Abstract
Cyclase-associated protein (CAP) is a multi-domain protein that promotes actin filament dynamics. The C-terminal region of CAP contains a CAP and X-linked retinitis pigmentosa 2 protein (CARP) domain (or a β-sheet domain), which binds to actin monomer and is essential for enhancing exchange of actin-bound nucleotides. However, how the CARP domain binds to actin is not clearly understood. Here, we report that conserved hydrophobic residues in the CARP domain play important roles in the function of CAP to regulate actin dynamics. Single mutations of three conserved surface-exposed hydrophobic residues in the CARP domain of CAS-2, a Caenorhabditis elegans CAP, significantly reduce its binding to actin monomers and suppress its nucleotide exchange activity on actin. As a result, these mutants are weaker than wild-type to compete with ADF/cofilin to promote recycling of actin monomers for polymerization. A double mutation (V367A/I373A) eliminates these actin-regulatory functions of CAS-2. These hydrophobic residues and previously identified functional residues are scattered on a concave β-sheet of the CARP domain, suggesting that a wide area of the β-sheet is involved in binding to actin. These observations suggest that the CARP domain of CAP binds to actin in a distinct manner from other known actin-binding proteins.
Keywords: Actin dynamics, ADF/cofilin, nucleotide exchange
Introduction
Cyclase-associated protein (CAP) is highly conserved among eukaryotes, which regulates multiple aspects of actin filament turnover (Ono 2013). CAP is expressed in a variety of cell types and involved in a number of actin-dependent processes including cell migration (Bertling et al. 2004; Noegel et al. 1999; Yamazaki et al. 2009; Zhang et al. 2013; Zhang and Zhou 2016), myofibril assembly (Effendi et al. 2013; Field et al. 2015; Nomura et al. 2012; Peche et al. 2013), and plant development (Barrero et al. 2002; Deeks et al. 2007). CAP binds to actin monomers (G-actin) and enhances exchange of actin-bound ATP or ADP (Chaudhry et al. 2007; Moriyama and Yahara 2002). CAP also competitively dissociates actin depolymerizing factor (ADF)/cofilin from ADP-G-actin (Balcer et al. 2003; Moriyama and Yahara 2002). Because ADF/cofilin preferentially binds to ADP-G-actin and inhibits exchange of actin-bound nucleotides (Hawkins et al. 1993; Hayden et al. 1993; Nishida 1985), CAP regenerates polymerization-competent ATP-G-actin and promotes actin turnover in an ATP-dependent manner (Nomura and Ono 2013). Furthermore, CAP directly binds to actin filaments (F-actin) and promotes filament severing together with ADF/cofilin (Chaudhry et al. 2013; Jansen et al. 2014; Normoyle and Brieher 2012). In cooperation with twinfilin, CAP enhances dissociation of G-actin from filament ends (Johnston et al. 2015). Thus, these multiple actin-regulatory functions of CAP contribute to accelerating actin filament turnover or treadmilling. However, biochemical basis of the function of CAP is not completely understood.
CAP consists of multiple functional domains, which can be divided into N- and C-terminal halves (Ono 2013). The N-terminal half of CAP, containing a coiled-coil-like oligomerization motif (Nishida et al. 1998; Quintero-Monzon et al. 2009) and a helical-folded domain with six antiparallel α-helices (Ksiazek et al. 2003; Yusof et al. 2005), binds to ADF/cofilin-G-actin complexes (Moriyama and Yahara 2002; Quintero-Monzon et al. 2009) and ADF/cofilin-bound F-actin to promote severing (Chaudhry et al. 2013). The C-terminal half, containing, one or two proline-rich motifs, a WASP-homology 2 (WH2) domain, a CAP and X-linked retinitis pigmentosa 2 protein (CARP) domain (also known as a β-sheet domain), and a dimerization motif, possesses G-actin-regulating functions (Balcer et al. 2003; Freeman et al. 1995; Mattila et al. 2004; Moriyama and Yahara 2002). The G-actin regulating activity of CAP is suppressed or abolished by mutation or deletion of WH2 (Chaudhry et al. 2010; Makkonen et al. 2013), CARP (Jansen et al. 2014; Makkonen et al. 2013; Mattila et al. 2004), or a dimerization motif (Iwase and Ono 2016), indicating that these three elements play important roles in G-actin regulation. However, in the absence of structural information of a G-actin-CAP complex at an atomic level, how the CAP C-terminal half binds to G-actin remains unclear.
The CARP domain of CAP is composed of helically arranged β-strands forming two parallel β-sheets 1 and 2 (PB1 and PB2) (Dodatko et al. 2004). Two β-strands in the C-terminal dimerization motif participate in PB1 of another CAP molecule to stabilize a dimer (Dodatko et al. 2004; Hliscs et al. 2010). Concave PB1 and convex PB2 contribute to formation of an “S-shaped” dimer. Point mutations in the CARP domain of CAP reduce its affinity with G-actin but do not completely abolish its binding to G-actin (Jansen et al. 2014; Makkonen et al. 2013; Mattila et al. 2004). It has been unclear whether the residual G-actin binding by the CARP mutants is due to G-actin binding through the WH2 domain or incomplete suppression of the CARP-G-actin interaction by the previously reported mutations. Here, we report mutations of conserved hydrophobic residues in the CARP domain of CAP nearly completely abolish its G-actin regulatory activities. Distribution of functional residues suggest that a wide surface of the concave β-sheet in the CARP domain is an interface for binding to G-actin. Accordingly, the data suggest that CARP is a unique actin-binding domain that is essential for actin monomer regulation.
Results and Discussion
Interaction of CAS-2C with G-actin is resistant to high-salt conditions
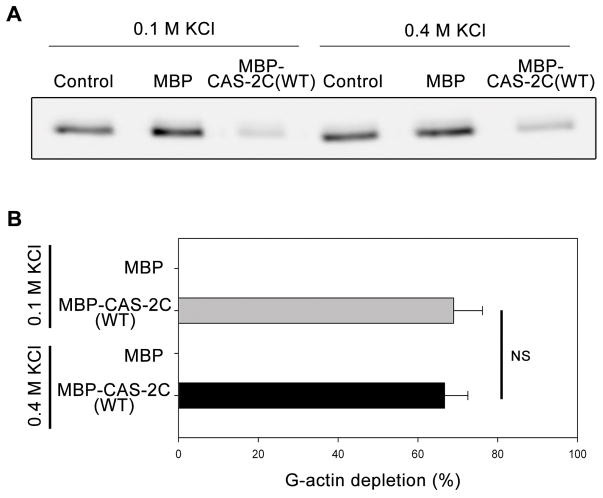
To characterize the mechanism of interaction between a C-terminal half of CAS-2 and G-actin, we tested salt-sensitivity of their binding using a supernatant-depletion assay (Iwase and Ono 2016) (Fig. 1). A C-terminal fragment of C. elegans CAS-2 (CAS-2C: residues 208–457, see Fig. 2F) containing a poly-proline motif, a WH2 domain, a CARP/β-sheet domain, and a dimerization motif, binds to G-actin and enhances nucleotide exchange on G-actin (Iwase and Ono 2016; Nomura and Ono 2013). CAS-2C was purified as a fusion protein with maltose-binding protein (MBP) and a polyhistidine tag (Nomura and Ono 2013) and incubated with DyLight 680-labeled ATP-G-actin (0.2 μM) in the presence of 0.1 or 0.4 M KCl. Then, MBP-CAS-2C and bound actin were pulled down with Ni-NTA agarose beads, and depletion of actin from the supernatants was examined by SDS-PAGE and quantified by infrared imaging (Fig. 1). Although MBP alone did not deplete G-actin, MBP-CAS-2C depleted G-actin from the supernatant, and the extent of depletion was not significantly different between 0.1 M and 0.4 M KCl (Fig. 1A and B), indicating that the interaction between CAS-2C and G-actin was resistant to high-salt conditions. These observations led us to hypothesize that hydrophobic interaction is involved in the binding of CAS-2C to G-actin.
Figure 1.
