Abstract
Background
Chagas disease, caused by the protozoan Trypanosoma cruzi, is the leading cause of heart failure in Latin America. The clinical treatment of Chagas disease is limited to two 60 year-old drugs, nifurtimox and benznidazole, that have variable efficacy against different strains of the parasite and may lead to severe side effects. CYP51 is an enzyme in the sterol biosynthesis pathway that has been exploited for the development of therapeutics for fungal and parasitic infections. In a target-based drug discovery program guided by x-ray crystallography, we identified the 4-aminopyridyl-based series of CYP51 inhibitors as being efficacious versus T.cruzi in vitro; two of the most potent leads, 9 and 12, have now been evaluated for toxicity and efficacy in mice.
Methodology/Principal findings
Both acute and chronic animal models infected with wild type or transgenic T. cruzi strains were evaluated. There was no evidence of toxicity in the 28-day dosing study of uninfected animals, as judged by the monitoring of multiple serum and histological parameters. In two acute models of Chagas disease, 9 and 12 drastically reduced parasitemia, increased survival of mice, and prevented liver and heart injury. None of the compounds produced long term sterile cure. In the less severe acute model using the transgenic CL-Brenner strain of T.cruzi, parasitemia relapsed upon drug withdrawal. In the chronic model, parasitemia fell to a background level and, as evidenced by the bioluminescence detection of T. cruzi expressing the red-shifted luciferase marker, mice remained negative for 4 weeks after drug withdrawal. Two immunosuppression cycles with cyclophosphamide were required to re-activate the parasites. Although no sterile cure was achieved, the suppression of parasitemia in acutely infected mice resulted in drastically reduced inflammation in the heart.
Conclusions/Significance
The positive outcomes achieved in the absence of sterile cure suggest that the target product profile in anti-Chagasic drug discovery should be revised in favor of safe re-administration of the medication during the lifespan of a Chagas disease patient. A medication that reduces parasite burden may halt or slow progression of cardiomyopathy and therefore improve both life expectancy and quality of life.
Author summary
Chagas disease is a parasitic disease caused by the Trypanosoma cruzi. The infection may result in gastrointestinal manifestations and cardiomyopathy. Benznidazole, the current treatment, has limited efficacy and often leads to serious side effects. Aiming to develop new treatments, our group has identified new inhibitors that block the synthesis of parasitic lipids, resulting in parasite death. In this work, we evaluated the safety and efficacy of two of these compounds, 9 and 12, in mouse models of T. cruzi infection. Both compounds were well-tolerated by animals throughout the 28-day administration. In acutely infected mice, the compounds drastically reduced bloodstream parasites and increased survival. When treatment was initiated during the chronic phase, parasitemia dropped to background levels and remained undetectable for 4 weeks after drug withdrawal; parasites were re-activated by chemically-induced immunosuppression. Thus, the experimental compounds tested in these studies had an acceptable safety profile, achieved a marked reduction in parasite load and prevented heart injury due to inflammation, even in the absence of sterile cure. We conclude that the development of non-toxic medications capable of slowing the progression of cardiomyopathy is a valuable treatment option for Chagas disease patients because it could enhance the quality of life.
Introduction
Chagas disease afflicts about 7 million people in South and Central America [1], where it is the leading cause of heart failure. More than 10,000 deaths are estimated to occur annually due to this disease. Despite joint efforts in Latin America to eradicate the transmission of Trypanosoma cruzi through screening of blood banks and control of triatomine vectors, Chagas disease presents a risk to 70 million people living in endemic countries [1,2]. International travel, infected blood transfusions, co-infection with HIV, mother to fetus transmission, and northward migration of the “kissing bug” insect vector [3], all help to drive up the number of cases and push the incidence outside its historic range. Chagas disease is now seen in Europe, North America and Asia and seems set to become an urgent public health issues in countries far beyond its focal source in South America [4,5]. An annual economic burden due to Chagas disease, calculated by simulation models as overall cost, reaches 7.19 billion US dollars, largely from the loss of productivity and premature mortality caused by cardiomyopathy [6,7].
Human infections by T. cruzi result in a significant mortality rate in children in the acute phase, or may lead to cardiomyopathy in chronically infected adults [8,9]. About 40% of infected individuals develop chronic manifestations of the disease: ten percent of patients develop gastrointestinal symptoms (e.g., mega colon and mega esophagus); and 30% of patients develop cardiac disease characterized by cardiomyopathy, arrhythmias and interstitial fibrosis accompanied by cardiac inflammation[9]. The clinical treatment of Chagas disease is limited to two drugs: nifurtimox and benznidazole, developed about 60 years ago. Nifurtimox is now discontinued in several countries [10,11], while benznidazole has been recently FDA-approved only for use in children of 2 to 12 years old [12,13].
Both benznidazole and nifurtimox are about 80% effective in the acute stage of Chagas disease [14]. Limitations of current therapy include variable efficacy against T. cruzi of different genetic backgrounds and elevated toxicity with severe side effects, including widespread dermatitis, digestive intolerance, polyneuritis and bone marrow depression, leading to poor patient compliance [10,11]. Both drugs are used in cases of new infections, congenital infections, reactivation and/or re-aggravation associated with immunosuppression and as a preventive measure against laboratory accidents [8]. The efficacy of benznidazole against the more prevalent chronic stage of Chagas dissease was investigated in the BENEFIT clinical trial [15,16]—the first randomized, placebo controlled, clinical study on the effects of benznidazole on the clinical progression of chronic Chagas disease patients with compromised cardiac function. Drug treatment led to a marked reduction of the circulating parasite load in patients from Brazil (strain TcII) and Argentina and Bolivia (strains TcV and TcVI), but not in patients from Colombia or El Salvador (strain TcI). In all cases, benznidazole failed to reduce cardiac function deterioration when evaluated at the 5–7 year follow-up. [15]. These results suggest that benznidazole has limited clinical utility in patients with moderate to advanced cardiac compromise (class I or II heart failure, New York Heart Association terminology). However, an important qualification is that previous observational, not randomized, studies [17] suggest that the drug is effective in patients in the asymptomatic (indeterminate) stage or those with incipient cardiac compromise. A combination of benznidazole with posaconazole in the treatment of asymptomatic patients (the STOP-Chagas clinical trial) also showed no advantage over benznidazole monotherapy, as judged by the PCR test alone. Clinical disease as evidenced by decreased cardiac function or other cardiomyopathy signs were not assessed in this study [18]. In either case, there is an urgent need for safer and more efficacious drugs and drug combinations to meet the etiological challenges of this complex disease.
As an alternative to the use of benznidazole in patients with chronic Chagas disease [19], significant efforts have been made to repurpose antifungal azole drugs targeting sterol biosynthesis. Among validated sterol biosynthetic targets, CYP51 is one of the most extensively exploited for the development of new therapeutics for fungal and parasitic infections [20,21]. The CYP51 inhibitors posaconazole (Noxafil, Merck) and ravuconazole (E1224, Eisai, Tokyo), which have undergone extensive pharmacological and toxicological optimization in antifungal programs, have demonstrated efficacy and curative activity in animal models of Chagas disease [22], and alleviated chronic Chagas disease in a patient with systemic lupus erythematosus [23,24]. Both drugs have been tested in controlled clinical trials for Chagas disease [18,25,26]. The perceived inferiority of both drugs to the current standard-of-care drug, benznidazole, [25,27] was due to their failure to produce sterile cure (PCR negative), and triggered discussions in the Chagas research community about the validity of CYP51 as a target [28,29]. Two concerns have been expressed: (i) differential activity of CYP51 inhibitors against different strains of T. cruzi or between the replicative (amastigote) and non-replicative (trypomastigote) stages of the parasite and (ii) the slow-acting mechanism of CYP51 versus fast-acting benznidazole [28–30]. A third factor that may have affected the outcomes of the clinical trials is that the repurposed antifungal drugs, including posaconazole and ravuconazole, were not optimized to target T. cruzi CYP51.
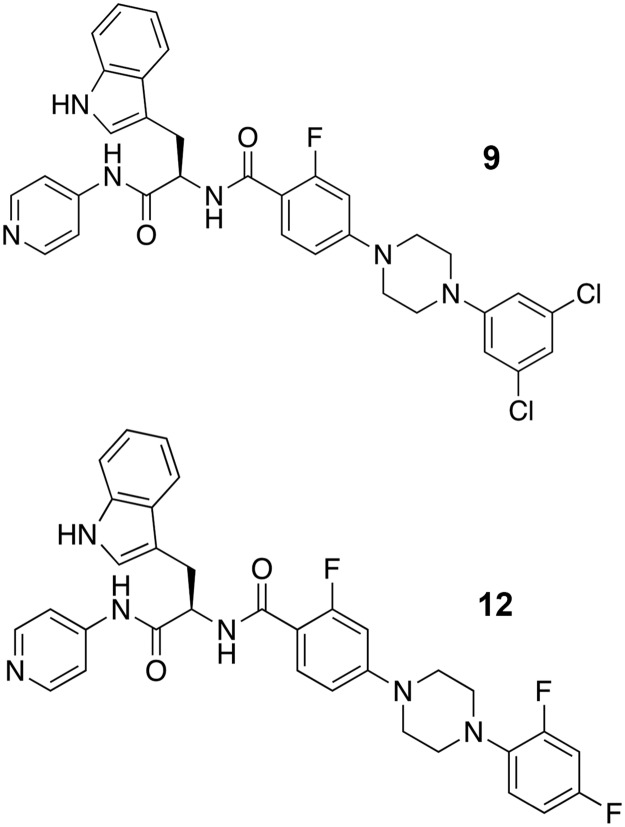
In parallel with the clinical trials, a number of laboratories pursued novel chemical scaffolds specifically targeting T. cruzi CYP51 (reviewed in [21]). Using a target-based structure-aided drug discovery approach, a 4-aminopyridyl-based scaffold was identified as efficacious and further developed into a series of lead compounds active against T. cruzi both in vitro and in vivo [31–37] (Fig 1). Two optimized leads of the series, 9 [37] and 12 [35] (Fig 1, compound numbers correspond to those in the cited references), have now been evaluated for both toxicity and parasitological cure in the acute and chronic animal models of T. cruzi infection. Although a sterile cure was not achieved, 9 and 12 were proven safe for long term administration in mice and suppressed parasitemia in both the acute and chronic phases. In the acute model, these lead compounds improved survival, protected mice from hepatic injury and drastically reduced cardiac inflammation. In the chronic phase, these lead compounds prevented spontaneous T. cruzi relapse for up to 4 weeks post-treatment.
