Abstract
Background:
Vitamin D is an environmental and dietary agent with known anticarcinogenic effects, but protection against breast cancer has not been established.
Objective:
We evaluated the association between baseline serum 25-hydroxyvitamin D [25(OH)D] levels, supplemental vitamin D use, and breast cancer incidence over the subsequent 5 y of follow-up.
Methods:
From 2003–2009, the Sister Study enrolled 50,884 U.S. women 35–74 y old who had a sister with breast cancer but had never had breast cancer themselves. Using liquid chromatography–mass spectrometry, we measured 25(OH)D in serum samples from 1,611 women who later developed breast cancer and from 1,843 randomly selected cohort participants. We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for the risk of developing breast cancer using Cox proportional hazards models.
Results:
We found that 25(OH)D levels were associated with a 21% lower breast cancer hazard (highest versus lowest quartile: adjusted ; CI: 0.63, 0.98). Analysis of the first 5 y of follow-up for all 50,884 Sister Study participants showed that self-reported vitamin D supplementation was associated with an 11% lower hazard [ (CI: 0.81, 0.99)]. These associations were particularly strong among postmenopausal women [ (CI: 0.57, 0.93) and (CI: 0.74, 0.93), respectively].
Conclusions:
In this cohort of women with elevated risk, high serum 25(OH)D levels and regular vitamin D supplement use were associated with lower rates of incident, postmenopausal breast cancer over 5 y of follow-up. These results may help to establish clinical benchmarks for 25(OH)D levels; in addition, they support the hypothesis that vitamin D supplementation is useful in breast cancer prevention. https://doi.org/10.1289/EHP943
Introduction
Vitamin D is acquired through both sun exposure and dietary sources. Vitamin is synthesized from cutaneous 7-dehydrocholesterol upon exposure to ultraviolet B radiation (Feldman et al. 2014; Holick 2006). Dietary sources of vitamin D include oily fish, fortified milks and cereals, and oral supplements. Vitamin D is metabolized into 25-hydroxyvitamin D [25(OH)D] by the liver and then converted to 1,25-dihydroxyvitamin D [] by the kidney and other tissues, including the breast (Welsh et al. 2003).
It is known that has potential anticarcinogenic effects, including regulation of cell growth and proliferation, stimulation of apoptosis, and down-regulation of estrogen receptors (Feldman et al. 2014; Holick 2006; Krishnan et al. 2010; Welsh et al. 2003). In animal models, and slowed the growth of existing cancer cells and mammary tumors (Feldman et al. 2014). Levels of are under tight physiologic control, but levels of the inactive precursor—25(OH)D—vary widely and reflect overall available vitamin D (Holick 2006).
Despite widespread fortification, of U.S. women have “insufficient” 25(OH)D levels (Forrest and Stuhldreher 2011) ( (Institute of Medicine of the National Academies 2010)). Extremely high intake of vitamin D [ international units (IU) daily] for an extended period can cause tissue damage, but adverse effects are extremely rare when intake is (Institute of Medicine of the National Academies 2010). Therefore, if vitamin D has antineoplastic effects, supplementation could offer a safe way to prevent breast cancer, a disease that affects approximately one in eight U.S. women during their lifetimes (Surveillance, Epidemiology, and End Results Program 2015).
The effect of vitamin D supplementation on breast cancer risk was investigated in a clinical trial of 36,282 postmenopausal women randomized to receive placebo or vitamin plus calcium daily (Chlebowski et al. 2008). During a mean of 7 y of follow-up, there was no difference in breast cancer rates between treatment arms [ (95% confidence interval (CI): 0.85, 1.09)]. However, off-protocol self-supplementation was common, and in a reanalysis limited to the 43% of women not taking personal supplements, women randomized to treatment had a statistically significant 18% lower breast cancer rate than women randomized to placebo (Bolland et al. 2011).
Using an alternative approach that considers total vitamin D exposure, numerous case–control (Abbas et al. 2008; Abbas et al. 2009; Chen et al. 2013; Colston et al. 2006; Crew et al. 2009; Janowsky et al. 1999) and cohort (Almquist et al. 2010; Amir et al. 2012; Bertone-Johnson et al. 2005; Chlebowski et al. 2008; Deschasaux et al. 2016; Eliassen et al. 2011; Engel et al. 2010; Freedman et al. 2008; Kim et al. 2014; Kühn et al. 2013; McCullough et al. 2009; Mohr et al. 2013; Neuhouser et al. 2012; Ordóñez-Mena et al. 2013; Rejnmark et al. 2009; Scarmo et al. 2013; Skaaby et al. 2014) studies have evaluated the association between 25(OH)D and breast cancer risk. The estimated strength of association in these observational studies differs across study designs. Case–control studies have reported inverse associations (Abbas et al. 2008; Abbas et al. 2009; Chen et al. 2013; Colston et al. 2006; Crew et al. 2009). Although some prospective cohort studies have also observed inverse associations (Bertone-Johnson et al. 2005; Chlebowski et al. 2008; Engel et al. 2010; Kim et al. 2014; Mohr et al. 2013; Rejnmark et al. 2009), the effects tended to be weaker and not statistically significant. Other prospective studies have reported null results (Almquist et al. 2010; Amir et al. 2012; Deschasaux et al. 2016; Eliassen et al. 2011; Freedman et al. 2008; Kühn et al. 2013; McCullough et al. 2009; Neuhouser et al. 2012; Ordóñez-Mena et al. 2013; Scarmo et al. 2013; Skaaby et al. 2014).
Because 25(OH)D levels vary over time (Bertrand et al. 2012; Scarmo et al. 2013), these differences may be related to the timing of sample collection relative to disease development and to the nature of vitamin D’s anticarcinogenic effects. Case–control studies, where levels in cases are assessed soon after diagnosis, should be useful for evaluating the relationship between recent 25(OH)D levels and breast cancer risk. However, it is possible that 25(OH)D levels in cases are affected by the disease, by its treatment, or by disease-related behavioral changes. Bias from reverse causation is avoided by prospective studies, but because many such studies have a 10- to 15-y gap between enrollment and the end of follow-up with no repeated measurements (Almquist et al. 2010; Eliassen et al. 2011; Freedman et al. 2008; Kühn et al. 2013; Neuhouser et al. 2012; Skaaby et al. 2014), their relative risk assessment is most relevant to the relationship between past 25(OH)D and breast cancer risk, and their results could be misleading if the most potent protective mechanism of 25(OH)D is inhibiting growth of existing cancer cells.
Based on the existing epidemiologic and biologic evidence, we hypothesized that recent vitamin D intake is associated with breast cancer risk. We examined this hypothesis prospectively using a large cohort of women with a family history of breast cancer to estimate the association between serum 25(OH)D and breast cancer risk within 5 y.
Methods
Study Population
We assessed this hypothesis in the Sister Study (data release 4.1, updated July 2014), a prospective cohort of 50,884 women who had never had breast cancer, but who had a sister diagnosed with the disease. U.S. women 35–74 y old were enrolled from 2003 through 2009. Further details are provided elsewhere (Niehoff et al. 2016). These sister participants have, on average, approximately twice the risk of breast cancer as similar women with no first-degree family history (Collaborative Group on Hormonal Factors in Breast Cancer 2001), allowing prospective and rapid accrual of incident cases. The Sister Study was approved by the institutional review boards of the National Institute of Environmental Health Sciences and the Copernicus Group.
