Abstract
Earth's future carbon balance and regional carbon exchange dynamics are inextricably linked to plant photosynthesis. Spectral vegetation indices are widely used as proxies for vegetation greenness and to estimate state variables such as vegetation cover and leaf area index. However, the capacity of green leaves to take up carbon can change throughout the season. We quantify photosynthetic capacity as the maximum rate of RuBP carboxylation (Vcmax) and regeneration (Jmax). Vcmax and Jmax vary within-season due to interactions between ontogenetic processes and meteorological variables. Remote sensing-based estimation of Vcmax and Jmax using leaf reflectance spectra is promising, but temporal variation in relationships between these key determinants of photosynthetic capacity, leaf reflectance spectra, and the models that link these variables has not been evaluated. To address this issue, we studied hybrid poplar (Populus spp.) during a 7-week mid-summer period to quantify seasonally-dynamic relationships between Vcmax, Jmax, and leaf spectra. We compared in situ estimates of Vcmax and Jmax from gas exchange measurements to estimates of Vcmax and Jmax derived from partial least squares regression (PLSR) and fresh-leaf reflectance spectroscopy. PLSR models were robust despite dynamic temporal variation in Vcmax and Jmax throughout the study period. Within-population variation in plant stress modestly reduced PLSR model predictive capacity. Hyperspectral vegetation indices were well-correlated to Vcmax and Jmax, including the widely-used Normalized Difference Vegetation Index. Our results show that hyperspectral estimation of plant physiological traits using PLSR may be robust to temporal variation. Additionally, hyperspectral vegetation indices may be sufficient to detect temporal changes in photosynthetic capacity in contexts similar to those studied here. Overall, our results highlight the potential for hyperspectral remote sensing to estimate determinants of photosynthetic capacity during periods with dynamic temporal variations related to seasonality and plant stress, thereby improving estimates of plant productivity and characterization of the associated carbon budget.
Introduction
Photosynthesis by land plants plays a critical role in regional and global carbon balance [1–3]. Globally, photosynthesis in the terrestrial biosphere, combined with photosynthetic and non-photosynthetic processes in the oceans, offsets 45% of anthropogenic carbon emissions annually [4]. Terrestrial carbon uptake by plants varies annually and seasonally based on climate conditions [5–9], water and nutrient availability [10,11], and plant physiological properties such as water use efficiency [12] and photosynthetic capacity [13]. The size and future of the terrestrial carbon sink remains a critical uncertainty in global climate models [14]. To accurately predict terrestrial carbon uptake, improved quantification of spatial and temporal variation in photosynthesis is necessary.
Spatial and temporal variation in plant photosynthesis can be estimated using remote sensing-derived spectral indices. Spectral estimates of green vegetation, including vegetation indices such as the Normalized Difference Vegetation Index (NDVI), are widely used to estimate photosynthesis and vegetation productivity across spatial and temporal scales [15–18]. Estimates of gross primary productivity derived from greenness measures rely on relationships between vegetation use of light energy and photosynthesis [19–22]. However, the capacity of green leaves to use absorbed light to convert CO2 into biomass varies dynamically throughout the season and with plant stress [23–26]. Many terrestrial biosphere models use static values of determinants of photosynthetic capacity [27]; however, there is some evidence that allowing photosynthetic capacity to vary temporally could improve representation of carbon dynamics [28]. The photochemical reflectance index (PRI) is a hyperspectral vegetation index that detects diurnal changes in xanthophyll cycle activity and responds to seasonal shifts in leaf pigment concentrations [29]. PRI is used to estimate photosynthetic light-use efficiency [30]; however, its relationship to photosynthetic capacity is unclear. Spectral methods that capture dynamic temporal changes in photosynthetic capacity could yield more accurate estimates of vegetation productivity and associated carbon uptake.
Photosynthetic capacity represents the potential of vegetation to fix CO2 under optimal light and water conditions. In this study, we estimate photosynthetic capacity through measurements of the maximum rate of carboxylation of RuBP by the enzyme rubisco (Vcmax) and the maximum rate of electron transport driving RuBP regeneration (Jmax)[31]. Some terrestrial biosphere models treat Vcmax and Jmax as fixed parameters [27]. However, these photosynthetic parameters vary in response to climate conditions [32–35], atmospheric CO2 concentrations [36], plant stress [37], and seasonally in response to ontogenetic processes [38–40]. This seasonal variation can be important, given that process-based biosphere models that account for within-season variation in photosynthetic capacity show improved predictions of carbon flux dynamics [28].
Although hyperspectral remote sensing shows promise for predicting photosynthetic capacity based on leaf optical properties, questions remain regarding temporal variability. Estimates of photosynthetic capacity from remote sensing methods are desirable because of their potential to map Vcmax and Jmax across space and constrain terrestrial biosphere model estimates of plant function. Predictive models of photosynthetic capacity, conditioned based on leaf reflectance metrics, have been developed using partial least squares regression (PLSR) for multiple tree species across glasshouse temperature regimes [29], in C4 crop species[41], and in C3 species at the canopy level [33]. Emergent studies on crop species using high-throughput phenotyping approaches further justify a better understanding of hyperspectral characterization of photosynthetic capacity [41–43]. Still unknown is whether hyperspectral methods of estimating Vcmax and Jmax are robust to temporal variation in these key determinants of photosynthetic capacity. Hyperspectral leaf reflectance correlates with leaf characteristics likely to cause seasonal variation in photosynthetic capacity such as chlorophyll content [44], nitrogen content [45], light-use efficiency [46], and water status [47], while spectral vegetation indices capture some but not all of these factors. However, previous studies have generally used variation in reflectance and plant function across space rather than time to derive these relationships so it remains unknown whether hyperspectral methods of estimating Vcmax and Jmax are robust to temporal variation in these key determinants of photosynthetic capacity. We hypothesize that if seasonal variation in photosynthetic capacity is caused by a combination of leaf changes detectable by reflectance in bands outside of narrowband multispectral bands, then PLSR models that utilize the full reflectance spectrum will predict seasonal changes in Vcmax and Jmax.
In this study, we evaluated the ability of hyperspectral data to represent and predict within-season temporal variation in Vcmax and Jmax and examined the influence of water stress on the robustness of these estimates of photosynthetic capacity. We compared leaf reflectance spectra from hybrid poplar (Populus spp.) to Vcmax and Jmax estimates throughout a 7-week period in the middle part of the growing season. We discuss our results in the context of emerging hyperspectral remote sensing methods and terrestrial biosphere models of global carbon dynamics.
Methods
Experimental site
Our study was conducted using poplar trees, grown outdoors at the Biosphere 2 Research Center near Oracle, AZ, USA (32° 34’ 51” N 110° 50’ 57” W; 1189 m). Biosphere 2 is leased to the University of Arizona. Authors RKM, DJPM, and GABG were responsible for the poplar stand at Biosphere 2. No additional permission was required to carry out this study, which did not involve endangered or protected species. We studied the relationship between spectral reflectance and photosynthetic capacity in 12 individual Populus deltoides hybrid poplar trees. Trees were planted in random arrangement with 1 x 1 m spacing in January 2013. Each year during the dormant season, the trees were coppiced and destructively sampled for biomass. Study trees were randomly selected before the start of the experiment in May 2016. During the study period (5/24/2016–7/5/2016), the mean high temperature was 34.4°C and the mean low temperature was 21.3°C. We applied 38 liters of water per day per tree during the pre-dawn period for 2 weeks prior to the start of the experiment (5/1/2016–5/14/2016) using an irrigation system to begin the study in well-watered conditions. We also fertilized the trees on 5/14/2016 using tree and shrub food (Arizona’s Best) to ensure the trees were not nutrient-limited at the start of the induced dry-down. The trees were exposed to ambient climate conditions and had no irrigation except for supplemental watering from 6/2/2016 through 6/6/2016 and additional watering on 6/20/2016, which avoided senescence and caused variance in the water status of the trees. On sampling dates, we measured predawn leaf water potential (Ψpd), leaf gas exchange, and hyperspectral leaf reflectance for each tree. The full suite of measurements was conducted on all 12 trees on consecutive sampling dates (6 trees per day), except for 6/30 when we measured all 12 trees in one day.
