Abstract
Background
Primary adrenal insufficiency (PAI) is a rare, potentially life-threatening condition. There are few Korean studies on PAI, and most have had small sample sizes. We aimed to examine the etiology, clinical characteristics, treatment, and mortality of PAI in Korean patients.
Methods
A nationwide, multicenter, registry-based survey was conducted to identify adults diagnosed with or treated for PAI at 30 secondary or tertiary care institutions in Korea between 2000 and 2014.
Results
A total of 269 patients with PAI were identified. The prevalence of PAI was 4.17 per million. The estimated incidence was 0.45 per million per year. The mean age at diagnosis was 49.0 years, and PAI was more prevalent in men. Adrenal tuberculosis was the most common cause of PAI in patients diagnosed before 2000; for those diagnosed thereafter, adrenal metastasis and tuberculosis were comparable leading causes. The etiology of PAI was not identified in 34.9% of cases. Of the patients receiving glucocorticoid replacement therapy, prednisolone was more frequently administered than hydrocortisone (69.4% vs. 26.5%, respectively), and only 27.1% of all patients received fludrocortisone. We observed an increased prevalence of metabolic disease and osteoporosis during the follow-up period (median, 60.2 months). The observed overall mortality and disease-specific mortality rates were 11.9% and 3.1%, respectively.
Conclusion
The prevalence of PAI is significantly lower in Koreans than in reports from Western countries. The high frequency undetermined etiology in patients with PAI suggests the need to reveal accurate etiology of PAI in Korea.
Keywords: Addison disease, Autoimmune diseases, Glucocorticoids, Mortality
INTRODUCTION
Primary adrenal insufficiency (PAI), commonly termed Addison's disease, is a severe and potentially life-threatening condition [1]. It is defined as the inability of the adrenal cortex to produce sufficient concentrations of glucocorticoids and/or mineralocorticoids. Traditionally, adrenal tuberculosis was the most common cause of PAI; however, in industrialized countries autoimmune disorders are now the predominant cause (~90%) [2]. PAI is rare, with a reported prevalence of approximately 100 to 221 cases per million inhabitants in European countries and, particularly high in Scandinavia [3,4,5,6,7], and a significantly low incidence in other countries, including Japan [8].
Patients with PAI require glucocorticoid and/or mineralocorticoid replacement therapy. However, long-term glucocorticoid replacement therapy can lead to several complications, including reduced bone mineral density, an increased risk of fractures and metabolic disease, and impaired health-related quality of life [2,9]. Registry studies have reported high mortality rates in patients with PAI; moreover, mortality is significantly associated with younger age at diagnosis [9].
Due to the rarity of the condition, there are few studies on PAI in Korean adults, and such studies are limited to case reports [10,11,12] and a clinical review of a small number of patients [13]. To understand the population-based epidemiology of Korean patients with PAI, a nationwide multicenter study is required. Therefore, we performed the first registry-based nationwide survey to obtain data on the epidemiology, clinical characteristics, treatment strategy, and mortality rate of patients with PAI in Korea.
METHODS
Data collection
We aimed to identify all patients aged ≥18 years diagnosed with or treated for PAI by endocrinologists in Korea. Of all the secondary and tertiary care institutions in Korea, 30 hospitals participated in the search for data from patients with PAI managed at these institutions between January 2000 and December 2014. The registration process took place between June 2015 and February 2016. Patients with PAI were identified based on the following International Classification of Diseases, 10th revision (ICD-10) codes: A18.7 (adrenal tuberculosis), C79.7 (metastatic cancer to the adrenal gland), E27.1–4 and E27.8 (primary adrenocortical insufficiency, adrenal crisis, adrenal gland hypofunction, and other adrenal disease), E31.0 (polyglandular autoimmune syndrome), E37.5 (Addison's disease in tuberculosis), and E71.3 (adrenoleukodystrophy). Patients with congenital adrenal hyperplasia, iatrogenic adrenal insufficiency, postoperative adrenal insufficiency, and hypothalamic and/or hypopituitary adrenal dysfunction were excluded.
The medical records of all patients registered in the study were reviewed by multiple endocrinologists to validate the diagnosis of PAI, based on the following criteria: (1) simultaneous plasma levels of adrenocorticotropic hormone (ACTH) above the reference range and of cortisol below the reference range; or (2) a suboptimal response to the corticotropin stimulation test; or (3) clinical symptoms and signs suggestive of PAI, such as hyperpigmentation, typical electrolyte disturbances, salt craving, and chronic treatment with glucocorticoids and/or fludrocortisone.
The following data were collected from the registered patients: age at diagnosis, date of last visit, managing institution, sex, etiology, clinical symptoms, and signs related to adrenal insufficiency, comorbidities, combined autoimmune diseases, results of biochemical tests, glucocorticoid and mineralocorticoid replacement therapy, and mortality. We also collected the patients' initials, date of birth, and address to exclude duplicated patients.
The study was approved by the Institutional Review Board of each institution participating in the study, based on the same research protocol of Seoul National University Hospital (No. H-1505-051-671), and was conducted in accordance with the Declaration of Helsinki. The need to obtain informed consent from the study participants was waived due to the study's retrospective nature.
