Abstract
Background:
Postnatal exposure to perfluorinated alkylate substances (PFASs) is associated with lower serum concentrations of specific antibodies against certain childhood vaccines at 7 y.
Objectives:
We prospectively followed a Faroese birth cohort to determine these associations at 13 y.
Methods:
In 516 subjects (79% of eligible cohort members) who were 13 years old, serum concentrations of PFASs and of antibodies against diphtheria and tetanus were measured and were compared with data from the previous examination at 7 y. Multiple regression analyses and structural equation models were applied to determine the association between postnatal PFAS exposures and antibody concentrations.
Results:
Serum concentrations of PFASs and antibodies generally declined from 7 y to 13 y. However, 68 subjects had visited the emergency room and had likely received a vaccination booster, and a total of 202 children showed higher vaccine antibody concentrations at 13 y than at 7 y. Therefore, separate analyses were conducted after exclusion of these two subgroups. Diphtheria antibody concentrations decreased at elevated PFAS concentrations at 13 y and 7 y; the associations were statistically significant for perfluorodecanoate (PFDA) at 7 y and for perfluorooctanoate (PFOA) at 13 y, both suggesting a decrease by for each doubling of exposure. Structural equation models showed that a doubling in PFAS exposure at 7 y was associated with losses in diphtheria antibody concentrations at 13 y of 10–30% for the five PFASs. Few associations were observed for anti-tetanus concentrations.
Conclusions:
These results are in accord with previous findings of PFAS immunotoxicity at current exposure levels. https://doi.org/10.1289/EHP275
Introduction
Perfluorinated alkylate substances (PFASs) have a wide range of applications in water-, soil-, and stain-resistant coatings for clothing and other textiles and in oil-resistant coatings for food wrapping materials, and their use for has resulted in worldwide exposure to these persistent compounds (Lindstrom et al. 2011). Epidemiological research on possible adverse effects in exposed populations has intensified only in recent years (Grandjean and Clapp 2015; Steenland et al. 2014).
As a measure of depressed immune function, antibody response to routine childhood vaccinations has been identified as a sensitive indicator of elevated PFAS exposures in children (Grandjean et al. 2012; Granum et al. 2013). Whereas pre-booster antibody concentrations at 5 y of age appeared to be affected by both current and prenatal exposures (Grandjean et al. 2012), concentrations at 7 y of age showed inverse associations mainly with postnatal exposures (Grandjean et al. 2012; Mogensen et al. 2015). Decreased antibody responses to vaccinations have also been reported in PFAS-exposed adults (Kielsen et al. 2015; Looker et al. 2014). In addition, elevated PFAS exposures seem to increase the risk of common infections in children (Granum et al. 2013). Prenatal exposures did not appear to affect childhood hospitalization rates (Fei et al. 2010), but the quality of the exposure assessment in the study has recently been called into doubt (Bach et al. 2015). Because serum PFAS concentrations are often highly correlated, epidemiological studies have not identified which PFASs are mainly responsible for the phenomenon, and some studies have therefore modeled total PFAS exposure (Grandjean et al. 2012; Mogensen et al. 2015). Results from in vitro studies of human leukocytes support the immunotoxic potential of several PFASs, including perfluorooctane sulfonate (PFOS), perfluorooctanoate (PFOA), and perfluorodecanoate (PFDA) (Corsini et al. 2012), and animal models suggest that immune depression may occur at serum concentrations of PFOA and PFOS similar to or slightly above those reported in exposed human populations (DeWitt et al. 2012).
Immune functions are affected by many different kinds of stimuli (MacGillivray and Kollmann 2014), and specific antibody concentrations vary between children with similar vaccination records; the main determinant of increased concentrations is recent booster vaccinations (Capua et al. 2013). Although developmental exposure to polychlorinated biphenyls (PCBs) can also reduce vaccine responses (Heilmann et al. 2010), PCB concentrations were only weakly associated with PFASs, and adjustment for prenatal and early postnatal PCB exposure did not materially affect the PFAS associations with antibody concentrations (Grandjean et al. 2012).
We have extended our follow-up of the Faroese birth cohort to the age of 13 y, where we most recently observed strong negative associations between postnatal serum PFAS concentrations and antibody concentrations at the age of 7 y (Grandjean et al. 2012; Mogensen et al. 2015). We maintained our focus on tetanus and diphtheria because these vaccines are toxoids that trigger complex immune system responses involving both T cells and B cells (Schatz et al. 1998). Our previous research showed that prenatal exposures were particularly linked to the pre-booster antibody concentration at 5 y of age, and the concomitant exposure was associated with the response to the 5-y booster, whereas antibody concentrations at 7 y depended mainly on the current exposure levels (Grandjean et al. 2012; Mogensen et al. 2015). We therefore focused on the PFAS exposures reflected in serum concentrations from the two most recent examinations.
Methods
Study Population
A birth cohort of 656 children was compiled from births at the National Hospital in Tórshavn in the Faroe Islands during 1997–2000 to explore childhood immune function and the impact on vaccination efficacy (Grandjean et al. 2012). Faroese children receive vaccinations against diphtheria and tetanus at 3 mo, 5 mo, and 12 mo, with a booster at 5 y, as part of the government-supported health care system. All children received the same amount of vaccine and associated alum adjuvant from the same source, although additional vaccines (pertussis and polio) were added to the booster during the project period (Grandjean et al. 2012). The study protocol was approved by the Faroese ethics review committee and by the institutional review board at Harvard T.H. Chan School of Public Health; written informed consent was obtained from all mothers.
