Abstract
Biomarkers are biological characteristics that can be used to indicate health or disease. This paper reviews studies on biomarkers of low back pain (LBP) in human subjects. LBP is the leading cause of disability, caused by various spine-related disorders, including intervertebral disc degeneration, disc herniation, spinal stenosis, and facet arthritis. The focus of these studies is inflammatory mediators, because inflammation contributes to the pathogenesis of disc degeneration and associated pain mechanisms. Increasingly, studies suggest that the presence of inflammatory mediators can be measured systemically in the blood. These biomarkers may serve as novel tools for directing patient care. Currently, patient response to treatment is unpredictable with a significant rate of recurrence, and, while surgical treatments may provide anatomical correction and pain relief, they are invasive and costly. The review covers studies performed on populations with specific diagnoses and undefined origins of LBP. Since the natural history of LBP is progressive, the temporal nature of studies is categorized by duration of symptomology/disease. Related studies on changes in biomarkers with treatment are also reviewed. Ultimately, diagnostic biomarkers of LBP and spinal degeneration have the potential to shepherd an era of individualized spine medicine for personalized therapeutics in the treatment of LBP.
Keywords: spine, intervertebral disc degeneration, inflammation, back pain, biomarkers
Introduction
Low back pain (LBP) is a global healthcare concern causing more global disability than any other medical condition.1–4 It is estimated that up to 84% of adults have LBP at some time in their lives.5,6 The vast majority of patients seen in primary care (> 85%) will have undefined LBP, meaning that the patient has back pain in the absence of a specific underlying condition that can be reliably identified.7–9 For most of these individuals, episodes of LBP are self-limited. Patients who continue to have LBP beyond the acute period (4 weeks) have subacute back pain (lasting between 4 and 12 weeks), and some may go on to develop chronic back pain (lasting >12 weeks).10 The direct and indirect costs associated with LBP in the United States are estimated to be upwards of $100–200 billion per year, more than half of which is due to loss of income and lack of productivity.11,12 This immense economic burden is reciprocal to the pain, disability, and psychological and social consequences on patients. As the global population ages, the encumbrance associated with LBP will increase exponentially. The detrimental effects associated with LBP are extensive, highlighting the need for novel therapeutic strategies to prevent and treat this condition and its concurrent symptoms.12
Clinically, LBP can be a symptom of multiple diseases, such as degenerative disc disease, intervertebral disc herniation, spinal stenosis, hypertrophy or ossification of the facets, spinal malalignment, pinched nerves, and peripheral neuropathy. Multifactorial processes, including genetics, lifestyle (occupation, smoking, alcohol consumption), and comorbidities (diabetes, obesity) all may contribute to worsening pathology and disease states. The multiple potential triggers of LBP manifest with overlapping clinical presentation, and thus physical examination is necessary but not typically diagnostic. Pinpointing the cause of LBP presents the biggest challenge to physicians in this field.
Intervertebral disc (IVD) degeneration
The intervertebral disc (IVD) is the soft connective tissue of the spine that interfaces with the vertebral bodies (Fig. 1). The primary function of the IVD is mechanical, transmitting loads arising from body weight and muscle activity through the spinal column.13 The IVD is a composite tissue, composed of the nucleus pulposus (NP),the annulus fibrosis (AF), and the cartilaginous end plate (EP). NP cells exist in a gelatinous matrix composed of collagen 2 and proteoglycans, vital in resistance to compressive axial forces and pressure on the spine.14,15 AF cells exist in a collagen 1–rich matrix that resists transverse expansion of the IVD during spinal loading. EP cells are chondrocytes embedded in a hyaline cartilage matrix that binds the disc to the overlaying vertebral bones.14 The mechanical functions of the disc are governed by the extracellular matrix, composed primarily of two major macromolecules, collagen and aggrecan. Collagen provides tensile strength to the disc and anchors the tissue to the bone. Aggrecan, the major proteoglycan of the disc, is responsible for maintaining tissue hydration through osmotic pressure regulation.13 Maintaining the integrity of the extracellular matrix is essential for a healthy, normal disc.
Figure 1.
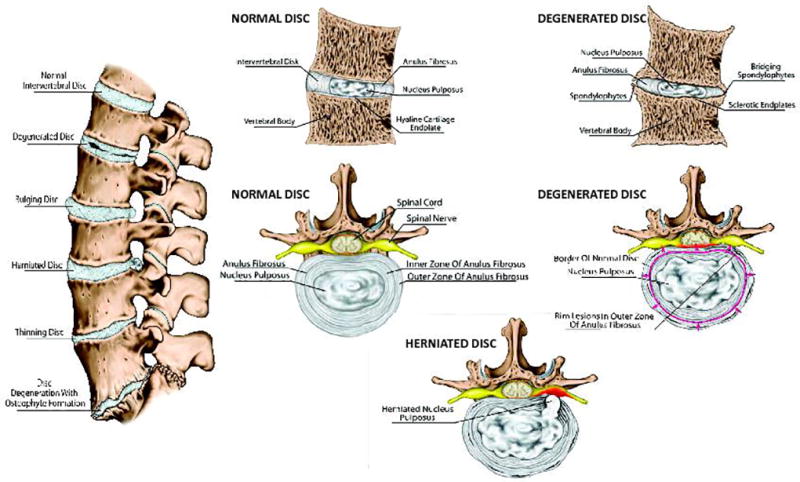
Spine and intervertebral disc anatomy in sagittal and cross-sectional views of healthy, degenerate, and herniated discs. Other potential disc changes are also shown in the sagittal view.
Disc degeneration results from changes in the architecture and biochemical configuration of the disc, altering the disc’s ability to bear load. The disc, which normally acts as a shock-absorbing cushion between the vertebrae, becomes more compressed and loses flexibility with degeneration. A noteworthy biochemical modification that occurs with disc degeneration is the degradation of aggrecan, resulting in the loss of proteoglycan and tissue hydration. This results in the loss of glycosaminoglycans, which in turn results in the decrease of osmotic pressure of the disc matrix. In the degraded state, because of the lack of hydration, the disc’s load-bearing function is altered.13 These changes in the matrix lead to reduced proteoglycan synthesis, increased collagen synthesis with a switch to fibrillated tissue quality, and an increase in synthesis and activity of matrix degrading enzymes, such as metalloproteinases (MMPs) and A disintegrin and metalloproteinase with thromobosopondin motifs (ADAMTS). Degradative changes in the AF include delamination of the lamellae and increased likelihood for radial fissures.16 Consequently, degenerated discs have less disc height and aberrant mechanical responses to loading. Though normally avascular, changes in tissue integrity allow for increased vascular and neural in-growth of the disc, which can become a source of peripheral neuropathy producing pain, weakness, and numbness due to nerve damage.17 This phenomenon progressively worsens with advancing age and has a strong influence on other spinal structures, predisposing them to injury. Tears in the AF caused by degeneration or an unrelated trauma lead to disc bulge or herniation, in which inner disc material protrudes into the spinal canal, eliciting pain as disc tissue compresses nerve roots.17 Nerve compression can also be a source of pain upon narrowing of the spinal canal. This narrowing may occur because of hypertrophy of surrounding soft tissue structures or ossification of the facet joints. Narrowing may also occur owing to bone spur formation (i.e., osteophytes). Entrapment of the nerve roots due to spinal stenosis may also be a cause of LBP, whereas compression of the microvasculature of the nerve roots can result in ischemia.18 However, the full complexity of the pain mechanisms underlying these conditions remains unclear.
In light of the limitations with the current standards of care, there has been a great deal of interest in the field of biomarkers over the past few decades. These biomarkers represent novel tools for directing patient care. In addition, if they are found to be sensitive indicators of true pain, they may allow us to separate those with psychological and secondary gain issues from those with a true lumbar etiology of pain. Biomarkers are characteristics that are objectively measured and evaluated as indicators of either normal or pathogenic biological processes or of response to therapeutic intervention.19 Though there are many types of biomarkers that can be indicative of health or disease, this review focuses on molecular biomarkers. Biomarkers can be identified in tissue, blood, urine, and other bodily fluids. The use of biomarkers can lead to individualized diagnosis and treatment. While markers of disease may be indicative or predictive of disease, they are not strictly required to be mediators of the disease process. Nevertheless, understanding the expression, function and role of certain proteins in cells, tissues, and fluids is typically a common starting point for evaluating potential biomarkers. Defined as alterations in the constituents of tissues or body fluids, biomarkers offer the means for homogeneous classification of a disease and risk factors, and they can extend our understanding of the underlying pathogenesis of disease. Biomarkers can also reflect the entire spectrum of disease from the earliest manifestations to the terminal stages and serve to reduce disease heterogeneity in epidemiologic studies or clinical trials. Lastly, biomarkers may serve to target certain individuals with specific therapies that may be efficacious for their specific state or type of disease.