Statistical analysis
The prevalence and annual incidence were calculated based on population census results obtained from the Korean National Statistical Office's database. Data are presented as the mean±standard deviation (SD), median and interquartile range (IQR), or number (%) as appropriate. A P value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS statistics for Windows version 21 (IBM Co., Armonk, NY, USA).
RESULTS
Epidemiology
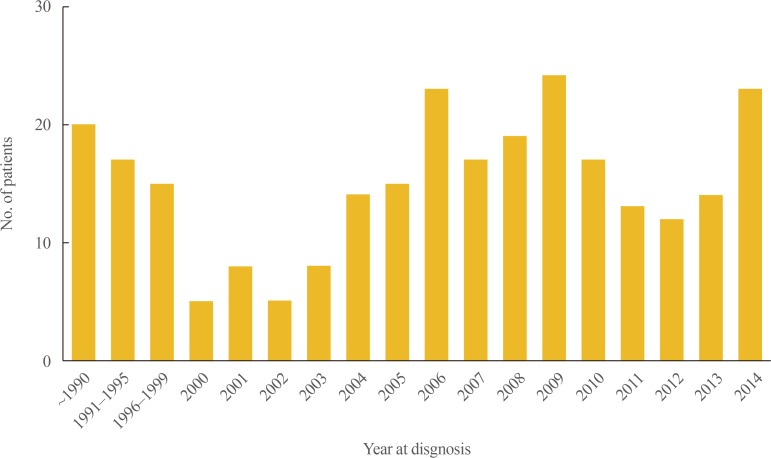
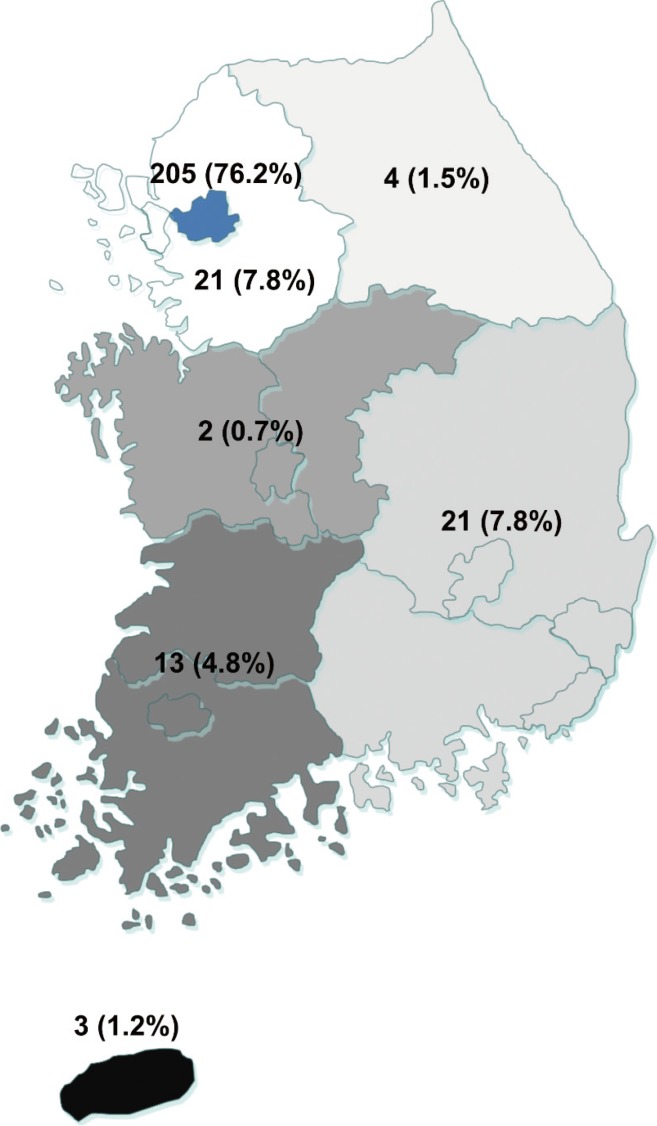
A total of 269 patients were diagnosed with or treated for PAI between 2000 and 2014. The estimated prevalence of PAI was 4.17 cases per million inhabitants and the estimated incidence was 0.45 cases per million inhabitants per year. Fig. 1 shows the observed incidence of PAI over time; no significant change in trend was observed. Fig. 2 demonstrates the regional distribution of patients according to the location of each hospital where the patients were diagnosed or treated for PAI. There was a marked difference between the numbers of patients in the different regions, with the majority of patients (76.2%) diagnosed with or treated for PAI in institutions located in Seoul.
Fig. 1. Observed incidence of primary adrenal insufficiency in Korea over time.
Fig. 2. Geographic distribution of patients with primary adrenal insufficiency in Korea.
Demographics of study subjects
During the study period, the number of patients newly diagnosed with PAI was four times higher between 2000 and 2014 than before 2000. The overall mean age at diagnosis was 49.0±19.7 years. However, patients diagnosed with PAI before 2000 were significantly younger than those diagnosed between 2000 and 2014 (30.7±21.0 years vs. 53.4±16.6 years, respectively; P<0.001). PAI was more prevalent in men than in women except for patients aged 70 to 79 years at diagnosis (1.68:1) (Table 1, Supplemental Fig. S1).
