Abstract
Background
Concurrent alcohol use among cocaine abusers is common but the behavioral variables that promote co-abuse are not well understood. The present study examined the effects of intragastric (i.g.) ethanol (EtOH) administration in monkeys responding under a schedule of cocaine reinforcement in which extensive drug seeking was maintained by conditioned stimuli.
Methods
Four adult male cynomolgus monkeys (Macaca fascicularis) were trained to respond under a second-order fixed-interval (FI) 600-s (fixed-ratio (FR) 30:S) schedule of cocaine (0.003–0.56 mg/kg/injection) presentation. Sessions ended after 5 injections or 90 min had elapsed. Different EtOH doses (0.5–2.0 g/kg, i.g.) were administered 30 min before the session, typically on Tuesdays and Fridays. Blood ethanlol concentrations (BECs) were also assessed. Pattern of FI responding was assessed by determining quarter-life (QL) values.
Results
Cocaine self-administration was characterized as an inverted U-shaped function of dose; QL values increased monotonically with dose. EtOH pretreatments dose-dependently decreased self-administration at several cocaine doses in 3 of 4 monkeys. In one animal, EtOH increased low-dose cocaine-maintained responding. For all monkeys, QL values were increased by EtOH when low- and high-cocaine doses were self-administered, suggesting additive effects of EtOH and cocaine. Furthermore, BECs were not altered following cocaine self-administration.
Conclusions
The reductions in cocaine self-administration and the increases QL values following EtOH, suggest that EtOH was enhancing cocaine-related conditioned reinforcement. A better understanding of the behavioral mechanisms that mediate the co-abuse of alcohol and cocaine will lead to improved treatments for both drugs.
Keywords: Cocaine, Ethanol, Second-order schedules, Conditioned stimuli, Cynomolgus monkey
1. INTRODUCTION
The ubiquity of poly-substance use among drug abusers is a well-documented characteristic that creates additional challenges related to psychosocial functioning and treatment outcome compared to patients abusing only one drug. Epidemiological evidence indicates that the majority of cocaine abusers report concurrent (past month) and simultaneous alcohol use (Grant and Harford, 1990; Helzer and Pryzbeck, 1988; Regier et al., 1990; SAMSHA, 2013; Tziortzis et al., 2011). In addition, 60–85 percent of treatment-seeking cocaine abusers also meet diagnostic criteria for lifetime alcohol dependence (Heil et al., 2001; Higgins et al., 1994; SAMSHA, 2013). Furthermore, clinical evidence indicates that alcohol consumption can induce relapse among cocaine abusers during abstinence (McKay et al., 1999) and treatment outcomes of patients with alcohol and cocaine co-dependence are often less successful than those of patients only dependent on cocaine (Anderson et al., 2009; Heil et al., 2001; Schmitz et al., 1997).
Despite the widespread prevalence of alcohol and cocaine co-abuse, the underlying behavioral mechanisms are not clear. In fact, preclinical studies using nonhuman primates have shown that ethanol (EtOH) either has no effect on cocaine self-administration (Czoty, 2015; Winger et al., 2007) or results in an increase in cocaine self-administration in only a subset of subjects (Aspen and Winger, 1997). In contrast, data from studies involving human subjects have shown that the combination of alcohol and cocaine produced more pleasurable subjective effects compared to either drug alone, as well as an increase in cocaine preference over alternative monetary reinforcers (Farré et al., 1997; Higgins et al., 1996).
A potentially important difference between these human and animal studies is the route of EtOH administration, which was oral in people but intravenous in monkeys. Pharmacokinetic studies in humans demonstrate that orally administered EtOH prior to cocaine increases blood cocaine levels, decreases cocaine clearance, and produces the active metabolite cocaethylene (Farré et al. 1993, 1997; McCance-Katz et al. 1993, 1998; Perez-Reyes and Jeffcoat, 1992). Thus, the insensitivity of cocaine self-administration to EtOH pretreatment in previous preclinical studies may have been due to a dissimilar pharmacokinetic interaction between cocaine and intravenous alcohol. In order to provide a more translational assessment, the first aim of the present study was to examine the effects of intragastric (i.g.) administration of EtOH on cocaine self-administration. Surprisingly, the effects of IG EtOH on cocaine self-administration have not been examined in animals.
Another aim of the present study was to extend the findings of Czoty (2015), which examined the effects of EtOH on cocaine self-administration under a fixed-interval (FI) schedule to include the role of behavior maintained by conditioned stimuli in EtOH-induced changes in cocaine-maintained behavior. To better understand how drugs affect FI responding, investigators can measure pattern of responding by calculating quarter-life (QL) values. QL indicates where within the FI the animal emitted 25% of its total responses. For example, QL values of 25% indicate a constant rate of responding throughout the interval and values above 25% indicate FI schedule-appropriate responding. Drugs that increase or decrease FI responding with an accompanying decrease in QL would indicate disruptions in schedule-appropriate responding. To examine behavior maintained by conditioned stimuli, monkeys were trained to self-administer cocaine under a second-order schedule of reinforcement. Responding maintained under these schedules can be used to examine the effects of drug pretreatments on self-administration that are less influenced by the reinforcer because extended sequences of behavior can be maintained by relatively few drug injections through presentations of a stimulus associated with the drug reinforcer (Kelleher, 1966). Behavior controlled under these conditions is analogous to the effectiveness of drugs in maintaining the long sequences of behaviors that are characteristic of human drug abusers involving the purchase, preparation and administration of the drug itself. Thus, examining the effects of EtOH on cocaine self-administration under a second-order schedule may lead to a better understanding of the environmental-pharmacological interactions that may promote co-abuse of alcohol and cocaine.
2. MATERIAL AND METHODS
2.1. Subjects
Four adult male cynomolgus monkeys (Macaca fascicularis), with an extensive history of cocaine self-administration (~ 8 years) served as subjects. Monkeys were pair-housed in stainless-steel cages (76 x 60 x 70 cm), except during operant behavioral sessions and feeding, when the monkeys were housed individually. Each monkey was fitted with an aluminum collar (Model B008, Primate Products, Redwood City, CA) and trained to sit calmly in a primate restraint chair (Primate Products). All subjects were fed enough food (Purina LabDiet 5045, St Louis, MO) to maintain healthy body weights as determined by veterinary staff; body weights ranged from 5.3–6.3 kg (Table 1) and did not change significantly during this study. Animal housing and handling and all experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by the Animal Care and Use Committee of Wake Forest University.
