Abstract
Diabetic retinopathy (DR) is a leading cause of blindness among working-age adults. Early diagnosis through effective screening programs is likely to improve vision outcomes. The ETDRS seven-standard-field 35-mm stereoscopic color retinal imaging (ETDRS) of the dilated eye is elaborate and requires mydriasis, and is unsuitable for screening. We evaluated an image analysis application for the automated diagnosis of DR from non-mydriatic single-field images. Patients suffering from diabetes for at least 5 years were included if they were 18 years or older. Patients already diagnosed with DR were excluded. Physiologic mydriasis was achieved by placing the subjects in a dark room. Images were captured using a Bosch Mobile Eye Care fundus camera. The images were analyzed by the Retinal Imaging Bosch DR Algorithm for the diagnosis of DR. All subjects also subsequently underwent pharmacological mydriasis and ETDRS imaging. Non-mydriatic and mydriatic images were read by ophthalmologists. The ETDRS readings were used as the gold standard for calculating the sensitivity and specificity for the software. 564 consecutive subjects (1128 eyes) were recruited from six centers in India. Each subject was evaluated at a single outpatient visit. Forty-four of 1128 images (3.9%) could not be read by the algorithm, and were categorized as inconclusive. In four subjects, neither eye provided an acceptable image: these four subjects were excluded from the analysis. This left 560 subjects for analysis (1084 eyes). The algorithm correctly diagnosed 531 of 560 cases. The sensitivity, specificity, and positive and negative predictive values were 91%, 97%, 94%, and 95% respectively. The Bosch DR Algorithm shows favorable sensitivity and specificity in diagnosing DR from non-mydriatic images, and can greatly simplify screening for DR. This also has major implications for telemedicine in the use of screening for retinopathy in patients with diabetes mellitus.
Introduction
Diabetic retinopathy (DR) in time affects nearly all individuals with type I,[1] and most patients with type II diabetes melllitus.[2] DR may progress to blindness; in fact, DR is the leading cause of new blindness among working-age adults[3]. About 4% of persons with early-onset diabetes are blind, and nearly all blindness in this age group is related to the complications of DR.[3] About 2–3% of late-onset diabetics are blind.[4]–in this group about a third of blindness is caused by diabetes.[3]
The risk of DR can be reduced by careful control of blood sugar levels and blood pressure.[5, 6] Early diagnosis of DR through effective screening programs, should, therefore, be expected to improve vision outcomes. DR has a long latent phase, and screening and timely intervention for DR has been shown to be cost-effective when compared with the disability loss of blindness.[7, 8] Indeed, Ferris[9] estimated that blindness would be reduced ten-fold with appropriate early intervention.
Fundus photography is a simple and cost-effective method of making a diagnosis in suspected retinopathy. One of the major advantages is that the photograph can be examined by others, at various locations, much as an X-ray can.[10] The first photographs of the retina were published in 1886, but commercial fundus cameras appeared only forty years later.[11]
The gold standard for grading DR is the expert interpretation of the ETDRS seven-standard-field 35-mm stereoscopic color retinal image (ETDRS) of the dilated eye.[12, 13] Traditional fundus cameras provide excellent pictures, but are typically large, bulky, difficult to use. They are also expensive, and require time-consuming and uncomfortable mydriasis, and are therefore clearly not designed for screening. Since screening for DR is often inadequate, there is a need for easily-available, inexpensive methods for diagnosing the condition.[14] Over the years, progress has been directed towards non-mydriatic photography, simplifying cameras, diagnosis by general practitioners, and automated diagnosis. Non-mydriatic image acquisition methods take less than half the time[15] and, when interpreted by ophthalmologists, show good correlation with the gold standard.[15, 16] Telemedicine has also helped overcome the shortage of qualified ophthalmologists.[17],[18]
Despite these endeavors, screening still may be inadequate.[19, 20] A screening method that does not require trained persons would be expected to significantly improve availability and reduce costs.[21]
We have been working on a medical image analysis application for ophthalmology professionals. This application can capture retinal images and transfer them to a computer (and to a cloud database), and can classify images as healthy, inconclusive, or DR affected. In the last year, we have prospectively evaluated this system for the diagnosis of DR. The objective of the paper is to present the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of this instrument, with respect to 7-field ETDRS imaging.
Methods
The study was a prospective, open-label trial. Non-mydriatic images were captured using the Bosch camera and were evaluated by the Bosch DR Algorithm software for the diagnosis of DR. The results were compared with those obtained by 7-field ETDRS images in the same subjects.
Patients
The study was conducted on patients recruited at one of 6 centers in India (Table 1).
Table 1. Participating centers in India.
| 1 | Padmashree Dr. DY Patil Medical College Hospital and Research Centre, Mumbai, Maharashtra. |
| 2 | JN Medical College, KLE University, Belgavi, Karnataka. |
| 3 | Dr. Virendra Laser, Phaco Surgery Centre Pvt. Ltd., Jaipur, Rajasthan (SEAROC Ethics Committee, Jaipur, Rajasthan). |
| 4 | Sri Sankaradeva Nethralaya, Guwahati, Assam. |
| 5 | Deenanath Mangeshkar Hospital and Research Centre, Pune, Maharashtra. |
| 6 | NKP Salve Institute of Medical Sciences and Lata Mangeshkar Hospital, Nagpur, Maharashtra. |
The study was approved by the ethics committees of each of the participating hospitals and medical centers. For the center at Jaipur, the approval was provided by the ethics committee of the SEAROC Cancer Center, Jaipur. The study was registered on the Clinical Trials Registry–India (CTRI), with the registration number CTRI/2017/01/007709 (the trial protocol can be viewed at the following url: http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=16105&EncHid=&userName=thinki). Informed written consent was taken, and Good Clinical Practice norms were followed at all times.