Predawn leaf water potential and A/Ci curves
Predawn leaf water potential (Ψpd) was measured using a pressure chamber (PMS Instruments, Albany, OR, USA). Leaves were collected before sunrise, transported to the lab in a cooler, and measured within 30 minutes of collection. One leaf per plant was measured per time point.
Leaf gas exchange was measured with two LI-COR portable photosynthesis systems (LI-COR Biosciences, Lincoln, NE, USA) equipped with a 6400-02B LED light source. Gas exchange measurements were performed on the youngest, most fully-expanded leaf on the south-facing side of each tree. Leaves were acclimated to the chamber at 25°C, a chamber-air CO2 concentration of 400 ppm, and a saturated photosynthetic photon flux density (PPFD) of 1800μmol m-2 s-1 until the photosynthetic rate (A) stabilized. Gas exchange curves were conducted only on leaves with an initial A ≥ 10 μmol CO2 m-2 s-1 to ensure that the leaves were active enough to yield appropriate estimates for photosynthetic capacity. Each curve consisted of 13 different intercellular CO2 concentrations (Ci) starting at the ambient CO2 concentration of 400 ppm and then decreasing to 300, 200, 100, 50 to 0 ppm before increasing to 400, 400, 600, 800, 1200, 1600, 2000 ppm. We used the Predictive Ecosystem Analyzer (PEcAn) photosynthesis package to perform quality control of CO2 response data before fitting A/Ci curves (https://github.com/PecanProject/pecan). In total, 86 CO2 response curves passed quality control. We fit A/Ci curves using the ‘fitaci’ function in ‘plantecophys’ package in R [48]. The ‘fitaci’ function fits the Farquhar-Berry-Von Caemmerer Model of leaf photosynthesis [31] to measurements of photosynthesis and intercellular CO2 and estimates Vcmax and Jmax along with their standard errors.
Hyperspectral measurements
Reflectance was measured on the same leaves as the gas exchange measurements using a high-spectral resolution ASD FieldSpec® 3 Full-Range (350–2500 nm) spectroradiometer (Analytical Spectral Devices, Boulder, CO, USA). Reflectance measurements were taken with a leaf-clip assembly containing an internal calibrated light source and a black background panel face. The relative leaf reflectance data were standardized prior to measurements of each leaf by measuring a standard white reference reflectance target. ViewSpec Pro® software was used to convert binary data to ASCII data. The spectral resolution is 3nm at 700 nm, 10nm at 1400 and 2100nm across the full spectrum. The hyperspectral data are sampled at every 1nm. The spectroradiometer was turned on for at least 30 minutes before reflectance measurements were taken. The white panel reference reflectance was captured every 2–5 minutes. All measurements were taken from the leaf adaxial surface, avoiding the midrib. Three reflectance measurements were obtained on three different areas of each leaf lamina, resulting in nine spectra per leaf. Each measurement required no more than 5 s. Spectral reflectance measurements were quality-checked by removing negative reflectance values. We then averaged the nine spectra to determine mean optical properties for each leaf. Measures of leaf optical properties generally occurred between 10:30am and 11:30am and followed gas exchange measurements by no more than 2 hours.
Partial least squares regression models
Partial least-squares regression (PLSR) was performed to generate predictive models using the package ‘PLS’ in R [49]. PLSR is a multivariate regression method commonly used in spectroscopy because it can account for many related predictor variables and relatively few observations. PLSR identifies key components that explain variation in a trait variable and generates a linear model to transform full-spectrum data based on these components. The package ‘PLSROpt’ in R was used for pre-processing the spectral data in the order of standard normal variate, a second-derivative Savitzky-Golay smoother, auto-scaling, and mean centering (https://github.com/uwadaira/plsropt). The model with the number of components that minimized the Root Mean Squared Error of Prediction (RMSEP) was selected as the most parsimonious PLSR model. Each PLSR model was generated independently for Vcmax and for Jmax. The spectrum range for all models was 450–2500 nm. Performance parameters were generated to assess the predictive ability of each model including the coefficient of determination (R2).
Three different evaluations of the PLSR model were performed in increasing order of statistical rigor. The first test was a "leave-one-out" cross-validation approach, which trains the model on all but one observation, and then makes a prediction based on the single remaining observation [50]. The second test was performed with a 20% holdout dataset; a random 80/20% split of the data divided it into a training and testing dataset, respectively. New PLSR models were generated based on calibration of the training dataset and validated based on the remaining 20%. A 100x cross validation of training/calibration splits was performed to assess model and data stability across different proportions of testing and training data (S1 Fig). The third and most rigorous test of model stability was testing the model on the sampling dates with greatest variation in the population in terms of water stress (Ψpd). The two consecutive sampling dates with the largest individual variation in Ψpd, (6/23/2016 and 6/24/2016, n = 12) were held out. The model was trained on all data except for the holdout dataset and was tested on the 12 observations with large variation in Ψpd. The purpose of this test was to assess model predictive capacity in a situation with large within-population variation in environmental stress. For each PLSR model, Selectivity Ratio (SR) scores were calculated to enable comparison of the relative significance of each wavelength in its contribution to the final model. Although the Variable Importance of the Projection (VIP) score is more widely used in the current scientific literature, SR has been found to be more reliable for model predictions and is thus presented in this study [51].
Spectral vegetation indices
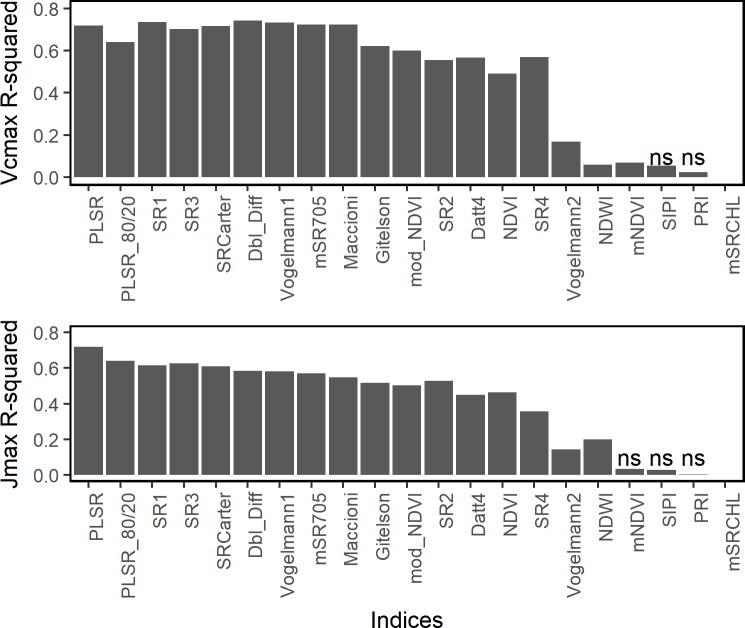
Spectral vegetation indices for estimating chlorophyll content, water stress, and carotenoid pigments were tested using the full set of observations (Table 1); these are all published indices used to estimate plant physiological status. The Normalized Difference Water Index (NDWI) was tested based on its correlation with plant water content in conifers [47], and PRI was tested based on known relationships with photosynthetic functioning [52,53] and environmental stress [54,55]. We also calculated ‘MODIS-like’ NDVI for all spectra. MODIS (Moderate Resolution Imaging Spectroradiometer) NDVI uses the red band (band 1; 620–670 nm) and the near-infrared (NIR) band (band 2; 841–876 nm). For both the red and NIR, we calculated the full-width- half-maximum for the subset of wavelengths corresponding to the MODIS bandwidths using the ‘peakshape’ function in the ‘pavo’ package [56]. We then took the mean of all reflectance values between the endpoints of the width of the spectrum curve. We then applied the standard NDVI equation (see Table 1) to get a ‘MODIS-like’ NDVI. Pairwise correlations between Vcmax and Jmax estimated using standard gas exchange techniques and spectral indices were tested and R2 reported.