Table 1.
Body weights and range of volume of ethanol delivered for each monkey
| Subject | Weight (kg) | Ethanol volume (ml)¶ |
|---|---|---|
| C-6525 | 5.3 | 13.3–53.0 |
| C-7078 | 6.3 | 15.8–63.0 |
| C-7084 | 5.7 | 14.3–57.0 |
| C-7427 | 6.0 | 6.0–60.0 |
The range was based on lowest and highest EtOH doses tested
2.2. Surgery
Subjects were prepared with chronic indwelling venous catheters under aseptic conditions. Monkeys were initially anesthetized with ketamine (10 mg/kg, i.m.) and maintained with isoflurane throughout surgery. A catheter was inserted into a major vein (femoral, internal or external jugular) to the level of the posterior vena cava. The distal end of the catheter was passed subcutaneously to an incision made slightly off the midline of the back and attached to a subcutaneous vascular access port (Access Technologies, Skokie, IL). After surgery, an analgesic dose of ketoprofen (5.0 mg/kg, i.m.) was administered SID for three days.
2.3. Apparatus
Behavioral sessions were carried out in ventilated, sound-attenuating chambers (1.5 x 0.74 x 0.76 m; Med Associates, East Fairfield, VT) designed to accommodate a primate chair. An intelligence panel (48 x 69 cm) was located on the right side of the chamber that contained two photo-optic finger-poke apertures (Model 117–1007; Stewart Ergonomics, Inc., Furlong, PA) on each side with a horizontal row of three stimulus lights positioned 14 cm above each finger poke. For these studies, cocaine was available by responding on one of the two finger-pokes (switches) and the active switch was counterbalanced between monkeys. A food receptacle was located between the switches and connected with a Tygon tube to a pellet dispenser (Med Associates) located on the top of the chamber (food-reinforced responding was not examined in these monkeys). A peristaltic infusion pump (7531–10, Cole-Parmer Co., Chicago, IL) for delivering drug injections at a rate of approximately 1.5 ml/10 sec was also located on the top of the chamber. White noise was continuously present in the room to mask extraneous noise.
2.4. Cocaine self-administration procedure
Monkeys were trained to self-administer cocaine under a second-order fixed-interval (FI) 600-s [fixed-ratio (FR) 30:S] schedule of i.v. drug injection. First, responding was maintained by 0.1 mg/kg cocaine under an FI 60-s schedule of reinforcement. Completion of the first response after 60 sec resulted in extinction of the white light above the finger poke, illumination of a red light and delivery of cocaine over 10-sec. The pairing of the red light with the cocaine injection was designed to make the red light a conditioned stimulus (CS). Over a 2–3 week period, the FI value was gradually increased to 600 sec and the schedule changed to a second-order FI 600-sec (FR 30:S), such that completion of every 30th response (FR 30) during the 600-s FI resulted in extinction of the white light and illumination of the red light for 2-s. Once the interval elapsed, the first FR 30 completed produced an intravenous injection of cocaine delivered over 10 s paired with the red light. A 60-s time out followed each injection, during which all lights were off and responses had no scheduled consequences. Daily sessions ended after the completion of five cycles of the second-order schedule or 90 min elapsed, whichever occurred first.
Once responding at the training dose of cocaine (0.1 mg/kg/injection) was deemed stable (overall response rates for 3 consecutive sessions were within ± 20% of the mean rate for those sessions with no trends), saline was substituted for cocaine and the CS was removed for at least five consecutive sessions and until responding declined to < 20% of baseline rates and was deemed stable. After re-establishing cocaine self-administration (0.1 mg/kg/injection) in the presence of the CS, different doses of cocaine (0.003–0.56 mg/kg/injection) were substituted for the training dose for at least five consecutive sessions and until responding was stable. Cocaine doses were tested in random order for each animal and there was a minimum 3-session return to the training dose between each substitution.
After the completion of the cocaine dose-response curve, monkeys were adapted to the passing of an infant feeding tube (5 French, 1.7 mmx381 mm) down the nose, through the esophagus, and into the stomach for intragastric (i.g.) intubation of EtOH. Training occurred two to three times per week, for at least two months during which water (5 ml/kg) was administered 30 min prior to the self-administration session. Following training, EtOH (0.5 – 2.0 g/kg; see Table 1 for range of volumes administered) or a comparable volume of water (5 ml/kg) was administered i.g. 30 min prior to the start of the cocaine self-administration session. This pretreatment time was selected on the basis of the time course for the discriminative stimulus effects of i.g. EtOH in male cynomolgus monkeys (Helms et al., 2009). EtOH test sessions were typically conducted on Tuesdays and Fridays and vehicle (water) pretreatments were typically on Thursdays. All doses of EtOH were tested on each point of the cocaine self-administration dose-response curve in random order and each dose was determined two to three times in each monkey.
2.5. Blood Ethanol Concentrations (BECs)
Blood samples (20 μl) were collected from the saphenous vein in alert animals while seated in the primate chair. Samples were collected at 30 and 120 min after 2.0 g/kg (i.g.) EtOH was administered when there was no cocaine self-administration sessions conducted (baseline) and on days when cocaine self-administration (0.1 mg/kg/injection) was studied. The order of these two conditions was counterbalanced between monkeys. Each sample was sealed in airtight vials containing 500 μl of distilled water and 20 μl of isopropanol (10%; internal standard) and stored at −4°C until assayed using gas chromatography (Agilent 7890A GC system with G1888 Network Headspace Autosampler Santa Clara, CA) supplied with a flame ionization detector and Agilent ChemStation integrator.