Male and female patients suffering from type I or type II diabetes mellitus for at least 5 years were included if they were 18 years or older. We excluded patients who were already diagnosed to have DR, and those who had associated intraocular disorders (Table 2).
Table 2. Exclusion criteria.
| Inability or unwillingness to provide an informed consent |
| History of known retinal disease |
| History of intraocular surgery (other than cataract surgery), or of ocular laser or injection treatment for any retinal disease |
| Extremely small pupil that affected image capture, or an opacity or other condition in either eye that precluded good bilateral retinal photography |
| Conjunctivitis, red eye, or any other inflammatory condition with photophobia |
| Gestational diabetes mellitus |
| Inability or unwillingness to provide an informed consent |
Diagnosis of DR
The algorithm diagnosed the eyes as “healthy”, “DR affected” or “inconclusive”.
The investigators who read the images diagnosed the eyes as “DR present” or “DR absent”. They used the American Academy of Ophthalmology guidelines[22] as the standard for making the diagnosis. Any findings falling under the nonproliferative DR or proliferative DR category of ETDRS[12] were categorized as “DR present”.
Fundus image collection
Once subjects were enrolled, a medical history was obtained and a clinical examination carried out. The subjects were placed in a dark room for at least two minutes to achieve physiological mydriasis before they underwent retinal imaging.
A single external fundus image of each eye was acquired using the Bosch nonmydriatic fundus camera. This camera is a physical hand-held device that offers both non-mydriatic and mydriatic modes for faster and accurate detection. We captured non-mydriatic single-field color fundus images with effective spatial resolutions equal or more than 2.6 MP. Images were uploaded to the cloud using the telemedicine software, Medibilder Lite. There were first pre-processed by cropping (to get approximately square images), re-scaled to a lower resolution of 512*512, and then normalized and transferred to the Bosch algorithm for DR evaluation. The results from the algorithm were documented. Investigators were kept blinded from the results.
After the nonmydriatic images were taken, the patients underwent 7-Standard Field stereoscopic Digital Colour Fundus (EDTRS) imaging[12] after mydriasis with tropicamide. These images were evaluated by the investigators.
Deep learning algorithm for DR classification
The fundus images captured from the patients were analyzed by the Bosch DR Algorithm, which performs classification. The image data acquired was processed and the output generated using MediBilder as a user-interface. Medibilder is a DICOM compliant client software for picture archiving and communication systems, capable of image operations such as marking, annotation, and layering. (DICOM is an acronym for Digital Imaging and Communications in Medicine standard for handling, storing, printing, and transmitting information in medical imaging.) This algorithm classifies each fundus image as DR affected, healthy, or inconclusive. The data were recorded into electronic case record forms (CRFs). Fig 1 describes the process flow.
Fig 1. Process flow of subjects.
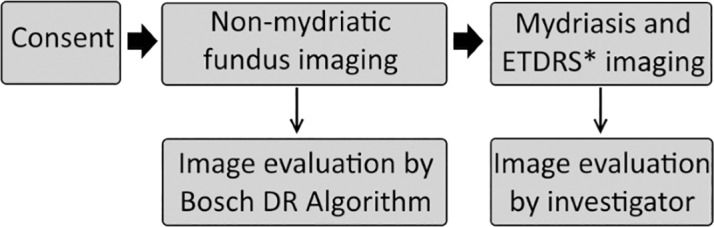
*ETDRS: ETDRS seven-standard-field 35-mm stereoscopic color retinal imaging.
The Bosch DR Algorithm uses deep convolutional neural network to automatically detect whether a fundus image has DR. Deep learning is an artificial intelligence method in which the input image passes through many types of filters in order to automatically extract the best features.[23] The computational models involve numerous convolutional layers, and the automatically generated features have markedly improved the state-of-the-art in visual object recognition.[24] Our deep learning architecture configuration consists of 13 convolutional layers. The optimal number of layers was based on experimental analysis, and we found that increasing the layers did not raise the performance significantly. We have used cross-entropy as the cost function to be minimized. The output layer consists of a single output neuron (for specifying the result of binary classification).
The Bosch algorithm differs from one developed by the Google team[25] in two main ways.
One, the Google network uses a more complex architecture that consists of 11 blocks, and, totally, there are more than 80 convolutional layers. They had used an ensemble of 10 such networks. We have used a different scheme of a single network, which consists of 13 convolutional layers, and yet it provides sufficient accuracy.
Two, in our network, to avoid the problem of vanishing gradients (wherein the gradient update tends to zero), we adopted the technique of adding random noise to the gradients during optimization, as described by Neelakantan et al.[26] This approach also helps to avoid overfitting, and results in lower training loss. On the other hand, this addition of random noise was not found in Google’s paper.
Inconclusive cases: The proportion of inconclusive cases is constrained to be around 10%-20% of the test data by optimizing the thresholds (which is ensured during training).
Training database: For the training phase, we have used a large dataset of nearly 80,000 images. These consist of challenging cases from (i) Open-source data EyePACS-1, that comprises mydriatic and non-mydriatic fundus images, and (ii) About 5000 Bosch Eye Camera images collected from various camps across India, verified by 3 ophthalmologists. The training set was derived from completely different patients, sites, and operators, when compared to the testing images of the actual study. This ensures external validity of the algorithm, as the test set is different.