All cohort members were invited for a follow-up examination at 13 y that included a physical examination and blood sampling. Information from the children’s mandatory vaccination cards was copied, and a questionnaire on past medical history and current health status also included questions about vaccinations administered since the previous examination and whether the child had visited the emergency room. Because tetanus vaccination (which includes the diphtheria toxoid) in the emergency room is a routine procedure that may not have been recorded, hospital records did not contain further information on vaccinations that could be used for the purposes of the present study.
The PFAS concentrations were measured in serum that was obtained at the clinical examinations and then frozen at shortly after separation. We performed online solid-phase extraction and analysis using high-pressure liquid chromatography with tandem mass spectrometry (Grandjean et al. 2012). Within-batch and between-batch imprecision levels (assessed by the coefficient of variation) were or better for all analytes. Results with excellent accuracy have been obtained in regular comparisons organized by the German Society of Occupational Medicine. The PFASs quantified were perfluorohexanesulfonic acid (PFHxS), PFOA, PFOS, perfluorononanoate (PFNA), and PFDA.
Serum concentrations of major polychlorinated biphenyl congeners were available from maternal pregnancy serum; the sum of the three major congeners was used as an indicator of the total PCB exposure as on previous occasions (Grandjean et al. 2012).
Serum concentrations of immunoglobulin G (IgG) antibodies were measured by the vaccine producer (Statens Serum Institut, Copenhagen, Denmark) using an enzyme-linked immunosorbent assay for tetanus and, for diphtheria, a Vero cell–based neutralization assay using 2-fold dilutions of the serum. For both assays, calibration was performed using both international and local standard antitoxins. The methods were the same as those used for previous examinations (Grandjean et al. 2012).
Statistical Methods
Owing to skewed distributions, the antibody concentrations and the serum PFAS concentrations measured at 7 y and 13 y were all -transformed before the models were applied. Initial analyses were based on separate multiple linear regressions with age-13 antibody concentration as the dependent variable and serum PFAS concentration as a predictor along with age and sex as mandatory covariates while also considering age-5 booster type [i.e., co-immunization with other vaccines (Grandjean et al. 2012)]. We included adjustment for prenatal PCB exposure in separate analyses. We also performed covariate-adjusted logistic regression analyses to determine the odds ratios for each age-13 vaccine antibody concentration being below the clinically protective level of 0.1 IU/mL with a doubled PFAS exposure. Using structural equation models (Mogensen et al. 2015), we determined the associations of the age-7 PFAS concentrations with the specific antibody levels at 13 y. We modeled these associations as an indirect effect (via the antibody result at 7 y) and as a total effect. The total effect has the same interpretation as a linear regression model without the adjustment for the antibody level at 7 y, but it provides a more stable measure with better error control. The indirect effect is the loss in antibody concentration at 13 y due to increases in PFAS at 7 y being associated with lower antibody concentrations at that age, with these antibody concentrations being associated with the levels at 13 y. In addition, in a separate model, we ignored the direct path from PFAS exposure at 7 y to the antibody concentration at 13 y. All models were adjusted for sex and age as covariates, and incomplete observations were included assuming that information was missing at random, thus allowing calculations based on the maximum likelihood principle (Rubin 1987).
For comparison, we also applied linear mixed models to ascertain the effects of age-7 serum PFAS concentrations on the antibody outcomes at 7 and 13 y. Interaction between exposure and age was included, and the results were again adjusted for both age and sex. The assumption of linear dose–response associations was verified by allowing for a more flexible relationship between the PFAS and antibody concentrations in generalized additive models using cubic regression splines with three knots (Hastie and Tibshirani 1990); no significant deviation from linearity was found. Because both PFAS concentrations and antibody outcomes were -transformed, we expressed the regression coefficients as the change in the antibody concentration in percent for each doubling of the exposure.
Results
A total of 516 children (78.7% of the cohort members) participated in the age-13 examinations, and 587 (89.0%) participated either at 7 y or at 13 y or both. The characteristics of the children who provided sufficient serum for analyses at 13 y are shown in Table 1. PFOS was by far the most prevalent PFAS, with a median serum concentration at 13 y of , a 56% decrease since 7 y. PFOA and PFNA decreased to a similar extent. Although the correlation between the age-7 and age-13 concentrations of the same PFAS was even closer than the correlation between the different PFASs at each age (), the close correlations prevented meaningful adjustment for concomitant PFAS exposures. In contrast, the PCB concentration in maternal pregnancy serum correlated poorly with the child’s serum PFAS concentrations at 7 y (r between and 0.15) and 13 y (r between 0.11 and 0.20).
Table 1.
