Abstract
Background
Thrombosis within a Left Ventricular Assist Device (LVAD) is a devastating complication that often necessitates device exchange. Few studies have evaluated the relationship between patient anatomy and pump thrombosis. We hypothesize that lateral displacement of the left ventricular (LV) apex increases risk for pump thrombosis.
Methods
All patients who underwent primary implantation of a Thoratec HeartMate II (HM2) device at a single center (2009-2015) were evaluated. Operative mortalities and patients without imaging were excluded. The angle of the LV apex relative to the midline was measured on preoperative CT scans by two independent surgeons. Pump thrombosis was defined as LDH>700 with clinical symptoms of hemolysis or LVAD malfunction. Univariate and Cox Proportional Hazards analysis was performed to evaluate the impact of LV apex angle on long-term freedom from pump exchange for thrombosis.
Results
Of 122 patients who met inclusion criteria for this study, 16 (13.1%) underwent exchange for presumed pump thrombosis. Of these patients, 6 (37.5%) required more than one exchange. Patients undergoing exchange for thrombosis had greater LV angle (43.8±9.7 vs 49.5±11.2, p=0.037) with LV apex angle being a significant predictor of LVAD exchange for thrombosis (HR= 1.047, p=0.046). Additionally, when surgeon measurements were compared there was good inter-observer reliability (Pearson Correlation= 0.89).
Conclusion
A laterally displaced left ventricular apex correlates with a higher risk of pump thrombosis in patients undergoing HM2 implantation. LV apex angle is an easily obtained, reproducible measurement that should be considered when selecting a ventricular assist device.
Keywords: LVAD, pump thrombosis, LV angle, complications
INTRODUCTION
Left Ventricular Assist Devices (LVADs) are an essential component of treatment for patients with advanced heart failure.1 However, device related complications continue to limit the potential of this therapy.2 Pump thrombosis is one of the most devastating complications associated with LVADs. The estimated rate of occurrence is between 12.3% and 18.3% at 24 months of support.3, 4 Management of pump thrombosis requires high dose anticoagulation, pump exchange, or urgent transplantation.5, 6 In those patients who are unable to undergo an exchange or transplantation there is an observed mortality of 48.2% at 6 months.1
In 2011, there was a significant increase in the rate of pump thrombosis associated with the HeartMate II device (HM2).3, 5 As a result, close attention has been given to factors that may lead to pump thrombosis including implantation technique. Careful intraoperative positioning has been highlighted as a key component of optimal therapy. Several studies have demonstrated that pump malposition may contribute to pump thrombosis.7, 8 The ideal inflow cannula placement is parallel to the septum and oriented to the central left ventricle. Sorensen et al note that in post-implantation imaging over 50% of patients exhibit some form of cannula malposition.9
Most studies have concluded that improved surgical technique is the answer to appropriate pump placement.7, 10, 11 However, even with meticulous technique, there are some patients in whom anatomic factors make it more difficult to obtain and subsequently maintain the ideal pump placement with the HeartMate II device.9 The purpose of this study was to determine the reproducibility and impact of Left Ventricular Angle (LV Angle) measurement in predicting pump thrombosis after HM2 implantation. We hypothesized that a laterally displaced left ventricular apex would correlate with increased risk of pump thrombosis and need for exchange.
METHODS
Patient Data
Medical records for all primary HeartMate II (Thoratec, Pleasanton, CA) LVAD implantations from January 2009 through December 2015 at a single academic center were reviewed. Of the 156 patients, 34 were excluded due to lack of preoperative computed tomography (CT) scan or death during the index hospitalization. Chart review of the remaining 122 patients was utilized to identify all patients who developed presumed thrombus in their device requiring pump exchange. Additionally, preoperative computed tomography (CT) scans were reviewed to calculate the effective LV Angle. The University of Virginia Institutional Review Board approved this study with a waiver of patient consent due to low risk of its retrospective nature (IRB Protocol # 19247).
