Abstract
Background:
Age-related macular degeneration (AMD) is a leading cause of blindness in developed countries. Few studies have investigated its relationship to environmental neurotoxicants. In previous cross-sectional studies, we found an association between pesticide use and self-reported retinal degeneration.
Objective:
We evaluated the association of pesticide use with physician-confirmed incident AMD.
Methods:
The Agricultural Health Study (AHS) is a prospective cohort of pesticide applicators and their spouses enrolled from 1993–1997 in Iowa and North Carolina. Cohort members reported lifetime use of 50 specific pesticides at enrollment. Self-reports of incident AMD during follow-up through 2007 were confirmed by reports from participants’ physicians and by independent evaluation of retinal photographs provided by the physicians. Confirmed cases () were compared with AHS cohort members without AMD (). We estimated odds ratios (ORs) and 95% confidence intervals (CIs) by logistic regression with adjustment for age, gender, and smoking.
Results:
AMD was associated with ever use of organochlorine [ (95% )] and organophosphate [ (95% CI: 1.3, 3.0)] insecticides and phenoxyacetate herbicides [ (95% )]. Specific pesticides consistently associated with AMD included chlordane, dichlorodiphenyltrichloroethane (DDT), malathion, and captan; others with notable but slightly less consistent associations were heptachlor, diazinon, phorate, 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), and 2,4-dichlorophenoxyacetic acid (2,4-D). Results were similar for men and women. Some specific pesticides were associated with both early- and late-stage AMD, but others were associated with only one stage.
Conclusions:
Exposures to specific pesticides may be modifiable risk factors for AMD. https://doi.org/10.1289/EHP793
Introduction
Age-related macular degeneration (AMD) is a degenerative condition of the central portion of the retina, the macula (Velez-Montoya et al. 2014). AMD is the leading cause of blindness in older individuals in developed countries, affecting million U.S. residents. The early stage of the disease is often asymptomatic, but late AMD, either geographic atrophy (“dry” AMD) or the neovascular form (“wet” AMD), results in the loss of central, high-acuity vision. Factors affecting risk of early AMD may differ from those affecting progression to late-stage disease (Evans and Lawrenson 2012a; Evans and Lawrenson 2012b).
Both genetic and environmental factors play a role in the etiology of AMD (Sobrin and Seddon 2014). AMD is associated with polymorphisms in approximately 20 genes, most notably complement factor H (CFH) (Sofat et al. 2012) and the age-related maculopathy susceptibility 2/HtrA serine peptidase (ARMS2/HTRA1) locus at chromosome 10q26 (Tong et al. 2010). Smoking is associated with increased risk of AMD, and adiposity may also be important (Chakravarthy et al. 2010). However, these factors do not explain all cases of AMD.
Limited evidence suggests an association of pesticide exposure with retinal dysfunction. Several case series reported signs of macular degeneration in pesticide workers (Dementi 1994; Misra et al. 1985), and experimental studies of rodents have shown biochemical, morphological, and functional changes in the retina after systemic (Imai et al. 1983) or intraocular (Zhang et al. 2006) treatment with pesticides. Nevertheless, few epidemiologic studies have addressed this issue. The Agricultural Health Study (AHS) is a study of licensed pesticide applicators and their spouses who have been followed since enrollment in the mid-1990s. In a cross-sectional analysis of AHS data collected at enrollment, we found that self-reported prevalent retinal or macular degeneration was associated with use of fungicides and organochlorine and organophosphate insecticides in pesticide applicators (Kamel et al. 2000) and with use of fungicides in AHS spouses (Kirrane et al. 2005).
The present study extends these findings. We exploited the prospective design of the AHS to evaluate the association of pesticide use with medically confirmed incident cases of AMD, thus overcoming some limitations of our previous studies.
Methods
Population
The AHS cohort includes 52,394 private pesticide applicators (mostly farmers) and 32,345 of their spouses enrolled between 1993 and 1997 in Iowa and North Carolina. Most applicators were men (97%), most spouses were women (99%), and the race/ethnicity of most cohort members was non-Hispanic white (97%). At enrollment, participants completed self-administered questionnaires that collected information on demographics, lifestyle characteristics, medical history, lifetime pesticide use, and other farming practices. Follow-up telephone interviews were conducted in 1999–2003 and 2005–2010.
To investigate the relationship of pesticide use to AMD incidence, we conducted a case–control study nested within the AHS cohort. We used information from the two follow-up interviews to identify potential incident cases through 1 September 2007. Among 84,739 AHS cohort members, 6 had requested no further contact, 26,002 had not completed either follow-up interview, and 2,554 had died. We also excluded 13,975 persons who were y old on 1 September 2007 because AMD is rare before that age, 324 who had reported retinal or macular degeneration at enrollment, and 15 for other reasons. Thus, 41,863 cohort members were eligible to participate.
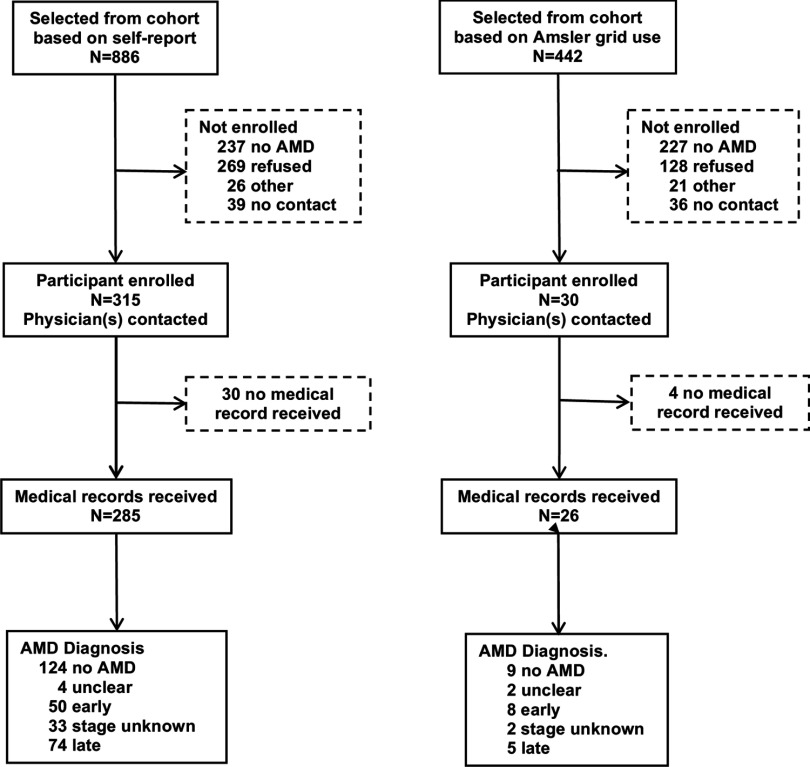
Medical histories collected in the follow-up interviews included self-report of physician-diagnosed retinal or macular degeneration. We verified self-reports using information from participants’ eye-care physicians (Figure 1). We screened 552 of 886 participants who reported AMD at either follow-up; of these, 315 affirmed their diagnosis and provided permission for retrieval of medical records. In addition, we screened 257 of 442 AHS cohort members who, although not reporting AMD, did report using an Amsler grid, a self-test of vision loss sometimes recommended for patients with early signs of AMD; of these, 30 affirmed a diagnosis of AMD and provided permission for retrieval of medical records. We contacted one or more physicians for 345 potential AMD cases and obtained diagnostic information for 311 study participants. Physicians either completed a short questionnaire on AMD diagnosis, retinal pathology, and treatment or provided relevant medical records. In addition, some physicians provided retinal photographs for one or both eyes for 101 participants. An optometrist abstracted medical records, and the study ophthalmologist (E.P.) evaluated retinal photographs.
Figure 1.
Recruitment and validation of AMD cases.
Case Definition
Cases were participants for whom the treating physician confirmed the diagnosis of AMD with supporting pathology or for whom the study ophthalmologist diagnosed AMD from retinal photographs. Early AMD was defined by the presence of large, soft, or confluent drusen, with or without pigmentary changes. Individuals with physician reports indicating small, hard drusen as the only sign of AMD were not included as cases unless the retinal photograph provided additional evidence to support the diagnosis. Late AMD was defined by the presence of at least one of the following pathological signs: geographic atrophy, disciform scar, retinal pigment epithelial detachment, and subretinal hemorrhage. AMD unknown stage was assigned when both the physician report and the retinal photograph indicated AMD but disagreed regarding stage. Overall, there was 76% agreement between diagnoses of AMD assigned with or without the photographs; the major difference was the identification of 19 additional early cases using supporting pathology from photographs. We used the more severe diagnosis from either eye to assign a diagnosis to the participant. The final assignments were unclear diagnosis (6), no AMD (133), AMD early stage (58), AMD late stage (79), and AMD unknown stage (35), with a total of 172 AMD cases of any stage.