Fig 1. The 4-aminopyridyl-based lead compounds.
The lead compounds optimized by the structure-aided rational drug design guided by x-ray crystallography and efficacy in the in vitro and in vivo T. cruzi infection models. Both compounds have EC50 in low nanomolar range against cultured amastigotes, are orally bioavailable and decrease 99% parasitemia in mice after 4-day oral dosing [35,37].
Results
Toxicity evaluation in uninfected mice
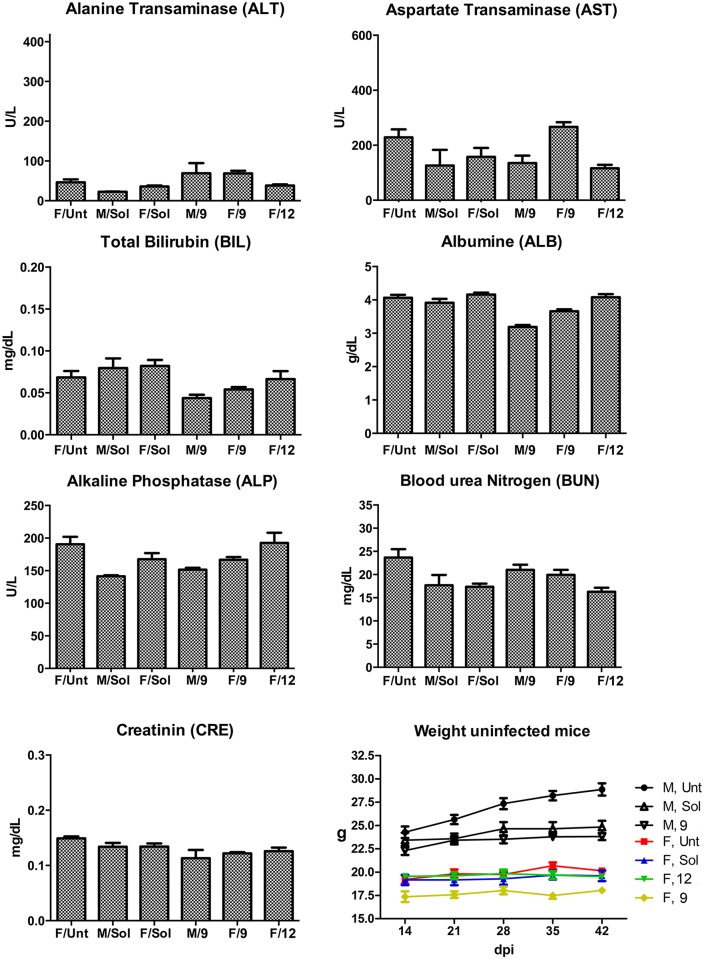
The No Observed Adverse Effect Level (NOAEL), of 9 and 12 was evaluated according to the Organization for Economic Cooperation and Development (OECD) guidelines. Escalating doses of 12 were administered orally to male and female Swiss mice every hour; adverse effects were observed only at concentrations higher than 300 mg/kg for male and 250 mg/kg for female mice. Cumulative in vivo effects were analyzed using uninfected BALB/c male and female mice treated with 9 or 12 at 25 mg/kg orally for up to 28 days, b.i.d. No adverse clinical signs (such as ruffled fur, hunched posture, reduced mobility, or tremor) or alteration in general health were observed in any of the mice. Blood was collected after the end of treatment and serum was evaluated in a chemistry panel that included liver enzymes and markers of renal function. No alteration of blood levels for alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin (BIL), albumin (ALB), blood urea nitrogen (BUN) or creatinine (CRE) was detected after the course of treatment (Fig 2). Histological analysis of brain, heart, liver, kidney, GI tract and lungs did not show any alteration of tissue morphology. The weights of the animals remained steady throughout the treatment.
Fig 2. Cumulative in vivo effect of 9 and 12.
No alteration in the liver (ALT, AST, BIL, ALB, ALP) or kidney (BUN, CRE) markers were observed in the course of mice treated with 9 or 12 at 25 mg/kg, for 28 days, b.i.d. Treatment groups are labeled along the x-axis by the gender/drug combination.
Effect of compounds on severe acute infections with the lethal T. cruzi Y strain
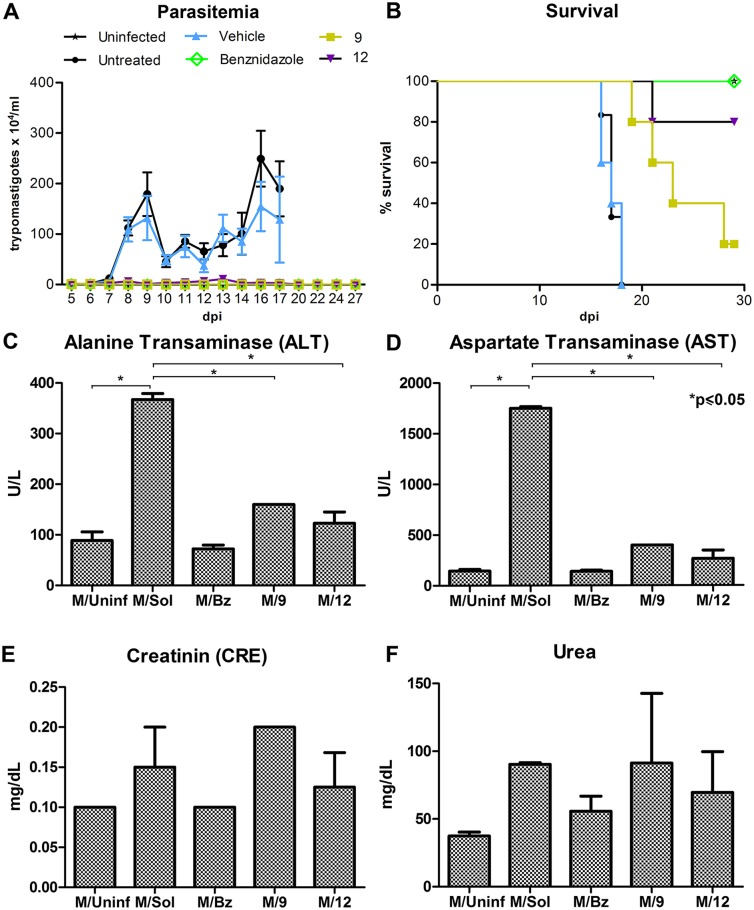
Since no toxicity was detected in uninfected mice, we performed a 28-day oral treatment at 25 mg/kg of compounds in Swiss male mice infected with T. cruzi Y strain (104 inoculum), an established model of acute infection recommended for drug screening and development by the Fiocruz Program for Research and Technological Development on Chagas Disease (PIDC/Fiocruz) and the Drugs for Neglected Diseases Initiative (DNDi) [38]. Treatment with 9 or 12 at 25 mg/kg significantly reduced parasitemia, reaching the minimum limit of detection by the Pizzi-Brener method at 9 days post infection (dpi), with inhibition levels of 99.9% for 9 and 99.3% for 12. Parasitemia remained undetectable in the treated mice, while untreated and vehicle-treated mice showed high parasitemia and all of these mice died by 18 dpi (Fig 3A). While parasites were not detected, only 20% of the infected mice treated with 9 and 80% of the infected mice treated with 12 survived the entire 30 day study (Fig 3B). 100% of benznidazole-treated mice survived. All mice were euthanized at the end of treatment. Compared to the negative controls, treatment with the test compounds did provide partial protection and delayed the death of the mice. Death of the treated animals in this model of acute disease with Y strain parasites may be due to yet unknown T. cruzi Y strain-specific factor(s) that could interfere with the compound effect in the infected mice. That effect with Y strain parasites was not seen in uninfected animals or animals infected with T. cruzi CL-luc strain.
Fig 3. The effects of 4-aminopyridyl lead compounds on mice with acute T. cruzi infection.
(A) Parasitemia levels in Swiss male mice infected with T. cruzi Y strain (104 inoculum) and treated for 28 days with 9 or 12 (25 mg/Kg) are undetectable. (B) Survival curve shows partial protection and delayed death of mice treated with 9 and 12 (25 mg/Kg) in the acute phase of the infection. (C-F) Biochemical analysis of serum from infected mice treated with 9 or 12 (25 mg/Kg) was comparable to serum from unifected controls and shows normal levels of liver enzymes—alanine aminotransferase (ALT;C) and aspartate aminotransferase (AST;D)–as well as the renal function markers—creatinine (CRE; E) and urea (F). dpi—days post infection. *Statistically significant by t test, p≤0.05.
Upon study completion, mice were euthanized and serum was assessed for hepatic enzymes and renal function markers (Fig 3C–3F). Data from the blood chemistry analysis showed that T. cruzi Y infection induced elevated serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), indicating infection-induced liver injury. The animals treated with 9 or 12 had reduced levels of these enzymes when compared to untreated controls. Urea and creatinine (CRE) levels, markers of renal function, were also analyzed; treated animals show normal levels similar to that of untreated controls, suggesting no renal toxicity was caused by 9 or 12.