Measures
Patients were stratified by development of presumed pump thrombosis: Thrombosis (n= 16) versus No Thrombosis (n= 106). Pump thrombosis was defined by a LDH>700 with clinical symptoms of hemolysis or LVAD malfunction. The effective LV Angle was measured from midline to the apex of the left ventricle using the picture archiving and communication system (Carestream Health, Rochester, NY) (Figure 1A). These measurements were calculated by two independent and blinded, attending cardiac surgeons. The LV apex angle was dichotomized into medial and lateral angles based on the 75th percentile (51.8 degrees). Additionally, initial postoperative chest radiographs were reviewed using PREVENT guidelines to confirm proper positioning. Variable definitions for baseline demographics, comorbidities, operative characteristics and short-term outcomes are available for each data version (2.61–2.83) in the STS Adult Cardiac Database Data Specifications.12
Figure 1.
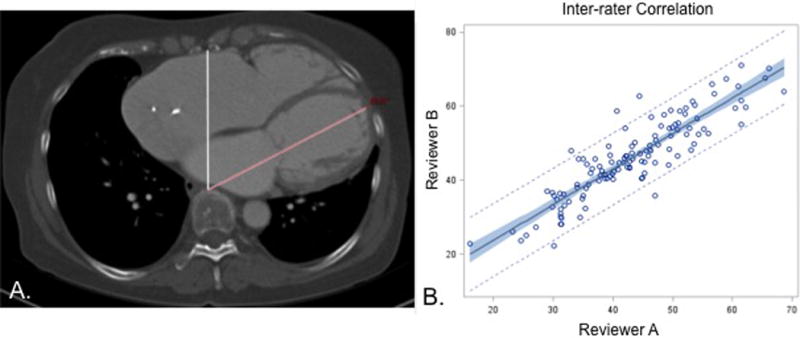
A. The LV apex angle measurement is demonstrated with midline in white and LV apex in red. B. The inter-observer correlation between surgeon measurements had a Pearson’s correlation coefficient of 0.89 demonstrating good reproducibility.
Statistical Analysis
Categorical variables are presented as counts and percentages while continuous variables are shown as mean ± standard deviation (SD) or median [IQR as 25th, 75th percentiles] based on normality. Baseline characteristics and short-term outcomes were assessed by univariate analysis. The Student’s T-Test or Mann-Whitney U Test was utilized for continuous variables and Chi-Square Test for categorical variables. LV angle was compared across of single, multiple and no thrombosis by analysis of variance (ANOVA) with Tukey correction for multiple comparisons. Linear regression was used to analyze the association between LV apex angle and pump thrombosis. Correlation between independent observer measurements was assessed by Pearson’s correlation coefficient. Finally, Cox proportional hazards assessment analyzed the impact of LV apex angle on risk for pump exchange due to thrombosis. All analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC) with statistical significant set at a p-value of 0.05.
RESULTS
Measurements
The mean LV apex angle calculated by surgeon A was 46.0 ± 10.7 degrees while the mean angle for surgeon B was 43.1 ± 10.0 degrees. These demonstrated a high level of inter-observer reliability as seen in Figure 1B with Pearson Correlation Coefficient of 0.893.
Patient Characteristics
Patient demographics and preoperative characteristics were largely similar between cohorts as presented in Table 1. The median age was 59, 24.6% were female and the mean LV angle was 44.6 degrees. There were no statistically different preoperative characteristics between those with pump thrombosis compared to those without thrombosis, except for LV angle, which was significantly greater in the pump thrombosis cohort (49.5 ± 11.2 versus 43.8 ± 9.7, p=0.037). Operative characteristics were also similar between groups as shown in Table 2. When we defined early thrombosis as those patients who required a pump exchange within a year of primary implant, we found that the patients with the greater preoperative LV angle were more likely to require early pump exchange (Figure 2). This was statistically significant when compared to the group with no pump thrombosis as well as when compared to those with a late pump thrombosis. Lastly, Figure 3 demonstrates the mean angle for patients with multiple thrombosis events was significantly greater compared to those without pump thrombosis (56.3 vs 43.9, p=0.02).
Table 1.