Table 1. Hyperspectral vegetation indices that were compared to estimated Vcmax and Jmax values.
| Index | Formula | Reference | R2 Vcmax | R2 Jmax |
|---|---|---|---|---|
| SR1 | ρ750/ρ700 | Gitelson and Merzlyak 1997 [57] | 0.74 | 0.61 |
| Double Difference | (ρ749-ρ720)-(ρ701-ρ672) | le Maire et al. 2004 [58] | 0.74 | 0.58 |
| Vogelmann1 | ρ740/ρ720 | Vogelmann et al. 1993 [59] | 0.73 | 0.58 |
| mSR705 | (ρ750-ρ445)/(ρ705-ρ445) | Sims and Gamon 2002 [44] | 0.73 | 0.57 |
| SRCarter | ρ760/ρ695 | Carter et al. 1994 [60] | 0.72 | 0.61 |
| Maccioni | (ρ780-ρ710)/(ρ780-ρ680) | Maccioni et al. 2001 [61] | 0.72 | 0.55 |
| SR3 | ρ750/ρ550 | Gitelson and Merzlyak 1997 [57] | 0.70 | 0.63 |
| Gitelson | 1/ρ700 | Gitelson et al. 1999 [62] | 0.62 | 0.52 |
| NDVI (MODIS-like) | (ρ NIRMODIS - ρ RedMODIS)/(ρ NIRMODIS + ρ RedMODIS) | see methods | 0.60 | 0.50 |
| Datt4 | ρ672/(ρ550*ρ708) | Datt (1998) [63] | 0.57 | 0.45 |
| SR4 | ρ700/ρ670 | McMurtey et al. (1994) | 0.57 | 0.36 |
| SR2 | ρ752/ρ690 | Gitelson and Merzlyak 1997 [42] | 0.56 | 0.53 |
| NDVI (hyperspectral) | (ρ860-ρ690)/(ρ860+ρ690) | Stimson et al. 2005 [47] | 0.49 | 0.46 |
| Vogelmann2 | (ρ734-ρ747)-(ρ715+ρ726) | Vogelmann et al. 1993 [59] | 0.17 | 0.14 |
| mNDVI | (ρ800-ρ680)/(ρ800+ρ680–2ρ445) | Sims and Gamon 2002 [44] | 0.07 | 0.03ns |
| NDWI | (ρ860-ρ1240)/(ρ860+ρ1240) | Gao 1996 [64] | 0.06 | 0.20 |
| SIPI | (ρ800-ρ445) | Penuelas et al. 1995 [65] | 0.06 | 0.03ns |
| PRI | (ρ531-ρ570)/(ρ531+ρ570) | Gamon 1997 [52] | 0.02ns | 0.00ns |
| mSRCHL | (ρ800-ρ445)/(ρ680-ρ445) | Sims and Gamon 2002 [44] | 0.00ns | 0.00ns |
The predictive formula (ρ = spectral reflectance) and reference for each index is shown in addition to the coefficient of determination for Vcmax and Jmax. Nonsignificant correlations are indicated with an “ns” superscript. Values of R2 above 0.50 are bolded.
Results
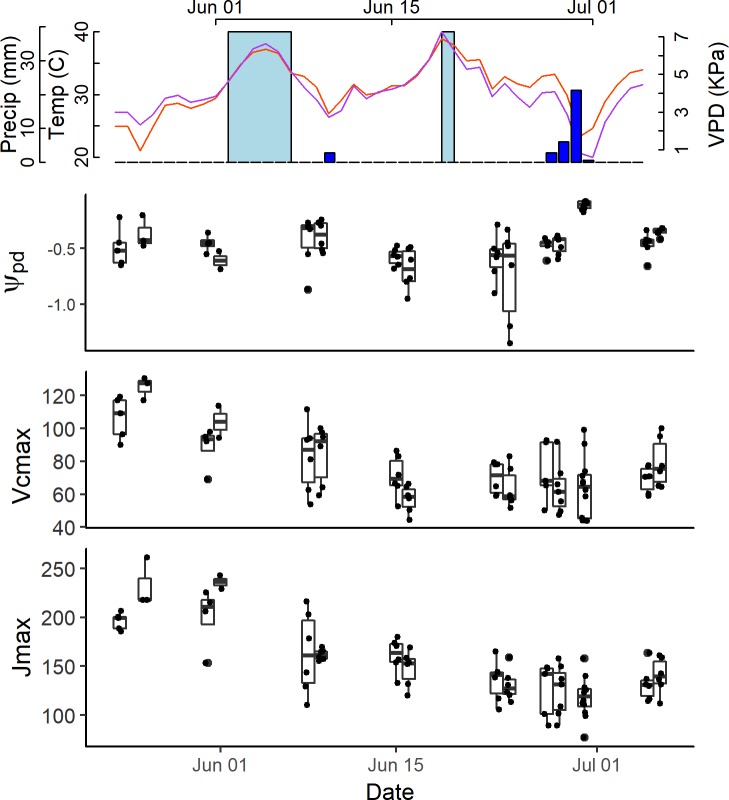
Weather conditions, photosynthetic capacity, and pre-dawn water potential (Ψpd) varied over the course of our study (Fig 1). Conditions were generally hot and dry throughout the study period, with high daytime temperatures and Vapor Pressure Deficit (VPD) and low precipitation (Fig 1A). Mean daytime temperature (06:00 to 18:00 MST) ranged from 20.73°C to 38.90°C throughout the study period with a mean of 30.1 ±4.3°C. Peak VPD (10:00 to 14:00 MST) ranged from 0.60 kPa to 7.2 kPa with a mean of 3.9 ±1.3 kPa. There was little precipitation throughout the study period with most (30.3 mm) of the total rainfall (36.1 mm) occurring between 6-28-2016 and 6-30-2016 (Fig 1A). Vcmax and Jmax both varied throughout the study period. Vcmax ranged from 43.9 to 130.4 with a mean of 75.7 ±20.8 μmol m−2 s−1. Jmax ranged from 76.7 to 261.2 with a mean of 150.1 ±37.5 μmol m−2 s−1. Vcmax and Jmax declined by approximately two-fold throughout the 7-week study period (Fig 1C and 1D). Vcmax and Jmax appeared to stabilize following the rain events between 6-28-2016 and 6-30-2016 which relieved water stress (Fig 1B). Pre-dawn water potential varied seasonally, with the lowest water potentials (most stressed) generally occurring in the middle of the study period (6–23 and 6–24, Fig 1B). Water potential values ranged from -1.35 to -0.8 MPa with a mean of -0.45 ±0.23 MPa. Leaf reflectance varied between individuals and temporally (Fig 2A). We evaluated our results first in terms of variability in leaf reflectance spectra in the study period, then evaluated estimates of Vcmax and Jmax from three different variations of PLSR models with increasing statistical rigor, and finally assessed relationships between Vcmax and Jmax and published hyperspectral indices.
Fig 1. Temporal variability in meteorological and physiological conditions throughout the study period.
A) shows mean daytime temperature in °C (orange line; 06:00 to 18:00), peak VPD in kPa (purple line; 10:00 to 14:00), precipitation in mm and supplemental watering throughout the study period with dark blue bars representing precipitation and light blue representing supplemental watering days. B) Shows the time series of predawn water potential (MPa) throughout the study period. C) and D) show the time series of estimated Vcmax (μmol m-2 s-1) and Jmax (μmol m-2 s-1), respectively.
