Abstract
Sporadic Creutzfeldt-Jakob disease (sCJD), the most common human prion disease, is transmissible through iatrogenic routes due to abundant infectious prions [misfolded forms of the prion protein (PrPSc)] in the central nervous system (CNS). Some epidemiological studies have associated sCJD risk with non-CNS surgeries. We explored the potential prion seeding activity and infectivity of skin from sCJD patients. Autopsy or biopsy skin samples from 38 patients [21 sCJD, 2 variant CJD (vCJD), and 15 non-CJD] were analyzed by Western blotting and real-time quaking-induced conversion (RT-QuIC) for PrPSc. Skin samples from two patients were further examined for prion infectivity by bioassay using two lines of humanized transgenic mice. Western blotting revealed dermal PrPSc in one of five deceased sCJD patients and one of two vCJD patients. However, the more sensitive RT-QuIC assay detected prion seeding activity in skin from all 23 CJD decedents but not in skin from any non-CJD control individuals (with other neurological conditions or other diseases) during blinded testing. Although sCJD patient skin contained ~103- to 105-fold lower prion seeding activity than did sCJD patient brain tissue, all 12 mice from two transgenic mouse lines inoculated with sCJD skin homogenates from two sCJD patients succumbed to prion disease within 564 days after inoculation. Our study demonstrates that the skin of sCJD patients contains both prion seeding activity and infectivity, which raises concerns about the potential for iatrogenic sCJD transmission via skin.
INTRODUCTION
Prion diseases are fatal, transmissible spongiform encephalopathies (TSEs) that affect humans and other animals. They are associated with the deposition of misfolded forms of the prion protein (PrPSc) in the central nervous system (CNS) that propagate by seeding the multimerization and conformational conversion of normal cellular prion protein (1). These misfolded PrP conformers are the primary constituent of infectious TSE agents (prions).
Human prion diseases include Creutzfeldt-Jakob disease (CJD), fatal insomnia, Gerstmann-Sträussler-Scheinker syndrome, kuru, and variably protease-sensitive prionopathy (2). The etiology can be sporadic, acquired by infection, or inherited. Although phenotypically heterogeneous, prion diseases are clinically characterized by varying degrees of dementia, myoclonus, extrapyramidal signs, cerebellar ataxia, and pyramidal signs and pathologically characterized by spongiform degeneration, neuronal loss, and astrocytosis in the brain. The most common form of human prion disease, sporadic CJD (sCJD), usually has a rapid and dramatic clinical course with a mean duration of less than 6 months. More than six subtypes of sCJD can be differentiated on the basis of their clinical phenotypes, together with the polymorphic residue at codon 129 of PrPSc [Met/Met (MM), Val/Val (VV), or Met/Val (MV)], and distinct or coexisting PrPSc types (1 or 2) associated with different amino terminal protease cleavage sites (3).
sCJD is transmissible iatrogenically, for example, via CNS-associated or cornea-associated surgical operations, or injections of brain-derived contaminated growth hormone and gonadotropins (4). Some epidemiological observations have suggested that the risk of sCJD may even be associated with a history of having various non-CNS–related surgeries, the number of surgeries, and the age at the time of the first surgery (5–8). A case-control study also reported that statistically significant odds ratios were obtained for injury to (or surgery on) the head, face, or neck and trauma to other parts of the body (9). However, other studies reported little correlation between surgeries and sCJD incidence (10, 11).
Although the involvement of the skin in sCJD infections remains unclear, proteinase K (PK)–resistant PrPSc was detected in the skin of a single patient with variant CJD (vCJD) (12), a distinct CJD strain that originated from exposure to bovine spongiform encephalopathy. Here, using Western blotting (13), real-time quaking-induced conversion (RT-QuIC) assay (14), and bioassay with humanized transgenic (Tg) mice (15), we tested for PrPSc and prion infectivity in skin samples from patients with sCJD.
RESULTS
CJD patient clinical information and detection of PrPSc in brain tissue
Skin tissues were collected at autopsy or by biopsy from 38 subjects, including patients with sCJD or vCJD, as well as from non-CJD control individuals with or without other neurological conditions (Table 1 and table S1) (16, 17). Both skin and brain tissues were collected from 30 of the 38 subjects, including cases with sCJD (n = 21), vCJD (n = 2), and non-CJD patients with other neurological conditions (n = 7). The demographic and clinical features of the patients with either sCJD or vCJD are summarized in Table 1. The demographic characteristics and diagnoses of non-CJD controls are provided in table S1. Patients with sCJD had either MM, MV, or VV polymorphisms at residue 129 of the PrP sequence and either type 1, type 2, or coexisting types 1 and 2 PrPSc (sCJDMM1, sCJDMM2, sCJDMV1, sCJDMV2, sCJDVV1, sCJDVV2, sCJDMV1-2, sCJDVV1-2, or sCJDMM1-2), as determined by Western blotting and histology of brain tissues (3). Western blotting and neurohistology detected PrPSc and spongiform degeneration in the brains of all of the CJD cases but not in the non-CJD controls (fig. S1 shows examples of brain tissue from five sCJD, two vCJD, and two non-CJD patients).
Table 1. Characteristics of sCJD and vCJD patients.
sCJD, sporadic Creutzfeldt-Jakob disease; vCJD, variant CJD; MRI, magnetic resonance imaging; CSF, cerebrospinal fluid.
| Mean age, years (SD) | 59.4 (15.3) |
| Mean duration, months (SD) | 10.7 (10.8) |
| Male/female | 10:13 (43.5%:56.5%) |
| Race, n (%) | |
| White | 19 (82.6%) |
| Black | 0 (0) |
| Hispanic | 1 (4.3%) |
| Mixed | 1 (4.3%) |
| Unknown | 2 (8.7%) |
| Cognitive symptoms, n (% with known symptoms) | 20 (100%) |
| Myoclonus, n (% with known symptoms) | 10 (52.6%) |
| Cerebellar symptoms, n (% with known symptoms) | 18 (94.7%) |
| Visual symptoms, n (% with known symptoms) | 11 (57.9%) |
| Pyramidal symptoms, n (% with known symptoms) | 8 (42.1%) |
| Extrapyramidal symptoms, n (% with known symptoms) | 8 (42.1%) |
| PSWCs on EEG, n (%)* | 0 (0) |
| Brain MRI suggestive of prion disease, n (%)† | 16 (88.9%) |
| CSF 14-3-3 positive, n (%) | 13 (59.1%) |
| Mean CSF τ concentration, pg/ml (SD) | 7136.8 (6237.0) |
| Prion disease subtype, n (%) | |
| MM1 | 6 (26.1%) |
| MM2 | 3 (13%) |
| MV1 | 1 (4.3%) |
| VV1 | 1 (4.3%) |
| VV2 | 4 (17.4) |
| MV1-2 | 3 (13%) |
| VV1-2 | 1 (4.3) |
| MM1-2 | 2 (8.7) |
| vCJD‡ | 2 (8.7) |
Detection of PrPSc in the skin by Western blotting and immunohistochemistry
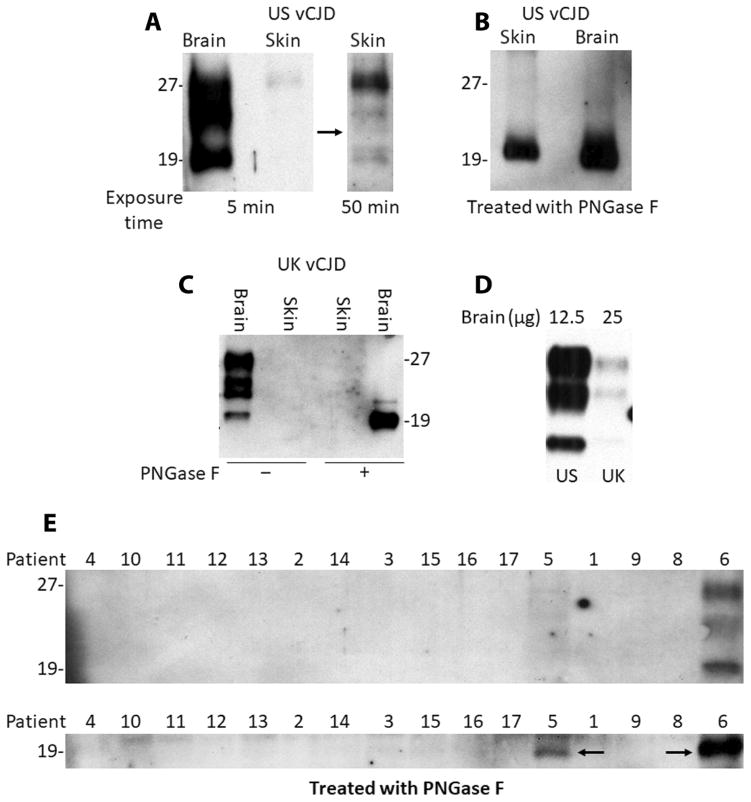
Previously, PrPSc was barely detectable by Western blotting in a skin sample collected postmortem from the torso area of a vCJD patient after enrichment with sodium phosphotungstate precipitation (12, 17). We first examined skin samples from the torso of the same vCJD patient from the United States (US vCJD) and from the forearm of another vCJD patient from the United Kingdom (UK vCJD) using an approach that previously succeeded in enriching and detecting PrPSc in the skin of animals naturally and experimentally infected with scrapie (13). After a series of ultracentrifugation steps followed by PK treatment, the PK-treated, PrPSc-concentrated fraction (termed P4) was obtained. PrPSc was detectable in the US vCJD patient P4 sample on Western blots probed with the anti-PrP antibody 3F4 against human PrP(96–112) or anti-C antibody against human PrP(220–231) after treatment with PK with or without peptide N-glycosidase F (PNGase F) (Fig. 1, A and B), consistent with the previous study (12). However, no PrPSc was detected in the skin of the UK vCJD patient after the same treatment (Fig. 1C). The amount of brain PrPSc in the two vCJD cases was compared by Western blotting, and a six- to sevenfold lower amount was found in the UK patient than in the U.S. patient by quantitative densitometric analysis (Fig. 1D).
