Abstract
Intensive selection for milk production has led to reduced reproductive efficiency in high-producing dairy cattle. The impact of intensive milk production on oocyte quality as well as early embryo development has been established but few analyses have addressed this question at the initiation of implantation, a critical milestone ensuring a successful pregnancy and normal post-natal development. Our study aimed to determine if contrasted maternal metabolism affects the previously described sensory properties of the endometrium to the conceptus in cattle. Following embryo transfer at Day 7 post-oestrus, endometrial caruncular (CAR) and intercaruncular (ICAR) areas were collected at Day 19 from primiparous postpartum Holstein-Friesian cows that were dried-off immediately after parturition (i.e., never milked; DRY) or milked twice daily (LACT). Gene quantification indicated no significant impact of lactation on endometrial expression of transcripts previously reported as conceptus-regulated (PLET1, PTGS2, SOCS6) and interferon-tau stimulated (RSAD2, SOCS1, SOCS3, STAT1) factors or known as female hormone-regulated genes (FOXL2, SCARA5, PTGS2). Compared with LACT cows, DRY cows exhibited mRNA levels with increased expression for FOXL2 transcription factor and decreased expression for oxidative stress-related genes (CAT, SOD1, SOD2). In vivo and in vitro experiments highlighted that neither interferon-tau nor FOXL2 were involved in transcriptional regulation of CAT, SOD1 and SOD2. In addition, our data showed that variations in maternal metabolism had a higher impact on gene expression in ICAR areas. Collectively, our findings prompt the need to fully understand the extent to which modifications in endometrial physiology drive the trajectory of conceptus development from implantation onwards when maternal metabolism is altered.
Introduction
During recent decades, dairy cattle have been selected in order to improve milk production, leading to an associated decrease in fertility [1]. Greater milk production has been associated with physiological disturbances that accentuate negative energy balance during early lactation [2]. In high-producing dairy cows, fertilization rate has been shown to be as high as 80–90%, but only about 40% of inseminations result in the birth of a calf, with 70% of pregnancy failures occurring during the pre-implantation period [3]. Indeed, in a review of fertilisation and embryo quality in dairy cows, it was reported that only approximately 50% of embryos are still viable by Day 7 [4].Milk production has been shown to affect not only the production of oocytes but also embryo metabolism during conceptus free-floating life [5]. When the impact of lactation on oocyte quality is circumvented by the use of embryo transfer in lactating recipient females, pregnancy rate is improved [6–8]. Other experiments in which embryos were transferred at Day 7 post-oestrus have shown that pregnancy rate is reduced in lactating cows compared with heifers or dry cows [9–11]. In addition to decreasing fertility, alterations in maternal metabolism during pregnancy have also been reported to affect antenatal programming of production traits in offspring [12]. As a biological dynamic interface, the endometrium acts as a sensor of embryo quality that drives the developmental trajectory of the conceptus [13].Thus, endometrial quality appears critical for pregnancy as well as post-natal issues.
Implantation is a critical step of pregnancy [14] and is defined as the establishment of cellular and permanent interactions between a receptive endometrium and a competent and synchronised embryo [15]. At implantation, regulation of endometrial gene expression result from a combination of biological processes that involve actions of maternal signals, impact of embryo-secreted factors and cellular interactions between the conceptus and the endometrium. In ruminants including cattle, interferon-tau (IFNT) has been reported as the major signal of maternal recognition of pregnancy [16,17]. The spatio-temporal secretion of IFNT is restricted to the trophectoderm during the elongation phase of the conceptus [18]. In the ovine and bovine species, expression patterns of endometrial genes have been intensively investigated by our laboratory and others using in vitro and in vivo experimental models associated with high-throughput analyses and candidate gene approaches. Determining variations in gene expression during estrous cycle and early pregnancy allow a classification of endometrial genes in three major categories namely (i) conceptus regulated gene expression that involves IFNT, making these genes members of classical and non-classical interferon-regulated genes such as the STAT1-SOCS pathway thought to play critical functions during implantation in cattle [19,20], (ii) conceptus regulated and IFNT-independent expression that is modified by still unknown or already identified conceptus-produced factors (e. g. cortisol, prostaglandins [21]) such as SOCS6 [22] and PLET1 [23], two genes highly expressed in the luminal epithelial cells when apposition initiates, and (iii) expression that is not affected by the presence of the conceptus but is variable across the estrous cycle (e. g. under the influence of female hormones including ovarian steroids [16,24,25] such as the scavenger receptor SCARA5 [23], the key enzyme in prostaglandin biosynthesis PTGS2 [26,27], and FOXL2, a key transcription factor of the ovary and recently characterized in the bovine endometrium [28]. These three categories of regulated genes contribute to endometrial receptivity, conceptus elongation and implantation and their perturbed expression may lead to detrimental effects on establishment, progression and outcome of pregnancy [13].
After calving, dairy cows undergo metabolic adjustments that are associated with the transition from pregnancy to lactation. In order to meet the increasing demand for energy in early lactation, shifts in nutrient distribution have been described [29]. In this context, the imbalance between reactive oxygen species (ROS) production and the availability of antioxidant defences during early lactation may expose cows to oxidative stress observed in blood plasma [30]. In mice, ROS may negatively impact embryonic metabolism leading to pregnancy complications and long-term modifications of offspring metabolism [31]. Activity of antioxidant enzymes involved in the regulation of oxidative stress (catalase, CAT; super oxide dismutase 1, SOD1; super oxide dismutase 2, SOD2) has been reported to vary in ovine endometrium during the peri-implantation period [32] and with endocrine milieu in the endometrium of beef cows at Day 7 of the oestrous cycle [33]. Interestingly, in immortalized granulosa cells, SOD2 gene expression has been shown to be affected by FOXL2 [34]. Nevertheless, when variations in maternal metabolism are considered, data relating to the regulation of these enzymes in the endometrium are lacking.
Considering this background, our study aimed to determine if maternal metabolism has distinct effects on the two areas that constitute the bovine endometrium at the time of implantation. In ruminants, endometrial morphology is characterised by caruncular (CAR) and intercaruncular areas (ICAR), both essential for supporting pregnancy. Caruncular endometrium is represented by small sparse aglandular structures, whereas intercaruncular endometrium contains endometrial glands the secretions of which are critical for conceptus elongation [35]. High-throughput data have highlighted that endometrial CAR and ICAR areas exhibit a site-specific response to the presence of the implanting conceptus in normal or perturbed pregnancies [22,23,28,36–38]. In this study, we used a novel model of age-matched pregnant primiparous postpartum lactating or non-lactating dairy cows combined with embryo transfer [39]. At Day 19 post-oestrus, we analysed the expression of genes known to be deeply affected in the endometrium when implantation occurs (i) conceptus-regulated and IFNT-Stimulated genes (ISG) namely RSAD2 [23,40] and three members of the STAT-SOCS transduction pathway (STAT1, SOCS1, SOCS3) [22,41]; (ii) conceptus-regulated but IFNT-independent genes, namely PLET1, PTGS2 and SOCS6, and (iii) female hormone-regulated genes including SCARA5 and FOXL2 as well as CAT, SOD1 and SOD2, three genes encoding enzymes with critical functions in oxidative stress regulation. For these three genes, expression in the bovine endometrium during implantation process and regulation by IFNT was first documented.
Materials and methods
Animal models
All experiments were conducted in accordance with the European Community Directive 2010/63/EU revising Directive 86/609/EEC on the protection of animals used for scientific purposes. Animal procedures described in Experiments 1 and 2 were licensed by the Department of Health and Children, Ireland, in accordance with the Cruelty to Animals Act (Ireland 1876) and were sanctioned by the Animal Research Ethics Committee of University College Dublin. Animals in experiments 1, 2 and 4 were slaughtered and processed as part of the normal work of a commercial abattoir. Animal procedures reported for endometrial biopsy collection were approved by the Ethical Committee of Animal Experimentation of INRA and AgroParisTech (CEEA 45 “COMETHEA”; reference APAFIS#596) with authorization subsequently granted by the French Ministry of Education and Scientific Research (reference 2015050417061568).
