Abstract
Introduction
Deployment of rotavirus vaccines has contributed to significant declines in diarrheal morbidity and mortality globally. Unfortunately, vaccine performance in low-middle income countries (LMICs) is generally lower than in developed countries. The cause for this has been associated with several host and maternal factors including poor water sanitation and hygiene (WASH) status, which are predominant in LMICs. More recently, environmental enteric dysfunction (EED) has specifically been hypothesized to contribute to poor vaccine uptake and response. The aim of this study was to examine the association between serological biomarkers of EED and seroconversion to rotavirus vaccine in Zambian infants.
Methods
This was a retrospective cohort study of 142 infants who had been fully immunized with Rotarix™, and had known seroconversion status. Seroconversion was defined as 4-fold or more increase in rotavirus-specific IgA titres between pre-vaccination and one month post-dose two vaccination. We performed ELISA assays to assess soluble CD14 (sCD14), Endotoxin Core IgG Antibodies (EndoCAb), intestinal fatty acid binding protein (i-FABP) and Zonulin according to the manufacturers protocols. Generalised linear model with family-poisson, link-log and robust standard error was used to estimate the independent effects of biomarkers on seroconversion adjusting for important cofounders.
Results
The median concentration of Zonulin, Soluble CD14, EndoCaB, and IFABP were 209.3 (IQR = 39.7, 395.1), 21.5 (IQR = 21.5, 21.5), 0.3 (IQR = 0.3, 0.3), and 107.7 (IQR = 6.4, 1141.4) respectively. In multivariable analyses adjusting for the independent effect of other biomarkers and confounders (i.e. age of child at vaccination, breast-milk anti-rotavirus IgA, infant serum anti-rotavirus IgG, and IgA seropositivity at baseline), there was strong evidence of about 24% increase in seroconversion due to doubling Zonulin concentration (Adjusted risk ratio (aRR) = 1.24; 95% CI = 1.12 to1.37; p<0.0001). Similarly, we found about 7% increase in seroconversion due to doubling IFABP concentration (aRR = 1.07; 95% CI = 1.02 to 1.13; p = 0.006).
Conclusion
We found that high levels of zonulin and IFABP played a role in seroconversion. It is plausible that increased gut permeability in EED allows greater uptake of the live virus within the vaccine, but later consequences result in deleterious local structural distortions and malabsorption syndromes.
Introduction
Diarrhea is the second largest killer of children in the world and rotavirus is the most common cause of severe diarrhea among children <5 years of age globally [1]. Rotavirus caused an estimated 233,000 deaths of children in 2013 alone, with the majority of these deaths occurring in low and middle-income countries (LMICs) [2]. Zambia records over 3,600 rotavirus-related deaths per year among children under 5 years [3].
Many LMICs are adding oral rotavirus vaccines (RVs) to their national immunization schedules to reduce the burden of rotavirus diarrhea [4]; however, RVs are proving to have lower immunogenicity, efficacy, effectiveness, and duration of protection in LMIC children [5]. For example, while in US children, RV effectiveness against hospitalization for rotavirus diarrhea was 87% (95% CI, 71%, 94%), in sub-Saharan Africa, clinical trials of the pentavalent (RV5) and monovalent (RV1) RVs showed efficacies were 39% (95% CI, 19%, 55%) and 61% (95% CI, 44%, 73%), respectively [6–13].
Several factors such as micronutrient deficiency, co-administration with oral poliovirus vaccines and maternal breast milk factors have been suggested as reasons for low immunogenicity of oral vaccines in developing countries [14, 15]. However, there is little known on the effect of intestinal mucosal integrity on seroconversion following administration of rotavirus vaccine [16].
Environmental enteric dysfunction (EED) is a syndrome of mucosal and sub-mucosal inflammation, reduced intestinal absorptive capacity and reduced barrier function, which is widespread in both adults and children residing in low and middle-income countries (LMICs) [17, 18]. Chronic inflammation due to EED has been associated with non-specific responses to oral vaccine antigens and result in clearance of the vaccine before sufficient induction of adaptive immunity [19–21]. Studies that have investigated EED have demonstrated intestinal architectural alterations such as crypt hyperplasia, blunting of the villi, and lymphocytic infiltration of the lamina propria [20, 22, 23]. Individuals with EED can often be asymptomatic of gastrointestinal diseases but may demonstrate underlying malabsorption and low grade inflammation that may potentially result in failure of oral vaccines [20, 24, 25].