The controls were AHS cohort members remaining after excluding both the 172 AMD cases and 1,156 individuals identified as possible cases but not confirmed. We also excluded from analysis 10 cases whose physician-reported diagnoses occurred before enrollment in the AHS and 1 case and 1,427 controls with incomplete smoking data. The final sample included 161 incident AMD cases diagnosed between 1994 and 2007 and 39,108 controls.
Institutional review boards of the National Institutes of Health and its contractors approved the study, and all participants signified consent by completing questionnaires; written consent was obtained for release of medical records.
Pesticide Exposure
We used information on ever use of 50 specific pesticides provided by AHS cohort members at enrollment. We also combined information on specific pesticides to create four categories based on function (insecticides, herbicides, fungicides, and fumigants), three chemical classes of insecticides (organochlorines, organophosphates, and carbamates), and two chemical classes of herbicides (phenoxyacetate and triazine herbicides).
Pesticide applicators, but not spouses, provided additional information on the duration (years) and frequency (days per year) of use for 22 individual pesticides. Some applicators (53% of controls and 72% of cases included in this analysis) also completed a second questionnaire, providing information on the frequency and duration of use for 28 additional pesticides. We multiplied frequency and duration to calculate lifetime days of use for each specific pesticide and categorized the results (0, 0.01–10, 10.01–100, and ), combining categories for some pesticides with sparse data. Because of potential overlap in periods of use, we did not calculate lifetime days of use for pesticide groups.
Data Analysis
We examined the association of AMD with pesticide exposure using multivariable logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs). We included age on 1 September 2007 (50–69, 70–79, ), gender, and smoking at enrollment (ever/never) in models because all are important risk factors for AMD and are associated with pesticide use. Adjustment for age using a six-level categorical variable, continuous age, or continuous age plus age squared gave estimates virtually identical to those using the three-level categorical variable. We also evaluated associations in models stratified by age (); these models were further adjusted for age with a continuous variable. Adjusting for smoking status (never, former, or current smoking) gave results similar to those using smoking as a binary variable. We considered body mass index, education, and study site (North Carolina or Iowa) as potential confounders; none of these factors substantially altered effect estimates (), so our final models included only age, gender, and smoking. Because the results were generally similar for men and women when analyzed separately, we present the results for men and women together (adjusted for gender) as our main analysis. In additional analyses, we restricted cases to individuals diagnosed with either early- or late-stage AMD (excluding those of unknown stage) and used multinomial logistic regression to compare each case group with controls. Because sun exposure may increase AMD risk, we evaluated AMD–pesticide associations in models including a variable for hours per day of sun exposure ().
We evaluated the association of AMD with ever use of 47 pesticides for which at least five cases reported use. We also examined associations with lifetime days of use for 38 pesticides for which users could be categorized into at least two groups with at least five cases in each; these analyses were restricted to men because few women were applicators and because spouses were not asked to provide data on frequency or duration of pesticide use. We evaluated correlations of ever-use variables between pairs of pesticides. Whenever the correlation coefficient for a pair was and at least one member of the pair was associated with AMD risk, we ran an additional model including both pesticides.
To address concerns regarding possible selection bias, we conducted a quantitative bias analysis (Lash et al 2009) (for details, see Supplemental Material, “Quantitative Bias Analysis”). Briefly, individuals who were not screened were allocated to AMD case or control status based on covariate distribution and pesticide use. We then estimated ORs associated with pesticide exposure among the “complete” set of cases and controls. Separate analyses were performed for each pesticide.
We used SAS (version 9.2; SAS Institute Inc.) and data from AHS data releases REL201004.00, P2REL0506.03, P1REL0506.01, P2REL0506.03, and P3REL0707.01 (https://aghealth.nih.gov/) in our analyses.
Results
We attempted to screen 1,328 potential cases, individuals identified from the AHS cohort because they reported a diagnosis of AMD or use of an Amsler grid (Figure 1). Among those screened, 43% (237/552) of those initially reporting AMD and 88% (227/257) reporting Amsler grid use denied AMD. If all potential cases had been screened and similar proportions had denied AMD, we project that a total of 557 would have affirmed AMD. We enrolled 345 cases (62% of the projected 557), and approximately half of these were confirmed after evaluation of medical records. Comparing potential cases () with those included in the analysis (; AMD confirmed, diagnosis after enrollment, smoking data available), we found that the latter were older and more likely to be ever smokers (data not shown); they were also considerably more likely to have used pesticides lifetime days (66% and 74%, respectively) and to have ever used organochlorines (50% and 62%, respectively).
AMD risk was positively associated with age and smoking and was slightly elevated among women, those with more than a high school education, and those who consumed alcohol more frequently; AMD was not related to race/ethnicity, state, or body mass index (Table 1). Both early AMD (57 cases) and late AMD (72 cases) were associated with age and smoking; late AMD was also associated with residence in North Carolina and having more than a high school education (see Table S1). Comparing case groups with one another, late AMD cases were slightly older (, ) and more likely to be from North Carolina (, ) than early cases; other characteristics were similar.
Table 1.
Characteristics of incident AMD cases and controls among pesticide applicators and their spouses, AHS 1993-2007.
| Characteristic | Case | Control | ORa | 95% CI | |||
|---|---|---|---|---|---|---|---|
| % | % | ||||||
| Age at enrollment in AMD study, y | |||||||
| 50–69 | 36 | 22 | 28,801 | 74 | 1.0 | Reference | |
| 70–79 | 82 | 51 | 8,286 | 21 | 7.9 | 5.3 | 11.7 |
| 43 | 27 | 2,021 | 5 | 17.5 | 11.2 | 27.3 | |
| Gender | |||||||
| Men | 95 | 59 | 22,658 | 58 | 1.0 | Reference | |
| Women | 66 | 41 | 16,450 | 42 | 1.3 | 0.9 | 1.8 |
| Race/ethnicity | |||||||
| White, non-Hispanic | 158 | 98 | 37,984 | 97 | 1.0 | Reference | |
| Other | 3 | 2 | 1,124 | 3 | 0.5 | 0.2 | 1.6 |
| State | |||||||
| Iowa | 95 | 59 | 25,612 | 65 | 1.0 | Reference | |
| North Carolina | 66 | 41 | 13,496 | 35 | 0.9 | 0.7 | 1.3 |
| Education | |||||||
| 92 | 59 | 19,877 | 54 | 1.0 | Reference | ||
| 64 | 41 | 17,046 | 46 | 1.3 | 0.9 | 1.8 | |
| Ever smoker | |||||||
| No | 75 | 47 | 23,292 | 60 | 1.0 | Reference | |
| Yes | 86 | 53 | 15,816 | 40 | 1.8 | 1.3 | 2.5 |
| Alcohol consumption (frequency) | |||||||
| Never | 77 | 49 | 16,100 | 42 | 1.0 | Reference | |
| to 3 times per mo | 41 | 26 | 13,471 | 35 | 1.0 | 0.7 | 1.4 |
| Once a week or more | 38 | 24 | 8,530 | 22 | 1.3 | 0.9 | 2.0 |
| BMI () | |||||||
| 54 | 34 | 12,123 | 32 | 1.0 | Reference | ||
| 25–30 | 78 | 50 | 17,139 | 45 | 1.0 | 0.7 | 1.5 |
| 25 | 16 | 87,98 | 23 | 0.7 | 0.5 | 1.2 | |
Note: AMD, age-related macular degeneration; BMI, body mass index; CI, confidence interval; OR, odds ratio.
All models include age, gender, and smoking.
AMD risk was elevated among ever users of insecticides and fungicides as classes but not among ever users of herbicides or fumigants (Table 2). Among chemical classes, AMD was associated with organochlorine and organophosphate insecticides and with phenoxyacetate herbicides. Specific organochlorines significantly associated with AMD were aldrin, chlordane, dichlorodiphenyltrichloroethane (DDT), dieldrin, heptachlor, and lindane; specific organophosphates significantly associated with AMD were diazinon, dichlorvos, malathion, parathion, and phorate; specific phenoxyacetate herbicides significantly associated with AMD were 2,4-dichlorophenoxyacetic acid (2,4-D), 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), and 2-propionic acid (fenoprop; 2,4,5-TP); specific chemicals in other classes significantly associated with AMD were the insecticide permethrin used on crops; the herbicide glyphosate; the fungicides benomyl and captan; and the fumigant ethylene dibromide. Adjustment for sun exposure () had no effect on the AMD–pesticide association.