Table 1. Demographic Characteristics of Patients with Primary Adrenal Insufficiency.
| Year of diagnosis | Overall patients | Before 2000 | 2000–2014 | P value |
|---|---|---|---|---|
| Number | 269 | 52 | 217 | |
| Age at diagnosis, yr | 49.0±19.7 | 30.7±21.0 | 53.4±16.6 | <0.001 |
| <10 | 14 (5.2) | 12 (23.1) | 2 (0.9) | |
| 10–19 | 14 (5.2) | 8 (15.4) | 6 (2.8) | |
| 20–29 | 17 (6.3) | 4 (7.7) | 13 (6.0) | |
| 30–39 | 29 (10.8) | 6 (11.5) | 23 (10.6) | |
| 40–49 | 49 (18.2) | 11 (21.2) | 38 (17.5) | |
| 50–59 | 57 (21.2) | 7 (13.5) | 50 (23.0) | |
| 60–69 | 51 (19.0) | 3 (5.8) | 48 (22.1) | |
| 70–79 | 34 (12.6) | 1 (1.9) | 33 (15.2) | |
| ≥80 | 4 (1.5) | - | 4 (1.8) | |
| Gender | 0.147 | |||
| Men | 169 (62.8) | 32 (61.5) | 137 (63.1) | |
| Women | 100 (37.2) | 20 (38.5) | 80 (36.9) |
Values are expressed as mean±SD or number (%).
Etiology
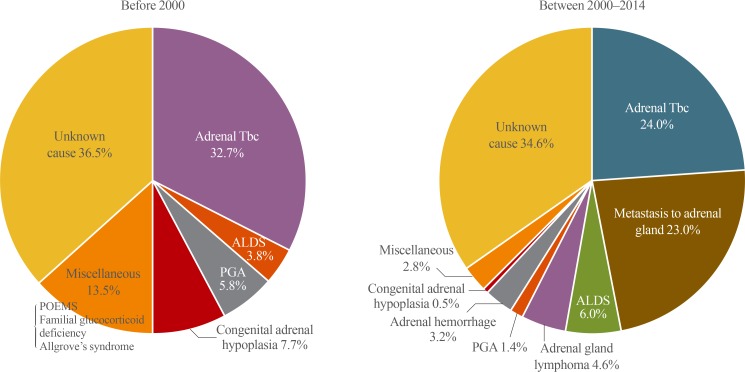
The cause of PAI was not identified in 34.9% of all patients. The most common cause of PAI among those for whom the etiology was confirmed, was adrenal tuberculosis (25.7%), followed by metastasis to adrenal gland (18.6%), adrenoleukodystrophy (5.6%), adrenal gland lymphoma (3.7%), adrenal hemorrhage (2.6%), POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes) syndrome (2.6%), polyglandular autoimmune syndrome (2.2%), congenital adrenal hypoplasia (1.9%), familial glucocorticoid deficiency (1.5%), and Allgrove's syndrome (0.8%). Fig. 3 shows the etiology of PAI according to the year of diagnosis. Before 2000, adrenal tuberculosis was the leading cause of PAI, whereas the frequency of metastasis to adrenal gland was comparable to that of adrenal tuberculosis between 2000 and 2014.
Fig. 3. Primary adrenal insufficiency etiologic pattern in patients diagnosed before 2000 and between 2000 and 2014. Tbc, tuberculosis; ALDS, adrenoleukodystrophy; PGA, polyglandular autoimmune syndrome; POEMS, polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes.
Clinical manifestations
Common symptoms at presentation were generalized weakness (53.5%), fatigue (41.3%), anorexia (36.8%), weight loss (29.7%), nausea (25.7%), and vomiting (21.2%). Abdominal pain (10.4%), arthralgia/myalgia (7.4%), salt craving (6.3%), diarrhea (4.5%), and constipation (3.7%) were also reported in a small number of patients. Electrolyte imbalance (37.2%) was the most common clinical sign at presentation. Hyperpigmentation (31.2%) and anemia (24.2%) were also common, while orthostatic hypotension (16.0%) and hypotension (15.6%) were reported in <20% of all patients.