Several biomarkers have been shown to be associated with, mucosal dysfunction of the small intestine in children under 5 years of age from developing countries [26]. Zonulin physiologically modulates tight junctions of enterocytes of the digestive tract [27]. This protein binds to a specific receptor on the surface of intestinal epithelia cells that induces tight junction disassembly and a subsequent increase in permeability of the intestinal epithelia [28]. Intestinal fatty acid binding protein (I-FABP) is a glycoprotein specifically secreted in circulation due to enterocyte damage [29]. Soluble CD14 (sCD14) is the glycoprotein expressed mainly on the surfaces of monocytes or macrophages which acts as a co-receptor along with Toll like receptor type 4 to which later binds lipopolysaccharides [30, 31]. Fourth, is Endotoxin Core IgG Antibodies (EndoCab); enhanced levels of this marker in serum may reflect a systemic inflammatory response [32].
We hypothesised that the levels of these intestinal inflammation biomarkers, measuring EED, may have an influence on seroconversion in infants receiving rotavirus vaccines. The objective of the study was to examine the association of Zonulin, I-FABP, sCD14 and EndoCab serum levels prior to vaccination with seroconversion in infants receiving rotavirus vaccine (Rotarix™ -GSK Biologicals, Belgium) in Lusaka, Zambia.
Methods
Study site and participants
In this study, serum samples collected from infants receiving rotavirus vaccination in a previously described study were used [5]. Briefly, the study was conducted at Kamwala clinic, a peri-urban health facility in Lusaka under which a prospective cohort of 420 infants aged between 6 to 12 weeks and receiving Rotarix™ vaccine was enrolled between April 2013 and March 2014. Blood was drawn from infants at baseline, before receiving the first dose of Rotarix™ and at one month post vaccine dose two at which seroconversion status was determined based on a four-fold or greater increase in rotavirus-specific IgA from baseline. Ethical approval was obtained from the University of Zambia Biomedical Research Ethics Committee as well as the Institutional Review Board of the University of North Carolina, at Chapel Hill USA. The study is registered at Clinical Trials.gov with NCT# 01886833.
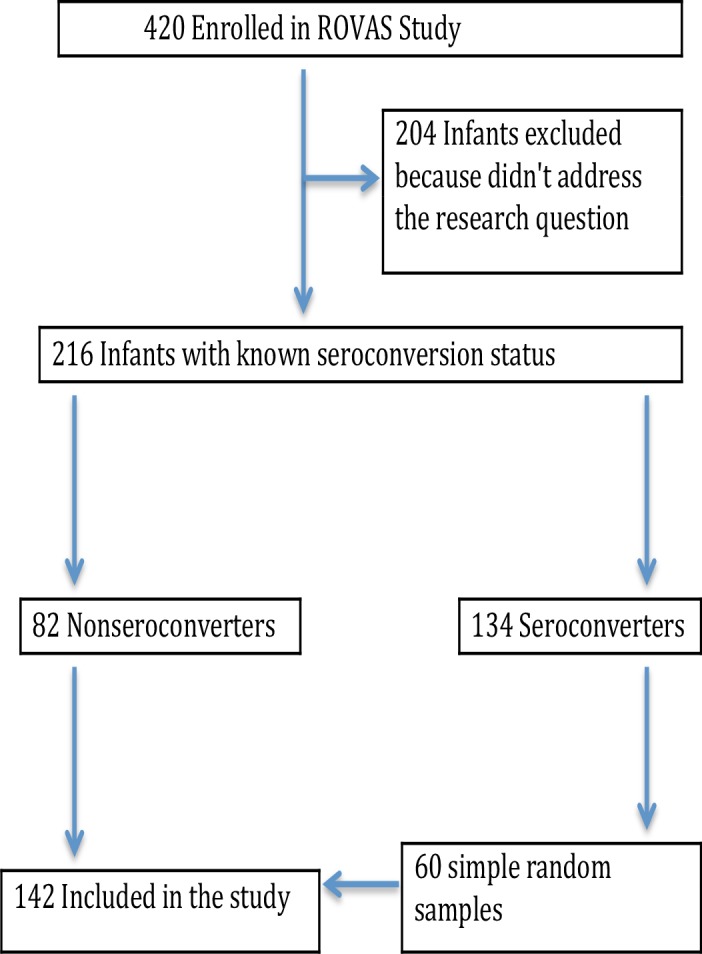
Baseline sera from infants with known seroconversion status were included and tested for presence of EED biomarkers. Of 420 enrolled infants, 216 had known seroconversion status: 82 classified as non-seroconverters, and 134 as seroconverters. Screening for EED biomarkers was done for all of the non-seroconverters and for 60 randomly selected seroconverters as illustrated in Fig 1.
