Abstract
An important function of emotion is that it motivates us to respond more effectively to threats in our environment. Accordingly, healthy emotional function depends on the ability to appropriately avoid, escape, or defend against threats we encounter. Thus, from a functional perspective, it is important to understand the emotional response to threat. However, prior work has largely focused on the emotional response in anticipation of threat, rather than the emotional response to the threat itself. The current review is focused on recent behavioral, psychophysiological, and neural findings from Pavlovian conditioning research that is centered on the expression and regulation of the emotional response to threat. The current evidence suggests that a neural network that includes the prefrontal cortex, hippocampus, and amygdala underlies learning, expression, and regulation processes that modulate emotional responses to threat. This line of research has important implications for our understanding of emotion regulation and stress resilience.
Keywords: Pavlovian conditioning, UCR, threat, learning, emotion, regulation, fMRI
Introduction
An important function of emotion is that it motivates adaptive responses to important events in our environment. For example, fear motivates defensive responses (e.g., fight or flight) and promotes the rapid formation of associations between warning signals and the threats they predict (i.e., associative learning). More specifically, the knowledge that a threat is imminent allows one to execute preparatory behaviors in anticipation of the impending threat. For example, imagine a traffic accident that suddenly appears, blocking the road ahead, as a driver reaches the crest of a hill on the highway. In a split second, an emotional response (e.g., increased heart rate, sweat secretion, respiration, and startle) is triggered by recognition of the accident ahead. If unchecked, the emotional reaction to this threat could cause the driver to lose control of the vehicle. Thus, an emotional response that may be adaptive in other circumstances is actually maladaptive when a driver must remain calm in order to maintain control of the vehicle. The driver’s ability to remain calm, however, benefits from prior knowledge of the threat. For example, imagine that the accident was reported on the radio, alerting the driver to the impending threat, before the accident appeared on the road ahead. In this alternate scenario, the driver’s emotional response (e.g., cardiac, sweat, respiration, and startle responses) to the accident is diminished by prior knowledge and anticipation of the impending threat. Although the driver must inhibit a maladaptive emotional response to the threat in either scenario, prior knowledge of the traffic accident provides a distinct advantage for maintaining control of the vehicle. Thus, adaptive emotional function is promoted when threats can be anticipated and effectively managed (Ochsner & Gross, 2005; Phan et al., 2005; Phillips, Drevets, Rauch, & Lane, 2003). Further, resilience to stress is mediated, in part, by our ability to predict and control impending threats in our environment (Chorpita & Barlow, 1998; Foa, Zinbarg, & Rothbaum, 1992; Maier & Seligman, 1976). However, the development of anxiety disorders appears to result from the dysfunction of normal fear processes. Specifically, maladaptive emotional responses produced in anticipation of threat, as well as emotional responses to the threat itself appear to be linked to anxiety and stress-related disorders (Rosen & Schulkin, 1998). Therefore, consideration of both the anticipatory response and the response to the threat itself are important for a better understanding of both healthy and dysfunctional emotion learning, expression, and regulation processes that mediate emotional disorders. Given that anticipation modifies the emotional response to threat, a key goal in the study of emotion is to elucidate the relationship between the anticipatory response and the response to the threat itself.
Pavlovian fear conditioning is an effective and popular paradigm often used in both animal model and human studies of emotional learning, memory, and regulation (Maren, 2001). Furthermore, Pavlovian conditioning is ideal for human studies of emotion because the neural mechanisms of fear conditioning are relatively well established across species (Kim & Jung, 2006). In a typical Pavlovian fear conditioning study, an originally neutral stimulus is repeatedly paired with an aversive threat (unconditioned stimulus; UCS) that elicits a reflexive emotional response (unconditioned response; UCR). Once an association between the signal and threat has formed, the neutral stimulus becomes a warning signal, called the conditioned stimulus (CS). The warning signal elicits an anticipatory response (conditioned response; CR) that is evoked in preparation of a threat and serves as an index of fear learning and emotional expression. Thus, CR expression during Pavlovian conditioning reflects anticipation of the forthcoming threat and serves as an objective measure of associative learning. During differential fear conditioning, one CS (CS+) is paired with the threat and serves as a warning signal, while a second CS (CS−) is presented alone and serves as a safety signal. Changes in response to the warning signal versus the safety signal serve as an index of fear learning and emotion regulation. For example, differential anticipatory responses to warning versus safety signals serve as an index of discriminatory fear learning. More specifically, the differential response demonstrates that the subject has learned the warning signal predicts the threat and the safety signal predicts the absence of the threat. Further, during extinction learning (i.e., the warning signal and threat are no longer paired), organisms must flexibly learn that a warning signal can become a safety signal. Thus, reductions in the anticipatory response during extinction assess inhibitory control of emotion and can be used to index emotion regulation (Raio & Phelps, 2015).
This review is focused on Pavlovian conditioned diminution of the emotional response to threat, an aspect of Pavlovian conditioning that has received relatively limited attention in the field. Following a brief introduction, conditioned diminution research will be described and then discussed as a valuable and novel approach to study emotional learning, expression, and regulation processes of interest to the field. Specifically, the field’s understanding of these emotional processes can be extended by Pavlovian conditioned diminution research that builds upon prior knowledge from studies that have focused on anticipatory responses, error detection, and traditional learning theory. Furthermore, this review will discuss novel insights into the neural processes that mediate emotional learning, expression, and regulation that have emerged from Pavlovian conditioned diminution approaches.
Conditioned diminution of the emotional response to threat
The majority of prior Pavlovian conditioning studies have emphasized learning-related changes in the anticipatory response prior to threat. However, the most important function of anticipatory learning outside of the laboratory is the impact learning has on the organism’s ability to successfully cope with the anticipated threat. Thus, from a functional perspective, the value of Pavlovian fear conditioning is the adaptive impact of the response to the threat itself (Domjan, 2005). Specifically, the ability to form an association between a warning signal and threat allows one to anticipate impending threats and initiate preparatory responses to avoid, escape, or reduce the impact of the threat and minimize harm (Domjan, 2005; Franchina, 1969; Helmstetter & Bellgowan, 1993; Kamin, 1954; Kim & Jung, 2006). For example, conditioned hypoalgesia (decreased sensitivity to painful stimuli) develops during fear conditioning, reducing the pain produced by noxious stimuli (Helmstetter, 1992; Helmstetter & Bellgowan, 1993). A similar process reduces the autonomic response to threat during fear conditioning (Baxter, 1966; Dunsmoor, Bandettini, & Knight, 2008; Knight, Lewis, & Wood, 2011; Knight, Waters, King, & Bandettini, 2010; Marcos & Redondo, 1999a; Marcos & Redondo, 1999b, 2001; Redondo, Fernandez-Rey, Padrón, & Alcaraz, 2015; Rust, 1976). Thus, learning-related reductions in the response to threat (called Pavlovian conditioned diminution) may offer new insights into emotion regulation processes.