Characteristics of children who contributed serum-antibody concentrations at the two follow-up examinations.
| Variable | Total cohort (n=587) | No ER visit (n=519) | No ER visits and no antibody increase (n=317) | |||
|---|---|---|---|---|---|---|
| Summary | Summary | Summary | ||||
| Sex, n (%) (Girls) | 587 (100%) | 278 (47.4%) | 519 (100%) | 238 (45.9%) | 317 (100%) | 147 (46.4%) |
| Booster type 1, (%) (yes) | 575 (98.0%) | 412 (71.7%) | 509 (98.1%) | 367 (72.1%) | 311 (98.1%) | 209 (67.2%) |
| Age, median (IQR) (years) | ||||||
| Current examination | 516 (87.9%) | 13.2 (12.9; 13.6) | 448 (86.3%) | 13.2 (12.9; 13.6) | 317 (100%) | 13.3 (13.0; 13.6) |
| Age-7 examination | 565 (96.3%) | 7.5 (7.5; 7.6) | 497 (95.8%) | 7.5 (7.5; 7.6) | 317 (100%) | 7.5 (7.5; 7.6) |
| Antibody concentrations, median (IQR) (IU/mL) | ||||||
| Anti-diphtheria at 13 y | 515 (87.7%) | 0.1 (0.0; 0.2) | 447 (86.1%) | 0.1 (0.0; 0.2) | 317 (100%) | 0.1 (0.0; 0.2) |
| Anti-diphtheria at 7 y | 459 (78.2%) | 0.8 (0.4; 1.6) | 391 (75.3%) | 0.8 (0.4; 1.6) | 317 (100%) | 0.8 (0.4; 1.6) |
| Anti-tetanus at 13 years | 515 (87.7%) | 0.6 (0.3; 1.8) | 447 (86.1%) | 0.5 (0.3; 1.2) | 317 (100%) | 0.5 (0.2; 1.0) |
| Anti-tetanus at 7 y | 459 (78.2%) | 1.8 (0.6; 4.5) | 391 (75.3%) | 2.1 (1.1; 5.2) | 317 (100%) | 2.3 (1.3; 5.5) |
| PFAS concentrations, median (IQR) (ng/mL) | ||||||
| PFOS at 13 years | 515 (87.7%) | 6.7 (5.2; 8.5) | 447 (86.1%) | 6.7 (5.3; 8.5) | 317 (100%) | 6.8 (5.4; 8.7) |
| PFOS at 7 y | 488 (83.1%) | 15.3 (12.4; 19.0) | 420 (80.9%) | 15.3 (12.4; 19.0) | 312 (98.4%) | 15.5 (12.9; 18.9) |
| PFOA at 13 y | 515 (87.7%) | 2.0 (1.6; 2.5) | 447 (86.1%) | 2.0 (1.5; 2.5) | 317 (100%) | 2.0 (1.6; 2.6) |
| PFOA at 7 y | 488 (83.1%) | 4.4 (3.5; 5.7) | 420 (80.9%) | 4.4 (3.6; 5.7) | 312 (98.4%) | 4.4 (3.5; 5.5) |
| PFHxS at 13 y | 515 (87.7%) | 0.4 (0.3; 0.5) | 447 (86.1%) | 0.4 (0.3; 0.5) | 317 (100%) | 0.4 (0.3; 0.5) |
| PFHxS at 7 y | 488 (83.1%) | 0.5 (0.4; 0.7) | 420 (80.9%) | 0.5 (0.4; 0.7) | 312 (98.4%) | 0.5 (0.4; 0.7) |
| PFNA at 13 y | 515 (87.7%) | 0.7 (0.6; 0.9) | 447 (86.1%) | 0.7 (0.6; 0.9) | 317 (100%) | 0.8 (0.6; 1.0) |
| PFNA at 7 y | 488 (83.1%) | 1.1 (0.9; 1.5) | 420 (80.9%) | 1.1 (0.9; 1.5) | 312 (98.4%) | 1.1 (0.9; 1.5) |
| PFDA at 13 y | 515 (87.7%) | 0.3 (0.2; 0.4) | 447 (86.1%) | 0.3 (0.2; 0.4) | 317 (100%) | 0.3 (0.2; 0.4) |
| PFDA at 7 y | 488 (83.1%) | 0.4 (0.2; 0.6) | 420 (80.9%) | 0.4 (0.2; 0.5) | 312 (98.4%) | 0.4 (0.2; 0.5) |
Note: ER, emergency room; IQR, interquartile range; IU, international units; PFAS, perflourinated alkylate substance; PFDA, perfluorodecanoate; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoate; PFOA, perflourooctanoate; PFOS, perfluorooctane sulfonate.