Effect of 9 and 12 on less severe acute infections with the transgenic CL-Brenner T. cruzi strain in mice
Given that neither 9 nor 12 produced sterile cure in the T. cruzi Y-infected animals, we next evaluated their effect in a less severe acute model using the transgenic CL-Brenner T. cruzi strain expressing “red-shifted” luciferase (CL-luc, a gift from Dr. John Kelly, UK). This strain carries a stable bioluminescent marker, which allows one to detect live parasites in tissues of a live mouse with a sensitivity limit exceeding that of the RT-PCR method for up to a year after infection [39,40]. Since mice gender influences the level of infection [41,42], we used both male and female mice for the 28 day b.i.d. treatment beginning 14 dpi. Males not treated with experimental or reference compounds showed levels of parasitemia higher than females (Figs 4, 5 and 6).
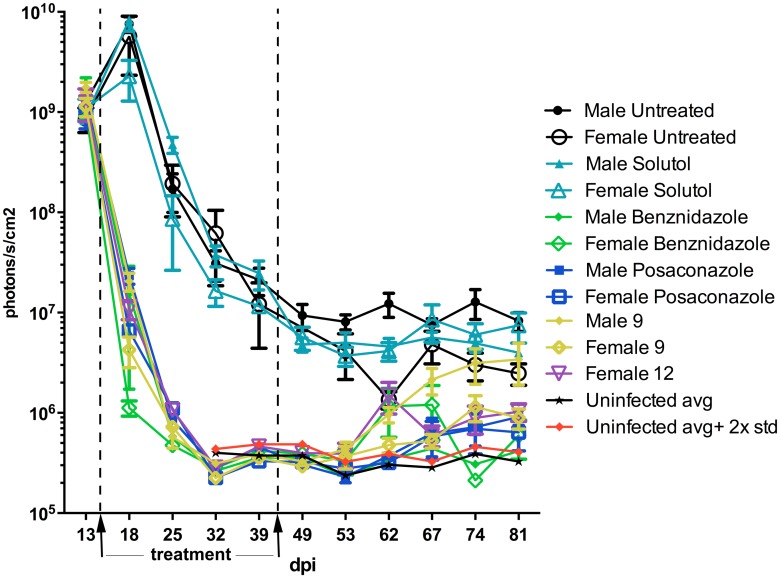
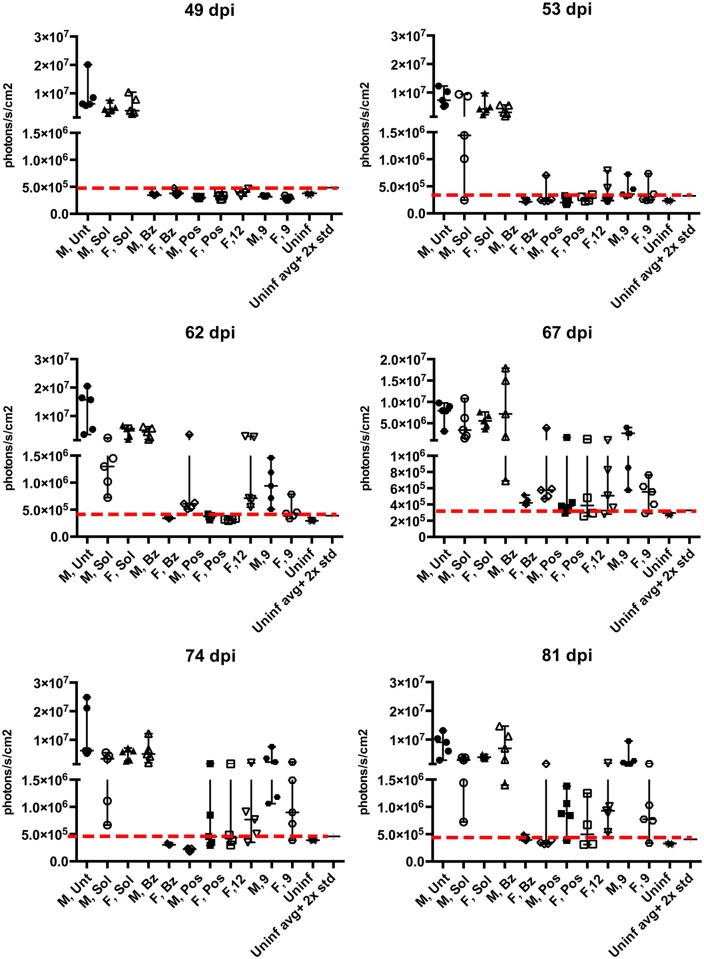
Fig 4. Monitoring parasitemia in acutely infected with T. cruzi CL-luc BALB/c mice over the course of 28-day treatment with 9 or 12.
Bioluminescence levels of infected and treated animals are indistinguishable from those of uninfected control groups between days 28 and 49 post-infection. Parasite relapse ocurred two weeks after the end of treatment, with males eventually reaching bioluminescence levels similar to untreated controls, while females display lower levels of parasitemia.
Fig 5. Development of parasitemia in acutely infected mice after the 28-day course of treatment with 9 or 12.
The photon count values are displayed for each individual animal up to 81 dpi, showing progressive parasite relapse. Post-treatment evolution of parasetimea in live animals is shown in Fig 6.
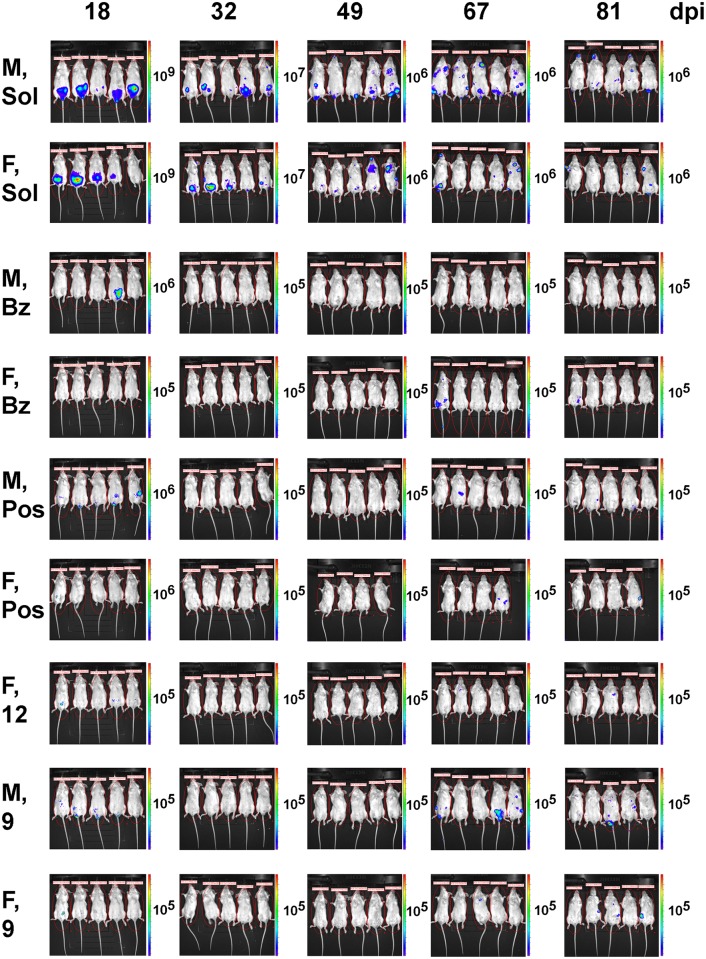
Fig 6. Post-treatment evolution of parasitemia in acutely infected mice monitored by bioluminescence imaging of live animals.
Stochastic profile of T. cruzi infection is shown. Abbreviations are M—male, F—female, Bz—benznidazole, Pos—posaconazole, Sol—solutol, 9 and 12 –lead compounds.
As judged by the bioluminescence (Fig 4), the level of CL-luc T. cruzi infection markedly decreased at 18 dpi, which corresponds to 4 days of treatment with the experimental or reference compounds, benznidazole or posaconazole. By 32 dpi (18 days of treatment), the parasite bioluminescence in all treated mice was reduced to the background level, with the photon count lower than that of the uninfected mice injected with luciferin. Both groups of negative control mice, including a group of infected but untreated mice, as well as a group of infected and vehicle-treated mice, had bioluminescence levels two orders of magnitude higher than the uninfected control mice. This trend was maintained up to 49 dpi (7 days after the end of treatment). However, by 53 dpi (11 days after the end of treatment) animals from the benznidazole (male and female), 12 (female), and 9 (male and female) groups all showed resurgence of parasites (Figs 4, 5 and 6). Posaconazole treated mice showed resurgence of parasites at 67 dpi.
By 81 dpi, the majority of the animals in the 9- and 12-treated groups were T. cruzi-positive as indicated by bioluminescence. All animals in the male group treated with 9 showed parasitemia as high as untreated controls at 81 days post infection. Both compounds reduced the parasite load in female mice, where bioluminescence levels were 10× lower than untreated controls in 4 out of 5 mice for both compounds at 81 dpi, with one 9-treated female negative (Table 1). Posaconazole reduced T. cruzi bioluminescence to undetectable levels in 1 out of 5 males and 2 out of 4 females, with the remaining mice showing reactivation of parasites. Posaconazole markedly reduced parasite load compared to the untreated controls. Finally, benznidazole was not able to suppress parasites in all the mice, with one female showing circulating parasites after treatment withdrawal (Figs 4, 5 and 6, Table 1).