In contrast to the anticipatory response, the response to the threat is an innate and automatic reaction that does not require learning. However, as described above, learning-related changes in the response to threat frequently develop during conditioning (Canli, Detmer, & Donegan, 1992; Canli & Donegan, 1995; Domjan, 2005; Dunsmoor et al., 2008; Knight et al., 2011; Knight et al., 2010; Wood, Kuykendall, Ver Hoef, & Knight, 2013; Wood, Ver Hoef, & Knight, 2012). Conditioned diminution of the emotional response to threat is a reduction in the emotional response to a threat that is predictable (e.g., preceded by the warning signal) compared to a threat that is unpredictable (e.g., presented alone or preceded by a safety signal). This conditioned reduction in the emotional response to predictable threat provides a continuous measure of the ability to regulate the emotional response to threat. Thus, differences in the emotional response to a predictable compared to an unpredictable threat serves as an index of regulatory control of emotion (Dunsmoor et al., 2008; Harnett et al., 2015; Knight et al., 2010; Wood et al., 2012).
Prior findings indicate that Pavlovian conditioned diminution of the emotional response to threat cannot be solely explained by a simple non-associative learning process (i.e., habituation). Instead, presentation of the warning signal modulates the response to the threat during Pavlovian conditioning. For example, the magnitude of the emotional response to threat decreases as the warning signal and threat are repeatedly paired (Baxter, 1966; Kimble & Ost, 1961). Further, the decrease in the response to threat is greater to paired compared to unpaired presentations of the warning signal and threat (Kimmel, 1967). More specifically, the predictability of threat modulates the magnitude of the emotional response to threat such that a diminished response is produced by predictable threat compared to unpredictable threat. Thus, there is a conditioned reduction in the response to predictable versus unpredictable threat (i.e., conditioned diminution of the emotional response to threat) (Baxter, 1966; Kimmel, 1967; Knight et al., 2011; Rust, 1976; Wood et al., 2012). Further, conditioned diminution of the response to threat cannot be explained by interference between the anticipatory response and the response to threat. Specifically, the amount of recovery following the anticipatory response does not vary with diminution of the emotional response to threat (Rust, 1976). Taken together, prior work indicates that conditioned diminution of the emotional response to threat is, in part, mediated by an associative learning process in which the warning signal gains discriminatory control over the emotional response to threat.
As described above, an enhanced anticipatory response to the warning versus safety signal demonstrates an association between the warning signal and threat has been learned. The anticipatory response also appears to influence the diminution of the emotional response to the threat itself. For example, as the magnitude of the anticipatory response increases, the magnitude of the response to predictable threat decreases (Figure 1) (Knight et al., 2011; Wood et al., 2012). However, a similar relationship is not observed when the threat is unpredictable. More specifically, the anticipatory response does not vary with the response to the threat itself when the threat unexpectedly follows a learned safety signal (Harnett, Wood, Wheelock, Knight, & Knight, 2017; Knight et al., 2011; Wood et al., 2012). These findings suggest that an anticipatory response specific to the warning signal is important for conditioned diminution of the emotional response to threat.
Figure 1.
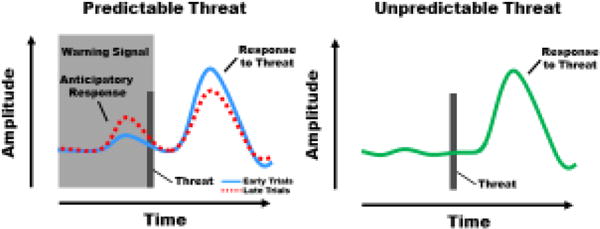
Illustration depicting anticipatory responses and emotional responses to threat during Pavlovian fear conditioning. A) During early conditioning trials (solid line), anticipatory responses to the warning signal are relatively small and the emotional response to the threat is relatively large. During late conditioning trials (dashed line), once the association between the warning signal and threat has formed, the warning signal elicits a relatively large anticipatory response and the predictable threat elicits a relatively small emotional response. Thus, the emotional response to threat is diminished on late compared to early conditioning trials. B) The emotional response to the unpredictable threat is similar to the response to predictable threat during early (solid line in A), but not late (dashed line in A), conditioning trials. Thus, the emotional response is larger for unpredictable (B) than for predictable (dashed line in A) threat.
Conscious expectations are another important process by which anticipation modifies the emotional response to threat (Dunsmoor et al., 2008; Knight et al., 2010; Rust, 1976). For example, greater diminution of the response to threat is observed when participants report a threat is expected compared to when the threat is unexpected (Dunsmoor et al., 2008; Knight et al., 2010). Further, as threat expectancy increases, the magnitude of the emotional response to threat decreases (Dunsmoor et al., 2008; Knight et al., 2010; Sarinopoulos et al., 2010; Wood et al., 2013) such that graded increases in threat expectancy are paralleled by graded decreases in the magnitude of the emotional response to the threat (Dunsmoor et al., 2008; Knight et al., 2010). Taken together, these prior findings suggest that conscious expectations of an impending threat modulate the expression of the emotional response to threat (Dunsmoor et al., 2008; Knight et al., 2010; Rust, 1976; Sarinopoulos et al., 2010; Wood et al., 2013). Although, conscious threat expectancies appear to modulate of the emotional response to threat (Dunsmoor et al., 2008; Knight et al., 2010; Rust, 1976), this process also appears to be mediated, in part, by associative learning mechanisms that are somewhat independent of conscious expectations. For example, prior work has observed diminution of the response to threat even when conditioning trials (i.e., predictable vs unpredictable threat) were matched on threat expectancy (Knight et al., 2011). These findings are consistent with prior work that demonstrates a differential conditioned anticipatory emotional response can be produced even when expectations of the threat do not differ (Balderston & Helmstetter, 2010; Knight, Nguyen, & Bandettini, 2003, 2006; Knight, Waters, & Bandettini, 2009; Schultz & Helmstetter, 2010). More specifically, anticipatory responses are elicited by auditory warning signals during conditioning even when the intensity (decibels) of the warning signal falls below the perceptual detection threshold. Although, subthreshold warning signals elicit anticipatory responses during conditioning, threat expectancy does not increase during subthreshold warning signals (Knight et al., 2003; Knight et al., 2006; Knight et al., 2009). However, conditioned diminution of the emotional response to threat is greater when the threat is preceded by a suprathreshold warning signal versus subthreshold warning signal (Knight et al., 2010). This finding indicates that conscious expectations of threat diminish the emotional response to threat above and beyond the diminution produced by anticipatory responses expressed in the absence of conscious expectations. However, this prior work did not assess the emotional response to threat in the absence of a warning signal (i.e., threat alone). Thus, it remains unclear whether subthreshold warning signals diminish the emotional response to threat compared to presentations of the threat alone. Regardless, prior work demonstrates conditioned diminution of the emotional response to threat develops to predictable versus unpredictable threat even when conscious expectations of the threat do not differ (Knight et al., 2011). However, conditioned diminution of the response to threat is greater when threats are expected vs. unexpected (Knight et al., 2010). Thus, although conscious expectations of threat are not necessary for conditioned diminution, greater diminution of the emotional response to threat develops when the warning signal elicits both an anticipatory emotional response and conscious expectation of the impending threat (Knight et al., 2010; Knight et al., 2011). Thus, prior results demonstrate that conditioned diminution of the emotional response to threat is influenced by both conscious threat expectations and an associative learning process that is at least somewhat independent of these expectations.