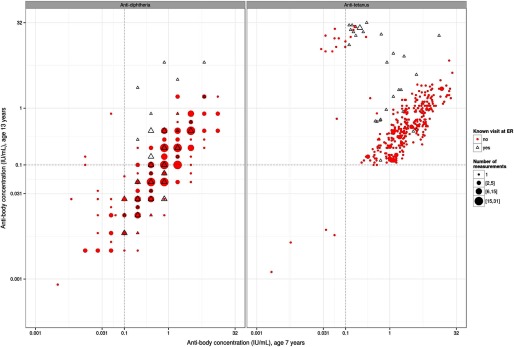
On average, both antibody concentrations showed clear decreases during the six-year period from 7 y to 13 y, although they were stronger for diphtheria (Table 1). At 13 y, 207 (39.4%) and 103 (19.6%) children had antibody concentrations for diphtheria and for tetanus, respectively. However, not all children showed the anticipated decrease in antibody concentrations. Scatter plots of the correlation between antibody concentrations at 7 y and 13 y show that concentrations increased between the two examinations in many cohort subjects (Figure 1). An increase in antibody concentration could likely be a result of additional antigen exposure, most likely because some subjects had received an unscheduled booster dose. Six of the children had received an additional booster at the project clinic because of their very low antibody concentrations after the age-5 booster. Questionnaire information further revealed that 68 cohort members had visited the emergency room where they likely received a tetanus booster shot, and indeed, many of them had elevated antibody concentrations at age 13 (Figure 1). Nevertheless, a total of 202 children did not show the anticipated decrease in antibody concentrations between 7 y and 13 y, most of whom were not known to have been vaccinated after 5 y. Statistical analyses were therefore performed on the whole group; after exclusion of subjects with a record of having visited the emergency room or otherwise having received an additional booster (no-ER group); and, for comparison purposes, after exclusion of the 202 subjects who for unknown reason did not show the anticipated decrease in antibody concentrations between 7 y and 13 y. These three groups were fairly similar with regard to sex, age, and PFAS exposure (Table 1).
Figure 1.
Scatter plot showing paired antibody concentrations for diphtheria (left) and tetanus (right) in children examined at both 7 y and 13 y.
Multiple regression analyses showed a uniformly inverse association between anti-diphtheria antibody concentrations at 13 y and the PFAS concentrations at either 7 y or 13 y (Table 2). The tendencies were the strongest after exclusion of subjects with a history of an additional booster, and an approximately 25% decrease for each doubling in serum PFOA concentration at 13 y and a 24% decrease for each doubling in serum PFDA concentration at 7 y were both statistically significant. However, tetanus antibody concentrations, which had decreased much less than diphtheria antibody concentrations, tended to show positive associations, one of which was statistically significant (Table 3). As expected (Grandjean et al. 2012), adjustment for developmental PCB exposure had no appreciable effect on these associations; therefore, PCB was not considered further. Logistic regression analyses of the results, many of which were close to the 0.1 IU/mL limit, in most cases showed odds close to 1 for the antibody concentrations being below the clinically protective level (data not shown). However, for diphtheria, a doubled concomitant serum PFOA concentration showed odds ratios of 1.47 (95% CI: 1.03, 2.14; ) and 1.71 (95% CI: 1.15, 2.55; ) for a nonprotective antibody level in the total study group and in the no-ER group, respectively. Further, PFDA at 7 y showed statistically significant odds ratios of 1.39 (95% CI: 1.05, 1.85; ) and 1.54 (95% CI: 1.13, 2.12; ) for diphtheria in the same two groups, respectively; no other associations reached statistical significance.
Table 2.
Linear regression models of changes in anti-diphtheria concentrations at 13 y associated with serum PFAS concentrations 13 y and 7 y adjusted for sex, age at antibody assessment, and booster type.
| |
Total cohort () |
No booster or ER visit () |
No booster or ER visit and no antibody increase () |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFAS (ng/mL) | Change | 95% CI | p-value | Change | 95% CI | p-value | Change | 95% CI | p-value | ||||||
| PFAS concentrations, 13 y | |||||||||||||||
| PFOS | 505 | , 15.6 | 0.454 | 439 | , 14.3 | 0.374 | 311 | , 30.9 | 0.965 | ||||||
| PFOA | 505 | , 5.8 | 0.129 | 439 | , | 0.029 | 311 | , 9.0 | 0.173 | ||||||
| PFHxS | 505 | , 15.8 | 0.583 | 439 | , 9.8 | 0.279 | 311 | , 25.0 | 0.984 | ||||||
| PFNA | 505 | , 20.2 | 0.693 | 439 | , 19.0 | 0.579 | 311 | , 25.2 | 0.780 | ||||||
| PFDA | 505 | , 18.9 | 0.726 | 439 | , 20.3 | 0.754 | 311 | , 21.8 | 0.716 | ||||||
| PFAS concentrations, 7 y | |||||||||||||||
| PFOS | 427 | , 2.3 | 0.070 | 361 | , 1.4 | 0.061 | 306 | , 23.5 | 0.490 | ||||||
| PFOA | 427 | , 23.3 | 0.742 | 361 | , 18.8 | 0.480 | 306 | , 28.5 | 0.845 | ||||||
| PFHxS | 427 | , 8.5 | 0.264 | 361 | , 2.0 | 0.077 | 306 | , 15.4 | 0.556 | ||||||
| PFNA | 427 | , 8.5 | 0.243 | 361 | , 7.5 | 0.190 | 306 | , 16.1 | 0.519 | ||||||
| PFDA | 427 | , | 0.008 | 361 | , | 0.005 | 306 | , | 0.029 | ||||||
Notes: The change in the antibody concentration is expressed in percent per doubling of the serum PFAS concentration at the two different ages. CI, confidence interval; ER, emergency room; PFAS, perflourinated alkylate substance; PFDA, perfluorodecanoate; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoate; PFOA, perflourooctanoate; PFOS, perfluorooctane sulfonate.
Table 3.