Experiment 1: Impact of contrasted maternal metabolism on endometrial gene expression modifications at implantation
Twenty-one primiparous Holstein-Friesian cows and six Holstein-Friesian heifers (HEIF) were enrolled onto this study as previously described [39]. At calving, cows were randomly assigned to one of two groups: lactating (LACT, n = 11) or non-lactating (DRY, n = 10). From calving, animals in the LACT group were milked twice per day (0700 and 1600 hours), while those in the DRY group were dried-off immediately after calving (i.e., never milked) as described by Forde et al. [39]. All animals were synchronised 65 to 75 days after calving using an EAZI-BREED™ CIDR® cattle insert in conjunction with prostaglandin F2α (Zoetis, Parsippany-Troy Hills, New Jersey, USA). Single grade 1 Day 7 embryos from superovulated donor heifers were transferred to LACT and DRY cows. As a control, HEIF were artificially inseminated. Blood samples were taken from the jugular vein into plain red-topped vacutainer tubes for serum collection. Progesterone serum concentrations on Day 19 were determined according to Carter et al. [42].
The day of oestrus was considered Day 0 and all females were slaughtered at Day 19, coinciding with the initiation of implantation in cattle. Uteri were collected and flushed and, when present, recovered concepti were observed by microscopy to confirm the stage of development [43]. From pregnant animals (HEIF n = 4; LACT n = 5; DRY n = 8), endometrial CAR and ICAR areas were dissected from the uterine horns ipsilateral to the corpus luteum as previously described [23]. For each type of endometrial area, a pool of tissue representative of the whole horn was used for protein or total RNA extraction. Tissue samples were snap frozen in liquid nitrogen and stored at -80°C prior to extraction.
Experiment 2: Expression of oxidative stress enzymes during the oestrous cycle and early pregnancy
In order to provide insights on gene expression of antioxidant enzymes in bovine endometrium, cross-bred heifers were collected at two days of the oestrous cycle (active luteal phase and luteolysis) as well as during pregnancy recognition and implantation as previously published [28]. Heifers were synchronized and artificially inseminated as formerly described [44]. The day of oestrus was considered Day 0 and heifers were slaughtered on Day 16 (cyclic: n = 5; pregnant: n = 4) or on Day 20 (cyclic: n = 6; pregnant: n = 5). Uteri were collected from pregnant and cyclic animals, they were flushed and, when present, recovered concepti were observed by microscopy to confirm the stage of development [43]. Endometrial CAR and ICAR areas were dissected from the uterine horns ipsilateral to the corpus luteum [23]. Tissue samples were snap frozen in liquid nitrogen and stored at -80°C prior to extraction.
Experiment 3: FOXL2 overexpression in primary cultures of bovine endometrial cells
In order to determine if FOXL2 regulates gene expression of enzymes involved in the regulation of oxidative stress, we collected endometrial biopsies during the active luteal phase, at Day 15 of the oestrous cycle when endometrial expression of FOXL2 was reported to be low [28]. Three Holstein heifers (n = 3) were sampled, that had been synchronized using Creastar method, EAZI-BREED™ CIDR® cattle insert (Zoetis). From each heifer, one endometrial biopsy was sampled and used for deriving primary cultures of fibroblasts and glandular epithelial cells as previously reported [23]. For each heifer, fibroblasts and epithelial cells were cultured separately, leading to 3 independent biological replicates for each cell type. Endometrial cells were cultured for 24 h on 12-well plates (TPP, Trasadingen, Switzerland). Fibroblasts and epithelial cells were transfected with 0.5 μg of pSG5 plasmid (mock) or pSG5-FOXL2 plasmid (kindly provided by M. Pannetier; INRA, Jouy-en-Josas, France; [45]), using CombiMag transient reagent (Oz Biosciences, Marseille, France) according to the manufacturer's instructions. Transfected cells were lysed after 24 h using Trizol reagent (Life Technologies, Carlsbad, California, USA). Cell lysates were frozen and stored at -80°C for gene expression analyses.
For determining FOXL2 localization by immunocytochemistry, fibroblasts and glandular epithelial cells were cultured using a Lab-tech chamber slide system (Sigma-Aldrich, St. Louis, Missouri, USA) and transiently transfected as described above. Cells were fixed for 10 min at room temperature in PAF 4% (Sigma-Aldrich) and washed in PBS (Sigma-Aldrich). Slides were incubated in citrate buffer (sodium citrate 0.01 M, pH 6) for 5 min at room temperature then for 10 min at 80°C. Endogenous peroxidase activity was quenched by 0.01% H2O2 (Sigma-Aldrich) treatment for 30 min. Cells were incubated with a rabbit anti-FOXL2 purified antibody (dilution 1:250; [28]) in phosphate buffer (0.1 M, pH 7.4 with 2% bovine serum albumin (BSA) and 1% Normal Donkey Serum) overnight at 4°C. After washes in phosphate buffer (0.1 M, pH 7.4 with 2% BSA), slides were incubated with an anti-rabbit biotinylated secondary antibody (dilution 1:1000; Ab6720, Abcam, Cambridge, UK) for 1 h at room temperature. Cells were washed in phosphate buffer containing 2% BSA and incubated for 1 h with ABC Vector kit (Vectastain Elite ABC kit, Vector Labs, Peterborough, UK) in Tris buffer (Tris 50 mM, NaCl 0.15 M, pH 7.5). Slides were washed, incubated for 5 min with Diaminobenzidine-Nickel in Tris buffer then they were mounted with Eukitt medium (Sigma-Aldrich). Nanozoomer Digital Pathology System was used to obtain images that were analysed using the NDP View software (Nanozoomer Digital Pathology Virtual Slide software, Hamamatsu, Japan).
Experiment 4: Treatment of bovine endometrial cell cultures with interferon-tau (IFNT)
In order to evaluate the impact of IFNT on the expression of genes encoding antioxidant enzymes, we used primary populations of epithelial and fibroblast cells that were isolated from bovine endometrium collected from cyclic mixed breed beef cows on Day 11–17 post-oestrus, as previously described [46]. During this period of the estrous cycle, endometrium has been shown to strongly react to conceptus secretions including IFNT [20,44]. The endometrial cells were cultured in medium containing RPMI-1640 Medium (Sigma-Aldrich, Saint-Quentin Fallavier, France), supplemented with 10% heat inactivated Fetal Bovine Serum (Sigma-Aldrich), 1% Penicillin-Streptomycin (Sigma-Aldrich), 1% Amphotericin B (Sigma-Aldrich). The epithelial and fibroblast cells were each treated with control medium or medium containing ovine recombinant IFNT (100 ng/ml or 1000 ng/ml) for 30 min, 2 h or 24 h [23,41]. Bovine endometrial cells were washed with Dulbecco’s Phosphate Buffered Saline with MgCl2 and CaCl2 (Sigma-Aldrich) and lysed using Trizol reagent (Life Technologies). Cell lysates were frozen and stored at -80°C before analysis. Data were generated using stromal and epithelial cells isolated from four independent animals.
Protein extraction and western blot analyses
FOXL2 protein level was investigated from endometrial ICAR and CAR protein extracts described in experiment 1 (n = 3 animals/condition). Frozen ground tissue (250 mg) was dispersed and sonicated in 1 ml of a cold lysis buffer at pH 7.4 containing 50 mM Hepes, 150 mM NaCl, 5 mM EDTA, 16 mM 3-((3-cholamidopropyl) dimethylammonio)-2-hydroxy-1-propanesulfonate, 1mM benzamidine-HCl, 1mM phenylmethylsulfonyl fluoride, 10 μg/ml soybean trypsin inhibitor, 10 μg/ml leupeptin and 10 μg/ml aprotinin. Protein quantification was performed with the Bio-rad protein assay (Bio-Rad, Hercules, California, USA) using BSA as the standard (Sigma Aldrich).