Salt sensitivity of CAS-2C binding to G-actin as determined by supernatant-depletion assay. DyLight 680-labeled ATP-G-actin (0.2 μM) was incubated with buffer only (control), 2 μM MBP, or 2 μM MBP-CAS-2C (WT) in the presence of 0.1 or 0.4 M KCl. MBP or MBP-CAS-2C and bound actin were pulled down by Ni-NTA resins, and DyLight 680-actin in the supernatants was analyzed by SDS-PAGE and infrared imaging (A). Percentages of G-actin depletion from the supernatants were quantified (B). Data are means ± standard deviations (n = 3). NS, not significant (p > 0.05) by Student’s t-test.
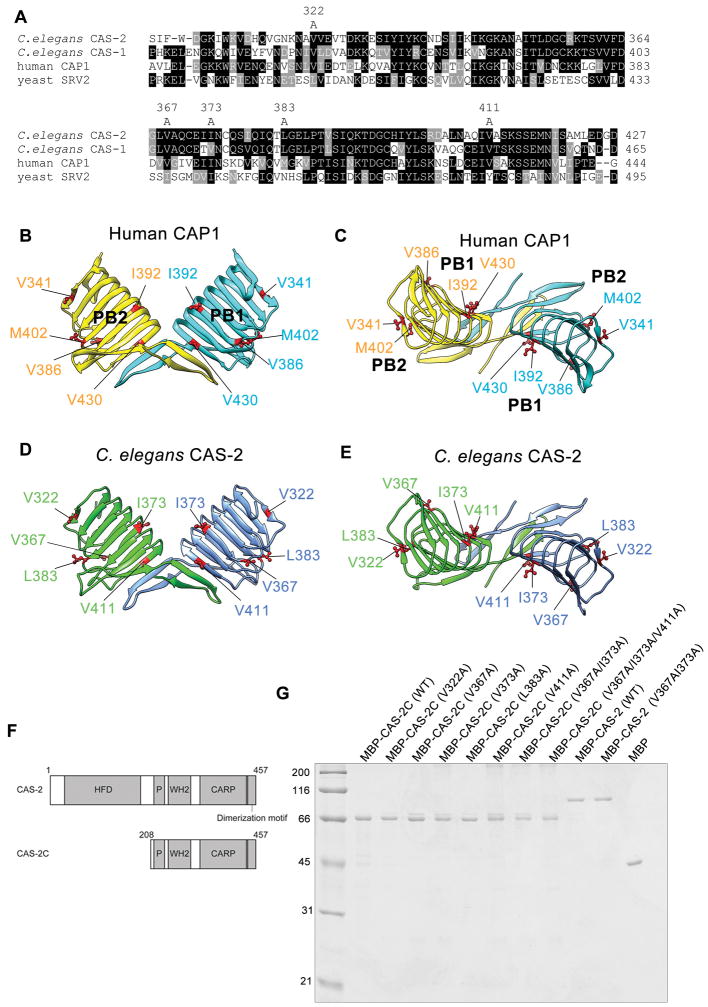
Figure 2.
Identification of surface-exposed hydrophobic residues in the CARP domain of CAPs and generation of C. elegans CAS-2 variants. (A) Alignment of CARP sequences from C. elegans CAS-2, C. elegans CAS-1, human CAP1, and budding yeast Srv2. Identical residues in majority are shown in black backgrounds. Similar residues are shown in gray backgrounds. Five hydrophobic residues that are examined in this study are shown on top (residue numbers for C. elegans CAS-2). (B, C) The crystal structure of the human CAP1 dimeric C-terminal fragment (residues 319 – 475) (Protein Data Bank accession number 1K8F) containing the CARP domain and the dimerization motif is shown as a side view perpendicular to the dimer axis (B) and a top view down the dimer axis (C). Each polypeptide chain is colored differently. Side chains of surface-exposed hydrophobic residues are shown in magenta. (D, E) A homology model of the CAS-2 dimeric C-terminal region (residues 306 – 457) (Iwase and Ono, 2016) is shown in a similar manner to human CAP1. Highlighted conserved hydrophobic residues are characterized in this study. (F) Domain structure of full-length CAS-2 and a CAS-2 C-terminal fragment (CAS-2C). (G) Purified MBP-tagged CAS-2C and CAS-2 variants (0.5 μg each) were examined by SDS-PAGE and visualized by Coomassie Brilliant Blue staining. Molecular mass markers in kDa are shown on the left of the gel.
Conserved hydrophobic residues are exposed on the surface of the CARP/β-sheet domain
To identify residues of CAS-2C, which might contribute to a hydrophobic interaction with actin, we examined the crystal structure of a C-terminal fragment of human CAP1 (Protein Data Bank accession number 1K8F) (Dodatko et al. 2004) and found that side chains of the following 11 hydrophobic amino acids are exposed on the surface of the molecule: (V321, V341, L347, V350, V357, V386, I388, I392, M402, V430, and L440). Many of the hydrophobic side chains are buried in the core of the helically arranged β-strands. Then, we compared the primary sequences of human CAP1 and C. elegans CAS-2 and found that 5 of corresponding residues in C. elegans CAS-2 (V322, V367, I373, L383, and V411) are also hydrophobic (Fig. 2A–E and Table 1). Therefore, we generated alanine substitutions for these five residues of C. elegans CAS-2C (Fig. 2D and E), purified as MBP-fusion proteins (Fig. 2G), and examined their effects on actin-regulatory functions in vitro.
Table 1.
Examined hydrophobic residues of C. elegans CAS-2 and corresponding residues in human CAP1 and yeast Srv2
| C. elegans CAS-2 | Human CAP1 | Yeast Srv2 |
|---|---|---|
| V322 | V341 | L391 |
| V367 | V386 | I436 |
| I373 | I392 | I442 |
| L383 | M402 | N452 |
| V411 | V430 | Y480 |
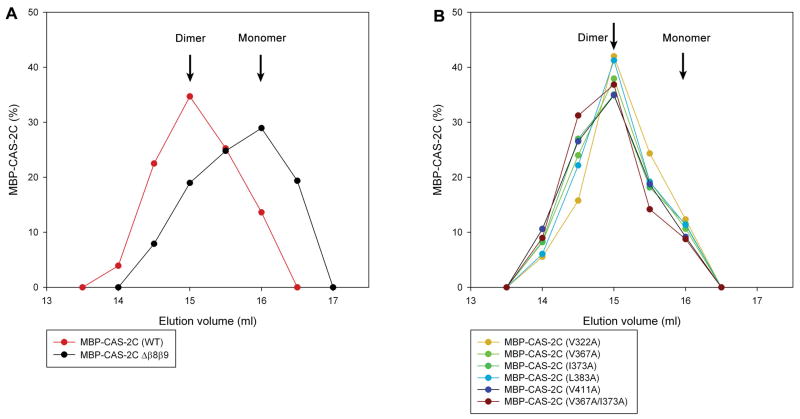
To test whether the mutations at hydrophobic residues cause major alterations in the dimerization state of CAS-2C, MBP-CAS-2C variants were analyzed by analytical gel filtration chromatography. CAS-2C dimerizes through the C-terminal dimerization motif, which is essential for its actin-monomer regulatory activities (Iwase and Ono 2016). MBP-CAS-2C (WT) was eluted faster than MBP-CAS-2CΔβ8β9 (a deletion of the dimerization motif) (Fig. 3A). We previously used dynamic light scattering analysis to estimate that the peak fractions of MBP-CAS-2C (WT) and MBP-CAS-2CΔβ8β9 were dimeric and monomeric, respectively (Iwase and Ono 2016). All examined MBP-CAS-2C single mutants were eluted in a similar manner to MBP-CAS-2C (WT) (Fig. 3B) with no detectable peaks of large aggregates, suggesting that these single mutations did not disrupt the dimeric state of CAS-2C.
Figure 3.
Estimation of dimeric states of MBP-CAS-2C variants by analytical gel filtration chromatography. (A) MBP-CAS-2C (WT) and MBP-CAS-2C Δβ8β9 (lacking the dimerization motif) were applied to Superose 6 gel filtration chromatography (24 ml bed), and eluted fractions were analyzed by SDS-PAGE. Relative amounts of eluted proteins are plotted on the graph. The graph shows the data in the elution volumes of 13 – 17.5 ml, and no other peaks were detected outside of this range. Peak positions of dimeric MBP-CAS-2C (WT) and monomeric MBP-CAS-2C Δβ8β9 are indicated. (B) Elution profiles of MBP-CAS-2C mutants were similar to that of dimeric MBP-CAS-2C (WT), suggesting that dimeric states were preserved in the examined mutants. No other peaks were detected outside of the range of 13 – 17.5 ml.