Fig. 1. Western blot analysis of skin tissue from CJD and non-CJD patients.
(A) Western blot analysis of proteinase K (PK)–resistant misfolded forms of the prion protein (PrPSc) in skin from the U.S. variant Creutzfeldt-Jakob disease (US vCJD) patient. The blot was probed with anti-C antibody against human PrP(220–231) with an exposure time of 5 or 50 min. The skin samples [PK-treated, PrPSc-concentrated fraction (P4) after enrichment by ultracentrifugation] were treated with PK (25 μg/ml) at 37°C for 1 hour, followed by addition of Roche complete protease inhibitor cocktail. A CJD brain homogenate (brain) was used as a positive control. The black arrow refers to the longer exposure time of the blot on the right corresponding to the right lane of the left panel (at normal exposure). (B) Western blot analysis of PrP from the US vCJD patient skin sample treated with PK and peptide N-glycosidase F (PNGase F) and probed with the 3F4 anti-PrP antibody. The CJD brain homogenate was used as a positive control. (C) Western blot analysis of PrP in skin or brain samples from the UK vCJD patient treated with PK or PK plus PNGase F and probed with the anti-PrP antibody 3F4. (D) Comparison of the amounts of PrPSc in the brain between the US vCJD and UK vCJD cases treated with PK. (E) Western blot analysis of PrPSc in skin samples from sCJD patient nos. 1 to 5, vCJD patient no. 6, and non-CJD patient nos. 8 to 17, treated with PK (top) or PK plus PNGase F (bottom). Skin samples from about 60 to 80 mg of skin tissues for each case (~6 mm × 6 mm in area) were used for PrPSc enrichment and loading onto the gel. The vCJD patient no. 6 skin sample was used as a positive control. The black arrows indicate the PK-resistant deglycosylated PrPSc. The results shown in (A) to (E) are representative of three independent experiments.
Using the same method, we next examined the PrPSc-enriched skin samples (P4 fraction) from the apex (top of the head), an area next to the ear, or other body areas including the forearm, torso, or leg of 5 confirmed sCJD patients and 10 non-CJD controls, using the US vCJD skin as a positive control. After PK treatment, no PK-resistant PrP was detectable in any of the non-CJD and sCJD skin samples except for one sCJD case (patient no. 5), who exhibited very faint bands that were much weaker than those shown in the vCJD skin sample positive control (patient no. 6) (Fig. 1E, top). To increase sensitivity, we treated enriched preparations with both PK and PNGase F before Western blot analysis using anti-C antibody, as previously described (18). Only the skin sample from patient no. 5 produced a deglycosylated PrPSc band that co-migrated with the corresponding vCJD band (Fig. 1E, bottom). Moreover, the intensity of this PrP band was about four- to fivefold lower in the sCJD skin sample than in the US vCJD skin sample positive control (Fig. 1E), as measured by densitometric analysis of the protein bands. These results indicated that the amount of PK-resistant PrPSc in the skin tissues of sCJD patients was either low or undetectable in most cases by Western blotting.
Compared to the US vCJD case, the UK vCJD patient exhibited no Western blotting–detectable PrPSc in the skin and also a six- to sevenfold lower amount of PrPSc in brain tissue (Fig. 1D), which may be associated with a shorter disease duration in the UK case (18 months for the UK vCJD patient versus 34 months for the US vCJD patient). In sCJD patients, it has been reported that the higher amounts of brain PrPSc in sCJD type 2 than in sCJD type 1 could be associated with the longer disease duration in the former than in the latter (19). Notably, the PrPSc from the UK case showed a doublet middle band and an unglycosylated PrP band that migrated slightly higher than that of the US vCJD case. A similar middle band variation has been reported in vCJD and sCJD by Head et al. (20). However, it is unclear why the lower PrP band migrated slower in the UK case than in the U.S. case, although the clinical history and neuropathology of this case were typical for the vCJD diagnosis. In addition, immunohistochemistry of skin samples from the two vCJD and two sCJD patients, as well as two non-CJD controls, also revealed no specific PrPSc staining with the anti-PrP antibody 3F4 (fig. S2 with examples of two vCJD and one non-CJD patient samples).