Table 2.
Incident AMD and ever use of specific pesticides in pesticide applicators and their spouses, AHS 1993-2007.
| Case | Control | ORa | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| % | % | ||||||
| Insecticides (any) | 126 | 78 | 28558 | 73 | 1.6 | 1.1 | 2.5 |
| Organochlorines (any) | 98 | 62 | 14870 | 38 | 2.7 | 1.8 | 4.0 |
| Aldrin | 37 | 26 | 5433 | 15 | 1.5 | 0.95 | 2.3 |
| Chlordane | 61 | 42 | 7696 | 21 | 2.4 | 1.7 | 3.6 |
| DDT | 70 | 47 | 7728 | 21 | 2.1 | 1.4 | 3.1 |
| Dieldrin | 21 | 15 | 1960 | 5 | 1.9 | 1.1 | 3.2 |
| Heptachlor | 36 | 26 | 4490 | 12 | 1.9 | 1.2 | 3.0 |
| Lindane | 32 | 22 | 5148 | 14 | 1.9 | 1.2 | 3.0 |
| Toxaphene | 27 | 19 | 3957 | 11 | 1.5 | 0.9 | 2.3 |
| Organophosphates (any) | 117 | 73 | 25157 | 64 | 2.0 | 1.3 | 3.0 |
| Chlorpyrifos | 45 | 29 | 10522 | 27 | 1.3 | 0.9 | 1.9 |
| Coumaphos | 10 | 7 | 2302 | 6 | 1.1 | 0.6 | 2.2 |
| Diazinon | 58 | 40 | 9404 | 26 | 2.0 | 1.4 | 2.9 |
| Dichlorvos | 18 | 12 | 3121 | 9 | 1.8 | 1.1 | 3.0 |
| Fonofos | 20 | 14 | 5463 | 15 | 1.0 | 0.6 | 1.7 |
| Malathion | 103 | 68 | 19889 | 53 | 2.2 | 1.5 | 3.3 |
| Parathion | 28 | 20 | 3877 | 11 | 1.9 | 1.2 | 3.0 |
| Phorate | 42 | 30 | 8070 | 22 | 1.7 | 1.1 | 2.6 |
| Terbufos | 35 | 24 | 9210 | 25 | 1.1 | 0.7 | 1.7 |
| Other insecticides | |||||||
| Aldicarb | 5 | 4 | 2511 | 7 | 0.5 | 0.2 | 1.3 |
| Carbaryl | 91 | 61 | 18890 | 51 | 1.4 | 0.99 | 2.0 |
| Carbofuran | 31 | 21 | 7231 | 20 | 1.1 | 0.7 | 1.7 |
| Permethrin (crops) | 16 | 11 | 3197 | 9 | 1.8 | 1.03 | 3.0 |
| Permethrin (animals) | 11 | 8 | 3541 | 10 | 1.3 | 0.7 | 2.4 |
| Herbicides (any) | 119 | 74 | 28973 | 74 | 1.2 | 0.8 | 1.9 |
| Phenoxyacetate (any) | 101 | 64 | 21222 | 55 | 1.9 | 1.2 | 2.8 |
| 2,4,5-T | 46 | 32 | 5841 | 16 | 2.0 | 1.3 | 3.0 |
| 2,4,5-TP | 18 | 13 | 2390 | 7 | 1.7 | 1.03 | 2.9 |
| 2,4-D | 98 | 62 | 20689 | 54 | 1.8 | 1.2 | 2.7 |
| Triazine (any) | 79 | 50 | 19414 | 50 | 1.2 | 0.7 | 1.8 |
| Atrazine | 74 | 47 | 17889 | 47 | 1.2 | 0.8 | 1.8 |
| Cyanazine | 41 | 28 | 10175 | 28 | 1.3 | 0.9 | 2.0 |
| Metribuzin | 40 | 28 | 10756 | 30 | 1.2 | 0.8 | 1.9 |
| Other herbicides | |||||||
| Alachlor | 59 | 39 | 13154 | 36 | 1.4 | 0.9 | 2.1 |
| Butylate | 30 | 21 | 7886 | 22 | 1.2 | 0.7 | 1.8 |
| Chlorimuron ethyl | 28 | 20 | 7887 | 22 | 1.2 | 0.7 | 1.9 |
| Dicamba | 44 | 30 | 12012 | 33 | 1.1 | 0.7 | 1.7 |
| EPTC | 17 | 12 | 4618 | 13 | 1.2 | 0.7 | 2.0 |
| Glyphosate | 103 | 64 | 23493 | 61 | 1.4 | 0.99 | 2.0 |
| Imazethapyr | 32 | 23 | 9503 | 26 | 1.1 | 0.7 | 1.8 |
| Metolachlor | 41 | 28 | 10728 | 29 | 1.2 | 0.8 | 1.8 |
| Paraquat | 30 | 20 | 5542 | 15 | 1.5 | 0.9 | 2.3 |
| Pendimethalin | 31 | 22 | 9912 | 27 | 0.9 | 0.6 | 1.4 |
| Petroleum oil | 39 | 27 | 11165 | 31 | 0.9 | 0.6 | 1.4 |
| Trifluralin | 51 | 36 | 12775 | 35 | 1.2 | 0.8 | 1.9 |
| Fungicides (any) | 51 | 32 | 9337 | 24 | 1.5 | 1.04 | 2.1 |
| Benomyl | 18 | 12 | 2548 | 7 | 1.7 | 0.99 | 2.8 |
| Captan | 20 | 14 | 3009 | 8 | 2.0 | 1.2 | 3.3 |
| Chlorothalonil | 11 | 7 | 1965 | 5 | 1.2 | 0.7 | 2.3 |
| Maneb | 13 | 9 | 2627 | 7 | 1.1 | 0.6 | 2.0 |
| Metalaxyl | 24 | 17 | 5459 | 15 | 1.1 | 0.7 | 1.8 |
| Fumigants (any) | 28 | 18 | 6032 | 16 | 1.0 | 0.6 | 1.5 |
| Carbon tetrachloride | 11 | 8 | 1542 | 4 | 1.4 | 0.7 | 2.6 |
| Ethylene dibromide | 10 | 7 | 884 | 2 | 2.8 | 1.5 | 5.6 |
| Methyl bromide | 17 | 11 | 4106 | 11 | 0.8 | 0.5 | 1.4 |
Note: AHS, Agricultrual Health Study; AMD, age-related macular degeneration; BMI, body mass index; CI, confidence interval; DDT, dichlorodiphenyltrichloroethane; EPTC, S-ethyl dipropylthiocarbamate; OR, odds ratio. 2,4-D, 2,4-dichlorophenoxyacetic acid; 2,4,5-T, 2,4,5-trichlorophenoxyacetic acid; 2,4,5,-TP, 2-propionic acid (fenoprop).
Adjusted for age, gender, and smoking.
In gender-stratified analyses, the results were generally similar for men (95 cases) and women (66 cases), although many chemicals were used by too few women to permit analysis (see Table S2). Only organochlorines as a class and the specific chemicals chlordane and malathion were significantly associated with AMD risk in both early and late AMD (see Table S3). Several other exposures—insecticides and organophosphates as classes, DDT, 2,4,5-T, and ethylene dibromide—were associated with both subtypes () but significantly so only in one subtype. Associations were weaker for late than for early AMD for organochlorines and phenoxyacetate and triazine herbicides as classes and for aldrin, dieldrin, 2,4-D, cyanazine, butylate, and metolachlor (). Associations were stronger for late than for early AMD for paraquat, petroleum oil, and benomyl.
In models stratified by age (), ORs for most pesticides were similarly elevated in both age groups, and most age-by-pesticide interactions were unimportant (; data not shown). The exception was phenoxyacetate herbicides: as a group, these pesticides were associated with AMD in those [ (95% )] but not in those [ (95% )]; .
Most pesticides were not strongly correlated with one another. We constructed models including each correlated pair (), one pair per model (see Table S4). The results suggested that some pesticides (chlordane, DDT, heptachlor, diazinon, malathion, parathion, 2,4,5-T) had strong independent effects: their associations with AMD persisted in models including other pesticides. Others (aldrin, toxaphene, carbaryl, 2,4,5-TP, glyphosate, paraquat, benomyl) did not have independent effects: when modeled with other pesticides, their associations with AMD became weaker and nonsignificant. Some (dieldrin, lindane, phorate, 2,4-D) were intermediate, affected by modeling with some pesticides but not with others. The remainder were not correlated with other pesticides; therefore, their effects were presumed to be independent.
