Abstract
Purpose of the Review: The age-related concomitant loss of skeletal muscle and accumulation of excess adipose tissue has been commonly referred to as sarcopenic obesity. While weight loss may help mitigate the metabolic abnormalities linked to obesity, low fitness levels and muscle atrophy complicate the effectiveness of lifestyle interventions. Due to low levels of compliance, sub-optimal economic efficiency and low functional capacity, there has been no consensus on optimal therapy. This includes the use of high protein diets that do not ensure muscle preservation during weight loss in this segment of the population. The primary objectives of this review are to discuss the relevance of sarcopenic obesity, examine the feasibility of weight loss in the elderly, and highlight new approaches to the problem.
Introduction
Obesity negatively affects the endocrine, gastrointestinal, cardiovascular and nervous systems of the body, and represents a tremendous clinical challenge to physicians, nurses, dieticians, and other healthcare providers [1]. This challenge is further complicated by the progressive loss of skeletal muscle, strength and function that has been commonly referred to as sarcopenia [2], leaving individuals at a lower level of function with advancing age. The transition from an active to a non-active lifestyle promotes muscle atrophy, and increases the risk of undesirable medical, economic and societal consequences [3]. While weight loss programs are usually employed to combat obesity in young to middle-aged populations [4], low fitness levels, economic challenges, and small allowable margins of skeletal muscle atrophy present our healthcare system with a clinical conundrum in older, obese individuals [5]. The purpose of this review is to highlight the significance and complexity of sarcopenic obesity, discuss the limitations of proposed lifestyle interventions, and discuss possible alternative solutions to the problem.
Obesity in the Elderly
A body mass index (BMI) of 30.0–34.9 kg/m2, 35.0–39.9 kg/m2, and ≥40 kg/m2 represents the classification of Grade I, II and III obesity, respectively. In conjunction with BMI, the increased risk of metabolic disease has been further delineated by waist circumferences measurements of > 40 in and > 35 in men and women, respectively [6].
Using these metrics, the existence of obesity and an elevated waist circumference dramatically increase the risk of hypertension, dyslipidemia and insulin resistance that may eventually lead to the development of type 2 diabetes (T2D) and cardiovascular disease (CVD) commonly referred to as metabolic syndrome [6]. Prevalence of the metabolic syndrome increases with age and exists in an alarming ~43% of people aged over 60 yrs [7]. The presence of obesity in individuals who may not present with traditional metabolic risk factors such as hypertension, dyslipidemia and insulin resistance [8] does not seem to reduce the increased risk of non-alcoholic fatty liver disease (NAFLD) and T2D when compared to individuals with a normal body weight [9].
Even though obesity has been strongly linked to metabolic disease in the elderly, it is somewhat counterintuitive that the existence of obesity has also been associated with a lower risk of mortality [10]. For example, the obesity paradox has been described in individuals with heart failure [11], chronic obstructive pulmonary disease (COPD) [12], and the residents of nursing homes [13]. There has been a small improvement in the clarity associated with this controversy as studies examining the link between all-cause mortality and obesity have linked grades 2 and 3 obesity to a higher risk of mortality but Grade 1 obesity was not [14].
The classification of obesity by BMI may be cost effective and expedient from a treatment perspective, but it does not take account of variations in lean body mass, fat mass or fluid retention [15]. Obesity may co-exist with relatively high levels of lean body mass, and this would provide physiological resilience against the risk of mortality [1]. Variations in the obesity phenotype indicative of visceral obesity as typically identified by waist/hip ratio or waist circumference measurements may be influenced physiologically by activation of inflammatory pathways that have a negative influence on metabolic health. Therefore, the grade of obesity and/or the presence of visceral obesity should be evaluated when considering the risk of metabolic disease and therapeutic options in the elderly [1].
Sarcopenia in the Elderly
The operational definition for sarcopenia was facilitated in 2009 by the European Working Group on Sarcopenia in Older People (EWGSOP). The consensus reached also included the European Society of Clinical Nutrition and Metabolism (ESPEN), the International Academy of Nutrition and Aging (IANA), and the International Association of Gerontology and Geriatrics – European Region (IAGG-ER). The diagnosis of sarcopenia requires documentation of low muscle mass and one of the two additional criteria: a) low muscle strength or b) low physical performance [16]. The cut-off points for low muscle mass by gender as measured by dual energy x-ray absorptiometry are 7.26 kg/m2 ad 5.25 kg/m2 for males and females, respectively.
These criteria are important since sarcopenia represents a condition that is linked to impaired mobility and ability to perform activities of daily living, an increased risk of injury, loss of independence, and increased mortality [17]. The loss or threshold that exceeds 30% in many physiological systems represents a breakpoint with regard to inadequate function [18], and highlights the significance of sarcopenia. Upon reaching the age of 80 years old, as many as 50% of the older population may be adversely affected by sarcopenia [19]. Maintaining or increasing body weight does not necessarily protect against sarcopenia [19–22] as much of the weight regained after weight loss may be adipose tissue [23].
The development of sarcopenia in obese elderly (i.e., sarcopenic obesity) is particularly problematic and complex, in that even normal activities of daily living require movement of a greater body mass despite limited muscle mass and functional capacity [24]. It would seem that loss of body weight in individuals with sarcopenic obesity would resolve both the physical and pathological issues associated with obesity. However, the reduction in caloric intake that is required for significant weight loss would likely to further accelerate the development of sarcopenia. When these factors are taken into consideration, the complexity of the condition exceeds that of marginal lean body mass due to the relevance overall body mass with respect to functional mobility. Thus, the benefits of reducing body mass may be offset by the debilitating effects of muscle atrophy and impaired function [25].
Consequences of Inactivity
The advantages of continued physical activity in the elderly include: retention of muscle strength, flexibility, gait, and protection against chronic disease that result in reduced function [26]. In fact, a regular, balanced and moderate physical activity program actually reduced the risk of major mobility disability of an elderly, vulnerable population [27]. Given the protective influence of aerobic fitness and lean body mass against the risk of mortality [28], an alteration in these variables during extended hospitalization in the elderly would be particularly problematic.