Linear regression models of changes in anti-tetanus concentrations at 13 y associated with serum-PFAS concentrations at 13 y and 7 y adjusted for sex, age at antibody assessment, and booster type.
| |
Total cohort () |
No ER visit () |
No ER visit and no antibody increase () |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFAS (ng/mL) | Change | 95% CI | p-value | Change | 95% CI | p-value | Change | 95% CI | p-value | |||||
| PFAS concentrations, 13 y | ||||||||||||||
| PFOS | 505 | 22.2 | , 70.3 | 0.237 | 439 | 23.4 | , 63.7 | 0.144 | 311 | 14.8 | , 44.4 | 0.236 | ||
| PFOA | 505 | 3.3 | , 46.9 | 0.856 | 439 | , 28.1 | 0.710 | 311 | , 6.3 | 0.145 | ||||
| PFHxS | 505 | 8.7 | , 45.0 | 0.568 | 439 | 19.3 | , 52.1 | 0.153 | 311 | 1.8 | , 22.9 | 0.851 | ||
| PFNA | 505 | 15.2 | , 59.7 | 0.394 | 439 | 5.1 | , 39.3 | 0.727 | 311 | 11.6 | , 38.8 | 0.324 | ||
| PFDA | 505 | 18.7 | , 59.8 | 0.258 | 439 | 6.9 | , 38.0 | 0.607 | 311 | 18.0 | , 44.4 | 0.106 | ||
| PFAS concentrations, 7 y | ||||||||||||||
| PFOS | 427 | 30.0 | , 101.4 | 0.240 | 361 | 45.4 | 1.2, 108.8 | 0.043 | 306 | 2.7 | , 34.8 | 0.849 | ||
| PFOA | 427 | 9.4 | , 58.9 | 0.637 | 361 | 2.9 | , 41.1 | 0.859 | 306 | , 20.0 | 0.671 | |||
| PFHxS | 427 | 14.8 | , 52.2 | 0.334 | 361 | 25.2 | , 57.7 | 0.057 | 306 | , 5.2 | 0.167 | |||
| PFNA | 427 | 31.0 | , 76.4 | 0.075 | 361 | 23.1 | , 59.0 | 0.110 | 306 | 11.9 | , 34.7 | 0.235 | ||
| PFDA | 427 | 36.8 | 4.7, 78.7 | 0.022 | 361 | 25.1 | , 57.0 | 0.054 | 306 | 3.5 | , 22.2 | 0.682 | ||
Notes: The change in the antibody concentration is expressed in percent per doubling of the serum PFAS concentration at the two different ages. CI, confidence interval; ER, emergency room; PFAS, perflourinated alkylate substance; PFDA, perfluorodecanoate; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoate; PFOA, perflourooctanoate; PFOS, perfluorooctane sulfonate.
In the first structural equation model, we included an indirect effect mediated by the exposure at 7 y via the antibody concentration at that age. Tables 4 and 5 show the indirect effect and the total effect observed in this model. For diphtheria, all associations were inverse, and all five PFASs showed statistically significant inverse associations in the no-ER group. Tendencies were weaker in the total group and after exclusion of subjects without decreasing antibody concentrations. For tetanus, some inverse associations were observed, although none was significant. A second model without a direct effect fitted the data equally well and showed a similar, though stronger, indirect effect for diphtheria via the age-7 antibody concentration. These tendencies also became clear for tetanus, where statistically significant indirect effects were now apparent in the no-ER group, both for PFOA (; 95% CI: , ) and for PFHxS (; 95% CI: , ). Finally, the results obtained with the linear mixed models were similar to those obtained with the first structural equation model.
Table 4.
Effects of serum-PFAS concentrations at 7 y on anti-diphtheria antibody concentrations at 7 y and 13 y adjusted for age and sex in structural equation models.
| |
|
Total cohort () |
No ER visit () |
No ER visit and no antibody increase () |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PFAS (ng/mL) | Effect | Change | 95% CI | p-value | Change | 95% CI | p-value | Change | 95% CI | p-value |
| PFOS | Indirect | , | 0.003 | , | 0.002 | , 1.2 | 0.061 | |||
| Total | , | 0.046 | , | 0.021 | , 15.4 | 0.282 | ||||
| PFOA | Indirect | , | 0.050 | , | 0.045 | , 5.9 | 0.147 | |||
| Total | , 23.8 | 0.739 | , 19.2 | 0.481 | , 22.9 | 0.661 | ||||
| PFHxS | Indirect | , 1.2 | 0.071 | , | 0.042 | , 7.9 | 0.289 | |||
| Total | , 7.5 | 0.211 | , | 0.043 | , 12.4 | 0.415 | ||||
| PFNA | Indirect | , | 0.003 | , | 0.002 | , | 0.030 | |||
| Total | , 3.2 | 0.099 | , 2.8 | 0.087 | , 12.5 | 0.371 | ||||
| PFDA | Indirect | , | , | 0.001 | , | 0.008 | ||||
| Total | , | 0.003 | , | 0.002 | , | 0.013 | ||||
Notes: The change in the anti-diphtheria concentration is expressed in percent per doubling of the age-7 serum PFAS concentration. CI, confidence interval; ER, emergency room; PFAS, perflourinated alkylate substance; PFDA, perfluorodecanoate; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoate; PFOA, perflourooctanoate; PFOS, perfluorooctane sulfonate.
Table 5.