Western blot immunoassays were processed with 25 μg of total protein extract electrophoresed in SDS–10% polyacrylamide gel and onto a Hybond-P, polyvinylidene difluoride membrane (Trans-blot® Turbo Midi Size PVDF Membrane; Bio-Rad) with Trans-blot® Turbo (Bio-Rad) system. Membranes were first incubated with a rabbit anti-FOXL2 purified antibody generated against a peptide (sequence: WDHDSKTGALHSRLDL) corresponding to the C-terminal conserved region of mammalian FOXL2 (CASLO Laboratory, Lyngby, Denmark; [28]) in a solution containing 20 mM Tris-HCl, 500 mM NaCl, (pH 7.6), 0.1% (wt/vol) Tween 20 containing 5% (wt/vol) low-fat milk (2 μg antibody/ml). Then, membranes were incubated with a biotinylated donkey anti-rabbit IgG antibody (Jackson ImmunoResearch, West Grove, Pennsylvania, USA). Blots were developed for 1 h using the Vectastain Elite ABC peroxidase complex 1:1000 diluted (Vectastain Elite ABC kit; Vector Labs). Immunoreactive signals were revealed with ECL2 western blotting detection kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA). After stripping, GAPDH protein was assessed as a loading control using a rabbit polyclonal anti-GAPDH antibody (Sigma-Aldrich) and donkey peroxidase-conjugated anti-rabbit antibody (Interchim, San Diego, California, USA). The molecular weights of the identified proteins were calculated using the ProSieve HyperPage Meridian (Bioline, London, UK).
Total RNA extraction
Total RNA were isolated from biological samples described in experiments 1, 2, 3 and 4 by homogenization using Trizol reagent (Life Technologies) as published previously [23]. All RNA samples were purified using Qiagen columns integrating a DNAse step (RNeasy mini kit; Qiagen, Hilden, Germany). Quality and integrity of purified total RNA were determined using an Agilent 2100 Bioanalyzer (@BRIDGe-ICE platform, INRA, Jouy-en-Josas, France). Total RNA samples were stored at -80°C prior to analysis.
Real time RT-PCR
Purified total RNA samples were used for gene quantification using Real Time-PCR (RT-qPCR). One μg of total RNA was reverse transcribed into cDNA with Superscript II enzyme (Life Technologie) in a 20 μl-volume. RT-qPCR reactions were carried out with Master Mix SYBR Green and Step One Plus system (Applied Biosystem, Foster city, California, USA). Primers (Eurogentec, Liège, Belgium) were designed (Primer express version 2.0 software; Applied Biosystem) to specifically amplify housekeeping genes (C2ORF29, GAPDH, RPL19, SLC30A6, SUZ12) and target genes (S1 Table).
Statistical analyses
Quantification of the amount of target mRNA relative to that of normalizer genes was calculated using Qbase plus software (Biogazelle; Zwijnaarde, Belgium) according to the relative standard curve method [47]. Normalized PCR data were processed using the Kruskal-Wallis method using the plug-in Coin (COnditional INference procedures in a Permutation Test Framework; Package Rcmdr) associated with pairewise t-test (R application V3.0.1). Principal Component Analysis (PCA) were carried out in Excel using the XLSTAT software (Addinsoft, Paris France).
Results
Endometrial expression of conceptus-regulated genes is not affected by maternal metabolism at implantation
During early pregnancy, the conceptus impacts endometrial physiology through cellular contact and secretions that include IFNT in ruminants. We investigated the impact of contrasted maternal metabolism on transcriptional expression of a selection of conceptus-regulated genes in endometrial CAR and ICAR areas. Amongst conceptus-regulated genes, RSAD2, STAT1, SOCS1 and SOCS3 are IFNT-dependent genes [22,41,48] whereas SOCS6 and PLET1 are IFNT-independent genes [22,23]. Variation in maternal metabolism did not significantly affect endometrial expression of these genes on Day 19 of pregnancy (Fig 1).
Fig 1. Expression of conceptus-regulated genes in bovine endometrium at implantation.
Caruncular (CAR) and intercaruncular (ICAR) endometrium was collected from Holstein heifers (HEIF, white; n = 3), lactating (LACT, light grey; n = 5) and non-lactating (DRY, dark grey; n = 8) cows at Day 19 post-oestrus. Gene expression was quantified by real-time RT-PCR using C2ORF29, GAPDH and SLC30A6 as housekeeping genes determined by Qbase plus software. Data are means ± SD.
FOXL2 gene expression at implantation varies with maternal metabolism
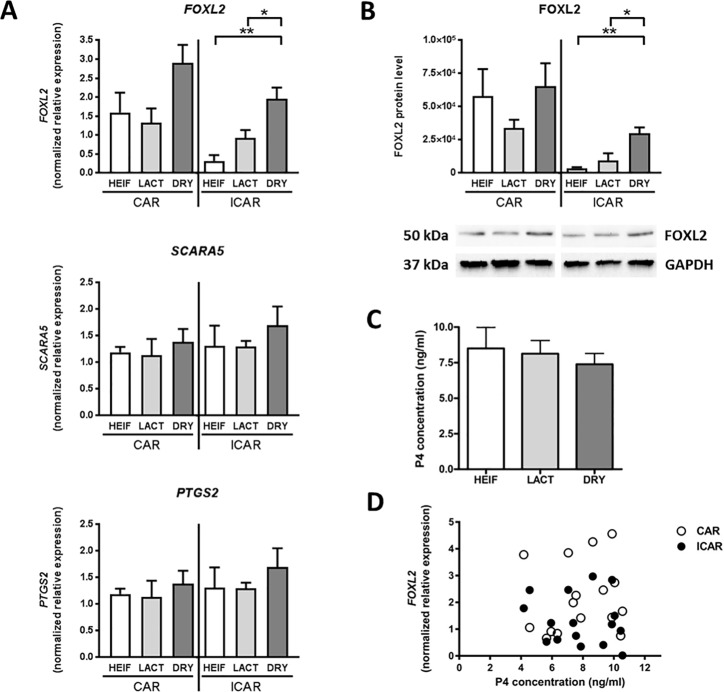
Maternal regulation of endometrial gene expression has been shown to be essential for endometrial receptivity and subsequent embryo implantation. In this model of contrasted metabolism, we investigated expression of three endometrial genes known to be regulated by maternal signals but not by the conceptus, namely SCARA5 [23], PTGS2 [26,27] and FOXL2 [28]. Maternal metabolism had no significant effect on SCARA5 and PTGS2 mRNA levels in bovine endometrium. FOXL2 transcript expression was significantly higher in ICAR areas of DRY cows compared with those of LACT cows (2.1 fold, P < 0.05) and HEIF (6.1 fold, P < 0.01) groups. No significant effect of the maternal metabolism on FOXL2 expression was noticeable in CAR areas (Fig 2A). Similarly, at the protein level, regulation of FOXL2 expression was seen with a significantly higher expression of FOXL2 in ICAR areas of the DRY group compared with the two other groups (DRY vs LACT: 3.2 fold, P < 0.05; DRY vs HEIF: 8.1 fold, P < 0.01; Fig 2B). There was no difference in plasma P4 concentrations between groups and FOXL2 mRNA expression in CAR and ICAR areas was not correlated with P4 blood concentrations (Fig 2C and 2D).
Fig 2. Expression of maternally-regulated genes in bovine endometrium at implantation.
Caruncular (CAR) and intercaruncular (ICAR) endometrium and blood samples for progesterone analysis were collected from Holstein heifers (HEIF, white; n = 3), lactating (LACT, light grey; n = 5) and non-lactating (DRY, dark grey; n = 8) cows on Day 19 post-oestrus. (A) SCARA5, PTGS2 and FOXL2 mRNA expression in bovine endometrium quantified by real-time RT-PCR using C2ORF29, GAPDH and SLC30A6 as housekeeping genes determined by Qbase plus software. (B) Quantification of FOXL2 protein by western blotting normalized to GAPDH protein level. (C) Circulating progesterone concentration in Holstein heifers, lactating and non-lactating cows. (D) Correlation between FOXL2 mRNA expression and circulating progesterone concentration in CAR (white circle; n = 16) and ICAR (black circle; n = 16) areas. Data are means ± SD (P<0.01 **; P<0.05 *).
