, Biedenbach DJ, Jones RN, India Antimicrobial Resistance Study Group, 2002. Evaluation of the in vitro activity of six broad-spectrum β-lactam antimicrobial agents tested against recent clinical isolates from India: a survey of ten medical center laboratories. Diagn Microbiol. Infect Dis 44(4):367–377, 10.1016/S0732-8893(02)00466-2. [DOI] [PubMed] [Google Scholar]
- Nelson JM, Chiller TM, Powers JH, Angulo FJ. 2007. Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story. Clin Infect Dis 44(7):977–980, PMID: 17342653, 10.1086/512369. [DOI] [PubMed] [Google Scholar]
- Rasheed MU, Thajuddin N, Ahamed P, Teklemariam Z, Jamil K. 2014. Antimicrobial drug resistance in strains of Escherichia coli isolated from food sources. Rev Inst Med Trop Sao Paulo 56(4):341–346, PMID: 25076436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat D, Nair D. 2010. Extended-spectrum β-lactamases in Gram Negative Bacteria. J Glob Infect Dis 2(3):263–274, PMID: 20927289, 10.4103/0974-777X.68531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu R, Saxena P. 2014. Antibiotics in Chicken Meat. New Delhi, India:Centre for Science and Environment; http://www.cseindia.org/userfiles/Antibiotics%20in%20Chicken_Lab%20Report_Final%2029%20July.pdf [accessed 3 August 2014]. [Google Scholar]
- Samanta I, Joardar SN, Das PK, Das P, Sar TK, Dutta TK, et al. 2014. Virulence repertoire, characterization, and antibiotic resistance pattern analysis of Escherichia coli isolated from backyard layers and their environment in India. Avian Dis 58(1):39–45, PMID: 24758111, 10.1637/10586-052913-Reg.1. [DOI] [PubMed] [Google Scholar]
- Sarma DK, Sambyal DS, Baxi KK. 1981. Drug susceptibility of Escherichia coli from domestic fowl. Zentralbl Veterinarmed B 28(4):333–335, PMID: 7027684. [DOI] [PubMed] [Google Scholar]
- Schmithausen RM, Schulze-Geisthoevel SV, Stemmer F, El-Jade M, Reif M, Hack S, et al. 2015. Analysis of transmission of MRSA and ESBL-E among pigs and farm personnel. PloS One 10(9):e0138173, PMID: 26422606, 10.1371/journal.pone.0138173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S, Kehrenberg C, Walsh TR. 2001. Use of antimicrobial agents in veterinary medicine and food animal production. Int J Antimicrob Agents 17(6):431–437, PMID: 11397611. [DOI] [PubMed] [Google Scholar]
- Shivachandra SB, Kumar AA, Biswas A, Ramakrishnan MA, Singh VP, Srivastava SK. 2004. Antibiotic sensitivity patterns among Indian strains of avian Pasteurella multocida. Trop Anim Health Prod 36(8):743–750, PMID: 15643810. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, Graham J, Price LB. 2008. Industrial food animal production, antimicrobial resistance, and human health. Annu Rev Public Health 29:151–169, PMID: 18348709, 10.1146/annurev.publhealth.29.020907.090904. [DOI] [PubMed] [Google Scholar]
- Sneeringer S, MacDonald JM, Key N, McBride W, Mathews K. 2015. Economics of Antibiotic Use in U.S. Livestock Production, ERR-200. Washington, DC:U.S. Department of Agriculture, Economic Research Service; https://www.ers.usda.gov/publications/pub-details/?pubid=45488 [accessed 14 July 2016]. [Google Scholar]
- Teillant A, Brower CH, Laxminarayan R. 2015. Economics of antibiotic growth promoters in livestock. Annu Rev Resour Econ 7:349–374, 10.1146/annurev-resource-100814-125015. [DOI] [Google Scholar]
- Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, et al. 2015. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci USA 112(18):5649–5654, PMID: 25792457, 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, et al. 2014. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 14(8):742–750, PMID: 25022435, 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- van den Bogaard AE, Willems R, London N, Top J, Stobberingh EE. 2002. Antibiotic resistance of faecal enterococci in poultry, poultry farmers and poultry slaughterers. J Antimicrob Chemother 49(3):497–505, PMID: 11864950. [DOI] [PubMed] [Google Scholar]
- You Y, Silbergeld EK. 2014. Learning from agriculture: understanding low-dose dantimicrobials as drivers of resistome expansion. Front Microbiol 5:284, 10.3389/fmicb.2014.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Ding LJ, Yue J. 2009. Occurrence and characteristics of class 1 and class 2 integrons in resistant Escherichia coli isolates from animals and farm workers in northeastern China. Microb Drug Resist 15(4):323–328, 10.1089/mdr.2009.0020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.