Abstract
Introduction
Persistent bacterial bronchitis (PBB) is a leading cause of chronic wet cough in young children. This study aimed to characterise the respiratory bacterial microbiota of healthy children and to assess the impact of the changes associated with the development of PBB.
Blind, protected brushings were obtained from 20 healthy controls and 24 children with PBB, with an additional directed sample obtained from PBB patients. DNA was extracted, quantified using a 16S rRNA gene quantitative PCR assay prior to microbial community analysis by 16S rRNA gene sequencing.
Results
No significant difference in bacterial diversity or community composition (R2 = 0.01, P = 0.36) was observed between paired blind and non-blind brushes, showing that blind brushings are a valid means of accessing the airway microbiota. This has important implications for collecting lower respiratory samples from healthy children. A significant decrease in bacterial diversity (P < 0.001) and change in community composition (R2 = 0.08, P = 0.004) was observed among controls, in comparison with patients. Bacterial communities within patients with PBB were dominated by Proteobacteria, and indicator species analysis showed that Haemophilus and Neisseria were significantly associated with the patient group. In 15 (52.9%) cases the dominant organism by sequencing was not identified by standard routine clinical culture.
Conclusion
The bacteria present in the lungs of patients with PBB were less diverse in terms of richness and evenness. The results validate the clinical diagnosis, and suggest that more attention to bacterial communities in children with chronic cough may lead to more rapid recognition of this condition with earlier treatment and reduction in disease burden.
Introduction
Persistent or protracted bacterial bronchitis (PBB) is a leading cause of chronic wet cough lasting more than 4 weeks in young children. PBB is particularly common in pre-school children, often developing after a viral lower respiratory infection [1, 2], but may present at any age. As a result, PBB is often either misdiagnosed as asthma or the symptoms are dismissed as being due to recurrent viral infection [1–3].
Standard treatment for PBB is high dose oral antibiotics, the cough typically taking 10–14 days to resolve [4]. Although the aim of therapy is to provide a definitive cure, reoccurrences are frequent and if not treated successfully may lead to bronchiectasis [1–4].
Our understanding of the role of bacteria in chronic respiratory diseases is changing rapidly. Until recently it was widely believed that the healthy lung was a sterile environment [5]. A growing body of evidence, however, indicates that the healthy airways have a resident microbiota which can vary between individuals [5–7] and can alter significantly as a result of respiratory diseases such as cystic fibrosis, COPD and asthma [8].
A previous study of children with PBB using 16S rRNA gene sequencing suggested that the bacterial communities present in their lungs demonstrated similarities to those seen in children with cystic fibrosis (CF) and non-CF bronchiectasis [9]. This study provided a useful insight into the bacterial community associated with PBB, although the control subjects were undergoing bronchoscopy for clinical indications and could not be considered to be healthy [9–11].
In this present study bronchial brushings were obtained from infants and children with a diagnosis of PBB and from healthy children who were free of any respiratory symptoms or significant previous lower respiratory tract illness. This has given the opportunity for a better understanding of the microbiota of the healthy airway in childhood, as well as insight into how it is perturbed in children with PBB. In addition, the validity of characterising the lower airways microbiome using blind brushings through an endotracheal tube as opposed to more invasive bronchoscope guided sampling has been investigated.
Methods
All study protocols were subject to ethical approval by the Local Health Research Authority, NRES Committee of Yorkshire & The Humber—South Yorkshire (Reference: 12/YH/0230). Full details of sampling, laboratory and analytical methods are given in S1 Appendix, Supplementary Methods.
The study subjects were all 17 years of age or younger. Written informed consent was taken from the parents or legally acceptable representatives of children aged 15 years and younger or from the patient themselves if 16 years or older. The study was conducted in accordance with the International Conference for Harmonisation of Good Clinical Practice and the guiding principles of the Declaration of Helsinki and the Research Governance Framework for Health and Social Care. None of these subjects had an identified significant immunodeficiency or other conditions.
Control subjects were recruited if they were undergoing an intervention requiring endotracheal intubation but were otherwise healthy without any history of acute or chronic upper or lower respiratory tracts symptoms.
Sixteen mothers of enrolled children aged ≤ 2 years had nasal and oropharyngeal (throat) swabs taken (S1 Appendix, S1 Table). Written informed consent was collected and stored in patient clinical records for all mothers enrolled in the study.