The results of recent longitudinal studies that followed 1,149 participants aged 65 years or older over a 9-year period have demonstrated that baseline levels of physical inactivity were directly linked to reductions in physical function and incident mobility disability [29]. Previous work from this same group of investigators has also shown that physical inactivity between the ages of 20–40 and 40–60 were associated with decreased functional ability and incident mobility disability. Only cumulative physical inactivity was linked to a higher risk of mortality in these ongoing studies [30]. From data collected in over 160,000 participants in the NIH-AARP Diet and Health project, physical inactivity (ie., individuals who watched television more than 5 hr/day) was linked to a 28% increase in mortality. With a 1–2 hr reduction in television viewing, there was a 15% reduction in the risk of mortality [31]. Data from these two studies highlight the influence of behavior, and how it interfaces with the link between physical inactivity and the risk of mortality. Even short-term physical inactivity promotes reductions in aerobic capacity and increased adiposity [5]. Poor fitness and obesity have been directly linked to an increased risk of mortality with age [32].
Exercise Recommendations
In 2009, the American College of Sports Medicine issued an updated position stand on the benefits of exercise and physical activity for older adults [33]. These guidelines included a strong statement regarding the detrimental consequences of physical inactivity and the protective benefits of maintaining physical activity against the risks of mortality as outlined in the previous section. In addition, the guidelines asserted that 150 min of moderate intensity exercise should be performed by aging adults “as conditions allow” [33]. While it is understandable that these general recommendations, if employed, may offset the age-related decline in structure and function, compliance is typically poor (ie., ~40%), and these recommendations would be unlikely to elicit the weight loss needed for improvements in cardiovascular and metabolic health [34].
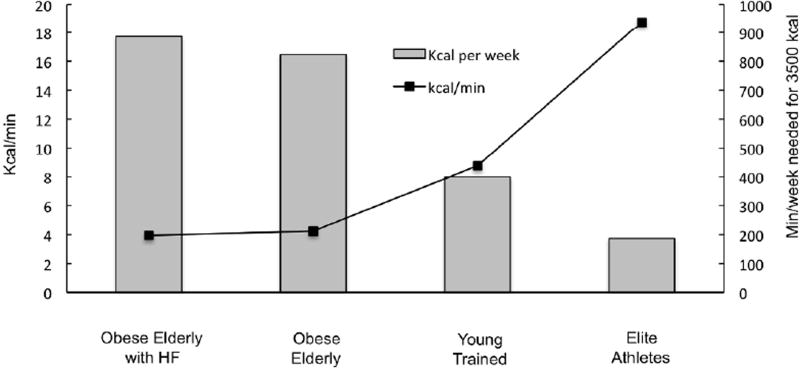
The concomitant importance of caloric restriction during exercise training to achieve significant weight loss in the elderly has been demonstrated in numerous studies over the past decade [19]. In fact, a systematic review of original research from 2005 – 2015 using PubMed, Cochrane Central Register of Controlled Trials, Web of Science, CINAHL, EMBASE, PsycINFO provided strong evidence that while exercise training did promote improvements in physical function, it did not lead to significant weight loss [19]. The studies included in this comprehensive review utilized a variety of exercise modalities and strategies, and the majority of them included 30–90 min of moderate aerobic and/or resistance exercise training 3–5 days/week. Unfit, older individuals only expend ~4 kcal/min at 50% of VO2 peak and this low level of energy expenditure would require ~900 min/week or 130 min/day to produce a 3500 kcal deficit (Figure 1). Even with a 25% increase in exercise intensity to 75% VO2peack, an unfit, older individual would need to exercise 549 min or ~9 hrs/week to elicit a 3500 kcal deficit.
Figure 1.
A comparison of the kcal/min and the min/week necessary for 3,500 kcal of energy expenditure at 50% of VO2peak in obese, elderly with heart failure, middle-aged overweight, and young trained individuals, and elite athletes.
To further illustrate the relevance of oxygen capacity or fitness levels when considering exercise training as a weight loss tool, it would be valuable to compare the theoretical caloric expenditures in obese elderly with heart failure and preserved ejection fraction [35], obese elderly without co-morbidities [36], young untrained individuals [37], and from Sir Matthew Pinsent (one of the highest recorded VO2peak values in history [38] (Figure 1). In his prime, the Olympic rower, Sir Pinsent would have utilized almost 20 kcal/min at 50% of his VO2peak, and required less than 200 min to elicit a 3500 kcal deficit/week. By comparison, master athletes who are 60 years of age have oxygen consumption rates that are 28% less than their fit counterparts who are 30 years younger [39]. Through the use of these illustrations based on actual data that are specifically relevant to the topic, it is clear that the ACSM exercise recommendations are not adequate to promote weight loss in older, overweight individuals. While the data from these calculations may be discouraging, they may shed light on the frustration experienced by many, especially the elderly when attempting to utilize exercise training as a tool for weight loss. It may also help clarify why the overwhelming majority of individuals choose dietary modification or caloric restriction as their method of weight loss [40].
Caloric Restriction: Pitfalls and Potential
Caloric restriction has been posited to increase lifespan [41]. Long-term caloric restriction has been shown to reduce the risk of atherosclerosis as well as promote efficacious changes in blood pressure, C-reactive protein, platelet-derived growth factor AB and carotid artery intima-media thickness. Total cholesterol, low density lipoprotein cholesterol, ratio of total cholesterol/high density lipoprotein cholesterol, triglycerides, and fasting glucose and insulin were also lowered with caloric restriction compared to the control group in the study [42]. While these results are encouraging, the individuals recruited for these studies were ~50±10 years of age with a range of 35–82 years of age [42]. The large variations in age are very important since the retention of skeletal muscle declines with aging [43–45]. Therefore, the benefits of caloric restriction drawn from studies in individuals aged 35–82, are difficult to interpret with respect to older individuals.