In the context of serum protein analysis, proteins from damaged or recovering cells can enter the bloodstream, and these alterations in the blood can easily be measured. Serum proteins can serve as indicator of pathogenesis, disease progression, and/or treatment response. In certain conditions, the mere presence of a biomarker may be sufficiently indicative of disease or diagnostic, whereas, in other cases, changes in the levels of a biomarker may be necessary to be diagnostic. Biomarkers used for screening or diagnosis also often represent surrogate manifestations of the disease. Depending on the target, serum biomarkers tend to have high sensitivity and specificity in measurement quality. Nevertheless, in order to have wide utility, biomarker analysis must be validated and standardized for reproducibility.20 These characteristics make blood biomarkers (e.g., serum, plasma) a candidate tool for diagnosis, with the key benefits of ease of sample collection (blood draw) and with accuracy, repeatability, and scalability of biomarker measurement to outweigh the complexity and invasiveness of obtaining a more organ-specific biological sample from patients (e.g., tissue biomarkers).
The use of serum biomarkers has revolutionized diagnosis and treatment of many diseases, including heart disease, rheumatoid arthritis, and osteoporosis. There is a growing body of literature suggesting the potential for the use of serum biomarkers for diagnosis and treatment of LBP due to intervertebral disc pathologies. Mature disc tissue is normally avascular and aneural. As the disc begins to degenerate, the cells synthesize and release neurogenic factors that promote neural and vascular ingrowths into the disc tissue.21 The nerve growth factors also function as chemotactic agents for endothelial cells that promote vascular ingrowth and pain. Painful discs have a higher density of nerve fibers and capillaries penetrating deeper into the disc tissue.51 Radial fissures, proteoglycan loss, and reduced pressure in the NP are hallmarks of these innervated discs.22 Proteoglycans are proposed to act as a barrier to vascular and neural ingrowths into the NP, and studies have suggested that excessive metalloproteinase activity and matrix degradation of these proteoglycans act as stimuli for vascular and neural ingrowths into the disc.23 This increased vascularity in the degenerating disc may lead to biological and chemical changes, from disc remodeling to overstimulation of nociceptors within the granular tissue. Additionally, the intracellular signal transduction in response to disc cell stress from adverse mechanical forces leads to increased expression of genes and release of proteins into the circulation as well.24 Over the last decade, there has been great deal of interest in measuring these serum proteins as potential biomarkers for LBP pathologies.
This paper reviews literature studies on blood biomarkers of LBP performed on patients and other human subjects. The biological focus of these studies is on mediators of inflammation, because inflammation is central to pain and spinal degenerative mechanisms, as reviewed elsewhere;22,25 however, other non-inflammatory biomarkers are discussed when relevant. Figure 2 summarizes the current hypotheses concerning how inflammatory mediators contribute to LBP.22,25 A number of triggering events, including mechanical trauma, deformity, genetic disposition, infection, and smoking induce proinflammatory signaling in IVD cells. These initializing triggers result in morphologic changes in the disc tissue and surrounding structures, which propagate a cycle of further inflammatory signaling and degeneration. The activation of immune cell infiltration into the disc, along with nerve compression, nerve growth and sensitization, and ingrowth all contribute to the production of pain via mechanisms mediated if not triggered by inflammation. Since inflammation contributes to the pathogenesis of LBP, proinflammatory cytokines can serve as molecular biomarkers of pathological processes associated with disc degeneration, disc herniation, and LBP. A growing literature suggests that the presence of these inflammatory mediators can be measured systemically in the blood of patients with LBP. The identification of measurable serum biomarkers could revolutionize diagnostic and therapeutic processes for LBP across diagnoses, especially for those with undefined origins of LBP symptoms. Studies in the literature are presented for patients/subjects who have been diagnosed with a specific source of LBP. However, a significant number of historical studies have been published that do not define the diagnosis of back pain. Therefore, the studies are subdivided into those performed on populations with undefined origins of LBP and well as those performed on patients who are diagnosed with specific disease conditions (e.g., disc herniation, spinal stenosis, facet osteoarthritis, degenerated intervertebral disc). Since the natural history of back pain and associated disease mechanisms are dynamic, the temporal nature of literature studies are categorized based on duration of symptomology/disease (i.e., acute versus chronic).
Figure 2.
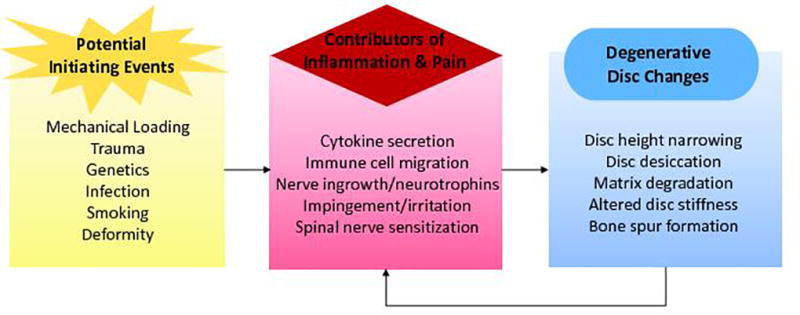
Summary of potential triggers, mediators, and disc consequences of inflammation and degradation contributing to LBP.
Results
Studies of LBP in patients with undefined diagnoses
Acute and subacute
High-sensitivity C-reactive protein (hsCRP) was the first described acute phase protein and is a systemic marker of inflammation. HsCRP serum levels have been positively associated with pain levels in acute LBP profiles.26,27 Sturmer et al. reported a difference in mean hsCRP levels in acute LBP patients by pain category; those in higher pain categories exhibited higher hsCRP levels.26 Gebhardt et al. found an analogous relationship between pain and hsCRP in which a decrease in both CRP and pain level was recorded in the initial clinical period, with an increase in functional capacity. However, this effect was not preserved beyond the acute phase, as a long-term reversal of this effect was observed at a 6-month follow-up, reinforcing the role of hsCRP as an acute phase marker as opposed to being involved in chronic inflammation.27
In addition to hsCRP, tumor necrosis factor α(TNF-α), soluble TNF receptor 1 (sTNFR1), and interleukin 6 (IL-6) have been identified as possible regulators of different aspects of acute LBP. TNF-α and IL-6 are both proinflammatory cytokines that have long been studied in mechanisms of disc degeneration and are also well-established mediators of nociception.28–30 IL-6 helps mediate the acute-phase response to injury by promoting monocyte differentiation into macrophages and activating maturation of lymphocytes.31 TNF-α is a cytokine that can stimulate inflammatory responses, induce nerve swelling and neuropathic pain, and promote cellular apoptosis via its cytotoxic effect.30 In a study by Queiroz et al., which assessed associations between plasma levels of inflammatory cytokines and pain and disability, it was found that TNF-α, sTNFR1, and IL-6 were all positively correlated with measures of pain intensity or severity. Additionally, IL-6 and sTNFR1 were correlated with LBP frequency, and IL-6 and TNF-α were correlated with disability due to LBP. These inflammatory markers, therefore, may be appropriate for assessment of acute LBP. However, this study population was formed from a subsample of elderly women, which limits the generalizability of these findings.32
Muscle damage and injury, which can be concurrent with instances of acute undefined LBP, have been shown to be associated with metabolic and inflammatory responses. The biochemical milieu of select inflammatory mediators, neuropeptides, and cytokines were found to be higher in subjects with painful muscle injury compared with those with latent injury or absent any pain.33–36 Moreover, exercise-induced injury models have found elevated serum levels of cytokines (e.g., IL-6) released by injured skeletal muscle fibers;33–36 however these levels were highly correlated with muscle metabolic products (e.g., creatine kinase). Thus, the combination of inflammatory cytokines measured with muscle metabolism biomarkers may be used to differentiate the specific contributions of acute muscle injury to undefined back pain.