Detection of prion seeding activity in CJD patient skin samples by RT-QuIC assay
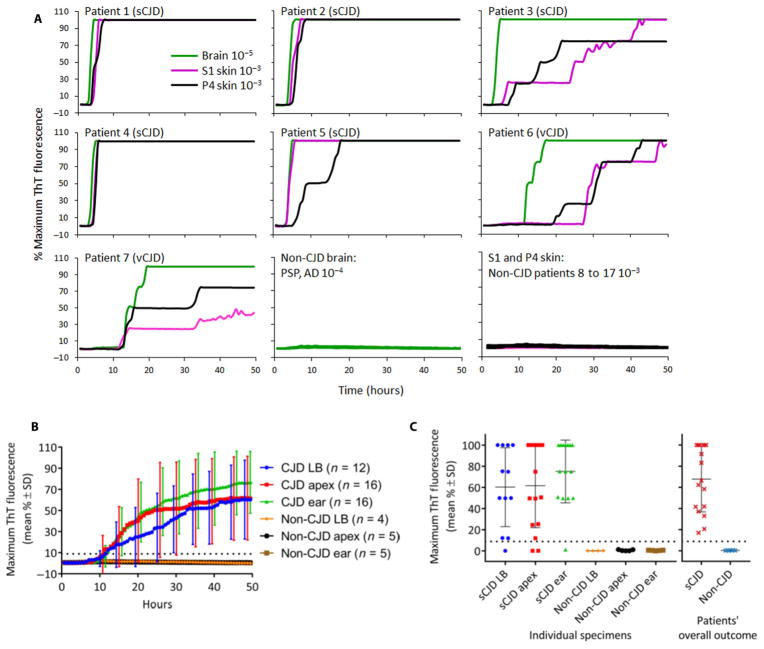
As another approach to detect PrPSc in sCJD skin samples, we used the ultrasensitive RT-QuIC assay. This assay uses the amyloid-sensitive fluorescent dye, thioflavin T (ThT), to monitor PrPSc-seeded formation of amyloid fibrils from a purified recombinant PrP substrate (14, 21–23). The same skin extracts, prepared and tested by Western blotting at the Case Western Reserve University (CWRU), were coded and examined blinded by RT-QuIC at the Rocky Mountain Laboratories. A 10−3 dilution of either the first supernatant (S1) or the final PK-digested and PrPSc-enriched pellet (P4) of the skin extraction sample, as well as a 10−5 dilution of brain homogenate from the same patients, was assayed using recombinant bank vole PrP (rPrPsen) as the substrate (Fig. 2A). As expected (14, 22, 23), the sCJD and vCJD brain samples all elicited strong and rapid positive RT-QuIC responses. Clear seeding activity was also observed in all skin S1 and P4 fractions from the CJD patients. By contrast, no prion seeding activity was observed in the brain or the skin S1 or P4 extracts from any of the 10 non-CJD control patients, showing that the seeding activity in the CJD patients’ brain and skin samples was disease-specific (Fig. 2A). These findings were further confirmed by blinded testing of S1 fractions from skin collected from the lower back, skull apex, and an area near the ear of each of 21 additional cases that included 16 sCJD and 5 non-CJD cases (Fig. 2, B and C). Our results showed that all of the CJD patients had at least one RT-QuIC–positive skin sample (Fig. 2, B and C, and Table 2). However, no single skin location was always RT-QuIC–positive. Of the three skin areas, the area near the ear had the highest and most consistent prion seeding activity (Fig. 2, B and C), that is, in 94% of sCJD cases (Table 2).
Fig. 2. RT-QuIC testing of sCJD and vCJD brain and skin samples.
(A) Final tissue dilutions of 10−5 brain (green) and 10−3 skin tissue [first supernatant (S1) fraction in magenta and P4 fraction in black] from sporadic CJD (sCJD) patient nos. 1 to 7 were used to seed quadruplicate real-time quaking-induced conversion (RT-QuIC) reactions. Negative control reactions were seeded in quadruplicate with a 10−4 dilution of brain tissue from patients with Alzheimer’s disease (AD) or progressive supranuclear palsy (PSP), and a 10−3 dilution of S1 or P4 skin fractions from non-CJD patient nos. 8 to 17. A final SDS concentration of 0.001% in combination with 300 mM NaCl was used with recombinant bank vole PrP (rPrPsen) as the substrate. Similar results were seen in at least two independent experiments. Traces from representative RT-QuIC experiments are the average of percent thioflavin T (ThT) fluorescence readings from all four replicate wells, regardless of the intensity of their signal (y axis) plotted as a function of time (x axis). (B) Average of percent ThT fluorescence readings from four replicate reactions, skin samples from the lower back (LB; blue), skull apex (red), and an area near the left ear (ear; green) from patients with CJD and from those without CJD (non-CJD) as control. For visual clarity, SDs of the averages are only shown every 5 hours. (C) Dot plot of the final mean relative ThT fluorescence readings for each skin sample obtained from the lower back, skull apex, and ear of 16 additional sCJD patients, and 5 additional non-CJD patients were examined with the RT-QuIC assay. The horizontal and vertical black lines indicate the means and SD of 60 ± 37%, 62 ± 40%, and 75 ± 30% ThT fluorescence for sCJD patient skin samples from the lower back, apex, and ear, respectively. The dotted line in (B) and (C) indicates the 8.8% calculated ThT fluorescence threshold. All samples were tested in a blinded fashion.
Table 2. Sensitivity and specificity of RT-QuIC assay for CJD skin samples.
NA, not available.
| Diagnosis | Lower back | Apex | Area near ear | Overall |
|---|---|---|---|---|
| sCJDVV1 | NA | + | NA | + |
| sCJDMM1 | NA | + | NA | + |
| sCJDVV1-2 | NA | + | NA | + |
| sCJDMM2 | NA | + | NA | + |
| sCJDMV2 | NA | + | NA | + |
| sCJDMM1 | NA | + | + | + |
| sCJDMM1 | +/−* | + | + | + |
| sCJDMM1 | +† | + | + | + |
| sCJDMM1 | −‡ | + | + | + |
| sCJDMM1 | + | +/− | + | + |
| sCJDVV2 | NA | + | + | + |
| sCJDVV2 | + | + | + | + |
| sCJDVV2 | NA | + | + | + |
| sCJDVV2 | + | + | + | + |
| sCJDMM2 | +/− | − | + | + |
| sCJDMM2 | NA | + | + | + |
| sCJDMM1-2 | + | + | + | + |
| sCJDMM1-2 | + | − | − | + |
| sCJDMV1-2 | + | + | + | + |
| sCJDMV1-2 | + | + | + | + |
| sCJDMV1 | + | + | + | + |
| Sensitivity (%) | 92 | 88 | 94 | 100§ |
| Non-CJD | − | − | − | − |
| Non-CJD | − | − | − | − |
| Non-CJD | NA | − | − | − |
| Non-CJD | − | − | − | − |
| Non-CJD | − | − | − | − |
| Non-CJD | − | − | − | − |
| Non-CJD | − | − | − | − |
| Specificity (%) | 100 | 100 | 100 | 100¶ |
Weak positive (+/−) means one of four or two of eight wells on two or more independent experiments.
Positive (+) RT-QuIC prion seeding activity.
Negative (−) RT-QuIC prion seeding activity.
RT-QuIC analysis of skin samples from the torso or forearm of the UK vCJD and US vCJD patients, respectively, was RT-QuIC–positive and was included in the overall sensitivity and confidence interval calculations.
RT-QuIC analysis of skin samples from the torso or forearm of eight non-CJD patients was RT-QuIC–negative and was included in the overall specificity and confidence interval calculations.
To assess the prion seeding activity in the S1 and P4 skin fractions compared to brain homogenates from the same patients, we performed RT-QuIC end point dilution analyses. Figure S3 shows representative data from vCJD and sCJD patients. Table 3 provides a summary of the calculated 50% seeding dose (SD50) per milligram of tissue from each brain and skin sample of five patients with sCJD and two patients with vCJD. Relative to brain concentrations of 108.0 to ≥109.7 SD50 per milligram of tissue, the S1 skin fractions for sCJD patients contained 105.0 to 106.9 SD50 per milligram of tissue equivalent, whereas the P4 fractions had 103.0 to 104.9 SD50 per milligram of tissue equivalent. Our analysis showed that the average prion seeding activity in sCJD S1 or P4 skin fractions was ~103- to 105-fold lower than in the corresponding brain tissue samples.
Table 3.
End point RT-QuIC quantitation of seeding activity in brain and skin samples from CJD patients.
| Log SD50 per milligram of tissue* | ||||
|---|---|---|---|---|
| Patient no. | Diagnosis | Brain | S1 skin | P4 skin |
| 1 | sCJDVV1 | >9.7 | 6.9 | 4.8 |
| 2 | sCJDMM1 | >8.5 | >6.8 | 4.9 |
| 3 | sCJDVV1-2 | 8.0 | 5.1 | ≤3.0 |
| 4 | sCJDMM2 | 9.2 | 6.8 | 4.7 |
| 5 | sCJDMV2 | >8.7 | 6.5 | 4.1 |
| 6 | vCJDMM2 | >8.2 | 5.0 | 3.6 |
| 7 | vCJDMM2 | 7.7 | ≤3.0 | ≤3.5 |
Log seeding dose 50 (SD50) was calculated, as described in Materials and Methods.