Participants completed a computer-assisted telephone interview, which included demographic information and reproductive, medical, and residential histories. Additionally, trained examiners visited participants’ homes to collect blood samples, take body measurements, and obtain written informed consent and self-completed questionnaires, including a modified Block 1998 Food Frequency Questionnaire (Block Dietary Data Systems, Berkeley, CA).
Sister Study participants are contacted annually to ascertain major health changes. Women who report a new breast cancer diagnosis are asked to authorize release of cancer-related medical records. Women with self-reported invasive or in situ breast cancer (excluding lobular carcinoma in situ) diagnosed within 5 y of the baseline blood draw were considered cases. Study compliance has been high: we retrieved medical records for 82% of self-reported breast cancer cases (with 99% confirmed as breast cancers), and 90% of women completed their most recently scheduled follow-up. Tumor characteristics, including estrogen receptor, progesterone receptor, human epidermal growth factor receptor-2, and invasiveness status, are based on medical record information when available, or self-report when not.
We selected 3,392 participants for the vitamin D substudy. We used a case–cohort design (Prentice 1986) and included 1,616 breast cancer cases and 1,844 participants randomly sampled from the Sister Study cohort (68 of whom were also cases). Because it was designed to overlap with preexisting genetic substudies that included only non-Hispanic white women, minority women were under-represented in the random subcohort. We corrected for this under-sampling by adjusting for race/ethnicity in all analyses.
Measurement of 25(OH)D
Baseline serum samples were stored in straws at before being shipped to Heartland Assays (Ames, IA) for analysis using liquid chromatography–mass spectrometry (LC/MS) with an Agilent 1290 Series High-Pressure Liquid Chromatography system and an Agilent 6460 Triple Quadruple LC/MS. Three vitamin D metabolites [, , and ] were measured for each participant. We summed the three metabolites and used that total, designated 25(OH)D, as our estimate of overall available serum vitamin D. Approximately 83% of the total consisted of . If an individual metabolite level was below the limit of detection () we imputed a value of (=1.5 divided by the square root of 2).
Of the 3,392 samples, 3,388 were successfully assayed. Cases were randomly allocated across 48 batches. Each batch included five quality-control samples: pooled specimens from both pre- and postmenopausal women and a National Institute of Standards and Technology (NIST) control for each metabolite. Interbatch coefficients of variation for total 25(OH)D were 11.0%, 8.5%, and 2.9% for the premenopausal, postmenopausal and NIST control samples, respectively. We excluded two participants with implausible values, leaving 1,611 cases and 1,775 noncase members of the subcohort.
Vitamin D Intake and Other Covariates
For all 50,884 Sister Study participants, we estimated average daily vitamin D intake in the year prior to enrollment. To do this, we combined information on frequency and portion size with data on the amount of vitamin D present in each type of food, accounting for gender preferences (Block Dietary Data Systems, weighted women-only method). Participants were also asked whether they took any vitamins, and if so, what type, how much, and how often. Questionnaires were completed at home so that the women could check bottles for information.
Sunlight-related variables (e.g., latitude, physical activity, and time spent outdoors) were assessed at baseline, along with other potentially relevant covariates such as exogenous hormone use, history of osteoporosis, education, and menopausal status. Body mass index (BMI) was computed from height and weight measured during the home visit. For consistency with the biomarker analysis, we only considered the first 5 y of follow-up when assessing the effects of diet and sunlight exposure on breast cancer risk. During this time, 1,699 women developed breast cancer (including 1,616 with blood samples).
Statistical Analysis
We adjusted 25(OH)D values for season of blood draw and batch effects as follows. We first modeled the effect of batch on 25(OH)D using a random effects model, then standardized across batches by subtracting the batch-specific coefficient estimate from each value in each batch. We then adjusted for season using LOESS regression (Borkowf et al. 2003), allowing seasonal variation to depend on race/ethnicity, latitude, and supplement use. Briefly, we used the LOESS to calculate how much each individual’s 25(OH)D level differed from the level expected given their date of blood draw and then added that residual back to the group-level mean to obtain season-adjusted values, which represented individuals’ average 25(OH)D over the entire year. The batch- and season-adjusted 25(OH)D levels were categorized as quartiles, with cut-points based on the distribution in the randomly sampled subcohort. We used quartiles to allow for nonlinearity (including a possible threshold effect) in the dose–response. We did not use the current cut-point for deficiency (; Institute of Medicine of the National Academies 2010) because this cut-point was determined based on bone health data and does not typically include .
We estimated HRs for the case–cohort design using Cox proportional hazards models, with age as the primary time scale. Using previously described methods (Barlow et al. 1999; Prentice 1986), women in the subcohort were followed from baseline until breast cancer, death, loss to follow-up, or 5 y, and cases not part of the random subcohort were statistically treated as if they were only at risk just prior to diagnosis. We calculated 95% CIs using robust variance estimates. Analyses were performed using SAS (v.9.3; SAS Institute Inc.) (Kulathinal et al. 2007).
All models included adjustment for the following covariates measured at baseline: race/ethnicity (categorical), education (categorical), current hormonal birth control use (yes/no), current hormone therapy use (none, estrogen plus progestin, or unopposed estrogen), menopausal status (pre- or postmenopausal), physical activity during the preceding year (categorical), BMI (continuous), history of osteoporosis (yes/no), alcohol consumption in the preceding year (never/former drinker, , ), parity (0, 1, 2, ), and a BMI × menopausal status interaction term. Women were considered postmenopausal if they had gone without menstruating, had had both ovaries removed, or had had a hysterectomy with ovarian retention and were old. These confounders were selected a priori based on assumed causal relationships among the variables (Greenland et al. 1999). For multivariate analyses, we excluded individuals with missing covariate information, leaving 1,600 cases and 1,822 random subcohort members in the final analysis.
We also examined whether the relationship between 25(OH)D and breast cancer was modified by time-varying menopausal status or selected covariates, assessing modification by calculating heterogeneity p-values from likelihood ratio tests (with considered evidence of heterogeneity). Additionally, we examined the influence of time since blood draw, and we tested for effect heterogeneity by tumor characteristics using case-only analyses (Begg and Zhang 1994).
Lastly, we evaluated the relationship between breast cancer and vitamin D sources, including supplements, diet, and sunlight exposure. Although these indicators provide an incomplete, crude assessment of vitamin D exposure, we could evaluate them in the entire cohort. We report adjusted HRs and CIs for the larger cohort based on Cox proportional hazards models with robust variance estimators to account for within-family clustering. Confounders were again selected based on a priori assumptions about causal relationships. For all models, we tested for violations of the proportional hazards assumption using age × exposure interaction terms.
Results
Most Sister Study participants are non-Hispanic white (84%) and well-educated (85% having had at least some college) (Table 1). At baseline, 54% reported that they regularly () took a vitamin D–containing supplement. The random subcohort was similar to the full Sister Study cohort. Compared with the subcohort, cases were more likely to be older, nonwhite, highly educated, postmenopausal and current hormone therapy users and to have high BMI, relatives with breast cancer, and no history of osteoporosis.
Table 1.