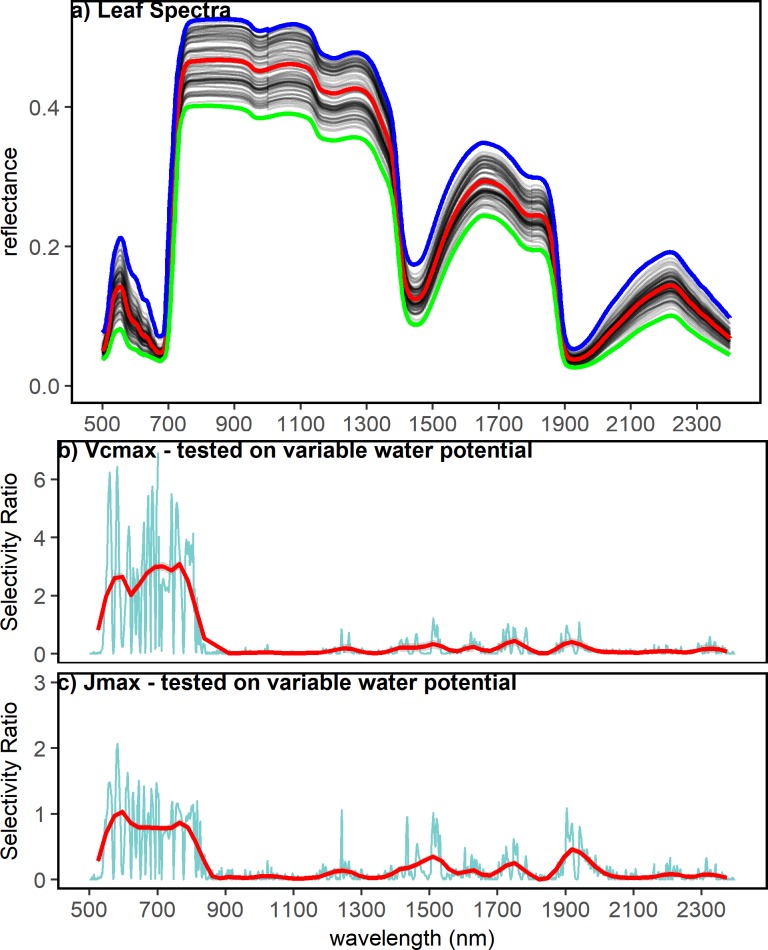
Fig 2. Leaf reflectance spectra and wavelength importance in PLSR models.
(A) Pooled reflectance spectra (λ = 450–2500 nm) for all plants studied throughout the course of the study period (5/24/2016 to 7/05/2017). The mean reflectance at each wavelength is shown by the red line, the maximum and minimum reflectance at each wavelength are shown by the blue and green lines, respectively. (B)&(C): selectivity ratio of each wavelength in the PLSR models tested on variable water potential. (B) Shows the selectivity ratio of each wavelength in the Vcmax PLSR model, and (C) shows the selectivity ratio of each wavelength in the Jmax PLSR model. The red lines in B&C are LOESS smoothers for visualization purposes.
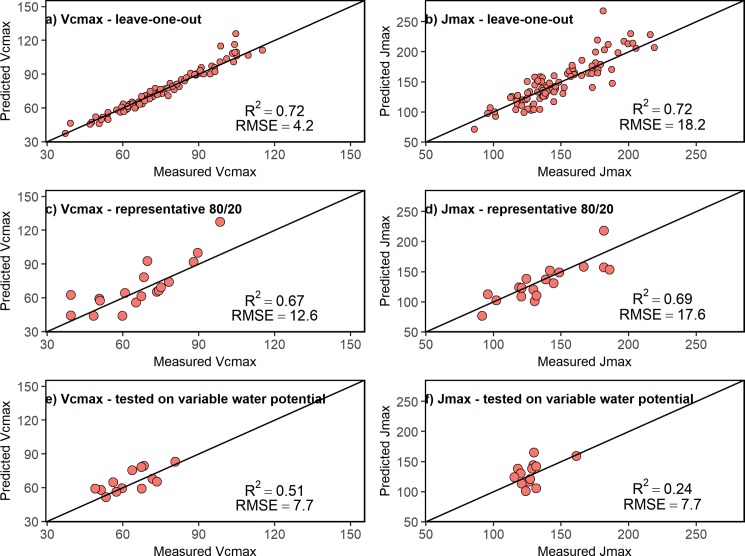
We performed three different evaluations of the PLSR models, presented in increasing order of statistical rigor. First, to assess the relationship between photosynthetic capacity and leaf spectra, PLSR models based on the complete dataset were developed. This least rigorous test of temporal stability used the "leave-one-out" cross validation approach to quantify the relationship between leaf reflectance spectra (450–2500 nm) and Vcmax and Jmax. PLSR models predicted photosynthetic capacity accurately, with comparable model predictive ability for Vcmax (R2 = 0.72; Fig 3A) and Jmax (R2 = 0.72; Fig 3B). The root mean squared error (RMSE) was lower for the Vcmax model (RMSE = 4.2, Fig 3A) than the Jmax model (RMSE = 18.2, Fig 3B). The predictive model for Vcmax only required two components to explain the variance, while the PLSR model for Jmax required four components.
Fig 3. Temporal stability of PLSR models.
All figures show observed vs. predicted values from PLS regression models. A&B: leave-one-out cross-validation procedure for (a) maximum rates of RuBP carboxylation (Vcmax, μmol m-2 s-1) and (b) RuBP regeneration Jmax (μmol m-2 s-1), n = 86. C&D: representative 80/20% split of the data for PLSR (C) Vcmax (μmol m-2 s-1) and (D) Jmax (μmol m-2 s-1). Each model was trained on 69 observations and tested on 17 observations. E & F: models tested on the period with the greatest between-individual variation in pre-dawn water potential (Ψpd) for (E) Vcmax (μmol m-2 s-1) and (F) Jmax (μmol m-2 s-1). Each model was trained on 74 observations and tested on the 12 observations on 6/23/2016 and 6/24/2016 when the population varied widely in Ψpd. Statistical tests of increase in rigor moving downward.
Second, to assess temporal stability of the model, the data were split into training and testing datasets to generate PLSR models. This second test of PLSR model stability used 80% of the observations for training and withheld a 20% holdout dataset for testing. The 20% holdout dataset was randomly selected from the full dataset and the remaining 80% were used to build PLSR models for Vcmax and Jmax. This procedure was performed 100 times with different randomly selected 20% holdout datasets (S1 Fig); the mean R2 for the 80/20 split was 0.64 ±0.03 for Vcmax and 0.64 ±0.07 for Jmax (S1 Table). Models with a 20% holdout dataset that were representative of mean predictive ability are shown in Fig 3C and 3D; predictive ability was comparable for Vcmax (R2 = 0.67; Fig 3C) and Jmax (R2 = 0.69; Fig 3D). The predictive capability of the 80% model was similar to the predictive capability of the full cross-validated model (Fig 3A and 3B). The RMSE was lower for the Vcmax model (RMSE = 12.6; Fig 3C) than the Jmax model (RMSE = 17.6; Fig 3D).
Third, to assess temporal stability in situations with large within-population variation in environmental stress, the data was split into a testing and training set based on variability in Ψpd. This third and most rigorous test of PLSR model stability trained the PLSR model on low environmental stress conditions (Ψpd) and tested it on the two consecutive sampling days with highest individual variation in Ψpd, (6/23 and 6/24; Fig 1C). PLSR models for Vcmax and Jmax in this third test differed in their predictive abilities. The predictive ability was only moderately reduced for Vcmax (R2 = 0.51; Fig 3E), whereas PLSR model predictive ability was substantially reduced for Jmax (R2 = 0.24; Fig 3F). The RSME was the same for both models (RMSE = 7.7; Fig 3E and 3F).
To compare the relative significance of different wavelengths in a given PLSR model, the selectivity ratio (SR) was used as a method of variable selection. The SR was used to assess relative contributions of different portions of the spectrum to the overall PLSR model. Both Vcmax and Jmax models were sensitive to variation in the visible wavelength and near infrared (Fig 2B, Fig 2C). Peak SR was at 703 nm for Vcmax and 583 nm for Jmax (Fig 2B and 2C). Contributions from the short-wave infrared regions were small to both Vcmax and Jmax models, however the SWIR contributed more to the Jmax models than Vcmax models (Fig 2B and 2C).