Discrimination of prion seeding activity by RT-QuIC in sCJD and vCJD patient skin samples
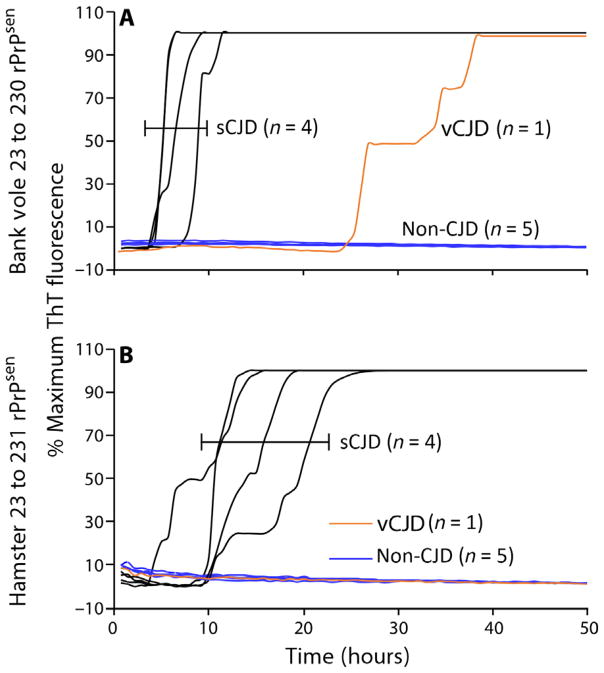
To further investigate the different seeding capabilities present in skin extracts from sCJD and vCJD patients, we analyzed P4 skin fractions from the sCJD and vCJD patients using a second rPrPsen substrate, hamster rPrPsen (residues 23 to 231), which was previously shown to help discriminate between these two human prion strains from brain tissues (21). PrPSc in both sCJD and vCJD skin samples could be amplified by bank vole rPrPsen substrate (Fig. 3A), but only the sCJD skin samples gave positive reactions with the hamster rPrPsen substrate (Fig. 3B). However, the P4 skin fractions from sCJD patient no. 3 and vCJD patient no. 7 were not included in this analysis because of the low amount of prion seeding activity that diminished the reliability of discrimination between the two strains. Nevertheless, these results provide further evidence that, as we described previously for brain samples, sCJD and vCJD seeding activities in skin could be discriminated by comparing differential RT-QuIC responses with bank vole and hamster rPrPsen substrates, provided that the seeding activities detected with the bank vole rPrPsen substrate were not close to the detection limit. Western blot analyses of the products of RT-QuIC reactions with the bank vole rPrPsen substrate revealed variable patterns of PK-resistant reaction products; however, it was not apparent that such patterns would aid additionally in CJD strain or type discrimination (fig. S4).
Fig. 3. RT-QuIC testing of prion seeding activity in P4 skin fractions from sCJD and vCJD patients.
P4 skin fractions from the sCJD and vCJD patients were analyzed using a second rPrPsen substrate, hamster rPrPsen (residues 23 to 231), which has been previously shown to help discriminate between these two human prion strains (21). Dilutions (10−3) of P4 skin fractions from four sCJD patients (nos. 1, 2, 4, and 5) and 1 vCJD patient (no. 6) were used to seed quadruplicate RT-QuIC reactions. Testing was performed using either bank vole rPrPsen in the presence of 300 mM NaCl and 0.001% SDS (A) or hamster rPrPsen in the presence of 300 mM NaCl and 0.002% SDS (B). Testing was performed twice with similar results. The data show the average fluorescence of replicate wells monitored over time.
Prion infectivity in skin samples from sCJD patients detected in humanized Tg mice
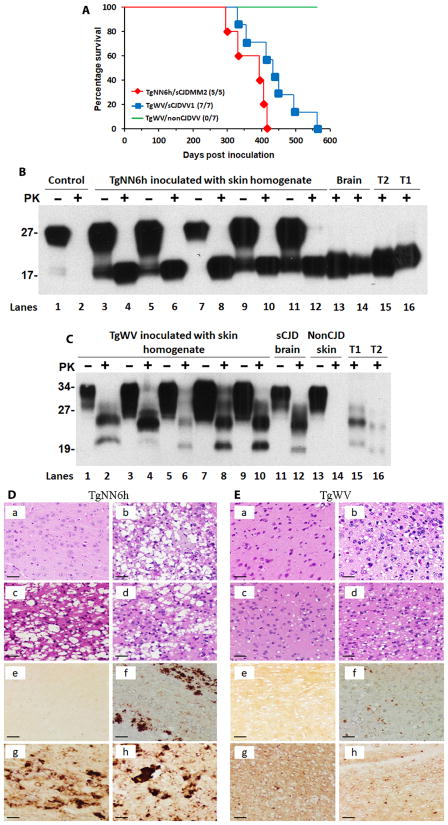
To determine whether skin from sCJD patients was infectious, we conducted a bioassay using two lines of humanized Tg mice (TgNN6h and TgWV), which were generated in our laboratories previously. TgNN6h mice express human PrP-129M with the two N-linked glycosylation sites mutated to eliminate glycosylation (24), whereas TgWV mice express wild-type human PrP-129V (25). We observed that TgNN6h and TgWV mice were susceptible to sCJDMM2 or sCJDVV2 prion strains, respectively, after an intracerebral inoculation of 129 polymorphism-matched sCJD brain homogenates; this was confirmed by Western blotting (fig. S5). TgNN6h mice (n = 7) each inoculated intracerebrally with 30 μl of 1% brain homogenate from a sCJDMM2 patient developed prion disease at an average of 263 ± 25 (SD) days post inoculation (dpi) (range, 164 to 368 dpi). By contrast, TgWV mice (n = 5) each inoculated intracerebrally with 30 μl of 1% brain homogenate from a sCJDVV2 patient succumbed to the disease at an average of 157 ± 11 (SD) dpi (range, 150 to 177 dpi). Given these studies, five TgNN6h mice and seven TgWV mice were inoculated intracerebrally with 30 μl of 5% skin tissue homogenate from a sCJDMM2 case (patient no. 4; Table 3) or a sCJDVV1 case (patient no. 1; Table 3), respectively. All five TgNN6h mice inoculated with the sCJDMM2 skin homogenate developed a hunched back, ruffled fur, and lethargy/slow movements after an average of 370 ± 51 (SD) dpi (range, 300 to 417 dpi) (Fig. 4A); these signs were very similar to those observed in TgNN6h mice inoculated with sCJDMM2 brain homogenate. We also detected PK-resistant PrPSc and spongiform degeneration in the brains of these TgNN6h mice (Fig. 4, B and D). All seven TgWV mice inoculated with sCJDVV1 skin homogenate became infected at an average of 436 ± 80 (SD) dpi (range, 332 to 564 dpi) (Fig. 4A), as confirmed by Western blotting and neurohistology (Fig. 4, C and E). The TgWV mice mostly exhibited slow movements and weight gain and had detectable PK-resistant PrPSc and spongiform degeneration in the brain (Fig. 4E). The gel migration pattern of PrPSc detected in the brains of mice inoculated with sCJD skin samples was very similar to that of PrPSc from mice inoculated with sCJD brain tissue, suggesting that at least some of the prion strain-specific biochemical features of the inocula were maintained (Fig. 4C). Moreover, the profiles of spongiform degeneration and PrP staining in the brains of skin homogenate–inoculated mice were also similar to those of brain homogenate–inoculated mice (Fig. 4, D and E). By contrast, all seven TgWV mice inoculated with normal skin homogenate from a non-CJD patient carrying the 129V/V genotype of PrP were overweight, but none of the mice became infected, and no PrPSc was detected in any of the mice at 564 dpi (Fig. 4, C and E). In addition, TgNN6h control mice that were not inoculated exhibited a normal life span and did not display any obvious abnormal phenotypes.
Fig. 4. Prion infectivity of skin samples from sCJD patients.