Comorbidities at diagnosis and during follow-up
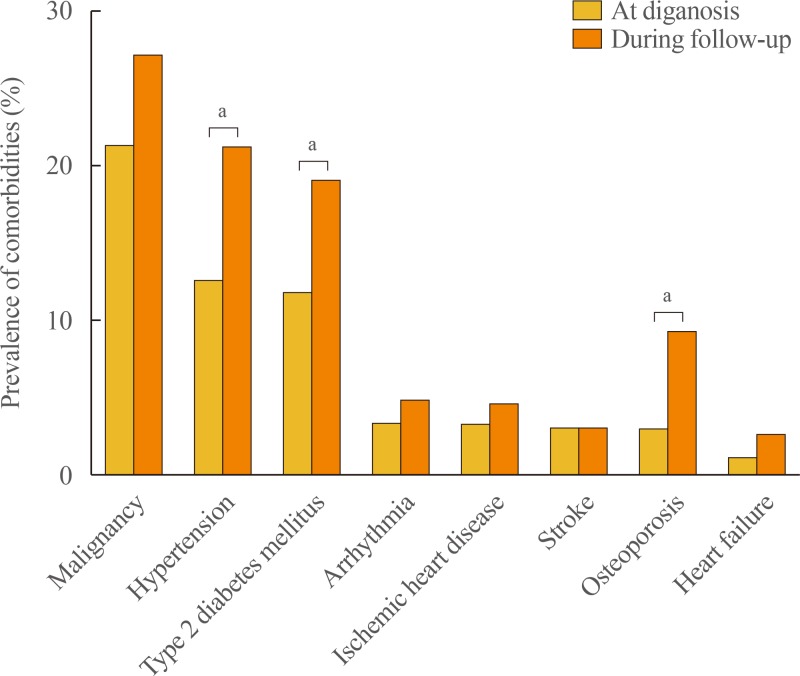
The prevalence of hypertension, type 2 diabetes mellitus, and osteoporosis increased during the follow-up period, from 12.6%, 11.9%, and 3.0%, respectively, to 21.2%, 19.0%, and 9.3%, respectively (all P<0.05). There was no significant difference in the prevalence of malignancy, arrhythmias, ischemic heart disease, stroke, and heart failure at diagnosis and during the period of follow-up (Fig. 4). The median duration of follow-up was 60.2 months (IQR, 8.0 to 138.1).
Fig. 4. Prevalence of comorbidities at diagnosis and during follow-up in patients with primary adrenal insufficiency. aP<0.05.
Combined autoimmune diseases
Hypothyroidism was the most common autoimmune disease observed in patients with PAI (13.8%). Other autoimmune disorders—including hyperthyroidism, premature ovarian failure, alopecia, autoimmune hepatitis, vitamin B12 deficiency, and type 1 diabetes mellitus—were reported in <3% of all patients. Vitiligo was not reported.
Biochemical measurements
The median plasma ACTH and serum cortisol levels were 202.0 pg/mL (IQR, 41.7 to 1,022.8) and 4.0 µg/dL (IQR, 1.6 to 8.6), respectively. The median plasma renin activity was 5.0 ng/mL/hr (IQR, 1.1 to 14.8) and the median plasma aldosterone concentration was 8.2 ng/dL (IQR, 2.8 to 19.9). The corticotropin stimulation test was performed in 72.5% of all patients; plasma aldosterone concentrations and plasma renin activity were measured in only 48.2% of those patients. The median peak cortisol level was 8.5 µg/dL (IQR, 3.0 to 12.9) and the median peak plasma aldosterone concentration was 11.1 ng/dL (IQR, 4.2 to 42.3).
Replacement therapy
Of the 269 patients, 245 (91.1%) received glucocorticoid replacement therapy (Table 2). The preferred glucocorticoid replacement regimen was prednisolone (69.4%), given at a median dose of 7.5 mg once daily. Hydrocortisone was only administered to 26.5% of all patients, at a median dose of 20 mg. Dexamethasone and deflazacort were used in 2.0% and 0.8% of patients, respectively. A significantly lower proportion of patients received mineralocorticoid (27.1%) than glucocorticoid (91.1%) therapy. The median dose of fludrocortisone was 0.1 mg daily.
Table 2. Glucocorticoid and Mineralocorticoid Replacement Therapy in Primary Adrenal Insufficiency Patients.
| Type of treatment | No. of patients (%) | Regimen | Median daily dose, mg | |
|---|---|---|---|---|
| Frequency/day | No. of patients (%) | |||
| Glucocorticoid replacement | 245 (91.1) | |||
| Prednisolone | 170 (69.4) | q.d. | 132 (77.6) | 7.5 |
| b.i.d. | 38 (22.4) | |||
| Hydrocortisone | 65 (26.5) | q.d. | 12 (18.5) | 20 |
| b.i.d. | 50 (76.9) | |||
| t.i.d. | 3 (4.6) | |||
| Dexamethasone | 5 (2.0) | q.d. | 3 (60.0) | 4 |
| b.i.d. | 2 (40.0) | |||
| Deflazacort | 2 (0.8) | q.d. | 2 (100.0) | 7.5 |
| Unknown | 3 (1.2) | - | - | - |
| Mineralocorticoid replacement | 73 (27.1) | q.d. | 73 (100.0) | 0.1 |
Values are expressed as number (%).
q.d., once daily; b.i.d., twice daily; t.i.d., 3 times daily.
Mortality
During the follow-up period, 32 patients (11.9%) died. The most common cause of death was malignancy (68.8%), followed by infection (9.4%), pulmonary disease (6.3%), gastrointestinal disease (6.3%), and unknown causes (6.3%). PAI disease-specific mortality was reported in only one case (3.1%).
