Abstract
Introduction
Sex hormones have been implicated in the etiology of a number of diseases. To better understand disease etiology and the mechanisms of disease-risk factor associations, this analysis aimed to investigate the associations of anthropometric, sociodemographic and behavioural factors with a range of circulating sex hormones and sex hormone-binding globulin.
Methods
Statistical analyses of individual participant data from 12,330 male controls aged 25–85 years from 25 studies involved in the Endogenous Hormones Nutritional Biomarkers and Prostate Cancer Collaborative Group. Analysis of variance was used to estimate geometric means adjusted for study and relevant covariates.
Results
Older age was associated with higher concentrations of sex hormone-binding globulin and dihydrotestosterone and lower concentrations of dehydroepiandrosterone sulfate, free testosterone, androstenedione, androstanediol glucuronide and free estradiol. Higher body mass index was associated with higher concentrations of free estradiol, androstanediol glucuronide, estradiol and estrone and lower concentrations of dihydrotestosterone, testosterone, sex hormone-binding globulin, free testosterone, androstenedione and dehydroepiandrosterone sulfate. Taller height was associated with lower concentrations of androstenedione, testosterone, free testosterone and sex hormone-binding globulin and higher concentrations of androstanediol glucuronide. Current smoking was associated with higher concentrations of androstenedione, sex hormone-binding globulin and testosterone. Alcohol consumption was associated with higher concentrations of dehydroepiandrosterone sulfate, androstenedione and androstanediol glucuronide. East Asians had lower concentrations of androstanediol glucuronide and African Americans had higher concentrations of estrogens. Education and marital status were modestly associated with a small number of hormones.
Conclusion
Circulating sex hormones in men are strongly associated with age and body mass index, and to a lesser extent with smoking status and alcohol consumption.
Introduction
Sex hormones in men are fundamental to many aspects of physiology. In the prostate, androgens modulate cell proliferation, differentiation and apoptosis and have been theorized to be involved in the etiology of prostate cancer. Although epidemiological studies have found no consistent association between circulating sex hormone concentrations and prostate cancer risk[1], nearly all metastatic prostate tumors overexpress deregulated androgen receptors[2] and androgen deprivation therapy is a common treatment for metastatic prostate cancer. Genome-wide association studies have also identified single nucleotide polymorphisms associated with prostate cancer risk that interact with, or are localized to, androgen receptors[3, 4]. In addition to their role in prostate cancer, sex hormones have been implicated in the development of other common health outcomes, including hypertension, obesity, diabetes, cardiovascular and renal disease[5–7]. By understanding the determinants of sex hormone concentrations, we may better comprehend disease etiology and the mechanisms of disease-risk factor associations.
The Endogenous Hormones, Nutritional Biomarkers and Prostate Cancer Collaborative Group (EHNBPCCG) was established to conduct collaborative pooled analyses of endogenous hormones and nutritional biomarkers in relation to subsequent prostate cancer risk. This large dataset also provides the opportunity to investigate the cross-sectional associations of anthropometric, sociodemographic, behavioural and other factors with circulating sex hormone concentrations. Although many of these associations have been previously studied[8–12], this collaborative dataset enables robust analyses to be performed for a range of hormones and participant characteristics using unified methods. The large size of this dataset also provides greater power to investigate the associations at extremes of the distribution for known relationships, as well as the opportunity to examine novel associations.
Subjects and methods
Data collection
Principal investigators were invited to join the EHNBPCCG if they had published studies on prostate cancer risk and circulating concentrations of sex hormones and/or nutritional biomarkers from blood samples collected prior to the diagnosis of prostate cancer. These were identified using literature search methods described previously[1]. Collaborators provided data on circulating concentrations for up to seven different sex hormones and sex hormone-binding globulin (SHBG) (Table 1) and a wide range of anthropometric, sociodemographic, behavioural and medical factors.
Table 1. Assay methods and geometric mean sex hormone and SHBG concentrations.
| Androstenedione (nmol/L) | A-diol-G (nmol/L) | DHEAS (nmol/L) | Testosterone (nmol/L) | DHT (nmol/L) | Estradiol (pmol/L) | Estrone (pmol/L) | SHBG (nmol/L) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method | CV % | Geometric mean (95% CI) | Method | CV % | Geometric mean (95% CI) | Method | CV % | Geometric mean (95% CI) | Method | CV (%) | Geometric mean (95% CI) | Method | CV % | Geometric mean (95% CI) | Method | CV % | Geometric mean (95% CI) | Method | CV % | Geometric mean (95% CI) | Method | CV % | Geometric mean (95% CI) | ||
| ATBC | E RIA | 7† | 4.7 (4.4–5.0) | E RIA | 12.3† | 5.7 (5.3–6.2) | E RIA | 11.3† | 3069 (2825–3335) | E RIA | 5.5† | 20.5 (19.5–21.5) | E RIA | 8.9† | 1.8 (1.7–2.0) | E RIA | 13.7† | 102 (97–107) | E RIA | 14.1† | 153 (146–159) | IRMA | 4.2† | 85.2 (80.3–90.4) | |
| BLSA | - | - | - | - | - | - | NE RIA | 2–6.6‡ | 3393 (3006–3830) | NE RIA | 3.3–6.4‡ | 15.3 (14.2–16.4) | - | - | - | - | - | - | - | - | - | NE RIA | 1.8–22‡ | 81.1 (74.5–88.3) | |
| CARET | E RIA | 5–9‡ | 2.7 (2.5–2.8) | NE RIA | 1.5–4.8‡ | 12.0 (11.3–12.8) | NE RIA | 0.8–1.9‡ | 1919 (1785–2064) | E RIA | 1–12‡ | 14.1 (13.5–14.7) | - | - | - | E RIA | 5–13‡ | 194 (186–201) | - | - | - | IRMA | 2–7‡ | 27.1 (25.8–28.6) | |
| CHDS | - | - | - | - | - | - | - | - | - | E RIA | 9–11a | 22.0 (21.2–22.8) | - | - | - | E RIA | 8–10a | 162 (156–167) | - | - | - | IA | 4–6a | 32.9 (31.5–34.4) | |
| EPIC | NE RIA | 3.5–11.1† | 4.7 (4.5–4.8) | NE RIA | 4.1–9.9† | 12.9 (12.3–13.5) | - | - | - | NE RIA | 10.8–14.8† | 15.7 (15.3–16.2) | - | - | - | - | - | - | - | - | - | IRMA | 7.7–12.2† | 42.8 (41.2–44.4) | |
| EPIC Norfolk | - | - | - | N/S | 12.1 (11.6–12.6) | N/S | 2378 (2268–2493) | N/S | 15.2 (14.7–15.6) | - | - | - | - | - | - | - | - | - | N/S | 42.0 (40.6–43.5) | |||||
| FMC | NE RIA | 9.5–11.7 a | 5.9 (5.6–6.2) | - | - | - | - | - | - | NE RIA | 4.5–7.2a | 22.9 (21.9–23.9) | - | - | - | - | - | - | - | - | - | IMF | 6.6–8.7 a | 50.7 (48.1–53.4) | |
| HHS | - | - | - | - | - | - | - | - | - | IMF | 5.5–13‡ | 19.7 (18.9–20.6) | - | - | - | - | - | - | - | - | - | IMF | 1.3–10.1‡ | 50.2 (47.6–52.9) | |
| HPFS | - | - | - | NE RIA | 6.7† | 10.7 (10.2–11.2) | - | - | - | ECIA | 4.9† | 15.4 (15.0–15.9) | NE RIA | 9.7† | 1.2 (1.2–1.3) | NE RIA | 5.2† | 111 (108–114) | - | - | - | IRMA | 10.7† | 56.9 (55.0–58.9) | |
| JACC | - | - | - | - | - | - | - | - | - | NE RIA | 5–12b | 15.6 (14.4–16.8) | - | - | - | - | - | - | - | - | - | IRMA | 5.6–6.9 b | 43.5 (39.7–47.6) | |
| Janus | - | - | - | - | - | - | - | - | - | E RIA | 5–15‡ | 22.4 (21.9–22.9) | - | - | - | - | - | - | - | - | - | Precipitation | 5–15‡ | 49.5 (48.2–50.8) | |
| JHCS 1988 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | E RIA | 9–15‡ | 66 (62–71) | E RIA | 9–15‡ | 109 (102–116) | Precipitation | 5–15‡ | 37.1 (33.9–40.6) | |
| JHCS 1996 | E RIA | 5–15‡ | 4.9 (4.5–5.3) | E RIA | 5–15‡ | 9.0 (8.1–9.9) | - | - | - | E RIA | 5–15‡ | 17.4 (16.3–18.6) | E RIA | 5–15‡ | 1.8 (1.6–2.0) | - | - | - | - | - | - | - | - | - | |
| JPHC | - | - | - | - | - | - | - | - | - | ECIA | 1–3‡ | 15.7 (15.1–16.3) | - | - | - | - | - | - | - | - | - | IRMA | 2–8‡ | 47.5 (45.4–49.7) | |
| KPMCP | - | - | - | - | - | - | NE RIA | N/S | 2467 (2263–2690) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| MCCS | NE RIA | 3.3b | 3.6 (3.5–3.7) | NE RIA | 4.3b | 13.8 (13.3–14.3) | CIA | 12.4b | 2812 (2704–2924) | ECIA | 1.6b | 15.4 (15.0–15.8) | - | - | - | ECIA | 11.1 b | 107 (105–109) | - | - | - | IA | 6 b | 36.6 (35.6–37.6) | |
| MEC | - | - | - | NE RIA | 3.4† | 9.5 (9.2–9.9) | - | - | - | E RIA | 3.5† | 18.3 (17.8–18.7) | E RIA | 3.8† | 1.9 (1.9–2.0) | - | - | - | - | - | - | ECIA | 3† | 35.9 (34.9–37.0) | |
| MMAS | E RIA | 8.6–10‡ | 2.9 (2.8–3.0) | - | - | - | NE RIA | 4.1–8.9‡ | 5375 (5116–5647) | E RIA | 4.6–7.2‡ | 16.4 (15.9–16.9) | NE RIA | 10.9–12.2‡ | 0.7 (0.7–0.8) | NE RIA | 3.6–7.1‡ | 140 (136–143) | - | - | - | Filtration assay | 8–10.9‡ | 30.4 (29.4–31.5) | |
| NSHDC androg | - | - | - | N/S | 11.7 (11.1–12.4) | - | - | - | N/S | 19.6 (18.9–20.4) | - | - | - | - | - | - | - | - | - | IRMA | N/S | 41.1 (39.2–43.1) | |||
| NSHDC E2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | N/S | - | 77 (74–79) | - | - | - | - | - | - | |
| PCPT | E RIA | 6.9–8.6 a | 2.0 (1.9–2.1) | NE RIA | 2.7–14.0‡ | 12.0 (11.6–12.4) | - | - | - | ECIA | 7.6–11.9 ‡ | 12.4 (12.2–12.7) | - | - | - | E RIA | 10–14.9‡ | 118 (116–121) | E RIA | 9–15.2 ‡ | 158 (155–161) | ECIA | 5.2–12.2‡ | 36.6 (35.6–37.6) | |
| PHS | - | - | - | NE RIA | 7.6† | 13.5 (12.7–14.3) | - | - | - | NE RIA | 8.7† | 16.0 (15.4–16.6) | E RIA | 5.3† | 1.2 (1.1–1.3) | - | - | - | E RIA | 10† | 122 (118–126) | IRMA | 8.9† | 20.6 (19.6–21.6) | |
| PLCO | NE RIA | 14† | 4.1 (4.0–4.2) | NE RIA | 11† | 14.2 (13.7–14.8) | - | - | - | NE RIA | 14† | 15.9 (15.5–16.3) | - | - | - | - | - | - | - | - | - | IRMA | 18† | 43.9 (42.6–45.2) | |
| ProtecT | - | - | - | - | - | - | - | - | - | EIA | N/S | 13.9 (13.4–14.3) | - | - | - | - | - | - | - | - | - | EIA | - | 37.2 (35.8–38.7) | |
| RBS | - | - | - | - | - | - | NE RIA | 3.1–7.3‡ | 2054 (1910–2208) | E RIA | 4.1–10‡ | 10.4 (9.9–10.8) | E RIA | 7.5‡ | 1.4 (1.3–1.5) | E RIA | 8–12‡ | 72 (70–75) | - | - | - | - | - | - | |
Abbreviations: A-diol-G = Androstanediol glucuronide; ATBC = The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; BLSA = The Baltimore Longitudinal Study of Aging; CARET = The Carotene and Retinol Efficacy Trial; CHDS = Child Health and Development Studies; CI = confidence interval; CIA = competitive immunoassay; CV = coefficient of variation; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; E RIA = extraction radioimmunoassay; ECIA = electrochemiluminescence immunoassay; EIA = enzyme immunoassay; EPIC = European Prospective Investigation into Cancer and Nutrition; FMC = Finnish Mobile Clinic Health Examination Survey; HHS = Helsinki Heart Study; HPFS = Health Professionals Follow-Up Study; IA = immunoassay; IMF = immunofluorometry; IRMA = immunoradiometric assay; JACC = Japan Collaborative Cohort Study; JPHC = Japan Public Health Center-based Prospective Study; JHCS = Japan-Hawaii Cancer Study; KPMCP = Kaiser Permanente Medical Care Program; MCCS = Melbourne Collaborative Cohort Study; MEC = Multiethnic Cohort Study of Diet and Cancer; MMAS = Massachusetts Male Aging Study; NE RIA = non-extraction radioimmunoassay; NSHDC = Northern Sweden Health and Disease Cohort (androg = androgens; E2 = estradiol); PCPT = Prostate Cancer Prevention Trial; PHS = Physicians' Health Study; PLCO = Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; ProtecT = Prostate Testing for Cancer and Treatment; RBS = Rancho Bernardo Study; SHBG = Sex hormone binding globulin
†Intra-assay.