(A) Survival curves of TgNN6h or TgWV humanized Tg mice inoculated with skin homogenate from sCJD or non-CJD patients. Six- to 8-week-old TgNN6h (n = 5) or TgWV (n = 7) mice were intracerebrally inoculated with 30 μl of 5% skin homogenate from patients with sCJDMM2 or sCJDVV1. Five TgNN6h mice were intracerebrally inoculated with skin homogenate from a patient with sCJDMM2 (red diamonds), and seven TgWV mice were intracerebrally inoculated with skin homogenate from a patient with sCJDVV1 (blue squares). Seven TgWV mice were intra-cerebrally inoculated with skin homogenate from a non-CJD patient as a negative control (green line). (B) Western blot analysis of PrPSc in the brains of TgNN6h humanized Tg mice inoculated with skin homogenate from a sCJDMM2 patient. Lanes 1 and 2 show samples of uninoculated mouse brain (control); lanes 3 to 12 show brain samples from five mice inoculated with a skin homogenate from a sCJDMM2 patient; lanes 13 and 14 show brain samples from two mice inoculated with sCJD brain homogenate (brain) as a positive control; lanes 15 and 16 show brain homogenates from sCJD type 2 (T2) and type 1 (T1) patients treated with PK and PNGase F that were used as a reference for PrPSc banding patterns. Samples in lanes 1, 3, 5, 7, 9, and 11 were not PK treated, whereas samples in the other lanes were treated with PK (50 μg/ml) for 1 hour at 37°C. The immunoblot was probed with the anti-PrP antibody 3F4. (C) Western blot analysis of PrPSc in the brains of TgWV mice inoculated with skin homogenate from a patient with sCJDVV1. Lanes 1 to 10 show brain samples from five humanized Tg mice inoculated with skin homogenate from the sCJDVV1 patient; lanes 11 and 12 show brain samples from a mouse inoculated with brain homogenate from a sCJDVV2 patient (brain); lanes 13 and 14 show brain samples from a mouse inoculated with skin homogenate from a non-CJD patient as a negative control (non-CJD skin); lanes 15 and 16 show brain homogenates from patients with sCJD type 1 or type 2 as a positive control. Samples in lanes 1, 3, 5, 7, 9, 11, and 13 were not treated with PK, whereas samples in the other lanes were treated with PK (50 μg/ml) for 1 hour at 37°C. Because of the lower amounts of PrPSc in the brain samples of mice shown in lanes 2 and 4, more PK-treated mouse brain homogenate was loaded onto the gel for these two mice compared to brain homogenate for the other mice. This could have resulted in the slower migration of the PK-resistant PrPSc in mouse brain samples in lanes 2 and 4 compared to the other lanes. The blot was probed with the anti-PrP antibody 3F4. (D) Hematoxylin and eosin (H&E) staining and PrP immunohistochemistry of the cerebral cortex of TgNN6h humanized Tg mice before and after inoculation with sCJD patient brain homogenate. (a and e) Brain sections from uninoculated mice as a negative control; (b and f) brain sections from mice inoculated with sCJD brain homogenate as a positive control; (c, d, g, and h) brain sections from two mice inoculated with skin homogenate from a patient with sCJDMM2. (E) H&E staining and PrP immunohistochemistry using the anti-PrP 3F4 antibody of the cerebral cortex of TgWV Tg mice inoculated with sCJD or non-CJD patient skin or brain homogenate. (a and e) Brain sections from a Tg mouse inoculated with skin homogenate from a non-CJD patient as a negative control; (b and f) brain sections from a Tg mouse inoculated with brain homogenate from a sCJD patient as a positive control; (c, d, g, and h) brain sections from two mice inoculated with skin homogenate from a sCJDVV1 patient. Scale bars, 50 μm.
DISCUSSION
Our study demonstrates the presence of prion infectivity, seeding activity, and, in one case, PK-resistant PrPSc in the skin of sCJD patients. Although we have not completed the direct comparison of the infectivity of PrPSc from sCJD brain and skin by bioassay in humanized Tg mice, our RT-QuIC assays indicated that prion seeding activity was approximately 1000-fold lower in the skin than in the brain of the same patient. These seed concentrations are similar to those found in nasal brushings from CJD patients (26). Given the observed partial correlation between seeding activity and infectivity (14, 27), we predict that sCJD infectivity is also orders of magnitude lower in the skin than in the brain of CJD patients.
The tissue distribution of prions can differ markedly between prion diseases, and prion detection in various tissues depends in part on the sensitivity of the assays used. For example, using Western blot analysis, PrPSc was found to be more widely distributed in the periphery of patients with vCJD than sCJD patients (28, 29). However, using the more sensitive RT-QuIC assay, sCJD seeding activity was recently detected in nonneuronal tissues such as the spleen, kidney, lung, liver, and adrenal glands of four sCJD patients (30). We have detected roughly comparable RT-QuIC prion seeding activity, as well as prion infectivity, in the skin of cases with two sCJD subtypes (MM2 and VV1). Whether PrPSc in skin from cases of the most common sCJD subtype (MM1) is also transmissible to experimental animals remains to be determined; however, our RT-QuIC analyses indicated at least equivalent seeding activity in sCJDMM1 skin (Table 3). Our current results raise concerns about iatrogenic transmission of prions harbored in sCJD skin. For example, the presence of transmissible skin prions raises the question of the presence of other possible iatrogenic routes of CJD, although the epidemiologic evidence for this varies widely and has largely been inconclusive (5–11).
We should emphasize that in no way does our study imply that prion transmission can occur via casual contact. The bioassays that we have performed involved inoculation of relatively large amounts of skin extracts directly into the brains of humanized Tg mice, which is not likely to be recapitulated in humans in any sort of routine event. Prion transmission from skin in humans, if even possible, likely requires additional specific inoculation scenarios that have existed in documented iatrogenic transmissions of human prion disease, that is, CNS injection, hematogenous spread, or oral consumption with sufficient quantities of infectious material (4). The apparent rarity of such scenarios in routine clinical care may be responsible for the lower than expected hazard ratios of certain procedures discussed in some epidemiological studies compared to the much higher risks associated with known modes of iatrogenic CJD transmission. In addition, the rate of skin-associated transmission, if present, would likely be much lower than that of CNS-associated transmission, given the much lower RT-QuIC prion seeding activity in the skin than in the brains of sCJD cases. Nevertheless, further scrutiny is necessary to determine whether extra precautions should be taken during non-CNS surgeries of sCJD patients, especially in the case of procedures where surgical instruments are reused.
As noted above, the detection of prions and PrPSc in skin is not altogether unprecedented. PrPSc has been detected in the skin of experimentally or naturally scrapie-infected hamsters and sheep (13), as well as in the skin of a single patient with vCJD (12). Prion infectivity has also been reported in the skin of infected greater kudu (31). In addition, mouse prion inoculation by skin scarification has been found to have almost the same efficiency as inoculation by the intraperitoneal, intravenous, or perivenous routes (32). The inoculated prions first propagate in the skin and then spread to the brain to cause prion disease (33, 34). All of these modes of prion spread between the brain and the skin are believed to occur via the peripheral nervous system. PrPSc has been detected in the trigeminal ganglion in sCJD and vCJD patients by immunohistochemistry and Western blotting (35) and in the nerve fibers within the skin of hamsters inoculated with the 263K prion strain by immunohistochemistry (13). The dermatomes examined in our study including the skull apex, the area near the ear, the forearm, and the lower back are innervated by the oculo-motor nerve III ophthalmic division, cervical nerves 2 to 3 (C2-3), C5, or thoracic nerves 10 to 11, respectively. It is possible that prions spread from the brain to the dermatomes through these spinal nerves.