Table 1. Parasite load on 81 day post-infection by the luminescence level.
| Compound/vehicle | Mice sex | Photons/s/cm2 ≥106 |
Photons/s/cm2 ≥105 |
Negative (≤ uninfected) |
|---|---|---|---|---|
| -/- | Male | 5/5 | 0/5 | 0/5 |
| -/- | Female | 3/5 | 2/5 | 0/5 |
| -/solutol | Male | 5/5 | 0/5 | 0/5 |
| -/solutol | Female | 4/5 | 1/5 | 0/5 |
| BZ/solutol | Male | 0/5 | 0/5 | 5/5 |
| BZ/solutol | Female | 1/5 | 0/5 | 4/5 |
| POS/solutol | Male | 0/5 | 4/5 | 1/5 |
| POS/solutol | Female | 0/4 | 2/4 | 2/4 |
| 9/solutol | Male | 5/5 | 0/5 | 0/5 |
| 9/solutol | Female | 1/5 | 3/5 | 1/5 |
| 12/solutol | Female | 1/5 | 4/5 | 0/5 |
BZ—benznidazole, POS—posaconazole
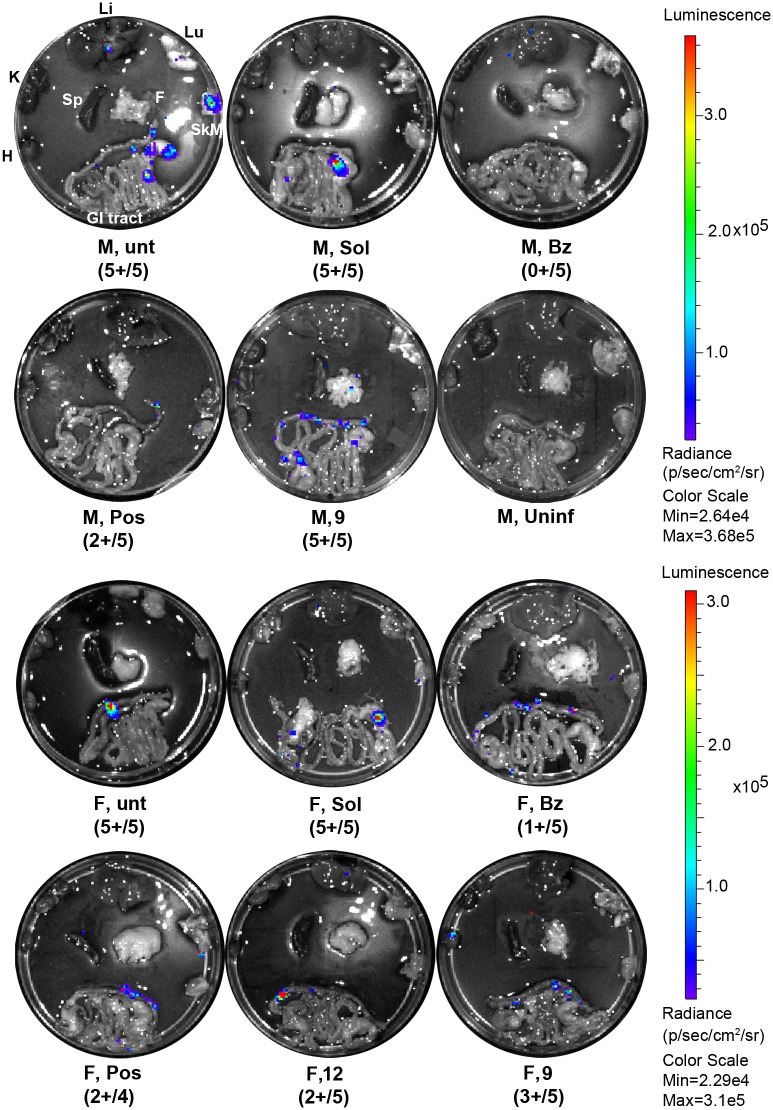
Ex vivo bioluminescence imaging of internal organs was performed at 81 dpi. As previously described for this model [39,43], parasites were detected consistently in the gastro-intestinal (GI) tract in all T. cruzi-positive mice (Figs 7 and 8). Moreover, bioluminescence above the background level was also observed randomly in heart, skeletal muscles, liver and mesenteric fat in T. cruzi-positive mice throughout the groups, suggesting a dynamic infection process (Fig 7). Ex vivo quantification of the parasite burden in the organs analyzed (heart, liver, kidneys, lungs, spleen, gastro-intestinal tract, skeletal muscle and mesenteric fat) was consistent with the whole-mouse imaging. One female mouse treated with benznidazole showed traces of infection in the GI tract, while posaconazole-treated animals revealed T. cruzi in the GI tract and lung. For the experimental inhibitors, no parasites were detected in two females treated with 12 and one female treated with 9. All other animals in the 9- or 12-treated groups showed T. cruzi bioluminescence in GI tract, liver, lung and mesenteric fat (Figs 7 and 8).
Fig 7. Ex vivo bioluminescence imaging of the internal organs of chronically-infected mice.
Gastro-intestinal (GI) tract, heart (H), skeletal muscle (SkM), liver (Li), lung (Lu), spleen (Sp) and mesenteric fat (F) are shown uniformly positioned on a petri dish surface as indicated in the top left image. Each group of mice is represented by one image labeled with the animal gender, compound name and a number of T. cruzi-positive animals in the group of five. Collectively, bioluminescence was detected in the GI of all T. cruzi-positive animals and randomly in heart, skeletal muscle, liver and mesenteric fat. Animals in the 9- or 12-treated groups showed T. cruzi bioluminescence in the GI tract, liver, lung and mesenteric fat. Bioluminescence was not detected in one female treated with 9 and two females treated with 12. Quantitative analysis of all animals is shown in Fig 8.
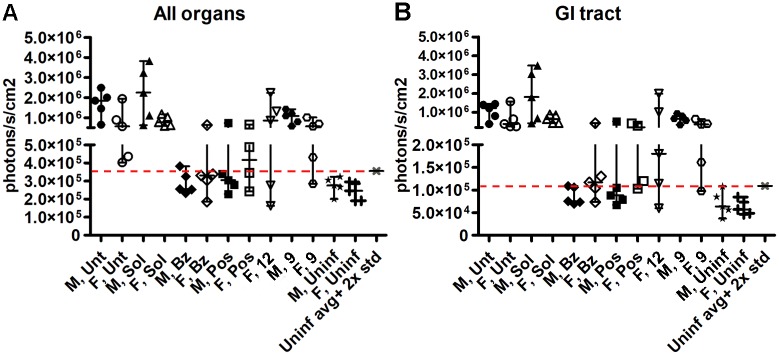
Fig 8. Quantitative analysis of ex vivo bioluminescence in the internal organs of chronically-infected mice.
Each animal is represented by an individual data point. Treatment groups are labeled along the x-axis by the gender and drug name. (A) Cumulative signal from all the organs in each individual animal. (B) Signal from the GI tract alone. The majority of the bioluminescent parasites are associated with the GI tract.
Histopathological analysis
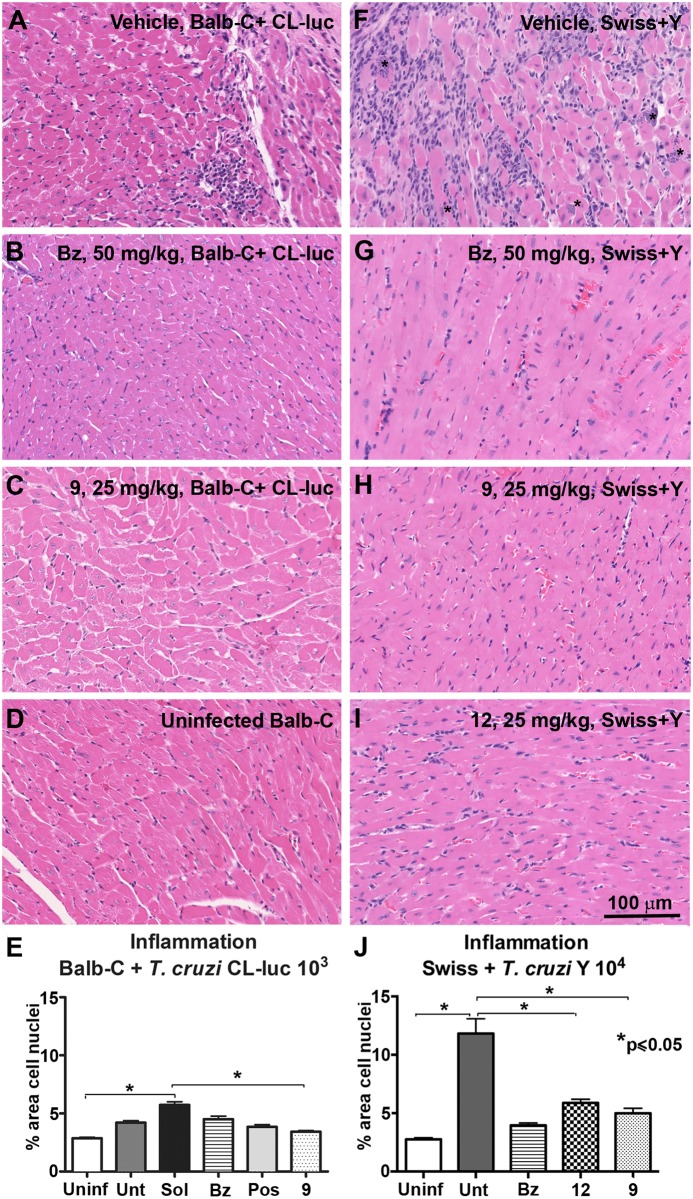
Tissue architecture and inflammation in the heart was evaluated through conventional histology and H&E staining from both lethal acute and bioluminescent models, with the levels of inflammation being quantified using FIJI software [44]. Uninfected mice had normal cardiac tissue, as expected (Fig 9D). T. cruzi infection in untreated mice resulted in marked inflammation in heart tissue with inflammatory infiltrates and interstitial fibrosis in both acute models (Fig 9A and 9F). BALB/c mice infected with CL-luc showed mild cardiac inflammation when compared to Swiss mice infected with T. cruzi Y strain; the latter showed levels of inflammation at least 2× higher than the former and included the presence of amastigote nests, which were not observed in BALB/c mice infected with CL-luc (Fig 9A, 9F, 9E and 9J). In both models, mice treated with benznidazole (Fig 9B and 9G), 9 (Fig 9C and 9H), or 12 (Fig 9I) had normal heart tissue, with a significant reduction of inflammatory cells (Fig 9E and 9J) compared to vehicle treated controls, and no signs of interstitial fibrosis or amastigote nests, suggesting that the reduction of parasite load induced by treatment with the CYP51 inhibitors improved cardiac pathology, even without sterile cure.