Characteristics of the Sister Study cohort (2003–2009) and the case–cohort sample included in the 25-hydroxyvitamin D [25(OH)D] substudy.
| Characteristic | All participants (); (%) | Included in 25(OH)D substudy | |
|---|---|---|---|
| Random subcohorta (); (%) | Breast cancer casesa (); (%) | ||
| Age at blood drawb; mean (SD) | 55.6 (9.0) | 55.3 (8.9) | 57.4 (8.9) |
| Follow-up timec; mean (SD) | 6.5 (1.9) | 4.7 (0.8) | 2.5 (1.3) |
| Race/Ethnicity | |||
| Non-Hispanic white | 42,557 (84) | 1,589 (86) | 1375 (85) |
| Non-Hispanic black | 4,461 (9) | 134 (7) | 122 (8) |
| Hispanic | 2,515 (5) | 82 (4) | 63 (4) |
| Other | 1,334 (3) | 38 (2) | 50 (3) |
| Education level | |||
| High school or less | 7,804 (15) | 294 (16) | 248 (15) |
| Some college | 17,181 (34) | 645 (35) | 516 (32) |
| Bachelor’s degree | 13,714 (27) | 471 (26) | 423 (26) |
| Graduate degree | 12,171 (24) | 433 (23) | 423 (26) |
| Menopausal status | |||
| Premenopausal | 16,782 (33) | 616 (33) | 465 (29) |
| Postmenopausal | 34,093 (67) | 1,226 (67) | 1,146 (71) |
| Body mass index (BMI) | |||
| 19,634 (39) | 705 (38) | 588 (37) | |
| 25–29.9 | 16,064 (32) | 586 (32) | 508 (32) |
| 15,167 (30) | 549 (30) | 515 (32) | |
| Hormonal birth control use | |||
| Current user | 2,023 (4) | 75 (4) | 66 (4) |
| Former user | 41,090 (81) | 1,495 (81) | 1,286 (80) |
| Never user | 7,486 (15) | 265 (14) | 248 (16) |
| Hormone therapy use | |||
| Current, estrogen plus progestin | 1,700 (3) | 67 (4) | 84 (5) |
| Current, unopposed estrogen | 3,576 (7) | 125 (7) | 134 (8) |
| Former user | 17,637 (35) | 626 (34) | 576 (36) |
| Never user | 27,797 (55) | 1,019 (55) | 812 (51) |
| Physical activity (in last year) | |||
| 0–1 h/wk | 17,238 (34) | 640 (35) | 541 (34) |
| 1.1–3 h/wk | 15,616 (31) | 563 (31) | 520 (32) |
| 17,982 (35) | 640 (35) | 550 (34) | |
| History of osteoporosis | |||
| No | 39,370 (77) | 1,408 (76) | 1,267 (79) |
| Yes | 11,474 (23) | 434 (24) | 344 (21) |
| Alcohol consumption in last year | |||
| Never/former drinker | 9,679 (19) | 342 (19) | 300 (19) |
| Current drinker, | 34,255 (67) | 1,241 (68) | 1,079 (67) |
| Current drinker, | 6,861 (14) | 255 (14) | 231 (14) |
| Parity | |||
| 0 births | 9,207 (18) | 343 (19) | 299 (19) |
| 1 birth | 7,348 (14) | 277 (15) | 236 (15) |
| 2 births | 18,689 (37) | 669 (36) | 579 (36) |
| 15,603 (31) | 553 (30) | 497 (31) | |
| Regular vitamin D supplement use () | |||
| None | 23,278 (47) | 847 (47) | 736 (46) |
| Multivitamin, no extra vitamin D | 20,399 (41) | 736 (41) | 662 (42) |
| Multivitamin and vitamin D | 3,773 (8) | 134 (7) | 122 (8) |
| Vitamin D and calcium | 1,930 (4) | 76 (4) | 54 (3) |
| Vitamin D only | 359 (1) | 10 (1) | 9 (1) |
| Family history of breast cancer | |||
| Affected sister or half-sister only | 38,086 (75) | 1,368 (74) | 1,053 (65) |
| first degree relative | 12,793 (25) | 475 (26) | 558 (35) |
Note: Missing values: race (17 overall, 1 case), education (14 overall, 1 case), menopausal status (9 overall, 1 from subcohort), current BMI (19 overall, 3 from subcohort), hormonal birth control use (285 overall, 8 from subcohort, 11 cases), hormone therapy use (174 overall, 6 from subcohort, 5 cases), physical activity (48 overall), alcohol (89 overall, 5 from subcohort, 1 case), parity (37 overall, 1 from subcohort), supplement use (1,145 overall, 40 from subcohort, 28 cases), family history of breast cancer (5 overall). SD, Standard deviation.
The subcohort includes 68 women who became cases and 1,775 women who did not. The 68 cases are included in both columns (total ).
A total of 453 Sister Study participants did not provide blood samples and were ineligible for the case–cohort sample. For these women, we substituted the age at which they completed the baseline interviews when calculating the mean age.
For the full cohort, follow-up time includes all person-time accrued through 1 July 2014. For subcohort members, follow-up time is the time from baseline blood draw until breast cancer diagnosis, death, end of follow-up, or 5 y, whichever occurred first. For cases, the follow-up time described here is the time between baseline blood draw and breast cancer diagnosis, although the cases only contributed person-time just prior to their age at diagnosis in the case–cohort analysis.
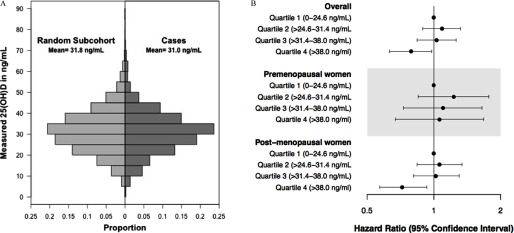
Batch- and season-corrected 25(OH)D was approximately normally distributed with a mean of in the subcohort and in cases (Figure 1A). After adjusting for confounders, (fourth quartile) was associated with a 21% lower breast cancer hazard when compared with levels (first quartile) [Figure 1B and Table 2; (CI: 0.63, 0.98)]. The HRs for the second and third quartiles were indistinguishable from 1.00 [ (CI: 0.89, 1.32) and (CI: 0.84, 1.27)], but a trend test across categories (1–4) showed evidence for lower risk (). A restricted cubic spline modeling the association between 25(OH)D and incident breast cancer relative to (see Figure S1) suggested below , near-null HRs for , and above . When we stratified by time-varying menopausal status, we found that although the inverse association between breast cancer and 25(OH)D was driven by postmenopause [ (CI: 0.56, 0.92) for the fourth vs. first quartile compared with (CI: 0.67, 1.68) in premenopause], the two estimates were not statistically distinguishable (). The proportional hazards assumption was not violated for any of these models.
Figure 1.
Distribution of 25(OH)D in cases and random subcohort (A). Hazard ratios and 95% confidence intervals for the association between 25(OH)D and breast cancer incidence within 5 y in the Sister Study (B). The hazard ratios are adjusted for batch, seasonal trends, race, education level, current hormonal birth control use, current hormone therapy use and type, menopausal status, physical activity, body mass index (BMI), osteoporosis, and a BMI × menopausal status interaction term. There are 1,600 cases and 1,822 participants in the random subcohort (including 67 cases) with complete covariate information (total ).
Table 2.