In conclusion, we have detected prions in the skin of the sCJD cases that we have tested but not in non-CJD control individuals. However, our study does not establish when and where within the skin prions appear in the course of disease. Moreover, other than the indication that prion seeding activity is ~1000-fold lower in the skin than in brain tissue, our data do not clarify the basis of seemingly divergent epidemiologic reports on sCJD risks, or lack thereof, associated with surgical procedures involving the skin. We have not yet validated that RT-QuIC analysis of skin would have practical utility in the diagnosis of sCJD. Further studies are needed to clarify the risks associated with skin samples from sCJD patients and to increase our understanding of prion transmissibility.
MATERIALS AND METHODS
Study design
The aim of the study was to determine whether there are infectious prions in the skin of sCJD patients. The skin samples were collected at autopsy or biopsy from different body areas of 38 patients including 21 sCJD, 2 vCJD, and 15 non-CJD. The abnormal PrP from the skin was enriched by ultracentrifugation before Western blotting analysis. Moreover, concentrated or unconcentrated PrP from skin and brain samples was further subjected to the highly sensitive RT-QuIC assay in a blinded fashion. Finally, to determine whether the sCJD skin is infectious, we conducted a bioassay with two lines of humanized Tg mice inoculated intracerebrally with skin homogenates from two sCJD patients and one non-CJD patient. In addition to monitoring the clinical signs of the inoculated animals, the brain samples of the mice were examined by Western blotting and neurohistology. Samples were collected randomly according to the diagnosis by Western blotting and histology. All data on Western blotting and RT-QuIC assay of human brain and skin samples were repeated at least two to three times. To ensure that the bioassay data were reliable, we used two different sCJD cases and one non-CJD case, as well as two different humanized Tg mouse models.
Patients
Thirty-seven patients including 21 sCJD, 1 vCJD (US vCJD), and 15 non-CJD individuals were obtained through our National Prion Disease Pathology Surveillance Center and Human Tissue Procurement Facility in the Department of Pathology at CWRU. An additional vCJD case (UK vCJD) was obtained from the National CJD Research and Surveillance Unit, University of Edinburgh, United Kingdom (part of the Medical Research Council Edinburgh Brain Bank). The study was monitored and approved by the University Hospitals Case Medical Center Institutional Review Board. The diagnosis of CJD was confirmed by Western blotting and neurohistology of the brain samples obtained at autopsy. CJD included 10 males and 13 females [mean age, 59.4 ± 15.3 (SD) years] (Table 1). The 15 non-CJD control patients comprised nine males and six females [mean age, 65.1 ± 14.2 (SD) years] (table S1).
Brain tissue and preparation
Brain tissues were obtained at autopsy from 30 patients including 21 sCJD, 2 vCJD, and 7 non-CJD individuals and processed for Western blotting with anti-PrP antibody 3F4 against human PrP(106–112) (18, 36) or RT-QuIC analysis, as described previously (14, 21, 37) with minor modifications (see the Supplementary Materials for details).
Skin tissues and preparation
Thirty-five of the 38 skin samples were obtained at autopsy, and the remaining three samples were biopsied. The skin samples (~60 to 80 mg each in weight, ~6 mm × 6 mm each in size) contained all three layers including epidermis, dermis, and hypodermis from different parts of the body such as the apex, area next to left ear (~1 cm away from the ear), forearm, chest, lower back, and knee. During the autopsy process, special care was taken to assure that there was no cross contamination between the skin and the brain samples and from case to case. All skin tissues were taken before the skull was opened. The instruments were either virgin or used only for collecting skin after decontamination each time. The autopsied or biopsied tissues were taken at nine different facilities and frozen in dry ice first and then transferred to a freezer at −80°C. The frozen skin samples were prepared for Western blot analysis probing with anti-PrP antibody 3F4 or anti-C against human PrP(220–231) (12, 13, 18, 36), for RT-QuIC analysis (14), or as inocula for bioassay, as described previously (15) with minor modifications (see the Supplementary Materials for details).
Molecular genetic analysis
These analyses were performed on genomic DNA, as described previously (15, 38) and in the Supplementary Materials.
RT-QuIC analysis
The RT-QuIC analysis of skin or brain samples was conducted, as described previously (14, 21, 26, 37) and in the Supplementary Materials.
Generation, screening, and characterization of Tg humanized TgWV and TgNN6h mice
The generation of Tg mice expressing human PrP-129V or PrP-129M from transgene constructs based on the murine half-genomic PrP clone in plasmid pHGPRP was performed, as described previously (15, 24, 25) and in the Supplementary Materials.
Humanized Tg mouse–based bioassay of skin or brain samples from sCJD patients
Infectivity of sCJD skin samples was evaluated by intracerebral inoculation of TgNN6h or TgWV mice with 5% skin tissue homogenate from one patient with sCJDMM2 or sCJDVV1, respectively. The reason that the two lines of humanized Tg mice were chosen for testing the two sCJD skin samples was because our preliminary study exhibited that they were highly susceptible to the 129 polymorphism-matched sCJD brain inocula. After anesthetization with isoflurane, 30 μl of 5% skin homogenate or 30 μl of 1% brain homogenate (in phosphate-buffered saline) was injected into each mouse intracerebrally with a 26-gauge needle inserted to a depth of 4 mm at the left parietal region of the cranium, as described previously (15). The mice were monitored for prion-related signs such as hunched back, ruffled fur, rigid tails, and slow movement every other day and sacrificed within 3 days after the appearance of severe signs or at death. The brains were collected and sliced sagittally—half of which was frozen for Western blotting with the anti-PrP antibody 3F4 (18, 36), and the other half was fixed in 10% formalin for histological and immunohistochemical staining analysis (see below). Total PrP, as well as PK-treated PrPSc, was determined by immunoblotting in SDS–polyacrylamide gels, as described in the Supplementary Materials.
Statistical analysis
Statistical comparisons of mean relative ThT fluorescence responses in samples from CJD and non-CJD patients were performed using unpaired Student’s t tests.
Supplementary Material
Fig. S1. Western blotting and neurohistology of autopsy brain samples from sCJD and vCJD patients.
Fig. S2. Representative immunohistochemistry of skin samples from two vCJD patients and a non-CJD patient.
Fig. S3. Representative RT-QuIC end-point dilution analysis of brain and skin fractions from two CJD patients.
Fig. S4. Western blot analysis of bank vole rPrPres RT-QuIC products from reactions seeded with brain or skin samples.
Fig. S5. Western blot analysis of brain samples from TgNN6h or TgWV mice inoculated intracerebrally with sCJDMM2 or sCJDVV2 brain homogenate, respectively.
Table S1. Summary of non-CJD cases.
Acknowledgments
We thank all donors for skin tissues, the families affected by CJD, and the physicians for support. We also thank the CJD Foundation for its invaluable help; M. Warren and D. Kofskey for skillful histologic and immunohistochemical preparations; L. Qing for help with some control transmission experiments; Y. Cohen and W. Chen for diagnostic Western blot preparations; N. Maurer and O. Taliaferro for animal care; J. Negrey, D. Jordan, J. Blevins, M. H. Rizk, V. Spencer, and A. Webb for coordinating tissue procurements; K. Glisic for helping to collect clinical history; and A. Athman for graphical assistance.
Funding: This work was supported by the CJD Foundation, the NIH (NS062787 and NS087588 to W.-Q.Z., NS096626 to W.-Q.Z., Q.K., and J.G.S., and NS088604 to Q.K.), and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, NIH (B.C.), as well as the Centers for Disease Control and Prevention (contract UR8/CCU515004 to J.G.S.).