Baseline Demographics
| Variable | No Pump Thrombosis | Pump Thrombosis | p-value |
|---|---|---|---|
| Age (years; median, IQR) | 59 [49-64 | 54 [53-59] | 0.216 |
| Female | 27 (25.5%) | 3 (18.8%) | 0.561 |
| Body Mass Index (kg/m2; median, IQR) | 26.5 [23.0-29.4] | 27.8 [24.3-32.6] | 0.21 |
| Tobacco Use | 18 (17.0%) | 3 (18.8%) | 0.861 |
| Diabetes | 39 (36.8%) | 9 (56.3%) | 0.138 |
| Lung Disease (moderate-severe) | 15 (14.2%) | 3 (8.3%) | 0.629 |
| Hypertension | 50 (47.2%) | 6 (37.5%) | 0.469 |
| Cerebrovascular Disease | 15 (14.2%) | 1 (6.3%) | 0.383 |
| Atrial Fibrillation | 46 (45.1%) | 5 (38.5%) | 0.650 |
| Previous Cardiac Intervention | 98 (92.5%) | 13 (81.3%) | 0.145 |
| Previous CABG | 21 (19.8%) | 2 (12.5%) | 0.467 |
| Aortic Regurgitation (moderate-severe) | 5 (6.3%) | 0 (0.0%) | 0.437 |
| Mitral Regurgitation (moderate-severe) | 56 (53.9%) | 8 (50.0%) | 0.774 |
| Tricuspid Regurgitation (moderate-severe) | 34 (33.7%) | 6 (37.5%) | 0.764 |
| LV Apex Angle (degrees) | 43.8±9.7 | 49.5±11.2 | 0.037 |
CABG = Coronary Artery Bypass Grafting; IQR = Interquartile Range; LV = Left Ventricle
Table 2.
Operative Characteristics
| Variable | No Pump Thrombosis | Pump Thrombosis | p-value |
|---|---|---|---|
| Status | 0.402 | ||
| Elective | 10 (9.4%) | 0 | |
| Urgent | 95 (89.6%) | 16 (100%) | |
| Emergent/Salvage | 1 (0.9%) | 0 | |
| Preoperative IABP | 14 (13.2%) | 4 (25.0%) | 0.215 |
| Reoperative status | 28 (26.4%) | 2 (12.5%) | 0.228 |
| Concomitant Valve Surgery | 28 (26.4%) | 4 (25.0%) | 0.905 |
| CPB (min; median, IQR) | 117 [98-145] | 118 [108-134] | 1.000 |
CPB = cardiopulmonary bypass; IABP = Intraaortic Balloon Pump
Figure 2.
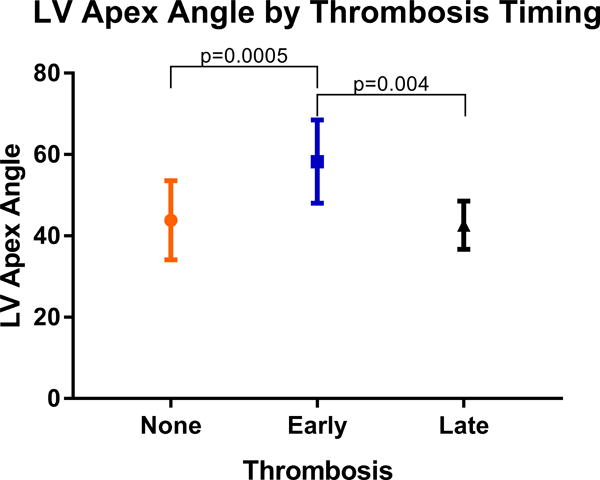
Patients who underwent a pump exchange for presumed pump thrombosis within 1 year of primary implantation had a significantly greater LV angle.
Figure 3.