Age-associated muscle impairments represent a multifactorial etiology, and muscle atrophy occurs with aging, even in the absence of caloric restriction. The factors that contribute to sarcopenia include insulin resistance, reductions in the interactive influence of macronutrients, signaling factors, oxidative stress, mitochondrial alterations, increased apoptosis, aberrant cytokines, and additional molecular pathways yet to be fully described [46]. It is clear that some of these factors are further complicated and challenged by reductions in nutrient delivery, and especially during the conditions of caloric restriction [47,48]. Even in middle aged individuals (ie., 58±3 years of age), modest caloric restriction-induced weight loss (ie., 4 kg) over a 12 week period using well-controlled metabolic feeding resulted in the significant loss of thigh lean tissue as determined by computed tomography (CT) scans [49]. Using a less specific measurement of skeletal muscle than CT scans, Kitzman et al. reported the loss of 2 kg of non-bone lean body mass in older, obese individuals (66±5 years of age) from dual energy x-ray absorptiometry (DXA) scans during a 20 week study employing caloric restriction-induced weight loss via dietary counseling [35]. Surprisingly, these investigators still linked intentional weight loss and the loss of lean tissue in another study to reductions in all-cause mortality despite almost 3 decades of variation in the age of participants [50]. Given the age-related differences in muscle atrophy, anabolic resistance and nutrient access across almost 1/2 to 1/3 of the human life span, associations between the loss of lean mass and improvements in all-cause mortality may present faulty conclusions.
It is not surprising that a recent exhaustive review of weight loss studies in older individuals concluded that caloric restriction or “dietary alone”-induced weight loss contributed to the development of sarcopenia and bone loss [19]. According to experts, intentional caloric restriction-induced weight loss in the elderly would contribute to significantly higher risk of functional impairment and incident disability [16,19,51]. The detrimental impact of caloric restriction-induced weight loss on skeletal muscle has been linked to the lower threshold of muscle/functional status at baseline, and greater anabolic resistance in older individuals compared to their younger counterparts [52,53]. These important differences are crucial in the evaluation of clinical efficacy when it comes to the health benefits of weight loss resulting from reduced caloric intake. Otherwise, the amount of skeletal muscle may fall below a threshold linked to dramatic reductions in their ability to perform activities of daily living, and increase their risk of morbidity and mortality [54–57]. A combined approach that employs dietary modification and physical activity seems to be more effective in maintaining lean body mass and bone mass, and physical function that caloric restriction alone [58]. This approach may also lead to a reduced decline in mobility in older adults as well [59,60], and remains effective when using a community-based approach [61].
Interventions and Economics
The expansive review conducted by Batsis et al., summarized the inherent risks of caloric restriction-induced weight loss in the elderly [19]. However, some investigative groups still contend that dietary mediated reductions in caloric intake an effective means of improving the health status of elderly individuals [35]. While this topic continues to be debated, the overall cost of these interventions should be evaluated. Within already established standards of care, the cost-effectiveness analysis or the cost per unit health outcome obtained is not a simplistic task [62]. Health utility scores are used to calculate quality-adjusted life years that serve as a metric for health outcomes [62]. Using this information, the cost-effectiveness of these interventions has not been consistent even when the beneficial influence of interventions has already been successfully demonstrated in absolute terms [63].
The reason for the lack of applicability from research to medical practice is likely due to the extensive resources (ie., infrastructure, medical personnel and staff) available to a funded research grant as opposed to resources supported by a third party system. This is easily understood when evaluating the cost effectiveness of a trial conducted by Kitzman et al., in which it took 20 weeks or 5 months for individuals to lose ~7 kg of total body weight and ~2 kg of lean body mass via caloric restriction-induced weight loss [35]. While minor improvements in VO2peak were noted, there was no significant impact on quality of life despite direct costs of ~$48,338/participant per NIH reporter [64]. In other studies that utilized caloric restriction-induced weight loss in older, overweight adults, direct costs/participants were even higher, and were accompanied by significant muscle loss and limited overall efficacy with respect to health outcomes [65,66]. When an intensive lifestyle intervention has been employed such as that utilized by the Look AHEAD Trial in individuals with type 2 diabetes, there was a 10-year cost savings of ~$5k in individuals without a history of cardiovascular disease. While potentially promising, it might be difficult to calculate the cost of treatment relative to the cost of the ILI due to the multi-site approach utilized for the study [67], and the costs of other studies seem excessive relative to overall benefit.
Consequence of Unanticipated Weight Loss
In aging individuals, muscle loss occurs with caloric restriction in a manner similar to but not as severe as a critical illness. While a critical illness can be particularly catastrophic with respect to the compromised maintenance of lean body mass/total body mass [68], an unanticipated event such as surgery may also reduce the quality of nutrient intake [69]. The increased risk of malnutrition under these circumstances may be further affected by metabolic abnormalities, lack of social interaction, reduced functional capacity, cognitive impairment and an array of potential morbidities in older individuals. For these reasons, inadequate nutrient and/or caloric intake has a dramatic and negative influence on the maintenance of the quality of life, independence, and overall longevity in older adults.
Older adults are likely to be uniquely susceptible to the consequences of malnutrition and/or caloric restriction due to established cut-off thresholds of 9.2 kg/m2 and 7.4 kg/m2 in males and females, respectively [53]. These thresholds may be all the more relevant during hospitalization, especially with unanticipated complications. For example, bed rest-reductions in protein synthesis and lean body mass in young (30±6 years of age) males were initially demonstrated ~20 years ago [70]. Since older adults lose lean body mass at twice the rate of younger individuals [71], the maintenance of skeletal muscle in healthy circumstances is vitally important. We have also demonstrated the detrimental influence of bed rest on metabolism and function in older individuals, even when these individuals have access to a well-controlled balanced diet that includes high quality nutrients [5,71,72]. Combined with the age-associated anabolic resistance [57,73], interventions that might further complicate bed rest induced-atrophy of skeletal muscle and substantially increase the risk and associated hazards of hospitalization in the elderly [74].
A Cochrane review conducted ~15 years ago examined data collected from thirty-one trials that utilized protein and energy supplementation in over 2,400 participants. The relative risk analysis indicated a lower risk of mortality with protein and energy supplementation. In this range of studies, protein intake varied from that 10–50 g of protein/day and energy intake fell between 175 and 1350 kcal/day [75]. Clearly, the beneficial role of nutrient and protein intake on long-term outcomes in the elderly has been recognized for quite some time but the variation in intake makes interpretation somewhat challenging.