Endometrial CAT, SOD1 and SOD2 expression varies with maternal metabolism at implantation
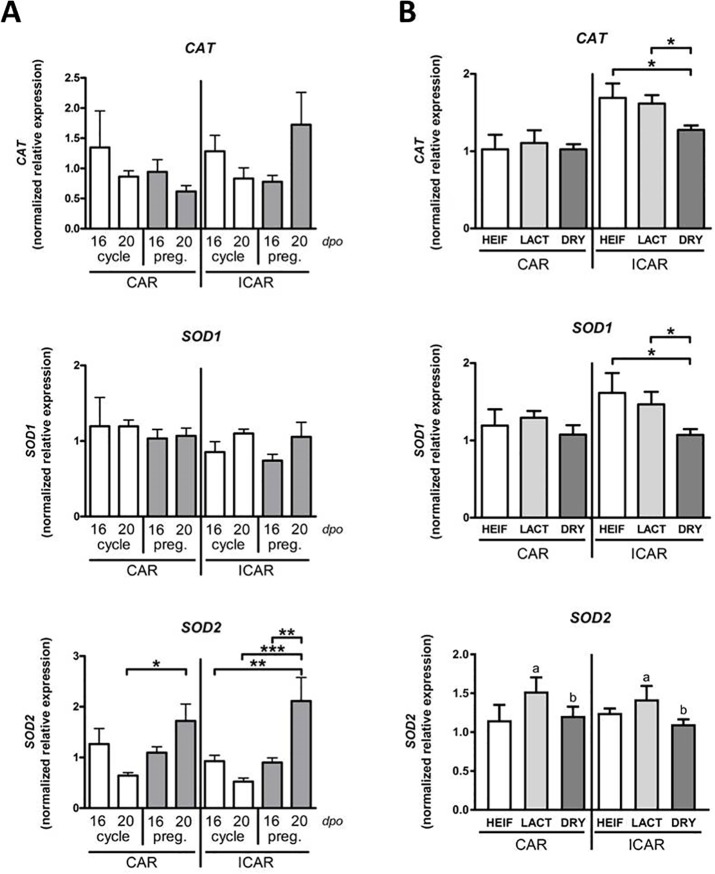
We first determined if CAT, SOD1 and SOD2 transcripts were expressed in bovine endometrium and regulated during oestrous cycle and early pregnancy (Fig 3A). In CAR and ICAR areas, endometrial expression of CAT and SOD1 mRNA did not significantly differ between Day 16 and Day 20 in cyclic or in pregnant heifers. At Day 20 of pregnancy, endometrial expression of SOD2 mRNA was significantly higher when compared with Day 20 of the oestrous cycle (4.0 fold, P < 0.001 in ICAR; 2.7 fold, P < 0.05 in CAR) or with Day 16 of pregnancy (2.3 fold, P < 0.01 in ICAR).
Fig 3. Expression of CAT, SOD1 and SOD2 genes in bovine endometrium.
(A) CAT, SOD1 and SOD2 mRNA expression in cyclic and pregnant endometrium on Days 16 and 20 post oestrous. Caruncular (CAR) and intercarncular (ICAR) endometrium was collected from cyclic (white; n = 5 at Day 16 and n = 6 at Day 20) and pregnant (grey; n = 4 at Day 16 and n = 5 at Day 20) cross-bred beef heifers. Gene expression was quantified by real-time RT-PCR using SUZ12 and SLC30A6 as housekeeping genes determined by Qbase plus software. (B) CAT, SOD1 and SOD2 mRNA expression in bovine endometrium on Day 19 post-oestrus. CAR and ICAR areas were collected from Holstein heifers (HEIF, white; n = 3), lactating (LACT, light grey; n = 5) and dried-off (DRY, dark grey; n = 8) cows at Day 19 post-oestrus. Gene expression was quantified by real-time RT-PCR using C2ORF29, GAPDH and SLC30A6 as housekeeping genes determined by Qbase plus software. Data are means ± SD (P<0.001 ***; P < 0.01 **; P<0.05 *).
In our model of contrasted maternal metabolism (Fig 3B), CAT and SOD1 mRNA expression was significantly lower in endometrial ICAR areas of DRY cows compared with those of LACT cows or heifers (0.78 and 0.73 fold respectively; P < 0.05). Similarly SOD2 mRNA expression was reduced in the DRY group compared with the LACT group when endometrial CAR and ICAR areas were considered simultaneously (0.78 fold, P < 0.05).
CAT, SOD1 and SOD2 mRNA levels are regulated neither by interferon-tau nor by FOXL2
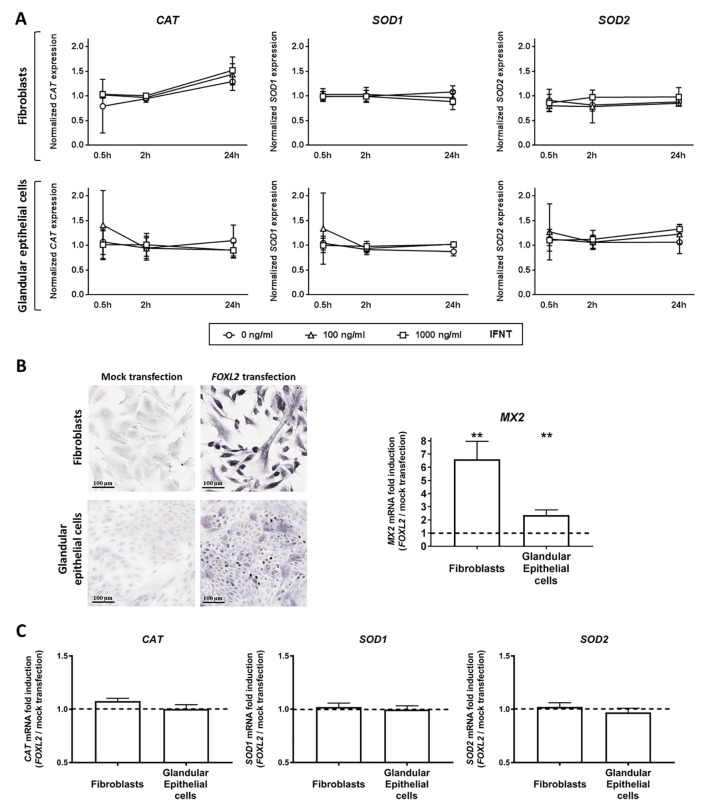
To determine if CAT, SOD1 and SOD2 gene expression was regulated by IFNT, primary cultures of bovine endometrial cells (fibroblasts and glandular epithelial cells) were treated in the presence of 0, 100 or 1000 μg/ml of IFNT for 30 min, 2 h or 24 h. In our previous report, these cultures were shown to be IFNT-responsive [41] but no significant effect of IFNT was detected on mRNA expression of antioxidant enzymes (Fig 4A).
Fig 4. Regulation of CAT, SOD1 and SOD2 gene expression by interferon-tau (IFNT) and FOXL2 in bovine endometrial cells.
(A) Regulation of CAT, SOD1 and SOD2 mRNA expression by IFNT in primary cultures of bovine endometrial cells. Fibroblasts or glandular epithelial cells were incubated with 0 (circle), 100 (triangle) or 1000 ng/ml (square) of IFNT for 30 min, 2 h and 24 h. (B) Left panel. FOXL2 sub-cellular localization in fibroblasts and glandular epithelial cells in mock-transfection and FOXL2-transfection. Scale bar: 100 μm. Right panel. MX2 mRNA expression in primary cultures of bovine endometrial cells overexpressing FOXL2 by transient transfection. (C) CAT, SOD1 and SOD2 mRNA expression in primary cultures of bovine endometrial cells overexpressing FOXL2 by transient transfection. Transcript expression is presented as a ratio between “FOXL2 transfection” condition and “mock transfection” condition (dashed line). Gene expression was quantified by real time RT-PCR using C2ORF29, RPL19 and SLC30A6 as housekeeping genes determined by Qbase plus software. Data are means ± SD.
Since FOXL2 was reported to regulate oxidative stress in ovary-related cells [34,49], the effect of FOXL2 on CAT, SOD1 and SOD2 gene expression was investigated. Overexpression of FOXL2 was reached by transiently transfecting primary cultures of bovine endometrial cells (fibroblasts and glandular epithelial cells). FOXL2 nuclear localization was confirmed by immunocytochemistry in primary cultures of transfected fibroblasts and glandular cells (Fig 4B, left panel). Biological effect of FOXL2 overexpression was confirmed by quantifying MX2 gene expression. Significant up-regulation of MX2 mRNA levels was detected in fibroblasts (6.61 fold; P < 0.01) and in glandular epithelial cells (2.36 fold, P < 0.01) (Fig 4B, right panel). FOXL2 overexpression did not significantly affect CAT, SOD1 or SOD2 mRNA levels in either cell type (Fig 4C).
