Abstract
Background:
Sustained exposure to ambient particulate matter (PM) is a global cause of mortality. Coal fly ash (CFA) is a byproduct of coal combustion and is a source of anthropogenic PM with worldwide health relevance. The airway epithelia are lined with fluid called airway surface liquid (ASL), which contains antimicrobial proteins and peptides (AMPs). Cationic AMPs bind negatively charged bacteria to exert their antimicrobial activity. PM arriving in the airways could potentially interact with AMPs in the ASL to affect their antimicrobial activity.
Objectives:
We hypothesized that PM can interact with ASL AMPs to impair their antimicrobial activity.
Methods:
We exposed pig and human airway explants, pig and human ASL, and the human cationic AMPs β-defensin-3, LL-37, and lysozyme to CFA or control. Thereafter, we assessed the antimicrobial activity of exposed airway samples using both bioluminescence and standard colony-forming unit assays. We investigated PM-AMP electrostatic interaction by attenuated total reflection Fourier-transform infrared spectroscopy and measuring the zeta potential. We also studied the adsorption of AMPs on PM.
Results:
We found increased bacterial survival in CFA-exposed airway explants, ASL, and AMPs. In addition, we report that PM with a negative surface charge can adsorb cationic AMPs and form negative particle–protein complexes.
Conclusion:
We propose that when CFA arrives at the airway, it rapidly adsorbs AMPs and creates negative complexes, thereby decreasing the functional amount of AMPs capable of killing pathogens. These results provide a novel translational insight into an early mechanism for how ambient PM increases the susceptibility of the airways to bacterial infection. https://doi.org/10.1289/EHP876
Introduction
Worldwide, 3.7 million deaths were attributable to ambient air pollution in 2012 [World Health Organization (WHO) 2014]. A major constituent of ambient air pollution is particulate matter (PM), a mixture of particles and liquid droplets. Coal combustion generates 32.1% of the world's electricity supply, and demand for it is growing (International Energy Agency 2013). A by-product of coal combustion is coal fly ash (CFA), an anthropogenic source of fine PM. In China, the world's largest coal burner, exposure to high levels of coal-combustion PM has decreased life expectancy by 5.5 y, partly because of respiratory diseases (Chen et al. 2013). Moreover, a transboundary air pollution transport simulation suggested that China could have contributed of the black carbon pollution in the western part of the United States in 2006 (Lin et al. 2014). Therefore, this type of pollutant is not confined to the place where it is produced and has global health implications.
The airways are constantly exposed to pollutants and microbes. Epidemiological data support the idea that continuous exposure to ambient air pollution, including PM generated by coal combustion, increases the risk of mortality and respiratory tract infections (Bruce et al. 2000; MacIntyre et al. 2014; Shen et al. 2009). The airway surface liquid (ASL) represents one of the first lines of defense against invading organisms. This thin layer of liquid covering the airways is rich in antimicrobial proteins and peptides (AMPs) that kill pathogens within minutes (Pezzulo et al. 2012). Cationic antimicrobial peptides, such as human β-defensins (HBDs) and LL-37, can rapidly interact with the negatively charged bacterial cell wall and permeabilize it (Ganz 2003; Lee et al. 2011; Travis et al. 1999). Impairment of cationic antimicrobial peptides such as HBDs has been linked with human airway bacterial colonization (Nurjadi et al. 2013; Tesse et al. 2008). Airway colonization with bacterial pathogens increases the risk of life-threatening airway infections (Gillet et al. 2002; Huang and Platt 2003; Rocha et al. 2013). Several epidemiological studies link air pollution and Staphylococcus aureus nasal carriage (Ataniiazova 2008; Kramar 2002; Leshchuk et al. 2011; Williams 1963). Given the major impact of PM on the risk of respiratory infections, is important to understand early mechanisms by which PM increases bacterial survival in the airways.
In addition to the size and concentration of ambient PM, other particle characteristics are relevant to their health hazard. Particles have intrinsic electrical charges dependent on their composition and on the surrounding medium. The presence of particles in the atmosphere can increase the atmosphere’s electrical potential gradient. The Kew Observatory measured atmospheric electrical potential, smoke, and sulfur dioxide () concentrations during the London Fog epidemics of 1950–1958. These factors were statistically evaluated as predictors of excess mortality. Atmospheric electrical potential, as a surrogate of inhalable aerosols in combination with , proved to be a better predictor of excess episodic mortality than smoke during these high-pollution episodes (Fisher et al. 1977). Therefore, the number of particles in the air and their electrical properties seem to relate to health outcomes in human populations.
CFA is a diverse mixture of particles containing aluminum, silicon, calcium, magnesium, and iron oxides that can readily adsorb ligands and proteins (Borcherding et al. 2013; Gieré et al. 2003; Vertegel et al. 2004; Vogler 2012). Therefore, we hypothesized that CFA interacts with ASL AMPs and impairs their antimicrobial activity. To test our hypothesis, we exposed pig and human airway epithelia, ASL, and AMPs to either CFA or to a control and assessed antimicrobial activity. We used pig airway samples for screening because their anatomy, biochemistry, physiology, and genetics are similar to those of humans (Rogers et al. 2008; Stoltz et al. 2010). To investigate the antimicrobial activity of the samples, we measured live bacteria within minutes after challenge. The short time points () were used as a strategy to control for any PM-induced bacterial growth (Borcherding et al. 2013) We also studied the interaction between CFA and AMPs by measuring the adsorption capacity of CFA and the surface charge of PM and AMPs, and we determined how this interaction was modified by the use of ethylene glycol tetraacetic acid (EGTA).
Methods
Ethical Statement
All animals were treated humanely, with due consideration for the alleviation of distress and discomfort, according to National Institutes of Health guidelines. The University of Iowa Institutional Animal Care and Use Committee approved all animal protocols.
The institutional review board at the University of Iowa approved the collection of human ASL (IRB No. 200607708), and all participants signed a written informed consent. This study is registered on ClinicalTrials.gov (NCT01967628).
Particulate Matter Sample Preparation
We used Standard Reference Materials® for CFA (2689, 2690, 2691) and urban PM (1648a) from the National Institute of Standards and Technology (NIST). We also used previously characterized volcanic ash from the Icelandic Eyjafjallajökull eruption in 2010 (Monick et al. 2013). For the majority of the experiments, we used CFA 2691 based on the results of preliminary studies in which we found increased Pseudomonas aeruginosa growth in vitro and decreased lung sterility in vivo in exposed mouse lungs challenged with Pseudomonas aeruginosa (Borcherding et al. 2013).
To expose airway explants, ASL, and AMPs to PM we suspended particles in sodium phosphate buffer , pH 7.4 ( buffer) at and sonicated the suspension for one hour to increase solution homogeneity and to avoid particle aggregation. We diluted the stock solution to the desired concentration to expose the airway explants, ASL, and AMP samples.
To modify the CFA surface, we sonicated for 1 h a separate stock of CFA 2691 particles () suspended with EGTA (; Sigma-Aldrich) or sodium citrate () in buffer (pH 7.4).