Recognizing the catabolic influence of bed rest on skeletal muscle and the relationship to decreased physical function in the elderly [71,73,76,77], unique nutritional approaches that provided specific profiles of essential amino acids (EAAs) have targeted this problem. Ferrando et al., demonstrated the beneficial influence of a blend of EAAs (15 gms; TID) on the preservation of skeletal muscle during bed rest [78]. In this study, EAAs were consumed twice per day in order to offset significant anabolic resistance in older adults [79]. While more investigation is potentially warranted with regard to timing and dosage of EAAs to protect skeletal muscle during catabolic scenarios, substantial evidence supports the efficacy of adequate EAA delivery in mitigating the risks of sarcopenia that will ultimately have a profound negative impact of functional independence [53].
What is the solution to the problem of obesity in the elderly?
The utilization of exercise training as a weight loss strategy has been complicated by lack of effectiveness in older individuals [19]. This has been largely due to their low levels of aerobic capacity that limit the potential for adequate caloric expenditure. The potential synergism between caloric restriction and exercise may offer a promising alternative with respect to significant weight loss, muscle preservation and improved mobility [58,59]. There is also a clear delineation between the importance of weight loss derived from caloric restriction and exercise training in the short term, and the long term benefits derived from weight maintenance [1]. Simply put, weight reduction through a combined approach may elicit a negative energy balance, but an effective weight maintenance period that maintains caloric balance is essential for long term efficacy[1] Despite the potentially promising potential of caloric restriction and exercise training, not all elderly individuals are capable of exercise training on their own, and the economic challenges of providing sufficient supervision using adequately trained personnel presents another concern [19,62]. For these reasons and other legitimate issues, the overwhelming majority of overweight individuals choose caloric restriction or dietary modification as their sole strategy [40]. With caloric restriction alone , both adipose tissue and skeletal muscle would be lost, and the reduction in muscle mass may have a detrimental influence of the functional status of older individuals who may be already classified with sarcopenia or at risk for the development of the syndrome.
Based on the guidelines provided by the PROT-AGE study group and the World Health Organization, it is well recognized that the protein RDA f 0.8 grams of protein per kilograms each day is not adequate to maintain lean body mass in older individuals [80,81]. An even greater consumption of dietary protein (ie., 1.2–1.5 gm/kg/day) may be required in older individuals because of age-associated alterations in metabolic regulation, immune status, and hormonal fluctuations that may promote an increased risk for the development of sarcopenia [80]. The beneficial influence of increased protein ingestion will be largely derived from the increase in amino acid availability [82], and increases muscle protein synthesis in response to amino acid intake are dose-dependent [83–85]. Recently, increased protein ingestion (up to 70 grams) in the context of a mixed meal was shown to result from a substantial increase in protein synthesis and reduction in protein breakdown resulting in greater overall whole body protein net balance [86]. Convincing data from these and other studies highlight the importance of increased dietary protein in older individuals that may delay or dramatically reduce the risk of sarcopenia [80,87,88].
A recent review of nineteen clinical trials employing caloric restriction-weight loss in younger individuals (>914 participants) demonstrated similar efficacy with respect to total weight loss regardless of protein intake [89,90]. The “success” of weight loss interventions in younger populations has been largely evaluated by the total amount of weight loss with little regard for concomitant reductions in skeletal muscle. This may be reasonable, since younger individuals have much greater resilience than older individuals with respect to the influence of inactivity or reduced protein intake on skeletal muscle [91]. However, these results cannot be directly extrapolated to older individuals due to the higher splanchnic extraction of amino acids and anabolic resistance with age that increase overall muscle atrophy during weight and contribute to the difficulty of replacing that muscle following weight loss [87].
Recently, the influence of increased protein intake on muscle preservation during caloric restriction-induced weight loss has been evaluated in older individuals [92]. In these studies, the investigators utilized a well-controlled 12-week, 25% reduction in energy intake with either high protein (HP; 1.7 g kg−1 per day) or normal protein (NP; 0.9 g kg−1 per day) intake with ~90% of the food intake provided and monitored by the research staff. In order to optimize dietary compliance, participants were allowed to choose 10% of their dietary intake from a restricted list of options. Both HP and NP lost ~9 kg of total body weight and ~2 kg of lean body mass [92]. The results were included in a meta-analysis that screened 1,542 articles and independently assessed the results of 24 eligible articles on the topic of protein intake in conjunction with caloric restriction-induced weight loss. While the results of the meta-analysis concluded that increased protein intake (>1.0 g kg−1 per day) reduced the amount of lean body mass associated with weight loss, significant reduction in lean body mass persisted despite increased protein intake [93]. In other words, ~20% of the weight loss represented a reduction in lean body mass despite greater consumption of dietary protein.
A central problem with relying on increased dietary protein during caloric restriction weight loss to slow the loss of muscle is that the diet does not generally include purified proteins, but rather protein food sources. Protein food sources consist of a significant amount of non-protein macronutrients. The caloric content of protein food sources may be more that 50% non-protein calories (USDA Nutrient Data Base), which makes achieving a truly high-protein diet in the context of a significantly reduced caloric intake very challenging. Furthermore, the protein of essential amino acids in dietary proteins may be less than optimal to overcome anabolic resistance [94].
Any loss of skeletal muscle in older individuals would narrow the threshold of muscle mass that might result in disability. In order to address the shortcomings of a caloric restriction approach that relies solely on increased dietary protein intake, we have previously investigated the efficacy of unique profile of EAAs as part of meal replacement strategy [95]. This approach was based on the greater stimulation of muscle protein synthesis by a unique profile of EAAs as compared to whey protein [96]. The total nitrogen intake was approximately matched in an EAA profile + protein-based meal replacement with the amount of nitrogen in a conventional meal replacement. The total nitrogen intake was dictated by the conventional meal replacement, which meant that the total protein intake was only about 0.8 g/kg/day. Consistent with other studies, this amount of protein intake in the meal replacement group was inadequate to preserve lean body mass during caloric restriction weight loss. In contrast, the ratio of lean body mass lost to fat mass lost was reduced significantly by inclusion of the EAAs. These results led to further inquiry on the topic as 40 gms of high quality protein 4 times/day (QID) would meet the recommendations for protein intake in an older individual weighing 90 kg [80], increase caloric intake from protein alone to 656 kcal and represent over 50% of the caloric intake in a conventional, low-calorie (1,200) diet [97]. On other hand, the QID consumption of 18 gms of EAAs which has been shown to elicit relatively identical responses in terms of feeding-induced changes in muscle protein synthesis in the elderly would only represent 295 kcal (almost 1/3 of the calories contained in 40 gms of whey protein), and ~33% of total caloric intake. A higher proportion of leucine that could activate mTOR and provide greater availability of EAA precursors as part of a meal replacement might maximize the anabolic response, and therefore lessen the intact dietary protein requirement [98]. This approach would allow normal carbohydrate and fat intake, and yet still allow significant weight loss (ie., 5–10%) necessary for improvements in metabolic risk factors and functional independence [87].
