Abstract
Hippocampal dendritic spine density rapidly increases following estradiol (E2) treatment, but the types of spines and trafficking of synaptic markers have received little investigation. We assessed rapid effects of E2 over time on the density of four spine types (stubby, filopodial, long thin and mushroom) and trafficking of AMPA receptor subunit GluA2 and PSD95 on tertiary, apical dendrites in CA1. Castrated male rats received 20 μg/kg of E2 or vehicle and were sacrificed 30 or 120 min later. Images of Golgi-Cox impregnated and PSD95/GluA2 stained dendrites were captured under the confocal microscope and quantified with IMARIS-XT. Stubby and filopodial spine densities did not change following treatment. Long-thin spines significantly decreased at 30 min while mushroom spines significantly increased at 120 min. GluA2, PSD95 and GluA2/PSD95 colocalization levels in stubby or long thin spines did not change, but filopodial spines had significantly reduced GluA2 levels at 30 min. Mushroom spines showed significantly increased levels for GluA2, PSD95 and GluA2/PSD95 colocalization at 120 min. Since GluA2 is important for memory consolidation, current results present novel data suggesting that trafficking of GluA2 to mushroom spines provides one mechanism contributing to estradiol's ability to enhance learning and memory by the PI3 signaling pathway.
That estrogens exert regulation on dendritic spine density in hippocampal CA1 pyramidal neurons has been well documented over the last thirty years, reviewed in (Luine and Frankfurt, 2012), but the types of spines affected and the molecular mechanisms underlying E2-induced spinogenesis still remain largely unclear. Recent demonstrations of rapid spinogenesis and synaptogensis, within 30-40 min of S.C. E2 injections to gonadectomized females (MacLusky et al., 2005; Inagaki et al., 2012; Phan et al., 2012) and males (Jacome et al., 2016), provide a novel model for understanding this process and how new spines may relate to enhanced spatial memory following E2 (Luine and Frankfurt, 2012)
In the current studies, a new 3-dimensional analysis of dendritic spines is combined with immunohistochemical staining allowing for co-localization of selected synaptic markers within various spine shapes. Our recent publication using this technique identifies rapid changes within particular spine shapes and changes in synaptic protein markers within these spines after stress and OP memory consolidation (Sebastian et al., 2013a; Iniguez et al., 2016). Thus, this powerful technique can be utilized to understand E2's role in promoting spinogenesis and the synaptic markers within these spines. We measured four distinct spine classes (filopodia, stubby, long-thin, and mushroom) characterized differentially by their head and neck ratio (Spiga et al., 2011; Rochefort and Konnerth, 2012). Filopodia and stubby spines are immature spines, while long-thin and mushroom spines have larger spine heads than immature spines and are considered mature spines (Bourne and Harris, 2007; Rochefort and Konnerth, 2012). Focusing on specific spine types allows for a more discrete evaluation of spine densities, which may otherwise be masked by only examining overall changes in spines. We determined the expression of functional synapses by the presence of the PSD-95 marker and the AMPA receptor subunit GluA2 within the four spine classes given their important role in the development of these spine types (Anggono and Huganir, 2012).
AMPA receptors are ionotropic glutamate receptors that have been widely implicated in synaptic plasticity, as well as learning and memory processes (Huganir and Nicoll, 2013). They are made up of 4 subunits that are composed of two identical heterodimers. Additionally, the GluA2 subunit is the rate-limiting factor for calcium influx after activation (Isaac et al., 2007), and thus plays an important role in modulating synaptic activity (Schmidt et al., 2010). The most predominant composition is the GluA1/2 subtype, followed by the GluA2/3 subtype (Anggono and Huganir, 2012) both of which are expressed in the hippocampus (Wenthold et al., 1996), and are important for learning (Joels and Lamprecht, 2010), and long-term memory processes (Migues et al., 2010; Henley and Wilkinson, 2013; Sebastian et al., 2013b; Braren et al., 2014), respectively. The mechanisms by which GluA2/3 subunits remain elevated in the synaptic membrane are dependent upon PKMζ (Ling et al., 2002). As the trafficking of the GluA2 receptor subunit to the post synaptic density increases during episodes of synaptic plasticity and memory, clusters of PKMζ/GluA2/PSD95 proteins have been identified (Shao et al., 2012), which prevent AMPA receptors from undergoing endocytosis, reviewed in (Sacktor, 2011). Stabilizing AMPA receptors within the synaptic membrane is important for memory consolidation (Migues et al., 2010), as well as enhancing the area of mushroom spine heads (mature spines), which are known to increase during memory (Sebastian et al., 2013a). Mushroom spines have been hypothesized to represent physical substrates of long-term memories, i.e., memory spines, while small or stubby spines represent the capacity for adaptive, experience-dependent rewiring of neuronal circuits, i.e., learning spines (Kasai et al., 2003; Bourne and Harris, 2008).