2.6. Data Analysis
The overall mean rate of responding was plotted as a function of the unit dose of cocaine for individual monkeys. The effect of EtOH pretreatment on cocaine self-administration for each subject was analyzed by two-way analysis of variance (ANOVA) followed by post-hoc Dunnett’s test to compare each dose of EtOH with vehicle pretreatment. Mean data were normalized to the percentage of responding maintained during baseline sessions (i.e., when no pretreatment was administered). However, because of individual differences in the sensitivity to the reinforcing effects of cocaine under the second-order schedule, the dose range over which drug effects were examined was not the same for each subject. Therefore, in order to ensure that average drug effects were analyzed on the same point of the dose-response curve for each subject, unit doses of cocaine were defined as low (i.e., lowest dose that maintained responding significantly above saline rates), peak (i.e., dose that maintained maximal rates of responding), and high (i.e., a dose that maintained responding significantly above saline rates, but on the descending limb of the cocaine dose-response curve). The peak cocaine dose was 0.1 mg/kg/injection for all subjects, the low cocaine dose was 0.003 (C-7078), 0.01 (C-7084), and 0.03 (C-6526 and C-7427) mg/kg/injection and the high cocaine dose was 0.3 (C-7078 and C-7084) and 0.56 (C-6526 and C-7427) mg/kg/injection.
Quarter-life (QL) values were also calculated in order to provide an index of the temporal pattern of FI responding (Catania and Reynolds, 1968; Gollub, 1964; Hernstein and Morse, 1957). To calculate QL values, the 10-min FI was divided into 10 bins (60 sec/bin) and the number of responses in each bin was recorded. To calculate effects of EtOH pretreatments on different cocaine doses, mean QL values for a session were expressed as a percent of baseline for each cocaine dose. Average changes in response rates and QL values were analyzed by two-way repeated measures ANOVA with cocaine dose and EtOH dose as the main factors. A significant ANOVA was followed by post hoc Dunnett’s test to compare test conditions with baseline conditions. For all analyses, p < 0.05 was considered statistically significant. In no case did 0.5 g/kg EtOH significantly affect responding and was therefore not included in the figures.
2.7. Drugs
(–)-Cocaine HCl (National Institute on Drug Abuse, Bethesda, MD) was dissolved in sterile 0.9 % saline. Ethanol (95% ethyl alcohol; Warner-Graham Co., Cockeysville, MD) was diluted with tap water to a concentration of 20% (w/v) and administered in a volume ranging from 2.5–10 ml/kg, depending on the EtOH dose (Table 1). Ethanol solutions were always prepared the day of administration.
3. RESULTS
3.1. Baseline cocaine self-administration
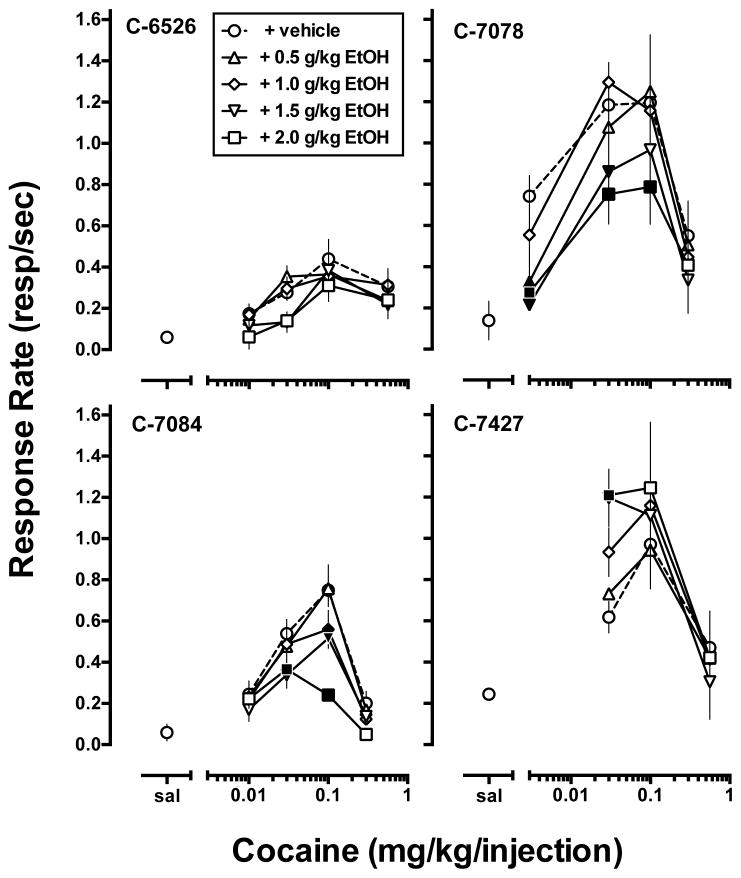
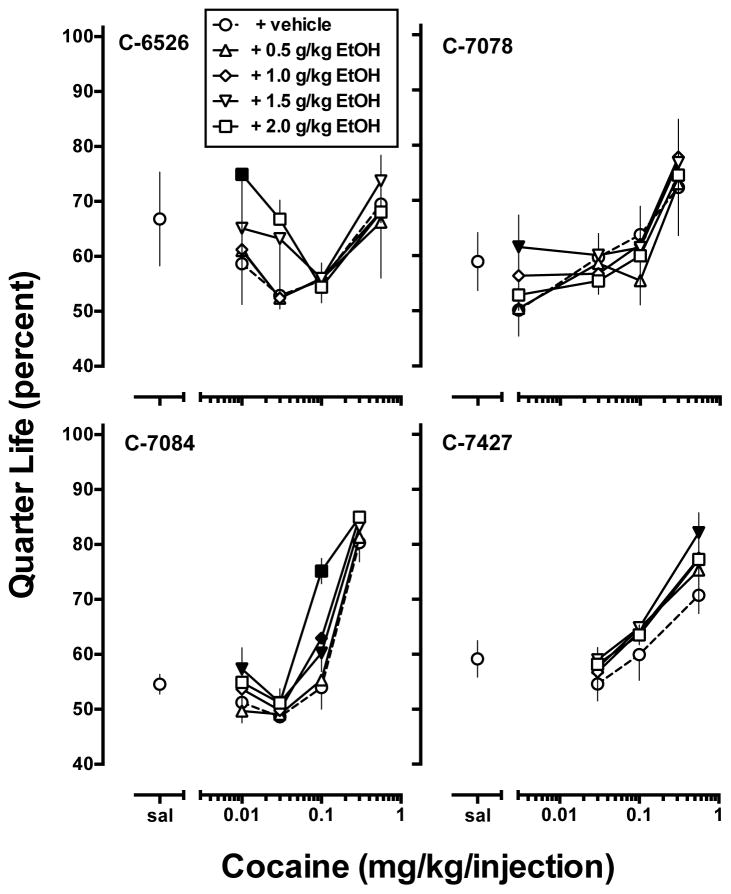
Cocaine maintained overall rates of responding that represented an inverted U-shaped function of dose (Fig. 1, open circles). When saline was substituted for cocaine and the CS was removed, responding rapidly decreased, with mean (± SEM) response rates of 0.13 ± 0.04 responses/sec. Peak responding occurred at 0.1 mg/kg/injection cocaine for all four monkeys with mean (± SEM) response rates of 0.78 ± 0.15 responses/sec. However, different cocaine doses were required for each monkey to generate an inverted U-shaped dose-response curve (Fig. 1). Over this dose range, QL values were linearly related to dose in three of four monkeys, with the lowest QL value associated with lowest reinforcing dose of cocaine and the highest QL value associated with the highest