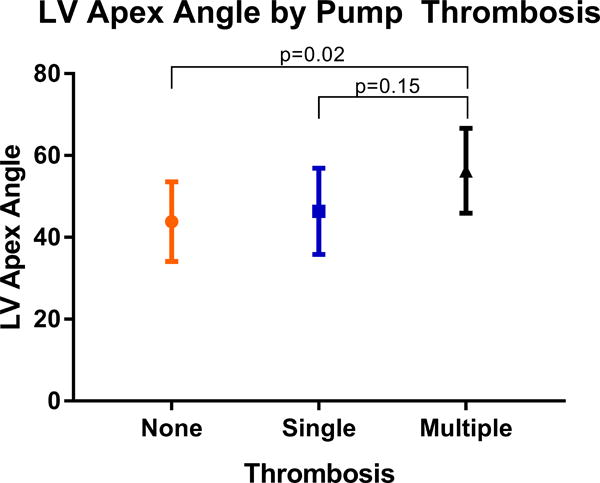
Patient who underwent multiple LVAD exchanges for pump thrombosis in this study were found to have the greatest LV angle.
Outcomes
For the entire cohort the operative mortality rate was 0.8%, major morbidity 48.7% and postoperative length of stay was a median of 20 days. Short-term outcomes were similar between groups without any statistically significant differences (Table 3). There was a trend towards a higher rate of reoperation in patients with pump thrombosis (43.8% vs 23.8%, p=0.092). Cox-Proportional Hazards analysis demonstrated that LV angle was significantly associated with risk of pump thrombosis (HR 1.05, p=0.047). This indicates a 1.05 times increase in pump thrombosis risk for every 1 degree increase in LV angle. Finally, review of immediate postoperative chest radiographs demonstrate compliance with the PREVENT guidelines in all but 1 (6.3%) in the pump thrombosis group and 4 (3.8%) in the non-thrombosis group (p=0.511).
Table 3.
Short-Term Outcomes
| Variable | No Pump Thrombosis | Pump Thrombosis | p-value |
|---|---|---|---|
| Operative Mortality | 1 (0.9%) | 0 (0%) | 0.697 |
| Major Morbidity | 50 (47.6%) | 9 (56.3%) | 0.520 |
| Permanent Stroke | 4 (3.8%) | 0 (0%) | 0.427 |
| Pneumonia | 9 (8.6%) | 1 (6.3%) | 0.753 |
| Prolonged Ventilation | 40 (38.1%) | 8 (50.0%) | 0.365 |
| Renal Failure | 7 (6.7%) | 3 (18.8%) | 0.102 |
| Reoperation | 25 (23.8%) | 7 (43.8%) | 0.092 |
| Reoperation for bleeding | 19 (15.7%) | 3 (18.8%) | 0.950 |
| Any Transfusion | 90 (84.9%) | 15 (93.8%) | 0.341 |
| PRBC Transfused | 89 (84.0%) | 15 (93.8%) | 0.304 |
| Atrial Fibrillation | 5 (4.8%) | 2 (12.5%) | 0.217 |
| Length of Stay (days; median, IQR) | 20 [16-25] | 19 [15-36] | 0.976 |
| ICU Length of Stay (hours; median, IQR) | 195 [161-312] | 190 [141-830] | 0.751 |
| Readmission | 14 (13.2%) | 2 (12.5%) | 0.938 |
ICU = Intensive Care Unit; IQR = Interquartile Range
DISCUSSION
This study demonstrates a more laterally displaced LV is correlated with pump thrombosis and need for pump exchange in patients with a HM2 LVAD. Furthermore these patients were more likely to require an early pump exchange within one year of primary implantation. Furthermore, review of immediate postoperative chest radiographs demonstrates almost all patients meet PREVENT guidelines for proper placement with no difference between those with and those without pump thrombosis. To our knowledge, this study is the first to identify a preoperative LV anatomically measure as a risk factor for early pump thrombosis. Pump thrombosis is a serious complication of LVAD therapy and is associated with increased morbidity and mortality.13 Strategies to prevent pump thrombosis are critical, but have been primarily limited to anti-coagulation.