Chronic
Much of the research surrounding LBP and associated inflammation focuses on chronic presentation of symptoms and are analyzed on the basis of symptom severity. Multiple studies have observed significant differences in proinflammatory cytokines (e.g., IL-6, TNF-α, IL-8, and IL-1β) in relation to pain intensity. By grouping patients on the basis of severity of patient-reported outcomes (e.g., pain), it was found that serum protein levels and serum mRNA levels of IL-6, IL-8, and TNF-α are all significantly higher in patients who experience categorically worse levels of LBP.37–39 Uçeyler et al. compared cytokine profiles of patients with painful versus painless neuropathies and identified higher levels of proinflammatory cytokines in the serum of patients experiencing pain.37 The study by Licciardone et al. found a similar trend and focused on IL-6 as a key player in LBP pathology, as it was seen to be significantly correlated with both pain severity and a measure of somatic dysfunction, along with IL-1β.39 Wang et al. further examined these trends, dividing the patient population into groups of mild and severe sciatica. IL-6 and IL-8 were both found to be greater in severe sciatica patients compared not only with controls but also with mild sciatica patients, suggesting that proinflammatory cytokine levels may contribute to sciatica intensity. IL-6 was also positively correlated with somatic dysfunction (r = 0.394, P = 0.013) as measured by the Oswestry Disability index (ODI).38
Conversely, anti-inflammatory markers, including IL-4 and IL-10, have been observed to be significantly lower in subjects experiencing lesser or no LBP. These cytokines are produced by activated macrophages and monocytes and considered to be anti-inflammatory because they can inhibit proinflammatory cytokine synthesis. In the study by Uçeyler et al. higher serum mRNA levels of IL-10 and IL-4 were found in painless neuropathy patients. Additionally, Wang et al. found elevated levels of IL-4 and IL-10 in mild sciatica patients as compared with severe cases. IL-10 was also negatively correlated with ODI. This suggests that there are analgesic effects of anti-inflammatory cytokines, which may also indicate a more favorable outcome to treatment.37,38
As a sensitive systemic marker of inflammation, which is upregulated by IL-6, hsCRP may also be involved in chronic LBP (cLBP), even though it is characterized as an acute-phase reactant. HsCRP has been studied in both surgical and non-surgical cLBP patients. Rannou et al. studied patients with cLBP on the basis of changes in vertebral endplate or modic changes40 observed on magnetic resonance imaging (MRI). Modic changes refer to pathological changes in the vertebrae, occurring both in the body of the vertebrae and in the endplate of the neighboring disc. Modic type 1 changes are indicative of inflammation and edema, without trabecular damage or marrow changes in the vertebra. Modic type 2 changes are indicative of bone marrow changes, where red cellular marrow gets substituted with fatty infiltration.41 Modic type 3 changes are less common and are indicative of fractures of the trabecular bone, along with trabecular shortening and widening. Rannou et al. found that serum hsCRP levels were higher in those patients with modic type 1 changes compared with those with modic type 2 changes or no modic changes. In addition, symptom duration and pain measures tended to be greater in these patients as well.42 In patients undergoing surgery, preoperative hsCRP levels have been positively correlated with worse postoperative outcomes measured by a Back Disability Score. This indicates that patients with higher preoperative hsCRP levels may show poor postoperative recovery due to persistent inflammation.43 This demonstrates great diagnostic potential for hsCRP. Nevertheless, other studies provide evidence that is counter to the notion of hsCRP as a marker of chronic degeneration. In a study of radicular back pain before and after epidural steroid injection, no correlation between hsCRP and visual analogue scale (VAS) score or clinical improvement was found. The authors thereby suggest that hsCRP is not associated with cLBP, and if any significant correlations were observed they could be explained by confounders of inflammation, such as body mass index (BMI), revealing no association after adjustment for such a covariate.44 In addition, although Gebhardt et al. supported hsCRP as an acute phase marker of LBP, they did not report hsCRP as having any major clinical relevance when evaluating cLBP.27
TNF-α has also been evaluated as another possible mediator in the pathology of cLBP. Serum protein levels and mRNA expression of TNF-α have been shown to be significantly higher in subjects experiencing greater intensity of cLBP. TNF-α has been associated with pain qualities, such as pain intensity and disability/ODI. A correlation between TNF-α and current pain intensity, as well as “severity of pain in the last week,” was observed through the use of the McGill Pain Questionnaire.32 Uçeyler et al. reported about twofold higher TNF protein levels in patients with painful neuropathies compared with painless neuropathies and healthy controls.37 In a study that categorized patients by severity of sciatica pain, those with severe sciatica showed twofold higher TNF-α serum levels than those without sciatica. In addition to its correlation to pain severity, TNF-α has been positively correlated with ODI and therefore disability related to LBP.38 Wang et al. performed a prospective comparative longitudinal study and found that there was a significantly higher proportion of TNF-α+ participants in the patient group than in the control group, which was sustained through multiple time points over a 6-month follow-up.45,46 They further investigated the cross-sectional associations of depressive symptoms as a comorbidity that can contribute to levels of cLBP and potentially to inflammation. They found that although elevated TNF-α is associated LBP, these levels were not modulated by depression in patients.45,46
Regulated on activation, normal T cell expressed and secreted (RANTES), another marker of inflammation, has also been investigated in relation to cLBP. RANTES is expressed in response to inflammatory stimuli and leads to catabolic activity. Sowa et al. found significant associations between levels of RANTES after activity and pain levels and pain-related functioning. They found that higher RANTES levels were correlated with higher affective scores, a measure of pain interpretation. This indicates an important role of this systemic biomarker in the experience of cLBP. Furthermore, it was concluded that higher levels of RANTES are associated with greater impairment and activity limitation, suggesting a link between inflammation and activity-related disability.47
In addition to studying systemic cytokines in the inflammatory response of cLBP, T lymphocytes have also been examined as key players in chronic pain. It was found that alterations in helper T cell subsets were associated with cLBP via pain and disability measures. CD4+ T cell lineages can be broken down into two cell subsets: TH17 and regulatory T (Treg) cells. Both T cell subsets have a role in the development of inflammatory diseases and were recently indicated to be involved in chronic pain.48 These immunological pathways exist in a paradigm in which the two T cell subsets counter one another in the formation (TH17) or the suppression (Treg) of autoimmunity.49 TH17 cells exert proinflammatory effects, while Treg cells function to restrain excessive effector T cell response. A decreased ratio between Treg cells and TH17 cells reported by Luchting et al. was characterized by elevated Treg counts with a decrease in TH17 cell counts. This imbalance led the authors to suggest that cLBP is associated with immune suppression, in contrast to the notion that immune system is overactivated in LBP.48
Disc herniation
Some LBP studies have been performed on subjects with undefined origins of pain, and thus the findings are presented in the context of one or more diagnostic codes. Disc herniation is the most commonly presenting diagnosis in lumbar spinal diseases. Disc herniation is broadly defined as localized or central displacement of disc tissue beyond the limits of the intervertebral disc, with local pain, with or without sciatica, due to mechanical compression and biochemical activity upon nerve roots.50 Disc herniation occurs most commonly when the NP protrudes through the surrounding AF. While, in most instances, degenerative changes must occur before disc herniation is initialized,51 studies on disc herniation biomarkers are typically performed on patients with incidental findings of painful herniated disc.