DISCUSSION
The present study is the first nationwide survey of patients with PAI in South Korea. We identified 269 registered adults diagnosed with or treated for PAI between 2000 and 2014. The estimated prevalence of PAI was 4.17 cases per million inhabitants; this is significantly lower than the prevalence reported in Western countries [7,14,15,16]. The estimated annual incidence of 0.45 cases per million inhabitants is approximately 10% of the incidence reported in national surveys conducted in Sweden and Norway [17,18]. The reason for the low incidence of PAI in Korea is unclear; however, it seems that certain racial or regional factors may influence the occurrence of the disease, considering that the incidence of PAI in Korea is similar to that reported in Japan (0.15 per million per year) [8].
Although 30 major secondary and tertiary care institutions participated in this survey, there were some regional differences in the participation rate. This could have resulted in underestimation of the actual incidence of PAI in particular regions with low participation rates. The sex distribution of the condition was somewhat different to that of previous reports, in which PAI was more prevalent in women than in men [8,17,18]. In the present survey, PAI was more frequently observed in men than in women, with an overall female to male ratio of 1:1.68. It is unclear whether the observed male preponderance is a characteristic of Korean PAI patients or a result of selection bias in this registry-based study.
In the present study, the etiology of PAI was not identified in >30% of cases. A recently published clinical practice guideline recommends using 21-hydroxylase antibody testing in the diagnostic approach to exclude autoimmunity, because in the majority (~90%) of cases of adult-onset PAI, the cause is an autoimmune disease [1]. This test is not commercially available in Korea; hence, many of the patients with an unknown etiology are presumed to have autoimmune PAI. The prevalence of combined autoimmune disorders was lower in the present study than in previous studies [3,19,20,21]. However, considering the retrospective design of this record-based study, it is possible that the prevalence of accompanying autoimmune disease was underestimated because of missing data. Therefore, to identify the exact etiologic pattern of PAI in Koreans, confirmation of autoimmunity by means of laboratory testing (including 21-hydroxylase autoantibody testing) is required as soon as possible.
Excluding cases with an unknown etiology, the most common cause of PAI diagnosed before the year 2000 was adrenal tuberculosis; thereafter, adrenal tuberculosis and adrenal gland metastases were both leading causes of PAI. This means that the etiology changed dramatically as the prevalence of tuberculosis declined. Compared with the high prevalence of adrenal tuberculosis and adrenal gland metastases in adult-onset PAI, in childhood-onset PAI—which rarely presented before 10 years of age—the most common causes were congenital adrenal hypoplasia, familial glucocorticoid deficiency, and Allgrove's syndrome, suggesting a close association with genetic defects. These findings show that the etiology of PAI varies according to the characteristics of the study population.
In the present survey, we observed that once-daily prednisolone was the preferred pharmacological replacement regimen (69.4%) for glucocorticoid deficiency. The median dose of prednisolone administered in Korea (7.5 mg/day) is higher than that recommended by current clinical practice guidelines (3 to 5 mg/day). In most industrialized countries, hydrocortisone was reported to be the most frequently used drug as replacement. Because of its short half-life (approximately 90 minutes), if multiple dosing is employed hydrocortisone can mimic physiologic conditions; this may be beneficial in avoiding long-term complications of glucocorticoid replacement. Moreover, hydrocortisone is a more potent drug for combined mineralocorticoid deficiency than are other replacement drugs, including prednisolone. Hence, the current guideline recommends the use of hydrocortisone (15 to 25 mg/day) as first-line glucocorticoid replacement therapy for patients with PAI [1]. The most likely reason for the preferred use of prednisolone is the decline in adherence to hydrocortisone therapy due to the need for multiple dosing. Another hypothesis is that the relatively low mineralocorticoid deficiency rate—a result of the high salt intake of Koreans—may have averted clinically significant problems with prednisolone treatment, which has less mineralocorticoid activity than hydrocortisone [22,23]. However, in the present study, laboratory tests for plasma aldosterone concentrations and plasma renin activity were performed in only half of the patients who underwent corticotropin stimulation testing. The low level of surveillance for mineralocorticoid deficiency could have resulted in suboptimal treatment of patients with combined mineralocorticoid deficiency, in particular, of those receiving prednisolone. Furthermore, the fludrocortisone treatment rate (27.1%) was significantly lower in this study than in studies from other countries (70% to 90%) [7,24,25]. Therefore, we recommend active surveillance for mineralocorticoid status at diagnosis. This should ensure that patients with confirmed mineralocorticoid deficiency receive appropriate treatment with fludrocortisone and/or glucocorticoid replacement with an agent with high mineralocorticoid activity, such as hydrocortisone.
The prevalence of type 2 diabetes mellitus, hypertension, and osteoporosis increased during the follow-up period, consistent with previous reports [26,27]. This seems to be associated with glucocorticoid replacement therapy. Long-term glucocorticoid treatment can result in several complications, including metabolic diseases, deterioration in bone strength, and decreased quality of life [28,29,30]. Fracture and quality of life assessments were not available in the present study. Although we could not compare the prevalence of comorbidities in PAI patients and in healthy subjects, the increased prevalence of such complications during the treatment period is itself meaningful. Because patients with PAI require long-term glucocorticoid replacement therapy, these findings serve to remind clinicians that it is important to pay close attention to the occurrence of long-term complications, in addition to the treatment outcome.