The current study assessed effects of acute E2 administration, 30 and 120 min, on density of the four spine subtypes described above and the levels of GluA2 and PSD-95 within the spines. Twenty adult (8 weeks old), castrated (ORX), male Sprague-Dawley rats (Harlan Sprague-Dawley, Inc) were used because gonadally intact males show increased CA1 spine densities as compared to ORX rats (Luine, 2015) due to the presence of circulating androgens and estrogens (Jacome et al., 2016). Subjects were housed two/cage at the Hunter College animal facility and kept on a 12/12 h light/dark cycle with food and water ad libitum as previously reported (Jacome et al., 2016), conforming to the Hunter College guidelines outlined by the Institutional Animal Care and Use Committee. One week following arrival to the Hunter College animal facility (two weeks following ORX surgery), rats were randomly assigned to one of three treatment conditions: Control (vehicle; n = 6), E2 (30 min; n = 7) or E2 (120 min, n=7). Subjects received a single sc injection at the nape of the neck of corn oil vehicle (1 ml/kg; Fisher Science education), or E2 (20 μg/kg; Sigma Aldrich Co). Animals were anesthetized and perfused either 30 or 120 min following E2 or vehicle treatment as previously reported (Jacome et al., 2016). Brains were prepared for Golgi-IHC staining and tertiary, apical spines in CA1 analyzed as previously reported (Sebastian et al., 2013a; Iniguez et al., 2016). Data were analyzed using 1-way ANOVAs across treatments (vehicle, E2 30 min, E2 120 min) x various spine types (stubby, filapodia, long-thin, mushroom) in combination with Tukey-corrected t tests for post-hoc analyses. Differences in the levels of the various synaptic markers within spine types were also tested by one-way ANOVAs across treatment conditions x individual synaptic markers (PSD-95, GluA2, or PSD-95/GluA2 colocalization) with post-hoc testing. Prism GraphPad 6.0 Statistical Package, (La Jolla, California) was used.
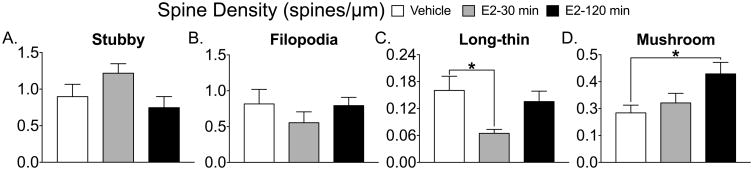
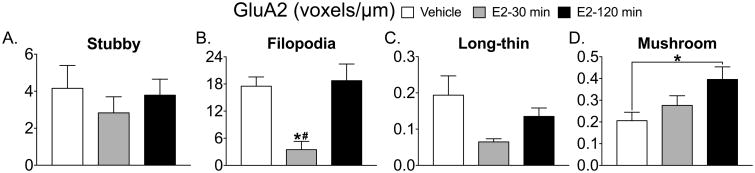
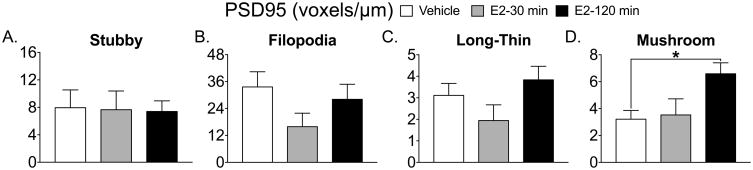
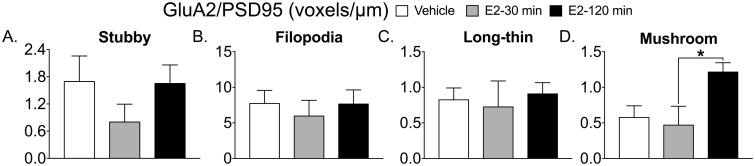