The association between total 25-hydroxyvitamin D [25(OH)D] and breast cancer incidence within 5 y in the sister study: hazard ratios (HRs) and 95% confidence intervals (CIs).
| 25(OH)D Level (ng/mL) | Overall | Premenopausal women | Postmenopausal women | |||
|---|---|---|---|---|---|---|
| a | HR (95% CI) | a | HR (95% CI) | a | HR (95% CI) | |
| 1st Quartile (0–24.6) | 451/395 | 1.00 | 197/101 | 1.00 | 254/294 | 1.00 |
| 2nd Quartile () | 454/440 | 1.09 (0.89, 1.32) | 166/113 | 1.24 (0.85, 1.79) | 288/327 | 1.06 (0.83, 1.34) |
| 3rd Quartile () | 464/432 | 1.04 (0.84, 1.27) | 139/78 | 1.13 (0.75, 1.70) | 325/354 | 1.03 (0.81, 1.30) |
| 4th Quartile () | 453/333 | 0.79 (0.63, 0.98) | 108/68 | 1.06 (0.67, 1.68) | 345/265 | 0.72 (0.56, 0.92) |
| p For category trend | 0.03 | 0.81 | 0.008 | |||
| 4th vs. Quartiles 1–3 | 0.75 (0.63, 0.89)b | 0.94 (0.63, 1.41)c | 0.70 (0.58, 0.85)c | |||
Note: Levels adjusted for batch and then season within categories of supplement use, latitude, and race. Model adjusted for age, race, education level, current hormonal birth control use, current hormone therapy use and type, menopausal status, physical activity, body mass index (BMI), alcohol consumption, parity, osteoporosis, and a BMI × menopausal status interaction term. A total of 1,600 cases (360 premenopausal, 1,240 postmenopausal) and 1,822 random subcohort members (610 premenopausal, 1,212 postmenopausal) had complete covariate information.
Subcohort members/cases (cases selected into subcohort counted in each category); frequencies based on complete case analysis and menopausal status at baseline.
Observed a violation of the proportional hazards assumption () for likelihood ratio test of an age-by-25(OH)D interaction term.
Evidence of heterogeneity across strata of menopausal status ().
To simplify exploratory analysis when examining effect measure modification, we used an a posteriori threshold model that combined the first three quartiles of 25(OH)D (Table 3). Here, we found evidence against the proportional hazards assumption (), with a stronger inverse association between breast cancer and 25(OH)D in y old than in women y old (heterogeneity ). Similarly, the effect was stronger in postmenopause than in premenopause ().
Table 3.
Stratum-specific hazard ratios (HRs), 95% confidence intervals (CIs), and p-values for test of heterogeneity for the association between 25-hydroxyvitamin D [25(OH)D] (4th quartiles vs. quartiles 1–3) and breast cancer within 5 y.
| Characteristic | Cases; (%) | Subcohort; (%) | Quartile 1–3 | Quartile 4 | p-Value for heterogeneity |
|---|---|---|---|---|---|
| Age, ya | |||||
| 35–59 | 946 (59) | 1,248 (69) | 1.00 | 0.92 (0.72, 1.17) | 0.01 |
| 654 (41) | 574 (32) | 1.00 | 0.62 (0.49, 0.78) | ||
| Menopausal statusa | |||||
| Premenopausal | 465 (29) | 610 (33) | 1.00 | 0.94 (0.63, 1.41) | 0.02 |
| Postmenopausal | 1,135 (71) | 1,212 (67) | 1.00 | 0.70 (0.58, 0.85) | |
| Race | |||||
| Non-African Americans | 1,479 (92) | 1,690 (93) | 1.00 | 0.75 (0.63, 0.90) | 0.90 |
| African Americans | 121 (8) | 132 (7) | 1.00 | 0.89 (0.30, 2.64) | |
| Obese () | |||||
| No | 1,089 (68) | 1,279 (70) | 1.00 | 0.83 (0.69, 1.01) | 0.04 |
| Yes | 511 (32) | 543 (30) | 1.00 | 0.45 (0.30, 0.69) | |
| Current hormone therapy user | |||||
| No | 1,384 (87) | 1,632 (90) | 1.00 | 0.78 (0.65, 0.94) | 0.12 |
| Estrogen plus progestin | 84 (5) | 66 (4) | 1.00 | 1.92 (0.84, 4.42) | |
| Estrogen only | 132 (8) | 124 (7) | 1.00 | 0.44 (0.23, 0.83) | |
| Regular vitamin D supplementation | |||||
| No | 733 (47) | 838 (47) | 1.00 | 0.62 (0.45, 0.84) | 0.14 |
| Yes | 839 (53) | 945 (53) | 1.00 | 0.83 (0.67, 1.04) | |
| Dietary intake of vitamin D | |||||
| 760 (48) | 876 (49) | 1.00 | 0.77 (0.60, 0.99) | 0.69 | |
| 812 (52) | 907 (51) | 1.00 | 0.74 (0.58, 0.94) | ||
| Time spent outdoors | |||||
| 830 (52) | 898 (50) | 1.00 | 0.73 (0.56, 0.94) | 0.69 | |
| 766 (48) | 914 (50) | 1.00 | 0.80 (0.63, 1.02) | ||
| Latitude of primary residence | |||||
| 0–39° | 823 (52) | 949 (52) | 1.00 | 0.84 (0.66, 1.07) | 0.07 |
| 775 (48) | 868 (48) | 1.00 | 0.66 (0.51, 0.85) | ||
| Physical activity | |||||
| 788 (49) | 931 (51) | 1.00 | 0.69 (0.52, 0.90) | 0.76 | |
| 818 (51) | 891 (49) | 1.00 | 0.78 (0.63, 0.97) | ||
Note: All models are adjusted for age, season, batch, race, education level, current hormonal birth control use, current hormone therapy type, menopausal status, physical activity, body mass index (BMI), osteoporosis, parity, alcohol consumption, and a BMI × menopausal status interaction term. IU, international units.
Age and menopausal status allowed to vary over time in the model. The provided frequencies are for age and menopausal status at the baseline interview. Menopausal status is based on information from both baseline and follow-up interviews.
We also observed a stronger inverse association between 25(OH)D and breast cancer among obese women () than among nonobese women (heterogeneity among all women and 0.02 among postmenopausal women), but there was little evidence of modification by any of the other examined covariates. The association with lower risk was apparent for all of the evaluated types of breast cancer (Table 4), and there was no evidence of heterogeneity by tumor type. We saw little change in the HRs if we excluded cases diagnosed in the first two or last 2 y of the 5-y follow-up interval (see Table S1), and the effect did not change over follow-up (p-value for ).
Table 4.
Subtype-specific hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between 25-hydroxyvitamin D [25(OH)D] (4th quartiles versus quartiles 1–3) and breast cancer within 5 y.
| Tumor characteristics | Cases; (%) | Quartiles 1–3 | HR for Quartile 4 | p-Value for heterogeneitya |
|---|---|---|---|---|
| Estrogen receptor (ER) status | ||||
| Positive | 1250 (82) | 1.00 | 0.78 (0.65, 0.94) | 0.42 |
| Negative | 281 (18) | 1.00 | 0.65 (0.46, 0.91) | |
| Subtypeb | ||||
| Triple-negative | 172 (12) | 1.00 | 0.60 (0.39, 0.93) | 0.25 |
| Not triple-negative | 1324 (89) | 1.00 | 0.78 (0.65, 0.94) | |
| Invasive status | ||||
| Invasive | 1208 (76) | 1.00 | 0.78 (0.64, 0.94) | 0.26 |
| In situ | 379 (24) | 1.00 | 0.67 (0.50, 0.89) |
Note: All models are adjusted for age, season, batch, race, education level, current hormonal birth control use, current hormone therapy type, menopausal status, physical activity, body mass index (BMI), osteoporosis, parity, alcohol consumption, and a menopausal status × BMI interaction term.
p-value for 25(OH)D effect in a case-only model.