Conclusions
Malnutrition, physical inactivity and/or unintentional/intentional weight loss will further increase the risk of sarcopenia as oroginally described by Baumgartner et al., [99] and further clarified by the European Society of Clinical Nutrition and Metabolism (ESPEN) [100], and complicate the functional status of older individuals. The epidemic of obesity and the increasing number of individuals who are reaching the age of 60 years presents a significant health crisis with respect to the risks of sarcopenic obesity and the large numbers of individuals who will be affected. While physical activity may be beneficial, the efficacy of its use as a weight loss strategy is limited by low functional capacity that reduces significant caloric expenditure. Moreover, sub-optimal compliance, functional limitations, economic challenges and limited availability may attenuate the effectiveness of exercise interventions. It is clear that increased protein intake is important towards maximizing functional independence but unique approaches that optimize the efficiency of protein utilization may be a vital aspect of any effective dietary approach to weight loss in older individuals.
What is already known about this subject?
The incidence of obesity in the elderly has been increasing.
Sarcopenia presents significant health risks for the elderly.
The proportion of elderly individuals/overall population is increasing dramatically.
What does this review add?
The risks of caloric restriction as it relates to muscle loss in the elderly.
The inadequacy of exercise training as a mono-therapy for obesity
The importance of amino acid delivery to offset muscle loss in the elderly
Acknowledgments
Funding: Research reported in this publication was supported by the National Institutes of Health Older American Independence Center Grant PG30-AG-028718, and by the National Institute of Diabetes and Digestive and Kidney Diseases Small Business Innovations in Research (R43 AG051298-01), the National Institute of General Medical Sciences (5 U54 GM104944), and by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103395. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH.
Footnotes
Disclosure: Drs. Coker and Wolfe are Managing Partners and Co-Owners of Essential Blends, LLC that has received funding from the National Institutes of Health to develop clinical nutrition products. The data presented in this manuscript are unrelated.
References
- 1.Bischoff S, Boirie Y, Cederholm t, Cuerda C, Delzenne NM, Deutz NE, et al. Towards a multidiscplinary approach to understand and manage obesity and related diseases. Clin Nutr. 2016 Nov 16; doi: 10.1016/j.clnu.2016.11.007. pii: S0261-5614(16)31323-1. [DOI] [PubMed] [Google Scholar]
- 2.Keller K, Engelhardt M. Strength and muscle loss with aging process. Age and strength loss. Muscle Ligaments Tendons. 2013;3:346–350. [PMC free article] [PubMed] [Google Scholar]
- 3.Beaudart C, Rizzoli R, Bruyere O, Reginster J-Y, Biver E. Sarcopenia: burden and challenges for public health. Arch Public Health. 2014 Dec 18;72(1):45. doi: 10.1186/2049-3258-72-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hays N, Galassetti P, Coker RH. Prevention and treatment of type 2 diabets: current role of lifestyle, natural product and pharmaocological interventions. Pharmacol Ther. 2008 May;118(2):181–91. doi: 10.1016/j.pharmthera.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coker R, Hays N, Williams RH, Wolfe RR, Evans WJ. Bed rest promotes reductions in walking speed, functional parameters, and aerobic fitness in older, healthy adults. J Gerontol A Biol Sci Med Sci. 2015 Jan;70(1):91–6. doi: 10.1093/gerona/glu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grundy S, Brewer H, Cleeman J, Smith S, Lenfant C. National Heart, Lung and Blood Institute, American Heart Association: Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/ American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 7.Ford E, Giles W, Dietz W. Prevalence of the metabolic syndrome among US adults: finding from the third National Health and Examination Survey. JAMA. 2002;16:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 8.Rey-Lope J, de Rezende LF, Pastor-Valero M, Tess BH. The prevalence of metabolically healthy obesity: a systematic review and critical evaluation of the definitions used. Obes Rev. 2014 Oct;15(10):781–90. doi: 10.1111/obr.12198. [DOI] [PubMed] [Google Scholar]
- 9.Heianza Y, Arase Y, Tsuji H, Fujihara K, Saito K, Hsieh SD, et al. Metabolically healthy obesity, presence or absence of fatty liver, and risk of type 2 diabetes in Japanese individuals: Toranomon Hospital Health Management Center Study 20 (TOPICS 20) J Clin Endocrinol Metab. 2014 Aug;99(8):2952–60. doi: 10.1210/jc.2013-4427. [DOI] [PubMed] [Google Scholar]
- 10.Oreopoulous A, Kalantar-Zadeh K, Sharma A, Fonarow G. The obesity paradox in the elderly: potential mechanisms and clinical implications. Clin Geriatr Med. 2009 Nov;25(4):643–59. doi: 10.1016/j.cger.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K, Block G, Horwich T, Fonarow G. Reverse epidemiology of conventional cardiovascular disease risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004 Apr 21;43(8):1439–44. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 12.Guo Y, Zhang T, Wang Z, Yu F, Xu Q, Guo W, et al. Body mass index and mortality in chronic obstructive pulmonary disease: A dose-response meta-analysis. Medicine (Baltimore) 2016 Jul;95(28):e4225. doi: 10.1097/MD.0000000000004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veronese N, Cereda E, Solmi M, Fowler SA, Mazato E, Maggi S, et al. Inverse relationship between body mass index and mortality in older nursing home residents: a meta-analysis of 19,538 elderly subjects. Obes Rev. 2015 Nov;16(11):1001–15. doi: 10.1111/obr.12309. [DOI] [PubMed] [Google Scholar]