Effects of serum-PFAS concentrations at 7 y on anti-tetanus antibody concentrations at 7 y and 13 y adjusted for age and sex in structural equation models.
| |
|
Total cohort () |
No ER visit () |
No ER visit and no antibody increase () |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PFAS (ng/mL) | Effect | Change | 95% CI | p-value | Change | 95% CI | p-value | Change | 95% CI | p-value |
| PFOS | Indirect | 3.2 | , 12.9 | 0.492 | , 2.4 | 0.347 | , 24.2 | 0.906 | ||
| Total | 26.3 | , 92.6 | 0.278 | 42.9 | , 110.0 | 0.069 | 2.1 | , 32.9 | 0.877 | |
| PFOA | Indirect | 6.0 | , 14.4 | 0.138 | , 1.5 | 0.181 | , 1.8 | 0.075 | ||
| Total | 11.3 | , 59.3 | 0.560 | 1.9 | , 42.6 | 0.915 | , 15.6 | 0.505 | ||
| PFHxS | Indirect | 7.7 | 0.6, 15.3 | 0.033 | , 0.8 | 0.107 | , 1.2 | 0.072 | ||
| Total | 14.1 | , 49.8 | 0.342 | 25.0 | , 59.8 | 0.075 | , 3.7 | 0.127 | ||
| PFNA | Indirect | 1.0 | , 6.9 | 0.739 | , 1.6 | 0.416 | 4.2 | , 21.9 | 0.604 | |
| Total | 28.9 | , 71.5 | 0.081 | 23.6 | , 62.5 | 0.129 | 12.7 | , 34.8 | 0.190 | |
| PFDA | Indirect | , 4.0 | 0.729 | 0.1 | , 2.0 | 0.936 | 7.8 | , 21.8 | 0.231 | |
| Total | 27.5 | , 62.9 | 0.053 | 16.7 | , 47.5 | 0.196 | 2.7 | , 18.5 | 0.721 | |
Notes: The change in the anti-tetanus concentration is expressed in percent per doubling of the age-7 serum PFAS concentration. CI, confidence interval; ER, emergency room; PFAS, perflourinated alkylate substance; PFDA, perfluorodecanoate; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoate; PFOA, perflourooctanoate; PFOS, perfluorooctane sulfonate.
Discussion
The present prospective study was performed in adolescents to ascertain PFAS-associated decreases in antibody responses to their childhood vaccines. Owing to numerous unscheduled booster vaccinations, the data were censored to remove cohort members with records of emergency room visits. In this subgroup, multiple regression results showed that diphtheria antibody concentrations decreased at elevated serum PFAS concentrations at 13 y and 7 y; the associations were statistically significant for PFDA at 7 y and for PFOA at 13 y, both suggesting a decrease by for each doubling of exposure. The findings after exclusion of all subjects without a decrease in antibody concentrations were less clear. Structural equation models showed that a doubling of PFAS exposure at 7 y was associated with statistically significant losses in diphtheria antibody concentrations at 13 y of 10–30% for the five PFASs. Few associations were observed for anti-tetanus concentrations. However, more advanced modeling showed negative associations for tetanus with regard to the age-7 PFAS exposure. Owing to the intercorrelations between the serum PFAS concentrations, further analysis of the possible role of individual PFASs was not pursued, and the observed associations may reflect the effects of the PFAS mixtures.
Although the present study aimed to obtain prospective data on the associations between PFAS exposure and vaccine antibody concentration over an extended period, an antibody concentration at a particular point in time does not represent the complete trajectory of changes and may not be representative of the protection against the specific disease in the long term. The present prospective study is apparently the first to elucidate the temporal changes in antibody concentrations in relation to PFAS immunotoxicity through to adolescence. A major obstacle in such observational studies is that children and adolescents who visit the emergency room for cuts and other injuries are routinely administered a tetanus booster, which also affects diphtheria because both toxoids are present in the vaccine, thereby interfering with the study design. We therefore chose to exclude subjects who had a record of an emergency room visit (to obtain no-ER group). For comparison, we performed parallel calculations after excluding subjects who had not revealed the anticipated decrease in antibody concentrations between 7 y and 13 y. With antibody concentrations that decrease to different degrees over time and with PFAS exposure levels that likewise show decreases, multiple regression analyses of the bivariate data sets need to be complemented by more-advanced modeling.
Our study is limited to PFAS exposure assessments at two points during childhood at an interval of approximately six years. Although the elimination half-lives of the PFASs are 2–5 y (Bartell et al. 2010; Olsen et al. 2007), the two widely separated measurements may not fully characterize the childhood exposure profile. Because exposures during childhood are likely to vary (Kato et al. 2009; Lindstrom et al. 2011), it is possible that serial serum PFAS analyses would provide stronger evidence for PFAS immunotoxicity. However, given that a decreased antibody concentration is most likely due to past exposures, rather than to current, lower exposures (Mogensen et al. 2015), we chose to rely on the age-7 exposure levels and to include both antibody measurements at 7 y and 13 y in the assessment. At age 7, concurrent serum PFAS concentrations showed strong inverse associations with vaccine antibody concentrations, and inclusion of age-5 PFAS concentrations slightly strengthened these tendencies (Mogensen et al. 2015).