Three hydrophobic residues of CAS-2 (V367, I373, and V411) are important for actin monomer binding
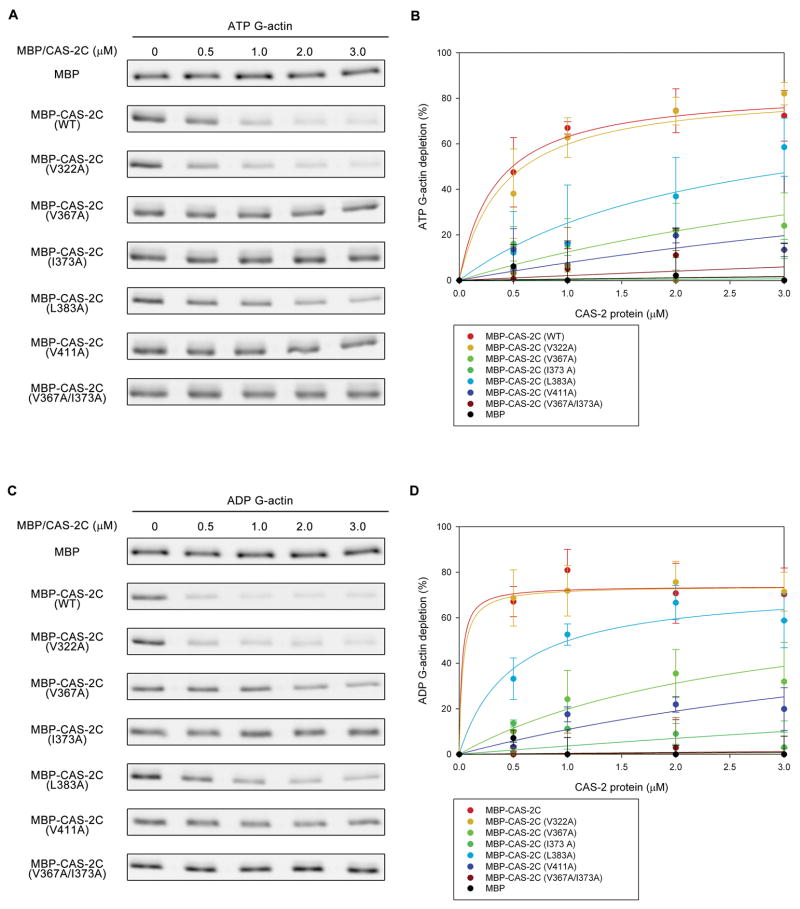
To determine residues responsible for the actin-CAS-2C interaction, we tested the CAS-2C single mutants for binding to ATP- or ADP-G-actin using the supernatant-depletion assays (Fig. 4). Similarly to the experiments in Fig. 1, ATP- or ADP-G-actin was incubated with various concentrations of MBP-CAS-2C variants under physiological conditions in the presence of 0.1 M KCl. MBP-CAS-2C and bound actin were pulled down, and depletion of G-actin from the supernatants was quantified by infrared imaging. As reported previously (Iwase and Ono 2016), MBP alone did not bind to either ATP- or ADP-G-actin (Fig. 4A–D), whereas MBP-CAS-2C (WT) bound to both ATP- and ADP-G-actin with higher affinity to ADP-G-actin (Fig. 4C and D, Table 2) than ATP-G-actin (Fig. 4A and B). The five examined single mutants exhibited variable affinity for binding to G-actin (Table 2). MBP-CAS-2C (V322A) bound to G-actin in an indistinguishable manner to MBP-CAS-2C (WT) (Fig. 4). MBP-CAS-2C (L383A) showed moderately weaker affinity with G-actin than MBP-CAS-2C (WT) (Fig. 4). However, MBP-CAS-2C (V367A), MBP-CAS-2C (I373A), and MBP-CAS-2C (V411A) showed strong defects in G-actin binding (Fig. 4) with MBP-CAS-2C (I373A) showing the lowest affinity for binding to either ATP-G-actin or ADP-G-actin (Fig. 4B and D, dark green).
Figure 4.
Binding of CAS-2C variants to ATP- and ADP-G-actin analyzed by supernatant-depletion assays. DyLight 680-labeled ATP-G-actin (0.2 μM) (A and B) or ADP-G-actin (0.2 μM) (C and D) was incubated with various concentrations of MBP or MBP-CAS-2C variants for 1 hr. The MBP-fusion proteins and associated G-actin were pulled down by Ni-NTA resins. DyLight 680-actin in the supernatants was analyzed by SDS-PAGE and infrared imaging (A and C). Percentages of G-actin depletion from the supernatants are plotted as a function of total concentrations of MBP-fusion proteins (B and D). Data are means ± standard deviations (n = 3).
Table 2.
Summary of biochemical properties of CAS-2 variants
| Protein | Kd forATP-actin binding (μM) | Kd forADP-actin binding (μM) | Nucleotide exchange activity | Actin recycling in competition with ADF/cofilin |
|---|---|---|---|---|
| MBP | No binding | No binding | - | - |
| MBP-CAS2C (WT) | 0.33 | 0.02 | +++ | +++ |
| MBP-CAS2C (V322A) | 0.41 | 0.03 | +++ | +++ |
| MBP-CAS2C (V367A) | 5.8* | 2.9* | + | + |
| MBP-CAS2C (I373A) | No binding | 19* | + | + |
| MBP-CAS2C (L383A) | 2.3* | 0.48 | +++ | ++ |
| MBP-CAS2C (V411A) | 9.8* | 5.8* | + | + |
| MBP-CAS2C (V367A/I373A) | No binding | No binding | - | - |
| MBP-CAS2 (WT) | ND1 | ND | +++ | +++ |
| MBP-CAS2 (V367A/I373A) | ND | ND | - | - |
Not determined.
Saturation of binding was not experimentally confirmed. Kd values were calculated by curve fitting assuming a saturation value that is reached by MBP-CAS-2C (WT).
Since even the most severe single-mutant MBP-CAS-2C (I373A) showed residual binding to ADP-G-actin, we generated a V367A/I373A double mutant and examined their binding to G-actin. In analytical gel filtration, the V367A/I373A double mutant was dimeric (Fig. 3B). We also generated a V367A/I373A/V411A triple mutant, but it was eluted broadly and not resolved in a clear peak, suggesting that the triple mutant was not properly folded (our unpublished observation). The V367A/I373A double mutant did not bind to either ATP- or ADP-G-actin (Fig. 4), indicating that the V367A/I373A double mutation is sufficient to cause nearly complete disruption of G-actin binding by CAS-2C. The V411A single mutation reduced G-actin binding of CAS-2C without affecting its dimeric state, suggesting that V411 also participates in G-actin binding. It should be noted that MBP-CAS-2C (V367A/I373A) contains intact WH2 domain and dimerization motif, indicating that neither of these is sufficient for G-actin binding.