a Inter-assay.
‡Intra-and inter-assay range.
b Not specified.
All studies were either of a prospective cohort design[13–30] or were a prospective observational study within a randomized trial[31–36]. This analysis used secondary data, therefore ethical approval for this analysis was not necessary. However, each study individually obtained ethical approval. Details of ethical approval, participant consent and study design can be found in the original publications[13–36]. Participants were considered eligible for this analysis if they had information on at least one of the circulating sex hormones or SHBG concentrations, had not been diagnosed with prostate cancer by the time of censoring, were 25 years or over at blood collection, had complete data on age at blood collection, height, and weight, and were not known to be on androgen therapy at blood collection. Overall, these exclusion criteria resulted in 12,330 participants (out of a total of 14,092; S1 Fig) from 25 studies being included in these analyses (Table 2).
Table 2. Participant characteristics by study.
| Study, country | N (% of total) | Mean age, years (SD) | Age range, years | Year of birth | Mean height, cm (SD) | Mean BMI, kg/m2 (SD) | % Current drinkers (median daily alcohol consumption, g alcohol) | % Current smokers (median number of daily cigarettes) | % White ethnic group | % Married/ cohabiting at blood collection | % University degree | % Family history of prostate cancer |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATBC, Finland | 231 (1.9%) | 60.5 (5.2) | 50–69 | 1915–1936 | 173.8 (6.8) | 25.9 (3.7) | 85.4 (8.5) | 100 (20) | - | 82.3 | 5.6 | 2.5 |
| BLSA, USA | 112 (0.9%) | 56.4 (12.8) | 30–84 | 1895–1948 | 176.8 (6.0) | 25.7 (3.0) | - | 21.4 | 95.5 | 91.8 | 57.1 | - |
| CARET, USA | 300 (2.5%) | 62.8 (6.0) | 47–77 | 1913–1945 | 174.9 (7.6) | 27.7 (4.4) | 68.2 (5) | 50.0 (20) | 93.7 | 85.9 | 24.2 | 3.3 |
| CHDS, USA | 396 (2.4%) | 34.9 (3.2) | 25–50 | 1912–1941 | 178.7 (6.8) | 24.7 (2.7) | 77.5 (6) | 50.6 (20) | 63.1 | 99.7 | 34.5 | - |
| EPIC, Europe | 638 (5.2%) | 60.9 (6.2) | 43–76 | 1918–1952 | 172.6 (7.0) | 27.0 (3.6) | 87.1 (13) | 27.8 (15) | 100 | 89.1 | 22.9 | - |
| EPIC Norfolk, Europe | 707 (5.7%) | 66.7 (6.5) | 45–78 | 1918–1950 | 172.9 (6.4) | 26.6 (3.2) | 73.8 (8) | 9.3 | 99.9 | 88.3 | 12.5 | 1.7 |
| FMC, Finland | 298 (2.4%) | 57.9 (9.9) | 33–80 | 1890–1937 | 169.6 (6.8) | 26.1 (3.5) | - | 34.2 (15) | 100 | 85.2 | - | - |
| HHS, Finland | 291 (2.4%) | 51.0 (3.8) | 40–56 | 1924–1940 | 174.2 (6.2) | 26.4 (3.1) | - | - | - | - | - | - |
| HPFS, USA | 682 (5.5%) | 65.1 (7.4) | 46–80 | 1913–1946 | 178.1 (6.4) | 26.0 (3.5) | 73.5 (6) | 3.9 (20) | 99.4 | 93.0 | 100 | 10.3 |
| JACC, Japan | 97 (0.8%) | 67.8 (5.8) | 58–83 | 1904–1934 | 159.5 (7.1) | 22.4 (2.7) | 52.6 (2) | 37.2 (20) | 0 | 93.8 | 2.7 | - |
| Janus, Norway | 1,153 (9.4%) | 46.4 (4.2) | 33–63 | 1910–1947 | 176.2 (6.8) | 25.1 (3.2) | - | 62.5 (12) | 100 | - | - | - |
| JHCS 1988, Japanese in Hawaii | 98 (0.8%) | 63.4 (5.6) | 52–74 | 1900–1919 | 162.2 (5.4) | 23.1 (2.8) | 74.5 (5.5) | 36.7 (20) | 0 | 90.8 | 8.2 | - |
| JHCS 1996, Japanese in Hawaii | 139 (1.1%) | 61.9 (5.7) | 52–74 | 1900–1919 | 162.8 (6.4) | 23.2 (2.8) | 78.4 (5) | 28.1 (20) | 0 | 94.2 | 10.1 | - |
| JPHC, Japan | 399 (3.2%) | 59.1 (6.6) | 41–71 | 1923–1949 | 162.0 (6.4) | 23.3 (2.6) | 71.2 (23) | 40.6 (20) | 0 | 91.0 | - | - |
| KPMCP, USA | 212 (1.7%) | 71.8 (4.5) | 60–85 | 1882–1906 | 169.9 (6.7) | 25.8 (3.1) | 69.7 (10) | 17.9 (30) | 98.6 | 82.7 | 5.4 | - |
| MCCS, Australia | 1,058 (8.6%) | 58.3 (7.2) | 40–72 | 1918–1954 | 172.2 (7.3) | 27.2 (3.7) | 78.3 (13) | 13.0 (20) | 100 | 81.0 | 22.2 | - |
| MEC, USA | 922 (7.5%) | 68.7 (7.2) | 48–84 | 1918–1948 | 174.2 (7.7) | 27.1 (4.2) | 90.9 (9) | 12.6 (15) | 13.2 | 78.1 | 32.2 | 9.0 |
| MMAS, USA | 651 (5.3%) | 57.6 (7.0) | 41–70 | 1916–1946 | 175.8 (7.2) | 27.5 (4.5) | - | 23.5 | 98.8 | 77.1 | 37.8 | - |
| NSHDC E2, Sweden | 465 (3.8%) | 57.6 (4.0) | 40–61 | 1927–1948 | 175.8 (5.9) | 26.6 (3.5) | - | 21.3 | 100 | - | - | - |
| NSHDC androg, Sweden | 382 (3.1%) | 57.2 (4.5) | 39–61 | 1927–1958 | 175.8 (6.2) | 26.5 (3.4) | 100.0 (4) | 17.4 | 100 | 85.6 | 12.4 | |
| PCPT, USA and Canada | 1,025 (8.3%) | 63.3 (5.5) | 55–83 | 1911–1940 | 177.4 (7.0) | 27.7 (4.0) | 68.9 (3) | 7.6 (20) | 83.9 | 87.7 | 37.1 | 20.7 |
| PHS, USA | 376 (3.0%) | 61.3 (7.5) | 41–77 | 1905–1941 | 177.9 (7.0) | 24.6 (2.5) | 84.4 (5) | 8.5 (20) | 96.5 | - | 100 | - |
| PLCO, USA | 858 (7.0%) | 64.8 (4.8) | 54–75 | 1919–1944 | 177.9 (6.5) | 27.4 (3.9) | 70.0 (4) | 9.1 (20) | 100 | 86.9 | 42.2 | 6.1 |
| ProtecT, UK | 539 (4.4%) | 61.5 (5.1) | 50–70 | 1930–1949 | 175.2 (6.5) | 26.6 (3.5) | 84.9 (18) | 10.5 | - | - | - | 4.1 |
| RBS, USA | 301 (2.4%) | 69.4 (9.0) | 47–85 | 1898–1938 | 174.4 (6.8) | 26.0 (3.6) | 68.8 (10) | 7.3 (20) | 100 | 90.4 | 22.6 | - |
| Missing data (%) | - | - | - | - | - | - | 31.1 | 12.2 | 9.0 | 26.3 | 27.3 | 55.5 |
| Overall | 12,330 | 59.9 (9.9) | 25–85 | 1882–1958 | 174.4 (7.8) | 26.4 (3.8) | 77.1 (8) | 23.7 (20) | 89.4 | 86.5 | 35.8 | 8.2 |
Abbreviations: ATBC = The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; BLSA = The Baltimore Longitudinal Study of Aging; BMI = body mass index; CARET = The Carotene and Retinol Efficacy Trial; CDHS = Child Health and Development Studies; EPIC = European Prospective Investigation into Cancer and Nutrition; FMC = Finnish Mobile Clinic Health Examination Survey; HHS = Helsinki Heart Study; HPFS = Health Professionals Follow-Up Study; JACC = Japan Collaborative Cohort Study; JPHC = Japan Public Health Center-based Prospective Study; JHCS = Japan-Hawaii Cancer Study; KPMCP = Kaiser Permanente Medical Care Program; MCCS = Melbourne Collaborative Cohort Study; MEC = Multiethnic Cohort; MMAS = Massachusetts Male Aging Study; NSHDC = Northern Sweden Health and Disease Cohort (androg = androgens; E2 = estradiol); PCPT = Prostate Cancer Prevention Trial; PHS = Physicians' Health Study; PLCO Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; ProtecT = Prostate Testing for Cancer and Treatment; RBS = Ranch Bernardo Study; SD = standard deviation
Free testosterone and free estradiol concentrations were calculated using the law of mass action[37, 38] based on recorded SHBG, testosterone and estradiol values and assuming a constant albumin concentration of 43 g/L.