Fig 9. Histopathological analysis of heart tissue.
H&E staining of heart tissue from acute models of T. cruzi infection. (A-D) BALB/c female mice infected with T. cruzi CL-luc. (F-I) Lethal infection of Swiss male mice infected with T. cruzi Y strain. In F, the parasite nests are labeled with the asterisks. Bar = 100μM. (E, J) Inflammation quantified using FIJI software.[44]. * Statistically significant by t test, p≤0.05.
Effect of compounds in chronic infection
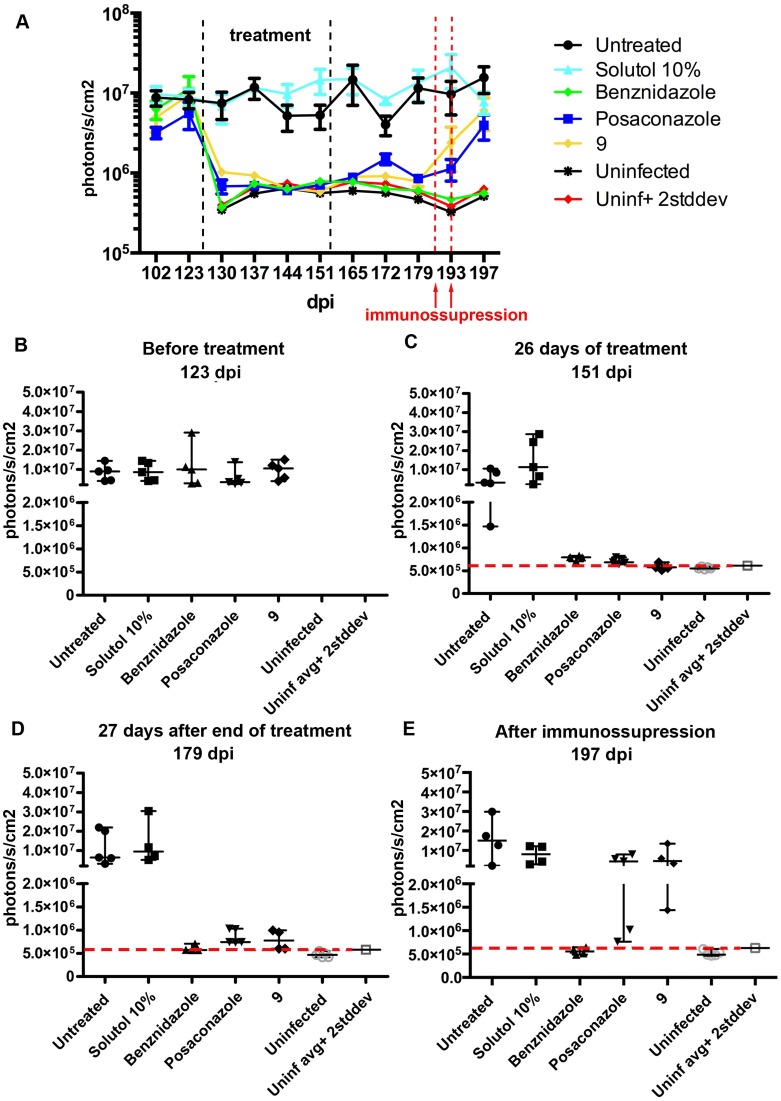
Most patients in need of treatment are in the chronic phase of Chagas disease. In this later stage, parasite load is low enough to require sensitive techniques for parasite detection [45]. To recapitulate these conditions, we evaluated performance of 9 in a chronic mouse model. Because males are more susceptible to infection than females, BALB/c males were infected with T. cruzi CL-luc strain allowing highly sensitive bioluminescence detection. Following the treatment scheme reported [46], the compounds were administered at 25 mg/kg for 28 days starting at 126 dpi, when chronic infection was established and the parasite signal was consistently detected. Similar to the acute model described above, T. cruzi bioluminescence levels dropped soon after the start of treatment. After 28 days, all treated groups, including 9, showed only background luminescence (Fig 10A–10C). Mice were then followed for 4 weeks after compound administration had ceased. For up to 27 days post-treatment, groups treated with posaconazole or 9 had bioluminescence levels slightly above the background defined by the uninfected mice and 10–100× lower than the untreated controls (Fig 10D and 10E). Since the parasite load was below the detection level 4 weeks post-treatment, the animals were immunossupressed with cyclophosphamide. After 2 rounds of immunossupression, parasites relapsed as evidenced by the bioluminescence levels similar to those of the untreated controls.
Fig 10. Effect of 4-aminopyridyl compounds in chronic infection.
Parasite levels measured by bioluminescence detection in BALB/c males infected with T. cruzi CL-luc strain. (A) Average levels from all groups from 102 to 197 dpi. Individual values for each mouse in the groups represented separately on 123 dpi (B), 151 dpi (C), 179 dpi (D) and 197 dpi (E).
Discussion
A similarity in sterol biosynthesis pathways between T. cruzi and fungi is that both produce ergosterol and ergosterol-like sterols as membrane building blocks [47]. This similarity encouraged the application of antifungal drugs for the treatment of Chagas disease. However, in human clinical trials for Chagas disease, both posaconazole and ravuconazole failed to demonstrate superiority to the current standard-of-care drug, benznidazole, using PCR as a marker of continued or reactivated T.cruzi infection [25,27]. The failure of posaconazole and ravuconazole to attain sterile cure in humans raised concerns about the CYP51 target. Differential activity of CYP51 inhibitors against the replicative (amastigote) and non-replicative (trypomastigote) stages of T. cruzi, a slow-acting mechanism of action, and the stochastic nature of T. cruzi infection with the non-replicating or rarely-replicating cryptic amastigotes ‘hidden’ inside the tissues [39], were listed as potential drawbacks of the CYP51 target [28,48]. On the other hand, it has been argued that both the dose and the duration of anti-fungal agents used in the clinical trials to treat human T. cruzi infection have been suboptimal. Urbina et al. noted that the plasma exposure in patients for the dose used in clinical trials corresponds to 10–20% of the curative dose in mice [29,49]. The post-clinical trial tendency to balance risks in the Chagas drug discovery portfolio, and to identify drug candidates aimed at other molecular targets is logical. At the same time, it is critical to not reject promising targets based on clinical studies with drugs not properly optimized, dosed, or clinically evaluated.
An important, but overlooked factor, that may have affected the performance of the anti-fungal drugs in Chagasic patients, is the loose drug-target fit demonstrated by posaconazole in co-crystal structures with Trypanosome CYP51. The electron density of the bound drug is poorly defined and its pendant phenyl-2-hydroxy-pentantriazolone group adopts alternative conformations to make multiple interactions outside of the active site [50,51]. Several novel CYP51 inhibitors developed in this collaboration [33–37] and elsewhere [51–53] demonstrated drug-target fits superior to posaconazole. The most potent 4-aminopyridyl-based inhibitors of the 4-aminopyridyl-based series bind entirely in the CYP51 target interior, making tight interactions with hydrophobic residues constituting the CYP51 active site [35,37]. Improved drug-target interactions may be responsible, at least in part, for the superior potency of the experimental inhibitors in the acute and chronic mouse models of infection [54].
Neither of the two lead compounds of the 4-aminopyridyl-based series evaluated in these studies attained a sterile cure. However, both leads were proven safe for long term administration in mice and, efficiently suppressed parasitemia in both acute and chronic models of infection. In the acute phase, compounds improved survival in highly stringent acute mouse models, protected mice from hepatic injury, and drastically reduced acute cardiac inflammation. In a model of chronic Chagas disease, 9 prevented spontaneous T. cruzi relapse for up to 4 weeks post-treatment. Similar results—supression of parasitemia, no spontaneous relapse after treatment withdrawal and parasite reactivation after immunossupression—were also achieved by other research groups using CYP51 inhibitors based on different molecular scaffolds [41,46,55].
Collectively, 9 is more efficacious in the treatment of the chronic phase of the disease with low parasite load. Although 9 did not eradicate cryptic reservoirs of parasites in vivo after 28 days of treatment, it successfully kept parasites under control and prevented the inflammation responsible, in part, for cardiac tissue damage. This outcome is not unique for inhibitors that target the ergosterol synthesis pathway. Treatment of chronically infected mice with N,N-dimethylsphingosine, an inhibitor of sphingosine kinase, also failed to produce a sterile cure, but reduced parasite load leading to a marked decrease in inflammation and fibrosis. Furthermore, there was a reduction of inflammatory mediators and an improvement of heart function measured as exercise capacity [56]. In addition, mice infected with resistant strains of T. cruzi showed decreased tissue parasitemia, reduced myocarditis and less electrocardiographical alterations after treatment with benznidazole, even though the drug failed to completely eliminate parasites in this model [57].
Several mechanisms have been proposed to explain the pathogenesis of Chagas’ cardiomyopathy, including parasite-dependent inflammation, autoimmunity, autonomic neuronal degeneration and damage of microvasculature [58,59]. Although more than one mechanism may be involved in Chagas disease pathogenesis, a consensus is that tissue damage is related to parasite persistence [58–60]. At the same time, 60–70% of infected individuals are asymptomatic. A balance between host and parasite in asymptomatic cases may be maintained by expression of the anti-inflammatory cytokine IL-10, while cardiomyopathy is associated with inflammation triggered by IFN-gamma and TNF-alpha [61]. Reducing the parasite burden diminishes inflammation even without complete elimination of the parasite [60]. In this regard, several non-randomized clinical trials have shown that etiological treatment of chronic patients with benznidazole resulted in slower progression to advanced stages of cardiomyopathy evaluated by electrocardiography and echocardiography [17,62].