To assess relationships between existing hyperspectral indices and photosynthetic capacity, correlations between Vcmax and Jmax and a suite of hyperspectral vegetation indices were compared (Fig 4). Estimated Vcmax and Jmax from hyperspectral chlorophyll and stress indices were well-correlated with measured values (Table 1). For Vcmax, four other metrics were all comparable to the full PLSR model (R2 = 0.72) based on the coefficient of determination: Maccioni (R2 = 0.72), Double Difference (R2 = 0.74), Vogelmann2 (R2 = 0.73), and SR1 (R2 = 0.74) (Fig 4A). The PLSR model for Jmax (R2 = 0.72) outperformed all tested hyperspectral indices, the best of which was SR3 (R2 = 0.63; Fig 4B). Several indices had very low or non-significant correlations with Vcmax, including PRI, NDWI, mNDVI, and SIPI (Table 1). The mNDVI, SIPI, PRI, and mSRCHL indices all had non-significant relationships with Jmax (Table 1). The hyperspectral normalized difference vegetation index (NDVI) had moderate predictive capability for Vcmax (R2 = 0.49) and Jmax (R2 = 0.46). The MODIS-like NDVI (“mod_NDVI”) performed better than the hyperspectral NDVI for both Vcmax (R2 = 0.60) and Jmax (R2 = 0.50) (Fig 4, Table 1).
Fig 4. Comparison of predictive abilities of hyperspectral indices and PLSR models.
Predictive capability for (A) maximum rates of RuBP carboxylation (Vcmax, μmol m-2 s-1) and (B) RuBP regeneration Jmax (μmol m-2 s-1). Pairwise correlations between Vcmax and Jmax estimated using standard gas exchange techniques and spectral indices were tested and R2 is reported. The R2 reported for the PLSR model is from the leave-one-out cross-validation approach. Indices with non-significant correlations are represented with “ns”.
Discussion
Our results show that hypers can predict photosynthetic parameters across time, suggesting that these hyperspectral remote sensing techniques have great potential to constrain model estimates of plant function. In this study, relationships between leaf reflectance spectra and photosynthetic capacity were robust throughout a 7-week period with dynamic change in photosynthetic capacity. Hyperspectral vegetation indices to estimate chlorophyll content were correlated with the key determinants of photosynthetic capacity, Vcmax and Jmax. Predictions of Vcmax and Jmax from PLSR models derived from leaf reflectance spectra were proportionally-sensitive to observed variation in Vcmax and Jmax. These results support our hypothesis that PLSR models that utilize the full spectrum can predict photosynthetic capacity through time. These findings highlight the potential of hyperspectral remote sensing methods to accurately predict Vcmax and Jmax despite dynamic temporal variation in photosynthetic capacity related to within-season variation and plant stress.
Leaf-level hyperspectral data have previously been used to estimate photosynthesis [66] and photosynthetic capacity across temperature regimes in glasshouse experiments [32], and in diverse agroecosystems [41,42], but our study is the first to assess the impacts of in situ within-season temporal variation on estimates of photosynthetic capacity derived from leaf reflectance spectra. As in previous studies, measured and PLSR-modeled leaf traits were significantly correlated [32,42,66,67]. Regression models predicted Vcmax and Jmax during a 7-week period with dynamic declines in photosynthetic capacity. This is confirmation that the spectral signals detected by leaf reflectance observations are accurately tracking seasonal metabolic adjustments made within the photosynthetic machinery of the leaf. When PLSR models were trained on only 80% of the data and tested on the remaining 20%, their mean predictive capability were similar to that of the full PLSR models. This shows that relationships between leaf reflectance spectra and Vcmax and Jmax are robust despite large variation in these values. Known hyperspectral vegetation indices had varying predictive capabilities. Hyperspectral vegetation indices developed for estimating chlorophyll content (e.g. SR1, Double Difference, Vogelmann 1) and plant stress (SRCarter) had the highest correlations with plant photosynthetic capacity (Table 1). The wavelengths that explained variance in photosynthetic capacity in the PLSR models (based on selectivity ratio) were consistent with the wavelengths that comprised the best-performing hyperspectral indices. The important wavelengths in PLSR models, based on Selectivity Ratio, for predicting Vcmax and Jmax largely fell in the visible and short wavelength end of the near-infrared (~500 to 850 nm), with minor contributions from wavelengths in the short-wave infrared. Important wavelengths in the visible region fell largely in the blue region (450–495 nm) and red regions (620–650 nm) for the Vcmax models, which is consistent with the chlorophyll absorption regions (i.e. ∼430–460 nm and 640–670 nm). The highest-performing hyperspectral vegetation indices generally leveraged diagnostic differences in the red-edge portion (680–750 nm) of the spectrum to estimate chlorophyll content. The red edge region of reflectance is known to be sensitive to differences in chlorophyll content, and chlorophyll content is generally positively correlated with photosynthetic capacity [68]. Overall, both hyperspectral indices and PLSR models had the capability to predict variation in within-season photosynthetic capacity.
Accurate representation of photosynthesis in terrestrial biosphere models is essential to predicting future carbon and global change dynamics [69]. Although modeled rates of photosynthesis are sensitive to Vcmax and Jmax [3,70,71], most terrestrial biosphere models use static values for these parameters [27]. Furthermore, Vcmax and Jmax values are often parameterized based on limited or poorly represented data sets [27]. Monthly optimization of Vcmax improved process-based biosphere model (Organizing Carbon and Hydrology in Dynamic Ecosystems; ORCHIDEE) representation of seasonal carbon dynamics (NEE; Net Ecosystem Exchange) in a tropical evergreen forest in Brazil, however, seasonal parameter variations could not be extrapolated spatially [28]. Remote sensing observations can improve model representation of photosynthesis across spatial and temporal scales [31,55,72]. Our results support the use of remotely sensed estimation of photosynthetic capacity using hyperspectral observations. Furthermore, hyperspectral remote sensing could be used to incorporate spatially explicit photosynthetic capacity/environment relationships in next-generation trait-based models. Our results highlight the potential of hyperspectral remote sensing to parameterize determinants of photosynthetic capacity and inform trait-environment relationships in terrestrial biosphere models, thereby improving model representation of photosynthesis and carbon dynamics.
Although PLSR model predictive capabilities for Vcmax and Jmax were generally similar, there were differences in PLSR model performance and sensitivity between the two variables. The mean predictive capability of the 80% PLSR models were comparable for Vcmax and Jmax (Table 1); however, the variance in Jmax was nearly twice that of the variance in Vcmax (Table 1, S1 Fig). A rigorous test of PLSR model performance in the population with individual variation in drought stress indicated modest reductions in predictive capability for Vcmax and substantial reductions in predictive capability for Jmax. This test of temporal stability indicates that predictive relationships between leaf reflectance spectra and photosynthetic capacity are reduced when there is within-population variation in environmental stress, particularly for Jmax. PLSR models for Jmax included more wavelengths in the short-wave infrared that are associated with leaf water content and internal structure. The performance improvement of the PLSR model for Jmax compared to simple indices underscores the importance of full spectral information for predicting this parameter (Fig 4B)[42]. Terrestrial biosphere models generally simulate Jmax as a function of Vcmax rather than as its own parameter [73]. However, our results suggest that Vcmax and Jmax may be differentially sensitive to within-population variation in environmental stress.
Quantifying temporal variation in relationships between photosynthetic capacity and leaf reflectance spectra is timely given the increasing availability of high resolution spectral remote sensing, such as hyperspectral overflights planned by the National Ecological Observatory Network (NEON) [74] and NASA’s Hyperspectral Infrared Imager (HyspIRI) mission [75]. NEON hyperspectral overflights, which may only occur once per year over a given region, may be used to develop predictive relationships between leaf reflectance spectra and photosynthetic capacity. Our results suggest that such predictive relationships developed from data in a short portion of the growing season could hold true throughout the growing season. The widely used NDVI had moderate predictive capacity for Vcmax and Jmax. Notably, our approximation of MODIS NDVI had higher predictive capacity than hyperspectral NDVI (Table 1). Detecting temporal changes in photosynthetic capacity using MODIS NDVI could improve model predictions of photosynthesis given its broad spatial and daily temporal coverage. Another important consideration in extrapolating the results of this study to aerial and satellite remote sensing is additional technical challenges posed by these approaches, such as view angle effects, canopy architecture, and atmospheric effects. More studies are needed to further test temporal stability of relationships between leaf reflectance spectra and photosynthetic capacity in other contexts before relationships from a single hyperspectral overflight can be extrapolated through the growing season. Nonetheless, our results suggest promise for this approach.