Fig 1. Infant recruitment and sample selection flow chart.
Post-hoc power calculation
With a seroconversion rate estimated at 60% in the general population [5], the study sample of 142 infants had 85% power to detect a reduction in seroconversion to 35% using a 2-sided Pearson Chi-Squared Test at 5% level of significance.
Laboratory procedures
Measurement of serum IgA and IgG
Rotavirus-specific serum IgA and IgG were determined by an antibody capture ELISA assay as previously described [5]. Plates were coated with rabbit hyperimmune serum to rhesus rotavirus (RRV) and incubated with diluted RV1 strain or blotto (5% skim milk in phosphate-buffered saline [PBS]). Following incubaton, plates were washed and serially diluted serum samples in diluent buffer (1% skim milk and 0.5% [v/v] of 10% polyoxyethylene ether W1 in PBS) were added to the wells together with biotin-conjugated goat antihuman IgA antibodies. This was followed by the second incubation stage and washing afterwards. Extravidin was then added to all wells and incubated. After incubation, reactions were developed with 3,3′,5,5′-tetramethylbenzidine) and stopped with 1N hydrogen chloride. Optical density (OD) were read at 450 nm with an enzyme immunoassay reader. Calculation of IgA titres in serum were as the reciprocal of the highest dilution that gave a mean OD greater than the cut-off value (3 standard deviations above the mean OD of the negative control serum wells). RV-specific IgG in serum samples was tested and analysed in a similar way as IgA the only difference was that 0.5% normal rabbit serum was added to the biotin-conjugated goat antihuman IgG antibody solution.
Measurement of serological biomarkers of EED
Commercial enzyme linked immunosorbent assays (ELISA) kits were used to measure levels of sCD14, EndoCAb, I-FABP (Hycult Biotech, Uden, Netherlands) and Zonulin (Immundiagnostik AG, Bensheim Germany) according to the manufacturer’s instructions with modifications on sample dilutions. Undiluted plasma samples were used for measurement of sCD14 whereas samples were diluted 1:20 for Zonulin, 1:50 for Endocab and 1:10 for IFABP. Optical density was measured at 450nm (ELX808 BioTek) and concentrations were determined using assay standard curve.
Statistical analysis
The primary outcome of interest was seroconversion defined as 4-fold or more increase in rotavirus-specific IgA titres between pre-vaccination and one month post-dose -2 vaccination. Rotavirus-specific IgA titres below limit of detection were imputed with 1 [5] before assessing the fold-increase. The exposures of interest were serological EED biomarkers of intestinal damage (IFABP), permeability (Zonulin) and microbial products of translocation (soluble CD14 and Endocab). We summarised the concentration of EED biomarkers using median and interquartile range. We used Fisher’s exact test to examine association of seroconversion with key infant and maternal factors. We used test for trend to assess dose-response relationship between the serological biomarkers of EED and seroconversion. The test for trend was performed by modelling the median concentration in each quartile of the biomarker concentration on seroconversion using a logit model.
Since this was a cohort study design we elected to estimate risk ratio using poisson with robust standard error. Therefore, Generalised linear model with family-poisson, link-log and robust standard error was used to estimate the independent effects of biomarkers on seroconversion adjusting for important cofounders. The biomarkers were modelled on log base 2 scale so that the effect would be doubling of the level of the biomarker. Values of biomarker concentrations below the limit of detection were imputed with half the lowest concentration for that biomarker before the log-transformation. The analysis was performed using Stata 15 (Statcorp, College Station, Texas, USA).
Results
A total of 142 infant plasma samples were assessed for serological EED biomarkers. The median concentration of Zonulin, Soluble CD14, EndoCaB, and IFABP were 209.3 (IQR = 39.7, 395.1), 21.5 (IQR = 21.5, 21.5), 0.3 (IQR = 0.3, 0.3), and 107.7 (IQR = 6.4, 1141.4) respectively (Table 1).