When looking at inflammatory biomarkers, earlier studies found that concentrations of IL-1β, IL-6, IFN-γ, and TNF-α in the serum and cerebrospinal fluid were within normal levels in patients with lumbar disc herniation at the time of surgery, when compared with historical reference levels of these cytokines.52 More recent studies, however, have challenged this concept. Park et al. found that mean serum concentrations of IL-2, IL-6, IL-8, TNF-α, soluble activation-inducible TNFR family receptor (AITR), and AITR ligand (AITRL) were all significantly higher in patients with lumbar disc herniation compared with control subjects.53 Kraychete et al. also measured IL-8, IL-1, TNF-α, IL-6, and sTNF-R levels in blood and cerebrospinal fluid in patients with chronic pain due to disc herniation. Despite the small number of subjects in this study (N = 23), the patients with cLBP and disc herniation exhibited significantly higher levels of TNF-α and IL-6 but not of IL-1 or sTNF-R.54
Pedersen et al. evaluated the serum levels of IL-6 and IL-8 in patients with lumbar radicular pain due to disc herniation longitudinally. Samples were collected at subject recruitment and at 6-week or 12-month follow-up time points, regardless of the treatment they received. They compared serum levels of IL-6 and IL-8 with patient-reported pain intensity on a 10-cm VAS. They found that chronic lumbar radicular pain may be associated with a persistent increase of the proinflammatory cytokines IL-6 and IL-8 in serum after disc herniation, suggesting that these cytokines may be associated with the mechanisms underlying development of chronic pain due to disc herniation.55
Since the disc is normally aneural and avascular, it is considered an immunoprivileged tissue in healthy conditions. When disc herniation or rupture occurs, the exposure of immunoprivileged tissue and cells with the systemic environment may result in a response that mimics autoimmune responses. Cheng et al. conducted a study investigating the involvement of TH17 lymphocytes in disc herniation through the study of peripheral blood from patients with ruptured and non-ruptured lumbar discs and healthy controls. The results demonstrated that patients with disc herniation exhibited a significant increase in peripheral TH17 frequency and IL-17 expression compared with healthy controls. Furthermore, peripheral TH17 frequency and IL-17 expression in patients with ruptured discs were much higher than in those with herniated discs. IL-17 is thought to be a major effector cytokine of TH17 cells, and may induce the production of inflammatory cytokines. Indeed, disc herniation initiates an autoimmune reaction accompanied by increased levels of TH17 cells and IL-17, leading to inflammation, further deterioration, and increased pain. This process is more pronounced in cases of disc rupture, which may be one of the reasons patients with a ruptured disc experience greater pain than those without rupture.56 IL-21 controls the functional activity of effector T helper cells and the differentiation of TH17 cells and promotes B cell differentiation. Xue et al. investigated the involvement of IL-21, IL-17, and COX-2 in disc herniation by evaluating peripheral blood and disc tissue samples from disc herniation patients and healthy controls. They demonstrated that disc herniation patients exhibited significantly higher levels of serum IL-21 and IL-17. Moreover, higher expression of IL-21, IL-17, and COX-2 was found in protein and mRNA levels in disc tissues from disc herniation patients than in normal disc tissues. VAS pain scores, IL-17, and COX-2 were positively correlated with the IL- 21 levels, implicating its role in the pathogenesis of lumbar disc herniation.57
Other novel mediators of disc inflammation and degeneration have also been investigated as biomarker targets. Xie et al. used proteomic analysis of blood samples to establish whether there are serum proteins associated with disc herniation, which may be useful in elucidating pathogenesis. Two-dimensional electrophoresis of blood samples from patients or control subjects was conducted, and distinct protein spots were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Results indicate that apolipoprotein-L1 (APO-L1), apolipoprotein M (APO-M), tetranectin (TN), and immunoglobulin light chain (IGL) differed in patients with lumbar disc herniation. They found that mean serum concentrations of APO-M, TN, and IGL were significantly lower in patients with disc herniation, whereas levels of APO-L1 were significantly higher.58 Extremely little is known with regard to APO-L, APO-M, and IGL in disc herniation. Although TNF-α is believed to play a key role in inflammation, certain studies have shown that TNF-α levels in the serum are not significantly different in herniation compared with healthy patients.52 In these circumstances, the level of APO-L may be a reflection of the TNF-α secreted into the serum and may serve as a biomarker for disc herniation. TN is a plasminogen-binding protein present in plasma and extracellular matrix and is thought to be associated with regeneration of the lumbar disc after initial insult.58
In a more comprehensive approach, Moen et al. also conducted a profiling study for serum levels of 92 inflammatory proteins in patients 1 year after lumbar disc herniation. Thirteen proteins were identified to be significantly upregulated in patients with severe pain 1 year after disc herniation, using a false discovery rate (FDR) of 0.05. They then looked at the profiles of patients with VAS > 6 at 12-month follow-up and defined them as a high-pain group, versus gender- and age-matched patients with VAS < 1 at 12-month follow-up, defined as a low-pain group. Using linear discriminant analysis, they showed a clear overall difference in the serum cytokine profile between the chronic and the recovered patients.59 These studies show that serum protein profiling of patients with differing LBP pathologies has the potential to be used to direct prognosis and choice of treatment.
Degenerative disc disease, spinal stenosis, and facet arthritis
Some degenerative disc changes are contained within the spinal column and are diagnosed by radiological indication of disc height loss, loss of water intensity on MRI, and/or formation of osteophytes/calcification indicated on X-ray or computed tomography (CT) scans. MRI remains the diagnostic modality of choice for imaging patients with LBP and suspected degenerative disc disease or disc herniation. MRI is noninvasive, requires no contrast agent, has superior soft tissue detail, and uses no ionizing radiation, unlike CT. In particular, T2-weighted (T2W) MRI is excellent at detecting the loss of normal disc space signal indicative of desiccation and degenerative disc disease. Pfirrmann et al. developed a grading system for gross morphology of disc degeneration based on standard T2W spin echo pulse sequences, which is one of the most commonly accepted methods of evaluating degenerative disc disease. This grading system ranks discs on a scale of I–V, with V being the most severely degenerated disc. The grading is based on MRI signal intensity, disc structure, distinction between nucleus and annulus, and disc height; with disc height being a discriminative feature between grade IV and V discs, but not between III and IV.60 Although this method is frequently used, it has historically been shown to be deficient at detecting early degenerative changes and does not always match with patient-reported symptoms.
From the Johnston County Osteoarthritis Project Database, Goode et al. investigated associations between radiographic features of degenerative disc disease, such as lumbosacral spine disc space narrowing and osteophytes with matrix degradation biomarkers. They found significant differences in mean biomarker levels across severities of degenerative disc disease for hyaluronic acid, collagen neoepitope, and urine C-terminal cross-linking telopeptide (CTX-II), with levels of these matrix biomarkers increasing with increasing disease severity.61 In a subsequent study, they went on to identify differences in two types of lumbar spine degeneration––facet joint arthritis versus lumbar spine osteoarthritis––and found that levels of hyaluronic acid were higher in facet joint arthritis and that CTX-II levels were greater in spine OA.61
By looking further at inflammation markers, Weber et al. found that serum levels of IL-6 were significantly higher in subjects with degenerative disc disease compared with control subjects, even when controlling for covariates, such as age and BMI.62 Interestingly, levels of IL-6 did not vary with severity of degeneration on MRI as indicated by Pfirmann grade. This was not particularly surprising, when taking into consideration that conventional MRI does not reliably diagnose early stages of disc degeneration, and the Pfirrmann grading system has poor sensitivity for the detection of early degenerative changes in the disc.63–65 Radiological imaging, while it helps, is not sufficient. Many asymptomatic patients may appear severally degenerated on MRI, while the opposite may be true for a symptomatic patient. Early stages of degenerative disc disease are characterized by biochemical changes rather than the morphological changes, such as loss of disc space height, that are more readily identified on standard non-contrast T2W66–70 and normal disc space signal on the long repetition time (TR) scans. More sensitive imaging modalities may serve to provide stronger relationships with systemic biomarkers. For example, T1ρ imaging is an emerging MRI imaging modality that may allow for the earlier detection of degenerative disc disease, as it is able to detect quantitative changes in the disc space matrix, specifically the loss of proteoglycans.71–74 In vivo studies have shown a correlation between T1ρ values and degenerative grade in an asymptomatic patient population at 1.5 T.75 A study on a small group of symptomatic patients at 3.0 T correlated T1ρ values and disc degeneration.71 A study by Filippi et al. also quantified T1ρ values and found them to be correlated with Pfirrmann grades and to significantly decrease with increasing age.72
Ye et al. found that IL-18, a regulatory cytokine that degrades the disc matrix, is significantly increased with increasing grade of IVD degeneration.76 There was a dramatic alteration in IL-18 levels between the advanced degeneration group, when subjects with grade III–V severity were grouped together, compared with the normal group. In another study, Grad et al. evaluated serum levels of the chemokines C-C motif ligand 5 (CCL5, also known as RANTES) and C-X-C motif ligand 6 (CXCL6, also known as granulocyte chemotactic protein 2). In the context of the disc, these chemoattractants can be released by damaged disc cells. The investigators found that systemic levels of both factors increased in patients with disc degeneration compared with controls without disc degeneration;77 however, a relationship with disease severity was not evaluated. Receiver operating curve analysis was performed to evaluate the diagnostic sensitivity and specificity of these two potential biomarkers. The area under the curve (AUC) was computed for both factors, and CXCL6 had slightly better diagnostic accuracy than CCL5, which had an AUC near the chance level of diagnosis (i.e., AUC = 0.5).77
Recent studies have also begun to question the possibility that serum cytokine levels vary between diagnoses of disc diseases or by subtype within a single diagnosis that represents classifications that are different from severity of disease. Deng et al. conducted a meta-analysis of literature studies to examine the relationship between IL-6 serum levels and intervertebral disc degeneration (IDD). Eight case-control studies met the inclusion criteria, with a total of 392 subjects, of which 263 were patients with IDD and 129 were healthy controls. A meta-analysis demonstrated that serum IL-6 protein expression levels may be associated with IDD; however, this was irrespective of IDD subtype.78 It should be noted, however, that the IDD classification used (bulging, protrusion, or sequestration) are more commonly accepted to be subtypes of disc herniation specifically, not IDD.50 Nevertheless, serum expression levels of the IL-6 protein were upregulated in protrusion subjects, as compared with normal disc subjects; thus suggesting that IL-6 may have an important role in a manner that depends on the subtype of degeneration or herniation.78
Weber et al. provided direct experimental evidence to support this notion that serum inflammatory cytokine levels vary by diagnosis subtype. In that study, serum cytokine levels were compared in patients with varying IDD pathologies. They found that serum levels of IL-6 were significantly higher in subjects with LBP compared with control subjects and further found that patients with degenerative disc disease and spinal stenosis had significantly higher IL-6 levels then patients with disc herniation, even when controlling for covariates, such as age and BMI.62 In a subsequent study, a more comprehensive profile was performed, which found that levels of many proinflammatory cytokines and growth factors were higher in stenosis and degenerative disc patients compared with disc herniation patients.79 These factors included IL-2, IL-3, IL-8, hepatocyte growth factor (HGF), interferon (IFN) α2 (IFN-α2), leukemia inhibitory factor (LIF), monocyte chemoattractant protein 3 (MCP-3), and TNF-β. The use of diagnostic profiling or biomarker panels may serve to enhance the specificity and accuracy of diagnosis.