Statistical analysis
Hormone and SHBG concentrations were logarithmically transformed to approximate normal distributions. Categories investigated were selected a priori and cut-points chosen based on sample size and the data distribution. The analyses examined associations of hormone concentrations with age (25–49 [mean age = 43.5], 50–54, 55–59, 60–64, 65–69, 70–74, 75+ years), height (<160.0, 160.0–164.9, 165.0–169.9, 170.0–174.9, 175.0–179.9, 180.0–184.9, 185.0–189.9, 190.0+ cm), BMI (<20.0, 20.0–22.4, 22.5–24.9, 25.0–27.4, 27.5–29.9, 30.0–32.4, 32.5–34.9, 35.0–37.4, 37.5+ kg/m2), waist circumference (<90.0, 90.0–94.9, 95.0–99.9, 100.0–104.9, 105.0+ cm) and waist-to-hip ratio (<0.900, 0.900–0.933, 0.934–0.966, 0.967–0.999, 1.000+). Health behaviours investigated included smoking status (never, former, current: <15, 15–29, 30+ cigarettes per day) and alcohol consumption (none, 1–9, 10–19, 20–29, 30–39, 40–49, 50–59, 60–69, 70+ g alcohol per day). Ethnic/racial group was categorised as white, African American/Caribbean, Hispanic/Latino, East Asian, and other ethnic/racial group. Other possible determinants that were examined included: time of day of blood collection (before 09:00, 09:00–11:59, 12:00–14:59, 15:00 onwards); educational status (no secondary/high school education, secondary school, college and university); marital status (currently married/cohabiting, not currently married/cohabiting) and family history of prostate cancer (no, yes: defined as a father and/or brother diagnosed with prostate cancer).
Partial correlations between the sex hormones and SHBG were calculated using study-specific standardized values: (xjk-mj)/sj, where mj and sj denote the mean and standard deviation of the log-transformed hormone concentrations in study j and xjk is an observation from that study. These standardized values were adjusted for age at blood collection and BMI (included as categorical variables, described above). Geometric mean concentrations of sex hormones and SHBG were calculated using predicted values from analysis of variance models scaled to the overall geometric mean concentration and adjusted for study, age at blood collection, and BMI (with the exception of when we analysed the associations of age and BMI with hormone concentrations, when these variables were not included as adjustment covariates). Analyses of smoking and alcohol consumption were mutually adjusted for each other. To investigate the relationship of hormone concentrations with ethnicity/race, studies were limited to the three studies that had sufficient representation from men across multiple ethnic/racial groups (Child Health and Development Studies, the Multi-Ethnic Cohort and the Prostate Cancer Prevention Trial). Heterogeneity of means by category of each characteristic was tested using the F test. Where appropriate, a test for trend was calculated using the analysis of variance test, with the categorical variables entered as linear values scored consecutively as 1, 2, 3 etc. Owing to the highly skewed distribution of alcohol consumption, the test for trend was calculated based on median values within each category. To test for trend by smoking status, never and former smokers were combined and coded as 0; light, medium and heavy smokers were coded as 1, 2 and 3, respectively.
Most variables had a small number of missing values (Table 2). Time of day at blood collection was missing for 44.8% of men and was therefore not included as a covariate in the analyses. To enable adjustment for study, each study had to contain observations in a minimum of two categories for each primary exposure to be included in the respective exposure analysis. Assay methods for each hormone varied between studies (Table 1); some studies used “extraction” assays to measure circulating sex hormones (immunoassays preceded by extraction into an organic solvent and celite column chromatography), which generally provide higher sensitivity and specificity than non-extraction or “direct” assays. Heterogeneity between studies and by assay method was tested using a study-by-factor and assay-by-factor interaction term (fitted separately) in the analysis of variance, and assessed using the F test.
All statistical tests were two-sided and due to the multiple tests the statistical significance threshold was p<0.01. Data analysis was carried out using Stata Statistical Software release 14.1 (Stata Corp., College Station, TX, USA).
Sensitivity analyses
To examine effect modification by these factors, separate analyses were performed stratified by: age (<55, 55–69, 70+ years); BMI (<20.0, 20.0–29.9, 30.0+ kg/m2); and time of day (morning and afternoon), in comparison to a restricted dataset. The analyses were also repeated after restricting the dataset to: i) white men to examine whether ethnicity/race was a confounder, ii) men with hormone concentrations that were within the range of [lower quartile– 3*interquartile range, upper quartile + 3*interquartile range] of each respective study to examine the effect of removing outliers (n = 63).
Results
25 studies contributed to the analysis, including 12,330 participants (Table 2). Participants were born between 1882–1958 and age at blood collection ranged from 25–85 years (mean = 59.9 years; standard deviation = 9.9 years). The men were predominantly white (89.4%).
Correlations between hormones
After taking into account age, BMI and study, most sex hormones were positively correlated with each other (Table 3). Testosterone was most strongly correlated with free testosterone (r = 0.77), DHT (r = 0.54) and SHBG (r = 0.52). DHEAS was correlated with androstenedione (r = 0.40) and A-diol-G (r = 0.23). Estradiol was strongly correlated with free estradiol (r = 0.88) and estrone (r = 0.54), and weakly correlated with SHBG (r = 0.10).
Table 3. Partial correlation coefficients in control subjects between log-transformed concentrations of sex hormones and SHBG.
| Androstenedione | A-diol-G | DHEAS | Testosterone | Free testosterone | DHT | Estradiol | Free estradiol | Estrone | SHBG | |
|---|---|---|---|---|---|---|---|---|---|---|
| Androstenedione | 1 | |||||||||
| A-diol-G | 0.23* | 1 | ||||||||
| DHEAS | 0.40* | 0.21* | 1 | |||||||
| Testosterone | 0.29* | 0.25* | 0.07* | 1 | ||||||
| Free testosterone | 0.29* | 0.28* | 0.15* | 0.77* | 1 | |||||
| DHT | 0.20* | 0.22* | 0.03 | 0.54* | 0.34* | 1 | ||||
| Estradiol | 0.13* | 0.14* | 0.06* | 0.33* | 0.34* | 0.19* | 1 | |||
| Free estradiol | 0.09* | 0.13* | 0.11* | 0.05* | 0.37* | -0.03 | 0.88* | 1 | ||
| Estrone | 0.45* | 0.18* | 0.15 | 0.27* | 0.26* | 0.19* | 0.54* | 0.50* | 1 | |
| SHBG | 0.10* | 0.04* | -0.08* | 0.52* | -0.11* | 0.36* | 0.10* | -0.36* | 0.09* | 1 |
Measurements are standardised by study and adjusted by age and BMI.
*P<0.01.
Abbreviations: A-diol-G = Androstanediol glucuronide; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
Age at blood collection
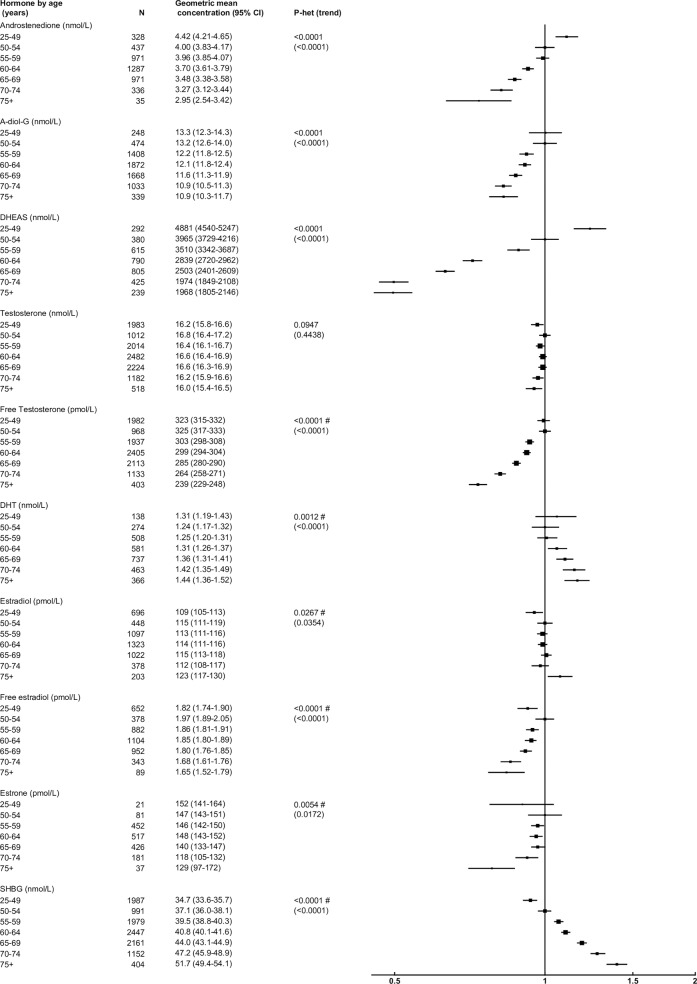
After adjusting for study and BMI, age was associated with concentrations of all hormones, except testosterone and estradiol (Fig 1). Compared to men aged 50–54 years, those aged 75+ years had circulating concentrations of DHEAS, free testosterone, androstenedione, A-diol-G and free estradiol that were 50%, 27%, 26%, 17%, 16% lower, respectively. In contrast, SHBG and DHT concentrations were 39% and 16% higher, respectively. There was significant heterogeneity (P<0.01) between studies in the associations of free testosterone, DHT, estradiol, free estradiol, estrone and SHBG.
Fig 1. Relative geometric mean concentrations* of male sex hormones by age category.
*relative to 50–54 years, adjusted for study and BMI. #significant interaction with study P<0.01. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
Although there was no evidence of a linear association between age and testosterone concentration, there was evidence of heterogeneity in the association of testosterone concentration with age by time of day of blood collection (F = 3.00; P = 0.0005). For men whose blood was collected in the morning, younger men (aged 50–54 years) had 10% higher circulating concentrations of total testosterone than those aged 75+ years, while age was not associated with total testosterone concentration in men whose blood was collected in the afternoon (S2 Fig).