Information on lifetime days of use was available for 38 pesticides for applicators but not spouses (Table 3). Because the data were sparse, we considered a trend to be notable if . Such trends were observed for the insecticides chlordane, DDT, lindane, malathion, parathion, and phorate; the herbicides 2,4-D, alachlor, and glyphosate; and the fungicides captan and maneb/mancozeb.
Table 3.
Dose–response trends for pesticide use and risk of incident AMD among male pesticide applicators, AHS 1993-2007.
| Cumulative days of use | Case | Control | ORa | 95% CI | p-Value for trend | |||
|---|---|---|---|---|---|---|---|---|
| % | % | |||||||
| Organochlorine insecticides | ||||||||
| Aldrinb | ||||||||
| 0 | 43 | 67 | 8,950 | 79 | 1.0 | Reference | ||
| 8 | 13 | 1,107 | 10 | 1.0 | 0.5 | 2.2 | ||
| 8 | 13 | 1,060 | 9 | 1.0 | 0.5 | 2.1 | ||
| 5 | 8 | 211 | 2 | 3.0 | 1.1 | 7.6 | 0.233 | |
| Chlordaneb | ||||||||
| 0 | 43 | 65 | 8,771 | 77 | 1.0 | Reference | ||
| 9 | 14 | 1,734 | 15 | 0.8 | 0.4 | 1.7 | ||
| 14 | 21 | 834 | 7 | 2.4 | 1.3 | 4.5 | 0.025 | |
| DDTb | ||||||||
| 0 | 30 | 45 | 8,308 | 73 | 1.0 | Reference | ||
| 9 | 14 | 1,396 | 12 | 0.9 | 0.4 | 1.8 | ||
| 19 | 29 | 1,033 | 9 | 2.3 | 1.3 | 4.2 | ||
| 8 | 12 | 575 | 5 | 1.9 | 0.8 | 4.2 | 0.011 | |
| Heptachlorb | ||||||||
| 0 | 54 | 81 | 9,708 | 85 | 1.0 | Reference | ||
| 4 | 6 | 885 | 8 | 0.6 | 0.2 | 1.6 | ||
| 9 | 13 | 819 | 7 | 1.4 | 0.7 | 2.8 | 0.670 | |
| Lindaneb | ||||||||
| 0 | 49 | 74 | 9,535 | 84 | 1.0 | Reference | ||
| 5 | 8 | 817 | 7 | 1.2 | 0.5 | 3.0 | ||
| 7 | 11 | 683 | 6 | 1.9 | 0.9 | 4.3 | ||
| 5 | 8 | 290 | 3 | 3.5 | 1.4 | 9.0 | 0.005 | |
| Toxapheneb | ||||||||
| 0 | 54 | 81 | 9,876 | 87 | 1.0 | Reference | ||
| 6 | 9 | 786 | 7 | 1.1 | 0.5 | 2.6 | ||
| 7 | 10 | 750 | 7 | 1.3 | 0.6 | 2.8 | 0.527 | |
| Organophosphate insecticides | ||||||||
| Chlorpyrifos | ||||||||
| 0 | 52 | 57 | 12,679 | 57 | 1.0 | Reference | ||
| 15 | 16 | 3,137 | 14 | 1.3 | 0.7 | 2.3 | ||
| 15 | 16 | 4,287 | 19 | 1.1 | 0.6 | 1.9 | ||
| 10 | 11 | 2,081 | 9 | 1.7 | 0.8 | 3.3 | 0.224 | |
| Diazinonb | ||||||||
| 0 | 46 | 71 | 8,782 | 78 | 1.0 | Reference | ||
| 7 | 11 | 1,196 | 11 | 1.1 | 0.5 | 2.5 | ||
| 12 | 18 | 1,326 | 12 | 1.6 | 0.8 | 3.0 | 0.174 | |
| Dichlorvos | ||||||||
| 0 | 70 | 86 | 17,938 | 88 | 1.0 | Reference | ||
| 5 | 6 | 736 | 4 | 1.9 | 0.8 | 4.8 | ||
| 6 | 7 | 1,779 | 9 | 1.1 | 0.5 | 2.5 | 0.558 | |
| Fonofos | ||||||||
| 0 | 63 | 77 | 15,648 | 76 | 1.0 | Reference | ||
| 4 | 5 | 1,500 | 7 | 0.8 | 0.3 | 2.2 | ||
| 15 | 18 | 3,451 | 17 | 1.2 | 0.7 | 2.2 | 0.522 | |
| Malathionb | ||||||||
| 0 | 21 | 32 | 3,720 | 33 | 1.0 | Reference | ||
| 12 | 18 | 2,961 | 26 | 0.8 | 0.4 | 1.5 | ||
| 15 | 23 | 3,247 | 29 | 0.9 | 0.4 | 1.7 | ||
| 17 | 26 | 1,352 | 12 | 2.0 | 1.1 | 3.9 | 0.093 | |
| Parathionb | ||||||||
| 0 | 52 | 81 | 10,353 | 92 | 1.0 | Reference | ||
| 8 | 13 | 403 | 4 | 3.3 | 1.6 | 7.1 | ||
| 4 | 6 | 548 | 5 | 1.3 | 0.5 | 3.8 | 0.087 | |
| Phorateb | ||||||||
| 0 | 39 | 61 | 7,570 | 67 | 1.0 | Reference | ||
| 6 | 9 | 1,552 | 14 | 0.8 | 0.3 | 1.9 | ||
| 9 | 14 | 1,664 | 15 | 1.0 | 0.5 | 2.2 | ||
| 10 | 16 | 557 | 5 | 3.5 | 1.7 | 7.2 | 0.020 | |
| Terbufos | ||||||||
| 0 | 49 | 61 | 12,154 | 59 | 1.0 | Reference | ||
| 4 | 5 | 2,047 | 10 | 0.6 | 0.2 | 1.6 | ||
| 27 | 34 | 6,371 | 31 | 1.3 | 0.8 | 2.0 | 0.394 | |
| Other insecticides | ||||||||
| Carbofuran | ||||||||
| 0 | 54 | 67 | 13,797 | 68 | 1.0 | Reference | ||
| 8 | 10 | 2,614 | 13 | 0.7 | 0.3 | 1.5 | ||
| 13 | 16 | 2,857 | 14 | 1.1 | 0.6 | 2.1 | ||
| 6 | 7 | 1,121 | 5 | 1.5 | 0.6 | 3.5 | 0.463 | |
| Carbarylb | ||||||||
| 0 | 27 | 40 | 6,178 | 55 | 1.0 | Reference | ||
| 15 | 22 | 2,042 | 18 | 1.5 | 0.8 | 2.9 | ||
| 11 | 16 | 1,728 | 15 | 1.1 | 0.5 | 2.3 | ||
| 15 | 22 | 1,355 | 12 | 1.9 | 1.0 | 3.6 | 0.101 | |
| Permethrin (crops) | ||||||||
| 0 | 67 | 84 | 17,630 | 87 | 1.0 | Reference | ||
| 8 | 10 | 1,394 | 7 | 2.1 | 0.99 | 4.4 | ||
| 5 | 6 | 1,287 | 6 | 1.4 | 0.6 | 3.5 | 0.150 | |
| Herbicides | ||||||||
| 2,4-D | ||||||||
| 0 | 13 | 14 | 4,580 | 21 | 1.0 | Reference | ||
| 10 | 11 | 2,502 | 11 | 1.4 | 0.6 | 3.2 | ||
| 26 | 28 | 6,877 | 31 | 1.4 | 0.7 | 2.8 | ||
| 44 | 47 | 8,116 | 37 | 2.2 | 1.2 | 4.1 | 0.011 | |
| 2,4,5,Tb | ||||||||
| 0 | 44 | 69 | 8,833 | 78 | 1.0 | Reference | ||
| 10 | 16 | 1,430 | 13 | 1.0 | 0.5 | 2.0 | ||
| 10 | 16 | 1,116 | 10 | 1.2 | 0.6 | 2.4 | 0.635 | |
| Alachlor | ||||||||
| 0 | 30 | 36 | 8,622 | 42 | 1.0 | Reference | ||
| 12 | 14 | 2,633 | 13 | 1.4 | 0.7 | 2.8 | ||
| 19 | 23 | 5,025 | 25 | 1.2 | 0.7 | 2.2 | ||
| 22 | 27 | 4,176 | 20 | 1.9 | 1.1 | 3.3 | 0.046 | |
| Atrazine | ||||||||
| 0 | 24 | 26 | 5,566 | 25 | 1.0 | Reference | ||