Triple-negative defined as estrogen receptor (ER) negative, progesterone receptor (PR) negative, and human epidermal growth factor receptor-2 (HER2) negative. The “not triple-negative” category includes all other individuals with nonmissing hormone receptor status ().
Neither the sunlight exposure variables nor daily dietary vitamin D intake was associated with breast cancer risk in the full cohort (Table 5), but self-reported regular ( at baseline) vitamin D supplement use was associated with a lower breast cancer hazard. However, after observing violations of the proportional hazards assumption for dietary and supplemental vitamin D intake, we stratified by time-varying menopausal status. For combined supplement use and dietary intake, we found an inverse association among postmenopausal women [see Table S2; (CI: 0.96, 1.00) per increase] and a positive association among premenopausal women [ (CI: 1.01, 1.10) per increase; ]. Similarly, we observed an inverse association for regular supplement use among postmenopausal women [ (CI: 0.75, 0.94)] and a positive, but statistically nonsignificant, association among premenopausal women [ (CI: 0.95, 1.43); ).
Table 5.
Hazard ratios (HR) and 95% confidence intervals (CIs) for the association between vitamin D–related exposures and breast cancer within 5 y (1,699 cases, 49,044 noncases).
| Characteristic | Noncases (%) | Cases (%) | HR (95% CI) |
|---|---|---|---|
| Total vitamin D intake ()a | |||
| 15,370 (33) | 507 (31) | 1.00 | |
| 8,095 (17) | 283 (17) | 1.01 (0.87, 1.17)b | |
| 14,621 (31) | 540 (33) | 0.99 (0.87, 1.12)b | |
| 8,999 (19) | 312 (19) | 0.90 (0.78–1.05)b | |
| Per | 0.99 (0.97, 1.01)b | ||
| Regular vitamin D supplementationc,d | |||
| No | 22,239 (47) | 778 (47) | 1.00 |
| Yes | 25,264 (53) | 876 (53) | 0.89 (0.81, 0.99)c |
| Hours per week doing any sports/exercise (including walking)e | |||
| 0–2 h/wk | 4,735 (10) | 177 (11) | 1.00 |
| 9,570 (20) | 315 (19) | 0.87 (0.73, 1.05) | |
| 17,720 (37) | 627 (37) | 0.93 (0.79, 1.10) | |
| 16,463 (34) | 562 (33) | 0.90 (0.76, 1.08) | |
| Per h/wk | 1.00 (0.99, 1.01) | ||
| Latitude, current residencef | |||
| 12,864 (26) | 427 (25) | 1.00 | |
| 35–39° | 12,885 (26) | 457 (27) | 1.05 (0.92, 1.20) |
| 40–42° | 14,380 (29) | 499 (29) | 1.03 (0.91, 1.18) |
| 8,754 (18) | 314 (19) | 1.06 (0.91, 1.23) | |
| Per degree | 1.01 (1.00, 1.01) | ||
| Hours spent outdoors per yearg | |||
| 0–320 | 12,498 (26) | 452 (27) | 1.00 |
| 321–530 | 11,195 (23) | 404 (24) | 1.02 (0.89, 1.16) |
| 531–850 | 12,424 (26) | 415 (25) | 0.94 (0.82, 1.08) |
| 12,222 (25) | 405 (24) | 0.93 (0.81, 1.07) | |
| Per 200 h/y | 0.99 (0.97, 1.02) |
Note: All covariates in the table were assessed during the baseline interview, unless otherwise specified. A total of 141 women were excluded because they were diagnosed with breast cancer before the completion of follow-up or because they provided no follow-up information beyond baseline. Numbers in columns are those with complete data for the specified analysis. IU, international units.
Adjusted for age, race, education, physical activity, body mass index (BMI), menopausal status, current birth control use, current hormone therapy type, current alcohol use, total energy intake, osteoporosis, parity, and a BMI × menopausal status interaction term. We excluded 1,137 women with missing dietary data (1,105 noncases, 32 cases) and 439 women with total energy intake or per day (427 noncases, 12 cases).
Observed a violation of the proportional hazards assumption () for likelihood ratio test of time interaction term.
Adjusted for age, race, education, physical activity, BMI, menopausal status, current birth control use, current hormone therapy type, current alcohol use, osteoporosis, parity, and a BMI × menopausal status interaction term.
Women who took a multivitamin or separate vitamin D supplement times per week were considered regular users.
Adjusted for age, race, education, BMI, menopausal status, current birth control use, current hormone therapy type, current alcohol use, osteoporosis, parity, and a BMI × menopausal status interaction term.
Adjusted for age, race, education.
Adjusted for age, race, education, physical activity, hours walked per week, BMI, current birth control use, current hormone therapy type, current alcohol use, menopausal status, parity, osteoporosis, and a BMI × menopausal status interaction term.
Discussion
In this prospective observational study of vitamin D and breast cancer, high 25(OH)D serum levels were associated with lower risk of developing breast cancer over the ensuing 5 y. The association with lower risk was only evident for women with serum levels in the highest quartile () and appeared to be strongest in postmenopausal women and obese women. The association remained strong even after excluding the first 2 y of follow-up, suggesting that it is unlikely to be explained by reverse-causal effects of occult tumors on 25(OH)D. Combined dietary plus supplemental vitamin D intake was associated with slightly higher breast cancer risk among premenopausal women but with lower risk among postmenopausal women. Regular supplement use was also associated with lower risk in postmenopausal women.
Our finding that high serum 25(OH)D was associated with lower breast cancer risk is consistent with findings from retrospective case–control studies (Abbas et al. 2008; Abbas et al. 2009; Chen et al. 2013; Colston et al. 2006; Crew et al. 2009). However, because we used serum drawn before diagnosis, our 25(OH)D findings are not subject to bias due to post-diagnostic changes. Our results are also generally consistent with those of previous prospective studies, most of which reported inverse, but nonsignificant, associations (Bertone-Johnson et al. 2005; Chlebowski et al. 2008; Engel et al. 2010; Kim et al. 2014; Mohr et al. 2013; Rejnmark et al. 2009). Some studies with relatively short follow-up periods showed the strongest evidence of protection. For example, a nested case–control study within the Nurses’ Health Study (Bertone-Johnson et al. 2005) reported a strong but statistically nonsignificant lower risk between high plasma 25(OH)D levels and breast cancer during 7 y of follow-up. Although a large proportion of their cases may have already had undiagnosed breast cancer at enrollment, a Danish study of women undergoing diagnostic mammography reported a statistically significant inverse association between serum 25(OH)D and breast cancer within 5 y (Rejnmark et al. 2009).