Principal component analysis (PCA) of endometrial gene expression in LACT, DRY and HEIF groups
A two-dimensional PCA was carried out when considering gene expression of the various genes that were analysed according to maternal metabolism. PCA highlighted distinct gene distribution for each endometrial area when maternal metabolic status varies (Fig 5A and 5B). Our PCA analysis did not allow the discrimination of LACT, DRY and HEIF groups in CAR areas (Fig 5A). In contrast, the F1 axis discriminates DRY cows from LACT cows and from heifers in ICAR areas (Fig 5B).
Fig 5. Gene clustering in the bovine endometrium.
Principal component analysis (PCA) was run to distinguish between Holstein heifers (circle; n = 3), lactating cows (square; n = 5) and non-lactating (triangle; n = 8) cows at Day 19 post-oestrus based on mRNA levels of genes expressed in CAR (A) and ICAR (B) areas. Gene expression was quantified by real-time RT-PCR using C2ORF29, GAPDH and SLC30A6 as housekeeping genes determined by Qbase plus software.
Discussion
In dairy cattle, poor reproductive performance results in greater economics losses for the farmer. Approximately two-thirds of pregnancy failures are believed to occur during the pre-implantation period [3]. As the maternal tissue layer dedicated to the molecular and cellular interactions with the implanting conceptus, the uterine endometrium drives development of the embryonic disk and elongation of the extra embryonic tissues [13,50]. Environmental stress or maternal physiology disturbances have been reported to affect endometrial function that may in turn impact conceptus development during the peri-implantation period with long-term consequences. In high-producing dairy cows, lactation has been shown to generate metabolic stress due to energy demands associated with milk production [39,51,52]. The present study aimed to determine if modifications in maternal metabolism observed between lactating and non-lactating primiparous cows [39,52,53] impact the expression of genes involved in the regulation of endometrial physiology at the initiation of implantation.
Mammalian endometrium has been demonstrated to act as a sensor that can adapt its physiology to variations in embryo quality [54,55]. Recent data in humans have suggested that maternal alterations in endometrial biosensor properties skew the biological response of this tissue to embryo signals [54]. We investigated the impact of a contrasted maternal metabolism on endometrial mRNA levels of conceptus-regulated genes we formerly classified as IFNT-stimulated genes (RSAD2, SOCS1, SOCS3 and STAT1,) or IFNT-independent genes (PLET1 and SOCS6) in cattle [23,37,41]. We analysed the caruncular (CAR; gland-free zones where placentation occurs) and intercaruncular (ICAR; containing endometrial glands that secrete histotroph critical for extra-embryonic tissue elongation; [35]) areas of the bovine endometrium that have been shown to respond differentially to the presence of the conceptus [22,23,36–38,41]. Our data showed that transcriptional expression of this set of conceptus-regulated genes was not significantly affected in either endometrial area when lactating cows were compared with non-lactating cows. Absence of significant RSAD2, STAT1 and SOCS1 transcript regulation in endometrium is consistent with formerly published analyses that were run with a dairy cattle model of contrasted metabolism with timed AI [53]. Our data support the notion that lactation-induced changes in metabolic status do not seem to significantly compromise the sensor property of endometrium and its ability to response to embryonic signals when implantation occurs.
Based on data generated in sheep, tight regulation of cellular reactive oxygen species (ROS) at the conceptus-endometrium interface appears critical for implantation and pregnancy outcome. SOD1, SOD2 and CAT enzymes have been suggested to control O2− and H2O2 generation as a means of limiting detrimental impact of oxidative and harmful ROS [32]. The present study showed that CAT, SOD1 and SOD2 transcripts are expressed in endometrial CAR and ICAR areas of cyclic and pregnant heifers and SOD2 mRNA levels were significantly up-regulated in ICAR areas of bovine endometrium at Day 20 of pregnancy. Our data are consistent with previous data reporting an increased amount of SOD2 in ovine intercaruncular endometrium at implantation [56]. Consequently, association between conceptus implantation and increased SOD2 gene expression or activity in the endometrium appears to be a conserved feature that could be necessary for the correct progression of pregnancy in ruminants. Interestingly, we showed that increase in SOD2 gene expression is not a consequence of IFNT action, demonstrating that neither SOD2 nor SOD1 or CAT can be classified as interferon-stimulated genes.
Oxidative stress results from the balance between the production of ROS and the capacity of antioxidant mechanisms to neutralize them in tissues and in blood. Continuous production of ROS results from normal metabolic processes, but it is known that this production rate varies with alterations of metabolic demand. In clinically healthy dairy cows, antioxidant status in the plasma compartment has been shown to reflect variations in maternal metabolism, during the periparturient and lactation periods [57,58]. Our findings show that transcript levels of endometrial CAT, SOD1 and SOD2 are similar between pregnant LACT cows and the control pregnancy group represented by inseminated heifers. Interestingly, immediate post calving drying-off leads to lower CAT and SOD1 mRNA levels in endometrial ICAR areas of pregnant DRY cows when compared with pregnant LACT cows. This reduced expression of genes encoding antioxidant enzymes suggests a reduction of ROS and oxidative stress in the endometrium of pregnant cows belonging to the DRY group. Our results provide a solid argument that variations in plasma oxidant status of dry or lactating cows during post-partum take also place in the endometrium. Small perturbations of oxidative stress balance through early fetal life can have profound implications for offspring metabolism and cause diseases in adulthood [31,59]. To understand how modifications in endometrial expression of oxidative stress-related genes impact on conceptus development requires longitudinal studies with comprehensive analyses of the redox environment including markers and enzyme activities at the conceptus-uterus interface when pregnancy establishes.
The forkhead transcription factor FOXL2 has been established as a master gene for folliculogenesis and maintenance of ovarian function in vertebrates [45,60]. Our previous data have shown that FOXL2 transcript and protein are expressed in bovine and ovine endometrium and are regulated by progesterone but not by oestradiol [28,61]. The current study demonstrated a significant increase in FOXL2 transcript and protein expression in endometrial ICAR areas of pregnant dry cows compared with those collected from pregnant lactating cows. Therefore regulation of FOXL2 gene expression appears to be specific to each endometrial area. Compared with our previous report [28], up-regulated expression of FOXL2 was not a consequence of a drop in progesterone circulating concentrations which were similar in the three experimental groups. Identifying molecular regulators of FOXL2 production according to the endometrial cell type in the context of metabolism variations will require complementary experiments.
After the pioneering report of Batista et al. [34] establishing FOXL2-stimulated SOD2 expression in a granulosa tumor cell line (KGN cells), subsequent findings have demonstrated that FOXL2 upregulation promotes granulosa cell accumulation in G1 phase and protects these cells from oxidative damage, notably by stimulating the production of antioxidant molecules [47,62]. Based on these results, we hypothesize a direct link between FOXL2 and oxidative-related factors in the endometrium of postpartum dairy cows displaying distinct metabolic status. In our bovine model, reduced levels of oxidative stress-related gene transcripts did not correlate with a decrease in FOXL2 gene expression. Under our experimental conditions, FOXL2 overexpression did not impact SOD2, SOD1 or CAT transcript levels in primary cultures of endometrial fibroblasts or glandular cells. Although interactions between FOXL2 and oxidative stress regulation cannot be ruled out in the endometrium, this connection is not obvious. A comprehensive approach will be necessary to bring new insights to the biological roles of FOXL2 in the endometrium.
Metabolic disorders have been shown to exert detrimental effects on reproduction as illustrated by impaired uterine receptivity and miscarriage in women [63]. Until recently, dairy cattle have been intensively selected to increase milk production with a significant decline in reproductive performances [64,65] that has been extensively investigated at the level of oocyte and embryo quality [66]. Nevertheless recent published data have shown an increased interest for determining the impact of selection on endometrial physiology of high producing dairy cattle [52,67,68]. These reports have focused on the analysis of endometrial ICAR area, whereas our present data have revealed that expression profiles of a selection of genes are distinct between the endometrial CAR and ICAR areas in the post-partum cow associated with metabolic status. In addition, principal component analyses of gene expression in ICAR areas have also revealed that the group of pregnant DRY cows was distinct from the groups of pregnant LACT cows and HEIF. Consequently, the impact of variations in maternal metabolism appears to be more profound on endometrial areas that contain endometrial glands. Endometrial glands are the main producers of histotroph, critical for conceptus elongation in cattle as well as for successful implantation and progression of pregnancy in mammals [69]. Endometrial cell-specific gene expression and histotroph composition will deserve further studies to shed light on the impact of metabolism on uterine secretions relatively to conceptus development.