Table 1. Seroconversion at post dose 2 by environmental enteric dysfunction status and key infant and maternal factors.
| Biomarkers and Characteristics | Number of Infants (% of total) | No. (%) seroconverted | 95% CI | Fisher's exact P-value |
|---|---|---|---|---|
| Zonulin titre—Quartiles (median titre) | ||||
| Median (IQR) | 209.3 (39.7, 395.1) | <0.0001* | ||
| 1 (3.8) | 36 (25) | 6 (17) | (8, 33) | |
| 2 (120.1) | 35 (25) | 16 (46) | (30, 62) | |
| 3 (272.9) | 35 (25) | 21 (60) | (43, 75) | |
| 4 (507.3) | 35 (25) | 23 (66) | (48, 80) | |
| Soluble CD14 titre—Quartiles (median titre) | ||||
| Median (IQR) | 21.5 (21.5, 21.5) | 0.189 * | ||
| 1 (21.5) | 130 (92) | 63 (48) | (40, 57) | |
| 2 (NIL) 2 | NIL | NIL | ||
| 3 (NIL) 2 | NIL | NIL | ||
| 4 (243) | 11 (8) | 3 (27) | (8, 60) | |
| EndoCaB titre—Quartiles (median titre) | ||||
| Median (IQR) | 0.3 (0.3, 0.3) | 0.073 * | ||
| 1 (0.3) | 125 (89) | 62 (50) | (41, 58) | |
| 2 (NIL) 2 | NIL | NIL | ||
| 3 (NIL) 2 | NIL | NIL | ||
| 4 (13.3) | 16 (11) | 4 (25) | (9, 52) | |
| IFABP titre—Quartiles (median titre) | ||||
| Median (IQR) | 107.7 (6.4, 1141.4) | 0.001 * | ||
| 1 (6.4) | 67 (47) | 17 (25) | (16, 37) | |
| 2 (15.6) | 4 (3) | 3 (75) | (18, 98) | |
| 3 (534.3) | 35 (25) | 22 (63) | (46, 77) | |
| 4 (2555.7) | 35 (25) | 24 (69) | (51, 82) | |
| Age of child at vaccination (Weeks) | ||||
| Median (IQR) | 6 (6, 7) | 0.034 | ||
| <7 | 91 (65) | 49 (54) | (43, 64) | |
| 7+ | 50 (35) | 17 (34) | (22, 48) | |
| Sex of child | ||||
| Female | 66 (47) | 28 (42) | (31, 55) | 0.398 |
| Male | 75 (53) | 38 (51) | (39, 62) | |
| Infant serum anti-rotavirus IgG titre—Quartiles (median titre) | ||||
| Median (IQR) | 5120 (2560, 10240) | 0.322 | ||
| 1 (2560) | 49 (35) | 27 (55) | (41, 69) | |
| 2 (5120) | 32 (23) | 15 (47) | (30, 64) | |
| 3&4 (10240) 1 | 59 (42) | 24 (41) | (29, 54) | |
| Seropositivity at baseline (IgA > = 1:40) | ||||
| No | 107 (76) | 54 (50) | (41, 60) | 0.167 |
| Yes | 34 (24) | 12 (35) | (21, 53) | |
| Age of mother (Years) | ||||
| Median (IQR) | 24 (22, 29) | 0.590 | ||
| 16–19 | 18 (13) | 7 (39) | (19, 63) | |
| 20–24 | 56 (40) | 28 (50) | (37, 63) | |
| 25–29 | 33 (23) | 13 (39) | (24, 57) | |
| 30–39 | 34 (24) | 18 (53) | (36, 69) | |
| Maternal HIV Status | ||||
| Negative | 88 (62) | 42 (48) | (37, 58) | 0.862 |
| Positive | 53 (38) | 24 (45) | (32, 59) | |
| Breast-milk anti-rotavirus IgA—Quartiles (median titre) | ||||
| Median (IQR) | 160 (80, 320) | 0.053 | ||
| 1 (80) | 54 (40) | 32 (59) | (46, 72) | |
| 2 (160) | 30 (22) | 11 (37) | (21, 55) | |
| 3 (320) | 28 (21) | 12 (43) | (26, 62) | |
| 4 (640+) | 24 (18) | 7 (29) | (14, 50) | |
| Total | 141 | 66 (47) | (39, 55) | |
1 3rd and 4th Quartiles were combined because the frequencies were small
2 Over 90% of titres were below limit of detection and therefore 25th, 50th, 75th percentiles were the same as shown in the interquartile range (IQR)
* P-values were calculated using logit model where median titres were used to test for trend
In univariable analyses, there was strong evidence that high levels of Zonulin (test for trend p<0.0001) and IFABP (test for trend p = 0.001) were associated with increased probability of seroconversion, while there was no evidence at 5% level of significance that high levels of sCD14 (p = 0.189) and EndoCab (p = 0.073) were associated with seroconversion (Table 1, Table 2).