Sample size calculations and statistics
We calculated the sample size as follows:
N = (Z2 * Sn * (1-Sn))/ (L2 * prevalence), where Z was 1.96 for alpha = 0.05, Sn = sensitivity, set at 0.9 from previous studies, L = margin of error, set at 0.05, and prevalence (of DR in diabetes) set at 0.25 from earlier studies.[27–29] This provided a requirement of 533 subjects, which we rounded off to 550.
Data analysis was carried out using SPSS® version 22. The sensitivity, specificity, PPV, and NPV were calculated for the algorithm using the investigators’ diagnoses on the 7 field ETDRS images as the gold standard. We excluded the cases classified as inconclusive by the algorithm.
Results
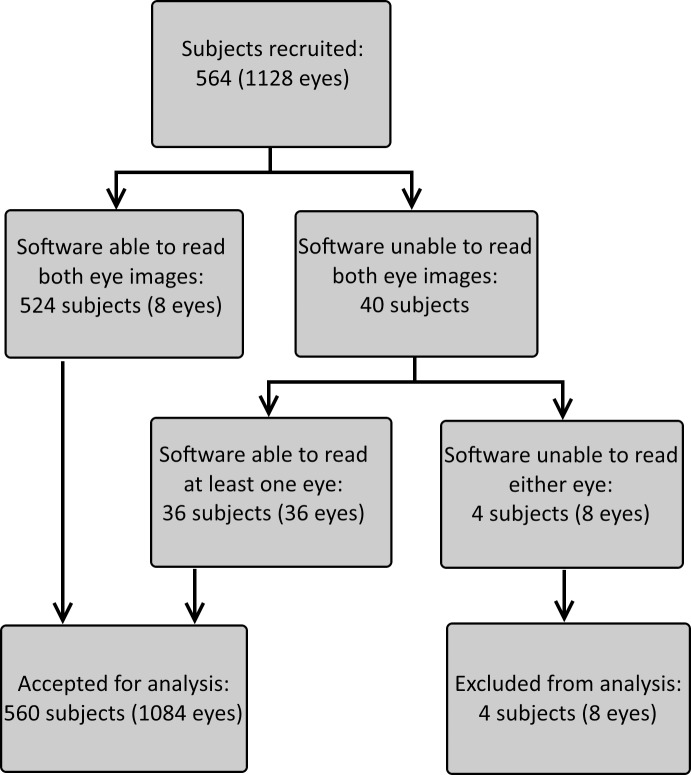
Subjects were recruited between October 2016 and January 2017. The study included 564 subjects, each of whom were evaluated at a single outpatient visit. Of the 1128 eyes studied, 44 images (3.9%) were categorized inconclusive by the algorithm. Eight of these were from the same four subjects, who were excluded because the algorithm categorized both eyes as inconclusive. This left 1084 eyes, from 560 subjects, for analysis (Fig 2). The subjects included 351 males and 209 females, with ages ranging from 20 to 85 years (median 58). They all had diabetes for at least five years.
Fig 2. Flow chart showing recruitment, inclusion, and exclusion of subjects.
Bosch DR Algorithm
The algorithm correctly diagnosed 531 of 560 cases (Table 3). The sensitivity, specificity, PPV, and NPV were 91.18%, 96.9%, 94.4%, and 95.0% respectively.
Table 3. Sensitivity and specificity of the Bosch Dr Algorithm.
| Bosch DR Algorithm | 7-field ETDRS imaging | Result | Cases (total: 560) |
|---|---|---|---|
| Positive | Positive | True positive | 186 |
| Negative | Positive | False negative | 18 |
| Negative | Negative | True negative | 345 |
| Positive | Negative | False positive | 11 |
Sensitivity 91.18% (86.41–94.69), Specificity 96.91% (94.54–98.45), PPV 94.4% (90.42–96.81), NPV 95.0% (92.5–96.75), positive likelihood ratio value 29.51 (16.47; 52.88), negative likelihood ratio value 0.09 (0.06; 0.14). Figures in parentheses represent 95% confidence limits.
Discussion
Non-mydriatic fundus photography became available in the 1980s.[30] It was, naturally, well-tolerated by patients, but the images sometimes lacked clarity, with 5–25% of pictures being unusable. Early studies showed that though the specificity rates were high, sensitivity rates for the diagnosis of DR were low, missing nearly half of cases.[14] Improvement in cameras was driven by the frequent occurrence of unusable images. An excellent review was published by Panwar et al,[11] detailing the technical specifications of fundus cameras for mydriatic and non-mydriatic photography. More recently, cameras have become smaller: indeed, some mobile phones are able to take pictures without the need for mydriasis.[11]
Image quality
In 1084 of 1128 cases the images were of acceptable quality for evaluation (96.1%). This compares favorably with the rates reported in earlier publications, where about 70–95% of non-mydriatic photographs were acceptable.[14, 31–34]
Our software has advantages over previously described work, for example that published recently by Google’s Gulshan and coworkers.[25] Unlike the California-based team, which used an ensemble of 10 networks, we have used a much simpler network and yet obtained comparable performance. Fewer layers require lesser memory and processing power. An additional novelty of our architecture is that we have added noise to the gradients (during optimization). This modification, which is included in the training model, helps us in providing robust results even for lower quality images, with variations such as color and visibility of lesion-affected regions.