Biomarkers and response to treatment
One potential utility for diagnostic biomarkers may be in precision medicine (i.e., to guide a more personalized therapeutic plan). To achieve this, changes in biomarkers in response to treatment must be demonstrated and validated. Several studies have extended the study of systemic cytokines to evaluate changes with various treatment modalities ranging from minimally invasive to surgical treatment. This concept is based on the premise that inflammatory cytokine levels may modulate treatment responses. For example, Schistad et al. found that high serum IL-6 levels were associated with less favorable recovery in patients with lumbar radicular pain when evaluating them using the Oswestry Disability Index (ODI) or VAS for LBP and leg pain.80
In cLPB patients, Licciardone et al. found that patients who underwent a treatment regimen of osteopathic manipulation therapy (OMT) exhibited reduced levels of TNF-α from pre- to postoperative measures compared with those patients who did not undergo OMT. This trend was reported as most evident in patients who achieved positive clinical outcomes with reference to pain severity and back-specific functioning, as measured by VAS and the Roland-Morris Disability Questionnaire and Medical Outcomes Study Short Form-36 Health Survey, respectively.39 Zu et al. measured serum levels of TNF-α and IL-4 in patients with lumbar radiculopathy at baseline and 1 and 12 months after microdisectomy. Cytokine levels were analyzed on the basis of pain intensity in the high-pain (VAS ≥ 3) group compared with the low-pain (VAS < 3) group. TNF-α blood protein levels were higher in the high-pain group than those in the low-pain group, while IL-4 was higher in the low-pain group. TNF-α decreased in both VAS groups over time. In contrast, IL-4 increased in both groups at 1 month and then decreased gradually until month 12. The changes in serum levels of TNF-α and IL-4 over time between the high-pain and low-pain groups were significantly different. This study suggests that elevated anti-inflammatory cytokines in patients with lumbar radiculopathy may be indicative of a favorable outcome.30 The subtle process of IL-4 elevation and decline during the follow-up period suggests a protective mechanism that is analgesic for neuropathic pain.
Weber et al. conducted an exploratory study to identify systemic biomarkers that correlate with patient-reported outcomes after epidural steroid injections. At early follow-up (7–10 days posttreatment), changes in systemic cytokine levels from pre- to posttreatment were dependent on the diagnosis cohort. In an effort to identify potential predictors of pain response to epidural steroid treatment, changes in systemic cytokines were analyzed with regard to change in pain in two diagnosis cohorts. Each cohort was associated with a unique profile of factors that significantly correlated with change in pain. Loss of pain in patients with spinal stenosis and degenerative disc disease correlated with decreases in chemokines (MCP-1, MIG) and factors that participate in mechanisms of angiogenesis (HGF, VEGF), inflammation (IL-1β, IL-1ra, IL-9, IL-12, TRAIL), and nociception (SCF, IFN-α2). On the other hand, disc herniation patients had an improvement in pain that correlated with decreases in factors that participate in hematopoiesis (SCGF-β, GM-CSF), nociception (SCF, IFN-α2), and inflammation (IL-6, IL-10, IL-18, IL-2Rα, IL-12p40).111
Conclusions
There have been a number of promising research findings in the field of biomarkers of LBP and disc diseases. Many potential targets have been explored and have the potential to guide diagnosis and therapeutics. Continued research and validation of relevant, accurate, and sensitive biomarkers of disc diseases is of high public health importance. Having the ability to discover pathologic processes in the disc at early stages will allow physicians to intervene earlier in the course of disease, offer more relevant treatment options, and decrease the likelihood of failed treatments, which are major potential benefits of biomarker development. This will have a significant impact on disease burden, relieving pain and avoiding surgical procedures that may not ultimately be found to be therapeutic. Blood biomarkers may help to stratify patients and optimize diagnostic processes. They also have the potential to identify the true physical causes of pain and eliminate psychological and secondary pain issues. In addition, the use of serum biomarkers has the potential to identify more individualized treatments with enhanced efficacy in certain patient populations. There are a number of specific anti-inflammatory drugs on the market that could be applied in a tailored treatment plan, including inhibitors of TNF-α (e.g., infliximab, adalimumab, and etanercept) or IL-6 (e.g., tocilizumab).81–83 The efficacy of these drugs in treating LBP is still being evaluated, but continued studies in biomarkers may be useful in selecting patients for these anti-inflammatory treatments.
One potential limitation of biomarker development remains the lack of sensitivity of existing MRI imaging modalities to identify disc disease changes early in the disease process. Developing quantitative, reliable, and non-invasive in vivo biomarkers or imaging markers of disc degeneration that correlate with patients’ subjective complaints of back pain are needed. Another potential limitation in the development and validation of systemic biomarkers is the contribution of disease covariates. Evidence exists that covariates, such as age, BMI, and depression, may contribute to pain status, especially for individuals affected by chronic pain. Moreover, some of these covariates present changes in disease mediators that overlap with disc disease (e.g., inflammation). Consequently, evaluation and control for these covariates is warranted in clinical studies of systemic biomarkers in this field. Nevertheless, biomarkers that allow for earlier diagnosis of disc degeneration are needed to provide evidenced-based metrics for preventative interventions and could potentially be used to monitor disease progression or responses to therapeutic interventions, both surgical and nonsurgical. By putting multiple targets together, biomarker profiling is a powerful technology that will greatly accelerate progress toward novel diagnostic and predictive tools to track early disease and tailor treatments to specific patients.
Table 1.
Summary of biomarker studies of LBP due to unspecified diagnoses
| Year | Study | Comparator groups |
Number of subjects (N) |
Study design | Summary of significant findings |
Reference |
|---|---|---|---|---|---|---|
|
| ||||||
| 1997 | Bruunsgaard et al | Eccentric vs. concentric exercise | 9 | Randomized control trial | IL-6 increased after eccentric exercise. | 34 |
|
| ||||||
| 1998 | Ostrowski et al. | Pre- and postintense prolonged exercise | 16 | Randomized control trail | IL-6 increased post exercise: | 35 |
| Pre: 1.5 ± 0.7 pg/mL | ||||||
| Post: 94.4 ± 12.6 pg/mL | ||||||
|
| ||||||
| 2005 | Stürmer et al. | Acute LBP/cLBP: low vs. high pain | 72 (41 chronic, 31 acute) | Longitudinal – prospective | Acute LBP: increasing CRP with increasing pain (CRP: 1.6× higher in high-pain group). | 26 |
|
| ||||||
| 2006 | Gebhardt et al. | Acute LBP cLBP | 72 (41 chronic, 32 acute) | Longitudinal – prospective | Lower CRP with decreasing pain in acute LBP | 27 |
|
| ||||||
| 2007 | Rannou et al. | Modic 0, I, II changes | 85 | Cross-sectional – prospective | Serum hsCRP higher in modic I than 0 or II | 42 |
|
| ||||||
| 2007 | Uçeyler et al. | Painless vs. painful neuropathy | 32 painful, 20 painless, 38 healthy control | Cross-sectional – prospective | Painful group had 2× higher blood IL-2 and TNF-α mRNA levels than painless and control groups; | 37 |
| IL-10 mRNA levels 2× higher in painless group than painful. | ||||||
|
| ||||||
| 2008 | Wang et al. | Chronic LBP and control | 120 per group | Longitudinal – prospective | Higher proportion of chronic LBP patients had TNF-α level higher than 2 pg/mL as compared with controls | 45 |
|
| ||||||
| 2010 | Wang et al. | Chronic LBP and control | 29 per group | Cross-sectional – prospective | TNF-α levels were 25× higher in cLBP than controls. | 46 |
|
| ||||||
| 2011 | Park & Lee | Pre- vs. posttreatment with ESI | 55 | Cross-sectional – prospective | No correlation between hsCRP and VAS; | 44 |
| hsCRP reduced posttreatment compared with pretreatment. | ||||||
|
| ||||||
| 2012 | Goode et al. | Graded scale of disc space narrowing (DSN), osteophyte formation (OST) in patients with and without LBP symptoms | 547 | Retrospective | Type 11 collagen (C2C) was associated with DSN; | 61 |
| COMP was associated with DSN among patientss. with symptoms; | ||||||
| HA and C2C are correlated with DSN severity. | ||||||
|
| ||||||
| 2012 | Licciardone et al. | Undefined chronic LBP by pain severity and number of osteopathic lesions | 70 | Substudy nested within random control trial | IL-6 and IL-1β correlated with number of osteopathic lesions; | 39 |
| IL-6 correlated with LBP severity. | ||||||
|
| ||||||
| 2014 | Luchting et al. | Chronic LBP vs. controls | 10 | Cross-sectional – prospective | Increased frequency of Treg cells in CLBP patients compared with controls; | 48 |
| decreased frequency of TH17 in cLBP compared with controls. | ||||||
|
| ||||||
| 2014 | Rathod et al. | Lumbar disc disease undergoing surgery and controls | 50 lumbar, 50 control | Longitudinal – prospective | hsCRP almost 30× higher in lumbar disc disease patients compared with controls | 43 |
|
| ||||||
| 2014 | Sowa et al. | Before vs. after exercise | 43 | Cross sectional – prospective | RANTES correlated with pain and pain-related function after activity | 47 |
|
| ||||||
| 2016 | de Queiroz et al. | Acute LBP by pain and disability | 155 | retrospective | Positive correlations: | 32 |
| TNF-α and pain severity; | ||||||
| IL-6 and pain severity | ||||||
|
| ||||||
| 2016 | Deng et al. | DH vs. control | 10 | Cross-sectional – prospective | IL-6 higher in DH than controls; | 78 |
| Col II and aggrecan lower in DH than controls. | ||||||
|
| ||||||
| 2016 | Grad et al. | Degenerated vs nondegenerated | 80 | Case control – prospective | CCL5 was 1.6× higher in degenerated group compared with nondegenerated controls; | 77 |
| CXCL6 was 1.3× higher in degenerated group compared with controls | ||||||
|
| ||||||
| 2016 | Wang et al. | Severe vs. mild sciatica | 58 severe, 50 mild, 30 healthy control | Cross sectional – prospective | IL-6: 1.5× higher in severe than mild or control; | 38 |
| IL-8: higher in severe than control; | ||||||
| TNF-a: 2× higher in severe than mild or control; | ||||||
| IL-4: 2× higher in mild than severe or control; | ||||||
| IL-6: TNF-αa, IL-10 correlated with ODI | ||||||
Table 2.