- 14.Fiegal K, Kit B, Orpana H, Graubard B. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013 Jan 2;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alagiakrishnan K, Banach M, Ahmed A, Aronow W. Complex relationship of obesity and obesity paradox in heart failure - higher risk of developing heart failure and better outcomes in established heart failure. Ann Med. 2016 Dec;48(8):603–613. doi: 10.1080/07853890.2016.1197415. [DOI] [PubMed] [Google Scholar]
- 16.Cruz-Jentoft A, Baeyens J, Bauer J, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010 Jul;39(4):412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruz-Jentoft AJ, Landi F, Schneider SM, Zuñiga C, Arai H, Boirie Y, et al. Prevalence of interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age and Ageing. 2014 Nov;43(6):748–59. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bortz W. A conceptual framework of frailty: a review. J Gerontol A Biol Sci Med Sci. 2002 May;57(5):M283–8. doi: 10.1093/gerona/57.5.m283. [DOI] [PubMed] [Google Scholar]
- 19.Batis J, Mackenzie TA, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obe.sity, and functional impairments in older adults: National Health and Nutrition Examination Surveys 1999–2004. Nutr Res. 2015 Dec;35(12):1031–9. doi: 10.1016/j.nutres.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogden C, Yanovski S, Carroll M, Flegal K. The epidemiology of obesity. Gastroenterology. 2007;13:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher D, Ruts E, Visser M. Weight stability masks sarcopenia in elderly men and women. Am J Physiol. 2000 Aug;279(2):E366–75. doi: 10.1152/ajpendo.2000.279.2.E366. [DOI] [PubMed] [Google Scholar]
- 22.Zamboni M, Zolco E, Scartezzini T. Body composition changes in stable-weight elderly subjects: the effect of sex. Aging Clin Exp Res. 15:321–327. doi: 10.1007/BF03324517. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Visser M, Tylavsky FA, Kritchevsky SB, Schwartz AV, Sahyoun N, et al. Weight Loss and Regain and Effects on Body Composition: The Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2010 Jan;65(1):78–83. doi: 10.1093/gerona/glp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janokowski C, Gozansky W, Van Pelt R. Relative contributions of adiposity and musculartiy to physical function in community-dwelling older adults. Obesity (Silver Spring) 2008;16:1039–1044. doi: 10.1038/oby.2007.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller S, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging. 2008;12:487–491. doi: 10.1007/BF02982710. [DOI] [PubMed] [Google Scholar]
- 26.Bouaziz W, Vogel T, Schmitt E, Kaltenbach G, Geny B, Lang PO. Health benefits of aerobic training programs in adults aged 70 and over: a systematic review. Arch Gerontol Geriatr. 2017 Mar-Apr;69:110–127. doi: 10.1016/j.archger.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014 Jun 18;311(23):2387–96. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kokkinos P. Physical activity, health benefits, and mortality risk. ISRN Cardiol. 2012:718789. doi: 10.5402/2012/718789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stenholm S, Koster A, Valkeinen H, Patel KV, Bandinelli S, Guralnik JM, et al. Association of physical activity history with physical function and mortality in old age. J Gerontol A Biol Sci Med Sci. 2016 Apr;71(4):496–501. doi: 10.1093/gerona/glv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel K, Coppin AK, Manini TM, Lauretani F, Bandinelli S, Ferrucci L, et al. Midlife physical activity and mobility in older age: The InCHIANTI study. Am J Prev Med. 2006 Sep;31(3):217–24. doi: 10.1016/j.amepre.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keadle S, Arem H, Moore S, Sampson J, Matthews C. Impact of changes in television viewing time and physical activity on longevity: a prospective cohort study. Int J Behav Nutr Phys Act. 2015 Dec 18;12:156. doi: 10.1186/s12966-015-0315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, et al. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007 Dec 5;298(21):2507–16. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1594–1599. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 34.Nicklas B, Ambrosius W, Messier SP, Miller GD, Penninx BW, Loeser RF, et al. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004 Apr;79(4):544–51. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 35.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016 Jan 5;315(1):36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davidson L, Hudson R, Kilpatrick K, Kuk JL, McMillan K, Janiszewski PM, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults. Arch Intern Med. 2009 Jan 26;169(2):122–31. doi: 10.1001/archinternmed.2008.558. [DOI] [PubMed] [Google Scholar]
- 37.Nordby P, Saltin B, Helge J. Whole-body fat oxidation determined by graded exercise and indirect calorimetry: a role for muscle oxidative capacity? Scand J Med Sci Sports. 2006 Jun;16(3):209–14. doi: 10.1111/j.1600-0838.2005.00480.x. [DOI] [PubMed] [Google Scholar]
- 38.Bailey J. 2017 http://www.q-power.co.