The mature spines, long-thin (Fig 1C) and mushroom (Fig 1D) both show an overall effect of treatment (long-thin; F(2, 14) = 3.853, p=0.046; mushroom F(2, 15) = 4.377 p=0.03) and a significant Tukey post-hoc test (long-thin, vehicle vs. E2 30 min, p= 0.04; mushroom, vehicle vs E2 120 min, p=0.03). Filopodia spines containing GluA2 show an overall effect of treatment (F(2, 11)=10.16, p=0.003) and significant Tukey post-hoc tests (E2 30 min vs. vehicle, *p=0.005; E2 30 min vs. E2 120 min #p=0.006, Fig 2B). The density of GluA2 containing mushroom spines show a significant overall effect of treatment (F(2, 16)= 4.1, p=0.03) and a significant Tukey post-hoc test (vehicle vs. E2 120 min, *p=0.027, Fig 2D). Only PSD95 levels within mushroom spines show an overall effect of treatment (F(2, 16)= 4.3, p=0.032) and a significant Tukey post-hoc test (vehicle vs. E2 120 min, *p=0.038, Fig 3D). GluA2/PSD95 colocalization within mushroom spines shows an overall effect of treatment (F(2,14)= 4.783, p=0.026) and a significant Tukey post-hoc test (E2 30 min vs. E2 120 min, *p=0.036, Fig 4D and Fig 5).
Figure 1. Estradiol treatment decreases the density of long-thin spines and increases mushroom spines.
Stubby (A) and filopodia (B) spines did not change as a consequence of E2 treatment. Long-thin spines show a significant reduction 30 min following E2 compared to vehicle (*p=0.04, C). Mushroom spines show a significant increase 120 min following E2 treatment compared to vehicle (*p=0.03, D).
Figure 2. Estradiol treatment decreases GluA2 levels in filopodia spines and increases GluA2 levels within mushroom spines.
GluA2 levels in stubby spines did not change with E2 treatment (A). 30 min following E2, GluA2 levels were significantly lower within filopodia spines compared to vehicle and compared to 120 min after E2 treatment (*p=0.005, #p=0.006 respectively, B). GluA2 levels within long-thin spines did not change as a consequence of E2 treatment (C). GluA2 levels within mushroom spines show a significant increase 120 min following E2 treatment compared to vehicle (*p=0.027, D).
Figure 3. Estradiol increases PSD95 levels within mushroom spines.
PSD95 levels within stubby (A), filopodia (B) and long-thin spines (C) did not change following E2 treatment. PSD95 levels within mushroom spines show a significant increase 120 min following E2 treatment compared to vehicle (*p=0.038, D).
Figure 4. Estradiol increases GluA2/PSD95 colocalization levels within mushroom spines.
GluA2/PSD95 colocalization levels within stubby (A), filopodia (B), and long-thin (C) spines did not significantly change following E2 treatment. GluA2/PSD95 colocalization levels significantly increase 120 min following E2 treatment compared to 30 min after E2 (*p=0.036; D).