Summary of biomarker studies of LBP due to disc herniation
| Year | Study | Comparator groups |
Number of subjects (N) |
Study design |
Summary of significant findings |
Reference |
|---|---|---|---|---|---|---|
|
| ||||||
| 2002 | Brisby et al. | DH | 39 | Cross-sectional – prospective | IL-1β, IL-6, IFN-γ and TNF-α were within normal ranges compared with historical control levels | 52 |
|
| ||||||
| 2007 | Park et al. | DH vs. controls | 20 | Cross-sectional – prospective | IL-2, IL-6, and IL-8 were 4× higher and TNF-α was 2× higher in DH compared with controls. | 53 |
|
| ||||||
| 2010 | Kraychete et al. | DH vs. controls | 23 | Cross-sectional – prospective | TNF-α: 6× higher in DH than controls; | 54 |
| IL-6: 4× higher in DH than controls | ||||||
|
| ||||||
| 2013 | Cheng et al. | DH vs. control; ruptured vs. non-ruptured disc | 34 | Cross-sectional – prospective | TH17 and IL-17 higher in DH vs. controls; | 56 |
| TH17 and IL-17 higher in ruptured vs. non-ruptured. | ||||||
|
| ||||||
| 2014 | Xie et al. | DH vs. control | 30 per group | Cross- sectional – prospective | APO-M, TN, and IGL lower in DH compared with controls, APO-L1 high in DH compared with controls | 58 |
|
| ||||||
| 2015 | Pedersen et al. | Low vs. high pain | 127 | Retrospective | IL-6 and IL-8 higher in high-pain group; | 55 |
| IL-6 decreased over time in high pain group. | ||||||
|
| ||||||
| 2015 | Weber et al. | DH vs. other Dx (DDD/SS); pre- vs. posttreatment | 16 | Longitudinal – prospective | SCGF-β and IL-2 were higher posttreatment in all groups compared with pretreatment; | 79 |
| IL-17 and VEGF decreased post treatment in DH; | ||||||
| change in pain correlated with change in MCP-1, SCF, IFN-α2, MIG, TRAIL, SCGF-β, IL-6, IL-10, HGF, IL-18; IL-2, IL-3, IL-8, HGF, IFN-α2, LIF, MCP-3, TNF-β, higher in other Dx compared with DH. | ||||||
|
| ||||||
| 2015 | Xueet al. | DH vs. control | 34 DH, 20 control | Cross-sectional – prospective | IL-21, IL-17: higher in DH vs. controls; IL-21 positively correlated with VAS (IL-17 & COX-2) in DH | 57 |
|
| ||||||
| 2016 | Moen et al. | High vs. low pain | 112 per group | Retrospective | MCP-3/CCL7 1.5×, M-CSF 1.2×, VEGF-A 1.47×, CXCL10 1.55×, MCP-2 1.56×, CXCL5 1.2×, CCL-4 1.4×, IL-15Ra 1.15×, MCP-4 1.7×, TGF-b1 1.5×, CASP-8 1.14×, EGF 1.14×, STAMPB 1.4× higher in high pain vs. low pain | 59 |
|
| ||||||
| 2016 | Weber et al. | LBP vs. control; DH vs. other Dx | 80 | Longitudinal – prospective | IL-6 1.57× lower in DH than other Dx | 62 |
Table 3.
Summary of biomarker studies of LBP due to disc degeneration
| Year | Study | Comparator groups |
Number of subjects (N) |
Study design | Summary of significant findings |
Reference |
|---|---|---|---|---|---|---|
|
| ||||||
| 2012 | Goode et al. | Graded scale of disc space narrowing (DSN), osteophyte formation (OST) in patients with and without LBP symptoms | 547 | Retrospective | Type II collagen (C2C) was associated with DSN; | 61 |
| COMP was associated with DSN among patients. with symptoms; | ||||||
| HA and C2C are correlated with DSN severity. | ||||||
|
| ||||||
| 2015 | Weber, et al. | DH vs. other Dx (DDD/SS); pre- vs. post treatment | 16 | Longitudinal – prospective | SCGF-β and IL-2 higher posttreatment in all groups compared with pretreatment; | 79 |
| IL-2Ra, IL-3, SCGF-β decreased posttreatment in other Dx; change in pain correlated with change in MCP-1, SCF, IFN-α2, MIG, TRAIL, SCGF-β, IL-6, IL-10, HGF, IL-18; IL-2, IL-3, IL-8, HGF, IFN-α2, LIF, MCP-3, TNF-β higher in other Dx compared with DH; | ||||||
|
| ||||||
| 2016 | Deng et al. | DH vs. control | 10 | Cross-sectional – prospective | IL-6 higher in DH than controls; | 78 |
| Col II and aggrecan lower in DH than controls. | ||||||
|
| ||||||
| 2016 | Grad et al. | Degenerate vs. nondegenerate | 80 | Case control –prospective | CCL5 was 1.6× higher in degenerated group compared with nondegenerated controls; | 77 |
| CXCL6 was 1.3× higher in degenerated group compared with controls | ||||||
|
| ||||||
| 2016 | Weber et al. | LBP vs. control; DH vs. other Dx | 80 | Longitudinal – prospective | IL-6 1.4×higher in LBP than controls; | 62 |
| MMP-1 1.56× higher in LBP than controls; | ||||||
| IL-2 2× lower in LBP than controls; | ||||||
| IL-4 1.5× higher in LBP than controls; | ||||||
|
| ||||||
| 2016 | Ye et al. | IVD degeneration vs. control | 40 | Cross-sectional – prospective | IL-18 increased with increased degeneration | 76 |
Acknowledgments
This study was supported in part by NIH R01AR069668 and NSF CAREER Award 1151605. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Hoy D, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(6):968–974. doi: 10.1136/annrheumdis-2013-204428. [DOI] [PubMed] [Google Scholar]
- 2.Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine (Phila Pa 1976) 2006;31(23):2724–2727. doi: 10.1097/01.brs.0000244618.06877.cd. [DOI] [PubMed] [Google Scholar]
- 3.Walker BF. The prevalence of low back pain: a systematic review of the literature from 1966 to 1998. J Spinal Disord. 2000;13(3):205–217. doi: 10.1097/00002517-200006000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Hoy D, Brooks P, Blyth F, Buchbinder R. The Epidemiology of low back pain. Best Pract Res Clin Rheumatol. 2010;24(6):769–781. doi: 10.1016/j.berh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Deyo RA, Tsui-Wu YJ. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine (Phila Pa 1976) 1987;12(3):264–268. doi: 10.1097/00007632-198704000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Cassidy JD, Carroll LJ, Côté P. The Saskatchewan health and back pain survey. The prevalence of low back pain and related disability in Saskatchewan adults. Spine (Phila Pa 1976) 1998;23(17):1860–1866. doi: 10.1097/00007632-199809010-00012. discussion 1867. [DOI] [PubMed] [Google Scholar]
- 7.Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344(5):363–370. doi: 10.1056/NEJM200102013440508. [DOI] [PubMed] [Google Scholar]
- 8.Qaseem A, Wilt TJ, McLean RM, Forciea MA, Physicians CGCotACo Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166(7):514–530. doi: 10.7326/M16-2367. [DOI] [PubMed] [Google Scholar]
- 9.Chou R, Qaseem A, Owens DK, Shekelle P, Physicians CGCotACo Diagnostic imaging for low back pain: advice for high-value health care from the American College of Physicians. Ann Intern Med. 2011;154(3):181–189. doi: 10.7326/0003-4819-154-3-201102010-00008. [DOI] [PubMed] [Google Scholar]
- 10.Chou R. In the clinic. Low back pain. Ann Intern Med. 2014;160(11):ITC6–1. doi: 10.7326/0003-4819-160-11-201406030-01006. [DOI] [PubMed] [Google Scholar]
- 11.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354(9178):581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 12.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 13.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5(3):120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes AJ, Benjamin M, Ralphs JR. Extracellular matrix in development of the intervertebral disc. Matrix Biol. 2001;20(2):107–121. doi: 10.1016/s0945-053x(01)00125-1. [DOI] [PubMed] [Google Scholar]
- 15.Hutton WC, et al. Does long-term compressive loading on the intervertebral disc cause degeneration? Spine (Phila Pa 1976) 2000;25(23):2993–3004. doi: 10.1097/00007632-200012010-00006. [DOI] [PubMed] [Google Scholar]
- 16.Roughley PJ, Alini M, Antoniou J. The role of proteoglycans in aging, degeneration and repair of the intervertebral disc. Biochem Soc Trans. 2002;30(Pt 6):869–874. doi: 10.1042/bst0300869. [DOI] [PubMed] [Google Scholar]