Bacterial Strains
We chose S. aureus Xen29 (Caliper LifeSciences Bioware™) because it is a human-isolated strain modified to integrate a single copy of the Photorhabdus luminescens luxABCDE operon on the bacterial chromosome, which allowed us to measure real-time luminescence. Xen29 was grown overnight from a bacterial glycerol stock on a tryptic soy agar plate containing kanamycin () (). On the next day, four to five colonies were grown overnight in tryptic soy broth medium (TSB) (BD Difco). Thereafter, a subculture of log-phase bacteria was centrifuged (1 min, , 16 × g) and washed twice with phosphate-buffered saline with no calcium or magnesium (PBS-/-) (Gibco® Life Technologies). The final bacterial pellet was suspended in minimal medium consisting of buffer supplemented with NaCl and 1% TSB to sustain bacterial luminescence as previously described (Abou Alaiwa et al. 2014).
Antimicrobial Activity Assays
Bacterial killing by pig airway explants.
Airway nasal septum was excised from newborn pigs (Exemplar Genetics) anesthetized with ketamine [ intramuscularly (IM)] (Phoenix Pharm) and xylazine ( IM) (Lloyd Laboratories) and euthanized with Euthasol® [ intravenous (IV)] (Virbac). Samples of nasal septum epithelia were obtained with a 6-mm biopsy punch (Miltex) and were placed in a 96-well plate (Optiplate™-96; Perkin Elmer). The samples () were exposed to of CFA (0.3, 3, ), CFA-EGTA (), CFA-sodium citrate (, sodium citrate), or buffer control for 1 h in an incubator at and supplemented with . Subsequently, the bacterial challenge was performed by injecting of Xen29 solution [ colony-forming units (CFUs)] onto the apical side using the precision peristaltic pump in a luminometer (Spectra Max L, Molecular Devices). We maintained the samples at and measured relative light units (RLU) at 2 min at a wavelength of 527 nm.
We confirmed our luminescence findings using a conventional CFU counting method. We exposed nasal septum samples to of CFA, CFA-EGTA (), or control for one hour. After 20 min of bacterial challenge, we added of PBS-/- at to the wells containing the samples to prevent bacterial replication. Subsequently, we placed a sterile 96-well rubber lid on top of the plate and vortexed for 1 min. We calculated CFUs by standard methods. Briefly, the liquid harvested from the samples was further diluted and plated on plates. The plates were cultured at , and the colonies were counted after overnight incubation.
Bacterial killing by human airway explants.
We used human lung lobes ( of ischemia) from transplanted recipients, donated by The University of Iowa Hospital and Clinics. A secondary bronchus was excised, and samples of the airways were obtained with a 6-mm biopsy punch. The tissue () was placed in an OptiPlate-96 and then exposed to of CFA (), CFA-EGTA (, EGTA), EGTA (), or control for 1 h in an incubator at and . The bacterial challenge was performed by injecting of bacteria on the apical side, and RLU were registered after 2 min.
Bacterial killing by ASL from pig airway explants.
To investigate the effects of CFA on the ASL from pig explants, we exposed pig nasal explants with and without ASL to CFA. First, we excised pig nasal epithelia with a biopsy punch (4 mm) and washed the ASL three times by submerging samples in PBS-/-, then drying the surface with sterile gauze. We exposed both washed and unwashed epithelia to of CFA () or control for 1 h in a 96-well plate. Subsequently, we performed a challenge with an inoculum of , and we harvested bacteria 20 min later using of PBS-/- (). We plated the liquid on and counted the number of CFUs after overnight incubation.
Bacterial killing by ex vivo ASL collected from pigs.
Tracheal secretions from pigs were collected as previously described (Hoegger et al. 2014). Briefly, newborn pigs were anesthetized with ketamine and xylazine and maintained with propofol ( IV) (Fresenius Kabe USA, LLC). Tracheas were surgically exposed in a 5% , 99% humidity incubator, accessed anteriorly and then stimulated using methacholine ( IV) (Sigma-Aldrich). The secretions were collected with strips of thin lens paper (VWR Scientific Products) fused to Parafilm M (Pechiney Plastic Packaging). These papers were placed in the tracheal lumen for 30 s, dried overnight, and stored at .
The day of the experiment, samples were obtained from the strips with a 4-mm biopsy punch (Miltex), placed in an OptiPlate-96, and rehydrated using of buffer or CFA solution ( final concentration) at for 1 h. We challenged the samples by injecting of bacterial solution (see Figure S1) and measured RLU two minutes after the challenge. We calculated the percentage of live bacteria using the following formula:
Bacterial killing by human ASL.
To collect human ASL, we recruited healthy nonsmoking participants as part of the trial “Human lung response to respiratory pathogens,” as previously described (Gerke et al. 2014). The subjects were 18–60 y old and were able to understand and sign a consent form. After providing their informed consent, women of childbearing age underwent urine pregnancy screening before each bronchoscopy.
A pulmonary physician performed a standard bronchoscopy at the University of Iowa Hospitals and Clinics (Iowa City, Iowa, USA) as previously described (Gerke et al. 2014). To collect the ASL, participants were premedicated with atropine ( IM) and either morphine ( IM) or meperidine (). Subsequently, the airways were numbed with topical 2–4% lidocaine. Under standard clinical monitoring, a flexible bronchoscope (model P160 or P180; Olympus) was introduced transnasally or transorally into the right lung. To collect the ASL, three sponges were placed on the airway for 30 s and were then transferred to a 1-mL tube. Thereafter, we eluted the ASL in the sponges by adding 1 mL of isotonic saline, vortexing for 1 min, and centrifuging for another minute. We stored the ASL samples at .
To perform the antimicrobial activity assay, we corrected the ASL for protein loading. Briefly, we thawed the samples at and measured the total protein concentration by Bradford assay (Bio-Rad). We further diluted the samples with isotonic saline for a total protein concentration of . Thereafter, we exposed of the human ASL to of CFA () or buffer for 1 h. We then injected of Xen29 solution () and measured RLU after 20 min.
Bacterial killing by ASL from porcine cell cultures.
We collected ASL from airway epithelium cell cultures grown at the air–liquid interface isolated from healthy newborn pig trachea and bronchi as previously described, with modifications (Karp et al. 2002; Pezzulo et al. 2012). Briefly, we washed with of PBS-/- by pipetting three times, the apical surface () of the cell cultures, which had been maintained in antibiotic-free media for wk. We exposed of the collected liquid to of CFA (), CFA-EGTA (; ), or buffer. We performed the bacterial challenge by injecting of Xen29 solution () into the ASL samples and measured RLU at 2 min normalized to control.
Bacterial killing by cationic AMPs.