In the present study, the overall mortality rate was 11.9%, with a disease-specific mortality rate of 3.1%. However, half of the registered patients were lost to follow-up; hence, the actual mortality rate may be higher than what was observed. Population-based studies reported more than a 2-fold mortality increase in patients with PAI, associated with malignancy, cardiovascular disease, and infectious conditions [31,32]. Contrary to previous studies, a recent survey in Norway showed no significant mortality increase among PAI patients. However, in a subgroup analysis, younger age (<40 years of age) was associated with a twofold increase in the standardized mortality ratio [3]. One possible reason for the inconsistent mortality data is that the duration and dose of glucocorticoid replacement therapy differs according to the population studied. Nevertheless, younger age certainly seems to be associated with high mortality in terms of acute adrenal failure, infections, and sudden death [3].
There are several regards to be discussed in the present study. First, because this study was a registry-based multicenter survey which retrospectively collected patients' medical records more than 15 years, there were considerable differences in methods for biochemical measurements such as cortisol and ACTH across the individual institution and even in the same institution over time. Furthermore, the diagnostic criteria for an optimal response to the corticotropin stimulation test were not consistent during the study period. There also may be some possibility that certain critical illness or drugs that could affect adrenal function were not excluded at the diagnosis. Given that limitations, we did not consider a specific cut-off cortisol value for the diagnosis of PAI, but included the diagnostic criteria of “a suboptimal response to the corticotropin stimulation test” to allow endocrinologists to clarify the diagnosis from the clinicians' perspectives. Second, the median basal cortisol level was not that low. Considering the sensitivity of cortisol measurements, it may be attributed to the various serum cortisol measurement methods, difference in normal serum cortisol range, and when cortisol was measured during the day. In addition, we could not collect the data on the specified malignancy history and more details regarding mortality. Because cause of death was analyzed based on the answers to surveys alone, it may not fully reflect actual mortality data in our study.
Notwithstanding several limitations attributed to the retrospective nature of the present study, it is valuable as the first multicenter, nationwide survey analyzing the epidemiology of PAI in South Korea. This survey provides a comprehensive overview of the epidemiology, clinical characteristics, treatment, and mortality of patients with PAI in Korea. Notably, the cause of PAI was not identified in >30% of patients, suggesting that efforts to clarify the exact etiology of PAI are needed. Furthermore, these data imply the need to improve the glucocorticoid and mineralocorticoid replacement strategies currently used, giving extra attention to countering the long-term complications associated with treatment. Given the low incidence of PAI in Korea, further follow-up studies of large, unbiased cohorts are needed to better understand the clinical impact of PAI.
ACKNOWLEDGMENTS
This study was supported by the Korean Endocrine Society of EnM Research Award 2016, and the Korean Adrenal Gland and Endocrine Hypertension Study Group.
We thank all the 30 hospitals and contributors for their cooperation in this study. The full list of all participating university hospitals and contributors is as follows: The Korean Adrenal Gland and Endocrine Hypertension Study Group, Korean Endocrine Society.
Eun-Hee Cho (Kangwon National University Hospital, Chuncheon), Dong Seop Choi (Korea University Anam Hospital, Seoul), Choon Hee Chung (Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju), Dong Jin Chung (Chonnam National University Hospital, Gwangju), Ho Yeon Chung (Kyung Hee University Hospital at Gangdong, Seoul), Ho-Cheol Kang (Chonnam National University Hwasun Hospital, Hwasun), Mi Yeon Kang (Saint Carollo Hospital, Suncheon), Chul Hee Kim (Soonchunhyang University Bucheon Hospital, Bucheon), Doo Man Kim (Hallym University Kangdong Sacred Heart Hospital, Seoul), Hye Sun Kim (Keimyung University Dongsan Medical Center, Daegu), In Joo Kim (Pusan National University Hospital, Busan), Jae Hyeon Kim (Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul), Jin Taek Kim (Nowon Eulji Medical Center, Eulji University School of Medicine, Seoul), Jung Guk Kim (Kyungpook National University Hospital, Daegu), Jung Hee Kim Kim (Seoul National University Hospital, Seoul), Kyoung Min Kim (Seoul National University Bundang Hospital, Seongnam), Kyung Ah Kim (Dongguk University Ilsan Hospital, Goyang), Sang Wan Kim (Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul), Seung-Hyun Ko (St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon), Sang Ah Lee (Jeju National University Hospital, Jeju), Seung Hun Lee (Asan Medical Center, University of Ulsan College of Medicine, Seoul), Dong-Mee Lim (Konyang University Hospital, Daejeon), Sung Dae Moon (Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Incheon), Jong Suk Park (Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul), Sang Youl Rhee (Kyung Hee University Medical Center, Seoul), Yumie Rhee (Severance Hospital, Yonsei University College of Medicine, Seoul), Ohk-Hyun Ryu (Hallym University Chuncheon Sacred Heart Hospital, Chuncheon), Kee Ho Song (Konkuk University Medical Center, Seoul), Min Ho Song (Chungnam National University Hospital, Daejeon), and Sung Hoon Yu (Hallym University Kangnam Sacred Heart Hospital, Seoul).