- 39.Tanaka H, Seals DR. Endurance exercise performance in Masters athletes: age-associated changes and underlying physiological mechanisms. J Physiol. 2008 Jan 1;586(1):55–63. doi: 10.1113/jphysiol.2007.141879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss E, Galuska D, Kettel-Khan L, Serdula M. Weight control practices among U.S. adults, 2001–2002. Am J Prev Med. 2006 Jul;31(1):18–24. doi: 10.1016/j.amepre.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Barzilai N, Huffman D, Muzumdar R, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fontana L, Meyer T, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA. 2004 Apr 27;101(17):6659–63. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balagopal P, Rooyrackers OE, Adey DB, Ades PA, Nair K. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol. 1997 Oct;273(4 Pt 1):E790–800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- 44.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001 Sep 12;286(10):1206–12. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar V, Atherton P, Smith K, Rennie MJ. Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol. 2009 Jun;106(6):2026–39. doi: 10.1152/japplphysiol.91481.2008. [DOI] [PubMed] [Google Scholar]
- 46.Atherton PJ, Greenhaff PL, Phillips SM, Bodine SC, Adams CM, Lang CH. Control of skeletal muscle atrophy in response to disuse: clinical/preclinical contentions and fallacies of evidence. Am J Physiol Endocrinol Metab. 2016 Sep 1;311(3):E594–604. doi: 10.1152/ajpendo.00257.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cruz-Jentoft AJ, Kiesswetter E, Drey M, Sieber CC. Nutrition, frailty, and sarcopenia. Aging Clin Exp Res. 2017 Feb;29(1):43–48. doi: 10.1007/s40520-016-0709-0. [DOI] [PubMed] [Google Scholar]
- 48.Barberi L, Scicchitano B, Musaro A. Molecular and cellular mechanisms of muscle aging and sarcopenia and effects of electrical stimulation in seniors. Eur J Transl Myol. 2015 Aug 25;25(4):231–6. doi: 10.4081/ejtm.2015.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coker RH, Williams RH, Yeo SE, Kortebein PM, Bodenner DL, Kern PA, et al. The impact of exercise training compared to caloric restriction on hepatic and peripheral insulin resistance in obesity. J Clin Endocrinol Metab. 2009 Nov;94(11):4258–66. doi: 10.1210/jc.2008-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kritchevsky S, Beavers KM, Miller SE, Shea MK, Houston DK, Kitzman DW, et al. Intentional weight loss and all-cause mortality: a meta-analysis of randomized clinical trials. PLoS One. 2015 Mar 20;10(3):e0121993. doi: 10.1371/journal.pone.0121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Studenski S, Peters K, Alley D, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014 May;69(5):547–58. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shad B, Thompson J, Breen L. Does the muscle protein synthetic response to exercise and amino acid-based nutrition diminish with advancing age? A systematic review. Am J Physiol Endocrinol Metab. 2016 Nov 1;311(5):E803–E817. doi: 10.1152/ajpendo.00213.2016. [DOI] [PubMed] [Google Scholar]
- 53.Bahat G, Tufan A, Fufan F, Kilic C, Akpinar TS, Kose M, et al. Cut-off points to identify sarcopenia according to European Working Group on Sarcopenia in Older People (EWGSOP) definition. Clin Nutr. 2016 Dec;35(6):1557–1563. doi: 10.1016/j.clnu.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Bosaeus I, Rothenberg E. Nutrition and physical activity for the prevention and treatment of age-related sarcopenia. Proc Nutr Soc. 2016 May;75(2):174–80. doi: 10.1017/S002966511500422X. [DOI] [PubMed] [Google Scholar]
- 55.Fearon K, Evans WJ, Anker SD. Myopenia-a new universal term for muscle wasting. J Cachexia Sarcopenia Muscle. 2011 Mar;2(1):1–3. doi: 10.1007/s13539-011-0025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roubenoff R. The pathophysiology of wasting in the elderly. J Nutr. 1999;129:256S–259S. doi: 10.1093/jn/129.1.256S. [DOI] [PubMed] [Google Scholar]
- 57.Marzetti E, Calvani R, Tosato M, Cesari M, Di Bari M, Cherubini A, et al. Sarcopenia: an overview. Aging Clin Exp Res. 2017 Feb;29(1):11–17. doi: 10.1007/s40520-016-0704-5. [DOI] [PubMed] [Google Scholar]
- 58.Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011 Mar 31;364(13):1218–29. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rejeski WJ, Ip EH, Bertoni AG, Bray GA, Evans G, Gregg EW, et al. Lifestyle change and mobility in obese Adults with type 2 diabetes. N Engl J Med. 2012 Mar 29;366(13):1209–17. doi: 10.1056/NEJMoa1110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murphy R, Patel KV, Kritchevsky SV, Houston DK, Newman AB, Koster A, et al. Weight change, body composition, and risk of mobility disability and mortality in older adults: a population-based cohort study. J Am Geriatr Soc. 2014 Aug;62(8):1476–83. doi: 10.1111/jgs.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rejeski W, Ambrosius W, Burdette J, Walkup M, Marsh AP. Community weight loss to combat obesity and disability in at-risk older adults. J Gerontol A Biol Sci Med Sci. 2017 Jan 6; doi: 10.1093/gerona/glw252. pii: glw252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gold M, Siegel J, Russell L, Weinstein M, editors. Cost-Effectiveness in Health and Medicine. Oxford University Press; 1996. [Google Scholar]
- 63.Rothberg A, Peeters A, Herman W. Handbook of Obesity Vol 2: Clinical Applications. Informa Healthcare; 2014. The Cost-Effectiveness of Obesity Prevention and Treatment. [Google Scholar]
- 64.NIH Reporter. [Accessed March 27, 2017];2017 https://projectreporter.nih.gov/reporter.cfm.
- 65.Chomentowski P, Dubé JJ, Amati F, Stefanovic-Racic M, Zhu S, Toledo FG, et al. Moderate exercise attenuates the loss of skeletal muscle mass that occurs with intentional caloric restriction-induced weight loss in older, overweight to obese adults. J Gerontol A Biol Sci Med Sci. 2009 May;64(5):575–80. doi: 10.1093/gerona/glp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bouchonville M, Armamento-Villareal R, Shah K, Napoli N, Sinacore DR, Qualis C, et al. Weight loss, exercise, or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int J Obes (Lond) 2014 Mar;38(3):423–31. doi: 10.1038/ijo.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Espeland M, Glick HA, Bertoni A, Brancati FL, Bray GA, Clark JM, et al. Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with type 2 diabetes: the action for health in diabetes. Diabetes Care. 2014 Sep;37(9):2548–56. doi: 10.2337/dc14-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koekkoek K, van Zanten AR. Nutrition in the critically ill patient. Curr Opin Anaesthesiol. 2017 Apr;30(2):178–185. doi: 10.1097/ACO.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 69.Holst M, Beermann T, Mortensen MN, Skadhauge LB, KØhler M, Lindorff-Larsen K, et al. Optimizing protein and energy intake in hospitals by improving individualized meal serving, hosting and the eating environment. Nutrition. 2017 Feb;34:14–20. doi: 10.1016/j.nut.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 70.Ferrando AA, Lane H, Stuart C, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996 Apr;270(4 Pt 1):E627–33. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- 71.Kortebein P, Symons TB, Ferrando A, Paddon-Jones D, Ronsen O, Protas E, et al. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol A Biol Sci Med Sci. 2008 Oct;63(10):1076–81. doi: 10.1093/gerona/63.10.1076. [DOI] [PubMed] [Google Scholar]