Assays were performed as previously described (Abou Alaiwa et al. 2014). Human β-defensin 3 (HBD-3) (PeproTech) and LL-37 (Anaspec) were diluted with the peptide diluent recommended by the manufacturer. We exposed of HBD-3 () or peptide diluent to of CFA solution (, final concentration), CFA-EGTA (; EGTA), or buffer in an opaque white 96-well plate. The plates were sealed with a rubber lid to avoid evaporation and incubated at for 1 h with constant shaking (200 rpm). We then performed a challenge with of Xen29 solution (). RLU were registered in the first 4 min, and a percentage was calculated using the peptide diluent as the control. We also exposed recombinant human lysozyme (, , of protein) (Sigma-Aldrich) to of CFA (10, 100, or ), CFA-EGTA (; EGTA), or buffer. The samples were incubated for 1 h at with constant shaking (200 rpm). Thereafter, we added of Xen29 solution () and measured RLU at 120 min. The percentage of live bacteria was calculated using the exposed buffer as the control.
Particle Analysis
Zeta potential measurement.
To investigate the role of CFA in particle–protein interactions, we assessed the zeta potential, which is a measurement of the magnitude of the electrostatic repulsion or attraction between particles. We mixed human lysozyme (, final concentration) or diluent with CFA solution (, final concentration) at for 1 h. We pipetted the samples into disposable capillary cells (DTS1070; Malvern) in a Zetasizer Nano ZS90 (Malvern). The Zetasizer applies an electric field and calculates electrophoretic mobility using phase analysis light scattering measurements. The zeta potential is then calculated with the Smoluchowski equation (Smoluchowski 1921). We used the automatic protein standard operating procedure to measure lysozyme charge. In the CFA samples, we set up the standard operating procedure to use 1.56 as the refractive index of CFA, as previously described (Jewell and Rathbone 2009).
AMP adsorption measurement by attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FTIR).
Experiments using ATR-FTIR were performed to determine if lysozyme was adsorbed onto CFA particle surfaces and whether the adsorption was inhibited by CFA pretreatment with EGTA. The ATR-FTIR flow system, equipped with a horizontal ATR crystal (AMTIR crystal element; Pike Technologies), was used in all of the experiments and has previously been described in detail (Borcherding et al. 2014). To perform these experiments, a thin layer of CFA particles was deposited onto an ATR crystal from a hydrosol suspension ( CFA/mL) and dried overnight. This process produced a uniform film of particles on the ATR crystal. After the thin layer of particles was formed, a flow of water was introduced into the ATR-FTIR cell to eliminate any loosely bound particles. Next, a background spectrum was collected. Then, a solution of lysozyme () was introduced into the ATR-FTIR cell. The infrared spectrum was then recorded as a function of time. Infrared absorption bands at 1,650 and , associated with amide I and amide II vibrational modes, were monitored.
The reversibility of lysozyme adsorption was also investigated. Following the adsorption experiments, ATR-FTIR spectra were recorded after introducing a flow of pure water at approximately pH 7 over the CFA particles to determine whether the infrared absorptions associated with lysozyme decreased.
We studied the inhibition of lysozyme adsorption by EGTA in a separate experiment. A thin film of CFA particles on the ATR crystal was exposed to a solution of EGTA () and allowed to equilibrate for 5 h. Following this incubation, the EGTA solution was removed, and the CFA particles were then exposed to the lysozyme solution. The absorption bands resulting from adsorbed lysozyme were recorded as a function of time. For solution-phase measurements only (i.e., in the absence of CFA), FTIR spectra were collected from the respective solutions in contact with the ATR element.
AMP bulk adsorption assay.
We examined the adsorption of lysozyme onto CFA using a batch depletion technique. We coincubated of lysozyme () with of CFA in solution (10, 100, or ) for 60 min at . A sample of the solution was withdrawn, and the remaining sample was centrifuged for 10 min at , after which we took a sample of the supernatant. The concentration of protein in both the total sample and the supernatant was measured by Bradford assay (Bio- Rad), interpolating from a lysozyme standard curve. The percentage of free protein was calculated using the following formula:
AMP desorption assay.
We also determined the amount of protein attached to the particle using a desorption assay. We coincubated 1 mL of lysozyme () with 1 mL of solution containing 10, 100, or of CFA for 1 h at . Tthe sample was then washed three times with PBS-/- to remove the nonadsorbed protein. The pellet was resuspended in of Laemmli buffer and heated for 10 min at to desorb the protein from the particle. The desorbed protein concentration was measured by Bradford assay (Bio-Rad), interpolating from a lysozyme standard curve.
To study the effects of EGTA on the CFA desorption of lysozyme (), we prepared the CFA suspension with EGTA diluted in buffer at pH 7.4 and followed the abovementioned protocols.
Statistical analysis.
Data are expressed as the . We normalized data as a percentage of the control using the formulas described above. Control conditions refer to samples with no antimicrobial activity (e.g., buffer) exposed to CFA at the same concentration as the sample with antimicrobial activity within the same experiment. We used the Kolmogorov–Smirnov test to calculate the normality of the data. To determine the statistical significance between two data sets, we used a paired Student t-test for parametric data and the Mann–Whitney and Wilcoxon matched-pairs signed rank tests for nonparametric data. We used analysis of variance (ANOVA) with Dunnett’s multiple comparison test to compare three or more groups for parametric data and the Kruskal–Wallis test for comparison of three or more groups with nonparametric distributions. Data analysis was performed using GraphPad Prism 6.00 for Windows (GraphPad Software, Inc.).
Results
CFA Impairs Bacterial Killing in Airway Epithelia
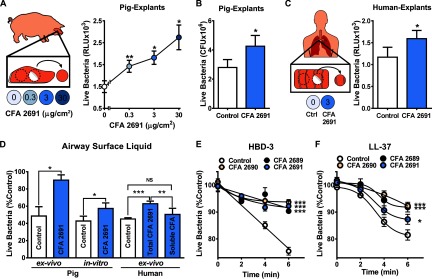
Nasal passages, lined with ASL AMPs, function as the first defense against inhalable particles and bacteria. We exposed explants of pig nasal epithelium (6 mm diameter) to CFA (0.3, 3, and ) for 1 h. After exposure, we loaded of a bioluminescent strain of S. aureus (Xen29) in of buffer onto the apical surface of the explants. This assay allowed us to interrogate bacterial viability after bacterial challenge by measuring RLU after 2 min. We interpreted reduced luminescence as a decrease in live bacteria due to killing (Travis et al. 1999). Exposure to increasing concentrations of CFA correlated with impaired bacterial killing (Figure 1A). As a control, we coincubated bacteria with CFA and observed no increase in bacterial luminescence (data not shown).
Figure 1.