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
SUPPLEMENTARY MATERIAL
Distribution of age at diagnosis in men (orange bars) and women (gray bars) patients with primary adrenal insufficiency.
References
- 1.Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:364–389. doi: 10.1210/jc.2015-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bensing S, Hulting AL, Husebye ES, Kampe O, Lovas K. Management of endocrine disease: epidemiology, quality of life and complications of primary adrenal insufficiency. A review. Eur J Endocrinol. 2016;175:R107–R116. doi: 10.1530/EJE-15-1242. [DOI] [PubMed] [Google Scholar]
- 3.Erichsen MM, Lovas K, Fougner KJ, Svartberg J, Hauge ER, Bollerslev J, et al. Normal overall mortality rate in Addison's disease, but young patients are at risk of premature death. Eur J Endocrinol. 2009;160:233–237. doi: 10.1530/EJE-08-0550. [DOI] [PubMed] [Google Scholar]
- 4.Wallace I, Cunningham S, Lindsay J. The diagnosis and investigation of adrenal insufficiency in adults. Ann Clin Biochem. 2009;46(Pt 5):351–367. doi: 10.1258/acb.2009.009101. [DOI] [PubMed] [Google Scholar]
- 5.Chakera AJ, Vaidya B. Addison disease in adults: diagnosis and management. Am J Med. 2010;123:409–413. doi: 10.1016/j.amjmed.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Laureti S, Vecchi L, Santeusanio F, Falorni A. Is the prevalence of Addison's disease underestimated? J Clin Endocrinol Metab. 1999;84:1762. doi: 10.1210/jcem.84.5.5677-7. [DOI] [PubMed] [Google Scholar]
- 7.Olafsson AS, Sigurjonsdottir HA. Increasing prevalence of Addison disease: results from a nationwide study. Endocr Pract. 2016;22:30–35. doi: 10.4158/EP15754.OR. [DOI] [PubMed] [Google Scholar]
- 8.Nomura K, Demura H, Saruta T. Addison's disease in Japan: characteristics and changes revealed in a nationwide survey. Intern Med. 1994;33:602–606. doi: 10.2169/internalmedicine.33.602. [DOI] [PubMed] [Google Scholar]
- 9.Mazziotti G, Formenti AM, Frara S, Roca E, Mortini P, Berruti A, et al. Management of endocrine disease: risk of overtreatment in patients with adrenal insufficiency. Current and emerging aspects. Eur J Endocrinol. 2017;177:R231–R248. doi: 10.1530/EJE-17-0154. [DOI] [PubMed] [Google Scholar]
- 10.Lee KS, Chung YS, Park KH, Kim HS, Kim HM. A case of primary bilateral adrenal lymphoma with partial adrenal insufficiency. Yonsei Med J. 1999;40:297–300. doi: 10.3349/ymj.1999.40.3.297. [DOI] [PubMed] [Google Scholar]
- 11.Park HD, Park SJ, Choi YM, Kang JH. Adrenomyeloneuropathy presenting with adrenal insufficiency. Ann Rehabil Med. 2013;37:563–566. doi: 10.5535/arm.2013.37.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YW, Won JC, Ki CS, Lee HY, Ahn HS, Lee YK, et al. Clinical and genetic analysis of a Korean patient with late-onset X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism: identification of a novel mutation in the NR0B1 gene. J Int Med Res. 2008;36:357–361. doi: 10.1177/147323000803600220. [DOI] [PubMed] [Google Scholar]
- 13.Sung SK, Kwon YJ, Lee BW, Kim DM, Yoo HJ. Clinical review of 20 cases of Addison's disease in Korea: including previously reported cases and 6 new cases at the National Medical Center. Korean J Intern Med. 1988;3:72–76. doi: 10.3904/kjim.1988.3.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer G, Neumann K, Badenhoop K, Linder R. Increasing prevalence of Addison's disease in German females: health insurance data 2008-2012. Eur J Endocrinol. 2014;170:367–373. doi: 10.1530/EJE-13-0756. [DOI] [PubMed] [Google Scholar]
- 15.Mason AS, Meade TW, Lee JA, Morris JN. Epidemiological and clinical picture of Addison's disease. Lancet. 1968;2:744–747. doi: 10.1016/s0140-6736(68)90948-3. [DOI] [PubMed] [Google Scholar]
- 16.Nerup J. Addison's disease: clinical studies. A report of 108 cases. Acta Endocrinol (Copenh) 1974;76:127–141. doi: 10.1530/acta.0.0760127. [DOI] [PubMed] [Google Scholar]
- 17.Bjornsdottir S, Sundstrom A, Ludvigsson JF, Blomqvist P, Kampe O, Bensing S. Drug prescription patterns in patients with Addison's disease: a Swedish population-based cohort study. J Clin Endocrinol Metab. 2013;98:2009–2018. doi: 10.1210/jc.2012-3561. [DOI] [PubMed] [Google Scholar]