- 72.Coker R, Hays NP, Williams RH, Xu L, Wolfe RR, Evans WJ. Bed rest worsens impairments in fat and glucose metabolism in older, overweight adults. J Gerontol A Biol Sci Med Sci. 2014 Mar;69(3):363–70. doi: 10.1093/gerona/glt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tanner RE, Brunker LB, Agergaard J, Barrows KM, Briggs RA, Kwon OS, et al. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J Physiol. 2015 Sep 15;593(18):4259–73. doi: 10.1113/JP270699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Creditor M. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;1:219–223. doi: 10.7326/0003-4819-118-3-199302010-00011. [DOI] [PubMed] [Google Scholar]
- 75.Milne A, Potter J, Avenell A. Protein and energy supplementation in elderly people at risk for malnutrition. Cochrane Database Syst Rev. 2002;3:CD003288. doi: 10.1002/14651858.CD003288. [DOI] [PubMed] [Google Scholar]
- 76.Wall B, Dirks M, van Loon LJ. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev. 2013 Sep;12(4):898–906. doi: 10.1016/j.arr.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 77.Coker R, Wolfe RR. Bedrest and sarcopenia. Curr Opin Clin Nutr Metab Care. 2012 Jan;15(1):7–11. doi: 10.1097/MCO.0b013e32834da629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferrando AA, Paddon-Jones D, Hays NP, Kortebein PM, Ronsen O, Williams RH, et al. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr. 2010 Feb;29(1):18–23. doi: 10.1016/j.clnu.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 79.Drummond MJ, Dickinson JM, Fry CS, Walker DK, Gundermann DM, Reidy PT, et al. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab. 2012 May 15;302(9):E1113–22. doi: 10.1152/ajpendo.00603.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JM, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013 Aug;14(8):542–59. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 81.Jankovic N, Geelen A, Streppel MT, de Groot LC, Kiefte-de Jong JC, Orfanos, et al. WHO guidelines for a healthy diet and mortality from cardiovascular disease in European and American elderly: the CHANCES project. Am J Clin Nutr. 2015 Oct;102(4):745–56. doi: 10.3945/ajcn.114.095117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Motil K, Matthews DE, Bier DM, Burke JF, Munro HN, Young VR. Whole-body leucine and lysine metabolism: response to dietary protein intake in young men. Am J Physiol Endocrinol Metab. 1981 Jun;240(6):E712–21. doi: 10.1152/ajpendo.1981.240.6.E712. [DOI] [PubMed] [Google Scholar]
- 83.Paddon-Jones D, Sheffield M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004 Mar;286(3):E321–8. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 84.Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular but not intracellular amino acid availability. J Physiol. 2003 Oct 1;552(Pt 1):315–324. doi: 10.1113/jphysiol.2003.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carroll C, Fluckey J, Williams RH, Sullivan D, Trappe T. Human soleus and vastus lateralis muscle protein metabolism with an amino acid infusion. Am J Physiol Endocrinol Metab. 2005;288:E479–485. doi: 10.1152/ajpendo.00393.2004. [DOI] [PubMed] [Google Scholar]
- 86.Kim I-Y, Schutzler S, Schrader A, Spencer HJ, Azhar G, Ferrando AA, et al. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. Am J Physiol Endocrinol Metab. 2016 Jan 1;310(1):E73–80. doi: 10.1152/ajpendo.00365.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wolfe R. The role of dietary protein in optimizing muscle mass, function and health outcomes in older individuals. Br J Nutr. 2012 Aug;108(Suppl 2):S88–93. doi: 10.1017/S0007114512002590. [DOI] [PubMed] [Google Scholar]
- 88.Wolfe RR, Miller SL, Miller KB. Optimal protein intake in the elderly. Clin Nutr. 2008;27(5):675–684. doi: 10.1016/j.clnu.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 89.Noakes M, Keogh J, Foster P, Clifton P. Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional high-carbohydrate, low-fat diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am J Clin Nutr. 2005 Jun;81(6):1298–306. doi: 10.1093/ajcn/81.6.1298. [DOI] [PubMed] [Google Scholar]
- 90.Sacks F, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Evans W. Skeletal muscle loss, cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S–1127S. doi: 10.3945/ajcn.2010.28608A. [DOI] [PubMed] [Google Scholar]
- 92.Backx E, Tieland M, Borgonjen-van den Berg KJ, Claessen PR, van Loon LJ, et al. Protein intake and lean body mass preservation during energy intake restriction in overweight older adults. Int J Obes (Lond) 2016 Feb;40(2):299–304. doi: 10.1038/ijo.2015.182. [DOI] [PubMed] [Google Scholar]
- 93.Kim JE, O’Connor L, Sands L, Slebodnik M, Campbell W. Effects of dietary protein intake on body composition changes after weight loss in older adults: a systematic review and meta-analysis. Nutrition Reviews. 2016 Mar;74(3):210–24. doi: 10.1093/nutrit/nuv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Katsanos C, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006 Aug;291(2):E381–7. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 95.Coker RH, Miller SL, Schutzler S, Deutz NE, Wolfe RR. Whey protein and essential amino acids promote the reduction of adipose tissue and increased muscle protein synthesis during caloric restriction-induced weight loss in elderly, obese individuals. Nutr J. 2012 Dec 11;11:105. doi: 10.1186/1475-2891-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paddon-Jones D, Sheffield-Moore M, Katsanos C, Zhang X-J, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006 Feb;41(2):215–9. doi: 10.1016/j.exger.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 97.Lean M, Han T, Prvan T, Richmond P, Avenell A. Weight loss with high and low carbohydrate 1200 kcal diets in free living women. Eur J Clin Nutr. 1997 Apr;51(4):243–8. doi: 10.1038/sj.ejcn.1600391. [DOI] [PubMed] [Google Scholar]
- 98.Graber T, Borack M, Reidy P, Volpi E, Rasmussen BB. Essential amino acid ingestion alters expression of genes associated with amino acid sensing, transport, and mTORC1 regulation in human skeletal muscle. Nutr Metab. 2017 May 11;14:35. doi: 10.1186/s12986-017-0187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998 Apr 15;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 100.Sanchez-Rodriguez D, Marco E, Ronquillo-Moreno N, Miralles R, Vázquez-Ibar O, Escalada F, et al. Prevalence of malnutrition and sarcopenia in a post-acute care geriatric unit: Applying the new ESPEN definition and EWGSOP criteria. Clin Nutr. 2016 Sep 9; doi: 10.1016/j.clnu.2016.08.024. pii: S0261-5614(16)30233-3. [DOI] [PubMed] [Google Scholar]