reinforcing cocaine dose (Fig. 2). More specifically, for all but monkey C-6526, the lowest and highest cocaine doses (Fig. 1), while maintaining similar response rates were associated with vastly different QL values (Fig. 2). For each monkey, three cocaine doses were designated low (L), peak (P) and high (H) and represent an inverted U-shaped function dose-response curve that was averaged in Fig. 3. For the group (Fig. 3, open bars), response rates (Fig. 3, top panel) and QL values (Fig. 3, bottom panel) during each of the five available FI 600-s components remained stable throughout the session, irrespective of the cocaine dose self-administered.
Figure 1.
Effects of acute ethanol pretreatment (0.5 – 2.0 g/kg, IG) on cocaine self-administration under a second-order [FI 10 min (FR 30:S)] schedule of reinforcement. Ordinate: mean overall rate of responding (responses/second). Abscissae: unit dose of cocaine (mg/kg/injection) available for self-administration. Data represent mean ± S.D. of at least two EtOH determinations for each subject. Filled symbols indicate a statistically significant difference (P < 0.05) compared to vehicle within a given unit cocaine dose.
Figure 2.
Effects of acute ethanol pretreatment (0.5 – 2.0 g/kg, IG) on cocaine self-administration under a second-order [FI 10 min (FR 30:S)] schedule of reinforcement. Abscissae: unit dose of cocaine available for self-administration representing the low (L), peak (P) and high (H) cocaine doses for each monkey. Left ordinate: overall rate of responding (responses/second). Right ordinate: mean overall quarter-life values (percent of interval). Data represent mean ± S.E.M. of N = 4. Filled symbols indicate a statistically significant difference (P < 0.05) compared to vehicle within a given unit cocaine dose.
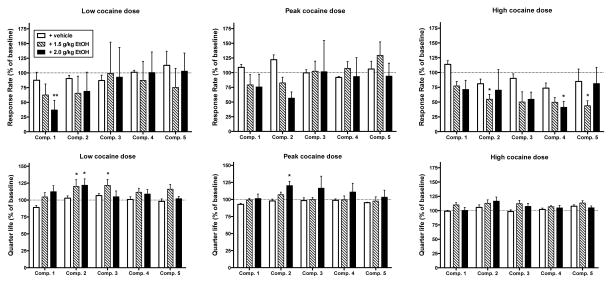
Figure 3.
Effects of ethanol pretreatment (0.5 – 2.0 g/kg, IG) during consecutive components of a second-order [FI 10 min (FR 30:S)] schedule of cocaine self-administration at three cocaine doses. Because the low EtOH doses (0.5 and 1.0 g/kg) did not produce significant effects on response rates or QL values, those data are not shown in the Figure. Abscissae: consecutive components during session. Ordinate (top panel): percent of baseline response rate. Ordinate (bottom panel): percent of baseline quarter-life value. Data represent mean ± S.E.M. of N = 4. * P < 0.05; ** P < 0.01 from baseline.
3.2. Effects of EtOH on cocaine self-administration
Two-way ANOVA performed on response rates for each subject showed a main effect of cocaine dose and a main effect of EtOH treatment for all four monkeys and a significant interaction for C-7084 and C-7427 (Table 2). In three of the four monkeys (C-6526, C-7078, C-7084), EtOH pretreatment produced a dose-dependent downward shift in the dose-response curve (Fig. 1). In contrast, EtOH pretreatment dose-dependently increased overall response rates of the low dose of cocaine in monkey C-7427 (Fig. 1). For QL values, two-way ANOVA showed a main effect of cocaine dose for all four monkeys, a main effect of EtOH treatment in three of the four monkeys, and a significant cocaine x EtOH interaction in C-7084 (Table 2). Post-hoc analysis revealed that EtOH pretreatment significantly increased QL values from vehicle pretreatment for all monkeys; for three subjects (C-6526, C-7078, C-7084) this occurred at a low cocaine dose, for C-7078 there was also a significant increase in QL value when the dose of cocaine that maintained peak rates of responding was available and in C-7427 a significant increase occurred when the highest cocaine dose was self-administered (Fig. 2).
Table 2.