Sterile cure is a highly desirable treatment outcome, however, it may not always be achieved, and drug discovery efforts are often hampered by deficiencies in understanding the nuances of disease pathogenesis. There is currently no sterile cure of HIV infection. The desirable outcome of the antiretroviral treatment is a long term plasma HIV-RNA count below 50 copies/ml [63]. The WHO recommends antiretrovirals in people of all ages, including pregnant women as soon as the diagnosis is made; once treatment is begun, it is recommended to continue throughout the entire life span without interruptions [63]. Benefits of treatment include a decreased risk of progression to AIDS and a decreased risk of death [64]. Highly active antiviral therapy options are available as drug ‘cocktails’ consisting of at least three medications belonging to at least two different classes of antiviral agents [65]. As of 2017, 19.5 million people are accessing antiretroviral therapy and more than half of all people living with HIV are on treatment [66].
By analogy with HIV/AIDS, the treatment option of non-toxic medications should be developed for Chagas patients to slow down progression to cardiomyopathy and to improve life expectancy and quality of life. With the scarce arsenal of anti-T. cruzi agents, the drug discovery community cannot afford to be prejudiced against CYP51, or any other target, if the inhibitors have acceptable safety profiles and achieve a marked reduction in parasite load, even in the absence of sterile cure. Regardless of the molecular target affected by the drug, development of an efficacious and safe treatment for Chagas disease would be a breakthrough for society, medicine and science.
Material and methods
Ethics statement
Research performed at UC San Diego was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to the principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 2011. The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Animal research was conducted under approved protocol S14187 from the Institutional Animal Care and Use Committee, University of California, San Diego. Research performed at Oswaldo Cruz Foundation—FIOCRUZ, Rio de Janeiro, Brazil, was approved by the Committee for Ethics in the Use of Animals of FIOCRUZ, under protocol number LW-37/13 and is in compliance with Brazilian Federal Law number 11794/08, Federal Brazilian Decree number 6899/09 and Brazilian Normative Resolution number 1 (July 9th, 2010) of the National Council for the Control of Animal Experimentation. Euthanasia was accomplished by CO2 inhalation or by sodium pentobarbital overdose (60 mg/kg), followed by cervical dislocation. These methods of euthanasia have been selected because they cause minimal pain and distress to animals, are relatively quick, and do not adversely impact interpretation of the results of studies. All methods are in accord with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association.
Materials
Compounds 9 and 12 were synthesized by following the procedures previously reported [35,37].
Animals
In vivo experiments were performed at the University of California San Diego (UCSD), La Jolla, California, USA and Oswaldo Cruz Foundation—FIOCRUZ, Rio de Janeiro, Brazil. At FIOCRUZ, Swiss Webster male and female mice weighting 18–20 g were obtained from CEMIB (Centro Multidisciplinar para Investigação Biológica), UNICAMP (Campinas, SP, Brazil). At UCSD, male and female 6 weeks old BALB/c mice, in the same weight range, were purchased from Jackson Laboratories (Farmington, CT, USA). Mice were housed in a maximum number of 5 animals per cage and kept in a conventional room at 20 to 24°C under a 12 h/12 h light/dark cycle. The animals were provided with sterilized water and chow ad libitum.
Toxicity assays
Acute toxicity was evaluated by administration of escalating doses of the compounds to male and female Swiss Webster mice (n = 2/group) orally by gavage, at 100 ul/hour 50 mg/kg dose formulated in 20% solutol (also known as Kolliphor HS15) (Sigma #42966). The general health of the animals was closely monitored for up to 48 h and the last dose before the onset of toxic symptoms were observed was defined as NOAEL according to the OECD guidelines. Cumulative toxicity after prolonged treatment was evaluated using BALB/c females (n = 5/group) treated orally by gavage with the experimental CYP51 inhibitors, 9 (25 mg/kg) or 12 (25 mg/kg), dissolved in 10% solutol, at 100 ul/dose, b.i.d, for 28 consecutive days. Mice were weighed once a week and their general health was assessed daily. After treatment, mice were euthanized, blood was collected for analysis of several biochemical markers of general health. Brain, heart, liver, kidney, gastro-intestinal (GI) tract and lungs were collected, briefly rinsed in PBS and fixed in buffered formalin solution including 10% formaldehyde, 33 mM NaH2PO4, 45 mM Na2HPO4 for histological evaluation.
Parasites
The T. cruzi Y parasites were obtained from the bloodstream of infected Swiss Webster mice at the peak of parasitemia, as previously described [67]. Transgenic T. cruzi CL Brener parasites expressing a red-shifted luciferase that emits light in the tissue-penetrating orange-red region of the spectrum (a gift from Dr. John Kelly, London School of Hygiene and Tropical Medicine, London, United Kingdom), were obtained as described previously [39]. Epimastigote forms were maintained at 28°C in LIT media supplemented with 10% FBS and 100 μg/ml of antibiotic G418 to keep selective pressure in favor of the luciferase marker [68]. Epimastigotes were induced to differentiate to trypomastigotes through metacyclogenesis as previously described [69]. Metacyclic trypomastigotes were used to infect C2C12 myoblasts monolayers. After 5–7 days, trypomastigotes released in supernatant were collected by centrifugation for 15 min at 3300 rpm, re-suspended in DMEM and used to infect mice.
Infection of mice
Swiss Webster male mice weighting 18–20 g were infected intraperitoneally with 104 bloodstream trypomastigote form of T. cruzi Y parasites. For bioluminescence imaging, six week old BALB/c male and female mice were infected by intraperitoneal injection with 103 T. cruzi CL-luc trypomastigotes derived from cell culture supernatant.
Treatment strategies
All drugs were solubilized in 10% solutol and administered orally, b.i.d, at previously optimized doses: 25 mg/kg for 9 and 12, 50 mg/kg for benznidazole, and 20 mg/kg for posaconazole. The treatment of Swiss mice acutely infected with T. cruzi Y strain was started with parasitemia onset at 5 days post-infection (dpi). The treatment of BALB/c mice infected with CL-luc parasites started at 14 dpi (acute phase), when parasitemia reached a peak as detected by bioluminescence, or at 126 dpi (chronic phase), when a chronic state of infection was established [46]. In all models, only parasite-positive mice (5 mice/group) were used in the treatment course lasting for 28 days. To assess if sterile cure was achieved, immunossupression was performed in the chronic model of infection 4 weeks after the end of treatment, with two doses of cyclophosphamide (200 mg/kg) by intraperitoneal (i.p.) injection at 3-day intervals.
Parasitemia determination
In Swiss mice acutely infected with T. cruzi Y strain, parasites in the blood of each animal were quantified by using the Pizzi-Brener method. The total number of parasites are counted in 50 fields under 400X magnification of freshly prepared blood samples (5 μl drops) obtained from the tail veins of mice, collected 3 times a week, starting at 5 dpi and continued until the end of treatment [70]. Mortality was monitored daily and % survival was calculated using GraphPad prism software.
Bioluminescent imaging
BALB/c mice infected with parasites carrying a bioluminescent marker were imaged at 13 dpi, before treatment was initiated, and then once a week, both during the 28-day treatment period and 39 days post-treatment, as previously described [35]. Briefly, mice were injected i.p. with 150 mg/kg D-luciferin potassium salt in PBS (Gold Biotechnology, St. Louis, MO), and 5 minutes later, anesthetized by isofluorane inhalation (3–5%) and imaged using IVIS Lumina in vivo imaging system (PerkinElmer, Waltham, MA) with 180s exposure time. Data acquisition and analysis were performed with the LivingImage V4.1 software (PerkinElmer, Waltham, MA). Uninfected controls were imaged in parallel to establish a negative threshold.
Ex-vivo imaging
To evaluate sites of parasite persistence in BALB/c mice infected with T. cruzi expressing luciferase, we performed ex vivo imaging of selected internal organs according to the protocol adapted from Lewis et al., 2014 [39]. Briefly, the animals were injected i.p. with 150 mg/kg of D-luciferin, euthanized in a CO2 chamber and perfused with 10 ml of D-luciferin. Then, heart, liver, kidneys, lungs, spleen, mesenteric fat, skeletal muscle (excised from left thigh) and the whole gastro-intestinal (GI) tract were removed, placed in a petri dish with PBS containing 300 μg/ml of D-luciferin, and imaged using the IVIS Lumina system.
Histology
Brain, heart, liver, kidney, GI tract and lungs from uninfected mice for toxicity analysis, and heart and GI tract from infected animals were removed and fixed as described above. Samples were processed for routine histologic examination in the Histology Core of Moore Cancer Center (UCSD), embedded in paraffin, sectioned and stained with hematoxylin and eosin. The slides were scanned using Nanozoomer Slide Scanner (Hamamatsu Photonics, NJ, USA) and images were obtained through NDP viewer software (Hamamatsu Photonics, NJ, USA).
Histopathology analysis
To quantify levels of inflammation, 5 random images of mouse hearts (10× magnification) were obtained from each animal, 5 animals/group. At this magnification, 5 fields comprise the majority of the area of the heart section. Image processing was performed using Fiji software [44], where cell nuclei was segmented through the Particle Analyzer plugin and the fraction of total area of the image occupied by all cell nuclei was then measured. Even though cardiomyocytes and cardiac fibroblasts cell nuclei are being counted together with inflammatory cells, uninfected heart sections were used as controls and provide a baseline number.
Blood analysis
Terminal blood collection was performed via cardiac cavity exsanguination in uninfected and T. cruzi-infected mice. Blood was collected in serum separator tubes (Microtainer, BD Biosciences), allowed to clot for 0.5–2.0 h and then centrifuged for 90 s at 10000 g. Serum was removed and analyzed at the Central Animal Facilities of the Oswaldo Cruz Foundation (Rio de Janeiro, Brazil, CECAL/Fiocruz platform) using Vitros 250 (Ortho Clinical-Johnson & Johnson), or at the UC Davis Comparative Pathology Laboratory (Davis, CA, USA), where samples were analyzed using Roche Cobas Integra 400 Plus clinical chemistry analyzer. In both facilities, tests were performed for electrolytes and enzyme metabolites indicative of liver, kidney and cardiac functions, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (BIL), albumin (ALB), alkaline phosphatase (ALP), blood urea nitrogen (BUN), creatinine (CRE), urea, calcium, phosphorus, glucose, total protein.