The 5-y booster vaccination is in principle the last booster vaccination that a child receives, and long-term protection is therefore intended. Our previous results (Grandjean et al. 2012) showed that at 7 y, many children had antibody concentrations below the level assumed to provide the desired protection. The number of children with antibody concentrations below the desired level had substantially increased by age 13 and was similar to the number of children with antibody concentrations below the level of protection at age 5 before the booster. These observations support the justification for routinely supplying a booster vaccination in the emergency room in connection with any injury that may have involved the slightest risk of tetanus. Unfortunately, such preventive measures add variance to the present study design, which relied on all children being vaccinated at the same age and with the same antigen dose. The study assumption was therefore violated, and it proved to be difficult to adjust for additional immunizations at different ages given the incomplete knowledge of booster administrations. Accordingly, the results clearly show the strongest associations between PFAS exposure and antibody concentrations in cohort subjects who were not known to have visited the emergency room or to otherwise have received a booster. However, Figure 1 also shows an increased antibody concentration at 13 y in many cohort members, possibly resulting from a booster that had not been recorded. Exclusion of all cohort members without an apparent decrease in antibody concentration at 13 y may have more than remedied this problem because it most likely excluded too many subjects, thereby attenuating the statistical power. As might be expected, the results for the most restricted subgroup are somewhat weaker than those for the less restricted cohort. Nevertheless, the increased antibody concentration in children who had received a booster suggests that any adverse influences of PFAS exposure could be remedied by repeated antigen challenge, although such intervention might not compensate for any other adverse effects associated with PFAS immunotoxicity.
As in previous studies (Grandjean et al. 2012; Mogensen et al. 2015), we found stronger associations for diphtheria than for tetanus. The former also exhibited much greater decreases from 7 y to 13 y, most likely because the diphtheria toxoid is a weaker antigen than tetanus and is therefore more easily affected by PFAS-depressed immune function. The exact magnitude of the serum antibody concentration may not be clinically important, but very low levels will result in poor or absent protection. With many antibody concentrations being close to the assumed clinically protective level of 0.1 IU/mL, logistic regression showed only weak tendencies for antibody levels below the limit to be associated with serum PFAS concentrations. Similarly, imprecision of antibody assessments, particularly at concentrations below or close to the clinically protective concentration, may have biased the regression analyses toward the null.
In support of our findings of PFAS immunotoxicity, a study of 99 Norwegian children at 3 y found that the maternal serum PFOA concentrations were associated with decreased vaccine responses in the children, particularly toward rubella vaccine, as well as increased frequencies of common cold and gastroenteritis (Granum et al. 2013). However, PFOS and PFOA concentrations in serum from 1,400 pregnant women from the Danish National Birth Cohort were not associated with the total hospitalization rate for a variety of infectious diseases in 363 of the children up to an average age of 8 y (Fei et al. 2010). However, the validity of this study has been questioned owing to the poor stability of the serum PFAS measurements (Bach et al. 2015); such imprecision of the exposure assessment could easily bias the results toward the null (Grandjean and Budtz-Jørgensen 2010).
In adults, PFOA exposure from contaminated drinking water was associated with lower serum concentrations of total IgA and IgE (in females only), although not of total IgG (Fletcher et al. 2009). More specifically, a reduced antibody titer increase after influenza vaccination was found in the most highly exposed subjects (Looker et al. 2014). Further, a small intervention study of 12 adults showed that the time-dependent increase in vaccine-specific antibody concentrations decreased at higher PFAS exposures (Kielsen et al. 2015). Overall, support is building for the notion that PFAS exposure is associated with deficient immune function. Although diphtheria and perhaps tetanus may not be likely hazards in the Faroese and in the residents of many other countries, the strongly decreased antibody concentrations likely reflect an immunological deficit. Because optimal immune system function is crucial for health, the associations identified should be regarded as adverse. We recently calculated benchmark dose levels to estimate the magnitude of exposure limits that would protect against the immunotoxicity observed. The results suggested that the present exposure limits may be too high by a factor of 100 (Grandjean and Budtz-Jørgensen 2013). The present study extends the previous findings of deficient antibody responses in this cohort at younger ages and therefore adds support to the notion that substantially strengthened prevention of PFAS exposure is indicated.
Conclusions
The results of the present study are in accord with previous findings of PFAS immunotoxicity at current exposure levels, although the results of this observational study are affected by concomitant decreases in concentrations of both PFASs and antibodies and by known and suspected booster vaccinations during the eight-year interval since the routine 5-y booster.
Acknowledgments
This study was supported by the National Institute of Environmental Health Sciences, National Institutes of Health (ES012199); the U.S. Environmental Protection Agency (R830758); the Danish Council for Strategic Research (09-063094); and the Danish Environmental Protection Agency as part of the environmental support program DANCEA (Danish Cooperation for Environment in the Arctic). The authors are solely responsible for all results and conclusions, which do not necessarily reflect the position of any of the funding agencies.