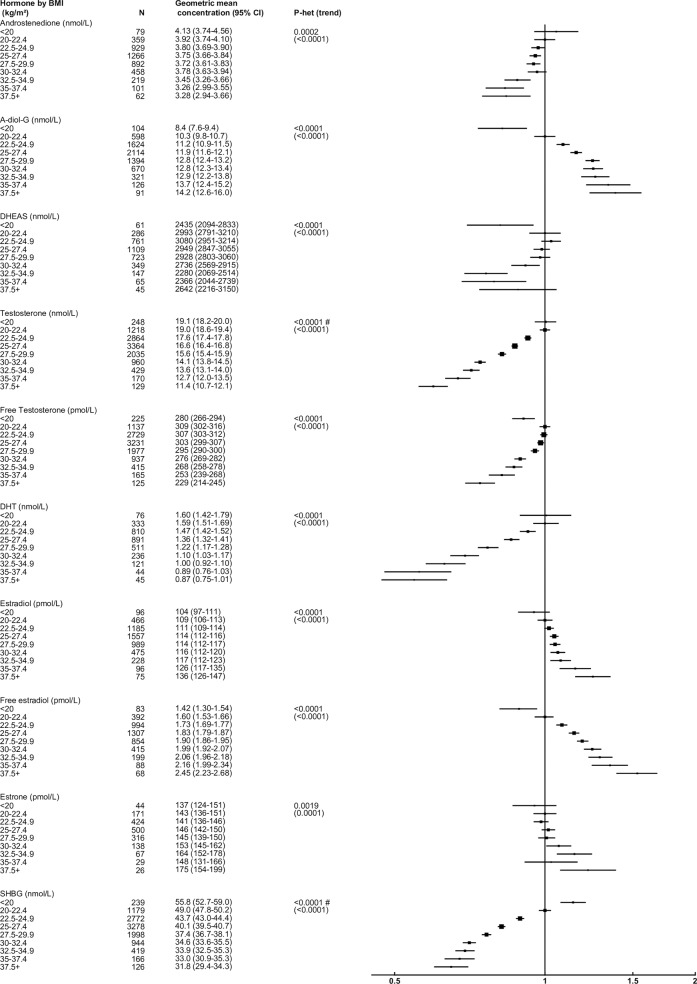
BMI
After adjusting for study and age, BMI was strongly associated with all sex hormones and SHBG (Fig 2). Compared to men with a BMI 20.0–22.4 kg/m2, those with a BMI ≥37.5 kg/m2 had concentrations of DHT, testosterone, SHBG, free testosterone, androstenedione and DHEAS that were 45%, 40%, 35%, 26%, 16% and 12% lower, respectively. In contrast, concentrations of free estradiol, A-diol-G, estradiol and estrone were 53%, 38%, 25% and 22% higher, respectively. These associations remained broadly similar when using waist circumference or WHR (S3 and S4 Figs), although when data were divided into fifths the magnitudes of the associations were smaller with WHR than with BMI or waist circumference (data not shown). Differences in geometric mean hormone concentrations by waist circumference and WHR were attenuated following additional adjustment for BMI, although testosterone and SHBG remained significantly associated with both waist circumference and WHR (S5 and S6 Figs).
Fig 2. Relative geometric mean concentrations* of male sex hormones by BMI category.
*relative to BMI 20.0–22.4 kg/m2, adjusted for study and age. #significant interaction with study P<0.01. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
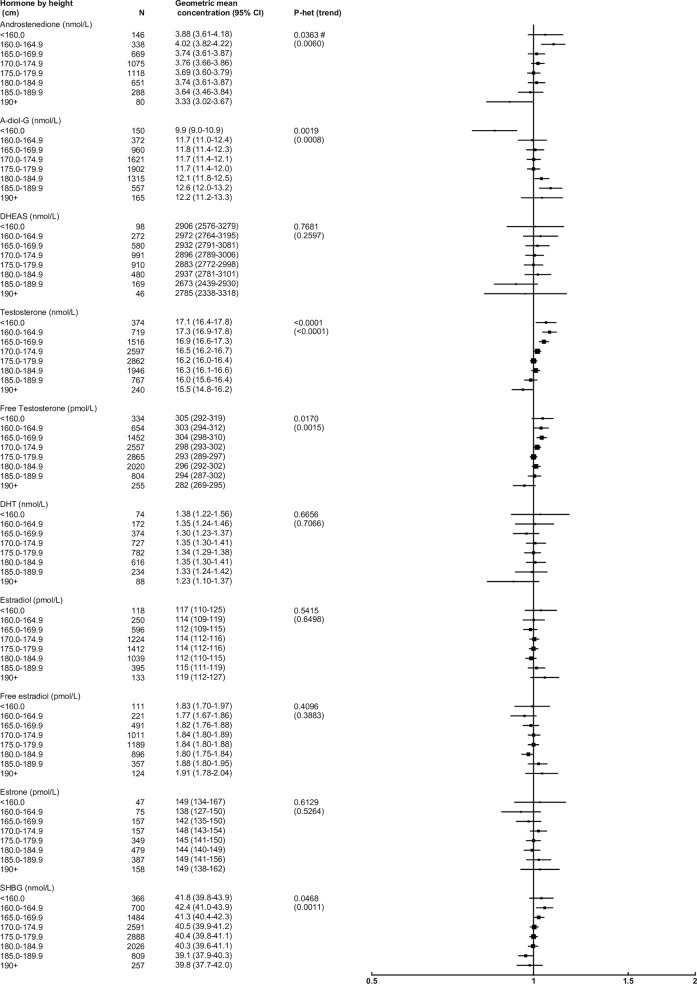
Height
After adjusting for study, age and BMI, height was associated with concentrations of several androgens and SHBG, but not estrogens (Fig 3). Compared to men with a height of 175–179 cm, those in the tallest category (≥190 cm) had concentrations of androstenedione, testosterone, free testosterone and SHBG that were 10%, 4%, 4%, and 2% lower, respectively, whilst A-diol-G concentration was 4% higher.
Fig 3. Relative geometric mean concentrations* of male sex hormones by height categories.
*relative to 175.0–179.9 cm, adjusted for study, age and BMI. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
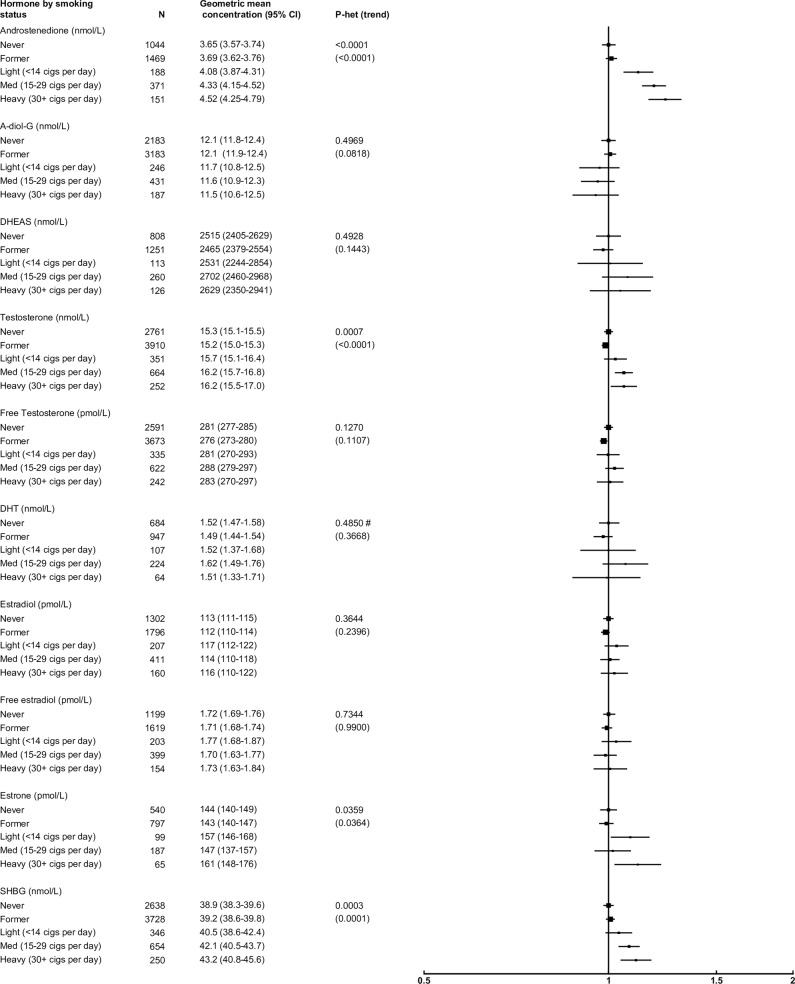
Smoking
After adjusting for study, age, BMI and alcohol consumption, smoking status was positively associated with concentrations of androgens and SHBG, but not estrogens (Fig 4). Compared with never smokers, heavy smokers (30+ cigarettes/day) had concentrations of androstenedione, SHBG and testosterone that were 24%, 11% and 6% higher, respectively.
Fig 4. Relative geometric mean concentrations* of male sex hormones by smoking status.
*relative to never smokers, adjusted for study, age, BMI and alcohol consumption. #significant interaction with study P<0.01. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
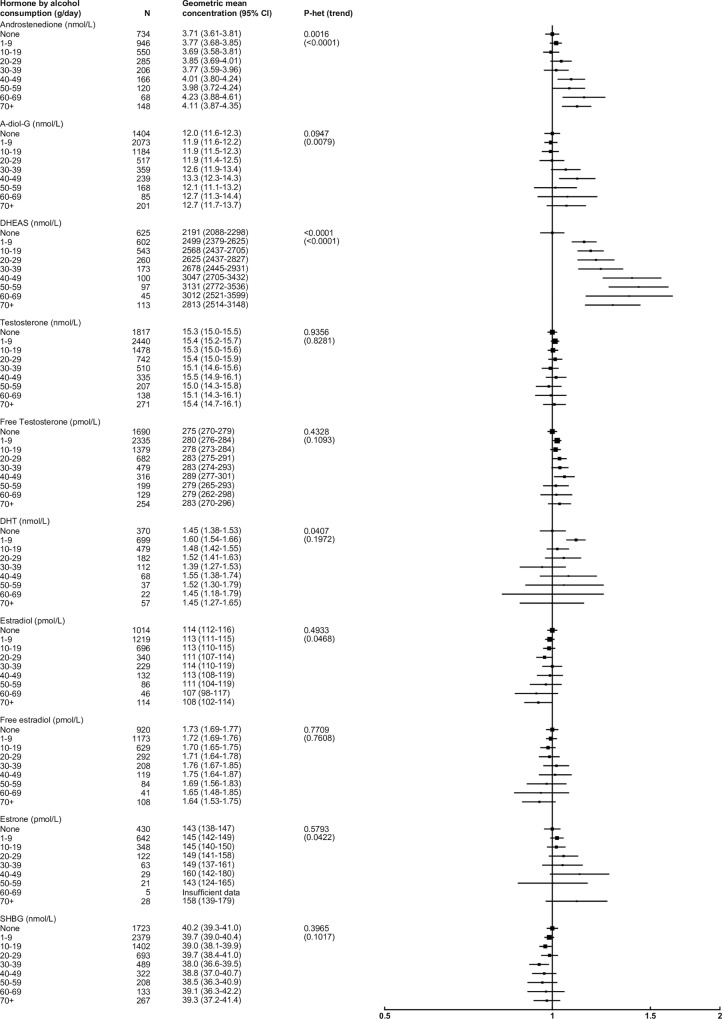
Alcohol
After adjusting for study, age, BMI and smoking, alcohol consumption was associated with higher concentrations of several androgens, but was not associated with estrogens (Fig 5). In particular, compared with non-drinkers, those who drank ≥70 g alcohol/day had a 28% higher concentration of DHEAS. High alcohol consumption was also moderately associated with higher concentrations of androstenedione and A-diol-G (11% and 6% higher, respectively). Restricting the analysis to non-smokers (n = 6,953) did not materially alter these results.
Fig 5. Relative geometric mean concentrations* of male sex hormones by alcohol consumption categories.