The three hydrophobic residues of CAS-2 are essential for nucleotide exchange on G-actin and ATP-dependent actin-monomer recycling for polymerization
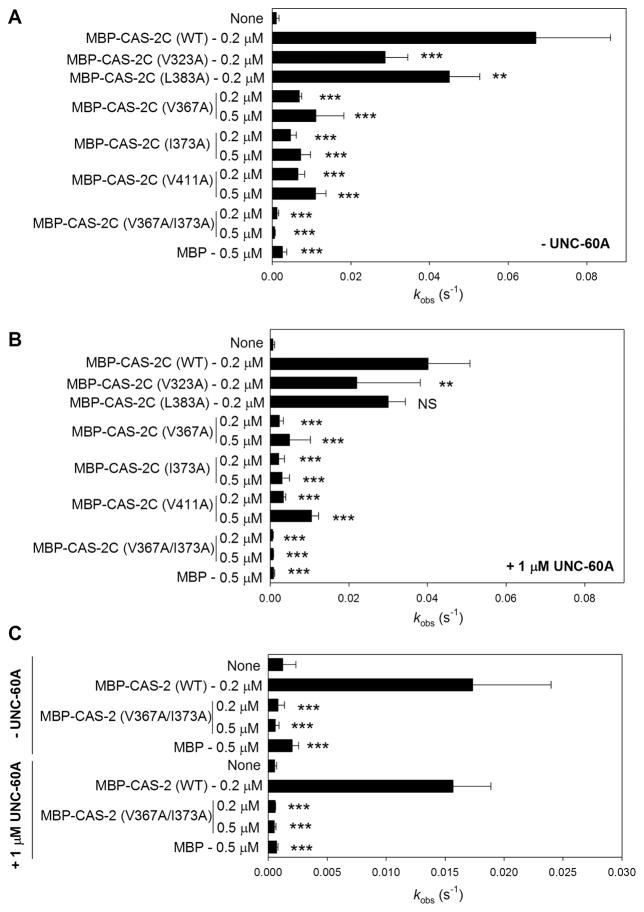
The defects of the MBP-CAS2C mutants in G-actin binding correlated with defects in enhancement of actin-bound nucleotide exchange (Fig. 5, Table 2). MBP-CAS-2C (WT) strongly enhanced exchange of nucleotides bound to ADP-G-actin either in the absence (Fig. 5A) or presence of UNC-60A (ADF/cofilin) (Fig. 5B). MBP had no effect on nucleotide exchange (Fig. 5A and B). MBP-CAS-2C (V322A), which bound normally to G-actin, exhibited somewhat weaker activity than MBP-CAS-2C (WT), but its activity was still strong either in the absence (Fig. 5A) or presence of UNC-60A (ADF/cofilin) (Fig. 5B). Interestingly, MBP-CAS-2C (L383A), which had a moderate defect in G-actin binding, exhibited only a minor decrease in the nucleotide exchange activity only in the absence of UNC-60A (Fig. 5A and B). In contrast, MBP-CAS-2C (V367A), MBP-CAS-2C (I373A), and MBP-CAS-2C (V411A), with severe defects in G-actin binding, showed much weaker nucleotide exchange activity than MBP-CAS-2C (WT) even when the concentrations of MBP-CAS-2C variants were increased up to 0.5 μM (Fig. 5A and B). The nucleotide exchange activity was abolished by V367A/I373A double mutation (Fig. 5A and B), which is consistent with the loss of G-actin binding by this double mutation. These results demonstrate that the hydrophobic residues of CAS-2 are essential for enhancing exchange of actin-bound nucleotides.
Figure 5.
Effects of CAS-2C and full-length CAS-2 variants on the exchange of actin-bound nucleotides in the presence or absence of UNC-60A. ADP-G-actin (1 μM) was incubated with etheno-ATP in the absence (A and C upper half) or presence of 1 μM UNC-60A (B and C lower half) without (none) or with MBP, MBP-CAS-2C variants (A and B), or MBP-CAS-2 full-length variants (C). The fluorescence of etheno-ATP was monitored over time, and rates of fluorescence increase [kobs (S−1)] are shown in the bar graphs. Data are means ± standard deviations (n = 3 – 7). Results of statistics (one-way ANOVA) are shown only for comparisons between wild-type (WT) and mutant in each group: NS, not significant (p > 0.05); *, 0.05 > p > 0.01; **, 0.01 > p > 0.001; ***, 0.001 > p.
We further tested whether these hydrophobic residues are essential for the nucleotide exchange activity in the context of full-length CAS-2. The N-terminal helical-folded domain is capable of binding to the G-actin-ADF/cofilin complex and cooperates with the C-terminal domains to promote actin turnover (Chaudhry et al. 2013; Chaudhry et al. 2014; Moriyama and Yahara 2002; Zhang et al. 2013). Because the V367A/I373A double mutation was sufficient to eliminate the nucleotide exchange activity in the CAS-2C fragment, effects of this double-mutation on MBP-tagged full-length CAS-2 (MBP-CAS-2) were examined. Our attempts to estimate oligomeric states of wild-type and mutant MBP-CAS-2 full-length protein failed because these proteins were not resolved in clear peaks in analytical gel filtration chromatography, suggesting that MBP-CAS-2 oligomers are unstable and may exist in multiple oligomeric states (our unpublished data). The MBP tag at the N-terminus, which is adjacent to the oligomerization motif (Quintero-Monzon et al. 2009), might destabilize the CAS-2 oligomers, but we could not cleave MBP at the Tobacco Etch Virus protease recognition site (our unpublished data). Nonetheless, MBP-CAS-2 (WT) enhanced nucleotide exchange in the absence or presence of UNC-60A (ADF/cofilin) (Fig. 5C). The activity of MBP-CAS-2 (WT) was lower than that of MBP-CAS-2C (WT), which might be due to a disturbance by the MBP tag. The V367A/I373A double mutation was sufficient to eliminate the nucleotide exchange activity in full-length CAS-2 in the absence or presence of UNC-60A (Fig. 5C). Therefore, although the activity of the MBP-tagged full-length CAS-2 may not be optimal, the results suggest that the conserved hydrophobic residues of CAS-2 play critical roles in actin monomer regulation even in the full-length CAS-2 protein.
Two conserved hydrophobic residues, V367 and I373, of CAS-2 are essential for ATP-dependent actin-monomer recycling for polymerization
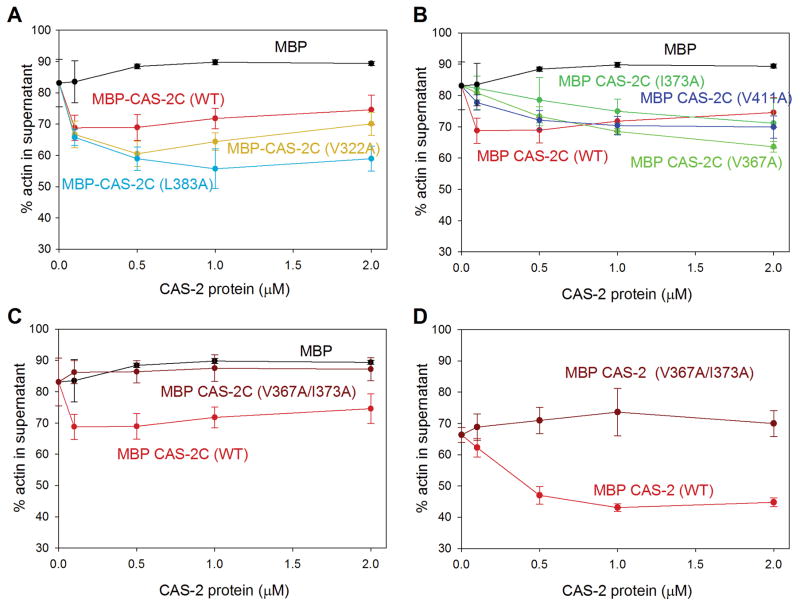
CAS-2 antagonizes UNC-60A-dependent actin-monomer sequestration in the presence of ATP (Nomura and Ono 2013). In this mechanism, CAS-2C is necessary and sufficient to enhance nucleotide exchange and increase ATP-G-actin, which has lower affinity with UNC-60A and is more competent for polymerization than ADP-G-actin, thereby promoting recycling of depolymerized actin monomers for polymerization. As expected from the effects on G-actin binding and nucleotide exchange activity, V367 and I373 of CAS-2 were critical for this mechanism (Fig. 6). In F-actin sedimentation assays using ultracentrifugation, most of F-actin by actin alone was recovered in the pellets (Supplementary Fig. S1), whereas UNC-60A kept >80 % of actin in the supernatants by sequestering actin monomers (Yamashiro et al. 2005) (Fig. 6A). In the presence of ATP, addition of 0.1–0.5 μM MBP-CAS-2C (WT) to UNC-60A-sequestered actin decreased actin in the supernatants (Fig. 6A, red), indicating that F-actin was increased by MBP-CAS-2C (WT). However, at higher concentrations of MBP-CAS-2C (WT) (1.0 – 2.0 μM), it did not further contribute to increase in F-actin because MBP-CAS-2C is capable of sequestering actin monomers (Nomura and Ono 2013). MBP did not have this effect (Fig. 6A, black). Two mutants, V322A and L383A, had similar effects to wild-type at 0.1 μM, although, at higher concentrations, these mutants decreased supernatant actin greater than wild-type probably due to weaker G-actin sequestration activity (Fig. 6A). Three MBP-CAS-2C single mutants, V367A, I373A, and V411A, had weaker effects than wild-type in a range of 0.1 – 0.5 μM and further decreased actin in the supernatants at > 1.0 μM (Fig. 6B) because of poor actin-monomer sequestering activity. In the presence of ADP or the absence of ATP or ADP, all MBP-CAS-2C variants did not alter the amounts of actin in the supernatants (Supplementary Fig. S2).