| 8 | 9 | 2,686 | 12 | 0.8 | 0.4 | 1.9 | ||
| 28 | 30 | 6,561 | 30 | 1.2 | 0.7 | 2.1 | ||
| 34 | 36 | 7,387 | 33 | 1.5 | 0.9 | 2.5 | 0.111 | |
| Butylateb | ||||||||
| 0 | 49 | 74 | 7,968 | 70 | 1.0 | Reference | ||
| 5 | 8 | 1,177 | 10 | 0.8 | 0.3 | 2.0 | ||
| 12 | 18 | 2,246 | 20 | 1.2 | 0.6 | 2.3 | 0.668 | |
| Chlorimuron ethylb | ||||||||
| 0 | 47 | 71 | 7,907 | 69 | 1.0 | Reference | ||
| 12 | 18 | 2,274 | 20 | 1.2 | 0.6 | 2.2 | ||
| 7 | 11 | 1,240 | 11 | 1.2 | 0.5 | 2.6 | 0.633 | |
| Cyanazine | ||||||||
| 0 | 41 | 52 | 11,217 | 55 | 1.0 | Reference | ||
| 11 | 14 | 2,816 | 14 | 1.3 | 0.6 | 2.5 | ||
| 17 | 22 | 3,986 | 19 | 1.4 | 0.8 | 2.5 | ||
| 10 | 13 | 2,556 | 12 | 1.5 | 0.8 | 3.1 | 0.141 | |
| Dicamba | ||||||||
| 0 | 39 | 49 | 9,454 | 46 | 1.0 | Reference | ||
| 10 | 13 | 3,089 | 15 | 1.0 | 0.5 | 2.0 | ||
| 16 | 20 | 4,934 | 24 | 1.1 | 0.6 | 1.9 | ||
| 15 | 19 | 2,887 | 14 | 1.9 | 1.03 | 3.5 | 0.112 | |
| EPTC | ||||||||
| 0 | 65 | 82 | 16,124 | 79 | 1.0 | Reference | ||
| 7 | 9 | 1,902 | 9 | 1.1 | 0.5 | 2.5 | ||
| 7 | 9 | 2,284 | 11 | 1.0 | 0.5 | 2.2 | 0.917 | |
| Glyphosate | ||||||||
| 0 | 15 | 16 | 5,104 | 23 | 1.0 | Reference | ||
| 18 | 19 | 4,929 | 22 | 1.3 | 0.6 | 2.5 | ||
| 33 | 35 | 7,403 | 33 | 1.7 | 0.9 | 3.1 | ||
| 28 | 30 | 4,783 | 22 | 2.6 | 1.4 | 4.9 | 0.002 | |
| Imazethapyr | ||||||||
| 0 | 45 | 59 | 11,670 | 57 | 1.0 | Reference | ||
| 13 | 17 | 3,957 | 19 | 1.1 | 0.6 | 2.1 | ||
| 18 | 24 | 4,748 | 23 | 1.5 | 0.9 | 2.6 | 0.167 | |
| Metolachlor | ||||||||
| 0 | 41 | 53 | 10,720 | 52 | 1.0 | Reference | ||
| 10 | 13 | 2,435 | 12 | 1.2 | 0.6 | 2.5 | ||
| 13 | 17 | 4,275 | 21 | 1.0 | 0.5 | 1.8 | ||
| 13 | 17 | 3,078 | 15 | 1.5 | 0.8 | 2.9 | 0.325 | |
| Metribuzinb | ||||||||
| 0 | 40 | 60 | 6,746 | 59 | 1.0 | Reference | ||
| 13 | 19 | 2,253 | 20 | 1.2 | 0.6 | 2.3 | ||
| 14 | 21 | 2,389 | 21 | 1.4 | 0.7 | 2.6 | 0.282 | |
| Paraquatb | ||||||||
| 0 | 53 | 80 | 9,560 | 84 | 1.0 | Reference | ||
| 7 | 11 | 1,032 | 9 | 1.2 | 0.5 | 2.6 | ||
| 6 | 9 | 829 | 7 | 1.4 | 0.6 | 3.2 | 0.413 | |
| Pendimethalinb | ||||||||
| 0 | 42 | 66 | 7,250 | 64 | 1.0 | Reference | ||
| 14 | 22 | 1,924 | 17 | 1.4 | 0.8 | 2.6 | ||
| 8 | 13 | 2,239 | 20 | 0.8 | 0.4 | 1.8 | 0.980 | |
| Petrolium oilb | ||||||||
| 0 | 48 | 79 | 8,894 | 78 | 1.0 | Reference | ||
| 7 | 11 | 1,771 | 16 | 0.8 | 0.4 | 1.8 | ||
| 6 | 10 | 679 | 6 | 1.9 | 0.8 | 4.5 | 0.370 | |
| Trifluralin | ||||||||
| 0 | 35 | 45 | 9,185 | 45 | 1.0 | Reference | ||
| 9 | 12 | 1,813 | 9 | 1.3 | 0.6 | 2.7 | ||
| 15 | 19 | 4,862 | 24 | 0.9 | 0.5 | 1.7 | ||
| 19 | 24 | 4,657 | 23 | 1.4 | 0.8 | 2.5 | 0.386 | |
| Fungicides | ||||||||
| Captan | ||||||||
| 0 | 66 | 81 | 17,971 | 89 | 1.0 | Reference | ||
| 7 | 9 | 1,436 | 7 | 1.8 | 0.8 | 3.9 | ||
| 8 | 10 | 677 | 3 | 2.9 | 1.4 | 6.2 | 0.002 | |
| Manebb | ||||||||
| 0 | 56 | 84 | 10,447 | 92 | 1.0 | Reference | ||
| 7 | 10 | 694 | 6 | 1.7 | 0.8 | 3.7 | ||
| 4 | 6 | 239 | 2 | 2.5 | 0.9 | 6.9 | 0.039 | |
| Metalaxylb | ||||||||
| 0 | 54 | 82 | 9,216 | 81 | 1.0 | Reference | ||
| 5 | 8 | 723 | 6 | 1.2 | 0.5 | 3.0 | ||
| 7 | 11 | 1,394 | 12 | 0.9 | 0.4 | 1.9 | 0.822 | |
| Fumigants | ||||||||
| Methyl bromide | ||||||||
| 0 | 77 | 82 | 18,556 | 84 | 1.0 | Reference | ||
| 6 | 6 | 923 | 4 | 1.2 | 0.5 | 2.9 | ||
| 11 | 12 | 2,728 | 12 | 0.8 | 0.4 | 1.5 | 0.582 | |
Note: AHS, Agricultrual Health Study; AMD, age-related macular degeneration; BMI, body mass index; CI, confidence interval; DDT, dichlorodiphenyltrichloroethane; EPTC, S-ethyl dipropylthiocarbamate; OR, odds ratio. 2,4-D, 2,4-dichlorophenoxyacetic acid; 2,4,5-T, 2,4,5-trichlorophenoxyacetic acid; 2,4,5,-TP, 2-propionic acid (fenoprop).
Adjusted for age and smoking.
Data available only for subset of applicators who completed the take-home questionnaire.
Table 4 summarizes results of the various analyses. We defined a consistent association of a pesticide with AMD as one that was evident in all five of the following: a) the ever use analysis (Table 2); b) the analysis with adjustment for correlated pesticides (see Table S4); c) the lifetime use analysis (Table 3); d) either men or women (see Table S2); and e) either early or late AMD (see Table S3). Four pesticides met these criteria fully: chlordane, DDT, malathion, and captan. Five additional pesticides met most of the criteria for consistency: phorate and 2,4-D were each significantly associated with AMD in one of two analyses with correlated pesticides, and each had evidence of dose–response; heptachlor, diazinon, and 2,4,5-T were each associated with AMD in analyses of correlated pesticides but did not have evidence of dose–response, possibly because small numbers of individuals were exposed.
Table 4.