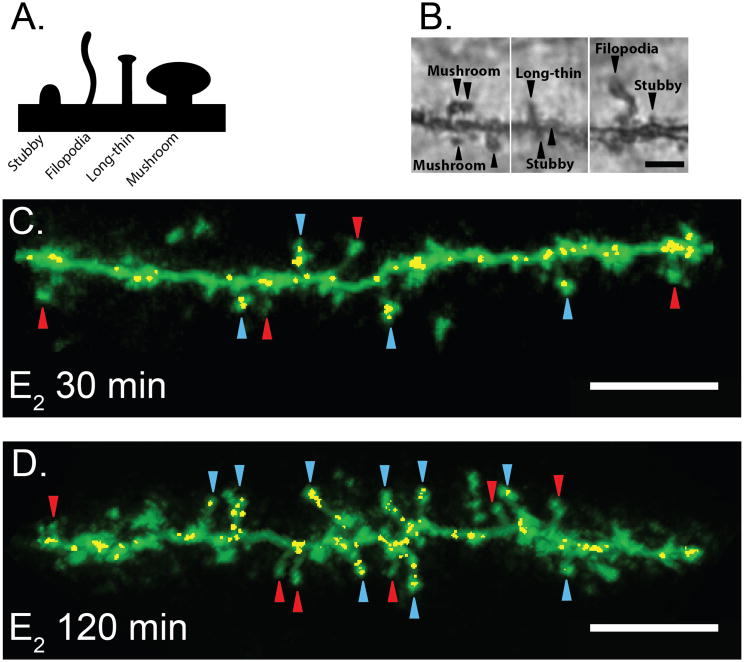
Figure 5. Identification of spine-specific changes in the CA1 region of the hippocampus following Estradiol administration.
(A) Classification of dendritic spine morphology. (B) Representative Golgi-impregnation of hippocampus CA1 spines captured at 100x magnification. Scale bar =3μm. (C, D) Estradiol increases colocalization of GluA2 and PSD95 within Mushroom spines. Representative dendrites from E30 min (C) and E120 min (D). Yellow voxels indicate colocalized GluA2 and PSD95 within Mushroom spines. All carats indicate mushroom spines. Red carats indicate mushroom spines that do not contain colocalized GluA2 and PSD95. Blue carats indicate mushroom spines that contain colocalized GluA2 and PSD95. Scale bar =5μm.
Our results show that the density of long-thin spines decreases 30 min after treatment with E2 while the density of mushroom spines increases 120 min after treatment. Further, the density of mushroom spines which show co-localization of GluA2 and PSD-95 increased threefold between 30 and 120 min following E2 treatment. Thus, this study is the first demonstration of the time course for shifts in the densities of various spine types in CA1 of the hippocampus following E2. We and others have previously reported that E2 increases overall dendritic spine density on pyramidal neurons in CA1 of the hippocampus and the medial prefrontal cortex (PFC) at 30 min, 2 h and 4 h following S.C. (Phan et al., 2011; Inagaki et al., 2012; Phan et al., 2012) or intra-hippocampal administration to ovariectomized (OVX) rats (Phan et al., 2015; Tuscher et al., 2016). In vitro studies support these findings using hippocampal sections from male or female rats that were treated with E2 resulting in an increase in spine density within 20 (Phan et al., 2015) to 120 min (Hasegawa et al., 2015), respectively. Similar, but more rapid, changes were reported in cultured cortical neurons where increased synaptogenesis and levels of PDS-95 were found 30 min following estradiol (Sellers et al., 2015). In the in vitro studies, specific types of spines were not identified, nor were decreases in spine densities as a consequence of treatment found in either the in vivo or in vitro studies. While these studies show increases in total spines following E2, only a few have identified changes in spine types. Mushroom spines were higher in OVX+E2 compared to OVX mice following 5 days of treatment, but thin and total spine counts were unchanged (Li et al., 2004). Christensen and colleagues (2011) also identified a dynamic switch in spine formation, showing increases in immature spines (filopodial spines) within 4h of E2 treatment followed by increases in mature spines and a return of filopodial spines to control levels 20 h after E2 treatment (long-thin spines were not measured) in the arcuate nucleus, an E2 sensitive nucleus regulating reproductive function (Christensen et al., 2011). Furthermore, Gonzalez and colleagues (2005) show higher levels of mushroom spines in the proestrus phase (high estrogen) in rats, while in estrus (low estrogen) thin spine levels are high (Gonzalez-Burgos et al., 2005). This result is consistent with our E2-dependent increase in mushroom spines with a concomitant decrease in long-thin spine types.