Figure 6.
Effects of CAS-2C and full-length CAS-2 variants on the amounts of pelletable F-actin in the presence of UNC-60A and ATP. F-actin (10 μM) was preincubated with 20 μM UNC-60A for 30 min in the presence of 0.5 mM ATP, and incubated with various concentrations of MBP, MBP-CAS-2C variants (A–C), or MBP-CAS-2 full-length variants (D) for 30 min. The mixtures were ultracentrifuged, and the supernatants and pellets were analyzed by SDS-PAGE (representative gel images are shown in Supplementary Fig. S1). Percentages of actin in the supernatants were quantified and plotted as a function of concentrations of the CAS-2 variants. Data are means ± standard deviations (n = 3). For clarity, CAS-2C single mutants are shown in two separate graphs: weakly affected mutants (V322A and L383A) in A and moderately affected mutants (V367A, I373A, and V411A) in B. A double CAS-2C mutant is shown in C. Common data for MBP and MBP-CAS-2C (WT) are used to generate graphs in A–C.
The function of CAS-2C to antagonize UNC-60A was nearly completely eliminated by the V367A/I373A double and V367A/I373A/V411A triple mutations (Fig. 6C). These double and triple mutants did not alter the amounts of UNC-60A-sequestered actin in the examined range of concentrations (0.1 – 2.0 μM) either in the presence of ATP (Fig. 6C) or ADP (Supplementary Fig. S2B) or in the absence of ATP/ADP (Supplementary Fig. S2D). These are consistent with the severe effects of these mutations on G-actin binding and nucleotide exchange activity.
The effect of V367A/I373A double mutation was further confirmed in the full-length CAS-2 protein. As reported previously (Nomura and Ono 2013), MBP-tagged full-length CAS-2 also antagonized UNC-60A to suppress actin-monomer sequestration (Fig. 6D) in a similar manner to MBP-CAS-2C. However, the V367A/I373A double mutation nearly completely abolished this activity (Fig. 6D). These results suggest that V367 and I373 are particularly important for full-length CAS-2 to regulate actin monomer recycling.
Distribution of functional CAP residues suggests that a wide surface of a concave β-sheet of the CARP domain is involved in G-actin binding
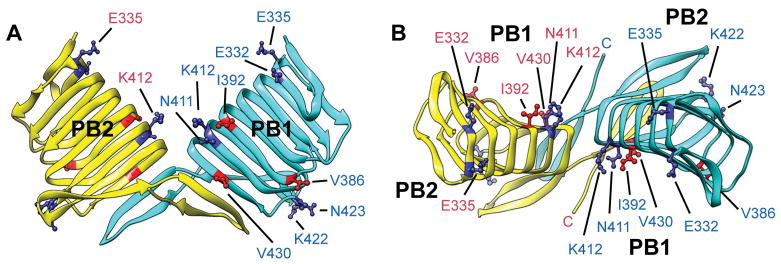
To deduce the mechanism of the G-actin-CARP interaction, three-dimensional relationships of functionally important conserved CAP residues were examined in the crystal structure of human CAP1 C-terminal fragment (Dodatko et al. 2004) (Fig. 7). The three functionally important hydrophobic residues, V367, I373, and V411A, of CAS-2 are equivalent to V386, I392, and V430 of human CAP1 (Table 1). These residues are distributed on or near the concave parallel β-sheet 1 (PB1) (Fig. 7A and B, shown in red). A previous mutagenesis study on yeast Srv2/CAP demonstrated that three sets of mutations (Srv2-104, Srv2-108, and Srv2-109) at charged residues in the CARP domain weaken binding of Srv2/CAP to G-actin (Mattila et al. 2004). Equivalent human CAP1 residues to these yeast Srv2/CAP residues are listed in Table 3, and the locations are shown in Fig. 7 (shown in dark blue). Two residues, N411 and K412 (equivalent to mutated residues in Srv2-108) are adjacent to I392 on PB1, and E332, one of residues mutated in Srv2-104, is also located on PB1 (Fig. 7). Intriguingly, E335, another mutated residue in Srv2-104, is located in the cleft between the two monomers and faces towards PB1 of another monomer (Fig. 7A and B). However, K422 and N423 (equivalent to mutated residues in Srv2-109) are located in a loop on the opposite side from PB1 (Fig. 7A and B). The mapping indicates that many of the functionally important residues of CAP are located on or near PB1. These residues are scattered on PB1, suggesting that a wide surface of PB1 participates in binding to G-actin. This view is consistent with a broad distribution of actin surface residues that are involved in interaction between actin and Srv2/CAP in yeast (Amberg et al. 1995).
Figure 7.
Three-dimensional distribution of functionally important amino acids in the human CAP1 dimeric C-terminal fragment. Side (A) and top (B) views of the human CAP1 dimeric C-terminal fragment (Protein Data Bank accession number 1K8F) are shown in a similar manner to Fig. 2B and C. Parallel β-sheets 1 and 2 are indicated as PB1 and PB2, respectively. Homologous residues in human CAP1 to the three functionally important hydrophobic residues in C. elegans CAS-2 are shown in red. Homologous residues in human CAP1 to the functionally important residues in yeast Srv2 are shown in dark blue.
Table 3.
Functionally important residues of yeast Srv2 and corresponding residues in human CAP1 and C. elegans CAS-21
| Residue number2 | |||
|---|---|---|---|
| Yeast allele | Yeast Srv2 | C. elegans CAS-2 | Human CAP1 |
| Srv2-1041 | E382/E385 | D313/V316 | E332/E335 |
| Srv2-108 | D461/K462 | Q392/K393 | N411/K412 |
| Srv2-109 | K472/E473 | R403/D404 | K422/N423 |
Yeast alleles were reported in Mattila et al. (2004).
Corresponding residues were determined by sequence alignment reported in Ono (2013).
The three functional residues of CAPs are evolutionarily conserved
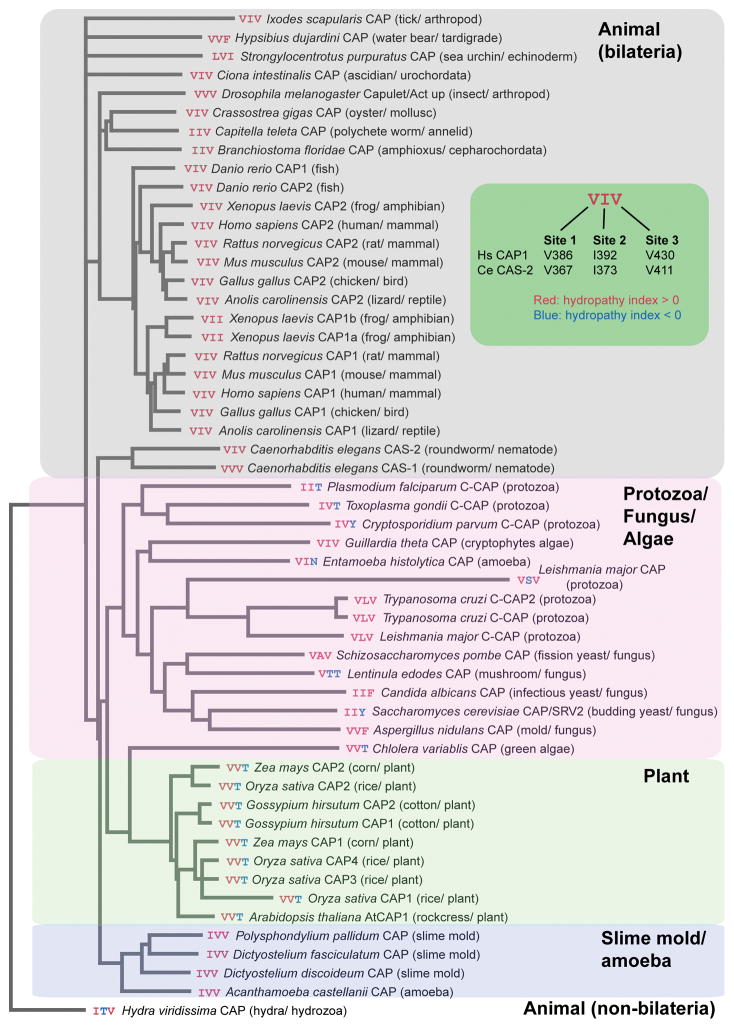
To examine evolutionary conservation of the three hydrophobic amino acids, we aligned 54 CARP domain sequences from CAPs and C-CAPs across eukaryotes and analyzed how well these residues are conserved (Fig. 8). The CARP sequences were aligned with Clustal Omega (Sievers et al. 2011) and analyzed based on the hydropathy index (Kyte and Doolittle 1982) (the entire alignment is presented in Supplementary Fig. S3). The three residues were designated as site 1 (V386 of human CAP1 and V367 of C. elegans CAS-2), site 2 (I392 of human CAP1 and I373 of C. elegans CAS-2), and site 3 (V430 of human CAP1 and V411 of C. elegans CAS-2), and color-coded for hydropathy: hydrophobic residues with hydropathy index > 0 were colored red, and other residues with hydropathy index < 0 were colored blue (Fig. 8). Phylogenetic relationships among the CARP sequences agreed well with classification of organisms with an exception of Entoamoeba CAP, which was sorted in the protozoa/fungus/algae clade (Fig. 8). However, the previous analysis of full-length proteins sorted Entoamoeba CAP into the slime mold/amoeba clade (Ono 2013). Remarkably, hydrophobicity is conserved at site 1 in all examined species, suggesting functional importance of this residue (Fig. 8). Hydrophobicity at site 2 is also highly conserved with exceptions of Lentinula (shiitake mushroom) and Hydra CAPs. Hydrophobicity at site 3 is conserved in CAPs of all examined animal and slime mold species, and some of protozoan, fungal and algal species (Fig. 8). Interestingly, threonine is conserved at site 3 in CAPs of all examined plant and green algal species, suggesting that plant actins may interact with site 3 in a different manner from animal actins. Thus, these comparative analyses support the idea that these three site are evolutionarily conserved because of their functional significance.