Recent vitamin D levels could plausibly affect breast cancer risk: is known to play a role in cell growth, proliferation, and apoptosis and in estrogen receptor regulation (Feldman et al. 2014; Holick 2006; Krishnan et al. 2010; Welsh et al. 2003). Although vitamin D seems to be most effective as a chemopreventive agent in murine models of early-stage cancer (Feldman et al. 2014), there is also evidence that it can inhibit growth of transplanted mammary tumor cells (Jeong et al. 2015) and xenografted breast tumors in mice (Swami et al. 2012).
We observed heterogeneity in risk, with more beneficial associations seen in postmenopausal women and obese women. The former finding is consistent with those of a recent meta-analysis (Bauer et al. 2013) and can be considered further evidence of etiologic heterogeneity in breast cancer by age or menopausal status (Anderson et al. 2007). The same meta-analysis corroborates our finding that the dose–response relationship between 25(OH)D and breast cancer is nonlinear, with a threshold near [excluding 3-epi-25(OH)D]. The literature is inconsistent regarding the modifying effects of BMI (Deschasaux et al. 2016; Kühn et al. 2013; McCullough et al. 2009; Scarmo et al. 2013), but because obesity modified the 25(OH)D–breast cancer association even among postmenopausal women in our data, the BMI effects cannot be explained by correlation with menopausal status. Because 25(OH)D is fat-soluble, heavier women tend to have lower circulating levels even with similar ultraviolet exposure and dietary intakes (Arunabh et al. 2003; Wortsman et al. 2000). Therefore, compared with nonobese women with similar serum levels, obese women in the highest quartile of serum 25(OH)D presumably have greater available reserves and improved protection.
We did observe a higher hazard of premenopausal breast cancer in association with higher supplemental plus dietary vitamin D intake (see Table S2). Although this is a potentially concerning finding, it was not seen in our own assessment of 25(OH)D levels and breast cancer in premenopausal women (Table 2) or in other studies of dietary vitamin D intake among premenopausal women (Abbas et al. 2013; Engel et al. 2011; Lin et al 2007; Shin et al. 2002).
To date, the randomized clinical trials of vitamin D supplementation have provided little evidence of benefit from supplementation. However, certain features, including small sample size (Lappe et al. 2007; Trivedi et al. 2003), nonadherence, and combined treatment regimens or off-protocol supplementation (Bolland et al. 2011; Chlebowski et al. 2008) made it difficult for those trials to establish causality or to identify effective dose levels. In principle, a randomized, placebo-controlled trial of vitamin D supplements alone among women who abstain from self-supplementation would be the best way to assess the effects of vitamin D on breast cancer risk.
Our data suggest that a high 25(OH)D serum level () may be needed to lower the risk of breast cancer. However, in the cohort as a whole, we also observed a lower risk associated with regular vitamin D supplement use (; ), where regular users had an average 25(OH)D level of (see Table S3). In fact, these results are fairly consistent with those of the Women’s Health Initiative trial, where was associated with a nonsignificant reduction in breast cancer risk [ (CI: 0.85, 1.09)] (Chlebowski et al. 2008), although the treatment effect did not vary by baseline 25(OH)D level (Chlebowski et al. 2012). However, intent-to-treat analyses that include women who take supplements off-protocol are subject to misclassification and bias toward the null (Bolland et al. 2012), meaning that the true effects may have been stronger than what was observed.
One limitation related to our analyses of factors that should influence 25(OH)D levels is that some variables are difficult to measure with questionnaires. We are particularly concerned about self-reported dietary vitamin D, supplement use, and time spent outdoors, because misclassification could have biased the observed associations toward the null. Although we observed the expected relationships between 25(OH)D levels and these variables (see Table S3), previous studies have demonstrated how these self-reported factors only weakly predict measured 25(OH)D (Bertrand et al. 2012; Millen et al. 2010). Without evidence of strong correlations, it is difficult to provide public health recommendations for how to effectively modify 25(OH)D levels.
Another limitation is our small number of minority participants, particularly because African Americans and Hispanics have lower average vitamin D levels than non-Hispanic whites (Forrest and Stuhldreher 2011). Our use of women with a first-degree family history of breast cancer may further limit the generalizability of our findings, although we do not expect it to bias our effect estimates (Weinberg et al. 2007). Potentially, women with a first-degree family history could make lifestyle changes aimed at reducing their risk, which could induce a correlation between strength of family history and vitamin D and lead to an HR estimate that was biased toward the null. However, we found the opposite: participants with stronger family histories had slightly lower 25(OH)D levels than those with only one affected sister (see Table S3). We also note that the identification of modifiable risk factors could have the greatest public health impact among women who know they are at elevated risk.
Strengths of this study include the prospective collection of serum specimens, the large sample size, detailed covariate information, and the use of LC/MS to measure 25(OH)D levels. Although unmeasured confounding may be present, detailed covariate information allowed us to adjust for important covariates. We were also able to evaluate important potential modifiers of the association and to assess associations with known vitamin D determinants. LC/MS is the current gold standard for measuring 25(OH)D, outperforming other methods in direct comparisons (Farrell et al. 2012) and allowing us to include 3-epi-25(OH)D. Although the baseline assessment of serum 25(OH)D levels was a single “snapshot” measure, our concomitant finding of lower risk among postmenopausal women who regularly took supplemental vitamin D provides additional evidence that increased 25(OH)D levels can reduce risk in that group. An additional strength of our study is that recruiting a cohort of women who had sisters with breast cancer enabled us to accrue a large number of cases within 5 y of blood draw with minimal loss to follow-up. Thus, the present study provides new information for evaluating the hypothesis that recent, prospectively measured 25(OH)D levels are relevant to breast cancer risk.
Conclusions
In this large, prospective cohort of women at elevated risk, recent serum 25(OH)D levels and regular vitamin D supplementation were associated with lower breast cancer hazard in postmenopausal women. Our results support the hypothesis that vitamin D supplementation could be effective for breast cancer prevention and may help to establish clinical benchmarks for beneficial 25(OH)D levels.
Supplemental Material
Acknowledgments
The authors thank C. Kleeberger, L. DeRoo, J. Keller, D. Shore, D. Scharf, M. House, A. D’Aloisio, N. Gonzalez, S. Halverson, Q. Harmon, and A. White for their contributions to this manuscript.
This work was supported by an Office of Dietary Supplement Research Scholars Program Grant (to K.M.O.) and by the Intramural Research Program of the National Institutes of Health/National Institute of Environmental Health Sciences (project Z01-ES044005 to D.P.S. and Z01- ES102245 to C.R.W.).