In Golgi studies using OVX (Li et al., 2004; Beltran-Campos et al., 2011) or ORX (current study), mushroom spines constitute a minority of the total spines. In contrast, use of serial section transmission electron microscopy or direct application of fluorescent dyes into the brain with confocal microscopy shows higher densities of mushroom spines (Kasai et al., 2003; Bourne and Harris, 2011) However, it should be noted that most, if not all, morphological studies utilize intact male rodents. Using gonadally intact female rats, Gonzalez-Burgos et al (2005) found that mushroom, compared to stubby or thin, spines were the majority spines during proestrus when estrogens are high, whereas, during diestrus, mushroom spines decreased and thin spines were the majority. Thus, the predominance of stubby and filopodial spines in the current study may relate to low circulating levels of gonadal hormones in the males. However, it remains possible that long thin spines may be underrepresented in Golgi-Cox stained material because of the lesser resolution of this technique.
Enhancements in memory by E2 have long been associated with E2-dependent increases in the number of dendritic spines on pyramidal neurons in CA1, a seminal finding in neuroendocrinology (Frankfurt et al., 1990; Gould et al., 1990; Woolley et al., 1990), which has been widely replicated (reviewed in (Luine and Frankfurt, 2012; Frankfurt and Luine, 2015). Our data thus provide evidence for a close link between E2 treatment, increases in mushroom spines and memory consolidation across both sexes (Jacome et al., 2016). Consistent with our results and the role of mushroom spines in memory, increased mushroom spine density in CA1 produced by water maze training is prevented by ovariectomy (Beltrán-Campos et al, 2011).
The decrease in mature, long-thin dendritic spine density in CA1 at 30 min post estradiol is different from previous results in female rodents (Li et al., 2004; Christensen et al., 2011) and may reflect a sex difference in the effects of estradiol. However, few in vivo studies have been conducted in either sex. Nonetheless, these findings may be consistent with ex-vivo findings that AMPA currents are rapidly reduced by estradiol in female PFC (Sellers et al., 2015) and CA1 (Phan et al., 2015)These rapidly induced spines are initially silent synapses whereas in males, a similar functional outcome may be achieved with a physical reduction in spines. Supporting this notion of sex differences in responses, a similar pattern of spine turnover as reported here was found in males following induction of long term potentiation (LTP), i.e. decreases in small thin spines in the first 30 min followed by an increase in PSD area at 2 h (Bourne and Harris, 2011). Since both LTP and estradiol promote memory formation, spine trafficking may be integral to the process, but the pattern may be somewhat different between the sexes.
Our analysis of GluA2 containing spines types reveal significantly lower densities of filopodial spines 30 min after E2 treatment followed by significantly higher GluA2, PSD95 and GluA2/PSD95 colocalization within mushroom spines at 120 min. These results highlight the dynamic and rapid effects of E2 on hippocampal spines and the trafficking of GluA2 in specific spines. E2 can act at membrane receptors located outside of the cell nucleus on cell bodies, axons, spines, presynaptic terminals and near post synaptic neurotransmitter receptors (Almey et al., 2015). The interaction between E2 and various synaptic receptors causes rapid changes in rodent sexual behavior, avian male sexual displays, nutrient ingestion, social learning, and more importantly, cognition (Christensen et al., 2011; Cornil et al., 2012; Luine and Frankfurt, 2012; Sinchak and Wagner, 2012; Ervin et al., 2013). Within seconds to minutes of binding, cell signaling cascades are activated including ERK-dependent mammalian target of rapamycin, a key protein synthesis pathway involved in spine remodeling, the transcription factor cAMP-response element-binding protein which mediates filopodial extension and spine formation, and the PI3 pathway involved in synapse formation (Frick, 2015). Our results suggest that E2 activates this PI3-GluA2/PKMζ signaling pathway resulting in the rapid growth of spines, particularly filopodia to mushroom spines and the trafficking of the GluA2 receptors in the spines. Based on current knowledge of synaptic dynamics in the hippocampus (Bourne and Harris, 2007; Bourne and Harris, 2008), these rapid changes initiated by estradiol may provide an important substrate for enhanced memory.