Figure 8.
Phylogenetic relationships of the CARP domain sequences from CAPs and C-CAPS across eukaryotes and conservation of the three functional hydrophobic residues. Sequences of the CARP domains were analyzed by Clustal Omega (Sievers et al. 2011). Database accession numbers for original sequences are listed in Supplementary Table S1. The three functional hydrophobic residues are designated as sites 1 – 3 (green inset) and color-coded based on the hydropathy index (Kyte and Doolittle 1982) (red, hydrophobic residues with hydropathy score > 0; blue, other residues with hydropathy score < 0).
Conclusion
In addition to identification of novel functional residues in CAP, our study extends our knowledge on a critical function of the CARP domain of CAP in actin monomer regulation. Our results agree with mutational studies by others showing that mutations in the CARP domain abolish or significantly weaken the activity of CAP to enhance nucleotide exchange on G-actin (Jansen et al. 2014; Makkonen et al. 2013; Mattila et al. 2004). However, the mutβ mutation in mouse CAP1, which contains six alanine substitutions (four mutations equivalent to Srv2-108/Srv2-109 plus two additional mutations), only affects its binding to ADP-G-actin but not to ATP-G-actin (Makkonen et al. 2013), which led them to suggest that the WH2 domain is sufficient to mediate binding of CAP to ATP-G-actin. Nonetheless, our V367A/I373A double mutation in the C. elegans CAS-2 CARP domain virtually eliminated its binding to both ATP-G-actin and ADP-G-actin even in the presence of an intact WH2 domain. Therefore, the mutβ mutation in mouse CAP1 may still have functional hydrophobic residues in the CARP domain, which could mediate binding of CAP1 to ATP-G-actin.
In the absence of structural information on the G-actin-CAP complex at an atomic level, roles of the WH2 domain, the CARP domain, and the C-terminal dimerization motif, suggest that CAP binds to G-actin in a distinct manner from other known G-actin-binding proteins (Dominguez and Holmes 2011; Paavilainen et al. 2004). The WH2 domain of CAP binds to G-actin (Chaudhry et al. 2010; Makkonen et al. 2013), most likely at the cleft between subdomains 1 and 3 of actin in an analogous manner to other known WH2 domains (Dominguez and Holmes 2011). This suggests that the CARP domain binds to other parts of the actin molecule. In addition, the C-terminal dimerization motif is essential for actin monomer regulation (Iwase and Ono 2016). Interestingly, the C-terminal end of the dimerization motif is located closely to PB1 of another monomer (Fig. 8, compare the locations of the C-terminus of yellow chain and PB1 of light blue chain) and may be involved in direct interaction with G-actin. Therefore, the three domains, WH2, CARP, and dimerization motif, should work together to bind to G-actin and enhance nucleotide exchange. Future structural analysis of the G-actin-CAP complex at an atomic resolution should provide a big step forward to understanding the mechanism of actin regulation by CAP.
Materials and Methods
Proteins
Rabbit muscle actin was purified from acetone powder (Pel-Freeze Biologicals) as described previously (Pardee and Spudich 1982). ADP-G-actin was prepared using hexokinase (Worthington Biochemical)-conjugated Sepharose beads as described previously (Makkonen et al. 2013). UNC-60A was expressed in Escherichia coli and purified as described previously (Ono and Benian 1998). DyLight 680-labeled actin was prepared as described for preparation of DyLight 549-labeled actin (Liu et al. 2010) using DyLight 680 NHS ester (Thermo Fisher Scientific). Histidine-tagged maltose-binding protein (MBP) was expressed from pDEST-HisMBP, which was developed by the group of David Waugh (National Cancer Institute, Frederick, MD) and obtained through Addgene, and purified as described (Nallamsetty and Waugh 2007).
Molecular graphics
Coordinates for the crystal structure of the human CAP1 C-terminal region (Dodatko et al. 2004) was obtained from the Protein Data Bank (accession number 1K8F). A homology model of the C. elegans CAS-2 C-terminal region (residues 306–457) was described previously (Iwase and Ono 2016). Molecular graphics were generated by UCSF Chimera (Pettersen et al. 2004).
Mutagenesis and preparation of recombinant CAS-2C and full-length CAS-2 proteins
Bacterial expression vectors for full-length CAS-2 (residues 1–457) and CAS-2C (residues 208–457) as fusion proteins with histidine-tagged maltose-binding protein (MBP) in pDEST-HisMBP were described previously (Nomura and Ono 2013). Point mutations were introduced with a Quickchange Site-directed Mutagenesis Kit (Agilent Technologies). The protein coding sequences were verified by DNA sequencing. E. coli BL21(DE3) cells were transformed with the expression vectors, and protein expression and purification were performed essentially as described previously for MBP-CAS-2C (Nomura and Ono 2013). Briefly, expression of recombinant MBP-fusion proteins were induced by 1 mM isopropyl-β-D-thiogalactoside for 3 hr at room temperature, and the cells were harvested and homogenized by sonication in phosphate-buffered saline. The lysates were centrifuged at 20,000 × g for 15 min, and the supernatants were applied to a HisPur™ Ni-NTA Resin column (3-ml bed) (Thermo Fisher Scientific) and washed with 150 mM NaCl, 3 mM imidazole, and 50 mM Na·PO4 (pH 7.9). The bound proteins were eluted with 150 mM NaCl, 150 mM imidazole, and 50 mM Na·PO4 (pH 7.9). The proteins were further purified with Q-Sepharose chromatography when purity was not sufficient for subsequent analysis. Protein concentrations were determined using a Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific).
Supernatant depletion assays for G-actin binding
DyLight 680-labeled ATP- or ADP-G-actin (0.2 μM, 10 % labeled) was incubated with various concentrations of MBP or MBP-CAS-2 variants in 0.1 M KCl, 2 mM MgCl2, 1 mM EGTA, 20 mM HEPES-KOH, 0.2 mM dithiothreitol, 0.2 mM ATP or ADP, 0.1 mg/ml bovine serum albumin, and 10 mM imidazole, pH 7.5 for 1 h at room temperature. Then, 20 μl of HisPur Ni-NTA resin (Thermo Fisher Scientific) was added to the mixture and incubated for 1 h at room temperature with agitation. The resin was removed by passing through a Pierce™ Micro-Spin column (Thermo Fisher Scientific), and the flow-through fraction was subjected to SDS-PAGE (12 % acrylamide gel). The gel was scanned by an Odyssey Infrared Imaging System (LI-COR) using a 700-nm channel. The fluorescence intensity of the actin bands was quantified by ImageJ, and the percentages of G-actin depletion from the supernatants were calculated.