The Kaggle diabetic retinopathy challenge[35] released a huge publicly available dataset, wherein several deep learning-based approaches wee proposed to classify different grades of retinopathy. In our work, we only focus on referral/no referral classification for DR versus healthy images. The diagnostic accuracy of computer detection of DR was reported by Abramoff and coworkers,[36] who also provided a detailed clinical study.[37] A detailed survey of DR detection algorithms from fundus images was presented in a review by Mookiah et al,[38] but these dealt with lesion detection-based approaches and considered only much smaller public databases such as DIARETDB1.[39]
Sensitivity and specificity
Non-mydriatic photography is convenient, but sensitivity and specificity have always been a concern.
The best results are obtained when an ophthalmologist reads the images. Reports show sensitivity rates varying from 78%[40] to 96%[41]. Specificity is typically higher, ranging from 86%[40] to 98%[41]. In a small study of 55 subjects, Vujosevic et al[42] reported a sensitivity of 99%, and a specificity of 100%.
Recognizing that an ophthalmologist is not always available in rural areas,[43] healthcare personnel have used trained general practitioners to interpret the images. Castro and coworkers[44] reported sensitivity rates of 67% for non-mydriatic examination, but more recent reports indicate that the sensitivity for diagnosis is between 83–97% when carried out by general practitioners, with most publications reporting rates close to 95%.[45, 46]
Over the years, progress has also been made in identifying retinal lesions using algorithms to diagnose changes in digitized images.[10, 47–49] Abramoff and coworkers[50] evaluated an algorithm-based system for automated detection of DR in retinal photographs. They achieved a sensitivity of 0.84, but a low specificity, and concluded that automated detection showed promise, even if it was not immediately suitable for clinical use. Recently, Besenczi et al,[10] Schuster et al,[51] and Rahim et al[52] have described the components of automated detection programs. The software is able to localize the optic disc and the macula, and distinguish the arteries from the veins.[53] It can detect retinal lesions such as microaneurysms, exudates, and others. Changes such as papilledema,[54, 55] hemorrhages,[56] neovascularization,[57] and exudates[58] can be identified, typically with sensitivity rates approaching 80% and overall accuracy rates greater than 90%.
Putting these programs together, attempts have been made for the automated diagnosis DR from images. Tufail et al[59] stated that the sensitivity of EyeArt, their automated DR image assessment systems (ARIAS) was 94.7%, when compared to manual graders. Unfortunately their program setting appeared to achieve this high sensitivity at the cost of specificity, and the false-positive rate approached 80%. It was also not clear from their papers[59, 60] whether the images were consistently non-mydriatic. Bhaskaranand et al[61] reported similar, but slightly less accurate, results, also using EyeArt. Using mydriasis, Hansen et al,[62] in Kenya, used the Iowa Detection Program and set an optimum balance between sensitivity and specificity, achieving rates of 91% and 70% respectively.
There are few studies that have specifically evaluated the role of automated image analysis of non-mydriatic photography using mydriatic photographs as a standard. Hansen et al,[63] from Denmark, reported a sensitivity of 90% and a specificity of 85.7% in a small group of 83 subjects, and recommended that automated systems could be used effectively for screening. In the present study, using the ophthalmologist’s diagnosis from 7-field ETDRS images as the gold standard, the Bosch DR Algorithm achieved sensitivity, specificity, PPV, and NPV rates of 91%, 96%, 94%, and 95% respectively. The British Diabetic Association recommends that screening programs for DR should reach sensitivity and specificity levels of 80% of higher,[64] and the Bosch DR Algorithm comfortably surpasses this requirement. Our results compare favorably with those obtained by other workers, and we consider that this algorithm can be an effective instrument for screening for DR.
Telemedicine benefits
Despite well-established guidelines for screening, patients often present with DR-related blindness. The cause is probably inadequate screening,[65] and telemedicine with remote interpretation may be an important strategy for tackling this problem. Fortunately, DR is a condition that lends itself easily to the benefits of telemedicine.[66, 67] Studies have repeatedly confirmed the importance of telemedicine as an important screening tool in different parts of the world,[68–70] and it is more effective than traditional surveillance.[71] In a country like India, where a third of the rural population of the needs to travel over 30 kilometers for access to basic medical treatment,[72] “tele-ophthalmology” can be particularly valuable.[73] Non-mydriatic imaging is highly acceptable to patients.[74] The main problems are those of image inconsistency,[75] which highlights the importance of any system that can provide quality images with a low rate of unreadable pictures.
Strengths and weaknesses
This study compares non-mydriatic imaging with an acceptable gold standard. We consider that our other strength is in the large number of subjects, which should provide valid measures of accuracy using this technology.
In our study, a subject was considered for analysis if even one eye had yielded an adequately clear image. In some of the eyes diagnosed as normal, the other eye may well have had evidence of early DR. Further, while the study notes the findings of DR, it would be useful to know how accurate this software is for individual lesions, such as exudates, microaneurysms, and macular edema.
Acknowledgments
We would like to thank Dr. Virendra Agrawal for significant contributions to the study in recruiting subjects. We are also grateful to Mr. Kamal Shahani, Ms. Deepti Goel, and Mr. Shaitan Singh, who managed the entire study, and to Ms. Bhaswati Mukherjee and Mr. Sushant Tyagi who helped with the data management and statistical analysis. We would like to thank Dr.Bhargavi Pawar, consultant ophthalmologist, for providing her expert suggestions and guidance with this project.
We are grateful to Robert Bosch Engineering and Business Solutions Private Limited for funding the study. We would like to extend our gratitude to the members of their technical and management team including, but not limited to, Mr. Girish Haritz and Mr. Thennarasu Palanisamy (Program Managers, Engineering), Drs Kumar Thirunellai Rajamani and Kiran Aatre (Engineering Managers), Dr Sahana Prabhu (Specialist), and Mr. Seetharam Srinivasan (Manager, Supply Chain).