Differences in the level of anxiety individuals experience may be another factor that can influence the emotional response to threat. Prior conditioning research indicates that individuals with high anxiety produce a larger emotional response to the warning signal than those with low levels of anxiety (Nielsen & Petersen, 1976; Pitman & Orr, 1986; Schwerdtfeger, 2006; Thayer, Friedman, Borkovec, Johnsen, & Molina, 2000). Others have demonstrated that an individual’s anxiety level affects their response to aversive events (Cook, Davis, Hawk, Spence, & Gautier, 1992; Grillon, Ameli, Foot, & Davis, 1993). For example, anxiety disorder patients show an exaggerated startle response to aversive stimuli (Butler et al., 1990; Grillon, Ameli, Goddard, Woods, & Davis, 1994; Grillon, Morgan, Davis, & Southwick, 1998). This prior work suggests that anxiety enhances the startle response during the anticipation of aversive events, but does not affect baseline startle (Grillon et al., 1993). Taken together, this line of research suggests that individuals with high anxiety levels may form a stronger association between the warning signal and threat during conditioning. In turn, this may lead high anxiety individuals to allocate greater attentional resources to threat-related stimuli (Mogg & Bradley, 1998; Mogg, Bradley, & Hallowell, 1994). In turn, increased attention to warning signals that predict the threat would be expected to diminish the emotional response to the threat. Consistent with this perspective, prior work indicates that emotional responses to warning signals are larger in those with high anxiety (Nielsen & Petersen, 1976; Sarinopoulos et al., 2010), and an individual’s anxiety level effects their response to aversive stimuli (Cook et al., 1992; Grillon et al., 1993; Grillon et al., 1998). Consistent with these prior findings, the emotional response to threat appears to vary with anxiety regardless of whether the threat is predictable or unpredictable. Specifically, responses to both predictable and unpredictable threat vary with anxiety (Knight et al., 2011; Wood et al., 2012). However, both studies also showed that conditioned diminution of the response to threat (predictable vs unpredictable) does not vary with anxiety. Together, these findings suggest that anxiety level influences the anticipatory response as well as the response to the threat itself (i.e., both predictable and unpredictable threats). However, anxiety level does not appear to modulate the differential response to predictable vs unpredictable threat. Thus, high anxiety levels may not be linked to reduced inhibitory control during fear conditioning, but rather the overall magnitude of the emotional response.
Conditioned diminution and other emotion regulation approaches
As noted above, extinction learning is a popular paradigm that is often used to assess emotion regulation. However, the utility of extinction learning procedures is dependent upon the success of prior conditioning phases of the study. For example, extinction learning cannot be assessed when a conditioned anticipatory response to the warning signal is not acquired (Grady, Bowen, Hyde, Totsch, & Knight, 2016). Thus, individuals that do not develop an anticipatory emotional response to the warning signal during conditioning (i.e., non-learners) cannot, by definition, show extinction. Accordingly, assessing emotion regulation only in individuals who acquire an anticipatory response (i.e., learners) may preclude the study of important populations (or individual differences within populations) associated with atypical fear learning. Further, the interpretation of extinction learning is complicated when populations (e.g., patient and control) differ in their propensity for acquiring a conditioned anticipatory response to the warning signal. Conditioned diminution of the emotional response to threat may serve as a useful alternative approach to extinction learning as a paradigm to assess emotion regulation. Like extinction learning, conditioned diminution procedures assess regulation of the emotional response. However, extinction learning assesses regulation of the anticipatory emotional response to a warning signal, while conditioned diminution procedures assess regulation of the emotional response to the threat itself. Although both extinction learning and conditioned diminution procedures have been used to assess emotion regulation, these assessments differ in their emphasis on changes in anticipatory responses and responses to threat, respectively. Investigations of conditioned diminution of the response to threat may provide insight into the dysfunction of healthy emotion learning, expression, and regulation that characterizes stress-related disorders (Dretsch et al., 2016; Linnman, Zeffiro, Pitman, & Milad, 2011; Wheelock et al., 2014; Wood et al., 2013; Wood et al., 2012). Thus, studies that assess conditioned diminution of the emotional response to threat constitute an easy to implement approach that may offer novel insight to the study of emotion regulation.
Outside of Pavlovian conditioning studies, cognitive reappraisal is another popular approach to study emotion regulation (Gross, 2002). In contrast to conditioned diminution of the emotional response to threat, cognitive reappraisal is an emotion regulation strategy that relies on effortful, top-down control over judgement and/or appraisal processes to modulate emotion (Ochsner & Gross, 2005). Cognitive reappraisal is considered an antecedent-based strategy that is focused on regulation of the emotional response before the response actually occurs (e.g., avoidance and selective attention) (Gross, 2002). Cognitive reappraisal strategies successfully reduce expression of the peripheral emotional response (i.e., heart rate and emotion modulated startle) (Gross, 1998; Jackson, Malmstadt, Larson, & Davidson, 2000). Similar to cognitive reappraisal, conditioned diminution of the response to threat relies upon anticipatory processes that modulate the emotional response. Specifically, during conditioned diminution of the emotional response to threat, anticipatory responses and conscious expectations are elicited by the warning signal prior to the threat, and in turn, modulate the emotional response to threat. Thus, findings from both cognitive reappraisal and conditioned diminution of the emotional response to threat suggest that anticipatory processes are important for emotion regulation. As described above, anticipatory processes that are elicited by warning signals during fear conditioning diminish the emotional response to threat. However, in contrast to conditioned diminution of the emotional response to threat, cognitive reappraisal strategies are goal-directed and thus more dependent on voluntary and effortful anticipatory emotion regulation than conditioned diminution procedures. Thus, the anticipatory processes that regulate the emotional response to threat during conditioned diminution procedures are less dependent on effortful emotion regulation. Together, studies of cognitive reappraisal and conditioned diminution of the emotional response provide a better understanding of anticipatory processes that reduce the emotional response to threat.