Effects of cocaine dose, ethanol dose and their Interactions in each monkey
| Subject | Cocaine Dose | EtOH Dose | Interaction |
|---|---|---|---|
| Response Rate | |||
| C-6526 | F(3,30)=25.46, P<0.001 | F(4, 30)=5.04, P<0.01 | N.S. |
| C-7078 | F(3, 39)=48.76, P<0.0001 | F(4 39)=9.13, P<0.0001 | N.S. |
| C-7084 | F(3, 29)=67.69, P<0.0001 | F(4, 29)=12.03, P<0.0001 | F(12, 29)=3.41; P<0.01 |
| C-7427 | F(2, 23)=54.99, P<0.0001 | F(4, 23)=3.73, P<0.05 | F(8, 23)=2.69, P<0.05 |
| QL Values | |||
| C-6526 | F(3,30)=12.70, P<0.0001 | F(4, 30)=2.85, P<0.05 | N.S. |
| C-7078 | F(3, 39)=46.89, P<0.0001 | N.S. | N.S. |
| C-7084 | F(3, 29)=262.5, P<0.0001 | F(4, 29)=13.20, P<0.0001 | F(12, 29)=4.58; P<0.001 |
| C-7427 | F(2, 23)=117.7, P<0.0001 | F(4, 23)=6.16, P<0.01 | N.S. |
N.S., Not Significant
The time course and magnitude of EtOH’s effects on cocaine-maintained responding depended on the dose of cocaine available for self-administration (Fig. 3, top panel). Repeated measures two-way ANOVA revealed a significant component X treatment interaction (F(60, 180)=1.56, P<0.05). For the average effects of EtOH on QL values for each FI component (Fig. 3, bottom panel), there was a main effect of EtOH treatment (F(15, 45)=3.27, P<0.01) but no main effect of component (F(4, 12)=2.95, P=0.07) or component X EtOH treatment interaction (F(60, 180)=0.69, P=0.95). For the low cocaine dose, 2.0 g/kg EtOH significantly decreased response rates in the first component; however, these decreases recovered to baseline levels during the second component and for the remainder of the session. The decrease in response rates seen in component 1 did not result in significant changes in QL value (Fig. 3, bottom left panel). In contrast, mean rates of responding across components at the peak of the cocaine dose-response curve were not altered by EtOH pretreatment. The non-significant reduction in response rate seen in component 2 was associated with a significant increase in QL value (Fig. 3, bottom middle panel). At the high cocaine dose, the effects of EtOH were larger and longer lasting. Post-hoc analysis revealed significant reductions in rates of responding during component 2 that persisted throughout the remainder of the session. These reductions in response rates were not associated with significant changes in QL values (Fig. 3 bottom right panel).
3.3. Blood Ethanol Concentrations
Under conditions in which cocaine self-administration was not studied, 30 min after administration of 2.0 g/kg EtOH (i.g.) resulted in BEC values of 61.79 (± 15.96 SEM) mg/dl, which rose to 140.40 (± 19.20) mg/dl 120 min after administration (Table 3). When 0.1 mg/kg/injection cocaine was made available for self-administration 30 minutes after 2.0 g/kg EtOH (i.g.), the average 30 and 120 min BECs were not significantly affected (P=0.62). Interestingly, the one monkey in whom EtOH pretreatment produced an increase in the cocaine-maintained responding had the lowest 120-min BEC after administration of 2.0 g/kg EtOH (Table 3).
Table 3.
Blood ethanol concentrations (BEC) following 2.0 g/kg (IG) EtOH at baseline and when cocaine self-administration (0.1 mg/kg/injection) was studied
| Baseline | Cocaine Self-Administration | |||
|---|---|---|---|---|
|
| ||||
| Monkey | 30-min BEC (mg/dl) | 120-min BEC (mg/dl) | 30-min BEC (mg/dl) | 120-min BEC (mg/dl) |
| C-7427 | 39.88 | 114.19 | 54.11 | 83.39 |
| C-6526 | 46.61 | 120.33 | 49.56 | 130.43 |
| C-7084 | 101.49 | 187.58 | N.D. | N.D. |
| C-7078 | 59.19 | 139.51 | 43.30 | 139.01 |
N.D., Not determined
4. DISCUSSION
The main finding from the present study was that intragastric administration of EtOH significantly decreased rates of cocaine self-administration under a second-order FI 10-min (FR 30:S) schedule of reinforcement. Examination of the time course of the effects of EtOH when the highest cocaine dose was available, indicates decreases in cocaine self-administration without significant changes in patter of responding, suggesting that the effects of EtOH were not due to EtOH-induced disruptions in schedule-appropriate responding. Furthermore, self-administered cocaine did not affect BECs, consistent with human studies (Farré et al. 1993, 1997; Higgins et al., 1993; McCance-Katz et al. 1993; Perez-Reyes and Jeffcoat 1992). These results extend previous preclinical animal research investigating the interactions of intravenous EtOH and cocaine (Aspen and Winger, 1997; Czoty, 2015, 2016; Winger et al., 2007) to intragastric administration of EtOH and to a schedule of cocaine self-administration that utilized conditioned stimuli to maintain drug seeking.
Czoty (2015) reported that in monkeys self-administering cocaine under an FI 5-min schedule of reinforcement, 1.0 g/kg EtOH (i.v.) significantly decreased reinforcement frequency when a low dose of cocaine was self-administered. Aspen and Winger (1997) studied self-administered cocaine under an FR schedule of reinforcement and only examined the ascending limb of the cocaine dose-response curve; they found modest (non-significant) EtOH-induced increases in responding. One possibility for a lack of EtOH-induced increases in low-dose cocaine-maintained responding in the present study was that under these schedule conditions, monkeys typically received the maximum number of cocaine injections per session, while that did not occur when cocaine doses on the ascending limb were available to monkeys in the Aspen and Winger (1997) and Czoty (2015) studies. This may suggest a direct pharmacological interaction between low cocaine doses and EtOH, rather than an interaction between EtOH and cocaine-associated conditioned stimuli.
This study also differed from previous studies in that EtOH pretreatment was administered via the intragastric route as opposed to the intravenous route. As a result, dissimilar effects of EtOH on cocaine self-administration may be due in part to the pharmacokinetic interaction between alcohol and cocaine as determined by the route of EtOH administration. Studies in humans examining the pharmacokinetic interaction of EtOH and cocaine co-administration showed the time course of effects first involved an initial increase in blood levels of cocaine compared to cocaine alone followed by the formation of cocaethylene, which potentiated the subjective and physiological effects of cocaine (Farré et al. 1993, 1997; Foltin and Fischman, 1988; Higgins et al., 1994; McCance-Katz et al. 1993, 1998; Perez-Reyes and Jeffcoat 1992;). However, by avoiding first-pass metabolism, intravenous alcohol administration likely resulted in lower amounts of cocaethylene thereby limiting or completely eliminating its effect on cocaine-maintained responding. Therefore, one possibility for EtOH’s rate-decreasing effects on cocaine self-administration in the present study was a more prominent effect of cocaethylene from intragastric-administered EtOH compared to intravenous EtOH. This hypothesis is supported by the fact that EtOH was most effective at decreasing cocaine-maintained responding across consecutive components of the session when the highest dose of cocaine was available for self-administration. Future studies that systematically examine the temporal contiguity of cocaethylene on cocaine self-administration would help to elucidate the pharmacokinetic factors that likely mediate the co-abuse between alcohol and cocaine.