In this study, spectral estimation of Vcmax and Jmax in hybrid poplar was robust to temporal variation of up to 200% in photosynthetic capacity. Applying remote sensing tools to predict photosynthetic capacity across a wider range of wildlands requires an in situ test of this method outside of agroecosystem or controlled glasshouse conditions across a time period of variable abiotic and biotic conditions. Our results show that relationships between photosynthetic capacity and leaf reflectance spectra developed from limited data can in some cases be extrapolated temporally. These results highlight the potential of hyperspectral remote sensing methods to detect dynamic temporal variations in Vcmax and Jmax related to seasonality and plant stress, thereby aiding improved estimates of plant productivity and associated carbon budget. Furthermore, our results suggest that terrestrial biosphere models could use hyperspectral remote sensing to parameterize Vcmax and Jmax within season to improve predictions of future carbon dynamics. Reliable and precise methods to estimate Vcmax and Jmax across spatial and temporal scales will improve understanding of ecosystem carbon uptake and the terrestrial carbon sink.
Supporting information
Each point represents the r-squared between predicted and actual Vcmax/Jmax values from PLSR using a random sample corresponding to the designated proportion of training data (each proportion was sampled 100 times).
(TIF)
The mean, median, and standard deviation in R2 of 100 PLSR models per training proportion are represented.
(DOCX)
Acknowledgments
The authors thank Kyle D. Morton for assistance in the field. We also thank John Adams and Ian M. Shiach for maintaining the poplar plantation.
Data Availability
All relevant data are available from Open Science Framework at the following DOI: 10.17605/OSF.IO/ZUR8E.
Funding Statement
This work was supported by the National Science Foundation Research Experience for Undergraduates: grant no. EAR-1263251 to ACF. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Foley JA, Prentice IC, Ramankutty N, Levis S, Pollard D, Sitch S, et al. An integrated biosphere model of land surface processes, terrestrial carbon balance, and vegetation dynamics. Glob Biogeochem Cycles. 1996;10: 603–628. doi: 10.1029/96GB02692 [Google Scholar]
- 2.Zaehle S, Friend AD. Carbon and nitrogen cycle dynamics in the O-CN land surface model: 1. Model description, site-scale evaluation, and sensitivity to parameter estimates. Glob Biogeochem Cycles. 2010;24: GB1005 doi: 10.1029/2009GB003521 [Google Scholar]
- 3.Bonan GB, Lawrence PJ, Oleson KW, Levis S, Jung M, Reichstein M, et al. Improving canopy processes in the Community Land Model version 4 (CLM4) using global flux fields empirically inferred from FLUXNET data. J Geophys Res Biogeosciences. 2011;116: G02014 doi: 10.1029/2010JG001593 [Google Scholar]
- 4.Quéré CL, Moriarty R, Andrew RM, Canadell JG, Sitch S, Korsbakken JI, et al. Global Carbon Budget 2015. Earth Syst Sci Data. 2015;7: 349–396. doi: 10.5194/essd-7-349-2015 [Google Scholar]
- 5.Cao M, Woodward FI. Dynamic responses of terrestrial ecosystem carbon cycling to global climate change. Nature. 1998;393: 249–252. doi: 10.1038/30460 [Google Scholar]
- 6.Schimel D, Melillo J, Tian H, McGuire AD, Kicklighter D, Kittel T, et al. Contribution of Increasing CO2 and Climate to Carbon Storage by Ecosystems in the United States. Science. 2000;287: 2004–2006. doi: 10.1126/science.287.5460.2004 [DOI] [PubMed] [Google Scholar]
- 7.Frank D, Reichstein M, Bahn M, Thonicke K, Frank D, Mahecha MD, et al. Effects of climate extremes on the terrestrial carbon cycle: concepts, processes and potential future impacts. Glob Change Biol. 2015;21: 2861–2880. doi: 10.1111/gcb.12916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia J, Chen J, Piao S, Ciais P, Luo Y, Wan S. Terrestrial carbon cycle affected by non-uniform climate warming. Nat Geosci. 2014;7: 173–180. doi: 10.1038/ngeo2093 [Google Scholar]
- 9.Beer C, Reichstein M, Tomelleri E, Ciais P, Jung M, Carvalhais N, et al. Terrestrial Gross Carbon Dioxide Uptake: Global Distribution and Covariation with Climate. Science. 2010;329: 834–838. doi: 10.1126/science.1184984 [DOI] [PubMed] [Google Scholar]
- 10.Nijp JJ, Limpens J, Metselaar K, van der Zee SEATM, Berendse F, Robroek BJM. Can frequent precipitation moderate the impact of drought on peatmoss carbon uptake in northern peatlands? New Phytol. 2014;203: 70–80. doi: 10.1111/nph.12792 [DOI] [PubMed] [Google Scholar]
- 11.Reich PB, Hobbie SE, Lee TD. Plant growth enhancement by elevated CO2 eliminated by joint water and nitrogen limitation. Nat Geosci. 2014;7: 920–924. doi: 10.1038/ngeo2284 [Google Scholar]
- 12.Keenan TF, Hollinger DY, Bohrer G, Dragoni D, Munger JW, Schmid HP, et al. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature. 2013;499: 324–327. doi: 10.1038/nature12291 [DOI] [PubMed] [Google Scholar]
- 13.Morecroft MD, Stokes VJ, Morison JIL. Seasonal changes in the photosynthetic capacity of canopy oak (Quercus robur) leaves: the impact of slow development on annual carbon uptake. Int J Biometeorol. 2003;47: 221–226. doi: 10.1007/s00484-003-0173-3 [DOI] [PubMed] [Google Scholar]
- 14.Friedlingstein P, Meinshausen M, Arora VK, Jones CD, Anav A, Liddicoat SK, et al. Uncertainties in CMIP5 Climate Projections due to Carbon Cycle Feedbacks. J Clim. 2013;27: 511–526. doi: 10.1175/JCLI-D-12-00579.1 [Google Scholar]
- 15.Running SW, Nemani RR. Relating seasonal patterns of the AVHRR vegetation index to simulated photosynthesis and transpiration of forests in different climates. Remote Sens Environ. 1988;24: 347–367. doi: 10.1016/0034-4257(88)90034-X [Google Scholar]
- 16.Zhou L, Tucker CJ, Kaufmann RK, Slayback D, Shabanov NV, Myneni RB. Variations in northern vegetation activity inferred from satellite data of vegetation index during 1981 to 1999. J Geophys Res Atmospheres. 2001;106: 20069–20083. doi: 10.1029/2000JD000115 [Google Scholar]
- 17.Barnes ML, Moran MS, Scott RL, Kolb TE, Ponce-Campos GE, Moore DJP, et al. Vegetation productivity responds to sub-annual climate conditions across semiarid biomes. Ecosphere. 2016;7: n/a-n/a. doi: 10.1002/ecs2.1339 [Google Scholar]
- 18.Shi H, Li L, Eamus D, Huete A, Cleverly J, Tian X, et al. Assessing the ability of MODIS EVI to estimate terrestrial ecosystem gross primary production of multiple land cover types. Ecol Indic. 2017;72: 153–164. doi: 10.1016/j.ecolind.2016.08.022 [Google Scholar]
- 19.Running SW, Nemani RR, Heinsch FA, Zhao M, Reeves M, Hashimoto H. A Continuous Satellite-Derived Measure of Global Terrestrial Primary Production. BioScience. 2004;54: 547–560. doi: 10.1641/0006-3568(2004)054[0547:ACSMOG]2.0.CO;2 [Google Scholar]