Conditioned diminution approaches can also provide unique insight into dysfunctional learning processes that may contribute to emotional dysregulation. For example, the relationship between anticipatory learning (increased response to warning signals) and conditioned diminution of the response to threat (decreased response to predictable threats) typically shows an inverse relationship (Knight et al., 2011; Wood et al., 2012). The inverse relationship between the anticipatory response and the response to the threat suggests that the strength of anticipatory learning modulates the magnitude of the emotional response to the threat (Figure 2). Accordingly, the inverse relationship typically observed between the anticipatory response and the emotional response to threat appears to reflect inhibitory control processes that arise during fear conditioning. Thus, understanding the relationship between anticipatory responses and the response to threat may extend our understanding of emotion regulation, and the influence of emotional learning on stress-susceptibility.
Figure 2.
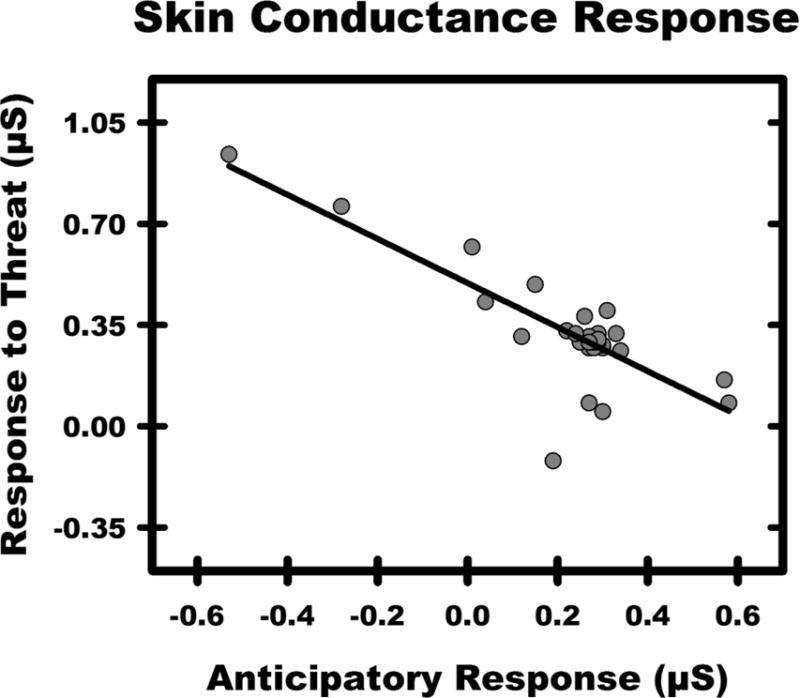
The relationship between the anticipatory response and the response to threat (unpublished mean centered data from Knight Laboratory). As the anticipatory response increases, the response to threat decreases. The black line reflects the negative correlation that is typically observed between anticipatory responses and the emotional response to threat in Pavlovian conditioned diminution studies.
Learning theory
Conditioned diminution of the emotional response to threat is consistent with traditional learning theory. For example, during Pavlovian fear conditioning, the warning signal gains discriminative control over threat-elicited behavior (Rescorla, 1988; Rescorla & Wagner, 1972). Furthermore, as an unpredictable threat becomes increasingly predictable during fear conditioning, there is a corresponding decrease in the emotional response to the threat (Rescorla, 1988; Rescorla & Wagner, 1972; Wagner, Brandon, Klein, & Mowrer, 1989). Accordingly, as the anticipatory response to the warning signal increases, the emotional response to the predictable threat decreases [Figures 1 and 2; (Harnett et al., 2015; Knight et al., 2011; Wood et al., 2013; Wood et al., 2012)].
In addition to anticipatory processes, conditioned diminution of the emotional response to threat may reflect the error detection that occurs during Pavlovian conditioning. Error detection (i.e., outcomes that violate expectations) plays a vital role in traditional learning theory. Error detection is the discrepancy between the expectation of threat and the actual occurrence of threat (Rescorla & Wagner, 1972). Thus, an unexpected threat modulates learning by generating a prediction error signal that is proportionate to the discrepancy between expectations and what actually occurs. This prediction error signal appears to act as a neuromodulator of learning by promoting the synaptic plasticity (e.g., within the amygdala) that underlies fear learning and memory (McNally, Johansen, & Blair, 2011). Similarly, changes in the conscious expectation of threat due to learning plays an important role in conditioned diminution of the threat-response (Dunsmoor et al., 2008; Knight et al., 2010; Rust, 1976; Sarinopoulos et al., 2010; Wood et al., 2013). Thus, the change in the discrepancy between expectations and outcomes (i.e., changes in error detection) that develops during fear conditioning may drive diminution of the emotional response to threat. Specifically, increases in expectation of the predictable threat are relatively large at the beginning of fear conditioning, but decrease as the warning signal and threat are repeatedly paired (reduced prediction errors). Thus, reductions in prediction errors may explain conditioned diminution of the emotional response to threat. Consistent with this view, conditioned diminution of the threat-response parallels increases in conscious expectations of threat (Dunsmoor et al., 2008; Knight et al., 2011; Wood et al., 2012). Likewise, error detection manipulations have shown that increased certainty (expectation) is associated with diminution of the response to threat (Li & McNally, 2014). Accordingly, as uncertainty of the threat decreases during fear learning, expectation of the predictable threat correspondingly increases. Thus, error detection and conditioned diminution of the emotional response to threat may represent the same learning process (i.e., changes in expectation that modulate the response to threat). However, error detection theory cannot solely account for the entirety of findings from conditioning studies.
Although error detection may play an important role in diminution of the emotional response to predictable threat, this associative learning process maybe somewhat independent of conscious expectations of threat. As mentioned previously, learning-related changes in the response to predictable threats are, at least in part, mediated by an associative learning process that is somewhat independent of conscious expectations (Knight et al., 2011). Specifically, conditioned diminution of the response to threat occurs even when there is no discrepancy in the conscious expectations between predictable and unpredictable threats (Knight et al., 2011). Furthermore, predictability of the threat has been shown to both diminish (Canli et al., 1992; Donegan, 1981) and potentiate (Grillon, Ameli, Woods, Merikangas, & Davis, 1991; Harnett et al., 2015; Wood et al., 2015) the response to threat. Prior work indicates that whether the response to threat is diminished or potentiated depends, in part, on the intensity (e.g., decibels, voltage) of the threat (Canli et al., 1992; Domjan, 2005; Donegan & Wagner, 1987). Regardless, increases in response magnitude associated with increases in expectancy are inconsistent with error detection theory, which suggests the response to threat should decrease as expectation of a predictable threat increases. Taken together, prior findings suggest that conditioned diminution of the emotional response to threat reflects an emotion regulation process that may be related to error detection. However, error detection may not explain the entirety of the observed conditioned changes in the response to threat.