Coal fly ash (CFA) impairs ASL antimicrobial activity. (A) Effects of CFA exposure on pig nasal epithelium explant antimicrobial activity assessed by luminescence. Explants exposed to increasing concentrations of CFA (0.3, 3, and ) had increased live bacteria in relative light units (RLU) (46.62%, 66.32%, and 112.9%, respectively) compared with control at 2 min after bacterial challenge (; ). (B) Effecst of CFA exposure on pig nasal epithelium explant antimicrobial activity assessed by standard colony-forming unit (CFU) counting. Explants exposed to CFA () that were harvested after 20 min of bacterial challenge had higher CFUs than the control (; ). (C) Effects of CFA exposure on human airway bronchial epithelium explant (6 mm) antimicrobial activity assessed by luminescence. Explants exposed to CFA had an increased number of live bacteria in RLU after bacterial challenge (; ). (D, E, F) Human β-defensin-3 (HBD-3) and LL-37 antimicrobial activity assessed by luminescence plotted as a function of time. CFAs 2689, 2690 and 2691 impaired (E) HBD-3 () and (F) LL-37 () antimicrobial activity in vitro compared with control at 6 min [A, E, and F were compared with control by analysis of variance (ANOVA). B, C, and D pig ASL ex vivo, and in vitro, were compared to control by paired Student’s t-test. D, human ex vivo samples were compared with each other by multiple comparisons ANOVA. * ; ** ; *** ].
We then investigated the effect of of CFA on bacterial killing on nasal epithelia using a standard bacterial dilution assay. Consistent with the luminescence assay, we recovered (20 min after bacterial challenge) an average of 52% more CFUs from the epithelia exposed to CFA than from the control (Fig. 1B). Finally, we tested the effects of CFA () on human bronchial airway epithelia and found that CFA exposure also impaired S. aureus killing (Fig. 1C). These results suggest that CFA impairs the antimicrobial activity of the airway epithelia.
CFA Impairs Bacterial Killing in ASL
We hypothesized that CFA would result in increased bacterial viability by impairing epithelial killing independently of bacterial growth. To test this hypothesis, we stripped the nasal epithelium from the cartilage and performed a bacterial challenge. We found that nasal cartilage had significantly higher bacterial viability in RLU (see Figure S2A) and CFU (see Figure S2B) than explants with intact epithelium, suggesting decreased antimicrobial activity in the cartilage. We then exposed nasal cartilage explants to CFA () and found that bacterial viability in RLU at 2 min was not significantly different from control without CFA ( vs. respectively, by paired t-test). These results suggest that in the absence of antimicrobial activity, CFA does not increase bacterial viability after challenge.
The bacterial killing observed in the epithelium is most likely due to ASL antimicrobial activity. We then investigated whether CFA impaired the ASL of the explants. We used both intact pig nasal explants and ASL-washed explants exposed to CFA () for 1 h, and we challenged them with bacteria. We harvested bacteria 20 min after the challenge and counted CFUs after overnight incubation. We found that only unwashed epithelia significantly decreased the inoculum. We also found that exposing intact epithelia to CFA increased the amount of bacteria recovered from the explants, similar to washing away the ASL. In addition, exposing washed epithelia to CFA did not increase the number of bacteria recovered from the nasal explants. These results suggest that CFA increased bacterial viability by impairing the ASL antimicrobial activity of pig airway explants and not by increasing bacterial growth (see Figure S2C).
To investigate whether CFA impairs isolated ASL antimicrobial activity, we collected pig and human tracheobronchial secretions and the ASL from pig primary airway epithelia cultures grown at the air–liquid interface as previously described. ASL was incubated with CFA () for 1 h at and then challenged with of S. aureus Xen29 (). After 20 min, we measured bacterial killing by luminescence. We found that exposure to CFA significantly impaired bacterial killing by pig and human tracheobronchial ASL and by pig airway ASL from cell cultures (Fig. 1D). These results suggest that CFA might have a direct effect on ASL AMPs.
CFA Impairs Cationic AMP Activity
The rapid bacterial killing seen in airway explants and ASL is consistent with the effect of ASL AMPs such as HBD-3 and LL-37 (Ganz 2003; Lee et al. 2011; Travis et al. 1999). To investigate whether exposure to CFA inhibited HBD-3 and LL-37 antimicrobial activity, we coincubated HBD-3 () or LL-37 () for 1 h with three CFAs () from different sources, challenged them with bacteria ( of S. aureus Xen29), and measured RLU over time for 6 min. We found that similarly to explants and ASL, exposure to CFA impaired the immediate antimicrobial activity of HBD-3 and LL-37 (Fig. 1E and F). We also investigated the effects of CFA on lysozyme antimicrobial activity because it is one of the most abundant cationic AMPs present in human secretions, including in the airways (Basbaum et al. 1990). We coincubated lysozyme () with increasing concentrations of CFA (10, 100, or ) for 1 h, performed a challenge with luminescent bacteria, and measured antimicrobial activity. We found that CFA also inhibited the antimicrobial activity of lysozyme in a concentration-dependent manner (Figure 2A). These results suggest that CFA impairs AMP activity.
Figure 2.
Coal fly ash (CFA) adsorbs lysozyme and inhibits its antimicrobial activity. (A) Effect of CFA exposure on lysozyme antimicrobial activity assessed by luminescence. CFA 2691 inhibited lysozyme antimicrobial activity in a concentration-dependent manner (). (B) CFA bulk adsorption assay of lysozyme. CFA, coincubated with lysozyme, decreased the percentage of available free protein in a concentration-dependent manner (). (C) CFA desorption assay. We recovered more lysozyme from the particles as we increased the amount of CFA in micrograms . (D) Attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FTIR) spectra of lysozyme on CFA. A lysozyme solution was flowed over the surface of a thin layer of CFA. Every 5 min, the surface was washed with water and spectra of the lysozyme amide groups were obtained using ATR-FTIR. Each line on the graph represents the spectra of lysozyme on the CFA particles every 5 min. The lysozyme absorbance on CFA increased as a function of time. (E) Integrated lysozyme absorbance () plotted as a function of time. Lysozyme adsorption onto CFA particles increased as a function of time. [A, B, and C were compared with the control by multiple comparison analysis of variance (ANOVA). * , ** , *** ].
CFA Particles Impair the Antimicrobial Activity
PM has several cationic elements on its surface that could leach into the ASL, impairing its antimicrobial activity. Divalent cations such as calcium and magnesium require lower concentrations to inhibit AMP activity compared with monovalent cations (Travis et al. 1999). We investigated the surface composition of three different CFA samples (2689, 2690, and 2691) by X-ray photoelectron spectroscopy. Table 1 shows the relative abundance of different elements on the particle surface, excluding carbon and oxygen (Chen et al. 2012). CFA 2691 had the highest proportion of divalent cations on the surface among all CFA studied. We hypothesized that divalent cations that dissolve into the ASL would impair HBD-3. However, the results did not prove this hypothesis. First, all CFAs tested impaired HBD-3 bacterial killing to the same extent (Figure 1E). Second, human ASL bacterial killing was not impaired when exposed to the supernatant from CFA 2691 (Figure 1D). Third, these elements are present as metal oxides and not as soluble salts; thus, they are less likely to leach as free ions (Catalfamo et al. 1997). These results suggest that the insoluble component (the CFA particle) is necessary to impair ASL antimicrobial activity.
Table 1.