Table 2. Independent effects of markers of environmetal enteric dysfunction on seroconversion post dose 2 among rotavirus vaccinated infants aged 6–11 weeks.
| Biomarkers | Crude RR (95%CI) | P-value | Adjusted RR (95%CI) 1 | Adjusted P-value |
|---|---|---|---|---|
| Zonulin | ||||
| Titre transformed to log base 2 | 1.26 (1.14, 1.40) | <0.0001 | 1.24 (1.12, 1.37) | <0.0001 |
| Soluble CD14 | ||||
| Titre transformed to log base 2 | 0.91 (0.71, 1.16) | 0.438 | 0.91 (0.73, 1.13) | 0.380 |
| EndoCaB | ||||
| Titre transformed to log base 2 | 0.92 (0.79, 1.08) | 0.307 | 0.98 (0.85, 1.14) | 0.807 |
| IFABP | ||||
| Titre transformed to log base 2 | 1.11 (1.06, 1.17) | <0.0001 | 1.07 (1.02, 1.13) | 0.006 |
1 Estimates were adjusted for biomarkers (transformed on log base 2); and Age of child at vaccination (binary); Breast-milk anti-rotavirus IgA (transformed on log base 2); Seropositivity at baseline (IgA > = 1:40) (binary); Infant serum anti-rotavirus IgG (transformed on log base 2)
In multivariable analyses adjusting for the independent effect of other biomarkers and confounders (i.e. age of child at vaccination, breast-milk anti-rotavirus IgA, infant serum anti-rotavirus IgG, and IgA seropositivity at baseline), there was strong evidence of about 24% increased in seroconversion due to doubling Zonulin concentration (Adjusted risk ratio (aRR) = 1.24; 95% CI = 1.12 to1.37; p<0.0001) (Table 2). Similarly, we found about 7% increase in seroconversion due to doubling IFABP concentration (aRR = 1.07; 95% CI = 1.02 to 1.13; p = 0.006).
Discussion
We report findings of the influence of intestinal inflammation biomarkers on seroconversion in infants receiving rotavirus vaccines. We found that high levels of Zonulin and IFABP were strongly associated with vaccine seroconversion. We also found very low levels of EndoCAb and sCD14, which were not associated with seroconversion.
Zonulin and IFABP biomarkers are indicators of the integrity of the “Gate” (intestinal barrier). Increased serum concentration reflects “Openness of the gate” and thus increased leaking from the intestinal lumen into lamina propria. We think this implied easy vaccine uptake and thus resulted into “better seroconversion”. Zonulin, as a tight junction modulator protein, is secreted due to antigen binding to receptor on intestinal epithelial cell surface, which later binds to EGF receptor (EGFR) via proteinase-activated receptor 2 (PAR2) [33]. Activation of the two receptors initiates the cascade reaction resulting in tight junction protein disengagement, this then enhances intercellular intestinal permeability [33, 34].
Elevated levels of zonulin in serum has been found to be associated with rotavirus infection in infants in Poland [35]. Further, it has been demonstrated that Dendritic Cells (DC) open the tight junctions between epithelial cells, send dendrites directly in the lumen for antigen sampling [36–38], suggesting that increased permeablility evidenced by high levels of Zonulin facilitates sending DC processes in lumen for antigen upatake. Also, antigen-nonspecific transport occurs through transcellular or paracellular pathways when the tight junction becomes more permeable or damaged by environmental factors. This increased uptake of antigens may occur as a result of allergic enteropathy, and other environmental factors that activate inflammatory cascades [39–41].
On the other hand, intestinal fatty-acid binding protein (I-FABP) is expressed in epithelial cells of the mucosal layer of the small intestine tissue [42]. When intestinal mucosal damage occurs due to presence of microbes or dietary antigens, gut barrier integrity can be disrupted leading to increased intestinal permeability [43]. This breach of intestinal barrier facilitates increased uptake of microbial products as well as live vaccines. These mechasims were supported by our findings that high levels of Zonulin and IFABP was associated with seroconversion. We think the high levels of Zonulin and IFABP implied early infant exposure to poor sanitation and hygiene, and therefore exposure to pathogens causing intestinal inflammation.
The timing of the samples just before the first vaccine dose was appropriate as it reflects the EED status of the internal milieu. We postulated that if the live oral vaccine is taken around such a time, the increased translocation of luminal microbes and their products actually facilitated better vaccine uptake. This thinking is consistent with the known mechanism for natural infections [17, 20, 21]. Our finding is consistent with that of a Bangladeshi study in which IFABP had positive association with oral chorela vaccine [44].