References
- Abbas S, Chang-Claude J, Linseisen J. 2009. Plasma 25-hydroxyvitamin D and premenopausal breast cancer risk in a German case-control study. Int J Cancer 124(1):250–255, PMID: 18839430, 10.1002/ijc.23904. [DOI] [PubMed] [Google Scholar]
- Abbas S, Linseisen J, Rohrmann S, Chang-Claude J, Peeters PH, Engel P, et al. 2013. Dietary intake of vitamin D and calcium and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition. Nutr Cancer 65(2):178–187, PMID: 23441605, 10.1080/01635581.2013.752018. [DOI] [PubMed] [Google Scholar]
- Abbas S, Linseisen J, Slanger T, Kropp S, Mutschelknauss EJ, Flesch-Janys D, et al. 2008. Serum 25-hydroxyvitamin D and risk of post-menopausal breast cancer–results of a large case-control study. Carcinogenesis 29(1):93–99, PMID: 17974532, 10.1093/carcin/bgm240. [DOI] [PubMed] [Google Scholar]
- Almquist M, Bondeson AG, Bondeson L, Malm J, Manjer J. 2010. Serum levels of vitamin D, PTH and calcium and breast cancer risk-a prospective nested case-control study. Int J Cancer 127(9):2159–2168, PMID: 20112341, 10.1002/ijc.25215. [DOI] [PubMed] [Google Scholar]
- Amir E, Cecchini RS, Ganz PA, Costantino JP, Beddows S, Hood N, et al. 2012. 25-hydroxy vitamin-D, obesity, and associated variables as predictors of breast cancer risk and tamoxifen benefit in NSABP-P1. Breast Cancer Res Treat 133(3):1077–1088, PMID: 22415479, 10.1007/s10549-012-2012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WF, Chen BE, Brinton LA, Devesa SS. 2007. Qualitative age interactions (or effect modification) suggest different cancer pathways for early-onset and late-onset breast cancers. Cancer Causes Control 18(10):1187–1198, PMID: 17823850, 10.1007/s10552-007-9057-x. [DOI] [PubMed] [Google Scholar]
- Arunabh S, Pollack S, Yeh J, Aloia JF. 2003. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab 88(1):157–161, PMID: 12519845, 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- Barlow WE, Ichikawa L, Rosner D, Izumi S. 1999. Analysis of case-cohort designs. J Clin Epidemiol 52(12):1165–1172, PMID: 10580779. [DOI] [PubMed] [Google Scholar]
- Bauer SR, Hankinson SE, Bertone-Johnson ER, Ding EL. 2013. Plasma vitamin D levels, menopause, and risk of breast cancer: Dose-response meta-analysis of prospective studies. Medicine (Baltimore) 92(3):123–131, PMID: 23625163, 10.1097/MD.0b013e3182943bc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C, Zhang Z. 1994. Statistical analysis of molecular epidemiology studies employing case-series. Cancer Epidemiol Biomarkers Prev 3(2):173–175, PMID: 8049640. [PubMed] [Google Scholar]
- Bertone-Johnson ER, Chen WY, Holick MF, Hollis BW, Colditz GA, Willett WC, et al. 2005. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 14(8):1991–1997, PMID: 16103450, 10.1158/1055-9965.EPI-04-0722. [DOI] [PubMed] [Google Scholar]
- Bertrand KA, Giovannucci E, Liu Y, Malspeis S, Eliassen AH, Wu K, et al. 2012. Determinants of plasma 25-hydroxyvitamin D and development of prediction models in three US cohorts. Br J Nutr 108(10):1889–1896, PMID: 22264926, 10.1017/S0007114511007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolland MJ, Grey A, Gamble GD, Reid IR. 2011. Calcium and vitamin D supplements and health outcomes: A reanalysis of the women's health initiative (WHI) limited-access data set. Am J Clin Nutr 94(4):1144–1149, PMID: 21880848, 10.3945/ajcn.111.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolland MJ, Grey A, Gamble GD, Reid IR. 2012. Reply to RT Chlebowski et al. Am J Clin Nutr 95(1):259, 10.3945/ajcn.111.028308. [DOI] [Google Scholar]
- Borkowf CB, Albert PS, Abnet CC. 2003. Using lowess to remove systematic trends over time in predictor variables prior to logistic regression with quantile categories. Stat Med 22(9):1477–1493, PMID: 12704611, 10.1002/sim.1507. [DOI] [PubMed] [Google Scholar]
- Chen P, Li M, Gu X, Liu Y, Li X, Li C, et al. 2013. Higher blood 25(OH)D level may reduce the breast cancer risk: Evidence from a Chinese population based case-control study and meta-analysis of the observational studies. PLoS One 8(1):e49312, PMID: 23382798, 10.1371/journal.pone.0049312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, et al. 2008. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst 100:1581–1591, PMID: 19001601, 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Pettinger M, Kooperberg C. 2012. Caution in reinterpreting the Women's Health Initiative (WHI) Calcium and Vitamin D Trial breast cancer results. Am J Clin Nutr 95(1):258–259, PMID: 22189262, 10.3945/ajcn.111.027664. [DOI] [PubMed] [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast Cancer, 2001. Familial breast cancer: Collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. The Lancet 358:1389–1399. [DOI] [PubMed] [Google Scholar]
- Colston KW, Lowe LC, Mansi JL, Campbell MJ. 2006. Vitamin D status and breast cancer risk. Anticancer Res 26(4A):2573–2580, PMID: 16886666. [PubMed] [Google Scholar]
- Crew KD, Gammon MD, Steck SE, Hershman DL, Cremers S, Dworakowski E, et al. 2009. Association between plasma 25-hydroxyvitamin D and breast cancer risk. Cancer Prev Res (Phila) 2(6):598–604, PMID: 19470790, 10.1158/1940-6207.CAPR-08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschasaux M, Souberbielle JC, Latino-Martel P, Sutton A, Charnaux N, Druesne-Pecollo N, et al. 2016. Weight status and alcohol intake modify the association between vitamin D and breast cancer risk. J Nutr. [DOI] [PubMed] [Google Scholar]
- Eliassen AH, Spiegelman D, Hollis BW, Horst RL, Willett WC, Hankinson SE. 2011. Plasma 25-hydroxyvitamin D and risk of breast cancer in the nurses' health study II. Breast Cancer Res 13(3):R50, PMID: 21569367, 10.1186/bcr2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel P, Fagherazzi G, Boutten A, Dupré T, Mesrine S, Boutron-Ruault MC, et al. 2010. Serum 25(OH) vitamin D and risk of breast cancer: A nested case-control study from the French E3N cohort . Cancer Epidemiol Biomarkers Prev 19(9):2341–2350, 10.1158/1055-9965.EPI-10-0264. [DOI] [PubMed] [Google Scholar]
- Engel P, Fagherazzi G, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F. 2011. Joint effects of dietary vitamin D and sun exposure on breast cancer risk: results from the French E3N cohort. Cancer Epidemiol Biomarkers Prev 20(1):187–198, 10.1158/1055-9965.EPI-10-1039. [DOI] [PubMed] [Google Scholar]
- Farrell CJ, Martin S, McWhinney B, Straub I, Williams P, Herrmann M. 2012. State-of-the-art vitamin D assays: A comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clin Chem 58(3):531–542, PMID: 22230812, 10.1373/clinchem.2011.172155. [DOI] [PubMed] [Google Scholar]
- Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. 2014. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 14:342–357, PMID: 24705652, 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- Forrest KY, Stuhldreher WL. 2011. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res 31(1):48–54, PMID: 21310306, 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Freedman DM, Chang SC, Falk RT, Purdue MP, Huang WY, McCarty CA, et al. 2008. Serum levels of vitamin D metabolites and breast cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev 17(4):889–894, PMID: 18381472, 10.1158/1055-9965.EPI-07-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, Pearl J, Robins JM. 1999. Causal diagrams for epidemiologic research. Epidemiology 10(1):37–48, PMID: 9888278. [PubMed] [Google Scholar]