In terms of behavioral mechanisms involved in this interaction, alcohol consumption has been reported to increase cocaine craving and has also been shown to be an antecedent to cocaine relapse (Marks et al., 2015; McKay et al., 1999). It is well established that cocaine-associated cues are involved in maintaining drug-taking behavior (Childress et al., 1999; Kosten et al., 2006; Sinha and Li, 2007; Volkow et al., 2008) and attentional bias towards such cues has been positively correlated with cocaine craving (Field et al., 2009; Leeman et al., 2014; Rosse et al., 1997). Thus, it is possible that one mechanism mediating co-abuse involve an increase in the salience of cocaine-associated cues via the pharmacological effects of alcohol. Examining responding in the first component of the second-order schedule, in which behavior was maintained by a conditioned stimulus in the absence of cocaine, allowed us to test this hypothesis. In the present study, responding during the first component was dose-dependently decreased following EtOH pretreatment only when the lowest cocaine dose was self-administered, presumably the dose that resulted in the weakest CS effects. Thus, alcohol pretreatments did not enhance the effects of weak cocaine-related conditioned stimuli. We hypothesized that decreases in FI responding and increases in schedule-appropriate responding (i.e., QL values) would be suggestive of an interaction between EtOH and cocaine-related CSs, since high cocaine doses maintained low response rates and high QL values. In the present study, when EtOH decreased cocaine self-administration, this was accompanied by either no change or increases in QL value.
While alcohol administration decreased cocaine-maintained responding in three monkeys, we did note individual differences such that intragastric EtOH administration dose-dependently increased responding maintained by a low dose of cocaine in one monkey (C-7427). Previous studies have shown that the stimulant effects of EtOH on schedule-controlled responding can be rate-dependent (Barrett and Stanley, 1980; Katz and Barrett, 1978, 1979; Leander et al., 1976), which offers one possibility for the individual differences observed here. However, another subject (C-7078) that had similar response rates at the same point on the cocaine dose-response curve did not demonstrate increases in responding following EtOH pretreatment. In addition, baseline QL values for the low cocaine dose were similar among all subjects suggesting that the increase in cocaine-maintained responding for the one subject was not due to differences in the rate and pattern of responding.
Individual differences in the pharmacokinetic interaction between alcohol and cocaine offer a more intriguing hypothesis for differential effects of EtOH. Interestingly, the one subject in which EtOH increased cocaine-maintained responding had the lowest baseline BEC values among the group at 30- and 120-min post 2.0 g/kg EtOH administration. Conversely, the subject that had the highest baseline BEC values demonstrated the largest reduction in rate of responding following 2.0 g/kg EtOH pretreatment. These data suggest that the behavioral effects of EtOH on cocaine self-administration could be related to individual differences in EtOH metabolism and the point on the BEC curve that cocaine self-administration occurred. Supporting this hypothesis, studies in humans indicated that alcohol produced differential subjective, behavioral, and physiological effects depending on whether measures were taken on the ascending or descending limb of the BEC curve (Babor et al., 1983; Conrod et al., 1997a, 1997b, 2001; Earleywine and Erblich, 1996; Hendler et al., 2013; Martin et al., 1993; Pohorecky, 1977). It is unknown how this biphasic pattern of alcohol-induced stimulation and sedation affects cocaine self-administration. In the present study, it is difficult to fully interpret this interaction without knowing the full BEC curve for each subject. Another study using the same species of nonhuman primates and the intragastric route of EtOH administration, found that the peak BEC values were reached at 60 min for all subjects, but the rate of EtOH elimination showed between-subject variability (Green et al., 1999). Studies in humans have also shown that EtOH elimination rates are highly variable, even using within-subject assessments (Thomasson, 1995; Wilson and Erwin, 1983). Thus, a confluence of factors involving time of administration, ascending vs. descending limb of BEC curve, and rate of change in BEC could all play a role in the individual differences in the effects of EtOH on cocaine self-administration. Future studies employing varying EtOH pretreatment times to systematically examine cocaine self-administration during different segments of the BEC curve would greatly enhance our understanding of the role pharmacokinetics play in co-abuse of alcohol and cocaine.
There are some limitations to this study. First, it should be noted that ethanol was experimenter-administered rather than self-administered. Previous research has shown that the neurochemical and behavioral effects of drugs, including ethanol, differ depending on whether they were administered contingently vs. non-contingently (Dworkin et al., 1995; Moolten and Kornetsky, 1990; Porrino et al., 1984; Smith et al., 1984). Therefore, the results from the present study should be considered in the context of these differences. Also, we only examined acute EtOH effects while the clinical situation typically involves more frequent alcohol use with cocaine. In addition, under the conditions of the present study, monkeys typically earned all cocaine injections (5 maximum injections per session), so it was not possible to observe EtOH-induced increases in cocaine intake. Finally, while we assessed BECs, we did not measure cocaethylene. In summary, these findings suggest that EtOH decreased cocaine self-administration in a manner similar to increasing the cocaine dose, suggesting that EtOH enhanced the strength of the cocaine-associated conditioned stimulus. A better understanding of the behavioral and pharmacological mechanisms that mediate the co-abuse between alcohol and cocaine should lead to improved treatments for these individuals.
Highlights.