However, soluble CD14 and Endocab are markers of responses to lipopolysaccharides (LPS), a product of gram negative bacterial cell wall, which indicate “Gut leakage”. It has been postulated that high levels of sCD14 in the plasma are reflective of LPS exposure [45]. The low levels of soluble CD14 and Endocab found in our study may suggest that infants were less exposed to intestinal bacterial infection.
We acknowledge some limitations in our study. First, stool biomarkers were not used to asses EED [46]. Second, we did not assess the intestinal microbiota, which has been shown to have an effect on vaccine responses among infants [47]. Third, there may be other unmeasured confounders that could impact immune responses. Despite these limitations, we believe that the findings are relevant to warrant future studies establishing the levels of Zonulin and IFABP at which immune activation due to increased permeability does not lead to negative association of vaccine seroconversion.
Conclusion
We found that high levels of zonulin and IFABP played a role in seroconversion. It is plausible that increased gut permeability in EED allows greater uptake of the live virus within the vaccine, but later consequences result in deleterious local structural distortions and malabsorption syndromes.
Supporting information
(ZIP)
(ZIP)
Acknowledgments
We are grateful to the parents of all the infants who have participated in this study.
Data Availability
The data underlying this study are restricted by the CIDRZ Ethics and Compliance Committee. To request data access, one must write to the Committee chair/Chief Scientific Officer, Dr. Roma Chilengi, (Roma.Chilengi@cidrz.org) or the Secretary to the Committee/Head of Research Operations, Ms. Hope Mwanyungwi (Hope.Mwanyungwi@cidrz.org). The request for data must include contact information, a research project title, and a description of the analysis being proposed as well as the format expected. The requested data should only be used for the purposes related to the original research or study. The CIDRZ Ethics and Compliance Committee will normally review all data requests within 48 – 72 hours (Monday - Friday), and provide notification if access has been granted or additional project information is needed, before access can be granted. If the requester chooses to use post mail, the following address must be used: The CIDRZ Ethics and Compliance Committee, Centre for Infectious Disease Research in Zambia, Plot # 34620, Off Alick Nkhata Road, next to Energy Regulation Board Offices, Opposite Football House, (FAZ), PO Box 34681, Lusaka, Zambia.
Funding Statement
This work was supported with funding from an NIH R01 grant #1R01AI099601 and DfID through the SHARE Consortium through grant # ITDCHA23-5. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.United Nations Children’s Fund, World, Health, Organization. Diarrhoea: why children are still dying and what can be done. Geneva, Switzerland: WHO, 2009. [Google Scholar]
- 2.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. Coordinated Global Rotavirus Surveillance Network Global, Regional, and National Estimates of Rotavirus Mortality in Children. CID. 2016;62(Suppl 2). [DOI] [PubMed] [Google Scholar]
- 3.PATH. Rotavirus disease and vaccines in Zambia. www.path.org/publications/files/VAD_rotavirus_zambia_fs.pdf. Accessed March 22, 2017.
- 4.Rudd C, Mwenda J, Chilengi R. Rotavirus landscape in Africa -Towards prevention and control: A report of the 8th African rotavirus symposium, Livingstone, Zambia. Vaccine. 2015;33(29):3263–7. doi: 10.1016/j.vaccine.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 5.Chilengi R, Simuyandi M, Beach L, Mwila K, Becker-Dreps S, Emperador D, et al. Association of Maternal Immunity with Rotavirus Vaccine Immunogenicity in Zambian Infants. PloS one. 2016;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armah G, Sow S, Breiman R, Dallas M, Tapia M, Feikin D. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–14. doi: 10.1016/S0140-6736(10)60889-6 [DOI] [PubMed] [Google Scholar]
- 7.Glass R, Parashar U, Bresee J, Turcios R, Fischer T, Widdowson M. Rotavirus vaccines: current prospects and future challenges. Lancet. 2006;368:323–32. doi: 10.1016/S0140-6736(06)68815-6 [DOI] [PubMed] [Google Scholar]