While pump thrombosis is clearly a multifactorial problem, several studies including that by Kauzui et al have concluded that pump malposition, at the time of surgical implantation, contributes to pump thrombosis.7, 8, 14 Meticulous surgical technique should be employed with every implantation, there are some patient specific factors, which may prevent appropriate device positioning intraoperatively.11 Several groups have demonstrated these anatomic factors, including shorter or heavier body habitus may influence pump migration.7, 15 Clinically, we recognized the challenge of correct HM2 positioning in those patients with a very lateral and posteriorly rotated left ventricle. Consequently, we began utilizing preoperative LV angle to guide decision making for device selection at our institution. The LV angle is relatively straightforward to obtain from a Chest CT and, in this study, had excellent inter-observer reliability (Pearson Correlation 0.893) by blinded surgical reviewers. Given its facility and reproducibility, preoperative LV angle is a readily available quantifiable measurement to guide LVAD selection and risk for pump thrombosis. Of note, patients with early pump thrombosis were more likely to have a more lateral LV. Conversely patients with late pump thrombosis had no significant difference in LV angle. We surmise that early pump thrombosis is primarily driven by pump positioning which is more sensitive to anatomic characteristics. Late pump thrombosis is primarily driven by pump migration and other hemodynamic factors. Of note, no increased hypercoaguability was noted in our patient cohort.
It is our practice to support the patient with cardiopulmonary bypass during LVAD placement. We only clamp the heart in the instance of aortic valve insufficiency or severe tricuspid regurgitation. Pre-existing thrombus is removed in all cases and a cross clamp is not specifically used for that purpose at our institution. This study population demonstrated no difference in preoperative atrial fibrillation but we do not perform atrial appendage ligation for these patients.
Pump exchange has been shown to decrease mortality associated with pump thrombosis and is the preferred strategy for management of pump thrombosis at our institution in view of the advantages and disadvantages of anticoagulation therapy in these high risk patients.16 Similar to other centers, we use an elevated LDH as well as signs of LVAD malfunction as criteria for pump exchange.13, 17–19 For HM2 exchange we use a subcostal incision for pump only exchange. We bleed the inflow and outflow grafts to ensure these are free of thrombus. In the early years of this study, not all devices were returned for inspection. However in those patients in whom the pump was returned to the company for inspection, all had a confirmed thrombus within the pump. In all patients in this cohort, HM2 devices were exchanged for another HM2. Given the findings of this study, in patients with a greater LV angle, consideration for exchange to a different device should be considered, as the risk for subsequent pump thrombosis may be high.
The limitations of this study include the single center retrospective nature in addition to only obtaining one anatomic measurement. While this was a statistically significant predictor of pump thrombosis there are a number of patients with a laterally displaced LV who did not have pump thrombosis. There are likely several anatomic factors such as central obesity as well as non-anatomic factors contributed to pump thrombosis.5, 8, 19 Finally, this study is limited to patients who underwent a pump exchange for presumed pump thrombosis. As a center we are very aggressive about early LVAD replacement for presumed pump thrombosis and if someone is admitted with clinical symptoms and elevated LDH refractory to medical therapy we almost always operate. For this reason we are unable to identify individuals who may have had pump thrombosis but were managed medically. Therefore our data may underestimate the incidence of pump thrombosis.
In conclusion the HM2 device has long term proven results and until recently, was one of the only durable left ventricular assist devices approved in the United States.20 For that reason, studies revolved around how to optimize this pump for all patients with advanced heart failure. The findings in this study demonstrate that there is a higher rate of pump thrombosis with the HM2 in those patients with the most laterally displaced LV apex despite proper positioning on postoperative chest radiograph. With the advent of implantable intrapericardial devices, there are now options for which device to implant. While, LVAD selection is still driven largely by a patient’s transplant status as well as insurance designation, we have used the data from this study as reason to consider patient anatomy when selecting a device. Continued follow-up is necessary to determine if a tailored approach based on patient anatomic features will decrease the incidence of pump thrombosis.