- 17.Raj PP. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008;8(1):18–44. doi: 10.1111/j.1533-2500.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez JA, Hardy RH. Lumbar spine stenosis: a common cause of back and leg pain. Am Fam Physician. 1998;57(8):1825–1834. 1839–1840. [PubMed] [Google Scholar]
- 19.Group. BDW. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 20.Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1(11):845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 21.García-Cosamalón J, et al. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anat. 2010;217(1):1–15. doi: 10.1111/j.1469-7580.2010.01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wuertz K, Haglund L. Inflammatory mediators in intervertebral disk degeneration and discogenic pain. Global Spine J. 2013;3(3):175–184. doi: 10.1055/s-0033-1347299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson WE, et al. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis Rheum. 2002;46(10):2658–2664. doi: 10.1002/art.10585. [DOI] [PubMed] [Google Scholar]
- 24.Peng B, et al. The pathogenesis of discogenic low back pain. J Bone Joint Surg Br. 2005;87(1):62–67. [PubMed] [Google Scholar]
- 25.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stürmer T, et al. Pain and high sensitivity C reactive protein in patients with chronic low back pain and acute sciatic pain. Ann Rheum Dis. 2005;64(6):921–925. doi: 10.1136/ard.2004.027045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gebhardt K, et al. The course of high-sensitive C-reactive protein in correlation with pain and clinical function in patients with acute lumbosciatic pain and chronic low back pain - a 6 months prospective longitudinal study. Eur J Pain. 2006;10(8):711–719. doi: 10.1016/j.ejpain.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Gadient RA, Otten UH. Interleukin-6 (IL-6)--a molecule with both beneficial and destructive potentials. Prog Neurobiol. 1997;52(5):379–390. doi: 10.1016/s0301-0082(97)00021-x. [DOI] [PubMed] [Google Scholar]
- 29.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107(3):660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zu B, Pan H, Zhang XJ, Yin ZS. Serum Levels of the Inflammatory Cytokines in Patients with Lumbar Radicular Pain Due to Disc Herniation. Asian Spine J. 2016;10(5):843–849. doi: 10.4184/asj.2016.10.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shamji MF, et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62(7):1974–1982. doi: 10.1002/art.27444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Queiroz BZ, et al. Association Between the Plasma Levels of Mediators of Inflammation With Pain and Disability in the Elderly With Acute Low Back Pain: Data From the Back Complaints in the Elders (BACE)-Brazil Study. Spine (Phila Pa 1976) 2016;41(3):197–203. doi: 10.1097/BRS.0000000000001214. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen BK, Steensberg A, Schjerling P. Muscle-derived interleukin-6: possible biological effects. J Physiol. 2001;536(Pt 2):329–337. doi: 10.1111/j.1469-7793.2001.0329c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruunsgaard H, et al. Exercise-induced increase in serum interleukin-6 in humans is related to muscle damage. J Physiol. 1997;499(Pt 3):833–841. doi: 10.1113/jphysiol.1997.sp021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol. 1998;508(Pt 3):949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah JP, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89(1):16–23. doi: 10.1016/j.apmr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Uçeyler N, Rogausch JP, Toyka KV, Sommer C. Differential expression of cytokines in painful and painless neuropathies. Neurology. 2007;69(1):42–49. doi: 10.1212/01.wnl.0000265062.92340.a5. [DOI] [PubMed] [Google Scholar]
- 38.Wang K, et al. A cohort study comparing the serum levels of pro- or anti-inflammatory cytokines in patients with lumbar radicular pain and healthy subjects. Eur Spine J. 2016;25(5):1428–1434. doi: 10.1007/s00586-015-4349-4. [DOI] [PubMed] [Google Scholar]
- 39.Licciardone JC, Kearns CM, Hodge LM, Bergamini MV. Associations of cytokine concentrations with key osteopathic lesions and clinical outcomes in patients with nonspecific chronic low back pain: results from the OSTEOPATHIC Trial. J Am Osteopath Assoc. 2012;112(9):596–605. doi: 10.7556/jaoa.2012.112.9.596. [DOI] [PubMed] [Google Scholar]
- 40.Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 41.Toyone T, et al. Vertebral bone-marrow changes in degenerative lumbar disc disease. An MRI study of 74 patients with low back pain. J Bone Joint Surg Br. 1994;76(5):757–764. [PubMed] [Google Scholar]
- 42.Rannou F, et al. High-sensitivity C-reactive protein in chronic low back pain with vertebral end-plate Modic signal changes. Arthritis Rheum. 2007;57(7):1311–1315. doi: 10.1002/art.22985. [DOI] [PubMed] [Google Scholar]
- 43.Rathod TN, et al. High sensitive C-reactive protein-Effective tool in determining postoperative recovery in lumbar disc disease. Indian J Orthop. 2014;48(4):354–359. doi: 10.4103/0019-5413.136216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park CH, Lee SH. Prognostic usefulness of high sensitivity C-reactive protein for transforaminal epidural steroid injection in patients with radicular pain. Pain Med. 2011;12(2):219–223. doi: 10.1111/j.1526-4637.2010.01039.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Schiltenwolf M, Buchner M. The role of TNF-alpha in patients with chronic low back pain-a prospective comparative longitudinal study. Clin J Pain. 2008;24(3):273–278. doi: 10.1097/AJP.0b013e31816111d3. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, et al. Influence of depression symptoms on serum tumor necrosis factor-α of patients with chronic low back pain. Arthritis Res Ther. 2010;12(5):R186. doi: 10.1186/ar3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sowa GA, et al. Associations between serum biomarkers and pain and pain-related function in older adults with low back pain: a pilot study. J Am Geriatr Soc. 2014;62(11):2047–2055. doi: 10.1111/jgs.13102. [DOI] [PubMed] [Google Scholar]
- 48.Luchting B, et al. Disrupted TH17/Treg balance in patients with chronic low back pain. PLoS One. 2014;9(8):e104883. doi: 10.1371/journal.pone.0104883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Homey B. After TH1/TH2 now comes Treg/TH17: significance of T helper cells in immune response organization. Hautarzt. 2006;57(8):730–732. doi: 10.1007/s00105-006-1199-3. [DOI] [PubMed] [Google Scholar]
- 50.Fardon DF, et al. Lumbar disc nomenclature: version 2.0: Recommendations of the combined task forces of the North American Spine Society, the American Society of Spine Radiology and the American Society of Neuroradiology. Spine J. 2014;14(11):2525–2545. doi: 10.1016/j.spinee.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 51.Moore RJ, Vernon-Roberts B, Fraser RD, Osti OL, Schembri M. The origin and fate of herniated lumbar intervertebral disc tissue. Spine (Phila Pa 1976) 1996;21(18):2149–2155. doi: 10.1097/00007632-199609150-00018. [DOI] [PubMed] [Google Scholar]