Conclusion
The present study has demonstrated that maternal metabolism rather than lactation perturbs endometrial function in cattle. ICAR areas have been identified as the endometrial regions more affected by perturbations in maternal metabolism in post-partum cows. We identified FOXL2 and oxidative stress-related enzymes as genes whose expression was modified by the absence of lactation. Based on our gene expression data in endometrial ICAR areas of these pregnant females, lactating cows were not significantly different from heifers whereas previously published analyses of circulating metabolites (insulin, IGF1, glucose, NEFA) in these same females indicated that lactating cows were distinct from heifers and dry cows during postpartum in the absence of pregnancy [39]. Pregnancy may have brought about modifications in expressed factors and biological functions in the endometrium of lactating cows that were not detected with our candidate gene approach. Certainly more genes and other biological functions in the endometrium are affected, and their identification prompts the need for more comprehensive molecular analyses of this tissue. Considering the driving property of the endometrium on the trajectory of embryo development [13], it appears critical to determine if molecular modifications detected at the endometrial level have an impact on conceptus features that have been shown to react to subtle modifications in dam energy metabolism [5]. These investigations may provide new approaches for nutritional strategies that could alleviate the negative impact of lactation on prenatal programming of adult performances when lactation and pregnancy overlap.
Supporting information
All primers were used at a concentration of 300 nM in a final reaction volume of 15 μl.
(XLSX)
Acknowledgments
We are grateful to Dr Isabelle Hue (INRA, Jouy-en-Josas, France) and graduate students, postdoctoral scientists, and technical staff at UCD and INRA for assistance with sample collection. We are grateful to the staff of the Unité Commune d’Expérimentation Animale (UCEA, INRA, Leudeville, France) for animal management. We thank Drs Eric Pailhoux and Maëlle Pannetier (INRA, Jouy-en-Josas, France) for providing us FOXL2-pSG5 and pSG5 plasmids. We thank Kaïs Al-Gubory for helpful discussions and Philippe Bolifraud (INRA, Jouy-en-Josas, France) for his technical help. We thank the ICE platform (Claudia Bevilacqua and Nicolas Crapart, INRA, Jouy-en-Josas, France) for access to the bioanalyzer.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funded by the European Union Seventh Framework Programme FP7/2007-2013 under grant agreement n° 312097 (‘FECUND’, http://www.fecund-project.eu/) to PL and OS; by UK Biotechnology and Biological Sciences Research Council (BBSRC; BB/I017240/1) to MS. AL, CE and AVC were PhD recipient from the French Ministry for Teaching and Research – Doctoral School ED568 Université Paris Saclay. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Butler WR. Energy balance relationships with follicular development, ovulation and fertility in postpartum dairy cows. Livest Prod Sci. 2003;83: 211–218. http://dx.doi.org/10.1016/S0301-6226(03)00112-X [Google Scholar]
- 2.Wathes DC, Fenwick M, Cheng Z, Bourne N, Llewellyn S, Morris DG, et al. Influence of negative energy balance on cyclicity and fertility in the high producing dairy cow. Theriogenology. 2007;68 doi: 10.1016/j.theriogenology.2007.04.006 [DOI] [PubMed] [Google Scholar]
- 3.Diskin MG, Morris DG. Embryonic and Early Foetal Losses in Cattle and Other Ruminants. Reprod Domest Anim. 2008;43: 260–267. doi: 10.1111/j.1439-0531.2008.01171.x [DOI] [PubMed] [Google Scholar]
- 4.Sartori R, Bastos MR, Wiltbank MC. Factors affecting fertilisation and early embryo quality in single- and superovulated dairy cattle. Reproduction, Fertility and Development. 2010. pp. 151–158. doi: 10.1071/RD09221 [DOI] [PubMed] [Google Scholar]
- 5.Valour D, Degrelle SA, Ponter AA, Giraud-Delville C, Campion E, Guyader-Joly C, et al. Energy and lipid metabolism gene expression of D18 embryos in dairy cows is related to dam physiological status. Physiol Genomics. 2014;46: 39–56. doi: 10.1152/physiolgenomics.00091.2013 [DOI] [PubMed] [Google Scholar]
- 6.Al-Katanani YM, Drost M, Monson RL, Rutledge JJ, Krininger CE, Block J, et al. Pregnancy rates following timed embryo transfer with fresh or vitrified in vitro produced embryos in lactating dairy cows under heat stress conditions. Theriogenology. 2002;58: 171–182. doi: 10.1016/S0093-691X(02)00916-0 [DOI] [PubMed] [Google Scholar]
- 7.Demetrio DGB, Santos RM, Demetrio CGB, Vasconcelos JLM. Factors Affecting Conception Rates Following Artificial Insemination or Embryo Transfer in Lactating Holstein Cows. J Dairy Sci. 2007;90: 5073–5082. doi: 10.3168/jds.2007-0223 [DOI] [PubMed] [Google Scholar]
- 8.Vasconcelos JLM, Demétrio DGB, Santos RM, Chiari JR, Rodrigues CA, Filho OGS. Factors potentially affecting fertility of lactating dairy cow recipients. Theriogenology. 2006;65: 192–200. doi: 10.1016/j.theriogenology.2005.09.030 [DOI] [PubMed] [Google Scholar]
- 9.Hasler JF. Factors affecting frozen and fresh embryo transfer pregnancy rates in cattle. Theriogenology. 2001;56: 1401–1415. doi: 10.1016/S0093-691X(01)00643-4 [DOI] [PubMed] [Google Scholar]
- 10.Chagas e Silva J, Lopes da Costa L, Robalo Silva J. Plasma progesterone profiles and factors affecting embryo-fetal mortality following embryo transfer in dairy cattle. Theriogenology. 2002;58: 51–59. doi: 10.1016/S0093-691X(02)00906-8 [DOI] [PubMed] [Google Scholar]
- 11.Wilson RD, Fricke PM, Leibfried-Rutledge ML, Rutledge JJ, Penfield CMS, Weigel KA. In vitro production of bovine embryos using sex-sorted sperm. Theriogenology. 2006;65: 1007–1015. doi: 10.1016/j.theriogenology.2005.07.007 [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Recio O, Ugarte E, Bach A. Trans-generational effect of maternal lactation during pregnancy: a Holstein cow model. PLoS One. United States; 2012;7: e51816 doi: 10.1371/journal.pone.0051816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandra O, Mansouri-Attia N, Lea RG. Novel aspects of endometrial function: A biological sensor of embryo quality and driver of pregnancy success. Reprod Fertil Dev. 2011;24: 68–79. doi: 10.1071/RD11908 [DOI] [PubMed] [Google Scholar]
- 14.Bazer FW, Wu G, Spencer TE, Johnson GA, Burghardt RC, Bayless K. Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol Hum Reprod. 2010;16: 135–152. doi: 10.1093/molehr/gap095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KY, DeMayo FJ. Animal models of implantation. Reproduction. 2004;128: 679–695. doi: 10.1530/rep.1.00340 [DOI] [PubMed] [Google Scholar]
- 16.Bazer FW, Burghardt RC, Johnson GA, Spencer TE, Wu G. Interferons and progesterone for establishment and maintenance of pregnancy: interactions among novel cell signaling pathways. Reprod Biol. 2008;8: 179–211. doi: 10.1016/S1642-431X(12)60012-6 [DOI] [PubMed] [Google Scholar]