Acknowledgments
Funding: This project was supported in part by the RCMI grant number RR003037 from the National Center for Research Resources (NCRR); Training Grants GM060665 to JA and NS080686 to BC; NIH 5R24DA012136-13 to PAS.
Footnotes
Conflict of Interest Statement: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions: PS, AA, JA and VL designed the experiments and wrote the manuscript. BC, RZ, and AA conducted the experiment. BC and JA carried out the Golgi staining, imaging, and IMARIS analyses. JA performed the statistical analyses. All authors approved the final manuscript for submission.
References
- Almey A, Milner TA, Brake WG. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav. 2015;74:125–138. doi: 10.1016/j.yhbeh.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol. 2012;22:461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran-Campos V, Prado-Alcalá R, Leon-Jacinto U, Aguilar-Vazquez A, Quirarte G, Ramirez-Amaya V, Diaz-Cintra S. Increase of mushroom spine density in CA1 apical dendrites produced by water maze training is prevented by ovariectomy. Brain research. 2011;1369:119–130. doi: 10.1016/j.brainres.2010.10.105. [DOI] [PubMed] [Google Scholar]
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17:381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP. Hippocampus. 2011;21:354–373. doi: 10.1002/hipo.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braren SH, Drapala D, Tulloch IK, Serrano PA. Methamphetamine-induced short-term increase and long-term decrease in spatial working memory affects protein Kinase M zeta (PKMzeta), dopamine, and glutamate receptors. Front Behav Neurosci. 2014;8:438. doi: 10.3389/fnbeh.2014.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A, Dewing P, Micevych P. Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity. Journal of Neuroscience. 2011;31:17583–17589. doi: 10.1523/JNEUROSCI.3030-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J. Rapid control of male typical behaviors by brain-derived estrogens. Front Neuroendocrinol. 2012;33:425–446. doi: 10.1016/j.yfrne.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervin KS, Phan A, Gabor CS, Choleris E. Rapid oestrogenic regulation of social and nonsocial learning. J Neuroendocrinol. 2013;25:1116–1132. doi: 10.1111/jne.12079. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, Luine V. The evolving role of dendritic spines and memory: Interaction(s) with estradiol. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankfurt M, Gould E, Woolley CS, McEwen BS. Gonadal steroids modify dendritic spine density in ventromedial hypothalamic neurons: a Golgi study in the adult rat. Neuroendocrinology. 1990;51:530–535. doi: 10.1159/000125387. [DOI] [PubMed] [Google Scholar]
- Frick KM. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav. 2015;74:4–18. doi: 10.1016/j.yhbeh.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos I, Alejandre-Gomez M, Cervantes M. Spine-type densities of hippocampal CA1 neurons vary in proestrus and estrus rats. Neurosci Lett. 2005;379:52–54. doi: 10.1016/j.neulet.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Hojo Y, Kojima H, Ikeda M, Hotta K, Sato R, Ooishi Y, Yoshiya M, Chung BC, Yamazaki T, Kawato S. Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: Involvement of kinase networks. Brain Res. 2015;1621:147–161. doi: 10.1016/j.brainres.2014.12.056. [DOI] [PubMed] [Google Scholar]
- Henley JM, Wilkinson KA. AMPA receptor trafficking and the mechanisms underlying synaptic plasticity and cognitive aging. Dialogues Clin Neurosci. 2013;15:11–27. doi: 10.31887/DCNS.2013.15.1/jhenley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80:704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Frankfurt M, Luine V. Estrogen-induced memory enhancements are blocked by acute bisphenol A in adult female rats: role of dendritic spines. Endocrinology. 2012;153:3357–3367. doi: 10.1210/en.2012-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez SD, Aubry A, Riggs LM, Alipio JB, Zanca RM, Flores-Ramirez FJ, Hernandez MA, Nieto SJ, Musheyev D, Serrano PA. Social defeat stress induces depression-like behavior and alters spine morphology in the hippocampus of adolescent male C57BL/6 mice. Neurobiol Stress. 2016;5:54–64. doi: 10.1016/j.ynstr.