- 20.Asrar G, Fuchs M, Kanemasu ET, Hatfield JL. Estimating Absorbed Photosynthetic Radiation and Leaf Area Index from Spectral Reflectance in Wheat. Agron J. 1984;76: 300–306. doi: 10.2134/agronj1984.00021962007600020029x [Google Scholar]
- 21.Fensholt R, Sandholt I, Rasmussen MS. Evaluation of MODIS LAI, fAPAR and the relation between fAPAR and NDVI in a semi-arid environment using in situ measurements. Remote Sens Environ. 2004;91: 490–507. doi: 10.1016/j.rse.2004.04.009 [Google Scholar]
- 22.Huete A, Ponce-Campos G, Zhang Y, Restrepo-Coupe N, Ma X, Moran MS. Monitoring Photosynthesis from Space [Internet]. CRC Press; 2016. Available: https://opus.lib.uts.edu.au/handle/10453/73083 [Google Scholar]
- 23.Ruimy A, Jarvis PG, Baldocchi DD, Saugier B. CO2 Fluxes over Plant Canopies and Solar Radiation: A Review. Adv Ecol Res. 1995;26: 1–68. doi: 10.1016/S0065-2504(08)60063-X [Google Scholar]
- 24.Turner DP, Urbanski S, Bremer D, Wofsy SC, Meyers T, Gower ST, et al. A cross-biome comparison of daily light use efficiency for gross primary production. Glob Change Biol. 2003;9: 383–395. doi: 10.1046/j.1365-2486.2003.00573.x [Google Scholar]
- 25.Sims DA, Rahman AF, Cordova VD, El-Masri BZ, Baldocchi DD, Flanagan LB, et al. On the use of MODIS EVI to assess gross primary productivity of North American ecosystems. J Geophys Res Biogeosciences. 2006;111: G04015 doi: 10.1029/2006JG000162 [Google Scholar]
- 26.Jenkins JP, Richardson AD, Braswell BH, Ollinger SV, Hollinger DY, Smith M-L. Refining light-use efficiency calculations for a deciduous forest canopy using simultaneous tower-based carbon flux and radiometric measurements. Agric For Meteorol. 2007;143: 64–79. doi: 10.1016/j.agrformet.2006.11.008 [Google Scholar]
- 27.Rogers A. The use and misuse of Vc,max in Earth System Models. Photosynth Res. 2014;119: 15–29. doi: 10.1007/s11120-013-9818-1 [DOI] [PubMed] [Google Scholar]
- 28.Verbeeck H, Peylin P, Bacour C, Bonal D, Steppe K, Ciais P. Seasonal patterns of CO2 fluxes in Amazon forests: Fusion of eddy covariance data and the ORCHIDEE model. J Geophys Res Biogeosciences. 2011;116: G02018 doi: 10.1029/2010JG001544 [Google Scholar]
- 29.Gitelson AA, Gamon JA, Solovchenko A. Multiple drivers of seasonal change in PRI: Implications for photosynthesis 1. Leaf level. Remote Sens Environ. 2017;191: 110–116. doi: 10.1016/j.rse.2016.12.014 [Google Scholar]
- 30.Gamon JA, Peñuelas J, Field CB. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens Environ. 1992;41: 35–44. doi: 10.1016/0034-4257(92)90059-S [Google Scholar]
- 31.Farquhar GD, Caemmerer S von, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 149: 78–90. doi: 10.1007/BF00386231 [DOI] [PubMed] [Google Scholar]
- 32.Serbin SP, Dillaway DN, Kruger EL, Townsend PA. Leaf optical properties reflect variation in photosynthetic metabolism and its sensitivity to temperature. J Exp Bot. 2012;63: 489–502. doi: 10.1093/jxb/err294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serbin SP, Singh A, Desai AR, Dubois SG, Jablonski AD, Kingdon CC, et al. Remotely estimating photosynthetic capacity, and its response to temperature, in vegetation canopies using imaging spectroscopy. Remote Sens Environ. 2015;167: 78–87. doi: 10.1016/j.rse.2015.05.024 [Google Scholar]
- 34.Kattge J, Knorr W. Temperature acclimation in a biochemical model of photosynthesis: a reanalysis of data from 36 species. Plant Cell Environ. 2007;30: 1176–1190. doi: 10.1111/j.1365-3040.2007.01690.x [DOI] [PubMed] [Google Scholar]
- 35.Dreyer E, Le Roux X, Montpied P, Daudet FA, Masson F. Temperature response of leaf photosynthetic capacity in seedlings from seven temperate tree species. Tree Physiol. 2001;21: 223–232. doi: 10.1093/treephys/21.4.223 [DOI] [PubMed] [Google Scholar]
- 36.Bernacchi CJ, Calfapietra C, Davey PA, Wittig VE, Scarascia-Mugnozza GE, Raines CA, et al. Photosynthesis and stomatal conductance responses of poplars to free-air CO2 enrichment (PopFACE) during the first growth cycle and immediately following coppice. New Phytol. 2003;159: 609–621. doi: 10.1046/j.1469-8137.2003.00850.x [DOI] [PubMed] [Google Scholar]
- 37.Limousin J-M, Misson L, Lavoir A-V, Martin NK, Rambal S. Do photosynthetic limitations of evergreen Quercus ilex leaves change with long-term increased drought severity? Plant Cell Environ. 2010;33: 863–875. doi: 10.1111/j.1365-3040.2009.02112.x [DOI] [PubMed] [Google Scholar]
- 38.Onoda Y, Hikosaka K, Hirose T. The balance between RuBP carboxylation and RuBP regeneration: a mechanism underlying the interspecific variation in acclimation of photosynthesis to seasonal change in temperature. Funct Plant Biol. 2005;32: 903–910. doi: 10.1071/FP05024 [DOI] [PubMed] [Google Scholar]
- 39.Xu L, Baldocchi DD. Seasonal trends in photosynthetic parameters and stomatal conductance of blue oak (Quercus douglasii) under prolonged summer drought and high temperature. Tree Physiol. 2003;23: 865–877. doi: 10.1093/treephys/23.13.865 [DOI] [PubMed] [Google Scholar]
- 40.Wilson KB, Baldocchi DD, Hanson PJ. Spatial and seasonal variability of photosynthetic parameters and their relationship to leaf nitrogen in a deciduous forest. Tree Physiol. 2000;20: 565–578. doi: 10.1093/treephys/20.9.565 [DOI] [PubMed] [Google Scholar]
- 41.Ainsworth EA, Serbin SP, Skoneczka JA, Townsend PA. Using leaf optical properties to detect ozone effects on foliar biochemistry. Photosynth Res. 2014;119: 65–76. doi: 10.1007/s11120-013-9837-y [DOI] [PubMed] [Google Scholar]
- 42.Yendrek C, Tomaz T, Montes CM, Cao Y, Morse AM, Brown PJ, et al. High-throughput phenotyping of maize leaf physiology and biochemistry using hyperspectral reflectance. Plant Physiol. 2016; pp.014472016. doi: 10.1104/pp.16.01447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heckmann D, Schlüter U, Weber APM. Machine Learning Techniques for Predicting Crop Photosynthetic Capacity from Leaf Reflectance Spectra. Mol Plant. 2017;10: 878–890. doi: 10.1016/j.molp.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 44.Sims DA, Gamon JA. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens Environ. 2002;81: 337–354. doi: 10.1016/S0034-4257(02)00010-X [Google Scholar]
- 45.Smith M-L, Ollinger SV, Martin ME, Aber JD, Hallett RA, Goodale CL. Direct Estimation of Aboveground Forest Productivity Through Hyperspectral Remote Sensing of Canopy Nitrogen. Ecol Appl. 2002;12: 1286–1302. doi: 10.1890/1051-0761(2002)012[1286:DEOAFP]2.0.CO;2 [Google Scholar]
- 46.Rahman AF, Gamon JA, Fuentes DA, Roberts DA, Prentiss D. Modeling spatially distributed ecosystem flux of boreal forest using hyperspectral indices from AVIRIS imagery. J Geophys Res Atmospheres. 2001;106: 33579–33591. doi: 10.1029/2001JD900157 [Google Scholar]
- 47.Stimson HC, Breshears DD, Ustin SL, Kefauver SC. Spectral sensing of foliar water conditions in two co-occurring conifer species: Pinus edulis and Juniperus monosperma. Remote Sens Environ. 2005;96: 108–118. doi: 10.1016/j.rse.2004.12.007 [Google Scholar]