Surface composition of three CFA samples.
| % Element | CFA 2689 | CFA 2690 | CFA 2691 |
|---|---|---|---|
| Si | 43.8 | 34.2 | 19.6 |
| Al | 19.7 | 18.7 | 8.3 |
| Fe | 8.7 | 7.3 | 3.7 |
| Mg | 2.5 | 16.4 | 9.2 |
| Ca | 6 | 8.1 | 23.2 |
| Na | 1.5 | 3.8 | 8.8 |
| K | 1.9 | 2 | 3.7 |
| S | 15.9 | 7.2 | 14.5 |
| P | NA | 2.3 | 4.7 |
| F | NA | NA | 4.3 |
| Area () |
Note: Al, aluminum; Ca, calcium; CFA, Coal Fly Ash; F, fluorine; Fe, iron; K, potassium; Mg, magnesium; NA, not available; Na, Sodium; P, phosphorus; S, sulfur; Si, silicon. We determined the surface composition of three CFA samples (CFA 2689, 2690, 2691) by X-ray photoelectron spectroscopy calculated the surface area (Chen et al. 2012).
CFA Adsorbs Antimicrobial Peptides
Our results suggest that either particle–AMP interaction or particle–bacterial interaction impaired the airway antimicrobial activity. The nature of particle–protein interactions can be governed by particle charge and surface sites as well as by ligand charge and exposed functional groups of the peptides and proteins (Vogler 2012). To investigate CFA-AMP interaction, we assessed the adsorption capacity of CFA through lysozyme adsorption–desorption assays. When lysozyme () was coincubated with several concentrations of CFA for 30 min, we found a concentration-dependent decrease in the fraction of free lysozyme (Figure 2B). Accordingly, via detergent-mediated desorption, we also recovered more lysozyme from particles as we increased CFA mass (Figure 2C).
To investigate the kinetics of lysozyme adsorption by CFA, we used ATR-FTIR. Briefly, we deposited a thin layer of CFA particles on the ATR element and flowed a lysozyme solution over the CFA. The resulting spectra showed two strong absorption bands at 1,650 and , corresponding to the amide I and II bands of lysozyme (Figure 2D). The intensity of these amide bands increased steadily in a time-dependent manner. Within 5 min, 50% of the maximal lysozyme adsorption had occurred, consistent with acute impairment of AMP. The intensities of the amide bands plateaued after 60 min, suggesting that the CFA surface was saturated with lysozyme (Figure 2E). Thereafter, particles were flushed with an aqueous solution to remove weakly adsorbed lysozyme. We then obtained spectra of lysozyme adsorbed on the CFA particles. After 5 h of rinsing, the lysozyme remained adsorbed to the CFA (data not shown), suggesting that the adsorption was irreversible. These data demonstrate that CFA can irreversibly adsorb lysozyme within minutes. Immobilization of AMPs on the CFA surface might decrease the amount of free protein capable of killing bacteria in the airway, thus impairing its antimicrobial activity.
CFA Adsorbs Lysozyme, Creating a Negative Particle–Protein Complex
Because CFA rapidly adsorbed cationic AMPs, we hypothesized that electrostatic interactions would contribute to CFA adsorption of AMP. To investigate this interaction, we studied zeta potential as a surrogate of surface charge. We found that CFA 2691 had a negative surface charge () and that HBD-3 had a positive charge (). Colloidal particles with a tight layer of negative charges attract complementary positive charges from the surrounding fluid (Hunter 1981). We hypothesized that PM would attract cationic AMPs to its surface. When we coincubated CFA with HBD-3, the average particle–protein complex zeta potential was less negative () than that of CFA alone (Figure 3A). We also measured the zeta potential of other PM, such as CFA from two other sources (NIST CFA 2690 and 2689), urban PM (NIST SRM 1648a), and volcanic ash from the Eyjafjallajökull eruption in 2010 (Monick et al. 2013). We found that all of these particles had a negative surface charge (Figure 3C-F). When different sources of PM were coincubated with increasing concentrations of lysozyme, we observed that they formed negatively charged complexes similar to CFA 2691 (Figure 3B-F). These results suggest that electrostatic interactions could contribute to the adsorption of cationic AMPs by CFA.
Figure 3.
Coal fly ash (CFA) creates a negative particle-protein complex. (A) Zeta potential measurements of CFA 2691, human β-defensin-3 (HBD-3), and HBD-3–CFA suspensions. CFA 2691 had a negative zeta potential, HBD-3 had a positive zeta potential. When they were coincubated, formed a less negative complex compared with CFA alone (). (B–F) Zeta potential measurements of several ambient particulate matters (PMs). (B) CFA 2691, (C) CFA 2690, (D) CFA 2689, (E) urban PM, and (F) volcanic ash all had a negative surface charge and formed negatively charged complexes when coincubated with increasing concentrations of lysozyme ().
CFA Passivation by EGTA
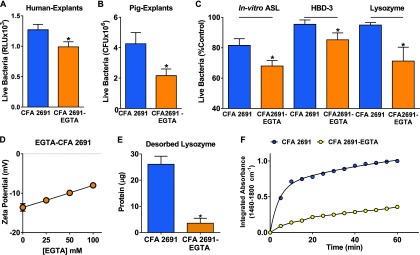
We originally speculated that divalent cations present in solution, particularly calcium, were responsible for the impairment of ASL bacterial killing by CFA. We used EGTA on human bronchial explants to chelate divalent cations, in the presence or absence of CFA. We found that EGTA, at the concentration used, did not enhance bacterial killing of human bronchial explants alone ( vs. control, ; ). However, when CFA 2691 was pretreated with EGTA, we prevented the airway antimicrobial activity impairment by CFA (Fig. 4A). We found similar effects of EGTA on pig airway explants, in vitro ASL, HBD-3, and lysozyme (Figure 4B, C). We also pretreated CFA particles with a different calcium chelator, sodium citrate, to investigate the role of calcium in CFA impairing the antimicrobial activity of the airways. When we exposed pig airway explants to CFA pretreated with sodium citrate and challenged them with bioluminescent bacteria (), we found no difference compared with CFA alone ( RLU vs. ; ).
Figure 4.
Ethylene glycol tetraacetic acid (EGTA) prevents the adsorption and the antimicrobial activity impaired by coal fly ash (CFA). (A) Effects of CFA exposure on the antimicrobial activity of human explants (; ), (B) pig explants (, ), (C) pig in vitro airway surface liquid (ASL) and antimicrobial proteins and peptides (AMPs) [human β-defensin-3 (HBD-3) and lysozyme]. CFA treated with EGTA prevented the impairment of antimicrobial activity by CFA. (D) Effects of increasing concentrations of EGTA on the zeta potential of CFA. Treatment of CFA with EGTA increased its surface charge in a concentration-dependent manner. (E) Lysozyme desorption assay for CFA treated with EGTA. CFA treated with EGTA bound less lysozyme per microgram . (F) Integrated absorbance of lysozyme ( to ) on CFA pretreated with EGTA, plotted as a function of time. CFA treated with EGTA showed decreased lysozyme uptake as a function of time (A, B, C, and E were compared with CFA 2691 by paired Student’s t-test. * ).