Ethanol decreased cocaine self-administration under a second-order schedule
Ethanol enhanced conditioned reinforcing effects of cocaine
Cocaine did not affect blood ethanol concentrations (BECs) following 2.0 g/kg ethanol
Individual differences were noted and related to ethanol pharmacokinetics
Acknowledgments
This work was supported by the National Institutes of Health grants R37 DA10584 (MAN), P50 DA06634 (MAN), F31 DA041825 (WSJ) and T32 AA-007565 (WSJ). BEC analyses were supported by P01 AA021099. The authors thank Dr. Paul Czoty for comments on an earlier version of the manuscript and April Davenport and Phillip Epperly for assistance with the BEC determinations.
Footnotes
CONTRIBUTIONS. WSJ and MAN designed the studies. WSJ conducted the experiments and analyzed the data. WSJ and MAN wrote the manuscript William S. John and Michael A. Nader certify that they have seen and approved the final version of the manuscript being submitted. They warrant that the article is the authors’ original work, hasn’t received prior publication and isn’t under consideration for publication elsewhere.
CONFLICT OF INTEREST. The authors declare no conflicts of interest.
Author Agreement/Declaration: William S. John and Michael A. Nader certify that they have seen and approved the final version of the manuscript being submitted. They warrant that the article is the authors’ original work, hasn’t received prior publication and isn’t under consideration for publication elsewhere.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC, 3rd, Elkashef AM. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspen JM, Winger G. Ethanol effects on self-administration of alfentanil, cocaine, and nomifensine in rhesus monkeys. Psychopharmacology. 1997;130:222–227. doi: 10.1007/s002130050232. [DOI] [PubMed] [Google Scholar]
- Barrett JE, Stanley JA. Effects of ethanol on multiple fixed-interval fixed-ratio schedule performances: dynamic interactions at different fixed-ratio values. J Exp Anal Behav. 1980;34:185–198. doi: 10.1901/jeab.1980.34-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Berglas S, Mendelson JH, Ellingboe J, Miller K. Alcohol, affect, and the disinhibition of verbal behavior. Psychopharmacology. 1983;80:53–60. doi: 10.1007/BF00427496. [DOI] [PubMed] [Google Scholar]
- Catania AC, Reynolds GS. A quantitative analysis of the responding maintained by interval schedules of reinforcement. J Exp Anal Behav. 1968;11:327–383. doi: 10.1901/jeab.1968.11-s327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO. Disinhibited personality and sensitivity to alcohol reinforcement as predictors of drinking behaviour. Alcohol Clin Exp Res. 1997a;21:1320–1332. [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO. Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology. 2001;157:20–30. doi: 10.1007/s002130100741. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO, Mankowski S. The bi-phasic effects of alcohol on heart rate are influenced by alcoholic family history and rate of alcohol ingestion. Alcohol Clin Exp Res. 1997b;21:140–149. [PubMed] [Google Scholar]
- Czoty PW. Effects of chronic binge-like ethanol consumption on cocaine self-administration in rhesus monkeys. Drug Alcohol Depend. 2015;153:278–285. doi: 10.1016/j.drugalcdep.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW. Lack of effect of ethanol on cocaine prime-induced reinstatement of extinguished cocaine self-administration in rhesus monkeys. Behav Pharmacol. 2016;27:633–636. doi: 10.1097/FBP.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology. 1995;117:262–266. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- Earleywine M, Erblich J. A confirmed factor structure for the biphasic alcohol effects scale. Exp Clin Psychopharmacol. 1996;4:107–113. [Google Scholar]
- Farré M, de la Torre R, Gonzalez ML, Teran MT, Roset PN, Menoyo E, Cami J. Cocaine and alcohol interactions in humans: neuroendocrine effects and cocaethylene metabolism. J Pharmacol Exp Ther. 1997;283:164–176. [PubMed] [Google Scholar]
- Farré M, de la Torre R, Llorente M, Lamas X, Ugena B, Segura J, Camí J. Alcohol and cocaine interactions in humans. J Pharmacol Exp Ther. 1993;266:1364–1373. [PubMed] [Google Scholar]
- Field M, Munafò MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull. 2009;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Ethanol and cocaine interactions in humans: cardiovascular consequences. Pharmacol Biochem Behav. 1988;31:877–883. doi: 10.1016/0091-3057(88)90399-1. [DOI] [PubMed] [Google Scholar]
- Gollub LR. The relations among measures of performance on fixed-interval schedules. J Exp Anal Behav. 1964;7:337–343. doi: 10.1901/jeab.1964.7-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Harford TC. Concurrent and simultaneous use of alcohol with cocaine: results of national survey. Drug Alcohol Depend. 1990;25:97–104. doi: 10.1016/0376-8716(90)90147-7. [DOI] [PubMed] [Google Scholar]
- Green KL, Szeliga KT, Bowen CA, Kautz MA, Azarov AV, Grant KA. Comparison of ethanol metabolism in male and female cynomolgus macaques (Macaca fascicularis) Alcohol Clin Exp Res. 1999;23:611–616. [PubMed] [Google Scholar]
- Heil SH, Badger GJ, Higgins ST. Alcohol dependence among cocaine-dependent outpatients: demographics, drug use, treatment outcome and other characteristics. J Stud Alcohol. 2001;62:14–22. doi: 10.15288/jsa.2001.62.14. [DOI] [PubMed] [Google Scholar]
- Helms CM, Rogers LS, Grant KA. Antagonism of the ethanol-like discriminative stimulus effects of ethanol, pentobarbital, and midazolam in cynomolgus monkeys reveals involvement of specific GABA(A) receptor subtypes. J Pharmacol Exp Ther. 2009;331:142–152. doi: 10.1124/jpet.109.156810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzer JE, Pryzbeck TR. The co-occurrence of alcoholism with other psychiatric disorders in the general population and its impact on treatment. J Stud Alcohol. 1988;49:219–224. doi: 10.15288/jsa.1988.49.219. [DOI] [PubMed] [Google Scholar]