Data Availability
All data files are available from the Harvard Dataverse repository database (10.7910/DVN/E5VNS4), at the following url: http://dx.doi.org/10.7910/DVN/E5VNS4.
Funding Statement
The study was funded by Robert Bosch Engineering and Business Solutions Private Limited. Investigators were given grant per completed subject by Think-i, Unit of Tenet Health Edutech Pvt. Ltd., the CRO conducting the study.
References
- 1.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102(4):520–6. . [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102(4):527–32. . [DOI] [PubMed] [Google Scholar]
- 3.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Diabetic retinopathy. Diabetes Care. 2003;26(1):226–9. . [DOI] [PubMed] [Google Scholar]
- 4.Idil A, Caliskan D, Ocaktan E. The prevalence of blindness and low vision in older onset diabetes mellitus and associated factors: a community-based study. European journal of ophthalmology. 2004;14(4):298–305. . [DOI] [PubMed] [Google Scholar]
- 5.Chew EY. There is level 1 evidence for intensive glycemic control for reducing the progression of diabetic retinopathy in persons with type 2 diabetes. Endocrine. 2015;49(1):1–3. doi: 10.1007/s12020-015-0553-6 . [DOI] [PubMed] [Google Scholar]
- 6.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ (Clinical research ed). 1998;317(7160):703–13. ; PubMed Central PMCID: PMCPMC28659. [PMC free article] [PubMed] [Google Scholar]
- 7.Vashist P, Singh S, Gupta N, Saxena R. Role of early screening for diabetic retinopathy in patients with diabetes mellitus: an overview. Indian J Community Med. 2011;36(4):247–52. doi: 10.4103/0970-0218.91324 ; PubMed Central PMCID: PMCPMC3263142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):766–85. . [PubMed] [Google Scholar]
- 9.Ferris FL 3rd. Results of 20 years of research on the treatment of diabetic retinopathy. Prev Med. 1994;23(5):740–2. doi: 10.1006/pmed.1994.1127 . [DOI] [PubMed] [Google Scholar]
- 10.Besenczi R, Toth J, Hajdu A. A review on automatic analysis techniques for color fundus photographs. Comput Struct Biotechnol J. 2016;14:371–84. doi: 10.1016/j.csbj.2016.10.001 ; PubMed Central PMCID: PMCPMC5072151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panwar N, Huang P, Lee J, Keane PA, Chuan TS, Richhariya A, et al. Fundus Photography in the 21st Century—A Review of Recent Technological Advances and Their Implications for Worldwide Healthcare. Telemedicine journal and e-health: the official journal of the American Telemedicine Association. 2016;22(3):198–208. doi: 10.1089/tmj.2015.0068 ; PubMed Central PMCID: PMCPMC4790203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):786–806. . [PubMed] [Google Scholar]
- 13.Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):823–33. . [PubMed] [Google Scholar]
- 14.Marks JB. Nonmydriatic fundus photography in screening for treatable diabetic retinopathy. Journal of diabetes and its complications. 1992;6(4):247–53. . [DOI] [PubMed] [Google Scholar]
- 15.Aiello LP. Nonmydriatic ultrawide field retinal imaging compared with dilated standard 7-field 35-mm photography and retinal specialist examination for evaluation of diabetic retinopathy. Telemedicine journal and e-health: the official journal of the American Telemedicine Association. 2012;154(3):549–59.e2. Epub 2012/07/26. doi: 10.1089/tmj.2011.0232 . [DOI] [PubMed] [Google Scholar]
- 16.Gupta V, Bansal R, Gupta A, Bhansali A. Sensitivity and specificity of nonmydriatic digital imaging in screening diabetic retinopathy in Indian eyes. Indian J Ophthalmol. 2014;62(8):851–6. doi: 10.4103/0301-4738.141039 ; PubMed Central PMCID: PMCPMC4185162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuypers M. A telemedical approach to the screening of diabetic retinopathy: digital fundus photography. Clinical & experimental ophthalmology. 2000;23(3):345–8. Epub 2000/09/12. . [DOI] [PubMed] [Google Scholar]
- 18.Aiello LP. Screening for diabetic retinopathy: the first telemedicine approach in a primary care setting in Bahrain. American journal of ophthalmology. 2012;19(3):295–8. Epub 2012/05/26. doi: 10.1016/j.ajo.2012.03.019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreton RB, Stratton IM, Chave SJ, Lipinski H, Scanlon PH. Factors determining uptake of diabetic retinopathy screening in Oxfordshire. Diabetic medicine: a journal of the British Diabetic Association. 2017. doi: 10.1111/dme.13350 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovshin JA, Shah BR. Inadequate screening for retinopathy among recent immigrants with type 2 diabetes despite universal health care: A population-based study. Journal of diabetes and its complications. 2017;31(4):664–8. Epub 2017/02/02. doi: 10.1016/j.jdiacomp.2016.12.011 . [DOI] [PubMed] [Google Scholar]
- 21.Sinthanayothin C, Boyce JF, Williamson TH, Cook HL, Mensah E, Lal S, et al. Automated detection of diabetic retinopathy on digital fundus images. Diabetic medicine: a journal of the British Diabetic Association. 2002;19(2):105–12. Epub 2002/03/05. . [DOI] [PubMed] [Google Scholar]
- 22.American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Pattern Guidelines Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology, 2016. Available at www.aao.org/ppp. [Google Scholar]
- 23.Krizhevsky A, Sutskever I, Hinton GE. ImageNet Classification with Deep Convolutional Neural Networks. Advances in Neural Information Processing Systems 25 (NIPS 2012). 2012:1106–14. [Google Scholar]
- 24.LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436–44. doi: 10.1038/nature14539 . [DOI] [PubMed] [Google Scholar]
- 25.Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA. 2016;316(22):2402–10. doi: 10.1001/jama.2016.17216 . [DOI] [PubMed] [Google Scholar]
- 26.Neelakantan A, Vilnis L, Le QV, Sutskever I, Kaiser L, Kurach K, et al. Adding gradient noise improves learning for very deep networks. arXiv preprint arXiv:1511.06807. 2015.