Summary of analyses of AMD and pesticide use.
| Ever use | Ever Use | Cumulative use | By Gender | By AMD stage | |||
|---|---|---|---|---|---|---|---|
| Adjusted for correlated pesticides | Men | Women | Early | Late | |||
| (Table 2) | (Table S4) | (Table 3) | (Table S2) | (Table S3) | |||
| Insecticides (any) | + | ND | ND | + | + | + | |
| Organochlorines (any) | + | ND | ND | + | + | + | + |
| Aldrin | + | + | + | ||||
| Chlordane | + | + | + | + | + | + | + |
| DDT | + | + | + | + | + | + | |
| Dieldrin | + | + | + | ||||
| Heptachlor | + | + | + | + | |||
| Lixane | + | + | + | + | |||
| Toxaphene | + | ||||||
| Organophosphates (any) | + | ND | ND | + | + | + | |
| Chlorpyrifos | |||||||
| Coumaphos | |||||||
| Diazinon | + | + | + | + | + | ||
| Dichlorvos | + | + | + | ||||
| Fonofos | + | ||||||
| Malathion | + | + | + | + | + | + | + |
| Parathion | + | + | |||||
| Phorate | + | + | + | + | |||
| Terbufos | |||||||
| Other insecticides | |||||||
| Aldicarb | |||||||
| Carbaryl | + | + | |||||
| Carbofuran | |||||||
| Permethrin (crops) | + | + | |||||
| Permethrin (animals) | + | ||||||
| Herbicides (any) | ND | ND | |||||
| Phenoxyacetate (any) | + | ND | ND | + | |||
| 2,4,5-T | + | + | + | + | |||
| 2,4,5-TP | + | + | |||||
| 2,4-D | + | + | + | + | + | ||
| Other herbicides | ND | ND | |||||
| Alachlor | + | ||||||
| Atrazine | + | ||||||
| Butylate | |||||||
| Chlorimuron ethyl | |||||||
| Cyanazine | |||||||
| Dicamba | |||||||
| EPTC | |||||||
| Glyphosate | + | + | + | ||||
| Imazethapyr | |||||||
| Metolachlor | + | ||||||
| Metribuzin | + | ||||||
| Paraquat | + | ||||||
| Peximethalin | |||||||
| Petroleum oil | |||||||
| Trifluralin | |||||||
| Fungicides (any) | + | ND | ND | + | |||
| Benomyl | + | + | |||||
| Captan | + | + | + | + | + | ||
| Chlorothalonil | |||||||
| Maneb | + | ||||||
| Metalaxyl | |||||||
| Fumigants (any) | ND | ND | |||||
| Carbon tetrachloride | |||||||
| Ethylene dibromide | + | + | + | ||||
| Methyl bromide | |||||||
Note: AMD, age-related macular degeneration; DDT, dichlorodiphenyltrichloroethane; EPTC, S-ethyl dipropylthiocarbamate; ND, not done. Blank cells indicate that no association was present. ND indicates that analyses of correlated pesticides and cumulative use could not be tested for grouped pesticides. +, AMD was associated with the pesticide in the indicated analysis; 2,4-D, 2,4-dichlorophenoxyacetic acid ; 2,4,5-T, 2,4,5-trichlorophenoxyacetic acid; 2,4,5,-TP, 2-propionic acid (fenoprop).
We performed a quantitative bias analysis in which individuals who were not screened were allocated to AMD case or control status based on covariate distribution and pesticide use (see Table S5). The ORs were somewhat attenuated but remained elevated, and our interpretation was not qualitatively affected.
Discussion
To our knowledge, this is the first epidemiologic study to examine the relationship between specific pesticides and physician-confirmed AMD. We found associations of incident AMD with specific pesticides in several functional and chemical groups. The results were strongest and most consistent for organochlorine and organophosphate insecticides and phenoxyacetate herbicides together with specific pesticides from these classes and the fungicide captan (Table 4). Results were qualitatively similar for men and women, but some differences were apparent between early and late AMD. The present findings are consistent with results from two previous cross-sectional analyses in the AHS, which evaluated cases prevalent at enrollment (not included in the present study). These earlier studies implicated organochlorine and organophosphate insecticides and fungicides as risk factors for AMD (Kamel et al. 2000; Kirrane et al. 2005).
The etiology of AMD likely involves both genetic susceptibility and environmental exposures. AMD has been associated with polymorphisms in approximately 20 genes (Fritsche et al. 2014; Sobrin and Seddon 2014) including CFH (Sofat et al. 2012), other genes in complement pathways (Schramm et al. 2014), and genes involved in inflammation and immune regulation, lipid metabolism and transport, maintenance of the extracellular matrix, and angiogenesis (Fritsche et al. 2014; Sobrin and Seddon 2014). Smoking is positively associated with AMD (Chakravarthy et al. 2010; Sobrin and Seddon 2014), and certain dietary factors, including vitamins, minerals, and omega-3 fatty acids, are inversely associated with AMD (Sobrin and Seddon 2014; Zampatti et al. 2014). A meta-analysis of 24 studies found consistent associations with adiposity, hypertension, and cardiovascular disease (Chakravarthy et al. 2010). Genetic variation may modify associations of environmental factors such as smoking with AMD (Seddon et al. 2006; Wang et al. 2008).
Two fundamental mechanisms critical to AMD pathogenesis are inflammation and oxidative stress. The importance of the former is supported by the genetic evidence cited above and by associations of AMD with changes in inflammation biomarkers (Hong et al. 2011). High levels of oxidative stress are normally present in the retina and may be further increased by aging or environmental factors such as smoking (Handa 2012). Biomarkers of oxidative stress are elevated in patients with AMD (Zafrilla et al. 2013), and dietary antioxidants may retard AMD progression (Evans and Lawrenson 2012b; Zampatti et al. 2014). Oxidative stress may provoke the innate immune system and further increase inflammation, perhaps particularly in the presence of genetic variation in complement factors (Handa 2012).
Many specific pesticides associated with AMD are polychlorinated cyclic hydrocarbons. Of 47 specific pesticides evaluated in the ever-use analysis, 11 of 14 (79%) polychlorinated cyclic hydrocarbons were associated with AMD compared with 10 of 33 (30%) pesticides having other structures (, ). Many polychlorinated cyclic hydrocarbons are persistent, perhaps because they are lipophilic, which might account both for the greater toxicity of these chemicals and for the inconsistent association of adiposity with AMD: perhaps the latter can be detected primarily in populations exposed to persistent lipophilic toxicants.
Polychlorinated cyclic hydrocarbons may activate mechanisms involved in AMD. Organochlorine insecticides and polychlorinated biphenyls (PCBs) increase both oxidative stress (Bagchi et al. 1995; Lee and Opanashuk 2004) and inflammation (Kim KS et al. 2012; Hayley et al. 2011). Pesticides from other chemical classes, including organophosphate and pyrethroid insecticides and the bipyridyl herbicide paraquat, increase oxidative stress in the retina (Cingolani et al. 2006; Rotstein et al. 2003; Yu et al. 2008). Further, many of the polycyclic pesticides associated with AMD are aromatic, and polycyclic aromatic hydrocarbons are prone to phototoxic reactions (Wielgus and Roberts 2012). These persistent lipophilic compounds could accumulate in the retina and increase oxidative stress in response to light exposure. Potentially of great importance to AMD are observations that complement pathways can be activated by some pesticides, including DDT (Dutta et al. 2008), other organochlorine insecticides (Kumar et al. 2014), malathion (Ayub et al. 2001), the pyrethroid fenvalerate (Dutta and Das 2011), and paraquat (Kim YS et al. 2012).
Risk of late AMD is greater in women than in men (Rudnicka et al. 2015), and risk factors may differ between the genders (Adams et al. 2011; Erke et al. 2014; Klein et al. 1998). We found, however, that the pesticide–AMD association was qualitatively similar in men and women, although fewer women used pesticides. Risk factors for early and late AMD may differ. For example, vitamin supplements do not appear to reduce the risk of early AMD but may delay progression to the late forms (Evans and Lawrenson 2012a; Evans and Lawrenson, 2012b). Similarly, smoking has a stronger association with late than with early AMD (McKay et al. 2011), as does adiposity (Adams et al. 2011; Klein et al. 2007). Differences in pesticide–AMD associations for early and late AMD might be attributable to chance or to confounding: for example, residual confounding by age-related factors. One would, however, expect the latter to produce greater discrepancies for pesticides with greater secular trends, such as organochlorine insecticides (which were banned in the United States beginning in the 1970s), but organophosphate insecticides and herbicides accounted for more of the differences between early and late AMD. Further, neither adjustment for age using finer-grained variables nor stratification by age affected the pesticide–AMD associations.
Because pesticides from several classes were associated with AMD, general farming-related exposures might confound pesticide–AMD associations. A possible confounder is sun exposure, which is a risk factor for AMD that is potentially related to pesticide use (Sui et al 2013). However, adjustment for hours per day of sun exposure did not alter pesticide–AMD associations.