- 17.Roberts RM, Chen Y, Ezashi T, Walker AM. Interferons and the maternal-conceptus dialog in mammals. Seminars in Cell and Developmental Biology. 2008. pp. 170–177. doi: 10.1016/j.semcdb.2007.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spencer TE, Johnson GA, Bazer FW, Burghardt RC. Implantation mechanisms: Insights from the sheep. Reproduction. 2004;128: 657–668. doi: 10.1530/rep.1.00398 [DOI] [PubMed] [Google Scholar]
- 19.Sandra O, Charpigny G, Galio L, Hue I. Preattachment Embryos of Domestic Animals: Insights into Development and Paracrine Secretions. Annu Rev Anim Biosci. United States; 2017;5: 205–228. doi: 10.1146/annurev-animal-022516-022900 [DOI] [PubMed] [Google Scholar]
- 20.Spencer TE, Sandra O, Wolf E. Genes involved in conceptus-endometrial interactions in ruminants: Insights from reductionism and thoughts on holistic approaches. Reproduction. 2008;135: 165–179. doi: 10.1530/REP-07-0327 [DOI] [PubMed] [Google Scholar]
- 21.Spencer TE, Forde N, Lonergan P. Insights into conceptus elongation and establishment of pregnancy in ruminants. Reprod Fertil Dev. 2017;29: 84 doi: 10.1071/RD16359 [DOI] [PubMed] [Google Scholar]
- 22.Carvalho AV, Reinaud P, Forde N, Healey GD, Eozenou C, Giraud-Delville C, et al. SOCS genes expression during physiological and perturbed implantation in bovine endometrium. Reproduction. 2014;148: 545–557. doi: 10.1530/REP-14-0214 [DOI] [PubMed] [Google Scholar]
- 23.Mansouri-Attia N, Aubert J, Reinaud P, Giraud-Delville C, Taghouti G, Galio L, et al. Gene expression profiles of bovine caruncular and intercaruncular endometrium at implantation. Physiol Genomics. 2009;39: 14–27. doi: 10.1152/physiolgenomics.90404.2008 [DOI] [PubMed] [Google Scholar]
- 24.Forde N, Carter F, Fair T, Crowe MA, Evans ACO, Spencer TE, et al. Progesterone-Regulated Changes in Endometrial Gene Expression Contribute to Advanced Conceptus Development in Cattle1. Biol Reprod. 2009;81: 784–794. doi: 10.1095/biolreprod.108.074336 [DOI] [PubMed] [Google Scholar]
- 25.Spencer TE, Forde N, Lonergan P. The role of progesterone and conceptus-derived factors in uterine biology during early pregnancy in ruminants. J Dairy Sci. 2016;99: 5941–5950. doi: 10.3168/jds.2015-10070 [DOI] [PubMed] [Google Scholar]
- 26.Charpigny G, Reinaud P, Tamby JP, Creminon C, Martal J, Maclouf J, et al. Expression of cyclooxygenase-1 and -2 in ovine endometrium during the estrous cycle and early pregnancy. Endocrinology. 1997;138: 2163–2171. doi: 10.1210/endo.138.5.5148 [DOI] [PubMed] [Google Scholar]
- 27.Arosh J a, Parent J, Chapdelaine P, Sirois J, Fortier M a. Expression of cyclooxygenases 1 and 2 and prostaglandin E synthase in bovine endometrial tissue during the estrous cycle. Biol Reprod. 2002;67: 161–169. doi: 10.1095/biolreprod67.1.161 [DOI] [PubMed] [Google Scholar]
- 28.Eozenou C, Carvalho A V., Forde N, Giraud-Delville C, Gall L, Lonergan P, et al. FOXL2 Is Regulated During the Bovine Estrous Cycle and Its Expression in the Endometrium Is Independent of Conceptus-Derived Interferon Tau. Biol Reprod. 2012;87: 32–32. doi: 10.1095/biolreprod.112.101584 [DOI] [PubMed] [Google Scholar]
- 29.Drackley JK. Biology of dairy cows during the transition period: The final frontier? J Dairy Sci. 1999;82: 2259–2273. doi: 10.3168/jds.S0022-0302(99)75474-3 [DOI] [PubMed] [Google Scholar]
- 30.Bernabucci U, Ronchi B, Lacetera N, Nardone A. Influence of Body Condition Score on Relationships Between Metabolic Status and Oxidative Stress in Periparturient Dairy Cows. J Dairy Sci. Elsevier; 2005;88: 2017–2026. doi: 10.3168/jds.S0022-0302(05)72878-2 [DOI] [PubMed] [Google Scholar]
- 31.Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, et al. Maternal Diet-Induced Obesity Alters Mitochondrial Activity and Redox Status in Mouse Oocytes and Zygotes. PLoS One. Public Library of Science; 2010;5: e10074 Available: doi: 10.1371/journal.pone.0010074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Gubory KH, Garrel C. Antioxidative signalling pathways regulate the level of reactive oxygen species at the endometrial-extraembryonic membranes interface during early pregnancy. Int J Biochem Cell Biol. Elsevier Ltd; 2012;44: 1511–1518. doi: 10.1016/j.biocel.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 33.Ramos RS, Oliveira ML, Izaguirry AP, Vargas LM, Soares MB, Mesquita FS, et al. The periovulatory endocrine milieu affects the uterine redox environment in beef cows. Reprod Biol Endocrinol. 2015;13: 39 doi: 10.1186/s12958-015-0036-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batista F, Vaiman D, Dausset J, Fellous M, Veitia R a. Potential targets of FOXL2, a transcription factor involved in craniofacial and follicular development, identified by transcriptomics. Proc Natl Acad Sci U S A. 2007;104: 3330–3335. doi: 10.1073/pnas.0611326104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gray CA, Taylor KM, Ramsey WS, Hill JR, Bazer FW, Bartol FF, et al. Endometrial glands are required for preimplantation conceptus elongation and survival. Biol Reprod. 2001;64: 1608–1613. doi: 10.1095/biolreprod64.6.1608 [DOI] [PubMed] [Google Scholar]
- 36.Biase FH, Rabel C, Guillomot M, Hue I, Andropolis K, Olmstead CA, et al. Massive dysregulation of genes involved in cell signaling and placental development in cloned cattle conceptus and maternal endometrium. Proc Natl Acad Sci. 2016;113: 201520945 doi: 10.1073/pnas.1520945114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mansouri-Attia N, Sandra O, Aubert J, Degrelle S, Everts RE, Giraud-Delville C, et al. Endometrium as an early sensor of in vitro embryo manipulation technologies. Proc Natl Acad Sci U S A. 2009;106: 5687–92. doi: 10.1073/pnas.0812722106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker CG, Meier S, Littlejohn MD, Lehnert K, Roche JR, Mitchell MD. Modulation of the maternal immune system by the pre-implantation embryo. BMC Genomics. 2010;11: 1–13. doi: 10.1186/1471-2164-11-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forde N, O’Gorman A, Whelan H, Duffy P, O’Hara L, Kelly AK, et al. Lactation-induced changes in metabolic status and follicular-fluid metabolomic profile in postpartum dairy cows. Reprod Fertil Dev. 2015;28: 1882–1892. doi: 10.1071/RD14348 [DOI] [PubMed] [Google Scholar]
- 40.Song G, Bazer FW, Spencer TE. Pregnancy and interferon tau regulate RSAD2 and IFIH1 expression in the ovine uterus. Reproduction. England; 2007;133: 285–295. doi: 10.1530/REP-06-0092 [DOI] [PubMed] [Google Scholar]
- 41.Carvalho AV, Eozenou C, Healey GD, Forde N, Reinaud P, Chebrout M, et al. Analysis of STAT1 expression and biological activity reveals interferon-tau-dependent STAT1-regulated SOCS genes in the bovine endometrium. Reprod Fertil Dev. 2016;28: 459–474. doi: 10.1071/RD14034 [DOI] [PubMed] [Google Scholar]
- 42.Carter F, Forde N, Duffy P, Wade M, Fair T, Crowe MA, et al. Effect of increasing progesterone concentration from Day 3 of pregnancy on subsequent embryo survival and development in beef heifers. Reprod Fertil Dev. Australia; 2008;20: 368–375. [DOI] [PubMed] [Google Scholar]
- 43.Degrelle SA, Campion E, Cabau C, Piumi F, Reinaud P, Richard C, et al. Molecular evidence for a critical period in mural trophoblast development in bovine blastocysts. Dev Biol. United States; 2005;288: 448–460. doi: 10.1016/j.ydbio.2005.09.043 [DOI] [PubMed] [Google Scholar]