Neural substrates
Neural circuitry that includes the prefrontal cortex (PFC), hippocampus, and amygdala supports fear learning and emotion regulation processes (Figure 3). Specifically, the hippocampus plays an important role in forming conscious expectations about the occurrence of imminent threats (Haritha, Wood, Ver Hoef, & Knight, 2013; Knight, Smith, Cheng, Stein, & Helmstetter, 2004; Knight et al., 2009). The hippocampus projects to the dlPFC and vmPFC, and in turn, anticipatory dlPFC function regulates dmPFC, vmPFC, and amygdala responses to the threat itself (Åhs, Kragel, Zielinski, Brady, & LaBar, 2015; Wheelock et al., 2014; Wood et al., 2012). Accordingly, anticipatory dlPFC activity prior to the threat modulates the dmPFC and vmPFC response to threat (Wood et al., 2012), and projections from the dmPFC and vmPFC appear to regulate amygdala activity (Delgado, Nearing, LeDoux, & Phelps, 2008; Wheelock et al., 2014). In turn, amygdala projections to midbrain regions (e.g., periaqueductal gray, hypothalamus, and ventral tegmental area) control the expression of the peripheral emotional response, including sympathetic and parasympathetic activity (Fendt & Fanselow, 1999). Thus, the amygdala is a critical component of the neural circuit that supports fear learning, memory, and expression (Davis, Walker, Miles, & Grillon, 2010; Dunsmoor et al., 2008; Helmstetter, 1992; Knight, Nguyen, & Bandettini, 2005; LeDoux, Cicchetti, Xagoraris, & Romanski, 1990; Wood, Ver Hoef, & Knight, 2014).
Figure 3.
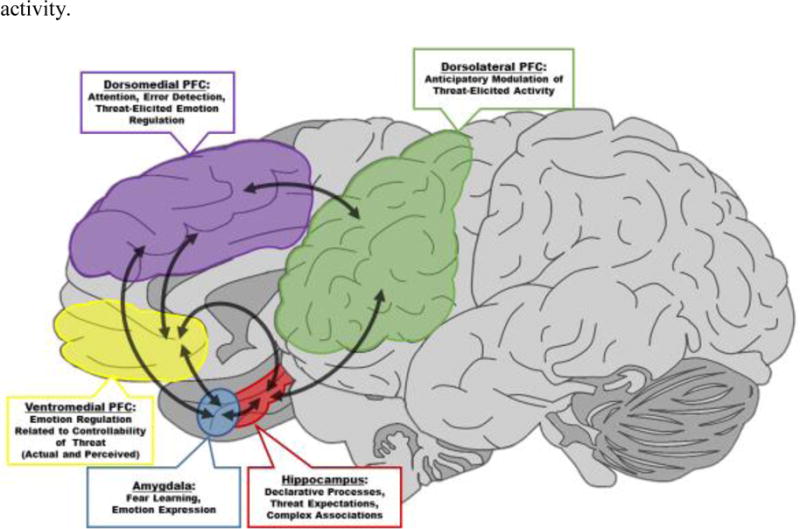
Regions that mediate the emotional response to threat. Arrows indicate ipsilateral connections between brain regions that regulate the emotional response to threat. The hippocampus (red) supports the development of conscious expectations of threat and projects to the dlPFC (green), vmPFC (yellow), and amygdala (blue). Anticipatory (i.e., conditioned response) dlPFC activity prior to the threat modulates the dmPFC (purple) and vmPFC response to threat (i.e., unconditioned response). Projections from the dmPFC and vmPFC regulate amygdala activity. In turn, amygdala projections to midbrain regions (e.g., the periaqueductal gray, hypothalamus, and ventral tegmental area) control the expression of the peripheral emotional response, including motor, autonomic, and hypothalamic-pituitary-adrenal axis activity.
Knowledge about the neural network that underlies conditioned diminution of the emotional response to threat provides insight into the threat-related processes that mediate healthy emotional function. For example, the dmPFC supports attention, error detection, and threat-elicited emotion regulation processes (Bishop, Duncan, Brett, & Lawrence, 2004; Carter et al., 1998; Furlong, Cole, Hamlin, & McNally, 2010; Milad, Quirk, et al., 2007; Ochsner & Gross, 2005; Ochsner, Hughes, Robertson, Cooper, & Gabrieli, 2009; Phan et al., 2005; Phelps, Delgado, Nearing, & LeDoux, 2004; Wheelock et al., 2014; Wood et al., 2012) that may mediate conditioned diminution of the emotional response to threat. Further, threat predictability and controllability appear to interact to influence the neural response to threat. Specifically, vmPFC and hippocampal activity varies with the predictability and controllability of threats (Amat, Matus-Amat, Watkins, & Maier, 1998; Baratta et al., 2009; Harnett et al., 2015; Wood et al., 2015). Taken together with findings that demonstrate the vmPFC and hippocampus are important components of the neural circuit that regulates emotion (Amat et al., 1998; Baratta et al., 2007; 2008; Hartley & Phelps, 2010; Milad et al., 2009; Milad, Wright, et al., 2007; Rauch, Shin, & Phelps, 2006; Schiller, Kanen, LeDoux, Monfils, & Phelps, 2013), these brain structures appear to support processes that mediate stress resilience and may be essential for healthy emotional function. Consequently, dysfunction of the PFC-hippocampus-amygdala network may result in maladaptive anticipatory processes that disrupt emotion regulation in the face of threat, and may be responsible for the emotional dysfunction associated with stress-related disorders (Harnett et al., 2017).
Conclusion
Defensive emotional responses (i.e., fight or flight) evolved as a healthy and adaptive reaction to threat. However, excessive emotional responses to threat can be maladaptive and may lead to adverse outcomes if unchecked. Therefore, regulation of the emotional response to threat is critical for healthy emotional function. Prior Pavlovian fear conditioning studies have largely focused on acquisition and extinction of conditioned anticipatory emotional responses to assess the association between a warning signal and the threat it predicts. However, from a functional perspective, this type of associative learning (i.e., Pavlovian conditioning) is primarily important because it allows one to cope with the threat more effectively. Relatively recent research has given renewed attention to the emotional response to the threat itself (i.e., conditioned diminution paradigm) (Table 1). Conditioned diminution of the emotional response to threat is a well-documented process, demonstrated by a diminished response to predictable compared to unpredictable threats.