- Holick MF. 2006. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 81(3):353–373, PMID: 16529140, 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine of the National Academies. 2010. Report Brief: Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC:National Academy of Sciences. [Google Scholar]
- Janowsky EC, Lester GE, Weinberg CR, Millikan RC, Schildkraut JM, Garrett PA, et al. 1999. Association between low levels of 1,25-dihydroxyvitamin D and breast cancer risk. Public Health Nutr 2(3):283–291, PMID: 10512563. [DOI] [PubMed] [Google Scholar]
- Jeong Y, Swami S, Krishnan AV, Williams JD, Martin S, Horst RL, et al. 2015. Inhibition of mouse breast tumor-initiating cells by calcitriol and dietary vitamin D. Mol Cancer Ther 14(8):1951–1961, PMID: 25934710, 10.1158/1535-7163.MCT-15-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Franke AA, Shvetsov YB, Wilkens LR, Cooney RV, Lurie G, et al. 2014. Plasma 25-hydroxyvitamin D3 is associated with decreased risk of postmenopausal breast cancer in whites: A nested case-control study in the multiethnic cohort study. BMC Cancer 14:29, PMID: 24438060, 10.1186/1471-2407-14-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan AV, Swami S, Feldman D. 2010. Vitamin D and breast cancer: Inhibition of estrogen synthesis and signaling. J Steroid Biochem Mol Biol 121(1–2):343–348, PMID: 20156557, 10.1016/j.jsbmb.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Kühn T, Kaaks R, Becker S, Eomois PP, Clavel-Chapelon F, Kvaskoff M, et al. 2013. Plasma 25-hydroxyvitamin D and the risk of breast cancer in the European prospective investigation into cancer and nutrition: A nested case-control study. Int J Cancer 133(7):1689–1700, PMID: 23526380, 10.1002/ijc.28172. [DOI] [PubMed] [Google Scholar]
- Kulathinal S, Karvanen J, Saarela O, Kuulasmaa K. 2007. Case-cohort design in practice - experiences from the MORGAM project. Epidemiol Perspect Innov: 4:15, PMID: 18053196, 10.1186/1742-5573-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. 2007. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am J Clin Nutr 85(6):1586–1591, PMID: 17556697. [DOI] [PubMed] [Google Scholar]
- Lin J, Manson JE, Lee IM, Cook NR, Buring JE, Zhang SM. 2007. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med 167(10):1050–1059, PMID: 17533208, 10.1001/archinte.167.10.1050. [DOI] [PubMed] [Google Scholar]
- McCullough ML, Stevens VL, Patel R, Jacobs EJ, Bain EB, Horst RL, et al. 2009. Serum 25-hydroxyvitamin D concentrations and postmenopausal breast cancer risk: A nested case control study in the Cancer Prevention Study-II Nutrition Cohort. Breast Cancer Res 11(4):R64, PMID: 19715600, 10.1186/bcr2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen AE, Wactawski-Wende J, Pettinger M, Melamed ML, Tylavsky FA, Liu S, et al. 2010. Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: the Women's Health Initiative Calcium plus Vitamin D clinical trial. Am J Clin Nutr 91(5):1324–1335, PMID: 20219959, 10.3945/ajcn.2009.28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr SB, Gorham ED, Alcaraz JE, Kane CI, Macera CA, Parsons JK, et al. 2013. Serum 25-hydroxyvitamin D and breast cancer in the military: A case-control study utilizing pre-diagnostic serum. Cancer Causes Control 24(3):495–504, PMID: 23296455, 10.1007/s10552-012-0140-6. [DOI] [PubMed] [Google Scholar]
- Neuhouser ML, Manson JE, Millen A, Pettinger M, Margolis K, Jacobs ET, et al. 2012. The influence of health and lifestyle characteristics on the relation of serum 25-hydroxyvitamin D with risk of colorectal and breast cancer in postmenopausal women. Am J Epidemiol 175(7):673–684, PMID: 22362582, 10.1093/aje/kwr350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehoff NM, Nichols HB, White AJ, Parks CG, D'Aloisio AA, Sandler DP. 2016. Childhood and adolescent pesticide exposure and breast cancer risk. Epidemiology 27(3):326–333, PMID: 26808595, 10.1097/EDE.0000000000000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordóñez-Mena JM, Schöttker B, Haug U, Müller H, Köhrle J, Schomburg L, et al. 2013. Serum 25-hydroxyvitamin D and cancer risk in older adults: Results from a large German prospective cohort study. Cancer Epidemiol Biomarkers Prev 22(5):905–916, PMID: 23462913, 10.1158/1055-9965.EPI-12-1332. [DOI] [PubMed] [Google Scholar]
- Prentice RL. 1986. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika 73(1):1–11, 10.1093/biomet/73.1.1. [DOI] [Google Scholar]
- Rejnmark L, Tietze A, Vestergaard P, Buhl L, Lehbrink M, Heickendorff L, et al. 2009. Reduced prediagnostic 25-hydroxyvitamin D levels in women with breast cancer: A nested case-control study. Cancer Epidemiol Biomarkers Prev 18(10):2655–2660, PMID: 19789365, 10.1158/1055-9965.EPI-09-0531. [DOI] [PubMed] [Google Scholar]
- Scarmo S, Afanasyeva Y, Lenner P, Koenig KL, Horst RL, Clendenen TV, et al. 2013. Circulating levels of 25-hydroxyvitamin D and risk of breast cancer: A nested case-control study. Breast Cancer Res 15(1):R15, PMID: 23442740, 10.1186/bcr3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. 2002. Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J Natl Cancer Inst 94(17):1301–1311, PMID: 12208895. [DOI] [PubMed] [Google Scholar]
- Skaaby T, Husemoen LL, Thuesen BH, Pisinger C, Jørgensen T, Roswall N, et al. 2014. Prospective population-based study of the association between serum 25-hydroxyvitamin-D levels and the incidence of specific types of cancer. Cancer Epidemiol Biomarkers Prev 23(7):1220–1229, PMID: 24789846, 10.1158/1055-9965.EPI-14-0007. [DOI] [PubMed] [Google Scholar]
- Surveillance, Epidemiology, and End Results Program. 2015. Cancer Stat Facts: Female Breast Cancer. Available: http://seer.cancer.gov/statfacts/html/breast.html [accessed 16 September 2015].
- Swami S, Krishnan AV, Wang JY, Jensen K, Horst R, Albertelli MA, et al. 2012. Dietary vitamin D3 and 1,25-dihydroxyvitamin D3 (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology 153(6):2576–2587, PMID: 22454149, 10.1210/en.2011-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi DP, Doll R, Khaw KT. 2003. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ, 326:469, 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg CR, Shore DL, Umbach DM, Sandler DP. 2007. Using risk-based sampling to enrich cohorts for endpoints, genes, and exposures. Am J Epidemiol 166(4):447–455, PMID: 17556763, 10.1093/aje/kwm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J, Wietzke JA, Zinser GM, Byrne B, Smith K, Narvaez CJ. 2003. Vitamin D-3 receptor as a target for breast cancer prevention. J Nutr 133(7 Suppl):2425S–2433S, PMID: 12840219. [DOI] [PubMed] [Google Scholar]
- Wortsman J, Matsuoka L, Chen T, Lu Z, Holick MF. 2000. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72(3):690–693, PMID: 10966885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.