Because we found that the soluble component of CFA did not impair AMP activity in ASL (Figure 1D) and that sodium citrate did not prevent the antimicrobial activity impairment by CFA in airway explants, we hypothesized that EGTA was passivating the CFA surface and was not necessarily related to calcium. EGTA can adsorb onto the particle surface and block binding sites, which limits particle–protein interaction. It has also been reported that organic zwitterionic coatings can reduce particle–protein interaction (Choi et al. 2007).
CFA treated with EGTA showed a concentration-dependent increase in CFA zeta potential (Spearman’s ; ) (Figure 4D). Furthermore, we found that EGTA treatment of CFA greatly decreased the adsorption of lysozyme, as assessed by desorption assays (Figure 4E). Finally, we investigated the adsorption kinetics of the system using ATR-FTIR and found decreased integrated absorbance and slower kinetics in the CFA pretreated with EGTA compared with untreated CFA (Figure 4F). These results suggest that EGTA decreased the interaction of CFA with AMPs, preventing the impairment of AMP activity by CFA.
Discussion
Ambient air pollution is a worldwide environmental problem that affects human health. CFA is a relevant component of anthropogenic air pollution and accounts for some of the mass and transition metals present in PM (Žibret et al. 2013). Sources of CFA include construction sites, smokestacks, and landfills. Aerosolization of these deposits can be generated by wind erosion, transportation, and handling procedures. In the United States, it is estimated that populations near landfills are exposed to CFA levels that exceed the U.S. National Ambient Air Quality Standards for heavy metals exposure, raising concerns about its impact on human and ecological wellbeing (U.S. Environmental Protection Agency 1998).
A wide variety of health effects have been reported as a consequence of CFA exposure (Borm 1997). In this study, we provide evidence that acute exposure to CFA can decrease airway antimicrobial activity. We propose that when PM is inhaled into the airway, the negative charge initially interacts with cationic AMPs via electrostatic forces, forming a particle–protein complex with negative charge. It has been reported that cationic AMP surface charge density is a major determinant of its ability to kill bacteria (Bai et al. 2009). We speculate that a decrease in the cationic AMP surface charge will decrease its incorporation into the negatively charged bacteria, thereby impairing this innate immune mechanism.
Impairment of ASL antimicrobial activity is essential in the development of airway infections in diseases such as cystic fibrosis (Pezzulo et al. 2012). We propose that decreased ASL antimicrobial activity by PM will increase the bacterial inoculum and will theoretically increase the likelihood of developing an airway infection (Camberlein et al. 2015). Nevertheless, we acknowledge that bacterial survival, as indicated by increased CFU/RLU after bacterial challenge, may not correlate with infectivity in humans. The development of an infection is multifactorial and does not only depend on the inoculum.
We found that different particles with different compositions had similar effects on antimicrobial peptides. Mortality from PM exposure is uniform, despite its chemical heterogeneity, suggesting that other general physicochemical characteristics, such as particle charge, might contribute to its toxicity. It has been reported that negative particle charge correlated with the release of interleukin 6, an inflammatory marker, in a cell line of bronchial epithelial cells exposed to PM, including CFA (Veronesi et al. 2002). In our study, all PM samples studied were negatively charged and formed negatively charged complexes when exposed to cationic AMPs. We did not find a correlation between the degree of AMP impairment and the charge of the particle. We propose that it is the differential surface charge between the cationic AMPs and CFA that influences the formation of these complexes. Once the AMPs are negatively charged, their function is affected. However, the kinetics of AMP adsorption might differ depending on the charge of the particle.
The formation of this PM–cationic AMP complex appears to be irreversible and cannot be explained solely by initial electrostatic interactions. Therefore, we propose that protein adsorption onto PM can also cause changes in protein structure or conformation that result in loss of activity. It has been reported that lysozyme adsorption onto silica nanoparticles results in loss of its activity owing to the loss of in its structure compared with that of “free” lysozyme (Vertegel et al. 2004). Conversely, for small peptides such as HBD-3 and LL-37, adsorption can impair their electrophoretic mobility into the bacterial cell wall.
We observed that treatment of PM with EGTA resulted in reduction of its negative charge, decreased protein adsorption, and restoration of airway antimicrobial activity that had been impaired by PM. One potential mechanism of EGTA is passivation of PM, resulting in more “free” and active cationic AMPs. We speculate that endogenous molecules such as alveolar surfactant proteins and the airway surfactant palate, lung, nasal epithelium clone (PLUNC) protein (Bartlett et al. 2011; Schleh and Hohlfeld 2009) could function as passivators in the lung.
Another mechanism by which PM increases the susceptibility of the lungs to pathogens is by decreasing the gene expression and protein abundance of antimicrobial peptides such as HBD-2 and HBD-3 (Rivas-Santiago et al. 2015). However, this mechanism is unlikely in our model given the short-term exposure and assessment of antimicrobial activity.
It is also possible that bacteria interact with PM to increase infectivity. We have previously described that PM can be used as a source of iron to promote P. aeruginosa growth and biofilm formation (Borcherding et al. 2014; Borcherding et al. 2013). In the present study, we did not find an effect on bacterial growth because most of our experiments were performed before the generation time of S. aureus. In addition, it has been shown that PM can harbor bacterial DNA, varying from nonpathogenic soil bacteria to antibiotic-resistant bacteria (Cao et al. 2014; McEachran et al. 2015). However, it is not known whether airborne microbes attached to PM continue to be infectious. We presume that bacterial species capable of sporulation may have higher chances of using PM as a carrier to cause infection. Therefore, further studies are needed to elucidate whether PM increases the infectivity or virulence of pathogens.
One limitation of our study is the sole use of Gram-positive bacteria (S. aureus). However, the proposed interaction is likely reproducible in other pathogens because cationic AMPs are also effective against Gram-negative bacteria (Travis et al. 1999). Another limitation is that most of the experiments were performed with CFA, which does not necessarily model the carbonaceous components of other PMs. However, we used CFA as a model to demonstrate a mechanism that could be applicable to other PMs. We found that when we exposed urban and volcanic ash to lysozyme, they had similar zeta potential changes to those of CFA. Furthermore, given the highly heterogeneous nature of CFA with regard to its particle size, shape, and elemental content, it is important to emphasize that measurements such as zeta potential only represent an ensemble measurement of the average of many different particle types. Therefore, it is challenging to identify which physical and chemical elements in PM are necessary, sufficient, or both to cause the observed effect. Nevertheless, the airway is always exposed to highly complex particles and not to a single particle type. To elucidate a more precise mechanism of impairment, future investigation is warranted to dissect these complex particle–protein interactions. The findings of this study will merit further confirmation with epidemiological studies that correlate particle charge and protein adsorption with their impact on human health.
Conclusion
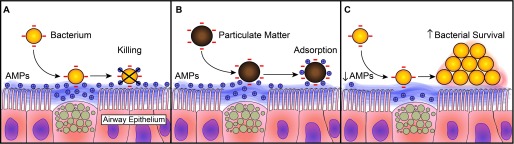
In summary, we propose that an acute exposure to ambient PM can impair airway antimicrobial activity by adsorption of cationic AMPs. This decrease of functional antimicrobial peptides will increase bacterial survival and potentially increase the risk of airway infections (Figure 5). Our results provide a novel translational insight into the initial mechanisms underlying ambient PM-induced respiratory infections.