Table 1.
Factors that influence the psychophysiological and neural response to threat
| Factor | Effect on response to threat | Studies |
|---|---|---|
| Increased anticipatory response | Decreased | Knight et al., 2011 |
| Wood et al., 2012 | ||
| Increased conscious expectation | Decreased | Rust, 1976 |
| Dunsmoor et al., 2008 | ||
| Knight et al., 2010 | ||
| Sarinopoulos et al., 2010 | ||
| Wood et al., 2013 | ||
| Decreased prediction error | Decreased | Li & McNally, 2014 |
| Decreased anxiety level | Decreased | Butler et al., 1990 |
| Cook et al., 1992 | ||
| Grillon, et al., 1993, 1994, 1998 | ||
| Knight et al., 2011 | ||
| Wood et al., 2012 |
A PFC-hippocampus-amygdala network underlies conditioned diminution of the emotional response to threat. In particular, hippocampal activity varies with conscious expectations of threat and projects expectancy information to regions of the PFC and amygdala. Anticipatory dlPFC activity appears to modulate threat-elicited activity within the dmPFC and vmPFC. In turn, the vmPFC and dmPFC modulate the amygdala response to threat, which controls the expression of the peripheral emotional response. Together, conditioned diminution findings suggest that PFC-hippocampus-amygdala circuitry is critical for emotional learning, expression, and regulation processes.
Pavlovian fear conditioning studies investigating conditioned diminution of the emotional response to threat have recently received renewed interest. This review highlights several recent findings and the reasons for the renewed interest in conditioned diminution approaches. Specifically, conditioned diminution assessments provide a valuable approach to study emotional learning, expression, and regulation processes. Furthermore, conditioned diminution approaches provide novel insights into the neural processes that mediate conditioning. In particular, the PFC-hippocampus-amygdala circuit that underlies conditioned diminution of the emotional response to threat may be essential for healthy emotional function.
Highlights.
Healthy emotional function depends on the ability to appropriately cope with threats.
Prior Pavlovian conditioning research has largely focused on anticipation of threat.
We review recent findings on the regulation of the emotional response to threat.
The PFC, hippocampus, and amygdala modulate threat-elicited emotional responses.
This research has important implications for emotion regulation and stress resilience.
Acknowledgments
This research was funded by the National Institute of Mental Health of the National Institutes of Health [grant number MH098348].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Åhs, Kragel, Zielinski, Brady, LaBar Medial prefrontal pathways for the contextual regulation of extinguished fear in humans. Neuroimage. 2015;122:262–271. doi: 10.1016/j.neuroimage.2015.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat, Matus-Amat, Watkins, Maier Escapable and inescapable stress differentially and selectively alter extracellular levels of 5-HT in the ventral hippocampus and dorsal periaqueductal gray of the rat. Brain research. 1998;797(1):12–22. doi: 10.1016/s0006-8993(98)00368-0. [DOI] [PubMed] [Google Scholar]
- Balderston, Helmstetter Conditioning with masked stimuli affects the timecourse of skin conductance responses. Behavioral neuroscience. 2010;124(4):478–489. doi: 10.1037/a0019927. doi: 2010-16138-005 [pii]10.1037/a0019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta, Christianson, Gomez, Zarza, Amat, Masini, Maier Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;146(4):1495–1503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta, Lucero, Amat, Watkins, Maier Role of the ventral medial prefrontal cortex in mediating behavioral control-induced reduction of later conditioned fear. Learn Mem. 2008;15(2):84–87. doi: 10.1101/lm.800308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta, Zarza, Gomez, Campeau, Watkins, Maier Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. European journal of neuroscience. 2009;30(6):1111–1116. doi: 10.1111/j.1460-9568.2009.06867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter Diminution and recovery of the UCR in delayed and trace classical GSR conditioning. Journal of Experimental Psychology. 1966;71(3):447. doi: 10.1037/h0022977. [DOI] [PubMed] [Google Scholar]
- Bishop, Duncan, Brett, Lawrence Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7(2):184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Butler, Braff, Rausch, Jenkins, Sprock, Geyer Physiological evidence of exaggerated startle response in a subgroup of Vietnam veterans with combat-related PTSD. American Journal of Psychiatry. 1990;147(10):1308–1312. doi: 10.1176/ajp.147.10.1308. [DOI] [PubMed] [Google Scholar]
- Canli, Detmer, Donegan Potentiation or diminution of discrete motor unconditioned responses (rabbit eyeblink) to an aversive pavlovian unconditioned stimulus by two associative processes: conditioned fear and a conditioned diminution of unconditioned stimulus processing. Behavioral neuroscience. 1992;106(3):498. doi: 10.1037//0735-7044.106.3.498. [DOI] [PubMed] [Google Scholar]
- Canli, Donegan Conditioned diminution of the unconditioned response in rabbit eyeblink conditioning: Identifying neural substrates in the cerebellum and brainstem. Behavioral neuroscience. 1995;109(5):874. doi: 10.1037//0735-7044.109.5.874. [DOI] [PubMed] [Google Scholar]
- Carter, Braver, Barch, Botvinick, Noll, Cohen Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Chorpita, Barlow The development of anxiety: the role of control in the early environment. Psychological Bulletin. 1998;124(1):3–21. doi: 10.1037/0033-2909.124.1.3. [DOI] [PubMed] [Google Scholar]
- Christoffel, Golden, Dumitriu, Robison, Janssen, Ahn, Russo IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31(1):314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, Davis, Hawk, Spence, Gautier Fearfulness and startle potentiation during aversive visual stimuli. Psychophysiology. 1992;29(6):633–645. doi: 10.1111/j.1469-8986.1992.tb02038.x. [DOI] [PubMed] [Google Scholar]
- Davis, Walker, Miles, Grillon Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35(1):105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado, Nearing, LeDoux, Phelps Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59(5):829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domjan Pavlovian conditioning: a functional perspective. Annu Rev Psychol. 2005;56:179–206. doi: 10.1146/annurev.psych.55.090902.141409. [DOI] [PubMed] [Google Scholar]
- Donegan Priming-produced facilitation or diminution of responding to a Pavlovian unconditioned stimulus. J Exp Psychol Anim Behav Process. 1981;7(4):295–312. [PubMed] [Google Scholar]
- Donegan, Wagner . Conditioned diminution and facilitation of the UR: A sometimes opponent-process interpretation. In: Gormezano I, Prokasy WF, Thompson RF, editors. Classical conditioning. 3rd. Hillsdale, NJ, US: Lawrence Erlbaum Associates, Inc; 1987. pp. 339–369. [Google Scholar]