*relative to non-drinkers, adjusted for study, age, BMI and smoking status. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
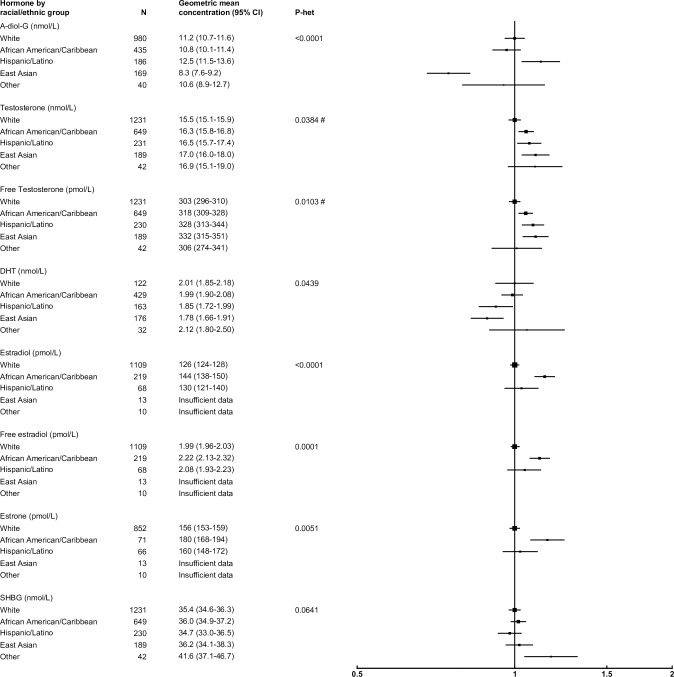
Ethnic/racial group
After adjusting for study, age and BMI, most sex hormones did not differ by ethnic/racial group, with the exception of A-diol-G and estrogens (Fig 6). Compared with white men, A-diol-G concentration was 25% lower in East Asian men and 12% higher in Hispanic/Latino men, while estrone, estradiol and free estradiol concentrations were 15%, 14% and 12% higher in African American men than whites.
Fig 6. Relative geometric mean concentrations* of male sex hormones by ethnic/racial group.
*relative to whites, adjusted for study, age and BMI. #significant interaction with study P<0.01. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
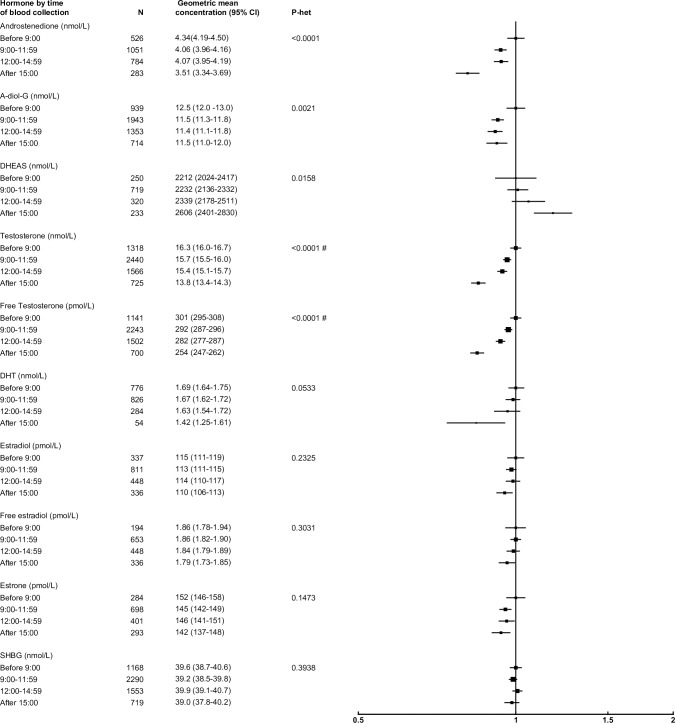
Time of blood collection
After adjusting for study, age and BMI, several sex hormones were associated with time of blood collection (Fig 7). Compared with men whose blood was collected before 09:00, participants whose blood was collected at 15:00 or later had concentrations of androstenedione, testosterone, free testosterone and A-diol-G that were 19%, 16%, 15% and 8% lower, respectively.
Fig 7. Relative geometric mean concentrations* of male sex hormones by time of blood collection.
*relative to before 09:00, adjusted for study, age and BMI. #significant interaction with study P<0.01. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
Sociodemographic factors and health
After adjusting for study, age and BMI several sex hormones had small associations with sociodemographic factors. In comparison to men with no secondary/high school qualifications, men with a university education had concentrations of SHBG, androstenedione and testosterone and that were 7%, 6% and 5% lower, respectively (S7 Fig). In comparison to men who were married/cohabiting, men who were not married/cohabiting had concentrations of estrone, androstenedione, free estradiol and estradiol and that were 12%, 7%, 6% and 6% higher, respectively (S8 Fig). Hormone concentrations were not associated with a family history of prostate cancer (S9 Fig).
Heterogeneity by study and assay
Some heterogeneity between studies in the associations of sex hormone concentrations with anthropometric and sociodemographic factors was observed (see Figs 1–7). Much of the observed heterogeneity was due to differences in the magnitude rather than the direction of associations (data not shown). Some of the heterogeneity between studies may have been caused by differences in assay type. There was heterogeneity by assay type in the associations of DHT and estradiol concentrations with age. There was no increase in DHT with age in studies that used assays without an extraction step prior to assay, whilst age was associated with higher DHT concentration in studies with an extraction step. For estradiol, the positive association with age was stronger in studies that used an extraction step than in those that did not.
Sensitivity analyses
Stratification by age, BMI and time of day did not materially affect the results (data not shown), with the exception of the association of total testosterone with age (see above). The results also remained broadly unchanged after restricting the dataset to white men and excluding within study hormone outliers (data not shown).
Discussion
This international collaboration has brought together and analysed data from over 12,300 men on the associations of various anthropometric, behavioural and sociodemographic factors with circulating sex hormone and SHBG concentrations. Our findings suggest that age, body composition, and to a lesser extent smoking status and alcohol consumption, may be important determinants of circulating sex hormone concentrations.
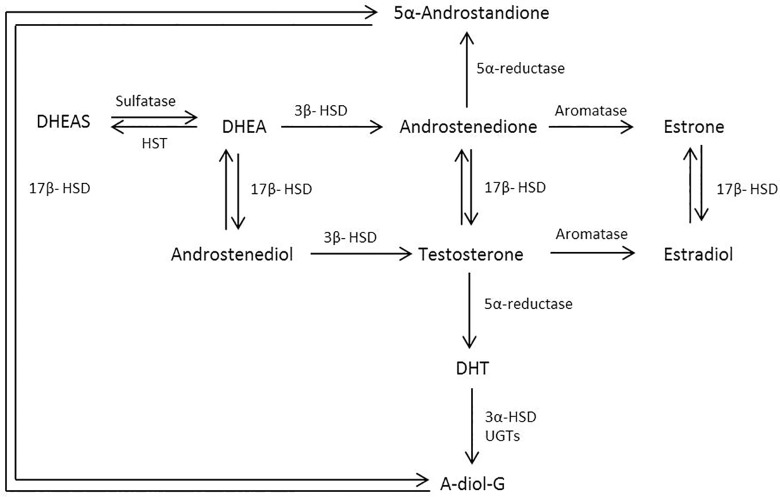
As expected, hormones were strongly correlated with other hormones close to them on the sex steroid pathway (Fig 8). Testosterone is primarily secreted by the testes. The adrenal glands are another source of androgens, including DHEAS and androstenedione, both of which can ultimately be converted to testosterone and/or DHT in peripheral tissues. Androgens can also be converted into estrogens, primarily in the testes and adipose tissue. <3% of circulating testosterone and estradiol is bioavailable to the tissues, or “free”, the remainder is primarily bound to either SHBG or albumin[39].
Fig 8. Simplified schematic of the biosynthesis pathway of sex hormones.
Abbreviations: A-diol-G = Androstanediol glucuronide; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; HSD = Hydroxysteroid dehydrogenase; HST = Hydroxysteroid sulfotransferase; UGT = Uridine 5'-diphospho-glucuronosyltransferase.
Age was strongly associated with circulating SHBG and androgens, with the exception of total testosterone concentration. Other studies have generally found that total testosterone declines with age[12, 40–44], although more recent research suggests that the observed decline may be partly due to confounding by obesity and comorbidities[45–49]. Our analyses found no significant overall association of total testosterone with age. However, stratification by time of day showed a significant linear decline with age only in men whose blood was collected in the morning; circulating testosterone concentrations are highest in the morning and decline throughout the day. However, this diurnal rhythm is blunted as men age[50, 51]. The marked positive association of SHBG concentration with age may be related to a lower insulin-like growth factor-1 concentration in older individuals[52]. Adrenal sensitivity to adrenocorticotrophic hormone has also been shown to decline with age[53], which results in a substantially reduced production of DHEAS and, to a lesser extent, androstenedione[54, 55]. Despite this age-associated decline in androstenedione, DHT concentration was higher in older men, which might be due to the reduced conversion of DHT to A-diol-G[56].
BMI was strongly associated with concentrations of all the sex hormones and SHBG. A higher BMI was associated with markedly lower SHBG concentration, which may be due to high insulin[57]. Some recent evidence indicates that this BMI-SHBG association may involve low grade inflammation and/or increased liver fat[58, 59]. The reduction in SHBG may lead to a fall in testosterone production via a negative feedback loop. Increasing adipose tissue also leads to higher aromatase activity[60], which converts androgens to estrogens, leading to higher concentrations of circulating estrogens. This, together with the low concentrations of SHBG, results in markedly higher concentrations of free estradiol in obese men. Our finding of a strong linear association of A-diol-G concentration with BMI suggests an increase in peripheral androgen metabolism with greater adiposity. Obesity leads to dysregulation of the hypothalamic-pituitary-adrenal axis[57, 61] and may therefore result in lower DHEAS and androstenedione production. After adjustment for BMI, waist circumference and WHR each remained significantly associated with testosterone and SHBG concentrations. This suggests that visceral fat, or other factors correlated with waist circumference and WHR, may be important predictors of androgen and SHBG concentrations[62].
Testosterone, free testosterone and SHBG were weakly inversely associated with height, and A-diol-G was positively associated with height. While estrogens are involved in the control of growth in height[63] and affect growth hormone secretion[64], there is some evidence to suggest that androgens may also be involved[65]. However, it is unclear to what extent circulating sex hormone concentrations during childhood and adolescence correlate with concentrations later in life.
The mechanisms through which tobacco may affect hormone concentrations are complex because cigarette smoke contains a wide range of endocrine disruptors, some of which exert opposing physiological effects[66, 67]. Smoking was associated with higher concentrations of testosterone and SHBG, which resulted in a relatively unchanged free testosterone concentration[68–70]. In our analysis smoking was also associated with elevated circulating androstenedione concentration.
Alcohol consumption was associated with moderately higher androgen concentrations, particularly for DHEAS, as observed previously[71–73], and to a lesser extent with androstenedione, possibly involving increased adrenal secretion and inhibition of hydroxysteroid dehydrogenase[73]. The large association with DHEAS may be related to the long half-life of this metabolite. Alcohol consumption was also moderately positively associated with A-diol-G, although findings from other studies are inconsistent[74, 75]. While chronic excessive alcohol consumption is a well-known cause of low testosterone in men[76], we did not find an inverse association of alcohol with testosterone concentration in the current analyses, perhaps because the levels of alcohol intake were not sufficient to induce a detrimental biological effect.
Three studies provided data from men across several ethnic/racial groups and we found that ethnic/racial group was significantly associated with circulating A-diol-G concentration, which was lower in East Asian men compared to all other ethnic/racial groups, as observed previously[77–79]. Estrone, estradiol and free estradiol concentration were also significantly higher in African Americans, as reported elsewhere[80], although the mechanism for this remains unclear. Further inference regarding the association between ethnicity/race and other hormone concentrations was not possible due to the relatively low representation of non-white men.