References
- Bach CC, Henriksen TB, Bossi R, Bech BH, Fuglsang J, Olsen J, et al. 2015. Perfluoroalkyl acid concentrations in blood samples subjected to transportation and processing delay. PLoS One 10(9):e0137768, PMID: 26356420, 10.1371/journal.pone.0137768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K. 2010. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ Health Perspect 118(2):222–228, PMID: 20123620, 10.1289/ehp.0901252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capua T, Katz JA, Bocchini JA Jr. 2013. Update on adolescent immunizations: Selected review of U.S. recommendations and literature. Curr Opin Pediatr 25(3):397–406, PMID: 23652687, 10.1097/MOP.0b013e328360dc63. [DOI] [PubMed] [Google Scholar]
- Corsini E, Sangiovanni E, Avogadro A, Galbiati V, Viviani B, Marinovich M, et al. 2012. In vitro characterization of the immunotoxic potential of several perfluorinated compounds (PFCs). Toxicol Appl Pharmacol 258(2):248–255, PMID: 22119708, 10.1016/j.taap.2011.11.004. [DOI] [PubMed] [Google Scholar]
- DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR. 2012. Immunotoxicity of perfluorinated compounds: Recent developments. Toxicol Pathol 40(2):300–311, PMID: 22109712, 10.1177/0192623311428473. [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L, Olsen J. 2010. Prenatal exposure to PFOA and PFOS and risk of hospitalization for infectious diseases in early childhood. Environ Res 110(8):773–777, PMID: 20800832, 10.1016/j.envres.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Fletcher T, Steenland K, Savitz D. 2009. Status report: PFOA and immune biomarkers in adults exposed to PFOA in drinking water in the mid Ohio Valley. In C8 Science Panel Report. http://www.c8sciencepanel.org/pdfs/Status_Report_C8_and_Immune_markers_March2009.pdf [accessed 5 July 2017].
- Grandjean P, Andersen EW, Budtz-Jørgensen E, Nielsen F, Mølbak K, Weihe P, et al. 2012. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 307(4):391–397, PMID: 22274686, 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Budtz-Jørgensen E. 2010. An ignored risk factor in toxicology: The total imprecision of exposure assessment. Pure Appl Chem 82(2):383–391, PMID: 20419070, 10.1351/PAC-CON-09-05-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Budtz-Jørgensen E. 2013. Immunotoxicity of perfluorinated alkylates: Calculation of benchmark doses based on serum concentrations in children. Environ Health 12(1):35, PMID: 23597293, 10.1186/1476-069X-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Clapp R. 2015. Perfluorinated alkyl substances: Emerging insights into health risks. New Solut 25(2):147–163, PMID: 26084549, 10.1177/1048291115590506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum B, Haug LS, Namork E, Stølevik SB, Thomsen C, Aaberge IS, et al. 2013. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol 10(4):373–379, PMID: 23350954, 10.3109/1547691x.2012.755580. [DOI] [PubMed] [Google Scholar]
- Hastie TJ, Tibshirani RJ. 1990. Generalized additive models (monographs on statistics and applied probability 43). Boca Raton, FL:Chapman and Hall/CRC Press. [Google Scholar]
- Heilmann C, Budtz-Jørgensen E, Nielsen F, Heinzow B, Weihe P, Grandjean P. 2010. Serum concentrations of antibodies against vaccine toxoids in children exposed perinatally to immunotoxicants. Environ Health Perspect 118(10):1434–1438, PMID: 20562056, 10.1289/ehp.1001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Calafat AM, Wong LY, Wanigatunga AA, Caudill SP, Needham LL. 2009. Polyfluoroalkyl compounds in pooled sera from children participating in the National Health and Nutrition Examination Survey 2001–2002. Environ Sci Technol 43(7):2641–2647, PMID: 19452929, 10.1021/es803156p. [DOI] [PubMed] [Google Scholar]
- Kielsen K, Shamim Z, Ryder LP, Nielsen F, Grandjean P, Budtz-Jorgensen E, et al. 2015. Antibody response to booster vaccination with tetanus and diphtheria in adults exposed to perfluorinated alkylates. J Immunotoxicol 12(2):270–273, PMID: 26181512, 10.3109/1547691X.2015.1067259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL. 2011. Polyfluorinated compounds: past, present, and future. Environ Sci Technol 45(19):7954–7961, PMID: 21866930, 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- Looker C, Luster MI, Calafat AM, Johnson VJ, Burleson GR, Burleson FG, et al. 2014. Influenza vaccine response in adults exposed to perfluorooctanoate and perfluorooctanesulfonate. Toxicol Sci 138(1):76–88, PMID: 24284791, 10.1093/toxsci/kft269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillivray DM, Kollmann TR. 2014. The role of environmental factors in modulating immune responses in early life. Front Immunol 5:434, PMID: 25309535, 10.3389/fimmu.2014.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen UB, Grandjean P, Heilmann C, Nielsen F, Weihe P, Budtz-Jørgensen E. 2015. Structural equation modeling of immunotoxicity associated with exposure to perfluorinated alkylates. Environ Health 14:47, PMID: 26041029, 10.1186/s12940-015-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305, PMID: 17805419, 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB. 1987. Multiple Imputation for Nonresponse in Surveys. New York, NY:John Wiley & Sons. [Google Scholar]
- Schatz D, Ellis T, Ottendorfer E, Jodoin E, Barrett D, Atkinson M. 1998. Aging and the immune response to tetanus toxoid: diminished frequency and level of cellular immune reactivity to antigenic stimulation. Clin Diagn Lab Immunol 5(6):894–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Savitz DA, Fletcher T. 2014. Commentary: class action lawsuits: can they advance epidemiologic research?. Epidemiology 25(2):167–169, PMID: 24487199, 10.1097/EDE.0000000000000067. [DOI] [PubMed] [Google Scholar]