That many potential cases were not included in the final analysis raises a concern of selection bias. Some nonparticipation may have resulted from individuals misreporting AMD on the original questionnaires because they misunderstood the question or for other reasons; this could explain why 43% of those initially reporting AMD subsequently denied the diagnosis on screening. However, we expected that most Amsler grid users would deny the diagnosis. We enrolled 62% of potential cases, but only approximately half of these were confirmed by evaluation of medical records. Overall, these results suggest primarily that self-reports are unreliable and underscore the importance of our effort to validate cases. Quantitative bias analysis (Lash et al 2009) indicated that the results were qualitatively similar after accounting for the possible case–control status of unscreened individuals, although the ORs were slightly weaker. Thus, our findings do not appear to be the result of selection bias.
A further concern is that 25% of individuals otherwise eligible for the study did not complete either follow-up interview and so could not be considered for screening. In a previous study, we found that reporting either a health condition or greater pesticide use at enrollment in the AHS was associated with slightly greater odds of participation at follow-up (Montgomery et al 2010). Thus, we are unlikely to have lost exposed cases from the study population, which might have biased our estimates to the null. Further, our previous study showed that associations of pesticide use with health conditions either in the full cohort or in those participating in the follow-up gave very similar results (Montgomery et al 2010), and in another study, we found that loss to follow-up created notable bias only in analyses of strongly related exposures and outcomes (e.g., smoking and lung cancer) and was not a general problem (Rinsky et al. 2016).
Differences in age and smoking between potential AMD cases and those included in the analysis are not surprising because these factors are known to be related to AMD. Differences in pesticide use are a greater concern; however, they undoubtedly reflect the greater age of AMD cases. Further, it is difficult to understand why greater exposure to pesticides would select for substantially greater participation. Although our previous study found a small association of ever use of pesticides with greater participation at follow-up, increasing frequency of use was associated with nonparticipation, and neither duration of use nor ever use of insecticides was related to participation, either positively or inversely (Montgomery et al 2010). Thus, greater use of pesticides among confirmed AMD cases may represent a real difference and not selection bias.
Based on the age structure and race/ethnicity of the AHS cohort, we expected to find incident late AMD cases compared with the 72 we identified (Rudnicka et al. 2015). There are few estimates of incidence for early AMD, but we are likely to have missed more of these cases. Early cases may be less aware of their disease or less likely to report it and so would not have been contacted for screening. Our inability to identify asymptomatic early AMD cases is unlikely to have produced spurious associations unless underascertainment of early cases was related to pesticide use: an unlikely event. Further, pesticide associations with early AMD were often stronger than those with late AMD, suggesting that underascertainment of early cases may have resulted in underestimation of pesticide–AMD associations.
Strengths of the present study include its prospective design and its uniquely detailed information on the use of specific pesticides. Reporting of pesticide use by AHS applicators is generally reliable (Blair et al. 2002), and remaining misclassification would likely be nondifferential and bias associations toward the null (Blair et al. 2011). Although virtually all applicators and approximately half of spouses had used some pesticides, the use of specific chemicals varied considerably, enabling us to compare exposed and unexposed individuals within the cohort. Information was available to control for confounding by many risk factors for AMD, and the use of cases and controls from the same cohort would tend to further minimize confounding. The study was sufficiently large to evaluate exposure–response trends for many pesticides and to compare findings for men and women and for early and late AMD.
Conclusion
We found associations of AMD with use of organochlorine and organophosphate insecticides and phenoxyacetate herbicides as classes as well as with individual pesticides. Specifically, there were consistent associations with chlordane, DDT, malathion, and captan. Additional pesticides with slightly less consistent but nevertheless notable associations were heptachlor, diazinon, phorate, 2,4,5-T and 2,4-D. Overall, these results are consistent with experimental studies of mechanisms underlying AMD, including oxidative stress, inflammation, and complement activation. Our study involved a relatively small number of cases, and its novel results require replication. Nevertheless, it suggests that use of specific pesticides may be a modifiable risk factor for AMD.
Supplemental Material
Acknowledgments
B. Wujciak evaluated medical records provided by physicians. This work was supported in part by the Intramural Research Program of the National Institutes of Health/National Institute of Environmental Health Sciences (Z01-ES049030) and the National Cancer Institute (Z01-CP-1-119).
References
- Adams MK, Simpson JA, Aung KZ, Makeyeva GA, Giles GG, English DR, et al. 2011. Abdominal obesity and age-related macular degeneration. Am J Epidemiol 173(11):1246–1255, PMID: 21422060, 10.1093/aje/kwr005. [DOI] [PubMed] [Google Scholar]
- Ayub S, Deb K, Sharma M, Das N. 2001. Activation of the human serum complement cascade by insecticides. Curr Sci 80(12):1592–1595. [Google Scholar]
- Bagchi D, Bagchi M, Hassoun EA, Stohs SJ. 1995. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology 104(1–3):129–140, PMID: 8560491. [DOI] [PubMed] [Google Scholar]
- Blair A, Tarone R, Sandler D, Lynch CF, Rowland A, Wintersteen W, et al. 2002. Reliability of reporting on life-style and agricultural factors by a sample of participants in the Agricultural Health Study from Iowa. Epidemiology 13(1):94–99, PMID: 11805592. [DOI] [PubMed] [Google Scholar]
- Blair A, Thomas K, Coble J, Sandler DP, Hines CJ, Lynch CF, et al. 2011. Impact of pesticide exposure misclassification on estimates of relative risks in the Agricultural Health Study. Occup Environ Med 68(7):537–541, PMID: 21257983, 10.1136/oem.2010.059469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, et al. 2010. Clinical risk factors for age-related macular degeneration: A systematic review and meta-analysis. BMC Ophthalmol 10:31, PMID: 21144031, 10.1186/1471-2415-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani C, Rogers B, Lu L, Kachi S, Shen J, Campochiaro PA. 2006. Retinal degeneration from oxidative damage. Free Radic Biol Med 40(4):660–669, PMID: 16458197, 10.1016/j.freeradbiomed.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Dementi B. 1994. Ocular effects of organophosphates - a historical perspective of Saku disease. J Appl Toxicol 14(2):119–129, PMID: 8027507. [DOI] [PubMed] [Google Scholar]
- Dutta R, Das N. 2011. Immunomodulation of serum complement (C3) and macrophages by synthetic pyrethroid fenvalerate: In vitro study. Toxicology 285(3):126–132, PMID: 21557984, 10.1016/j.tox.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Dutta R, Mondal AM, Arora V, Nag TC, Das N. 2008. Immunomodulatory effect of DDT bis[4-chlorophenyl]-1,1,1-trichloroethane on complement system and macrophages. Toxicology 252(1–3):78–85, PMID: 18755234, 10.1016/j.tox.2008.07.063. [DOI] [PubMed] [Google Scholar]
- Erke MG, Bertelsen G, Peto T, Sjolie AK, Lindekleiv H, Njolstad I. 2014. Cardiovascular risk factors associated with age-related macular degeneration: the Tromsø Study. Acta Ophthalmol 92(7):662–669, PMID: 24460653, 10.1111/aos.12346. [DOI] [PubMed] [Google Scholar]
- Evans JR, Lawrenson JG. 2012a. Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration. Cochrane Database Syst Rev 6: CD000253. [DOI] [PubMed] [Google Scholar]
- Evans JR, Lawrenson JG. 2012b. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev 11:CD000254. [DOI] [PubMed] [Google Scholar]