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Jacome LF, Barateli K, Buitrago D, Lema F, Frankfurt M, Luine VN. Gonadal Hormones Rapidly Enhance Spatial Memory and Increase Hippocampal Spine Density in Male Rats. Endocrinology. 2016;157:1357–1362. doi: 10.1210/en.2015-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels G, Lamprecht R. Interaction between N-ethylmaleimide-sensitive factor and GluR2 is essential for fear memory formation in lateral amygdala. J Neurosci. 2010;30:15981–15986. doi: 10.1523/JNEUROSCI.1872-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat Neurosci. 2002;5:295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- Luine V. Recognition memory tasks in neuroendocrine research. Behav Brain Res. 2015;285:158–164. doi: 10.1016/j.bbr.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Frankfurt M. Estrogens facilitate memory processing through membrane mediated mechanisms and alterations in spine density. Front Neuroendocrinol. 2012;33:388–402. doi: 10.1016/j.yfrne.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci. 2010;13:630–634. doi: 10.1038/nn.2531. [DOI] [PubMed] [Google Scholar]
- Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor alpha and beta selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152:1492–1502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- Phan A, Gabor CS, Favaro KJ, Kaschack S, Armstrong JN, MacLusky NJ, Choleris E. Low doses of 17beta-estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharmacology. 2012;37:2299–2309. doi: 10.1038/npp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan A, Suschkov S, Molinaro L, Reynolds K, Lymer JM, Bailey CD, Kow LM, MacLusky NJ, Pfaff DW, Choleris E. Rapid increases in immature synapses parallel estrogen-induced hippocampal learning enhancements. Proc Natl Acad Sci U S A. 2015;112:16018–16023. doi: 10.1073/pnas.1522150112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort NL, Konnerth A. Dendritic spines: from structure to in vivo function. EMBO Rep. 2012;13:699–708. doi: 10.1038/embor.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor TC. How does PKMzeta maintain long-term memory? Nat Rev Neurosci. 2011;12:9–15. doi: 10.1038/nrn2949. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Trumbach D, Weber P, Wagner K, Scharf SH, Liebl C, Datson N, Namendorf C, Gerlach T, Kuhne C, Uhr M, Deussing JM, Wurst W, Binder EB, Holsboer F, Muller MB. Individual stress vulnerability is predicted by short-term memory and AMPA receptor subunit ratio in the hippocampus. J Neurosci. 2010;30:16949–16958. doi: 10.1523/JNEUROSCI.4668-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian V, Estil JB, Chen D, Schrott LM, Serrano PA. Acute physiological stress promotes clustering of synaptic markers and alters spine morphology in the hippocampus. PLoS One. 2013a;8:e79077. doi: 10.1371/journal.pone.0079077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian V, Vergel T, Baig R, Schrott LM, Serrano PA. PKMzeta differentially utilized between sexes for remote long-term spatial memory. PLoS One. 2013b;8:e81121. [Google Scholar]
- Sellers KJ, Erli F, Raval P, Watson IA, Chen D, Srivastava DP. Rapid modulation of synaptogenesis and spinogenesis by 17β-estradiol in primary cortical neurons. Frontiers in cellular neuroscience. 2015;9 doi: 10.3389/fncel.2015.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao CY, Sondhi R, van de Nes PS, Sacktor TC. PKMzeta is necessary and sufficient for synaptic clustering of PSD-95. Hippocampus. 2012;22:1501–1507. doi: 10.1002/hipo.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Wagner EJ. Estradiol signaling in the regulation of reproduction and energy balance. Front Neuroendocrinol. 2012;33:342–363. doi: 10.1016/j.yfrne.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga S, Acquas E, Puddu MC, Mulas G, Lintas A, Diana M. Simultaneous Golgi-Cox and immunofluorescence using confocal microscopy. Brain Struct Funct. 2011;216:171–182. doi: 10.1007/s00429-011-0312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Luine V, Frankfurt M, Frick KM. Estradiol-mediated spine changes in the dorsal hippocampus and medial prefrontal cortex of ovariectomized female mice depend on ERK and mTOR activation in the dorsal hippocampus. Journal of Neuroscience. 2016;36:1483–1489. doi: 10.1523/JNEUROSCI.3135-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]