- Hendler RA, Ramchandani VA, Gilman J, Hommer DW. Stimulant and sedative effects of alcohol. Curr Top Behav Neurosci. 2013;13:489–509. doi: 10.1007/7854_2011_135. [DOI] [PubMed] [Google Scholar]
- Hernstein RJ, Morse WH. Effects of pentobarbital on reinforced behavior. Science. 1957;125:929–931. doi: 10.1126/science.125.3254.929-a. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Badger GJ. Alcohol dependence and simultaneous cocaine and alcohol use in cocaine-dependent patients. J Addict Dis. 1994;13:177–189. doi: 10.1300/j069v13n04_06. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Roll JM, Bickel WK. Alcohol pretreatment increases preference for cocaine over monetary reinforcement. Psychopharmacology. 1996;123:1–8. doi: 10.1007/BF02246274. [DOI] [PubMed] [Google Scholar]
- Katz JL, Barrett JE. Effects of ethanol on behavior under fixed-ratio, fixed-interval, and multiple fixed-ratio fixed-interval schedules in the pigeon. Arch Int Pharmacodyn Ther. 1978;234:88–96. [PubMed] [Google Scholar]
- Katz JL, Barrett JE. Effects of d-amphetamine and ethanol alone and in combination on schedule controlled responding of pigeons. Psychopharmacology. 1979;64:13–18. doi: 10.1007/BF00427338. [DOI] [PubMed] [Google Scholar]
- Kelleher RT. Conditioned reinforcement in second-order schedules. J Exp Anal Behav. 1966;9:475–485. doi: 10.1901/jeab.1966.9-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent subjects. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Leander JD, McMillan DE, Ellis FW. Ethanol and isopropanol effects on schedule controlled responding. Psychopharmacology. 1976;47:157–164. doi: 10.1007/BF00735815. [DOI] [PubMed] [Google Scholar]
- Leeman RF, Robinson CD, Waters AJ, Sofuoglu M. A critical review of the literature on attentional bias in cocaine use disorder and suggestions for future research. Exp Clin Psychopharmacol. 2014;22:469–483. doi: 10.1037/a0037806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks KR, Pike E, Stoops WW, Rush CR. Alcohol administration increases cocaine craving but not cocaine cue attentional bias. Alcohol Clin Exp Res. 2015;39:1823–1831. doi: 10.1111/acer.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. Concurrent use of cocaine and alcohol is more potent and potentially more toxic than use of either alone—a multiple-dose study. Biol Psychiatry. 1998;44:250–259. doi: 10.1016/s0006-3223(97)00426-5. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Price LH, McDougle CJ, Kosten TR, Black JE, Jatlow PI. Concurrent cocaine-ethanol ingestion in humans: pharmacology, physiology, behavior, and the role of cocaethylene. Psychopharmacology. 1993;111:39–46. doi: 10.1007/BF02257405. [DOI] [PubMed] [Google Scholar]
- McKay JR, Alterman AI, Rutherford MJ, Cacciola JS, McLellan AT. The relationship of alcohol use to cocaine relapse in cocaine dependent patients in an aftercare study. J Stud Alcohol. 1999;60:176–180. doi: 10.15288/jsa.1999.60.176. [DOI] [PubMed] [Google Scholar]
- Moolten M, Kornetsky C. Oral self-administration of ethanol and not experimenter-administered ethanol facilitates rewarding electrical brain stimulation. Alcohol. 1990;7:221–225. doi: 10.1016/0741-8329(90)90008-z. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Jeffcoat AR. Ethanol/cocaine interaction: cocaine and cocaethylene plasma concentrations and their relationship to subjective and cardiovascular effects. Life Sci. 1992;51:553–563. doi: 10.1016/0024-3205(92)90224-d. [DOI] [PubMed] [Google Scholar]
- Pohorecky L. Biphasic action of ethanol. Biobehav Rev. 1977;1:231–244. [Google Scholar]
- Porrino LJ, Esposito RU, Seeger TF, Crane AM, Pert A, Sokolof L. Metabolic mapping of the brain during rewarding self- stimulation. Science. 1984;224:306–309. doi: 10.1126/science.6710145. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Rosse RB, Johri S, Kendrick K, Hess AL, Alim TN, Miller M, Deutsch SI. Preattentive and attentive eye movement during visual scanning of a cocaine cue: correlation with intensity of cocaine cravings. J Neuropsychol Clin Neurosci. 1997;9:91–93. doi: 10.1176/jnp.9.1.91. [DOI] [PubMed] [Google Scholar]
- SAMSHA (Substance Abuse and Mental Health Services Administration) Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: 2014. NSDUH Series H-48, HHS Publication No. (SMA) 14–4863. [Google Scholar]
- Schmitz JM, Bordnick PS, Kearney ML, Fuller SM, Breckenridge JK. Treatment outcome of cocaine-alcohol dependent patients. Drug Alcohol Depend. 1997;47:55–61. doi: 10.1016/s0376-8716(97)00069-0. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Smith JE, Co C, Lane JD. Limbic muscarinic cholinergic and benzodiazepine receptor changes with chronic intravenous morphine and self-administration. Pharmacol Biochem Behav. 1984;20:443–450. doi: 10.1016/0091-3057(84)90283-1. [DOI] [PubMed] [Google Scholar]
- Thomasson HR. Gender differences in alcohol mctabolism: physiological responses to ethanol. In: Galantc M, editor. Recent Developments in Alcoholism. Vol. 6. Plenum Press; New York: 1995. pp. 163–179. [DOI] [PubMed] [Google Scholar]
- Tziortzis D, Mahoney JJ, 3rd, Kalechstein AD, Newton TF, De La Garza R., 2nd The relationship between impulsivity and craving in cocaine- and methamphetamine-dependent volunteers. Pharmacol Biochem Behav. 2011;98:196–202. doi: 10.1016/j.pbb.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Erwin VG. Rate of alcohol metabolism: do not “correct” the b60 estimate for comparisons among ethnic groups. J Stud Alcohol. 1983;44:1093–1096. doi: 10.15288/jsa.1983.44.1093. [DOI] [PubMed] [Google Scholar]
- Winger G, Galuska CM, Hursh SR. Modification of ethanol’s reinforcing effectiveness in rhesus monkeys by cocaine, flunitrazepam, or gamma-hydroxybutyrate. Psychopharmacology. 2007;193:587–598. doi: 10.1007/s00213-007-0809-9. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]