Footnotes
Author contributions: C.D.O., J.Y., Q.K., B.C., and W.-Q.Z. conceived and designed the study. J.Y., Y.L., Y.-A.Z., G.-X.W., Q.K., and W.-Q.Z. developed, performed, and interpreted protein chemistry analysis of PrPSc in the skin and brain tissues. C.D.O., B.R.G., and B.C. developed, performed, and interpreted the RT-QuIC analysis of the skin and brain samples and Western blotting of RT-QuIC products. C.D.O., J.Y., Y.L., M.R., J.R., R.E.W., T.J., J.W.I., J.G.S., B.C., and W.-Q.Z. prepared, developed, and performed preparation of skin samples for RT-QuIC analysis. J.Y., B.L., Q.K., and W.-Q.Z. designed and performed the bioassay of sCJD skin samples in humanized Tg mice. J.Y., Y.L., Y.-A.Z., R.B.P., Q.K., and W.-Q.Z. designed and performed generation of humanized Tg mouse lines (TgWV and TgNN6h). B.S.A., R.E.W., T.J., J.W.I., and W.-Q.Z. designed the clinical report forms and collected the epidemiological data. Z.W., M.L.C., J.W.I., and W.-Q.Z. developed, performed, and interpreted the histology data. J.Y., D.W., Q.K., S.Z., M.E.Q.-M., and W.-Q.Z. developed, performed, and interpreted DNA extraction and PrP genotype analysis. C.D.O., Q.K., B.C., and W.-Q.Z. wrote the first version of the paper. All authors critically reviewed and approved the final version of the manuscript.
Competing interests: B.S.A. is the medical director of the CJD Foundation. B.C. is a co-inventor on U.S. Patent no. 8,216,788 and European Patent no. EP 2554996 “Detection of infectious prion protein by seeded conversion of recombinant prion protein” pertaining to RT-QuIC testing. B.C., C.D.O., and B.R.G. are co-inventors on U.S. Provisional Patent no. 62/134, 476 “Bank vole prion protein as a broad-spectrum substrate for RT-QuIC–based detection and discrimination of prion strains.” All other authors declare that they have no competing interests.
Data and materials availability: All materials used in this study will be made available subject to a materials transfer agreement.
Exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works
REFERENCES AND NOTES
- 1.Kraus A, Groveman BR, Caughey B. Prions and the potential transmissibility of protein misfolding diseases. Annu Rev Microbiol. 2013;67:543–564. doi: 10.1146/annurev-micro-092412-155735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das AS, Zou W-Q. Prions: Beyond a single protein. Clin Microbiol Rev. 2016;29:633–658. doi: 10.1128/CMR.00046-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gambetti P, Cali I, Notari S, Kong Q, Zou W-Q, Surewicz WK. Molecular biology and pathology of prion strains in sporadic human prion diseases. Acta Neuropathol. 2011;121:79–90. doi: 10.1007/s00401-010-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown P, Brandel J-P, Sato T, Nakamura Y, MacKenzie J, Will RG, Ladogana A, Pocchiari M, Leschek EW, Schonberger LB. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Infect Dis. 2012;18:901–907. doi: 10.3201/eid1806.120116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins S, Law MG, Fletcher A, Boyd A, Kaldor J, Masters CL. Surgical treatment and risk of sporadic Creutzfeldt-Jakob disease: A case-control study. Lancet. 1999;353:693–697. doi: 10.1016/s0140-6736(98)08138-0. [DOI] [PubMed] [Google Scholar]
- 6.de Pedro-Cuesta J, Mahillo-Fernandez I, Calero M, Rábano A, Cruz M, Siden A, Martínez-Martín P, Laursen H, Ruiz-Tovar M, Mølbak K EUROSURGYCJD Research Group. Towards an age-dependent transmission model of acquired and sporadic Creutzfeldt-Jakob disease. PLOS ONE. 2014;9:e109412. doi: 10.1371/journal.pone.0109412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Pedro-Cuesta J, Mahillo-Fernández I, Rábano A, Calero M, Cruz M, Siden A, Laursen H, Falkenhorst G, Mølbak K EUROSURGYCJD Research Group. Nosocomial transmission of sporadic Creutzfeldt-Jakob disease: Results from a risk-based assessment of surgical interventions. J Neurol Neurosurg Psychiatry. 2011;82:204–212. doi: 10.1136/jnnp.2009.188425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward HJT, Everington D, Cousens SN, Smith-Bathgate B, Gillies M, Murray K, Knight RSG, Smith PG, Will RG. Risk factors for sporadic Creutzfeldt-Jakob disease. Ann Neurol. 2008;63:347–354. doi: 10.1002/ana.21294. [DOI] [PubMed] [Google Scholar]
- 9.Davanipour Z, Alter M, Sobel E, Asher D, Gajdusek DC. Creutzfeldt-Jakob disease: Possible medical risk factors. Neurology. 1985;35:1483–1486. doi: 10.1212/wnl.35.10.1483. [DOI] [PubMed] [Google Scholar]
- 10.Zerr I, Brandel J-P, Masullo C, Wientjens D, de Silva R, Zeidler M, Granieri E, Sampaolo S, van Duijn C, Delasnerie-Lauprêtre N, Will R, Poser S. European surveillance on Creutzfeldt-Jakob disease: A case-control study for medical risk factors. J Clin Epidemiol. 2000;53:747–754. doi: 10.1016/s0895-4356(99)00207-3. [DOI] [PubMed] [Google Scholar]
- 11.Hamaguchi T, Noguchi-Shinohara M, Nozaki I, Nakamura Y, Sato T, Kitamoto T, Mizusawa H, Yamada M. Medical procedures and risk for sporadic Creutzfeldt-Jakob disease, Japan, 1999–2008. Emerg Infect Dis. 2009;15:265–271. doi: 10.3201/eid1502.080749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Notari S, Moleres FJ, Hunter SB, Belay ED, Schonberger LB, Cali I, Parchi P, Shieh W-J, Brown P, Zaki S, Zou W-Q, Gambetti P. Multiorgan detection and characterization of protease-resistant prion protein in a case of variant CJD examined in the United States. PLOS ONE. 2010;5:e8765. doi: 10.1371/journal.pone.0008765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomzig A, Schulz-Schaeffer W, Wrede A, Wemheuer W, Brenig B, Kratzel C, Lemmer K, Beekes M. Accumulation of pathological prion protein PrPSc in the skin of animals with experimental and natural scrapie. PLOS Pathog. 2007;3:e66. doi: 10.1371/journal.ppat.0030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilham JM, Orrú CD, Bessen RA, Atarashi R, Sano K, Race B, Meade-White KD, Taubner LM, Timmes A, Caughey B. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLOS Pathog. 2010;6:e1001217. doi: 10.1371/journal.ppat.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong Q, Zheng M, Casalone C, Qing L, Huang S, Chakraborty B, Wang P, Chen F, Cali I, Corona C, Martucci F, Iulini B, Acutis P, Wang L, Liang L, Wang M, Li X, Monaco S, Zanusso G, Zou W-Q, Caramelli M, Gambetti P. Evaluation of the human transmission risk of an atypical bovine spongiform encephalopathy prion strain. J Virol. 2008;82:3697–3701. doi: 10.1128/JVI.02561-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zerr I, Kallenberg K, Summers DM, Romero C, Taratuto A, Heinemann U, Breithaupt M, Varges D, Meissner B, Ladogana A, Schuur M, Haik S, Collins SJ, Jansen GH, Stokin GB, Pimentel J, Hewer E, Collie D, Smith P, Roberts H, Brandel JP, van Duijn C, Pocchiari M, Begue C, Cras P, Will RG, Sanchez-Juan P. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain. 2009;132:2659–2668. doi: 10.1093/brain/awp191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belay ED, Sejvar JJ, Shieh WJ, Wiersma ST, Zou W-Q, Gambetti P, Hunter S, Maddox RA, Crockett L, Zaki SR, Schonberger LB. Variant Creutzfeldt-Jakob disease death, United States. Emerg Infect Dis. 2005;11:1351–1354. doi: 10.3201/eid1109.050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan J, Xiao X, McGeehan J, Dong Z, Cali I, Fujioka H, Kong Q, Kneale G, Gambetti P, Zou W-Q. Insoluble aggregates and protease-resistant conformers of prion protein in uninfected human brains. J Biol Chem. 2006;281:34848–34858. doi: 10.1074/jbc.M602238200. [DOI] [PubMed] [Google Scholar]