Acknowledgments
Conflict of Interest and Funding Sources
Dr. Ailawadi is a consultant for Abbott, Edwards, Medtronic, and a speaker for Atricure in last 3 years but not within the last year. The National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL007849 supported research reported in this publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 2.Kirklin JK, Naftel DC, Kormos RL, et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2014;33:12–22. doi: 10.1016/j.healun.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370:33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 4.Susen S, Rauch A, Van Belle E, Vincentelli A, Lenting PJ. Circulatory support devices: fundamental aspects and clinical management of bleeding and thrombosis. J Thromb Haemost. 2015;13:1757–1767. doi: 10.1111/jth.13120. [DOI] [PubMed] [Google Scholar]
- 5.Blitz A. Pump thrombosis-A riddle wrapped in a mystery inside an enigma. Ann Cardiothorac Surg. 2014;3:450–471. doi: 10.3978/j.issn.2225-319X.2014.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stulak JM, Cowger J, Haft JW, Romano MA, Aaronson KD, Pagani FD. Device exchange after primary left ventricular assist device implantation: indications and outcomes. Ann Thorac Surg. 2013;95:1262–1267. doi: 10.1016/j.athoracsur.2012.08.031. discussion 1267–1268. [DOI] [PubMed] [Google Scholar]
- 7.Kazui T, Zhang A, Greenberg J, et al. Left Ventricular Assist Device Inflow Angle and Pump Positional Change Over Time Adverse Impact on Left Ventricular Assist Device Function. Ann Thorac Surg. 2016;102:1933–1940. doi: 10.1016/j.athoracsur.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Uriel N, Han J, Morrison KA, et al. Device thrombosis in HeartMate II continuous-flow left ventricular assist devices: a multifactorial phenomenon. J Heart Lung Transplant. 2014;33:51–59. doi: 10.1016/j.healun.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Sorensen EN, Hiivala NJ, Jeudy J, Rajagopal K, Griffith BP. Computed tomography correlates of inflow cannula malposition in a continuous-flow ventricular-assist device. J Heart Lung Transplant. 2013;32:654–657. doi: 10.1016/j.healun.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Maltais S, Kilic A, Nathan S, et al. PREVENtion of HeartMate II Pump Thrombosis Through Clinical Management: The PREVENT multi-center study. J Heart Lung Transplant. 2017;36:1–12. doi: 10.1016/j.healun.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Adamson RM, Mangi AA, Kormos RL, Farrar DJ, Dembitsky WP. Principles of HeartMate II implantation to avoid pump malposition and migration. J Card Surg. 2015;30:296–299. doi: 10.1111/jocs.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Data Collection. STS.org2016
- 13.Levin AP, Saeed O, Willey JZ, et al. Watchful Waiting in Continuous-Flow Left Ventricular Assist Device Patients With Ongoing Hemolysis Is Associated With an Increased Risk for Cerebrovascular Accident or Death. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002896. [DOI] [PubMed] [Google Scholar]
- 14.Aliseda A, Chivukula VK, McGah P, et al. LVAD Outflow Graft Angle and Thrombosis Risk. ASAIO J. 2017;63:14–23. doi: 10.1097/MAT.0000000000000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adamson RM, Bower BL, Sundareswaran KS, Farrar DJ, Dembitsky WP. Radiologic assessment of HeartMate II position: Minimal pump migration after long-term support. J Heart Lung Transplant. 2015;34:1617–1623. doi: 10.1016/j.healun.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Basken R, Bazzell CM, Smith R, Janardhanan R, Khalpey Z. Advantages and disadvantages of using intravenous tissue Plasminogen activator as salvage therapy for inoperable HeartWare thrombosis. J Card Surg. 2017;32:443–446. doi: 10.1111/jocs.13165. [DOI] [PubMed] [Google Scholar]
- 17.Tran BC, Nijjar PS. Role of contrast CT for the diagnosis and the prognosis of suspected LVAD thrombosis. J Card Surg. 2017;32:162–165. doi: 10.1111/jocs.13094. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein DJ, John R, Salerno C, et al. Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant. 2013;32:667–670. doi: 10.1016/j.healun.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Bhamidipati CM, Ailawadi G, Bergin J, Kern JA. Early thrombus in a HeartMate II left ventricular assist device: a potential cause of hemolysis and diagnostic dilemma. J Thorac Cardiovasc Surg. 2010;140:e7–8. doi: 10.1016/j.jtcvs.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]