Figure 5.
Proposed mechanism of particulate matter (PM) impairment of airway surface liquid (ASL) antimicrobial activity. (A) Airborne bacteria are killed by the ASL cationic antimicrobial proteins and peptides (AMPs). (B) Inhaled PM can adsorb cationic AMPs, creating a negatively charged particle–protein complex. (C) The airway, with a decreased concentration of functional AMPs, has an increased probability of bacterial survival.
Supplemental Material
Acknowledgments
We acknowledge P. Taft for the revision of the manuscript, M. Hoegger for providing the ex vivo pig ASL samples, L. Powers for processing the human airway surface liquid (ASL) samples, and D. Stoltz for his valuable suggestions. We also thank X. Li for his technical assistance in antimicrobial activity assays and P. Karp, at the University of Iowa In vitro Models and Cell Culture Core, for his assistance with cell cultures. M. Monick provided the samples of particulate matter collected from the Icelandic Eyjafjallajökull volcano eruption in 2010. The Iowa Donor Network and the Lung Transplant Team at the University of Iowa provided the human donor lungs.
This project was made possible by the Center for Health Effects of Environmental Contamination Seed Grants, FY 2010; the National Center for Research Resources, a part of the National Institutes of Health (NIH) (UL1RR024979, KO1HL080966 and P30 ES005605); the National Science Foundation (NSF 1424502/1640936), and the National Institute of Allergy and Infectious Diseases (NO1-AI-30040-01).
References
- Abou Alaiwa MH, Reznikov LR, Gansemer ND, Sheets KA, Horswill AR, Stoltz DA, et al. 2014. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc Natl Acad Sci USA 111:18703–18708, PMID: 25512526, 10.1073/pnas.1422091112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataniiazova RA. 2008. Impact of atmospheric pollution on preschool children's health [in Russian]. Gig Sanit (2):87–89. [PubMed] [Google Scholar]
- Bai Y, Liu S, Jiang P, Zhou L, Li J, Tang C, et al. 2009. Structure-dependent charge density as a determinant of antimicrobial activity of peptide analogues of defensin. Biochemistry 48:7229–7239, PMID: 19580334, 10.1021/bi900670d. [DOI] [PubMed] [Google Scholar]
- Bartlett JA, Gakhar L, Penterman J, Singh PK, Mallampalli RK, Porter E, et al. 2011. PLUNC: A multifunctional surfactant of the airways. Biochem Soc Trans 39:1012–1016, PMID: 21787339, 10.1042/BST0391012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum CB, Jany B, Finkbeiner WE. 1990. The serous cell. Annu Rev Physiol 52:97–113, PMID: 2158772, 10.1146/annurev.ph.52.030190.000525. [DOI] [PubMed] [Google Scholar]
- Borcherding J, Baltrusaitis J, Chen H, Stebounova L, Wu CM, Rubasinghege G, et al. 2014. Iron oxide nanoparticles induce pseudomonas aeruginosa growth, induce biofilm formation, and inhibit antimicrobial peptide function. Environ Sci: Nano 1:123–132, PMID: 25221673, 10.1039/c3en00029j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borcherding JA, Chen H, Caraballo JC, Baltrusaitis J, Pezzulo AA, Zabner J, et al. 2013. Coal fly ash impairs airway antimicrobial peptides and increases bacterial growth. PLoS One 8:e57673, PMID: 23469047, 10.1371/journal.pone.0057673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borm PJ. 1997. Toxicity and occupational health hazards of coal fly ash (CFA). A review of data and comparison to coal mine dust. Ann Occup Hyg 41:659–676, PMID: 9375525, 10.1016/S0003-4878(97)00026-4. [DOI] [PubMed] [Google Scholar]
- Bruce N, Perez-Padilla R, Albalak R. 2000. Indoor air pollution in developing countries: A major environmental and public health challenge. Bull World Health Organ 78:1078–1092, PMID: 11019457, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2560841/. [PMC free article] [PubMed] [Google Scholar]
- Camberlein E, Cohen JM, José R, Hyams CJ, Callard R, Chimalapati S, et al. 2015. Importance of bacterial replication and alveolar macrophage-independent clearance mechanisms during early lung infection with streptococcus pneumoniae. Infect Immun 83:1181–1189, PMID: 25583525, 10.1128/IAI.02788-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Jiang W, Wang B, Fang J, Lang J, Tian G, et al. 2014. Inhalable microorganisms in Beijing's PM2.5 and PM10 pollutants during a severe smog event. Environ Sci Technol 48:1499–1507, PMID: 24456276, 10.1021/es4048472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalfamo P, Di Pasquale S, Corigliano F, Mavilia L. 1997. Influence of the calcium content on the coal fly ash features in some innovative applications. Resour Conserv Recycl 20:119–125, 10.1016/S0921-3449(97)00013-X. [DOI] [Google Scholar]
- Chen H, Laskin A, Baltrusaitis J, Gorski CA, Scherer MM, Grassian VH. 2012. Coal fly ash as a source of iron in atmospheric dust. Environ Sci Technol 46:2112–2120, PMID: 22260270, 10.1021/es204102f. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ebenstein A, Greenstone M, Li H. 2013. Evidence on the impact of sustained exposure to air pollution on life expectancy from China's Huai River policy. Proc Natl Acad Sci USA 110:12936–12941, PMID: 23836630, 10.1073/pnas.1300018110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, et al. 2007. Renal clearance of quantum dots. Nat Biotechnol 25:1165–1170, PMID: 17891134, 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R, Severs R, Hardy R. 1977. Parallelism of atmospheric electrical potential and mortality in London air pollution episodes. Int J Biometeorol 21:311–318, PMID: 598944, 10.1007/BF01555190. [DOI] [PubMed] [Google Scholar]
- Ganz T. 2003. Defensins: Antimicrobial peptides of innate immunity. Nat Rev Immunol 3:710–720, PMID: 12949495, 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Gerke AK, Pezzulo AA, Tang F, Cavanaugh JE, Bair TB, Phillips E, et al. 2014. Effects of vitamin D supplementation on alveolar macrophage gene expression: Preliminary results of a randomized, controlled trial. Multidiscip Respir Med 9:18, PMID: 24669961, 10.1186/2049-6958-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieré R, Carleton LE, Lumpkin GR. 2003. Micro- and nanochemistry of fly ash from a coal-fired power plant. Am Mineral 88:1853–1865, 10.2138/am-2003-11-1228. [DOI] [Google Scholar]
- Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, et al. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359:753–759, PMID: 11888586, 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, et al. 2014. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 345:818–822, PMID: 25124441, 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SS, Platt R. 2003. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis 36:281–285, PMID: 12539068, 10.1086/345955. [DOI] [PubMed] [Google Scholar]