- 48.Duursma RA. Plantecophys—An R Package for Analysing and Modelling Leaf Gas Exchange Data Struik PC, editor. PLOS ONE. 2015;10: e0143346 doi: 10.1371/journal.pone.0143346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wehrens R, Mevik B-H. The pls Package: Principal Component and Partial Least Squares Regression in R. 24 2007; Available: http://repository.ubn.ru.nl/handle/2066/36604 [Google Scholar]
- 50.Siegmann B, Jarmer T. Comparison of different regression models and validation techniques for the assessment of wheat leaf area index from hyperspectral data. Int J Remote Sens. 2015;36: 4519–4534. doi: 10.1080/01431161.2015.1084438 [Google Scholar]
- 51.Farrés M, Platikanov S, Tsakovski S, Tauler R. Comparison of the variable importance in projection (VIP) and of the selectivity ratio (SR) methods for variable selection and interpretation: Comparison of variable selection methods. J Chemom. 2015;29: 528–536. doi: 10.1002/cem.2736 [Google Scholar]
- 52.Gamon JA, Serrano L, Surfus JS. The photochemical reflectance index: an optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia. 1997;112: 492–501. doi: 10.1007/s004420050337 [DOI] [PubMed] [Google Scholar]
- 53.Drolet GG, Middleton EM, Huemmrich KF, Hall FG, Amiro BD, Barr AG, et al. Regional mapping of gross light-use efficiency using MODIS spectral indices. Remote Sens Environ. 2008;112: 3064–3078. doi: 10.1016/j.rse.2008.03.002 [Google Scholar]
- 54.Gray SB, Dermody O, DeLucia EH. Spectral reflectance from a soybean canopy exposed to elevated CO2 and O3. J Exp Bot. 2010;61: 4413–4422. doi: 10.1093/jxb/erq244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dobrowski SZ, Pushnik JC, Zarco-Tejada PJ, Ustin SL. Simple reflectance indices track heat and water stress-induced changes in steady-state chlorophyll fluorescence at the canopy scale. Remote Sens Environ. 2005;97: 403–414. doi: 10.1016/j.rse.2005.05.006 [Google Scholar]
- 56.Maia R, Eliason CM, Bitton P-P, Doucet SM, Shawkey MD. pavo: an R package for the analysis, visualization and organization of spectral data. Methods Ecol Evol. 2013;4: 906–913. doi: 10.1111/2041-210X.12069 [Google Scholar]
- 57.Gitelson AA, Merzlyak MN. Remote estimation of chlorophyll content in higher plant leaves. Int J Remote Sens. 1997;18: 2691–2697. doi: 10.1080/014311697217558 [Google Scholar]
- 58.le Maire G, François C, Dufrêne E. Towards universal broad leaf chlorophyll indices using PROSPECT simulated database and hyperspectral reflectance measurements. Remote Sens Environ. 2004;89: 1–28. doi: 10.1016/j.rse.2003.09.004 [Google Scholar]
- 59.Vogelmann JE, Rock BN, Moss DM. Red edge spectral measurements from sugar maple leaves. Int J Remote Sens. 1993;14: 1563–1575. doi: 10.1080/01431169308953986 [Google Scholar]
- 60.Carter GA. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int J Remote Sens. 1994;15: 697–703. doi: 10.1080/01431169408954109 [Google Scholar]
- 61.Maccioni A, Agati G, Mazzinghi P. New vegetation indices for remote measurement of chlorophylls based on leaf directional reflectance spectra. J Photochem Photobiol B. 2001;61: 52–61. doi: 10.1016/S1011-1344(01)00145-2 [DOI] [PubMed] [Google Scholar]
- 62.Gitelson AA, Buschmann C, Lichtenthaler HK. The Chlorophyll Fluorescence Ratio F735/F700 as an Accurate Measure of the Chlorophyll Content in Plants. Remote Sens Environ. 1999;69: 296–302. doi: 10.1016/S0034-4257(99)00023-1 [Google Scholar]
- 63.Datt B. Remote Sensing of Chlorophyll a, Chlorophyll b, Chlorophyll a+b, and Total Carotenoid Content in Eucalyptus Leaves. Remote Sens Environ. 1998;66: 111–121. doi: 10.1016/S0034-4257(98)00046-7 [Google Scholar]
- 64.Gao B. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens Environ. 1996;58: 257–266. doi: 10.1016/S0034-4257(96)00067-3 [Google Scholar]
- 65.Penuelas J. “Semi-empirical indices.” Photosynthetica 312 (1995): 221–230. APA. [Google Scholar]
- 66.Doughty CE, Asner GP, Martin RE. Predicting tropical plant physiology from leaf and canopy spectroscopy. Oecologia. 2011;165: 289–299. doi: 10.1007/s00442-010-1800-4 [DOI] [PubMed] [Google Scholar]
- 67.Hansen PM, Schjoerring JK. Reflectance measurement of canopy biomass and nitrogen status in wheat crops using normalized difference vegetation indices and partial least squares regression. Remote Sens Environ. 2003;86: 542–553. doi: 10.1016/S0034-4257(03)00131-7 [Google Scholar]
- 68.Evans JR. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia. 1989;78: 9–19. doi: 10.1007/BF00377192 [DOI] [PubMed] [Google Scholar]
- 69.Rogers A, Medlyn BE, Dukes JS, Bonan G, von Caemmerer S, Dietze MC, et al. A roadmap for improving the representation of photosynthesis in Earth system models. New Phytol. 2017;213: 22–42. doi: 10.1111/nph.14283 [DOI] [PubMed] [Google Scholar]
- 70.Zaehle S, Sitch S, Smith B, Hatterman F. Effects of parameter uncertainties on the modeling of terrestrial biosphere dynamics. Glob Biogeochem Cycles. 2005;19: GB3020 doi: 10.1029/2004GB002395 [Google Scholar]
- 71.Verheijen LM, Brovkin V, Aerts R, Bönish G, Cornelissen JHC, Kattge J, et al. Impacts of trait variation through observed trait-climate relationships on performance of a representative Earth System Model: a conceptual analysis. Biogeosciences. 2013;10: 5497–5515. doi: 10.5194/bg-10-5497-2013 [Google Scholar]
- 72.Schimel D, Pavlick R, Fisher JB, Asner GP, Saatchi S, Townsend P, et al. Observing terrestrial ecosystems and the carbon cycle from space. Glob Change Biol. 2015; n/a-n/a. doi: 10.1111/gcb.12822 [DOI] [PubMed] [Google Scholar]
- 73.Walker AP, Beckerman AP, Gu L, Kattge J, Cernusak LA, Domingues TF, et al. The relationship of leaf photosynthetic traits–Vcmax and Jmax–to leaf nitrogen, leaf phosphorus, and specific leaf area: a meta-analysis and modeling study. Ecol Evol. 2014;4: 3218–3235. doi: 10.1002/ece3.1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kampe TU, Johnson BR, Kuester M, Keller M. NEON: the first continental-scale ecological observatory with airborne remote sensing of vegetation canopy biochemistry and structure. J Appl Remote Sens. 2010;4: 043510-043510-24. doi: 10.1117/1.3501124 [Google Scholar]
- 75.Lee CM, Cable ML, Hook SJ, Green RO, Ustin SL, Mandl DJ, et al. An introduction to the NASA Hyperspectral InfraRed Imager (HyspIRI) mission and preparatory activities. Remote Sens Environ. 2015;167: 6–19. doi: 10.1016/j.rse.2015.06.012 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Each point represents the r-squared between predicted and actual Vcmax/Jmax values from PLSR using a random sample corresponding to the designated proportion of training data (each proportion was sampled 100 times).
(TIF)
The mean, median, and standard deviation in R2 of 100 PLSR models per training proportion are represented.
(DOCX)
Data Availability Statement
All relevant data are available from Open Science Framework at the following DOI: 10.17605/OSF.IO/ZUR8E.