- 19.Cali I, Castellani R, Alshekhlee A, Cohen Y, Blevins J, Yuan J, Langeveld JPM, Parchi P, Safar JG, Zou W-Q, Gambetti P. Co-existence of scrapie prion protein types 1 and 2 in sporadic Creutzfeldt-Jakob disease: Its effect on the phenotype and prion-type characteristics. Brain. 2009;132:2643–2658. doi: 10.1093/brain/awp196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Head MW, Bunn TJR, Bishop MT, McLoughlin V, Lowrie S, McKimmie CS, Williams MC, McCardle L, MacKenzie J, Knight R, Will RG, Ironside JW. Prion protein heterogeneity in sporadic but not variant Creutzfeldt–Jakob disease: UK cases 1991–2002. Ann Neurol. 2004;55:851–859. doi: 10.1002/ana.20127. [DOI] [PubMed] [Google Scholar]
- 21.Orrú CD, Groveman BR, Raymond LD, Hughson AG, Nonno R, Zou W, Ghetti B, Gambetti P, Caughey B. Bank vole prion protein as an apparently universal substrate for RT-QuIC-based detection and discrimination of prion strains. PLOS Pathog. 2015;11:e1004983. doi: 10.1371/journal.ppat.1004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atarashi R, Satoh K, Sano K, Fuse T, Yamaguchi N, Ishibashi D, Matsubara T, Nakagaki T, Yamanaka H, Shirabe S, Yamada M, Mizusawa H, Kitamoto T, Klug G, McGlade A, Collins SJ, Nishida N. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med. 2011;17:175–178. doi: 10.1038/nm.2294. [DOI] [PubMed] [Google Scholar]
- 23.Peden AH, McGuire LI, Appleford NEJ, Mallinson G, Wilham JM, Orrú CD, Caughey B, Ironside JW, Knight RS, Will RG, Green AJE, Head MW. Sensitive and specific detection of sporadic Creutzfeldt–Jakob disease brain prion protein using real-time quaking-induced conversion. J Gen Virol. 2012;93:438–449. doi: 10.1099/vir.0.033365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haldiman T, Kim C, Cohen Y, Chen W, Blevins J, Qing L, Cohen ML, Langeveld J, Telling GC, Kong Q, Safar JG. Co-existence of distinct prion types enables conformational evolution of human PrPSc by competitive selection. J Biol Chem. 2013;288:29846–29861. doi: 10.1074/jbc.M113.500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan J, Zhan Y-A, Abskharon R, Xiao X, Martinez MC, Zhou X, Kneale G, Mikol J, Lehmann S, Surewicz WK, Castilla J, Steyaert J, Zhang S, Kong Q, Petersen RB, Wohlkonig A, Zou W-Q. Recombinant human prion protein inhibits prion propagation in vitro. Sci Rep. 2013;3:2911. doi: 10.1038/srep02911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orrú CD, Bongianni M, Tonoli G, Ferrari S, Hughson AG, Groveman BR, Fiorini M, Pocchiari M, Monaco S, Caughey B, Zanusso G. A test for Creutzfeldt–Jakob disease using nasal brushings. N Engl J Med. 2014;371:519–529. doi: 10.1056/NEJMoa1315200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vascellari S, Orrù CD, Hughson AG, King D, Barron R, Wilham JM, Baron GS, Race B, Pani A, Caughey B. Prion seeding activities of mouse scrapie strains with divergent PrPSc protease sensitivities and amyloid plaque content using RT-QuIC and eQuIC. PLOS ONE. 2012;7:e48969. doi: 10.1371/journal.pone.0048969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wadsworth JDF, Joiner S, Hill AF, Campbell TA, Desbruslais M, Luthert PJ, Collinge J. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet. 2001;358:171–180. doi: 10.1016/s0140-6736(01)05403-4. [DOI] [PubMed] [Google Scholar]
- 29.Glatzel M, Abela E, Maissen M, Aguzzi A. Extraneural pathologic prion protein in sporadic Creutzfeldt–Jakob disease. N Engl J Med. 2003;349:1812–1820. doi: 10.1056/NEJMoa030351. [DOI] [PubMed] [Google Scholar]
- 30.Takatsuki H, Fuse T, Nakagaki T, Mori T, Mihara B, Takao M, Iwasaki Y, Yoshida M, Murayama S, Atarashi R, Nishida N, Satoh K. Prion-seeding activity is widely distributed in tissues of sporadic Creutzfeldt-Jakob disease patients. EBioMedicine. 2016;12:150–155. doi: 10.1016/j.ebiom.2016.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunningham AA, Kirkwood JK, Dawson M, Spencer YI, Green RB, Wells GA. Bovine spongiform encephalopathy infectivity in greater kudu (Tragelaphus strepsiceros) Emerg Infect Dis. 2004;10:1044–1049. doi: 10.3201/eid1006.030615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor DM, McConnell I, Fraser H. Scrapie infection can be established readily through skin scarification in immunocompetent but not immunodeficient mice. J Gen Virol. 1996;77:1595–1599. doi: 10.1099/0022-1317-77-7-1595. [DOI] [PubMed] [Google Scholar]
- 33.Mohan J, Hopkins J, Mabbott NA. Skin-derived dendritic cells acquire and degrade the scrapie agent following in vitro exposure. Immunology. 2005;116:122–133. doi: 10.1111/j.1365-2567.2005.02207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wathne GJ, Kissenpfennig A, Malissen B, Zurzolo C, Mabbott NA. Determining the role of mononuclear phagocytes in prion neuroinvasion from the skin. J Leukoc Biol. 2012;91:817–828. doi: 10.1189/jlb.1211633. [DOI] [PubMed] [Google Scholar]
- 35.Head MW, Ritchie D, Smith N, McLoughlin V, Nailon W, Samad S, Masson S, Bishop M, McCardle L, Ironside JW. Peripheral tissue involvement in sporadic, iatrogenic and variant Creutzfeldt-Jakob disease: An immunohistochemical, quantitative, and biochemical study. Am J Pathol. 2004;164:143–153. doi: 10.1016/S0002-9440(10)63105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou W-Q, Langeveld J, Xiao X, Chen S, McGeer PL, Yuan J, Payne MC, Kang H-E, McGeehan J, Sy M-S, Greenspan NS, Kaplan D, Wang G-X, Parchi P, Hoover E, Kneale G, Telling G, Surewicz WK, Kong Q, Guo J-P. PrP conformational transitions alter species preference of a PrP-specific antibody. J Biol Chem. 2010;285:13874–13884. doi: 10.1074/jbc.M109.088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orrú CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, Caughey B. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. MBio. 2015;6:e02451–14. doi: 10.1128/mBio.02451-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibson RM, Meyer AM, Winner D, Archer J, Feyertag F, Ruiz-Mateos E, Leal M, Robertson DL, Schmotzer CL, Quiñones-Mateu ME. Sensitive deep-sequencing-based HIV-1 genotyping assay to simultaneously determine susceptibility to protease, reverse transcriptase, integrase, and maturation inhibitors, as well as HIV-1 coreceptor tropism. Antimicrob Agents Chemother. 2014;58:2167–2185. doi: 10.1128/AAC.02710-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Western blotting and neurohistology of autopsy brain samples from sCJD and vCJD patients.
Fig. S2. Representative immunohistochemistry of skin samples from two vCJD patients and a non-CJD patient.
Fig. S3. Representative RT-QuIC end-point dilution analysis of brain and skin fractions from two CJD patients.
Fig. S4. Western blot analysis of bank vole rPrPres RT-QuIC products from reactions seeded with brain or skin samples.
Fig. S5. Western blot analysis of brain samples from TgNN6h or TgWV mice inoculated intracerebrally with sCJDMM2 or sCJDVV2 brain homogenate, respectively.
Table S1. Summary of non-CJD cases.