Heterogeneity between the included studies was present for several associations. Further investigation showed that this was generally caused by differences in the magnitude of association rather than the direction. Heterogeneity may have been caused by differences in sample populations, study design, and for testosterone distribution of time of day of blood draw. Additionally, some of the observed heterogeneity may have been attributable to differences in assay type. In this analysis, no study used mass spectrometry, often considered the gold standard method of measurement for sex hormones[81]. Instead, the majority of sex hormones were measured using extraction or non-extraction immunoassays. Non-extraction assays generally have a lower sensitivity and specificity for hormone concentrations than extraction assays, and differences in assay performance may explain some of the differences observed in hormone concentrations (Table 1).
The cross-sectional nature of this analysis means that it was not possible to determine whether the associations found here are causal or due to reverse causation. Furthermore, younger age groups are under-represented. Data regarding other potential confounders such as physical activity and dietary factors were not collected in this centralized dataset, although only weak associations with hormone concentrations have been reported[82, 83], therefore any related confounding of the studied associations is likely to be small.
In summary, this analysis of individual participant data from 25 studies shows that circulating sex hormones in men are strongly associated with age and BMI, and to a lesser extent, with smoking status and alcohol consumption. These associations may enable a greater understanding of how risk factors are associated with the etiology of hormone-related diseases.
Supporting information
Abbreviations: ATBC = The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; BLSA = The Baltimore Longitudinal Study of Aging; CARET = The Carotene and Retinol Efficacy Trial; CDHS = Child Health and Development Studies; EPIC = European Prospective Investigation into Cancer and Nutrition; FMC = Finnish Mobile Clinic Health Examination Survey; HHS = Helsinki Heart Study; HPFS = Health Professionals Follow-Up Study; JACC = Japan Collaborative Cohort Study; JPHC = Japan Public Health Center-based Prospective Study; JHCS = Japan-Hawaii Cancer Study; KPMCP = Kaiser Permanente Medical Care Program; MCCS = Melbourne Collaborative Cohort Study; MEC = Multiethnic Cohort; MMAS = Massachusetts Male Aging Study; NSHDC = Northern Sweden Health and Disease Cohort; PCPT = Prostate Cancer Prevention Trial; PHS = Physicians' Health Study; PLCO Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; ProtecT = Prostate Testing for Cancer and Treatment; RBS = Ranch Bernardo Study.
(DOCX)
(PNG)
*Relative to overall geometric mean of testosterone, adjusted for study and BMI. Test of heterogeneity between morning and afternoon blood collection: F = 3.00; P = 0.0005. Abbreviations: CI = confidence interval.
(EMF)
*relative to 90.0–94.9 cm, adjusted for study and age. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
(EMF)
*relative to 0.90–0.93, adjusted for study and age. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
(EMF)
*relative to 90.0–94.9 cm, adjusted for study, age and BMI. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
(EMF)
*relative to 0.90–0.93, adjusted for study, age and BMI. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
(EMF)
*relative to < secondary/high school, adjusted for study, age and BMI. Abbreviations: A-diol-g = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = dihydrotestosterone; SHBG = sex hormone-binding globulin.
(EMF)
*relative to married/cohabiting, adjusted for study, age and BMI. # significant interaction with study P<0.01. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
(EMF)
*relative to no family history of prostate cancer, adjusted for study, age and BMI. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
(EMF)
Acknowledgments
We thank the men who participated in the collaborating studies, the research staff, the collaborating laboratories and the funding agencies in each of the studies. We also wish to thank The Massachusetts Male Aging Study for contributing data for this analysis. The point of view and conclusions expressed in this study are those of the authors and do not necessarily represent the official position or policies of the funding agencies named below. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the National Cancer Institute and the Centers for Disease Control and Prevention or their Contractors and Subcontractors, or any of the funders of this research is not intended nor should be inferred.
Data Availability
Data are available via request through the Richard Doll Centenary Archive held in the Nuffield Department of Population Health at Oxford University. As the dataset contains confidential and potentially identifiable information, data access and sharing are monitored by the Oversight Committee. The Oversight Committee ensure that data access requests are in line with the RDCA Data Access and Sharing Policy, see https://www.ndph.ox.ac.uk/about/richard-doll-centenary-archive for more information. For information on how to submit an application for gaining access to data held by the Nuffield Department of Population Health at Oxford University please follow the instructions at https://www.ndph.ox.ac.uk/about/richard-doll-centenary-archive.
Funding Statement
Centralized pooling, checking and data analysis was supported by Cancer Research UK grants C8221/A19170 and C8221/A20986 (https://www.cancerresearchuk.org/). Details of funding for the original studies are in the relevant publications (see Subjects and methods section for individual study details). These include: Eunice Kennedy Shriver National Institute of Child Health and Development, National Institutes of Health and Department of Health and Human Services, grant number: HHSN275201100020C; California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program awarded to the Cancer Prevention Institute of California, grant number: HHSN261201000140C; the National Cancer Institute's Surveillance, Epidemiology and End Results Program awarded to the University of Southern California, HHSN261201000035C; National Cancer Institute's Surveillance, Epidemiology and End Results Program awarded to the Public Health Institute; the Centers for Disease Control and Prevention's National Program of Cancer Registries, grant number: HHSN261201000034C; the California Department of Public Health, grant number: U58DP003862-01; the Cancer Research Fund, under Interagency Agreement #97-12013 (University of California contract #98-00924V) with the Department of Health Services, Cancer Research Program, UM1 CA182883; the National Cancer Institute, National Institutes of Health/National Cancer Institute (grant numbers CA167552, CA055075, CA133891, CA141298, CA09001, CA131945, CA34944, CA40360, CA097193), National Institutes of Health/National Heart, Lung and Blood Institute (grant numbers HL26490, HL34595) and the Hellenic Health Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Endogenous Hormones and Prostate Cancer Collaborative Group. Endogenous Sex Hormones and Prostate Cancer: A Collaborative Analysis of 18 Prospective Studies. Journal of the National Cancer Institute. 2008;100(3):170–83. doi: 10.1093/jnci/djm323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer cell. 2010;18(1):11–22. Epub 2010/06/29. doi: 10.1016/j.ccr.2010.05.026 ; PubMed Central PMCID: PMCPmc3198787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bu H, Narisu N, Schlick B, Rainer J, Manke T, Schafer G, et al. Putative Prostate Cancer Risk SNP in an Androgen Receptor-Binding Site of the Melanophilin Gene Illustrates Enrichment of Risk SNPs in Androgen Receptor Target Sites. Human mutation. 2016;37(1):52–64. Epub 2015/09/29. doi: 10.1002/humu.22909 ; PubMed Central PMCID: PMCPmc4715509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas G, Jacobs KB, Yeager M, Kraft P, Wacholder S, Orr N, et al. Multiple loci identified in a genome-wide association study of prostate cancer. Nat Genet. 2008;40(3):310–5. doi: http://www.nature.com/ng/journal/v40/n3/suppinfo/ng.91_S1.html. doi: 10.1038/ng.91 [DOI] [PubMed] [Google Scholar]
- 5.Maggi M, Schulman C, Quinton R, Langham S, Uhl-Hochgraeber K. The burden of testosterone deficiency syndrome in adult men: economic and quality-of-life impact. J Sex Med. 2007;4(4 Pt 1):1056–69. Epub 2007/07/14. doi: 10.1111/j.1743-6109.2007.00531.x . [DOI] [PubMed] [Google Scholar]
- 6.Selvin E, Feinleib M, Zhang L, Rohrmann S, Rifai N, Nelson WG, et al. Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III). Diabetes Care. 2007;30(2):234–8. Epub 2007/01/30. doi: 10.2337/dc06-1579 . [DOI] [PubMed] [Google Scholar]
- 7.Kaltenboeck A, Foster S, Ivanova J, Diener M, Bergman R, Birnbaum H, et al. The direct and indirect costs among U.S. privately insured employees with hypogonadism. J Sex Med. 2012;9(9):2438–47. Epub 2012/06/29. doi: 10.1111/j.1743-6109.2012.02810.x . [DOI] [PubMed] [Google Scholar]
- 8.Rohrmann S, Shiels MS, Lopez DS, Rifai N, Nelson WG, Kanarek N, et al. Body fatness and sex steroid hormone concentrations in US men: results from NHANES III. Cancer Causes & Control. 2011;22(8):1141–51. doi: 10.1007/s10552-011-9790-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjørnerem As, Straume B, Midtby M, Fønnebø V, Sundsfjord J, Svartberg J, et al. Endogenous Sex Hormones in Relation to Age, Sex, Lifestyle Factors, and Chronic Diseases in a General Population: The Tromsø Study. The Journal of Clinical Endocrinology & Metabolism. 2004;89(12):6039–47. doi: 10.1210/jc.2004-0735 [DOI] [PubMed] [Google Scholar]
- 10.Gapstur SM, Gann PH, Kopp P, Colangelo L, Longcope C, Liu K. Serum Androgen Concentrations in Young Men: A Longitudinal Analysis of Associations with Age, Obesity, and Race. The CARDIA Male Hormone Study. 2002;11(10):1041–7. [PubMed] [Google Scholar]
- 11.Morley JE, Kaiser FE, Perry HM, Patrick P, Morley PM, Stauber PM, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46(4):410–3. [DOI] [PubMed] [Google Scholar]
- 12.Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol. 2003;149(6):583–9. Epub 2003/12/04. . [DOI] [PubMed] [Google Scholar]
- 13.Travis RC, Key TJ, Allen NE, Appleby PN, Roddam AW, Rinaldi S, et al. Serum androgens and prostate cancer among 643 cases and 643 controls in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2007;121(6):1331–8. Epub 2007/05/22. doi: 10.1002/ijc.22814 . [DOI] [PubMed] [Google Scholar]