- 27.Raman R, Vaitheeswaran K, Vinita K, Sharma T. Is prevalence of retinopathy related to the age of onset of diabetes? Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Report No. 5. Ophthalmic research. 2011;45(1):36–41. doi: 10.1159/000314720 . [DOI] [PubMed] [Google Scholar]
- 28.Gadkari SS, Maskati QB, Nayak BK. Prevalence of diabetic retinopathy in India: The All India Ophthalmological Society Diabetic Retinopathy Eye Screening Study 2014. Indian J Ophthalmol. 2016;64(1):38–44. doi: 10.4103/0301-4738.178144 ; PubMed Central PMCID: PMCPMC4821119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramavat PR, Ramavat MR, Ghugare BW, Vaishnav RG, Joshi MU. Prevalence of Diabetic Retinopathy in Western Indian Type 2 Diabetic Population: A Hospital—based Cross—Sectional Study. J Clin Diagn Res. 2013;7(7):1387–90. doi: 10.7860/JCDR/2013/5259.3146 ; PubMed Central PMCID: PMCPMC3749641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryder RE, Vora JP, Atiea JA, Owens DR, Hayes TM, Young S. Possible new method to improve detection of diabetic retinopathy: Polaroid non-mydriatic retinal photography. Br Med J (Clin Res Ed). 1985;291(6504):1256–7. ; PubMed Central PMCID: PMCPMC1417105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chin EK, Ventura BV, See KY, Seibles J, Park SS. Nonmydriatic fundus photography for teleophthalmology diabetic retinopathy screening in rural and urban clinics. Telemedicine journal and e-health: the official journal of the American Telemedicine Association. 2014;20(2):102–8. doi: 10.1089/tmj.2013.0042 ; PubMed Central PMCID: PMCPMC3910561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouyang Y, Heussen FM, Keane PA, Sadda SR, Walsh AC. The retinal disease screening study: retrospective comparison of nonmydriatic fundus photography and three-dimensional optical coherence tomography for detection of retinal irregularities. Invest Ophthalmol Vis Sci. 2013;54(8):5694–700. doi: 10.1167/iovs.13-12043 . [DOI] [PubMed] [Google Scholar]
- 33.Bruce BB, Thulasi P, Fraser CL, Keadey MT, Ward A, Heilpern KL, et al. Diagnostic accuracy and use of nonmydriatic ocular fundus photography by emergency physicians: phase II of the FOTO-ED study. Annals of emergency medicine. 2013;62(1):28–33 e1. doi: 10.1016/j.annemergmed.2013.01.010 ; PubMed Central PMCID: PMCPMC3722897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raman R, Rani PK, Mahajan S, Paul P, Gnanamoorthy P, Krishna MS, et al. The tele-screening model for diabetic retinopathy: evaluating the influence of mydriasis on the gradability of a single-field 45 degrees digital fundus image. Telemedicine journal and e-health: the official journal of the American Telemedicine Association. 2007;13(5):597–602. doi: 10.1089/tmj.2006.0084 . [DOI] [PubMed] [Google Scholar]
- 35.Diabetic Retinopathy Detection: Kaggle; [updated 2017]. Available from: https://www.kaggle.com/c/diabetic-retinopathy-detection.
- 36.Abramoff MD, Reinhardt JM, Russell SR, Folk JC, Mahajan VB, Niemeijer M, et al. Automated early detection of diabetic retinopathy. Ophthalmology. 2010;117(6):1147–54. doi: 10.1016/j.ophtha.2010.03.046 ; PubMed Central PMCID: PMCPMC2881172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abramoff MD, Folk JC, Han DP, Walker JD, Williams DF, Russell SR, et al. Automated analysis of retinal images for detection of referable diabetic retinopathy. JAMA Ophthalmol. 2013;131(3):351–7. doi: 10.1001/jamaophthalmol.2013.1743 . [DOI] [PubMed] [Google Scholar]
- 38.Mookiah MR, Acharya UR, Chua CK, Lim CM, Ng EY, Laude A. Computer-aided diagnosis of diabetic retinopathy: a review. Comput Biol Med. 2013;43(12):2136–55. doi: 10.1016/j.compbiomed.2013.10.007 . [DOI] [PubMed] [Google Scholar]
- 39.Kauppi T, Kalesnykiene V, Kamarainen J-K, Lensu L, Sorri I, Raninen A, et al. DIARETDB1—Standard Diabetic Retinopathy Database: Imageret; 2007. [11 August 2017]. Available from: http://www2.it.lut.fi/project/imageret/diaretdb1/. [Google Scholar]
- 40.Lin DY, Blumenkranz MS, Brothers RJ, Grosvenor DM. The sensitivity and specificity of single-field nonmydriatic monochromatic digital fundus photography with remote image interpretation for diabetic retinopathy screening: a comparison with ophthalmoscopy and standardized mydriatic color photography. American journal of ophthalmology. 2002;134(2):204–13. . [DOI] [PubMed] [Google Scholar]
- 41.Williams R, Nussey S, Humphry R, Thompson G. Assessment of non-mydriatic fundus photography in detection of diabetic retinopathy. Br Med J (Clin Res Ed). 1986;293(6555):1140–2. ; PubMed Central PMCID: PMCPMC1341851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vujosevic S, Benetti E, Massignan F, Pilotto E, Varano M, Cavarzeran F, et al. Screening for diabetic retinopathy: 1 and 3 nonmydriatic 45-degree digital fundus photographs vs 7 standard early treatment diabetic retinopathy study fields. American journal of ophthalmology. 2009;148(1):111–8. doi: 10.1016/j.ajo.2009.02.031 . [DOI] [PubMed] [Google Scholar]