Gel filtration chromatography
A Superose 6 Increase 10/300 GL gel filtration column (24 ml bed, GE Healthcare Life Sciences, Pittsburgh, PA) was equilibrated with F-buffer (0.1 M KCl, 2 mM MgCl2, 0.2 mM dithiothreitol, 20 mM HEPES-KOH, pH 7.5). Proteins at 1.0 mg/ml (500 μl) were filtrated through a Spin-X 0.22 mm filter (Corning Costar, Tewksbury, MA), applied to the column, and eluted with the same buffer. The eluates were collected in fractions of 0.5 ml each and analyzed by SDS-PAGE. The gels were stained with Coomassie Brilliant Blue R-250 (National Diagnostics), scanned by an Epson Perfection V700 Photo Scanner at 300 dots per inch, and analyzed by densitometry using ImageJ. Correlation of elution profiles and dimeric/monomeric states of MBP-CAS-2C and MBP-CAS-2CΔβ8β9 was determined previously by dynamic light scattering (Iwase and Ono 2016).
Assays for the exchange of actin-bound nucleotides
The exchange of G-actin-bound nucleotides was examined by monitoring the increase in the fluorescence of etheno-ATP. ADP-G-actin (1.3 μM) in 150 μl of G-buffer without ATP or ADP (2 mM Tris-HCl, 0.2 mM CaCl2, 0.2 mM dithiothreitol, pH 8.0) and 50 μl of 160 μM etheno-ATP with or without CAS-2 variants and/or UNC-60A were mixed. Then, the fluorescence of etheno-ATP (excitation at 360 nm and emission at 410 nm) was monitored for 20 min with an F-4500 fluorescence spectrophotometer (Hitachi High-Technologies). The exponential rates (kobs) were calculated by curve fitting using SigmaPlot 13 (Systat Software). The mean values were tested by one-way analysis of variance (ANOVA) using SigmaPlot 13 (Systat Software).
F-actin sedimentation assays
F-actin (10 μM) in F-buffer with or without 0.5 mM ATP or ADP was preincubated with 20 μM UNC-60A for 30 min at room temperature. Then, various concentrations of MBP or MBP-CAS-2 variants were added to the mixture and incubated for 30 min. The mixtures were ultracentrifuges at 42,000 rpm for 20 min using a Beckman 42.2Ti rotor. Supernatant and pellet fractions were adjusted to the same volumes and subjected to SDS-PAGE (12 % acrylamide gel) and staining with Coomassie Brilliant Blue R-250 (National Diagnostics). Protein markers (Catalog # 29458-24, Nacalai USA) were used as molecular mass markers. Gels were scanned by an Epson Perfection V700 scanner at 300 dots per inch, and band intensity was quantified using ImageJ.
Sequence alignment and phylogenetic analysis
Total 54 sequences of CAPs and C-CAPs across eukaryotes were obtained from the National Center for Biotechnology Information Database (accession numbers are listed in Supplementary Table S1). Sequences for the CARP domains were aligned with Clustal Omega (Sievers et al. 2011) using the MegAlign Pro module of the Lasergene software package (DNASTAR, Inc, Madison, WI). The aligned sequences were further analyzed for hydrophobicity using Jalview 2.10. (Waterhouse et al. 2009) based on the hydropathy index (Kyte and Doolittle 1982). A phylogenetic tree was generated with TreeVector (Pethica et al. 2010) and annotated with Adobe Illustrator.
Supplementary Material
Acknowledgments
We thank Kazumi Nomura for performing preliminary experiments. Molecular graphics were generated by UCSF Chimera, which is developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (Supported by NIH P41-GM103311). This work was supported by a grant from the National Institutes of Health (AR048615) to S. O. The authors declare no conflict of interest.
References
- Amberg DC, Basart E, Botstein D. Defining protein interactions with yeast actin in vivo. Nat Struct Biol. 1995;2:28–35. doi: 10.1038/nsb0195-28. [DOI] [PubMed] [Google Scholar]
- Balcer HI, Goodman AL, Rodal AA, Smith E, Kugler J, Heuser JE, Goode BL. Coordinated regulation of actin filament turnover by a high-molecular-weight Srv2/CAP complex, cofilin, profilin, and Aip1. Curr Biol. 2003;13:2159–2169. doi: 10.1016/j.cub.2003.11.051. [DOI] [PubMed] [Google Scholar]
- Barrero RA, Umeda M, Yamamura S, Uchimiya H. Arabidopsis CAP regulates the actin cytoskeleton necessary for plant cell elongation and division. Plant Cell. 2002;14:149–163. doi: 10.1105/tpc.010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertling E, Hotulainen P, Mattila PK, Matilainen T, Salminen M, Lappalainen P. Cyclase-associated protein 1 (CAP1) promotes cofilin-induced actin dynamics in mammalian nonmuscle cells. Mol Biol Cell. 2004;15:2324–2334. doi: 10.1091/mbc.E04-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry F, Breitsprecher D, Little K, Sharov G, Sokolova O, Goode BL. Srv2/cyclase-associated protein forms hexameric shurikens that directly catalyze actin filament severing by cofilin. Mol Biol Cell. 2013;24:31–41. doi: 10.1091/mbc.E12-08-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry F, Guerin C, von Witsch M, Blanchoin L, Staiger CJ. Identification of Arabidopsis cyclase-associated protein 1 as the first nucleotide exchange factor for plant actin. Mol Biol Cell. 2007;18:3002–3014. doi: 10.1091/mbc.E06-11-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry F, Jansen S, Little K, Suarez C, Boujemaa-Paterski R, Blanchoin L, Goode BL. Autonomous and in trans functions for the two halves of Srv2/CAP in promoting actin turnover. Cytoskeleton (Hoboken) 2014;71:351–360. doi: 10.1002/cm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry F, Little K, Talarico L, Quintero-Monzon O, Goode BL. A central role for the WH2 domain of Srv2/CAP in recharging actin monomers to drive actin turnover in vitro and in vivo. Cytoskeleton (Hoboken) 2010;67:120–133. doi: 10.1002/cm.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks MJ, Rodrigues C, Dimmock S, Ketelaar T, Maciver SK, Malho R, Hussey PJ. Arabidopsis CAP1 - a key regulator of actin organisation and development. J Cell Sci. 2007;120:2609–2618. doi: 10.1242/jcs.007302. [DOI] [PubMed] [Google Scholar]
- Dodatko T, Fedorov AA, Grynberg M, Patskovsky Y, Rozwarski DA, Jaroszewski L, Aronoff-Spencer E, Kondraskina E, Irving T, Godzik A, Almo SC. Crystal structure of the actin binding domain of the cyclase-associated protein. Biochemistry. 2004;43:10628–10641. doi: 10.1021/bi049071r. [DOI] [PubMed] [Google Scholar]
- Dominguez R, Holmes KC. Actin structure and function. Annu Rev Biophys. 2011;40:169–186. doi: 10.1146/annurev-biophys-042910-155359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effendi K, Yamazaki K, Mori T, Masugi Y, Makino S, Sakamoto M. Involvement of hepatocellular carcinoma biomarker, cyclase-associated protein 2 in zebrafish body development and cancer progression. Exp Cell Res. 2013;319:35–44. doi: 10.1016/j.yexcr.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Field J, Ye DZ, Shinde M, Liu F, Schillinger KJ, Lu M, Wang T, Skettini M, Xiong Y, Brice AK, Chung DC, Patel VV. CAP2 in cardiac conduction, sudden cardiac death and eye development. Sci Rep. 2015;5:17256. doi: 10.1038/srep17256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman NL, Chen Z, Horenstein J, Weber A, Field J. An actin monomer binding activity localizes to the carboxyl-terminal half of the Saccharomyces cerevisiae cyclase-associated protein. J Biol Chem. 1995;270:5680–5685. doi: 10.1074/jbc.270.10.5680. [DOI] [PubMed] [Google Scholar]
- Hawkins M, Pope B, Maciver SK, Weeds AG. Human actin depolymerizing factor mediates a pH-sensitive destruction of actin filaments. Biochemistry. 1993;32:9985–9993. doi: 10.1021/bi00089a014. [DOI] [PubMed] [Google Scholar]
- Hayden SM, Miller PS, Brauweiler A, Bamburg JR. Analysis of the interactions of actin depolymerizing factor with G- and F-actin. Biochemistry. 1993;32:9994–10004. doi: 10.1021/bi00089a015. [DOI] [PubMed] [Google Scholar]