- Hunter RJ. 1981. Charge and potential distribution at interfaces. In: Zeta Potential in Colloid Science. London, UK:Academic Press, 11–58. [Google Scholar]
- International Energy Agency. 2013. Coal information. Paris, France:IEA. [Google Scholar]
- Jewell RB, Rathbone RF. 2009. Optical properties of coal combustion byproducts for particle-size analysis by laser diffraction. CCGP 1:1–7, 10.4177/CCGP-D-09-00001. [DOI] [Google Scholar]
- Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, et al. 2002. Epithelial Cell Culture Protocols. Wise C, ed Totowa, NJ: Humana Press, Inc. [Google Scholar]
- Kramar LV. 2002. Ecological ranging of the Volgograd territory on the basis of Staphylococcus aureus resident carrier state determination [in Russian]. Zh Mikrobiol Epidemiol Immunobiol (4):29.–; , PMID: 12449694. [PubMed] [Google Scholar]
- Lee CC, Sun Y, Qian S, Huang HW. 2011. Transmembrane pores formed by human antimicrobial peptide LL-37. Biophys J 100:1688–1696, PMID: 21463582, 10.1016/j.bpj.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshchuk SI, Popkova SM, Budnikova ZI, Ochirzhapova D. 2011. Enteric microbiocenosis in the population of an industrial city [in Russian]. Gigiena i Sanitariia 2011:31–35, PMID: 21598641. [PubMed] [Google Scholar]
- Lin J, Pan D, Davis SJ, Zhang Q, He K, Wang C, et al. 2014. China's international trade and air pollution in the United States. Proc Natl Acad Sci USA 111:1736–1741, PMID: 24449863, 10.1073/pnas.1312860111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre EA, Gehring U, Mölter A, Fuertes E, Klümper C, Krämer U, et al. 2014. Air pollution and respiratory infections during early childhood: An analysis of 10 European birth cohorts within the Escape Project. Environ Health Perspect 122:107–113, PMID: 24149084, 10.1289/ehp.1306755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachran AD, Blackwell BR, Hanson JD, Wooten KJ, Mayer GD, Cox SB, et al. 2015. Antibiotics, bacteria, and antibiotic resistance genes: Aerial transport from cattle feed yards via particulate matter. Environ Health Perspect 123:337–343, PMID: 25633846, 10.1289/ehp.1408555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monick MM, Baltrusaitis J, Powers LS, Borcherding JA, Caraballo JC, Mudunkotuwa I, et al. 2013. Effects of Eyjafjallajökull volcanic ash on innate immune system responses and bacterial growth in vitro. Environ Health Perspect 121:691–698, PMID: 23478268, 10.1289/ehp.1206004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurjadi D, Herrmann E, Hinderberger I, Zanger P. 2013. Impaired β-defensin expression in human skin links DEFB1 promoter polymorphisms with persistent Staphylococcus aureus nasal carriage. J Infect Dis 207:666–674, PMID: 23204181, 10.1093/infdis/jis735. [DOI] [PubMed] [Google Scholar]
- Pezzulo AA, Tang XX, Hoegger MJ, Abou Alaiwa MH, Ramachandran S, Moninger TO, et al. 2012. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487:109–113, PMID: 22763554, 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-Santiago CE, Sarkar S, Cantarella P 4th, Osornio-Vargas Á, Quintana-Belmares R, Meng Q, et al. 2015. Air pollution particulate matter alters antimycobacterial respiratory epithelium innate immunity. Infect Immun 83:2507–2517, PMID: 25847963, 10.1128/IAI.03018-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha LA, Marques Ribas R, da Costa Darini AL, Gontijo Filho PP. 2013. Relationship between nasal colonization and ventilator-associated pneumonia and the role of the environment in transmission of Staphylococcus aureus in intensive care units. Am J Infect Control 41:1236–1240, PMID: 23890377, 10.1016/j.ajic.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Rogers CS, Abraham WM, Brogden KA, Engelhardt JF, Fisher JT, McCray PB Jr, et al. 2008. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 295:L240–L263, PMID: 18487356, 10.1152/ajplung.90203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleh C, Hohlfeld JM. 2009. Interaction of nanoparticles with the pulmonary surfactant system. Inhal Toxicol 21(Suppl 1):97–103, PMID: 19558240, 10.1080/08958370903005744. [DOI] [PubMed] [Google Scholar]
- Shen M, Chapman RS, Vermeulen R, Tian L, Zheng T, Chen BE, et al. 2009. Coal use, stove improvement, and adult pneumonia mortality in Xuanwei, China: A retrospective cohort study. Environ Health Perspect 117:261–266, PMID: 19270797, 10.1289/ehp.11521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoluchowski M. 1921. Handbuch der Electrizität und des Magnetismus, Vol. II Graetz L, ed Leipzig, Germany:Barth. [Google Scholar]
- Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, et al. 2010. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med 2:29ra31, PMID: 20427821, 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesse R, Cardinale F, Santostasi T, Polizzi A, Manca A, Mappa L, et al. 2008. Association of β-defensin-1 gene polymorphisms with Pseudomonas aeruginosa airway colonization in cystic fibrosis. Genes Immun 9:57–60, PMID: 17960157, 10.1038/sj.gene.6364440. [DOI] [PubMed] [Google Scholar]
- Travis SM, Conway BA, Zabner J, Smith JJ, Anderson NN, Singh PK, et al. 1999. Activity of abundant antimicrobials of the human airway. Am J Respir Cell Mol Biol 20:872–879, PMID: 10226057, 10.1165/ajrcmb.20.5.3572. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA). 1998. Non-groundwater Pathways, Human Health and Ecological Risk Analysis for Fossil Fuel Combustion Phase 2 (FFC2).Washington, DC:U.S. EPA. [Google Scholar]
- Veronesi B, de Haar C, Lee L, Oortgiesen M. 2002. The surface charge of visible particulate matter predicts biological activation in human bronchial epithelial cells. Toxicol Appl Pharmacol 178:144–154, PMID: 11858730, 10.1006/taap.2001.9341. [DOI] [PubMed] [Google Scholar]
- Vertegel AA, Siegel RW, Dordick JS. 2004. Silica nanoparticle size influences the structure and enzymatic activity of adsorbed lysozyme. Langmuir 20:6800–6807, PMID: 15274588, 10.1021/la0497200. [DOI] [PubMed] [Google Scholar]
- Vogler EA. 2012. Protein adsorption in three dimensions. Biomaterials 33:1201–1237, PMID: 22088888, 10.1016/j.biomaterials.2011.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2014. Burden of disease from ambient air pollution for 2012. http://www.who.int/phe/health_topics/outdoorair/databases/FINAL_HAP_AAP_BoD_24March2014.pdf [accessed 18 July 2016].
- Williams REO. 1963. Healthy carriage of Staphylococcus aureus: Its prevalence and importance. Bacteriol Rev 27:56–71, PMID: 14000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žibret G, Van Tonder D, Žibret L. 2013. Metal content in street dust as a reflection of atmospheric dust emissions from coal power plants, metal smelters, and traffic. Environ Sci Pollut Res Int 20:4455–4468, PMID: 23247531, 10.1007/s11356-012-1398-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.