- 43.Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic epidemiology. 2007;14(4):179–83. doi: 10.1080/09286580701396720 . [DOI] [PubMed] [Google Scholar]
- 44.Castro AF, Silva-Turnes JC, Gonzalez F. Evaluation of retinal digital images by a general practitioner. Telemedicine journal and e-health: the official journal of the American Telemedicine Association. 2007;13(3):287–92. doi: 10.1089/tmj.2006.0046 . [DOI] [PubMed] [Google Scholar]
- 45.Perez-de-Arcelus M, Andonegui J, Serrano L, Eguzkiza A, Maya JR. Diabetic retinopathy screening by general practitioners using non-mydriatic retinography. Current diabetes reviews. 2013;9(1):2–6. Epub 2012/09/15. . [PubMed] [Google Scholar]
- 46.Farley TF, Mandava N, Prall FR, Carsky C. Accuracy of primary care clinicians in screening for diabetic retinopathy using single-image retinal photography. Ann Fam Med. 2008;6(5):428–34. doi: 10.1370/afm.857 ; PubMed Central PMCID: PMCPMC2532778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoover A, Goldbaum M. Locating the optic nerve in a retinal image using the fuzzy convergence of the blood vessels. IEEE Trans Med Imaging. 2003;22(8):951–8. doi: 10.1109/TMI.2003.815900 . [DOI] [PubMed] [Google Scholar]
- 48.Hoover A, Kouznetsova V, Goldbaum M. Locating blood vessels in retinal images by piecewise threshold probing of a matched filter response. IEEE Trans Med Imaging. 2000;19(3):203–10. doi: 10.1109/42.845178 . [DOI] [PubMed] [Google Scholar]
- 49.Hipwell JH, Strachan F, Olson JA, McHardy KC, Sharp PF, Forrester JV. Automated detection of microaneurysms in digital red-free photographs: a diabetic retinopathy screening tool. Diabetic medicine: a journal of the British Diabetic Association. 2000;17(8):588–94. . [DOI] [PubMed] [Google Scholar]
- 50.Abramoff MD, Niemeijer M, Suttorp-Schulten MS, Viergever MA, Russell SR, van Ginneken B. Evaluation of a system for automatic detection of diabetic retinopathy from color fundus photographs in a large population of patients with diabetes. Diabetes Care. 2008;31(2):193–8. doi: 10.2337/dc08-0952 ; PubMed Central PMCID: PMCPMC2494619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuster AK, Fischer JE, Vossmerbaeumer U. Semi-automated retinal vessel analysis in nonmydriatic fundus photography. Acta ophthalmologica. 2014;92(1):e42–9. doi: 10.1111/aos.12242 . [DOI] [PubMed] [Google Scholar]
- 52.Rahim SS, Palade V, Shuttleworth J, Jayne C. Automatic screening and classification of diabetic retinopathy and maculopathy using fuzzy image processing. Brain Inform. 2016;3(4):249–67. doi: 10.1007/s40708-016-0045-3 ; PubMed Central PMCID: PMCPMC5106407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Y, Liu Y, Wu X, Harding SP, Zheng Y. Retinal vessel segmentation: an efficient graph cut approach with retinex and local phase. PLoS One. 2015;10(4):e0122332 doi: 10.1371/journal.pone.0122332 ; PubMed Central PMCID: PMCPMC4382050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akbar S, Akram MU, Sharif M, Tariq A, Yasin UU. Decision Support System for Detection of Papilledema through Fundus Retinal Images. J Med Syst. 2017;41(4):66 doi: 10.1007/s10916-017-0712-9 . [DOI] [PubMed] [Google Scholar]
- 55.Fatima KN, Hassan T, Akram MU, Akhtar M, Butt WH. Fully automated diagnosis of papilledema through robust extraction of vascular patterns and ocular pathology from fundus photographs. Biomed Opt Express. 2017;8(2):1005–24. doi: 10.1364/BOE.8.001005 ; PubMed Central PMCID: PMCPMC5330576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roychowdhury S. Classification of large-scale fundus image data sets: a cloud-computing framework. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:3256–9. doi: 10.1109/EMBC.2016.7591423 . [DOI] [PubMed] [Google Scholar]
- 57.Roychowdhury S, Koozekanani DD, Parhi KK. Automated detection of neovascularization for proliferative diabetic retinopathy screening. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:1300–3. Epub 2017/03/09. doi: 10.1109/EMBC.2016.7590945 . [DOI] [PubMed] [Google Scholar]
- 58.Jaafar HF, Nandi AK, Al-Nuaimy W. Decision support system for the detection and grading of hard exudates from color fundus photographs. J Biomed Opt. 2011;16(11):116001 doi: 10.1117/1.3643719 . [DOI] [PubMed] [Google Scholar]
- 59.Tufail A, Rudisill C, Egan C, Kapetanakis VV, Salas-Vega S, Owen CG, et al. Automated Diabetic Retinopathy Image Assessment Software: Diagnostic Accuracy and Cost-Effectiveness Compared with Human Graders. Ophthalmology. 2017;124(3):343–51. doi: 10.1016/j.ophtha.2016.11.014 . [DOI] [PubMed] [Google Scholar]