- 8.Madhi S, Cunliffe N, Steele D, Witte D, Kirsten M, Louw C. Effect of human rotavirus vaccine on severe diarrhea in African infants. The New England journal of medicine. 2010;362:289–98. doi: 10.1056/NEJMoa0904797 [DOI] [PubMed] [Google Scholar]
- 9.Salinas B, Perez S, Linhares A, Ruiz P, Guerrero M, Yarzabal J. Evaluation of safety, immunogenicity and efficacy of an attenuated rotavirus vaccine, RIX4414: A randomized, placebo-controlled trial in Latin American infants. The Pediatric infectious disease journal. 2005;24:807–16. [DOI] [PubMed] [Google Scholar]
- 10.Soares-Weiser K, Maclehose H, Bergman H, Ben-Aharon I, Nagpal S, Goldberg E. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane database of systematic reviews (Online). 2012;11:CD008521. [DOI] [PubMed] [Google Scholar]
- 11.Vesikari T, Karvonen A, Puustinen L, Zeng S-Q, Szakal E, Delem A. Efficacy of RIX4414 Live attenuated human rotavirus vaccine in Finnish infants. The Pediatric infectious disease journal. 2004;23. [DOI] [PubMed] [Google Scholar]
- 12.Vesikari T, Matson D, Dennehy P, Van Damme P, Santosham M, Rodriguez Z. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. Rotavirus Efficacy and Safety Trial (REST) Study Group. The New England journal of medicine. 2006;354:23–33. doi: 10.1056/NEJMoa052664 [DOI] [PubMed] [Google Scholar]
- 13.Zaman K, Dang D, Victor J, Shin S, Yunus M, Dallas M. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–23. doi: 10.1016/S0140-6736(10)60755-6 [DOI] [PubMed] [Google Scholar]
- 14.Mwila K, Chilengi R, Simuyandi M, Permar S, Becker-Dreps S. Contribution of maternal immunity to decreased rotavirus vaccine performance in low- and middle-income countries. Clin Vaccine Immunol. 2017;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker E, Kampmann B, Kang G, Grassly N. Influence of enteric infections on response to oral poliovirus vaccine: a systematic review and meta- analysis. J Infect Dis. 2014;210:853–64. doi: 10.1093/infdis/jiu182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petri W, Naylor C, Haque R. Environmental enteropathy and malnutrition: do we know enough to intervene? BMC Medicine. 2014;12:187 doi: 10.1186/s12916-014-0187-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korpe P, Petri W. Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med. 2012;18:328–36. doi: 10.1016/j.molmed.2012.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prendergast A, Kelly P. Enteropathies in the developing world: neglected effects on global health. Am J Trop Med Hyg. 2012;86:756–63. doi: 10.4269/ajtmh.2012.11-0743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerrant R, Oriá R, Moore S, Oriá M, Lima A. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutrition reviews. 2008;66(9):487–505. doi: 10.1111/j.1753-4887.2008.00082.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly P, Menzies I, Crane R, Zulu I, Nicoles C, Feakins R. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. The American journal of tropical medicine and hygiene. 2004;70(4):412–9. [PubMed] [Google Scholar]
- 21.Patriarca P, Wright P, John T. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Reviews of infectious diseases. 1991;13(5):926–39. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan P, Lunn P, Northrop-Clewes C, Crowe P, Marsh M, Neale G. Persistent diarrhea and malnutrition-the impact of treatment on small bowel structure and permeability. Journal of pediatric gastroenterology and nutrition. 1992;14(2):208–15. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan P, Marsh M, Mirakian R, Hill S, Milla P, Neale G. Chronic diarrhea and malnutrition histology of the small intestinal lesion. Journal of pediatric gastroenterology and nutrition. 1991;12(2):195–203. [DOI] [PubMed] [Google Scholar]
- 24.Keusch G, Denno D, Black R, Duggan C, Guerrant R, Lavery J. Environmental enteric dys- function: pathogenesis, diagnosis, and clinical consequences. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;59(4):207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mondal D, Minak J, Alam M, Liu Y, Dai J, Korpe P. Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;54:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denno D, VanBuskirk K, Nelson Z, Musser C, Tarr P. Environmental enteric dysfunction: Advancing current knowledge. St.Louis, MO.