- 14.Heikkila R, Aho K, Heliovaara M, Hakama M, Marniemi J, Reunanen A, et al. Serum testosterone and sex hormone-binding globulin concentrations and the risk of prostate carcinoma: a longitudinal study. Cancer. 1999;86(2):312–5. Epub 1999/07/27. . [PubMed] [Google Scholar]
- 15.Platz EA, Leitzmann MF, Rifai N, Kantoff PW, Chen YC, Stampfer MJ, et al. Sex steroid hormones and the androgen receptor gene CAG repeat and subsequent risk of prostate cancer in the prostate-specific antigen era. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1262–9. Epub 2005/05/17. doi: 10.1158/1055-9965.EPI-04-0371 . [DOI] [PubMed] [Google Scholar]
- 16.Ozasa K, Nakao M, Watanabe Y, Hayashi K, Miki T, Mikami K, et al. Serum phytoestrogens and prostate cancer risk in a nested case-control study among Japanese men. Cancer science. 2004;95(1):65–71. Epub 2004/01/15. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vatten LJ, Ursin G, Ross RK, Stanczyk FZ, Lobo RA, Harvei S, et al. Androgens in serum and the risk of prostate cancer: a nested case-control study from the Janus serum bank in Norway. Cancer Epidemiol Biomarkers Prev. 1997;6(11):967–9. Epub 1997/11/21. . [PubMed] [Google Scholar]
- 18.Nomura A, Heilbrun LK, Stemmermann GN, Judd HL. Prediagnostic serum hormones and the risk of prostate cancer. Cancer Res. 1988;48(12):3515–7. Epub 1988/06/15. . [PubMed] [Google Scholar]
- 19.Nomura AM, Stemmermann GN, Chyou PH, Henderson BE, Stanczyk FZ. Serum androgens and prostate cancer. Cancer Epidemiol Biomarkers Prev. 1996;5(8):621–5. Epub 1996/08/01. . [PubMed] [Google Scholar]
- 20.Sawada N, Iwasaki M, Inoue M, Sasazuki S, Yamaji T, Shimazu T, et al. Plasma testosterone and sex hormone-binding globulin concentrations and the risk of prostate cancer among Japanese men: a nested case-control study. Cancer science. 2010;101(12):2652–7. Epub 2010/10/15. doi: 10.1111/j.1349-7006.2010.01721.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Severi G, Morris HA, MacInnis RJ, English DR, Tilley W, Hopper JL, et al. Circulating steroid hormones and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(1):86–91. Epub 2006/01/26. doi: 10.1158/1055-9965.EPI-05-0633 . [DOI] [PubMed] [Google Scholar]
- 22.Gill JK, Wilkens LR, Pollak MN, Stanczyk FZ, Kolonel LN. Androgens, growth factors and risk of prostate cancer: the Multiethnic Cohort. The Prostate. 2010;70(8):906–15. doi: 10.1002/pros.21125 PMC2860643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohr BA, Feldman HA, Kalish LA, Longcope C, McKinlay JB. Are serum hormones associated with the risk of prostate cancer? Prospective results from the Massachusetts Male Aging Study. Urology. 2001;57(5):930–5. doi: http://dx.doi.org/10.1016/S0090-4295(00)01116-X [DOI] [PubMed] [Google Scholar]
- 24.Wirén S, Stocks T, Rinaldi S, Hallmans G, Bergh A, Stenman U-H, et al. Androgens and prostate cancer risk: A prospective study. The Prostate. 2007;67(11):1230–7. doi: 10.1002/pros.20588 [DOI] [PubMed] [Google Scholar]
- 25.Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88(16):1118–26. Epub 1996/08/21. . [DOI] [PubMed] [Google Scholar]
- 26.Barrett-Connor E, Garland C, McPhillips JB, Khaw K-T, Wingard DL. A Prospective, Population-based Study of Androstenedione, Estrogens, and Prostatic Cancer. Cancer Research. 1990;50(1):169–73. [PubMed] [Google Scholar]
- 27.Pierorazio PM, Ferrucci L, Kettermann A, Longo DL, Metter EJ, Carter HB. Serum testosterone is associated with aggressive prostate cancer in older men: results from the Baltimore Longitudinal Study of Aging. BJU international. 2010;105(6):824–9. doi: 10.1111/j.1464-410X.2009.08853.x PMC2848292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guess HA, Friedman GD, Sadler MC, Stanczyk FZ, Vogelman JH, Imperato-McGinley J, et al. 5 alpha-reductase activity and prostate cancer: a case-control study using stored sera. Cancer Epidemiol Biomarkers Prev. 1997;6(1):21–4. Epub 1997/01/01. . [PubMed] [Google Scholar]
- 29.Stattin P, Lumme S, Tenkanen L, Alfthan H, Jellum E, Hallmans G, et al. High levels of circulating testosterone are not associated with increased prostate cancer risk: a pooled prospective study. Int J Cancer. 2004;108(3):418–24. Epub 2003/12/04. doi: 10.1002/ijc.11572 . [DOI] [PubMed] [Google Scholar]
- 30.Tsai CJ, Cohn BA, Cirillo PM, Feldman D, Stanczyk FZ, Whittemore AS. Sex steroid hormones in young manhood and the risk of subsequent prostate cancer: a longitudinal study in African-Americans and Caucasians (United States). Cancer Causes & Control. 2006;17(10):1237–44. doi: 10.1007/s10552-006-0052-4 [DOI] [PubMed] [Google Scholar]
- 31.Dorgan JF, Albanes D, Virtamo J, Heinonen OP, Chandler DW, Galmarini M, et al. Relationships of serum androgens and estrogens to prostate cancer risk: results from a prospective study in Finland. Cancer Epidemiol Biomarkers Prev. 1998;7(12):1069–74. Epub 1998/12/29. . [PubMed] [Google Scholar]
- 32.Chen C, Weiss NS, Stanczyk FZ, Lewis SK, DiTommaso D, Etzioni R, et al. Endogenous sex hormones and prostate cancer risk: a case-control study nested within the Carotene and Retinol Efficacy Trial. Cancer Epidemiol Biomarkers Prev. 2003;12(12):1410–6. Epub 2003/12/25. . [PubMed] [Google Scholar]
- 33.Schenk JM, Till C, Hsing AW, Stanczyk FZ, Gong Z, Neuhouser ML, et al. Serum androgens and prostate cancer risk: results from the placebo arm of the Prostate Cancer Prevention Trial. Cancer causes & control: CCC. 2016;27(2):175–82. Epub 2015/11/22. doi: 10.1007/s10552-015-0695-0 ; PubMed Central PMCID: PMCPmc4724283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss JM, Huang WY, Rinaldi S, Fears TR, Chatterjee N, Hsing AW, et al. Endogenous sex hormones and the risk of prostate cancer: a prospective study. Int J Cancer. 2008;122(10):2345–50. Epub 2008/01/04. doi: 10.1002/ijc.23326 . [DOI] [PubMed] [Google Scholar]
- 35.Oliver SE, Gunnell D, Donovan J, Peters TJ, Persad R, Gillatt D, et al. Screen-detected prostate cancer and the insulin-like growth factor axis: results of a population-based case-control study. Int J Cancer. 2004;108(6):887–92. Epub 2004/01/09. doi: 10.1002/ijc.11631 . [DOI] [PubMed] [Google Scholar]
- 36.Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, et al. Helsinki Heart Study: Primary-Prevention Trial with Gemfibrozil in Middle-Aged Men with Dyslipidemia. New England Journal of Medicine. 1987;317(20):1237–45. doi: 10.1056/NEJM198711123172001 . [DOI] [PubMed] [Google Scholar]
- 37.Bartsch W. Interrelationships between sex hormone-binding globulin and testosterone, 5 alpha-dihydrotestosterone and oestradiol-17 beta in blood of normal men. Maturitas. 1980;2(2):109–18. Epub 1980/07/01. . [DOI] [PubMed] [Google Scholar]
- 38.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801–10. Epub 1982/06/01. . [DOI] [PubMed] [Google Scholar]
- 39.Dunn JF, Nisula BC, Rodbard D. Transport of Steroid Hormones: Binding of 21 Endogenous Steroids to Both Testosterone-Binding Globulin and Corticosteroid-Binding Globulin in Human Plasma. The Journal of Clinical Endocrinology & Metabolism. 1981;53(1):58–68. doi: 10.1210/jcem-53-1-58 [DOI] [PubMed] [Google Scholar]
- 40.Lewis J, Ghanadian R, Chisholm G. Serum 5α-dihydrotestosterone and testosterone changes with age in man. Acta endocrinologica. 1976;82(2):444–8. [DOI] [PubMed] [Google Scholar]
- 41.Pirke KM, Doerr P. Age related changes and interrelationships between plasma testosterone, oestradiol and testosterone-binding globulin in normal adult males. Acta endocrinologica. 1973;74(4):792–800. [DOI] [PubMed] [Google Scholar]
- 42.Morley JE, Kaiser FE, Perry HM Iii, Patrick P, Morley PMK, Stauber PM, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46(4):410–3. doi: https://doi.org/10.1016/S0026-0495(97)90057-3 [DOI] [PubMed] [Google Scholar]
- 43.Travison TG, Araujo AB, O'Donnell AB, Kupelian V, McKinlay JB. A population-level decline in serum testosterone levels in American men. J Clin Endocrinol Metab. 2007;92(1):196–202. doi: 10.1210/jc.2006-1375 [DOI] [PubMed] [Google Scholar]
- 44.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87(2):589–98. doi: 10.1210/jcem.87.2.8201 [DOI] [PubMed] [Google Scholar]
- 45.Shi Z, Araujo AB, Martin S, O'Loughlin P, Wittert GA. Longitudinal changes in testosterone over five years in community-dwelling men. J Clin Endocrinol Metab. 2013;98(8):3289–97. Epub 2013/06/19. doi: 10.1210/jc.2012-3842 . [DOI] [PubMed] [Google Scholar]
- 46.Camacho EM, Huhtaniemi IT, O'Neill TW, Finn JD, Pye SR, Lee DM, et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168(3):445–55. Epub 2013/02/22. doi: 10.1530/EJE-12-0890 . [DOI] [PubMed] [Google Scholar]
- 47.Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neill TW, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737–45. doi: 10.1210/jc.2007-1972 [DOI] [PubMed] [Google Scholar]
- 48.Kelsey TW, Li LQ, Mitchell RT, Whelan A, Anderson RA, Wallace WHB. A Validated Age-Related Normative Model for Male Total Testosterone Shows Increasing Variance but No Decline after Age 40 Years. PLoS ONE. 2014;9(10):e109346 doi: 10.1371/journal.pone.0109346 PMC4190174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frost M, Wraae K, Nielsen TL, Hougaard DM, Brixen K, Hagen C, et al. Similar reference intervals for total testosterone in healthy young and elderly men: results from the Odense Androgen Study. Clin Endocrinol (Oxf). 2013;78(5):743–51. Epub 2012/10/31. doi: 10.1111/cen.12042 . [DOI] [PubMed] [Google Scholar]
- 50.Marrama P, Carani C, Baraghini GF, Volpe A, Zini D, Celani MF, et al. Circadian rhythm of testosterone and prolactin in the ageing. Maturitas. 1982;4(2):131–8. Epub 1982/08/01. . [DOI] [PubMed] [Google Scholar]
- 51.Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56(6):1278–81. Epub 1983/06/01. doi: 10.1210/jcem-56-6-1278 . [DOI] [PubMed] [Google Scholar]
- 52.Lecomte P, Lecureuil N, Lecureuil M, Lemonnier Y, Mariotte N, Valat C, et al. Sex differences in the control of sex-hormone-binding globulin in the elderly: role of insulin-like growth factor-I and insulin. Eur J Endocrinol. 1998;139(2):178–83. Epub 1998/09/02. . [DOI] [PubMed] [Google Scholar]
- 53.Parker L, Gral T, Perrigo V, Skowksy R. Decreased adrenal androgen sensitivity to ACTH during aging. Metabolism. 1981;30(6):601–4. Epub 1981/06/01. . [DOI] [PubMed] [Google Scholar]
- 54.Orentreich N, Brind JL, Vogelman JH, Andres R, Baldwin H. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J Clin Endocrinol Metab. 1992;75(4):1002–4. Epub 1992/10/01. doi: 10.1210/jcem.75.4.1400863 . [DOI] [PubMed] [Google Scholar]
- 55.Belanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez JL, et al. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab. 1994;79(4):1086–90. Epub 1994/10/01. doi: 10.1210/jcem.79.4.7962278 . [DOI] [PubMed] [Google Scholar]