- 52.Brisby H, Olmarker K, Larsson K, Nutu M, Rydevik B. Proinflammatory cytokines in cerebrospinal fluid and serum in patients with disc herniation and sciatica. Eur Spine J. 2002;11(1):62–66. doi: 10.1007/s005860100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park MS, et al. The association of the activation-inducible tumor necrosis factor receptor and ligand with lumbar disc herniation. Yonsei Med J. 2007;48(5):839–846. doi: 10.3349/ymj.2007.48.5.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kraychete DC, et al. Serum cytokine levels in patients with chronic low back pain due to herniated disc: analytical cross-sectional study. Sao Paulo Med J. 2010;128(5):259–262. doi: 10.1590/S1516-31802010000500003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pedersen LM, Schistad E, Jacobsen LM, Roe C, Gjerstad J. Serum levels of the pro-inflammatory interleukins 6 (IL-6) and-8 (IL-8) in patients with lumbar radicular pain due to disc herniation: A 12-month prospective study. Brain Behav Immun. 2015;46:132–136. doi: 10.1016/j.bbi.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Cheng L, Fan W, Liu B, Wang X, Nie L. Th17 lymphocyte levels are higher in patients with ruptured than non-ruptured lumbar discs, and are correlated with pain intensity. Injury. 2013;44(12):1805–1810. doi: 10.1016/j.injury.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 57.Xue H, et al. Interleukin-21 Is Associated with the Pathogenesis of Lumbar Disc Herniation. Iran J Allergy Asthma Immunol. 2015;14(5):509–518. [PubMed] [Google Scholar]
- 58.Xie P, et al. Comparative analysis of serum proteomes: Identification of proteins associated with sciatica due to lumbar intervertebral disc herniation. Biomed Rep. 2014;2(5):693–698. doi: 10.3892/br.2014.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moen A, et al. Inflammatory Serum Protein Profiling of Patients with Lumbar Radicular Pain One Year after Disc Herniation. Int J Inflam. 2016;2016:3874964. doi: 10.1155/2016/3874964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26(17):1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 61.Goode AP, et al. Association between serum and urine biomarkers and lumbar spine individual radiographic features: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage. 2012;20(11):1286–1293. doi: 10.1016/j.joca.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weber KT, et al. Serum levels of the proinflammatory cytokine interleukin-6 vary based on diagnoses in individuals with lumbar intervertebral disc diseases. Arthritis Res Ther. 2016;18:3. doi: 10.1186/s13075-015-0887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luoma K, Vehmas T, Riihimäki H, Raininko R. Disc height and signal intensity of the nucleus pulposus on magnetic resonance imaging as indicators of lumbar disc degeneration. Spine (Phila Pa 1976) 2001;26(6):680–686. doi: 10.1097/00007632-200103150-00026. [DOI] [PubMed] [Google Scholar]
- 64.Antoniou J, et al. Quantitative magnetic resonance imaging in the assessment of degenerative disc disease. Magn Reson Med. 1998;40(6):900–907. doi: 10.1002/mrm.1910400616. [DOI] [PubMed] [Google Scholar]
- 65.Kettler A, Wilke HJ. Review of existing grading systems for cervical or lumbar disc and facet joint degeneration. Eur Spine J. 2006;15(6):705–718. doi: 10.1007/s00586-005-0954-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tertti M, et al. Disc degeneration in magnetic resonance imaging. A comparative biochemical, histologic, and radiologic study in cadaver spines. Spine (Phila Pa 1976) 1991;16(6):629–634. doi: 10.1097/00007632-199106000-00006. [DOI] [PubMed] [Google Scholar]
- 67.Pearce RH, Thompson JP, Bebault GM, Flak B. Magnetic resonance imaging reflects the chemical changes of aging degeneration in the human intervertebral disk. J Rheumatol Suppl. 1991;27:42–43. [PubMed] [Google Scholar]
- 68.Southern EP, Fye MA, Panjabi MM, Patel TC, Cholewicki J. Disc degeneration: a human cadaveric study correlating magnetic resonance imaging and quantitative discomanometry. Spine (Phila Pa 1976) 2000;25(17):2171–2175. doi: 10.1097/00007632-200009010-00005. [DOI] [PubMed] [Google Scholar]
- 69.Weidenbaum M, et al. Correlating magnetic resonance imaging with the biochemical content of the normal human intervertebral disc. J Orthop Res. 1992;10(4):552–561. doi: 10.1002/jor.1100100410. [DOI] [PubMed] [Google Scholar]
- 70.Schiebler ML, et al. In vivo and ex vivo magnetic resonance imaging evaluation of early disc degeneration with histopathologic correlation. Spine (Phila Pa 1976) 1991;16(6):635–640. doi: 10.1097/00007632-199106000-00007. [DOI] [PubMed] [Google Scholar]
- 71.Blumenkrantz G, et al. In vivo 3.0-tesla magnetic resonance T1rho and T2 relaxation mapping in subjects with intervertebral disc degeneration and clinical symptoms. Magn Reson Med. 2010;63(5):1193–1200. doi: 10.1002/mrm.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taylor TK, et al. Spinal biomechanics and aging are major determinants of the proteoglycan metabolism of intervertebral disc cells. Spine (Phila Pa 1976) 2000;25(23):3014–3020. doi: 10.1097/00007632-200012010-00008. [DOI] [PubMed] [Google Scholar]
- 73.Iatridis JC, MacLean JJ, O'Brien M, Stokes IA. Measurements of proteoglycan and water content distribution in human lumbar intervertebral discs. Spine (Phila Pa 1976) 2007;32(14):1493–1497. doi: 10.1097/BRS.0b013e318067dd3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Filippi CG, et al. In vivo quantification of T1ρ in lumbar spine disk spaces at 3 T using parallel transmission MRI. AJR Am J Roentgenol. 2013;201(1):W110–116. doi: 10.2214/AJR.12.9523. [DOI] [PubMed] [Google Scholar]
- 75.Auerbach JD, et al. In vivo quantification of human lumbar disc degeneration using T(1rho)-weighted magnetic resonance imaging. Eur Spine J. 2006;15(Suppl 3):S338–344. doi: 10.1007/s00586-006-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ye S, Ju B, Wang H, Lee KB. Bone morphogenetic protein-2 provokes interleukin-18-induced human intervertebral disc degeneration. Bone Joint Res. 2016;5(9):412–418. doi: 10.1302/2046-3758.59.BJR-2016-0032.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grad S, et al. Systemic blood plasma CCL5 and CXCL6: Potential biomarkers for human lumbar disc degeneration. Eur Cell Mater. 2016;31:1–10. doi: 10.22203/ecm.v031a01. [DOI] [PubMed] [Google Scholar]
- 78.Deng X, Zhao F, Kang B, Zhang X. Elevated interleukin-6 expression levels are associated with intervertebral disc degeneration. Exp Ther Med. 2016;11(4):1425–1432. doi: 10.3892/etm.2016.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weber KT, et al. Exploratory study for identifying systemic biomarkers that correlate with pain response in patients with intervertebral disc disorders. Immunol Res. 2015;63(1–3):170–180. doi: 10.1007/s12026-015-8709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schistad EI, et al. Association between baseline IL-6 and 1-year recovery in lumbar radicular pain. Eur J Pain. 2014;18(10):1394–1401. doi: 10.1002/j.1532-2149.2014.502.x. [DOI] [PubMed] [Google Scholar]
- 81.Pimentel DC, et al. Anti-tumor necrosis factor antagonists in the treatment of low back pain and radiculopathy: a systematic review and meta-analysis. Pain Physician. 2014;17(1):E27–44. [PubMed] [Google Scholar]
- 82.Andrade P, et al. Tumor necrosis factor-α inhibitors alleviation of experimentally induced neuropathic pain is associated with modulation of TNF receptor expression. J Neurosci Res. 2014;92(11):1490–1498. doi: 10.1002/jnr.23432. [DOI] [PubMed] [Google Scholar]
- 83.Ohtori S, et al. Efficacy of epidural administration of anti-interleukin-6 receptor antibody onto spinal nerve for treatment of sciatica. Eur Spine J. 2012;21(10):2079–2084. doi: 10.1007/s00586-012-2183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