- 27.Fasano A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin Gastroenterol Hepatol. 2012;10(10):1096–100. doi: 10.1016/j.cgh.2012.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Uzzau S, Goldblum S, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions Journal of cell science. 2000;113(24):4435–40. [DOI] [PubMed] [Google Scholar]
- 29.Pelsers M, Namiot Z, Kisielewski W, Namiot A, Januszkiewicz M, Hermens W. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem. 2003;36:529–35. [DOI] [PubMed] [Google Scholar]
- 30.Kitchens R. "Role of CD14 in cellular recognition of bacterial lipopolysaccharides". Chemical immunology. 2000;74:61–82. [DOI] [PubMed] [Google Scholar]
- 31.Tapping R, Tobias P. "Soluble CD14-mediated cellular responses to lipopolysaccharide". Chemical immunology. 2000;74:108–21. [DOI] [PubMed] [Google Scholar]
- 32.Barclay G. Endogenous endotoxin-core antibody (EndoCAb) as a marker of endotoxin exposure and a prognostic indicator: a review. Prog Clin Biol Res. 1995;392:263–72. [PubMed] [Google Scholar]
- 33.Lammers K, Lu R, Brownley J, Lu B, Gerard C, Thomas K. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135:194–204. doi: 10.1053/j.gastro.2008.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tripathi A, Lammers K, Goldblum S, Shea-Donohue T, Yang S, Arrietta M. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci USA. 2009;106:16799–804. doi: 10.1073/pnas.0906773106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarko A, Suchojad A, Michalec M, Majcherczyk M, Brzozowska A, Maruniak-Chudek I. Zonulin: A Potential Marker of Intestine Injury in Newborns. Hindawi Disease Markers 2017;Article ID 2413437:6 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rescigno M, Ubrano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2(4):361–7. doi: 10.1038/86373 [DOI] [PubMed] [Google Scholar]
- 37.Soderrholm J, Streutker C, Yang P, Perdue M. Increased epithelial uptake of proteins among Crohns disease mediated by tumor necrosis factor alpha. Gut. 2004;53(12):1817–24. doi: 10.1136/gut.2004.041426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tennant S, Muhsen K. Gut Immunology and oral vaccination. Molecular vaccines. 2013;1:61. [Google Scholar]
- 39.Aristo V. The Assessment of Intestinal Permeability, Size Matters. Alternative Therapies. 2013;19(1). [PubMed] [Google Scholar]
- 40.Ford R, Menzies I, Philips A, Walker-Smith J, Turner M. Intestinal sugar permeability: relationship to diarrhoeal disease and small bowel morphology. Journal of pediatric gastroenterology and nutrition. 1985;4(4):568–74. [PubMed] [Google Scholar]
- 41.Juvonen P, Jakobssson I, Lindberg T. Macromolecular absorption and cows’ milk allergy. Arch Dis Child. 1991;66(3):300–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gajda A, Storch J. Enterocyte fatty acid-binding proteins (FABPs): Different functions of liver and intestinal FABPs in the intestine. Prostaglandins Leukot Essent Fatty Acids. 2014;93:9–16. doi: 10.1016/j.plefa.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lau E, Marques C, Pestana D, Santoalha M, Carvalho D, Freitas P. Conceição Calhau. The role of I-FABP as a biomarker of intestinal barrier dysfunction driven by gut microbiota changes in obesity. Nutrition & Metabolism. 2016;13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uddin M, Islam S, Nishat N, Hossain M, Rafique T, Rashu R. Biomarkers of Environmental Enteropathy are Positively Associated with Immune Responses to an Oral Cholera Vaccine in Bangladeshi Children. PLoS Negl Trop Dis. 2016;10(11):e0005039 doi: 10.1371/journal.pntd.0005039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durieux J, Vita N, Popescu O, Guette F, Calzada-wack J, Munker R. The two soluble forms of the lipopolysaccharide receptor, CD14: characterization and release by normal human monocytes. European journal of immunology. 1994;24:2006–12. doi: 10.1002/eji.1830240911 [DOI] [PubMed] [Google Scholar]
- 46.Kosek M, Haque R, Lima A, Babji S, Shrestha S, Quereshi S. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg. 2013;88:390–6. doi: 10.4269/ajtmh.2012.12-0549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huda M, Lewis Z, Kalanetra K, Rashid M, Ahmad S, Raqid R. Stool microbiota and vaccine responses of infants. Pediatrics. 2014;134:362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(ZIP)
(ZIP)
Data Availability Statement
The data underlying this study are restricted by the CIDRZ Ethics and Compliance Committee. To request data access, one must write to the Committee chair/Chief Scientific Officer, Dr. Roma Chilengi, (Roma.Chilengi@cidrz.org) or the Secretary to the Committee/Head of Research Operations, Ms. Hope Mwanyungwi (Hope.Mwanyungwi@cidrz.org). The request for data must include contact information, a research project title, and a description of the analysis being proposed as well as the format expected. The requested data should only be used for the purposes related to the original research or study. The CIDRZ Ethics and Compliance Committee will normally review all data requests within 48 – 72 hours (Monday - Friday), and provide notification if access has been granted or additional project information is needed, before access can be granted. If the requester chooses to use post mail, the following address must be used: The CIDRZ Ethics and Compliance Committee, Centre for Infectious Disease Research in Zambia, Plot # 34620, Off Alick Nkhata Road, next to Energy Regulation Board Offices, Opposite Football House, (FAZ), PO Box 34681, Lusaka, Zambia.