- Dretsch, Wood, Daniel, Katz, Deshpande, Goodman, Knight Exploring the Neurocircuitry Underpinning Predictability of Threat in Soldiers with PTSD Compared to Deployment Exposed Controls. Open Neuroimag J. 2016;10:111–124. doi: 10.2174/1874440001610010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor, Bandettini, Knight Neural correlates of unconditioned response diminution during Pavlovian conditioning. Neuroimage. 2008;40(2):811–817. doi: 10.1016/j.neuroimage.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt, Fanselow The neuroanatomical and neurochemical basis of conditioned fear. Neuroscience & Biobehavioral Reviews. 1999;23(5):743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Foa, Zinbarg, Rothbaum Uncontrollability and unpredictability in post-traumatic stress disorder: an animal model. Psychological Bulletin. 1992;112(2):218–238. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- Franchina Escape behavior and shock intensity: within-subject versus between-groups comparisons. Journal of comparative and physiological psychology. 1969;69(2):241. doi: 10.1037/h0028224. [DOI] [PubMed] [Google Scholar]
- Furlong, Cole, Hamlin, McNally The role of prefrontal cortex in predictive fear learning. Behav Neurosci. 2010;124(5):574–586. doi: 10.1037/a0020739. [DOI] [PubMed] [Google Scholar]
- Grady, Bowen, Hyde, Totsch, Knight Effect of continuous and partial reinforcement on the acquisition and extinction of human conditioned fear. Behav Neurosci. 2016;130(1):36–43. doi: 10.1037/bne0000121. [DOI] [PubMed] [Google Scholar]
- Grillon, Ameli, Foot, Davis Fear-potentiated startle: relationship to the level of state/trait anxiety in healthy subjects. Biological Psychiatry. 1993;33(8–9):566–574. doi: 10.1016/0006-3223(93)90094-t. [DOI] [PubMed] [Google Scholar]
- Grillon, Ameli, Goddard, Woods, Davis Baseline and fear-potentiated startle in panic disorder patients. Biological Psychiatry. 1994;35(7):431–439. doi: 10.1016/0006-3223(94)90040-x. doi: 0006-3223(94)90040-X [pii] [DOI] [PubMed] [Google Scholar]
- Grillon, Ameli, Woods, Merikangas, Davis Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology. 1991;28(5):588–595. doi: 10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Grillon, Morgan, Davis, Southwick Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biological Psychiatry. 1998;44(10):1027–1036. doi: 10.1016/s0006-3223(98)00034-1. doi: S0006-3223(98)00034-1 [pii] [DOI] [PubMed] [Google Scholar]
- Gross Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 1998;74(1):224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39(3):281–291. doi: 10.1017/s0048577201393198. doi: 10.1017.S0048577201393198. [DOI] [PubMed] [Google Scholar]
- Haritha, Wood, Ver Hoef, Knight Human trace fear conditioning: right-lateralized cortical activity supports trace-interval processes. Cogn Affect Behav Neurosci. 2013;13(2):225–237. doi: 10.3758/s13415-012-0142-6. [DOI] [PubMed] [Google Scholar]
- Harnett, Wheelock, Wood, Ladnier, Mrug, Knight Affective state and locus of control modulate the neural response to threat. Neuroimage. 2015;121:217–226. doi: 10.1016/j.neuroimage.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett, Wood, Wheelock, Knight, Knight . Anticipation and the Neural Response to Threat. In: Nadin M, editor. Anticipation and Medicine. Cham: Springer International Publishing; 2017. pp. 219–228. [Google Scholar]
- Hartley, Phelps Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35(1):136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter The amygdala is essential for the expression of conditional hypoalgesia. Behavioral neuroscience. 1992;106(3):518. doi: 10.1037//0735-7044.106.3.518. [DOI] [PubMed] [Google Scholar]
- Helmstetter, Bellgowan Lesions of the amygdala block conditional hypoalgesia on the tail flick test. Brain research. 1993;612(1):253–257. doi: 10.1016/0006-8993(93)91669-j. [DOI] [PubMed] [Google Scholar]
- Jackson, Malmstadt, Larson, Davidson Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37(4):515–522. [PubMed] [Google Scholar]
- Kamin Traumatic avoidance learning: the effects of CS-US interval with a trace-conditioning procedure. Journal of comparative and physiological psychology. 1954;47(1):65. doi: 10.1037/h0061380. [DOI] [PubMed] [Google Scholar]
- Kim, Jung Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30(2):188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble, Ost A conditioned inhibitory process in eyelid conditioning. J Exp Psychol. 1961;61:150–156. doi: 10.1037/h0044932. [DOI] [PubMed] [Google Scholar]
- Kimmel Judgments of UCS intensity and diminution of the UCR in classical GSR conditioning. Journal of Experimental Psychology. 1967;73(4):532–543. doi: 10.1037/h0024333. [DOI] [PubMed] [Google Scholar]
- Knight, Lewis, Wood Conditioned diminution of the unconditioned skin conductance response. Behavioral neuroscience. 2011;125(4):626. doi: 10.1037/a0024324. [DOI] [PubMed] [Google Scholar]
- Knight, Nguyen, Bandettini Expression of conditional fear with and without awareness. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(25):15280–15283. doi: 10.1073/pnas.25357801002535780100. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, Nguyen, Bandettini The role of the human amygdala in the production of conditioned fear responses. Neuroimage. 2005;26(4):1193–1200. doi: 10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Knight, Nguyen, Bandettini The role of awareness in delay and trace fear conditioning in humans. Cognitive, Affective and Behavioral Neuroscience. 2006;6(2):157–162. doi: 10.3758/cabn.6.2.157. [DOI] [PubMed] [Google Scholar]
- Knight, Smith, Cheng, Stein, Helmstetter Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cognitive, Affective, & Behavioral Neuroscience. 2004;4(3):317–325. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- Knight, Waters, Bandettini Neural substrates of explicit and implicit fear memory. Neuroimage. 2009;45(1):208–214. doi: 10.1016/j.neuroimage.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, Waters, King, Bandettini Learning-related diminution of unconditioned SCR and fMRI signal responses. Neuroimage. 2010;49(1):843–848. doi: 10.1016/j.neuroimage.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux, Cicchetti, Xagoraris, Romanski The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. The Journal of neuroscience. 1990;10(4):1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, McNally The conditions that promote fear learning: prediction error and Pavlovian fear conditioning. Neurobiol Learn Mem. 2014;108:14–21. doi: 10.1016/j.nlm.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Linnman, Zeffiro, Pitman, Milad An fMRI study of unconditioned responses in post-traumatic stress disorder. Biol Mood Anxiety Disord. 2011;1(1):8. doi: 10.1186/2045-5380-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier, Seligman Learned helplessness: Theory and evidence. Journal of Experimental Psychology: General. 1976;105(1):3–46. doi: 10.1037//0096-3445.105.1.3. [DOI] [Google Scholar]