- 60.Kapetanakis VV, Rudnicka AR, Liew G, Owen CG, Lee A, Louw V, et al. A study of whether automated Diabetic Retinopathy Image Assessment could replace manual grading steps in the English National Screening Programme. J Med Screen. 2015;22(3):112–8. doi: 10.1177/0969141315571953 . [DOI] [PubMed] [Google Scholar]
- 61.Bhaskaranand M, Ramachandra C, Bhat S, Cuadros J, Nittala MG, Sadda S, et al. Automated Diabetic Retinopathy Screening and Monitoring Using Retinal Fundus Image Analysis. Journal of diabetes science and technology. 2016;10(2):254–61. doi: 10.1177/1932296816628546 ; PubMed Central PMCID: PMCPMC4773978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hansen MB, Abramoff MD, Folk JC, Mathenge W, Bastawrous A, Peto T. Results of Automated Retinal Image Analysis for Detection of Diabetic Retinopathy from the Nakuru Study, Kenya. PLoS One. 2015;10(10):e0139148 doi: 10.1371/journal.pone.0139148 ; PubMed Central PMCID: PMCPMC4591009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hansen AB, Hartvig NV, Jensen MS, Borch-Johnsen K, Lund-Andersen H, Larsen M. Diabetic retinopathy screening using digital non-mydriatic fundus photography and automated image analysis. Acta ophthalmologica Scandinavica. 2004;82(6):666–72. doi: 10.1111/j.1600-0420.2004.00350.x . [DOI] [PubMed] [Google Scholar]
- 64.Squirrell DM, Talbot JF. Screening for diabetic retinopathy. J R Soc Med. 2003;96(6):273–6. ; PubMed Central PMCID: PMCPMC539506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garg S. Diabetic Retinopathy Screening With Telemedicine: A Potential Strategy to Engage Our Youth. JAMA Ophthalmol. 2017. doi: 10.1001/jamaophthalmol.2017.0150 . [DOI] [PubMed] [Google Scholar]
- 66.Das T, Raman R, Ramasamy K, Rani PK. Telemedicine in diabetic retinopathy: current status and future directions. Middle East African journal of ophthalmology. 2015;22(2):174–8. doi: 10.4103/0974-9233.154391 ; PubMed Central PMCID: PMCPMC4411613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Al Alawi E, Ahmed AA. Screening for diabetic retinopathy: the first telemedicine approach in a primary care setting in Bahrain. Middle East African journal of ophthalmology. 2012;19(3):295–8. doi: 10.4103/0974-9233.97928 ; PubMed Central PMCID: PMCPMC3401798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vujosevic S, Midena E. Diabetic Retinopathy in Italy: Epidemiology Data and Telemedicine Screening Programs. Journal of diabetes research. 2016;2016:3627465 doi: 10.1155/2016/3627465 ; PubMed Central PMCID: PMCPMC5136623 publication of this paper. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eszes DJ, Szabo DJ, Russell G, Kirby P, Paulik E, Nagymajtenyi L, et al. Diabetic Retinopathy Screening Using Telemedicine Tools: Pilot Study in Hungary. Journal of diabetes research. 2016;2016:4529824 doi: 10.1155/2016/4529824 ; PubMed Central PMCID: PMCPMC5204085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nguyen HV, Tan GS, Tapp RJ, Mital S, Ting DS, Wong HT, et al. Cost-effectiveness of a National Telemedicine Diabetic Retinopathy Screening Program in Singapore. Ophthalmology. 2016;123(12):2571–80. doi: 10.1016/j.ophtha.2016.08.021 . [DOI] [PubMed] [Google Scholar]
- 71.Mansberger SL, Gleitsmann K, Gardiner S, Sheppler C, Demirel S, Wooten K, et al. Comparing the effectiveness of telemedicine and traditional surveillance in providing diabetic retinopathy screening examinations: a randomized controlled trial. Telemedicine journal and e-health: the official journal of the American Telemedicine Association. 2013;19(12):942–8. doi: 10.1089/tmj.2012.0313 ; PubMed Central PMCID: PMCPMC3850428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Issac R, Sahasranamam S. Tele-consulting through rural health centres for tribal community—a case study from Wayanad 2014. [11 April 2017]. Available from: http://ieeexplore.ieee.org/stamp/stamp.jsp?arnumber=6970261. [Google Scholar]
- 73.Das T, Pappuru RR. Telemedicine in diabetic retinopathy: Access to rural India. Indian J Ophthalmol. 2016;64(1):84–6. doi: 10.4103/0301-4738.178151 ; PubMed Central PMCID: PMCPMC4821127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boucher MC, Nguyen QT, Angioi K. Mass community screening for diabetic retinopathy using a nonmydriatic camera with telemedicine. Canadian journal of ophthalmology Journal canadien d'ophtalmologie. 2005;40(6):734–42. doi: 10.1016/S0008-4182(05)80091-2 . [DOI] [PubMed] [Google Scholar]
- 75.Choremis J, Chow DR. Use of telemedicine in screening for diabetic retinopathy. Canadian journal of ophthalmology Journal canadien d'ophtalmologie. 2003;38(7):575–9. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data files are available from the Harvard Dataverse repository database (10.7910/DVN/E5VNS4), at the following url: http://dx.doi.org/10.7910/DVN/E5VNS4.