- 56.Morimoto I, Edmiston A, Hawks D, Horton R. Studies on the origin of androstanediol and androstanediol glucuronide in young and elderly men. J Clin Endocrinol Metab. 1981;52(4):772–8. Epub 1981/04/01. doi: 10.1210/jcem-52-4-772 . [DOI] [PubMed] [Google Scholar]
- 57.Hautanen A. Synthesis and regulation of sex hormone-binding globulin in obesity. Int J Obes Relat Metab Disord. 2000;24 Suppl 2:S64–70. Epub 2000/09/21. . [DOI] [PubMed] [Google Scholar]
- 58.Simo R, Saez-Lopez C, Barbosa-Desongles A, Hernandez C, Selva DM. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol Metab. 2015;26(7):376–83. Epub 2015/06/06. doi: 10.1016/j.tem.2015.05.001 . [DOI] [PubMed] [Google Scholar]
- 59.Wang Q, Kangas AJ, Soininen P, Tiainen M, Tynkkynen T, Puukka K, et al. Sex hormone-binding globulin associations with circulating lipids and metabolites and the risk for type 2 diabetes: observational and causal effect estimates. International journal of epidemiology. 2015;44(2):623–37. Epub 2015/06/08. doi: 10.1093/ije/dyv093 . [DOI] [PubMed] [Google Scholar]
- 60.Cohen PG. Aromatase, adiposity, aging and disease. The hypogonadal-metabolic-atherogenic-disease and aging connection. Med Hypotheses. 2001;56(6):702–8. doi: 10.1054/mehy.2000.1169 [DOI] [PubMed] [Google Scholar]
- 61.Pasquali R, Vicennati V. Activity of the hypothalamic-pituitary-adrenal axis in different obesity phenotypes. Int J Obes Relat Metab Disord. 2000;24 Suppl 2:S47–9. Epub 2000/09/21. . [PubMed] [Google Scholar]
- 62.Peter A, Kantartzis K, Machann J, Schick F, Staiger H, Machicao F, et al. Relationships of circulating sex hormone-binding globulin with metabolic traits in humans. Diabetes. 2010;59(12):3167–73. doi: 10.2337/db10-0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rochira V, Kara E, Carani C. The Endocrine Role of Estrogens on Human Male Skeleton. International Journal of Endocrinology. 2015;2015:15 doi: 10.1155/2015/165215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perry RJ, Farquharson C, Ahmed SF. The role of sex steroids in controlling pubertal growth. Clin Endocrinol (Oxf). 2008;68(1):4–15. Epub 2007/07/25. doi: 10.1111/j.1365-2265.2007.02960.x . [DOI] [PubMed] [Google Scholar]
- 65.Manolagas SC, O’Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nature reviews Endocrinology. 2013;9(12):699–712. doi: 10.1038/nrendo.2013.179 PMC3971652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dechanet C, Anahory T, Mathieu Daude JC, Quantin X, Reyftmann L, Hamamah S, et al. Effects of cigarette smoking on reproduction. Human Reproduction Update. 2011;17(1):76–95. doi: 10.1093/humupd/dmq033 [DOI] [PubMed] [Google Scholar]
- 67.Mlynarcikova A, Fickova M, Scsukova S. Ovarian intrafollicular processes as a target for cigarette smoke components and selected environmental reproductive disruptors. Endocr Regul. 2005;39(1):21–32. [PubMed] [Google Scholar]
- 68.De Ronde W, Van Der Schouw YT, Pierik FH, Pols HAP, Muller M, Grobbee DE, et al. Serum levels of sex hormone-binding globulin (SHBG) are not associated with lower levels of non-SHBG-bound testosterone in male newborns and healthy adult men. Clinical Endocrinology. 2005;62(4):498–503. doi: 10.1111/j.1365-2265.2005.02252.x [DOI] [PubMed] [Google Scholar]
- 69.English KM, Pugh PJ, Parry H, Scutt NE, Channer KS, Jones TH. Effect of cigarette smoking on levels of bioavailable testosterone in healthy men. Clinical science (London, England: 1979). 2001;100(6):661–5. Epub 2001/05/16. . [PubMed] [Google Scholar]
- 70.Svartberg J, Jorde R. Endogenous testosterone levels and smoking in men. The fifth Tromsø study. International Journal of Andrology. 2007;30(3):137–43. doi: 10.1111/j.1365-2605.2006.00720.x [DOI] [PubMed] [Google Scholar]
- 71.Kiechl S, Willeit J, Bonora E, Schwarz S, Xu Q. No association between dehydroepiandrosterone sulfate and development of atherosclerosis in a prospective population study (Bruneck Study). Arteriosclerosis, thrombosis, and vascular biology. 2000;20(4):1094–100. Epub 2000/04/15. . [DOI] [PubMed] [Google Scholar]
- 72.Ravaglia G, Forti P, Maioli F, Sacchetti L, Nativio V, Scali CR, et al. Dehydroepiandrosterone-sulfate serum levels and common age-related diseases: results from a cross-sectional Italian study of a general elderly population. Experimental gerontology. 2002;37(5):701–12. Epub 2002/03/23. . [DOI] [PubMed] [Google Scholar]
- 73.Frias J, Torres JM, Miranda MT, Ruiz E, Ortega E. Effects of acute alcohol intoxication on pituitary-gonadal axis hormones, pituitary-adrenal axis hormones, β-endorphin and prolactin in human adults of both sexes Alcohol and Alcoholism. 2002;37(2):169–73. doi: 10.1093/alcalc/37.2.169 [DOI] [PubMed] [Google Scholar]
- 74.Ukkola O, Gagnon J, Rankinen T, Thompson PA, Hong Y, Leon AS, et al. Age, body mass index, race and other determinants of steroid hormone variability: the HERITAGE Family Study. Eur J Endocrinol. 2001;145(1):1–9. Epub 2001/06/21. . [DOI] [PubMed] [Google Scholar]
- 75.Wu AH, Whittemore AS, Kolonel LN, John EM, Gallagher RP, West DW, et al. Serum androgens and sex hormone-binding globulins in relation to lifestyle factors in older African-American, white, and Asian men in the United States and Canada. Cancer Epidemiol Biomarkers Prev. 1995;4(7):735–41. Epub 1995/10/01. . [PubMed] [Google Scholar]
- 76.Sparrow D, Bosse R, Rowe JW. The influence of age, alcohol consumption, and body build on gonadal function in men. J Clin Endocrinol Metab. 1980;51(3):508–12. doi: 10.1210/jcem-51-3-508 [DOI] [PubMed] [Google Scholar]
- 77.Ross RK, Bernstein L, Pike MC, Henderson BE, Lobo RA, Stanczyk FZ, et al. 5-alpha-reductase activity and risk of prostate cancer among Japanese and US white and black males. The Lancet. 1992;339(8798):887–9. doi: https://doi.org/10.1016/0140-6736(92)90927-U [DOI] [PubMed] [Google Scholar]
- 78.Lookingbill DP, Demers LM, Wang C, Leung A, Rittmaster RS, Santen RJ. Clinical and biochemical parameters of androgen action in normal healthy Caucasian versus Chinese subjects. J Clin Endocrinol Metab. 1991;72(6):1242–8. Epub 1991/06/01. doi: 10.1210/jcem-72-6-1242 . [DOI] [PubMed] [Google Scholar]
- 79.Wu AH, Whittemore AS, Kolonel LN, Stanczyk FZ, John EM, Gallagher RP, et al. Lifestyle determinants of 5alpha-reductase metabolites in older African-American, white, and Asian-American men. Cancer Epidemiol Biomarkers Prev. 2001;10(5):533–8. Epub 2001/05/16. . [PubMed] [Google Scholar]
- 80.Rohrmann S, Nelson WG, Rifai N, Brown TR, Dobs A, Kanarek N, et al. Serum Estrogen, But Not Testosterone, Levels Differ between Black and White Men in a Nationally Representative Sample of Americans. The Journal of Clinical Endocrinology & Metabolism. 2007;92(7):2519–25. doi: 10.1210/jc.2007-0028 [DOI] [PubMed] [Google Scholar]
- 81.Handelsman DJ, Wartofsky L. Requirement for mass spectrometry sex steroid assays in the Journal of Clinical Endocrinology and Metabolism. J Clin Endocrinol Metab. 2013;98(10):3971–3. Epub 2013/10/08. doi: 10.1210/jc.2013-3375 . [DOI] [PubMed] [Google Scholar]
- 82.Hawkins VN, Foster-Schubert K, Chubak J, Sorensen B, Ulrich CM, Stancyzk FZ, et al. Effect of Exercise on Serum Sex Hormones in Men: A 12-Month Randomized Clinical Trial. Medicine and science in sports and exercise. 2008;40(2):223–33. doi: 10.1249/mss.0b013e31815bbba9 PMC3040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Allen NE, Appleby PN, Davey GK, Key TJ. Lifestyle and nutritional determinants of bioavailable androgens and related hormones in British men. Cancer causes & control: CCC. 2002;13(4):353–63. Epub 2002/06/21. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Abbreviations: ATBC = The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; BLSA = The Baltimore Longitudinal Study of Aging; CARET = The Carotene and Retinol Efficacy Trial; CDHS = Child Health and Development Studies; EPIC = European Prospective Investigation into Cancer and Nutrition; FMC = Finnish Mobile Clinic Health Examination Survey; HHS = Helsinki Heart Study; HPFS = Health Professionals Follow-Up Study; JACC = Japan Collaborative Cohort Study; JPHC = Japan Public Health Center-based Prospective Study; JHCS = Japan-Hawaii Cancer Study; KPMCP = Kaiser Permanente Medical Care Program; MCCS = Melbourne Collaborative Cohort Study; MEC = Multiethnic Cohort; MMAS = Massachusetts Male Aging Study; NSHDC = Northern Sweden Health and Disease Cohort; PCPT = Prostate Cancer Prevention Trial; PHS = Physicians' Health Study; PLCO Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; ProtecT = Prostate Testing for Cancer and Treatment; RBS = Ranch Bernardo Study.
(DOCX)
(PNG)
*Relative to overall geometric mean of testosterone, adjusted for study and BMI. Test of heterogeneity between morning and afternoon blood collection: F = 3.00; P = 0.0005. Abbreviations: CI = confidence interval.
(EMF)
*relative to 90.0–94.9 cm, adjusted for study and age. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
(EMF)
*relative to 0.90–0.93, adjusted for study and age. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
(EMF)
*relative to 90.0–94.9 cm, adjusted for study, age and BMI. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
(EMF)
*relative to 0.90–0.93, adjusted for study, age and BMI. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
(EMF)
*relative to < secondary/high school, adjusted for study, age and BMI. Abbreviations: A-diol-g = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = dihydrotestosterone; SHBG = sex hormone-binding globulin.
(EMF)
*relative to married/cohabiting, adjusted for study, age and BMI. # significant interaction with study P<0.01. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
(EMF)
*relative to no family history of prostate cancer, adjusted for study, age and BMI. Abbreviations: A-diol-G = Androstanediol glucuronide; CI = confidence interval; DHEAS = Dehydroepiandrosterone sulfate; DHT = Dihydrotestosterone; SHBG = sex hormone-binding globulin.
(EMF)
Data Availability Statement
Data are available via request through the Richard Doll Centenary Archive held in the Nuffield Department of Population Health at Oxford University. As the dataset contains confidential and potentially identifiable information, data access and sharing are monitored by the Oversight Committee. The Oversight Committee ensure that data access requests are in line with the RDCA Data Access and Sharing Policy, see https://www.ndph.ox.ac.uk/about/richard-doll-centenary-archive for more information. For information on how to submit an application for gaining access to data held by the Nuffield Department of Population Health at Oxford University please follow the instructions at https://www.ndph.ox.ac.uk/about/richard-doll-centenary-archive.