- 18.Lovas K, Husebye ES. High prevalence and increasing incidence of Addison's disease in western Norway. Clin Endocrinol (Oxf) 2002;56:787–791. doi: 10.1046/j.1365-2265.2002.t01-1-01552.x. [DOI] [PubMed] [Google Scholar]
- 19.Reato G, Morlin L, Chen S, Furmaniak J, Smith BR, Masiero S, et al. Premature ovarian failure in patients with autoimmune Addison's disease: clinical, genetic, and immunological evaluation. J Clin Endocrinol Metab. 2011;96:E1255–E1261. doi: 10.1210/jc.2011-0414. [DOI] [PubMed] [Google Scholar]
- 20.Fichna M, Fichna P, Gryczynska M, Walkowiak J, Zurawek M, Sowinski J. Screening for associated autoimmune disorders in Polish patients with Addison's disease. Endocrine. 2010;37:349–360. doi: 10.1007/s12020-010-9312-x. [DOI] [PubMed] [Google Scholar]
- 21.Betterle C, Scarpa R, Garelli S, Morlin L, Lazzarotto F, Presotto F, et al. Addison's disease: a survey on 633 patients in Padova. Eur J Endocrinol. 2013;169:773–784. doi: 10.1530/EJE-13-0528. [DOI] [PubMed] [Google Scholar]
- 22.Park SM, Joung JY, Cho YY, Sohn SY, Hur KY, Kim JH, et al. Effect of high dietary sodium on bone turnover markers and urinary calcium excretion in Korean postmenopausal women with low bone mass. Eur J Clin Nutr. 2015;69:361–366. doi: 10.1038/ejcn.2014.284. [DOI] [PubMed] [Google Scholar]
- 23.Kwon SJ, Ha YC, Park Y. High dietary sodium intake is associated with low bone mass in postmenopausal women: Korea National Health and Nutrition Examination Survey, 2008-2011. Osteoporos Int. 2017;28:1445–1452. doi: 10.1007/s00198-017-3904-8. [DOI] [PubMed] [Google Scholar]
- 24.Johannsson G, Filipsson H, Bergthorsdottir R, Lennernas H, Skrtic S. Long-acting hydrocortisone for glucocorticoid replacement therapy. Horm Res. 2007;68(Suppl 5):182–188. doi: 10.1159/000110621. [DOI] [PubMed] [Google Scholar]
- 25.Margulies PL, Mullen J. North American survey of individuals with Addison's disease (1997) [Internet] Great Neck: National Adrenal Diseases Foundation; c1987. [cited 2017 Oct 26]. Available from: http://www.nadf.us/contact-nadf/ [Google Scholar]
- 26.Lovas K, Gjesdal CG, Christensen M, Wolff AB, Almas B, Svartberg J, et al. Glucocorticoid replacement therapy and pharmacogenetics in Addison's disease: effects on bone. Eur J Endocrinol. 2009;160:993–1002. doi: 10.1530/EJE-08-0880. [DOI] [PubMed] [Google Scholar]
- 27.Giordano R, Marzotti S, Balbo M, Romagnoli S, Marinazzo E, Berardelli R, et al. Metabolic and cardiovascular profile in patients with Addison's disease under conventional glucocorticoid replacement. J Endocrinol Invest. 2009;32:917–923. doi: 10.1007/BF03345773. [DOI] [PubMed] [Google Scholar]
- 28.Lovas K, Loge JH, Husebye ES. Subjective health status in Norwegian patients with Addison's disease. Clin Endocrinol (Oxf) 2002;56:581–588. doi: 10.1046/j.1365-2265.2002.01466.x. [DOI] [PubMed] [Google Scholar]
- 29.Gurnell EM, Hunt PJ, Curran SE, Conway CL, Pullenayegum EM, Huppert FA, et al. Long-term DHEA replacement in primary adrenal insufficiency: a randomized, controlled trial. J Clin Endocrinol Metab. 2008;93:400–409. doi: 10.1210/jc.2007-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kluger N, Matikainen N, Sintonen H, Ranki A, Roine RP, Schalin-Jantti C. Impaired health-related quality of life in Addison's disease: impact of replacement therapy, comorbidities and socio-economic factors. Clin Endocrinol (Oxf) 2014;81:511–518. doi: 10.1111/cen.12484. [DOI] [PubMed] [Google Scholar]
- 31.Bergthorsdottir R, Leonsson-Zachrisson M, Oden A, Johannsson G. Premature mortality in patients with Addison's disease: a population-based study. J Clin Endocrinol Metab. 2006;91:4849–4853. doi: 10.1210/jc.2006-0076. [DOI] [PubMed] [Google Scholar]
- 32.Bensing S, Brandt L, Tabaroj F, Sjoberg O, Nilsson B, Ekbom A, et al. Increased death risk and altered cancer incidence pattern in patients with isolated or combined autoimmune primary adrenocortical insufficiency. Clin Endocrinol (Oxf) 2008;69:697–704. doi: 10.1111/j.1365-2265.2008.03340.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of age at diagnosis in men (orange bars) and women (gray bars) patients with primary adrenal insufficiency.