- 44.Forde N, Carter F, Spencer TE, Bazer FW, Sandra O, Mansouri-Attia N, et al. Conceptus-Induced Changes in the Endometrial Transcriptome: How Soon Does the Cow Know She Is Pregnant?1. Biol Reprod. 2011;85: 144–156. doi: 10.1095/biolreprod.110.090019 [DOI] [PubMed] [Google Scholar]
- 45.Pannetier M, Renault L, Jolivet G, Cotinot C, Pailhoux E. Ovarian-specific expression of a new gene regulated by the goat PIS region and transcribed by a FOXL2 bidirectional promoter. Genomics. 2005;85: 715–726. doi: 10.1016/j.ygeno.2005.02.011 [DOI] [PubMed] [Google Scholar]
- 46.Cronin JG, Turner ML, Goetze L, Bryant CE, Sheldon IM. Toll-like receptor 4 and MYD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium. Biol Reprod. 2012;86: 51 doi: 10.1095/biolreprod.111.092718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larionov A, Krause A, Miller W. A standard curve based method for relative real time PCR data processing. BMC Bioinformatics. 2005;6: 62 doi: 10.1186/1471-2105-6-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mansouri-Attia N, Oliveira LJ, Forde N, Fahey AG, Browne JA, Roche JF, et al. Pivotal role for monocytes/macrophages and dendritic cells in maternal immune response to the developing embryo in cattle. Biol Reprod. 2012. November 29;87(5):123 doi: 10.1095/biolreprod.112.101121 [DOI] [PubMed] [Google Scholar]
- 49.Benayoun BA, Batista F, Auer J, Dipietromaria A, L’Hôte D, De Baere E, et al. Positive and negative feedback regulates the transcription factor FOXL2 in response to cell stress: evidence for a regulatory imbalance induced by disease-causing mutations. Hum Mol Genet. 2009;18: 632–644. Available: http://dx.doi.org/10.1093/hmg/ddn389 [DOI] [PubMed] [Google Scholar]
- 50.Hue I. Determinant molecular markers for peri-gastrulating bovine embryo development. Reproduction, fertility, and development. 2016. pp. 51–65. doi: 10.1071/RD15355 [DOI] [PubMed] [Google Scholar]
- 51.Collard BL, Boettcher PJ, Dekkers JC, Petitclerc D, Schaeffer LR. Relationships between energy balance and health traits of dairy cattle in early lactation. J Dairy Sci. United States; 2000;83: 2683–2690. doi: 10.3168/jds.S0022-0302(00)75162-9 [DOI] [PubMed] [Google Scholar]
- 52.Lucy MC, Beck J, Staples CR, Head HH, De La Sota RL, Thatcher WW. Follicular dynamics, plasma metabolites, hormones and insulin-like growth factor I (IGF-I) in lactating cows with positive or negative energy balance during the preovulatory period. Reprod Nutr Dev. France; 1992;32: 331–341. [DOI] [PubMed] [Google Scholar]
- 53.Cerri RLA, Thompson IM, Kim IH, Ealy AD, Hansen PJ, Staples CR, et al. Effects of lactation and pregnancy on gene expression of endometrium of Holstein cows at day 17 of the estrous cycle or pregnancy. J Dairy Sci. 2012;95: 5657–5675. doi: 10.3168/jds.2011-5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Macklon NS, Brosens JJ. The Human Endometrium as a Sensor of Embryo Quality. Biol Reprod. 2014;91: 1–8. doi: 10.1095/biolreprod.114.122846 [DOI] [PubMed] [Google Scholar]
- 55.Sandra O, Constant F, Vitorino Carvalho A, Eozénou C, Valour D, Mauffré V, et al. Maternal organism and embryo biosensoring: Insights from ruminants. J Reprod Immunol. Elsevier Ireland Ltd; 2015;108: 105–113. doi: 10.1016/j.jri.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 56.Al-Gubory KH, Garrel C, Sugino N, Fowler PA. The conceptus induces a switch in protein expression and activities of superoxide dismutase 1 and 2 in the sheep endometrium during early pregnancy. Small Rumin Res. Elsevier B.V.; 2016;141: 77–83. doi: 10.1016/j.smallrumres.2016.07.006 [Google Scholar]
- 57.Castillo C, Hernandez J, Bravo A, Lopez-Alonso M, Pereira V, Benedito JL. Oxidative status during late pregnancy and early lactation in dairy cows. Vet J. 2005;169: 286–292. doi: 10.1016/j.tvjl.2004.02.001 [DOI] [PubMed] [Google Scholar]
- 58.Omidi A, Fathi MH, Parker MO. Alterations of antioxidant status markers in dairy cows during lactation and in the dry period. J Dairy Res. 2016; 1–5. doi: 10.1017/S0022029916000029 [DOI] [PubMed] [Google Scholar]
- 59.Jarvie E, Hauguel-de-Mouzon S, Nelson SM, Sattar N, Catalano PM, Freeman DJ. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin Sci. 2010;119: 123–129. doi: 10.1042/CS20090640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elzaiat M, Todeschini AL, Caburet S, Veitia RA. The genetic make-up of ovarian development and function: the focus on the transcription factor FOXL2. Clinical Genetics. 2017. pp. 173–182. doi: 10.1111/cge.12862 [DOI] [PubMed] [Google Scholar]
- 61.Eozenou C, Mauffre V, Reinaud P, Camous S, Bolifraud P, Albert JP, Shimizu T, Miyamoto A, Pannetier M, Constant F, Al-Gubory KH SO. FOXL2 is inhibited by progesterone in ruminant endometrium. Society for the Study of Reproduction, Biology of Reproduction. 2013. p. Suppl., pp.337, 2013. doi:<hal-01019549> [Google Scholar]
- 62.Benayoun BA, Georges AB, L'Hôtel D, Andersson N, Dipietromaria A, Todeschini A-LL, et al. Transcription factor FOXL2 protects granulosa cells from stress and delays cell cycle: role of its regulation by the SIRT1 deacetylase. Hum Mol Genet. 2011;20: 1673–1686. doi: 10.1093/hmg/ddr042 [DOI] [PubMed] [Google Scholar]
- 63.Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity’s impact. Fertil Steril. Elsevier; 2017; doi: 10.1016/j.fertnstert.2017.01.017 [DOI] [PubMed] [Google Scholar]
- 64.Friggens NC, Disenhaus C, Petit H V. Nutritional sub-fertility in the dairy cow: towards improved reproductive management through a better biological understanding. Animal. 2010;4: 1197–1213. doi: 10.1017/S1751731109991601 [DOI] [PubMed] [Google Scholar]
- 65.Norman HD, Wright JR, Hubbard SM, Miller RH, Hutchison JL. Reproductive status of Holstein and Jersey cows in the United States. J Dairy Sci. United States; 2009;92: 3517–3528. doi: 10.3168/jds.2008-1768 [DOI] [PubMed] [Google Scholar]
- 66.Leroy JLMR, Valckx SDM, Jordaens L, De Bie J, Desmet KLJ, Van Hoeck V, et al. Nutrition and maternal metabolic health in relation to oocyte and embryo quality: Critical views on what we learned from the dairy cow model. Reproduction, Fertility and Development. 2015. pp. 693–703. doi: 10.1071/RD14363 [DOI] [PubMed] [Google Scholar]
- 67.Thompson IM, Cerri RLA, Kim IH, Ealy AD, Hansen PJ, Staples CR, et al. Effects of lactation and pregnancy on metabolic and hormonal responses and expression of selected conceptus and endometrial genes of Holstein dairy cattle. J Dairy Sci. Elsevier; 2012;95: 5645–5656. doi: 10.3168/jds.2011-5113 [DOI] [PubMed] [Google Scholar]
- 68.Wathes DC, Cheng Z, Chowdhury W, Fenwick MA, Fitzpatrick R, Morris DG, et al. Negative energy balance alters global gene expression and immune responses in the uterus of postpartum dairy cows. Am Physiol Soc. 2009;39: 1–13. doi: 10.1152/physiolgenomics.00064.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spencer TE, Dunlap KA, Filant J. Comparative developmental biology of the uterus: Insights into mechanisms and developmental disruption. Mol Cell Endocrinol. Elsevier Ireland Ltd; 2012;354: 34–53. doi: 10.1016/j.mce.2011.09.035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All primers were used at a concentration of 300 nM in a final reaction volume of 15 μl.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.