Statistical analysis
Student’s t-test was used for evaluation of differences in experimental data between groups. Values were considered statistically significant when p≤ 0.05. Statistics were analyzed by GraphPad Prism Software.
Acknowledgments
The authors thank Dr. John Kelly, London School of Hygiene & Tropical Medicine, UK, for kindly providing the transgenic T. cruzi CL-luc strain; Jiri Gut, University of California San Francisco, for technical support in toxicity studies.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Institute of Health grant R01AI095437 (to L.M.P); Oswaldo Cruz Institute of the Oswaldo Cruz Foundation (Fiocruz), Rio de Janeiro, Brazil, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil, Grant 480328/2013-7 (to C.M.C.); Programa Estratégico de Apoio à Pesquisa em Saúde (Papes VI)/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil (grant 407729/2012-7 to M.C.S.P.) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (grant E-26/010.001548/2014 and E-26/110.553/2014 to M.C.S.P.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. Chagas disease (American trypanosomiasis). In: Fact Sheet [Internet]. 2017 [cited 1 Jan 2017]. http://www.who.int/mediacentre/factsheets/fs340/en/
- 2.WHO. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. 2015;90: 33–44. [PubMed] [Google Scholar]
- 3.Chagas C. Nova tripanozomiaze humana. Mem Inst Oswaldo Cruz. 1909;1, no:2: 0074–0276. [Google Scholar]
- 4.Andrade D V, Gollob KJ, Dutra WO. Acute chagas disease: new global challenges for an old neglected disease. PLoS Negl Trop Dis. 2014;8: e3010 doi: 10.1371/journal.pntd.0003010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas’ disease in the United States. Clin Microbiol Rev. 2011;24: 655–81. doi: 10.1128/CMR.00005-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee BY, Bacon KM, Bottazzi ME, Hotez PJ. Global economic burden of Chagas disease: a computational simulation model. Lancet Infect Dis. Elsevier Ltd; 2013;13: 342–8. doi: 10.1016/S1473-3099(13)70002-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanaway JD, Roth G. The Burden of Chagas Disease. Glob Heart. 2015;10: 139–144. doi: 10.1016/j.gheart.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 8.Coura JR, Dias JCP. Epidemiology, control and surveillance of Chagas disease: 100 years after its discovery. Mem Inst Oswaldo Cruz. 2009;104 Suppl: 31–40. [DOI] [PubMed] [Google Scholar]
- 9.Rassi A, Marcondes de Rezende J. American trypanosomiasis (Chagas disease). Infect Dis Clin North Am. 2012;26: 275–91. doi: 10.1016/j.idc.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 10.Bahia MT, Diniz L de F, Mosqueira VCF. Therapeutical approaches under investigation for treatment of Chagas disease. Expert Opin Investig Drugs. 2014;23: 1225–37. doi: 10.1517/13543784.2014.922952 [DOI] [PubMed] [Google Scholar]
- 11.Bermudez J, Davies C, Simonazzi A, Pablo Real J, Palma S. Current drug therapy and pharmaceutical challenges for Chagas Disease. Acta Trop. 2015;156: 1–16. doi: 10.1016/j.actatropica.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 12.FDA USF& DA. FDA approves first U.S. treatment for Chagas disease. In: Press Announcements [Internet]. 2017 [cited 5 Sep 2017]. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm573942.htm
- 13.CHEMO. U.S. FDA Approves Chemo Group’s Benznidazole to Treat Children with Chagas Disease. In: Press Room [Internet]. 2017 [cited 9 May 2017]. http://www.chemogroup.com/us-fda-approves-chemo-groups-benznidazole-treat-children-chagas-disease
- 14.Cançado JR. Long term evaluation of etiological treatment of chagas disease with benznidazole. Rev Inst Med Trop Sao Paulo. 44: 29–37. [PubMed] [Google Scholar]
- 15.Morillo C, Marin-Neto J, Avezum A, Sosa-Estani S, Rassi A Jr, Rosas F, et al. Randomized trial of Benznidazole for chronic Chagas’ cardiomyopathy. N Engl J Med. 2015;373: 1295–1306. doi: 10.1056/NEJMoa1507574 [DOI] [PubMed] [Google Scholar]
- 16.Marin-Neto JA, Rassi A Jr, Avezum A Jr, Mattos AC, Rassi A. The BENEFIT trial: testing the hypothesis that trypanocidal therapy is beneficial for patients with chronic Chagas heart disease. Mem Inst Oswaldo Cruz Rio Janeiro. 2009;104: 319–324. [DOI] [PubMed] [Google Scholar]
- 17.Viotti R, Vigliano C, Lococo B, Bertocchi G, Petti M, Alvarez MG, et al. Long-term cardiac outcomes of treating chronic Chagas disease with Benznidazole versus no treatment. Ann Intern Med. 2006;144: 724–734. [DOI] [PubMed] [Google Scholar]
- 18.Morillo CA, Waskin H, Sosa-Estani S, Bangher M del C, Cuneo C, Milesi R, et al. Benznidazole and posaconazole in eliminating parasites in asymptomatic T. cruzi carriers the STOP-Chagas trial. J Am Coll Cardiol. 2017;69: 949–947. doi: 10.1016/j.jacc.2016.12.023 [DOI] [PubMed] [Google Scholar]
- 19.Riarte A. TRAENA: Placebo-controlled evaluation of impact of benznidazole treatment on long-term disease progression in adults with chronic Chagas disease. 62nd Annual Meeting of the American 460 Society of Tropical Medicine and Hygiene, November 13–17, 2013, Washington, DC. 2013.
- 20.de Macedo-Silva ST, de Souza W, Rodrigues JCF. Sterol biosynthesis pathway as an alternative for the anti-protozoan parasite chemotherapy. Curr Med Chem. 2015;22: 2186–98. [DOI] [PubMed] [Google Scholar]
- 21.Choi JY, Podust LM, Roush WR. Drug strategies targeting CYP51 in neglected tropical diseases. Chem Rev. 2014;114: 11242–11271. doi: 10.1021/cr5003134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olivieri BP, Molina JT, de Castro SL, Pereira MC, Calvet CM, Urbina JA, et al. A comparative study of posaconazole and benznidazole in the prevention of heart damage and promotion of trypanocidal immune response in a murine model of Chagas disease. Int J Antimicrob Agents. 2010;36: 79–83. doi: 10.1016/j.ijantimicag.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 23.Pinazo M-J, Espinosa G, Gállego M, López-Chejade PL, Urbina JA, Gascón J. Successful treatment with posaconazole of a patient with chronic Chagas disease and systemic lupus erythematosus. Am J Trop Med Hyg. 2010;82: 583–7. doi: 10.4269/ajtmh.2010.09-0620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clayton J. Chagas disease: pushing through the pipeline. Nature. 2010;465: S12–5. doi: 10.1038/nature09224 [DOI] [PubMed] [Google Scholar]
- 25.Molina I, Gómez i Prat J, Salvador F, Treviño B, Sulleiro E, Serre N, et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N Engl J Med. 2014;370: 1899–908. doi: 10.1056/NEJMoa1313122 [DOI] [PubMed] [Google Scholar]
- 26.Torrico F. E1224–Results of proof of concept clinical trial in patients with chronic indeterminate Chagas disease. 62nd Annual Meeting of the American Society of Tropical Medicine and Hygiene, Washington, DC, November 13–17, 2013. 2013.