- Fritsche LG, Fariss RN, Stambolian D, Abecasis GR, Curcio CA, Swaroop A. 2014. A ge-related macular degeneration: Genetics and biology coming together. Annu Rev Genomics Hum Genet 15:151–171, PMID: 24773320, 10.1146/annurev-genom-090413-025610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa JT. 2012. How does the macula protect itself from oxidative stress?. Mol Aspects Med 33(4):418–435, PMID: 22503691, 10.1016/j.mam.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayley S, Mangano E, Crowe G, Li N, Bowers WJ. 2011. An in vivo animal study assessing long-term changes in hypothalamic cytokines following perinatal exposure to a chemical mixture based on Arctic maternal body burden. Environ Health 10:65, PMID: 21745392, 10.1186/1476-069X-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong T, Tan AG, Mitchell P, Wang JJ. 2011. A review and meta-analysis of the association between C-reactive protein and age-related macular degeneration. Surv Ophthalmol 56(3):184–194, PMID: 21420705, 10.1016/j.survophthal.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Imai H, Miyata M, Uga S, Ishikawa S. 1983. Retinal degeneration in rats exposed to an organophosphate pesticide fenthion. Environ Res 30(2):453–465, PMID: 6832126, 10.1016/0013-9351(83)90231-1. [DOI] [PubMed] [Google Scholar]
- Kamel F, Boyes WK, Gladen BC, Rowland AS, Alavanja MC, Blair A, et al. 2000. Retinal degeneration in licensed pesticide applicators. Am J Ind Med 37(6):618–628, PMID: 10797505. [DOI] [PubMed] [Google Scholar]
- Kim KS, Hong NS, Jacobs DR Jr, Lee DH. 2012. Interaction between persistent organic pollutants and C-reactive protein in estimating insulin resistance among non-diabetic adults. J Prev Med Public Health 45(2):62–69, 10.3961/jpmph.2012.45.2.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Jung H, Gil HW, Hong SY, Song HY. 2012. Proteomic analysis of changes in protein expression in serum from animals exposed to paraquat. Int J Mol Med 30(6):1521–1527, PMID: 23023206, 10.3892/ijmm.2012.1143. [DOI] [PubMed] [Google Scholar]
- Kirrane EF, Hoppin JA, Kamel F, Umbach DM, Boyes WK, Deroos AJ, Alavanja M, Sandler DP. 2005. Retinal degeneration and other eye disorders in wives of farmer pesticide applicators enrolled in the Agricultural Health Study. Am J Epidemiol 161(11):1020–1029, PMID: 15901622, 10.1093/aje/kwi140. [DOI] [PubMed] [Google Scholar]
- Klein R, Deng Y, Klein BEK, Hyman L, Seddon J, Frank RN, et al. 2007. Cardiovascular disease, its risk factors and treatment, and age-related macular degeneration: women's Health Initiative Sight Exam ancillary study. Am J Ophthalmol 143(3):473–483, PMID: 17317391, 10.1016/j.ajo.2006.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE. 1998. Relation of smoking to the incidence of age-related maculopathy. The Beaver Dam Eye Study. Am J Epidemiol 147(2):103–110, PMID: 9456998. [DOI] [PubMed] [Google Scholar]
- Kumar J, Lind PM, Salihovic S, van Bavel B, Ekdahl KN, Nilsson B, et al. 2014. Influence of persistent organic pollutants on the complement system in a population-based human sample. Environ Int 71:94–100, PMID: 24996157, 10.1016/j.envint.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Lash TL, Fox M, Fink AK. 2009. Applying Quantitative Bias Analysis to Epidemiologic Data. New York, NY:Springer, 43–58. [Google Scholar]
- Lee DW, Opanashuk LA. 2004. Polychlorinated biphenyl mixture aroclor 1254-induced oxidative stress plays a role in dopaminergic cell injury. Neurotoxicology 25(6):925–939, PMID: 15474611, 10.1016/j.neuro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- McKay GJ, Patterson CC, Chakravarthy U, Dasari S, Klaver CC, Vingerling JR, et al. 2011. Evidence of association of APOE with age-related macular degeneration: A pooled analysis of 15 studies. Hum Mutat 32(12):1407–1416, PMID: 21882290, 10.1002/humu.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra UK, Nag D, Misra NK, Mehr MK, Ray PK. 1985. Some observations on the macula of pesticide workers. Hum Toxicol 4(2):135–145, PMID: 4007878. [DOI] [PubMed] [Google Scholar]
- Montgomery MP, Kamel F, Hoppin JA, Beane Freeman LE, Alavanja MC, Sandler DP. 2010. Effects of self-reported health conditions and pesticide exposures on probability of follow-up in a prospective cohort study. Am J Ind Med 53(5):486–496, PMID: 20017198, 10.1002/ajim.20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinsky JL, Richardson DB, Wing S, Beard JD, Alavanja M, Beane Freeman LE, et al. 2016. Assessing the potential for bias from non-response to a study follow-up interview: An example from the Agricultural Health Study. Am J Epidemiol, PMID: 28486574, 10.1093/aje/kwx098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotstein NP, Politi LE, German OL, Girotti R. 2003. Protective effect of docosahexaenoic acid on oxidative stress-induced apoptosis of retina photoreceptors. Invest Ophthalmol Vis Sci 44(5):2252–2259, PMID: 12714668. [DOI] [PubMed] [Google Scholar]
- Rudnicka AR, Kapetanakis VV, Jarrar Z, Wathern AK, Wormald R, Fletcher AE, et al. 2015. Incidence of late-stage age-related macular degeneration in American whites: systematic review and meta-analysis. Am J Ophthalmol 160(1):85–93, PMID: 25857680, 10.1016/j.ajo.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Schramm EC, Clark SJ, Triebwasser MP, Raychaudhuri S, Seddon JM, Atkinson JP. 2014. Genetic variants in the complement system predisposing to age-related macular degeneration: a review. Mol Immunol 61(2):118–125, PMID: 25034031, 10.1016/j.molimm.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JM, George S, Rosner B, Klein ML. 2006. CFH gene variant, Y402H, and smoking, body mass index, environmental associations with advanced age-related macular degeneration. Hum Hered 61(3):157–165, PMID: 16816528, 10.1159/000094141. [DOI] [PubMed] [Google Scholar]
- Sobrin L, Seddon JM. 2014. Nature and nurture- genes and environment- predict onset and progression of macular degeneration. Prog Retin Eye Res 40:1–15, PMID: 24374240, 10.1016/j.preteyeres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofat R, Casas JP, Webster AR, Bird AC, Mann SS, Yates JR, et al. 2012. Complement factor H genetic variant and age-related macular degeneration: effect size, modifiers and relationship to disease subtype. Int J Epidemiol 41(1):250–262, 10.1093/ije/dyr204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui GY, Liu GC, Liu GY, Gao YY, Deng Y, Wang WY, et al. 2013. Is sunlight exposure a risk factor for age-related macular degeneration? A systematic review and meta-analysis. Br J Ophthalmol 97(4):389–394, PMID: 23143904, 10.1136/bjophthalmol-2012-302281. [DOI] [PubMed] [Google Scholar]
- Tong Y, Liao J, Zhang Y, Zhou J, Zhang H, Mao M. 2010. LOC387715/HTRA1 gene polymorphisms and susceptibility to age-related macular degeneration: A HuGE review, meta-analysis. Mol Vis 16:1958–1981, PMID: 21031019. [PMC free article] [PubMed] [Google Scholar]
- Velez-Montoya R, Oliver SC, Olson JL, Fine SL, Quiroz-Mercado H, Mandava N. 2014. Current knowledge and trends in age-related macular degeneration: genetics, epidemiology, and prevention. Retina 34(3):423–441, PMID: 24285245, 10.1097/IAE.0000000000000036. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Ross RJ, Tuo J, Burlutsky G, Tan AG, Chan CC, et al. 2008. The LOC387715 polymorphism, inflammatory markers, smoking, and age-related macular degeneration. A population-based case-control study. Ophthalmology 115(4):693–699, PMID: 17675241, 10.1016/j.ophtha.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielgus AR, Roberts JE. 2012. Retinal photodamage by endogenous and xenobiotic agents. Photochem Photobiol 88(6):1320–1345, PMID: 22582903, 10.1111/j.1751-1097.2012.01174.x. [DOI] [PubMed] [Google Scholar]
- Yu F, Wang Z, Ju B, Wang Y, Wang J, Bai D. 2008. Apoptotic effect of organophosphorus insecticide chlorpyrifos on mouse retina in vivo via oxidative stress and protection of combination of vitamins C and E. Exp Toxicol Pathol 59(6):415–423, 10.1016/j.etp.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Zafrilla P, Losada M, Perez A, Caravaca G, Mulero J. 2013. Biomarkers of oxidative stress in patients with wet age related macular degeneration. J Nutr Health Aging 17(3):219–222, PMID: 23459973, 10.1007/s12603-012-0095-z. [DOI] [PubMed] [Google Scholar]
- Zampatti S, Ricci F, Cusumano A, Marsella LT, Novelli G, Giardina E. 2014. Review of nutrient actions on age-related macular degeneration. Nutr Res 34(2):95–105, PMID: 24461310, 10.1016/j.nutres.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jones D, Gonzalez-Lima F. 2006. Neurodegeneration produced by rotenone in the mouse retina: A potential model to investigate environmental pesticide contributions to neurodegenerative diseases. J Toxicol Environ Health A 69(18):1681–1697, PMID: 16864419, 10.1080/15287390600630203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.