- Marcos, Redondo Effects of conditioned stimulus presentation on diminution of the unconditioned response in aversive classical conditioning. Biological psychology. 1999a;50(2):89–102. doi: 10.1016/s0301-0511(99)00007-1. [DOI] [PubMed] [Google Scholar]
- Marcos, Redondo Effects of CS-US interval modification on diminution of the unconditioned response in electrodermal classical conditioning. Biol Psychol. 1999b;50(3):191–201. doi: 10.1016/s0301-0511(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Marcos, Redondo Relation between conditioned stimulus-elicit responses and unconditioned response diminution in long-interval human heart-rate classical conditioning. Span J Psychol. 2001;4(1):11–18. doi: 10.1017/s1138741600005606. [DOI] [PubMed] [Google Scholar]
- Maren Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- McNally, Johansen, Blair Placing prediction into the fear circuit. Trends Neurosci. 2011;34(6):283–292. doi: 10.1016/j.tins.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad, Pitman, Ellis, Gold, Shin, Lasko, Rauch Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad, Quirk, Pitman, Orr, Fischl, Rauch A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62(10):1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Milad, Wright, Orr, Pitman, Quirk, Rauch Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Mogg, Bradley A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy. 1998;36(9):809–848. doi: 10.1016/s0005-7967(98)00063-1. doi: S0005-7967(98)00063-1 [pii] [DOI] [PubMed] [Google Scholar]
- Mogg, Bradley, Hallowell Attentional bias to threat: roles of trait anxiety, stressful events, and awareness. Quarterly Journal of Experimental Psychology A, Human Experimental Psychology. 1994;47(4):841–864. doi: 10.1080/14640749408401099. [DOI] [PubMed] [Google Scholar]
- Nielsen, Petersen Electrodermal correlates of extraversion, trait anxiety and schizophrenism. Scandinavian Journal of Psychology. 1976;17(2):73–80. doi: 10.1111/j.1467-9450.1976.tb00214.x. [DOI] [PubMed] [Google Scholar]
- Ochsner, Gross The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner, Hughes, Robertson, Cooper, Gabrieli Neural systems supporting the control of affective and cognitive conflicts. J Cogn Neurosci. 2009;21(9):1842–1855. doi: 10.1162/jocn.2009.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan, Fitzgerald, Nathan, Moore, Uhde, Tancer Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57(3):210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phelps, Delgado, Nearing, LeDoux Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phillips, Drevets, Rauch, Lane Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54(5):504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pitman, Orr Test of the conditioning model of neurosis: differential aversive conditioning of angry and neutral facial expressions in anxiety disorder patients. Journal of Abnormal Psychology. 1986;95(3):208–213. doi: 10.1037//0021-843x.95.3.208. [DOI] [PubMed] [Google Scholar]
- Raio, Phelps The influence of acute stress on the regulation of conditioned fear. Neurobiol Stress. 2015;1:134–146. doi: 10.1016/j.ynstr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch, Shin, Phelps Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research–past, present, and future. Biological Psychiatry. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Redondo, Fernandez-Rey, Padrón, Alcaraz Pavlovian conditioned diminution of the unconditioned SCR using unpleasant stimuli as the unconditioned stimulus. Learning and Motivation. 2015;52:32–35. doi: http://doi.org/10.1016/j.lmot.2015.09.001. [Google Scholar]
- Rescorla Pavlovian conditioning: It’s not what you think it is. American Psychologist. 1988;43(3):151. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- Rescorla, Wagner A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. Classical conditioning: current research and theory 1972 [Google Scholar]
- Rosen, Schulkin From normal fear to pathological anxiety. Psychol Rev. 1998;105(2):325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Rust Unconditioned response diminution in the skin resistance response. The Journal of general psychology. 1976;95(1):77–84. doi: 10.1080/00221309.1976.9710867. [DOI] [PubMed] [Google Scholar]
- Sarinopoulos, Grupe, Mackiewicz, Herrington, Lor, Steege, Nitschke Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cerebral Cortex. 2010;20(4):929–940. doi: 10.1093/cercor/bhp155. doi: bhp155 [pii]10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller, Kanen, LeDoux, Monfils, Phelps Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(50):20040–20045. doi: 10.1073/pnas.1320322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, Helmstetter Classical conditioning of autonomic fear responses is independent of contingency awareness. Journal of Experimental Psychology: Animal Behavior Processes. 2010;36(4):495–500. doi: 10.1037/a0020263. doi: 2010-21866-002 [pii]10.1037/a0020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerdtfeger Trait anxiety and autonomic indicators of the processing of threatening information: a cued S1–S2 paradigm. Biological psychology. 2006;72(1):59–66. doi: 10.1016/j.biopsycho.2005.07.008. doi: S0301-0511(05)00130-4 [pii]10.1016/j.biopsycho.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Thayer, Friedman, Borkovec, Johnsen, Molina Phasic heart period reactions to cued threat and nonthreat stimuli in generalized anxiety disorder. Psychophysiology. 2000;37(3):361–368. [PubMed] [Google Scholar]
- Wagner, Brandon, Klein, Mowrer Evolution of a structured connectionist model of Pavlovian conditioning (AESOP) Contemporary learning theories: Pavlovian conditioning and the status of traditional learning theory. 1989:149–189. [Google Scholar]
- Wheelock, Sreenivasan, Wood, Ver Hoef, Deshpande, Knight Threat-related learning relies on distinct dorsal prefrontal cortex network connectivity. Neuroimage. 2014;102:904–912. doi: 10.1016/j.neuroimage.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, Kuykendall, Ver Hoef, Knight Neural Substrates Underlying Learning-Related Changes of the Unconditioned Fear Response. The open neuroimaging journal. 2013;7:41. doi: 10.2174/1874440001307010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, Ver Hoef, Knight Neural mechanisms underlying the conditioned diminution of the unconditioned fear response. Neuroimage. 2012;60(1):787–799. doi: 10.1016/j.neuroimage.2011.12.048. [DOI] [PubMed] [Google Scholar]
- Wood, Ver Hoef, Knight The amygdala mediates the emotional modulation of threat-elicited skin conductance response. Emotion. 2014;14(4):693–700. doi: 10.1037/a0036636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, Wheelock, Shumen, Bowen, Ver Hoef, Knight Controllability modulates the neural response to predictable but not unpredictable threat in humans. Neuroimage. 2015;119:371–381. doi: 10.1016/j.neuroimage.2015.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]