- 27.DNDi. Drug trial for leading parasitic killer of the americas shows mixed results but provides new evidence for improved therapy. In: Press Release [Internet]. 2013 [cited 8 Feb 2017]. https://www.dndi.org/2013/media-centre/press-releases/e1224/
- 28.Chatelain E. Chagas disease drug discovery: toward a new era. J Biomol Screen. 2014;20: 22–35. doi: 10.1177/1087057114550585 [DOI] [PubMed] [Google Scholar]
- 29.Urbina JA. Recent clinical trials for the etiological treatment of chronic Chagas disease: advances, challenges and perspectives. J Eukaryot Microbiol. 2015;62: 149–156. doi: 10.1111/jeu.12184 [DOI] [PubMed] [Google Scholar]
- 30.Moraes CB, Giardini M a, Kim H, Franco CH, Araujo-Junior AM, Schenkman S, et al. Nitroheterocyclic compounds are more efficacious than CYP51 inhibitors against Trypanosoma cruzi: implications for Chagas disease drug discovery and development. Sci Rep. 2014;4: 4703 doi: 10.1038/srep04703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen C-K, Doyle PS, Yermalitskaya L V, Mackey ZB, Ang KKH, McKerrow JH, et al. Trypanosoma cruzi CYP51 inhibitor derived from a Mycobacterium tuberculosis screen hit. PLoS Negl Trop Dis. 2009;3: e372 doi: 10.1371/journal.pntd.0000372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doyle PS, Chen C-K, Johnston JB, Hopkins SD, Leung SSF, Jacobson MP, et al. A nonazole CYP51 inhibitor cures Chagas’ disease in a mouse model of acute infection. Antimicrob Agents Chemother. 2010;54: 2480–8. doi: 10.1128/AAC.00281-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi JY, Calvet CM, Gunatilleke SS, Ruiz C, Cameron MD, McKerrow JH, et al. Rational development of 4-aminopyridyl-based inhibitors targeting Trypanosoma cruzi CYP51 as anti-Chagas agents. J Med Chem. 2013;56: 7651–68. doi: 10.1021/jm401067s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi JY, Calvet CM, Vieira DF, Gunatilleke SS, Cameron MD, Mckerrow JH, et al. R -configuration of 4-aminopyridyl-based inhibitors of CYP51 confers superior efficacy against Trypanosoma cruzi. ACS Med Chem Lett. 2014;5: 434–439. doi: 10.1021/ml500010m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calvet CM, Vieira DF, Choi JY, Kellar D, Cameron MD, Siqueira-neto JL, et al. 4-aminopyridyl-based CYP51 inhibitors as anti- Trypanosoma cruzi drug leads with improved pharmacokinetic profile and in vivo potency. J Med Chem. 2014;57: 6989–7005. doi: 10.1021/jm500448u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vieira DF, Choi JY, Roush WR, Podust LM. Expanding the binding envelope of CYP51 inhibitors targeting Trypanosoma cruzi with 4-aminopyridyl-based sulfonamide derivatives. ChemBioChem. 2014;15: 1111–1120. doi: 10.1002/cbic.201402027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vieira DF, Choi JY, Calvet CM, Siqueira-neto JL, Johnston JB, Kellar D, et al. Binding mode and potency of n indolyloxopyridinyl-4- aminopropanyl-based inhibitors targeting Trypanosoma cruzi CYP51. J Med Chem. 2014;57: 10162–10175. doi: 10.1021/jm501568b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romanha AJ, De Castro SL, Soeiro MDNC, Lannes-Vieira J, Ribeiro I, Talvani A, et al. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem Inst Oswaldo Cruz. 2010;105: 233–238. doi: 10.1590/S0074-02762010000200022 [DOI] [PubMed] [Google Scholar]
- 39.Lewis MD, Fortes Francisco A, Taylor MC, Burrell-Saward H, McLatchie AP, Miles M a, et al. Bioluminescence imaging of chronic Trypanosoma cruzi infections reveals tissue-specific parasite dynamics and heart disease in the absence of locally persistent infection. Cell Microbiol. 2014;16: 1285–1300. doi: 10.1111/cmi.12297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis MD, Francisco AF, Taylor MC, Jayawardhana S, Kelly JM. Host and parasite genetics shape a link between Trypanosoma cruzi infection dynamics and chronic cardiomyopathy. Cell Microbiol. 2016;18: 1429–1443. doi: 10.1111/cmi.12584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guedes-Da-Silva FH, Batista DGJ, Da Silva CF, Meuser MB, Simões-Silva MR, De Araújo JS, et al. Different therapeutic outcomes of benznidazole and VNI treatments in different genders in mouse experimental models of Trypanosoma cruzi infection. Antimicrob Agents Chemother. 2015;59: 7564–7570. doi: 10.1128/AAC.01294-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Souza E, Rivera M, Araújo-Jorge T, De Castro SL. Modulation induced by estradiol in the acute phase of Trypanosoma cruzi infection in mice. Parasitol Res. 2001;87: 513–520. doi: 10.1007/s004360100376 [DOI] [PubMed] [Google Scholar]
- 43.Lewis MD, Francisco AF, Taylor MC, Kelly JM. A new experimental model for assessing drug efficacy against Trypanosoma cruzi infection based on highly sensitive in vivo imaging. J Biomol Screen. 2014;20: 36–43. doi: 10.1177/1087057114552623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9: 676–82. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramírez JC, Cura CI, da Cruz Moreira O, Lages-Silva E, Juiz N, Velázquez E, et al. Analytical validation of quantitative real-time PCR methods for quantification of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. J Mol Diagn. NIH Public Access; 2015;17: 605–15. doi: 10.1016/j.jmoldx.2015.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Francisco AF, Lewis MD, Jayawardhana S, Taylor MC, Kelly JM, Chatelain E, et al. Limited ability of posaconazole to cure both acute and chronic Trypanosoma cruzi infections revealed by highly sensitive in vivo imaging. Antimicrob Agents Chemother. 2015;59: 4653–4661. doi: 10.1128/AAC.00520-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liendo A, Visbal G, Piras MM, Piras R, Urbina JA. Sterol composition and biosynthesis in Trypanosoma cruzi amastigotes. Mol Biochem Parasitol. 1999;104: 81–91. [DOI] [PubMed] [Google Scholar]
- 48.Keenan M, Chaplin JH. A new era for Chagas disease drug discovery? Prog Med Chem. 2015;54: 185–230. doi: 10.1016/bs.pmch.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 49.Urbina JA, McKerrow JH. Drug susceptibility of genetically engineered Trypanosoma cruzi strains and sterile cure in animal models as a criterion for potential clinical efficacy of anti-T. cruzi drugs. Antimicrob Agents Chemother. 2015;59: 7923–7924. doi: 10.1128/AAC.01714-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen C-K, Leung SSF, Guilbert C, Jacobson MP, McKerrow JH, Podust LM. Structural characterization of CYP51 from Trypanosoma cruzi and Trypanosoma brucei bound to the antifungal drugs posaconazole and fluconazole. PLoS Negl Trop Dis. 2010;4: e651 doi: 10.1371/journal.pntd.0000651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lepesheva GI, Hargrove TY, Anderson S, Kleshchenko Y, Furtak V, Wawrzak Z, et al. Structural insights into inhibition of sterol 14alpha-demethylase in the human pathogen Trypanosoma cruzi. J Biol Chem. 2010;285: 25582–90. doi: 10.1074/jbc.M110.133215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lepesheva GI, Park H-W, Hargrove TY, Vanhollebeke B, Wawrzak Z, Harp JM, et al. Crystal structures of Trypanosoma brucei sterol 14alpha-demethylase and implications for selective treatment of human infections. J Biol Chem. 2010;285: 1773–80. doi: 10.1074/jbc.M109.067470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lepesheva GI, Hargrove TY, Rachakonda G, Wawrzak Z, Pomel S, Cojean S, et al. VFV as a new effective CYP51 structure-derived drug candidate for Chagas disease and visceral leishmaniasis. J Infect Dis. 2015;212: 1439–48. doi: 10.1093/infdis/jiv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villalta F, Dobish MC, Nde PN, Kleshchenko YY, Hargrove TY, Johnson CA, et al. VNI cures acute and chronic experimental Chagas disease. J Infect Dis. 2013; 1–8. doi: 10.1093/infdis/jit042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guedes-da-Silva FH, Batista DGJ, Da Silva CF, De Araújo JS, Pavão BP, Simões-Silva MR, et al. Anti-trypanosomal activity of sterol 14α-demethylase (CYP51) inhibitors VNI and VFV in the Swiss mouse models of Chagas disease induced by the Y strain Trypanosoma cruzi. Antimicrob Agents Chemother. 2017; AAC.02098-16. doi: 10.1128/AAC.02098-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fraga Vasconcelos J, Santana Meira C, Nascimento Silva D, Kymie Vasques Nonaka C, Santana Daltro P, Garcia Macambira S, et al. Therapeutic effects of sphingosine kinase inhibitor N,N- dimethylsphingosine (DMS) in experimental chronic Chagas disease cardiomyopathy. Sci Rep. 2017;7: 6171 doi: 10.1038/s41598-017-06275-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia S, Ramos CO, Senra JFV, Vilas-Boas F, Rodrigues MM, Campos-de-Carvalho AC, et al. Treatment with benznidazole during the chronic phase of experimental Chagas’ disease decreases cardiac alterations. Antimicrob Agents Chemother. 2005;49: 1521–1528. doi: 10.1128/AAC.49.4.1521-1528.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonney KM, Engman DM. Chagas heart disease pathogenesis: one mechanism or many? Curr Mol Med. 2008;8: 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rassi A Jr, Marin-Neto JA, Rassi A. Chronic Chagas cardiomyopathy: a review of the main pathogenic mechanisms and the efficacy of aetiological treatment following the BENznidazole Evaluation for Interrupting Trypanosomiasis (BENEFIT) trial. Mem Inst Oswaldo Cruz Rio Janeiro. 2017;112: 224–235. doi: 10.1590/0074-02760160334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang L, Tarleton RL. Parasite persistence correlates with disease severity and localization in chronic Chagas’ disease. J Infect Dis. 1999;180: 480–486. doi: 10.1086/314889 [DOI] [PubMed] [Google Scholar]
- 61.Dutra WO, Menezes CAS, Magalhães LMD, Gollob KJ. Immunoregulatory networks in human Chagas disease. Parasite Immunol. NIH Public Access; 2014;36: 377–387. doi: 10.1111/pim.12107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Viotti R, Vigliano C. Etiological treatment of chronic Chagas disease: neglected “evidence” by evidence-based medicine. Expert Rev Anti Infect Ther. 2007;5: 717–26. doi: 10.1586/14787210.5.4.717 [DOI] [PubMed] [Google Scholar]
- 63.Vogel M, Schwarze-Zander C, Wasmuth J-C, Spengler U, Sauerbruch T, Rockstroh JK. The treatment of patients with HIV. Dtsch Arztebl Int. Deutscher Arzte-Verlag GmbH; 2010;107: 507–15; quiz 516. doi: 10.3238/arztebl.2010.0507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.When To Start Consortium, Sterne JAC, May M, Costagliola D, de Wolf F, Phillips AN, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373: 1352–1363. doi: 10.1016/S0140-6736(09)60612-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.WHO. Antiretroviral therapy for HIV infection in adults and adolescents Recommendations for a public health approach. 2010 revision WHO Press; 2010. [PubMed] [Google Scholar]
- 66.UNAIDS. Ending AIDS: progress towards the 90–90–90 targets | UNAIDS [Internet]. 2017 [cited 9 Aug 2017]. http://www.unaids.org/en/resources/documents/2017/20170720_Global_AIDS_update_2017
- 67.Meirelles MNSL, Souto-Padrón T, De Souza W. Participation of cell surface anionic sites in the interaction between Trypanosoma cruzi and macrophages. J Submicrosc Cytol. 1984;16: 533–545. [PubMed] [Google Scholar]
- 68.Camargo EP. Growth and differentiation in Trypanosoma cruzi. I—Origin of metacyclic trypanosomes in liquid media. Rev Soc Bras Med Trop. 1964;6: 93–100. [PubMed] [Google Scholar]
- 69.Contreras VT, Araujo-Jorge TC, Bonaldo MC, Thomaz N, Barbosa HS, Meirelles M de N, et al. Biological aspects of the Dm 28c clone of Trypanosoma cruzi after metacyclogenesis in chemically defined media. Memorias do Instituto Oswaldo Cruz 1988. pp. 123–133. [DOI] [PubMed] [Google Scholar]
- 70.Brener Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo. 1962;4: 389–96. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.