- Hliscs M, Sattler JM, Tempel W, Artz JD, Dong A, Hui R, Matuschewski K, Schuler H. Structure and function of a G-actin sequestering protein with a vital role in malaria oocyst development inside the mosquito vector. J Biol Chem. 2010;285:11572–11583. doi: 10.1074/jbc.M109.054916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase S, Ono S. The C-terminal dimerization motif of cyclase-associated protein is essential for actin monomer regulation. Biochem J. 2016;473:4427–4441. doi: 10.1042/BCJ20160329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen S, Collins A, Golden L, Sokolova O, Goode BL. Structure and mechanism of mouse cyclase-associated protein (CAP1) in regulating actin dynamics. J Biol Chem. 2014;289:30732–30742. doi: 10.1074/jbc.M114.601765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AB, Collins A, Goode BL. High-speed depolymerization at actin filament ends jointly catalysed by Twinfilin and Srv2/CAP. Nat Cell Biol. 2015;17:1504–1511. doi: 10.1038/ncb3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek D, Brandstetter H, Israel L, Bourenkov GP, Katchalova G, Janssen KP, Bartunik HD, Noegel AA, Schleicher M, Holak TA. Structure of the N-terminal domain of the adenylyl cyclase-associated protein (CAP) from Dictyostelium discoideum. Structure. 2003;11:1171–1178. doi: 10.1016/s0969-2126(03)00180-1. [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Liu Z, Klaavuniemi T, Ono S. Distinct roles of four gelsolin-like domains of Caenorhabditis elegans gelsolin-like protein-1 in actin filament severing, barbed end capping, and phosphoinositide binding. Biochemistry. 2010;49:4349–4360. doi: 10.1021/bi100215b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkonen M, Bertling E, Chebotareva NA, Baum J, Lappalainen P. Mammalian and malaria parasite cyclase-associated proteins catalyze nucleotide exchange on G-actin through a conserved mechanism. J Biol Chem. 2013;288:984–994. doi: 10.1074/jbc.M112.435719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila PK, Quintero-Monzon O, Kugler J, Moseley JB, Almo SC, Lappalainen P, Goode BL. A high-affinity interaction with ADP-actin monomers underlies the mechanism and in vivo function of Srv2/cyclase-associated protein. Mol Biol Cell. 2004;15:5158–5171. doi: 10.1091/mbc.E04-06-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama K, Yahara I. Human CAP1 is a key factor in the recycling of cofilin and actin for rapid actin turnover. J Cell Sci. 2002;115:1591–1601. doi: 10.1242/jcs.115.8.1591. [DOI] [PubMed] [Google Scholar]
- Nallamsetty S, Waugh DS. A generic protocol for the expression and purification of recombinant proteins in Escherichia coli using a combinatorial His6-maltose binding protein fusion tag. Nat Protoc. 2007;2:383–391. doi: 10.1038/nprot.2007.50. [DOI] [PubMed] [Google Scholar]
- Nishida E. Opposite effects of cofilin and profilin from porcine brain on rate of exchange of actin-bound adenosine 5′-triphosphate. Biochemistry. 1985;24:1160–1164. doi: 10.1021/bi00326a015. [DOI] [PubMed] [Google Scholar]
- Nishida Y, Shima F, Sen H, Tanaka Y, Yanagihara C, Yamawaki-Kataoka Y, Kariya K, Kataoka T. Coiled-coil interaction of N-terminal 36 residues of cyclase-associated protein with adenylyl cyclase is sufficient for its function in Saccharomyces cerevisiae ras pathway. J Biol Chem. 1998;273:28019–28024. doi: 10.1074/jbc.273.43.28019. [DOI] [PubMed] [Google Scholar]
- Noegel AA, Rivero F, Albrecht R, Janssen KP, Kohler J, Parent CA, Schleicher M. Assessing the role of the ASP56/CAP homologue of Dictyostelium discoideum and the requirements for subcellular localization. J Cell Sci. 1999;112( Pt 19):3195–3203. doi: 10.1242/jcs.112.19.3195. [DOI] [PubMed] [Google Scholar]
- Nomura K, Ono K, Ono S. CAS-1, a C. elegans cyclase-associated protein, is required for sarcomeric actin assembly in striated muscle. J Cell Sci. 2012;125:4077–4089. doi: 10.1242/jcs.104950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura K, Ono S. ATP-dependent regulation of actin monomer-filament equilibrium by cyclase-associated protein and ADF/cofilin. Biochem J. 2013;453:249–259. doi: 10.1042/BJ20130491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normoyle KP, Brieher WM. Cyclase-associated protein (CAP) acts directly on F-actin to accelerate cofilin-mediated actin severing across the range of physiological pH. J Biol Chem. 2012;287:35722–35732. doi: 10.1074/jbc.M112.396051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S. The role of cyclase-associated protein in regulating actin filament dynamics - more than a monomer-sequestration factor. J Cell Sci. 2013;126:3249–3258. doi: 10.1242/jcs.128231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S, Benian GM. Two Caenorhabditis elegans actin depolymerizing factor/cofilin proteins, encoded by the unc-60 gene, differentially regulate actin filament dynamics. J Biol Chem. 1998;273:3778–3783. doi: 10.1074/jbc.273.6.3778. [DOI] [PubMed] [Google Scholar]
- Paavilainen VO, Bertling E, Falck S, Lappalainen P. Regulation of cytoskeletal dynamics by actin-monomer-binding proteins. Trends Cell Biol. 2004;14:386–394. doi: 10.1016/j.tcb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Pardee JD, Spudich JA. Purification of muscle actin. Methods Enzymol. 1982;85:164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- Peche VS, Holak TA, Burgute BD, Kosmas K, Kale SP, Wunderlich FT, Elhamine F, Stehle R, Pfitzer G, Nohroudi K, et al. Ablation of cyclase-associated protein 2 (CAP2) leads to cardiomyopathy. Cell Mol Life Sci. 2013;70:527–543. doi: 10.1007/s00018-012-1142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethica R, Barker G, Kovacs T, Gough J. TreeVector: scalable, interactive, phylogenetic trees for the web. PLoS One. 2010;5:e8934. doi: 10.1371/journal.pone.0008934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Quintero-Monzon O, Jonasson EM, Bertling E, Talarico L, Chaudhry F, Sihvo M, Lappalainen P, Goode BL. Reconstitution and dissection of the 600-kDa Srv2/CAP complex: roles for oligomerization and cofilin-actin binding in driving actin turnover. J Biol Chem. 2009;284:10923–10934. doi: 10.1074/jbc.M808760200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S, Mohri K, Ono S. The two Caenorhabditis elegans actin depolymerizing factor/cofilin proteins differently enhance actin filament severing and depolymerization. Biochemistry. 2005;44:14238–14247. doi: 10.1021/bi050933d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Takamura M, Masugi Y, Mori T, Du W, Hibi T, Hiraoka N, Ohta T, Ohki M, Hirohashi S, et al. Adenylate cyclase-associated protein 1 overexpressed in pancreatic cancers is involved in cancer cell motility. Lab Invest. 2009;89:425–432. doi: 10.1038/labinvest.2009.5. [DOI] [PubMed] [Google Scholar]
- Yusof AM, Hu NJ, Wlodawer A, Hofmann A. Structural evidence for variable oligomerization of the N-terminal domain of cyclase-associated protein (CAP) Proteins. 2005;58:255–262. doi: 10.1002/prot.20314. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ghai P, Wu H, Wang C, Field J, Zhou GL. Mammalian adenylyl cyclase-associated protein 1 (CAP1) regulates cofilin function, the actin cytoskeleton, and cell adhesion. J Biol Chem. 2013;288:20966–20977. doi: 10.1074/jbc.M113.484535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhou GL. CAP1 (Cyclase-Associated Protein 1) exerts distinct functions in the proliferation and metastatic potential of breast cancer cells mediated by ERK. Sci Rep. 2016;6:25933. doi: 10.1038/srep25933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.