Sample collection
Samples were obtained at the time of a diagnostic bronchoscopy for those with PBB and opportunistically from healthy subjects undergoing planned surgical procedures. Blind brushings were obtained in both groups using a protected cytology brush inserted into the airway through an ET tube. In the PBB subjects a second sample was obtained via the bronchoscope in order to compare the results from directed and blind brushings. After obtaining the brushings, BAL was collected from all children with PBB for routine bacterial, fungal and viral screening at the Sheffield children’s hospital clinical laboratories.
Once collected, brushes and swabs were immediately stored at -80°C prior to further processing.
DNA extractions
DNA was extracted from both swabs and brushes using the MPBio FastDNA™ spin kit for soil as per the manufacturer’s instructions.
qPCR
Prior to sequencing total bacterial burden was measured using a quantitative PCR assay targeting the V4 region of the 16S rRNA gene and using the primers 520F, 5’- AYTGGGYDTAAAGNG and 820R, 5’-TACNVGGGTATCTAATCC.
DNA sequencing
The bacterial community within each sample was assessed using 16S rRNA gene sequencing. Dual barcoded fusion primers were used to target the previously quantified V4 region of the gene (same primers as for qPCR assay but with appropriate barcoding. See S1 Appendix, S2 Table for barcode details). Samples were sequenced on the Illumina MiSeq platform using the Illumina V2 2x250bp cycle kit. Sequences were submitted to the European nucleotide database, project number PRJEB18478.
Downstream sequencing analysis was carried out using Quantitative Insights in Microbial Ecology (QIIME) Version 1.9.0 [12]. The QIIME recommended minimum threshold of 1,000 reads was applied and samples with less than 1,000 reads were removed from further analysis. All remaining samples were then rarefied to the same minimum number of reads present.
Statistics
All statistical analyses were carried out using R Version 3.2.2 [13]. Primary analysis and pre-processing was carried out in Phyloseq [13]. Non-parametric Wilcoxon sign ranked tests were used to test significant differences between means. DESeq2 [14] and Indicator species analysis [15] were used to identify Operational Taxonomic Units (OTUs) significantly associated with PBB.
Results
Twenty four children with PBB and 20 healthy controls were successfully recruited into the study (Table 1). Nasal and throat swabs were obtained from mothers for 16 of the children; 11 of the PBB children and 5 of the healthy child controls.
Table 1. Table of patient demographics for cases (PBB) and controls.
| Cases | Controls | |
|---|---|---|
| Number of subjects | 24 | 20 |
| Female | 14 | 12 |
| Blind brush | 24 | 21 |
| Non-blind brush | 24 | N/A |
| Age in years, mean (min, max) | 4.3 (0.8, 13.7) | 7.4 (1, 15.8) |
| Breastfed, count (min, max months) | 9 (0.07, 12) | 11 (0.5, 24) |
| Antibiotics, weeks since last dose (min, max) | 1, 25 | 4, 53 |
| Mother smokes | 2 | 4 |
| Father smokes | 5 | 10 |
| Both parents smoke | 2 | 4 |
| Mother sampled | 11 | 5 |
16S rRNA gene sequencing
A total of 146 samples were sequenced on the Illumina MiSeq. These included mock communities, PCR negative controls, kit controls and bronchoscopy brush controls (S2 Table). After quality control 143 samples were included for further analysis, comprising of a total of 8,833,294 reads from 1,393 distinct OTUs (61,771.29 +/- 85,954.18 [mean +/- SD] number of reads/OTU). Samples above the 1,000 read cut off recommended by QIIME were rarefied to the minimum number of reads found in the samples.
Blind versus non-blind brush
PBB patients were sampled using both blind and non-blind brushing methods to test for potential differences in the bacterial community due to sampling method. The difference in the bacterial community of 21 paired samples were assessed using both alpha and beta diversity measurements. Samples were rarefied to 1,067 reads. No significant difference in alpha diversity was observed between the blind and non-blind brushes using richness (observed number of species, Z = 1.843, P = 0.068), Shannon-Weiner (bias towards rare organisms, Z = -0.017, P = 1), Simpsons reciprocal (bias toward more dominant organisms, Z = 0.261, P = 0.812) and evenness (Z = -0.052, P = 0.973) (S1 Fig).
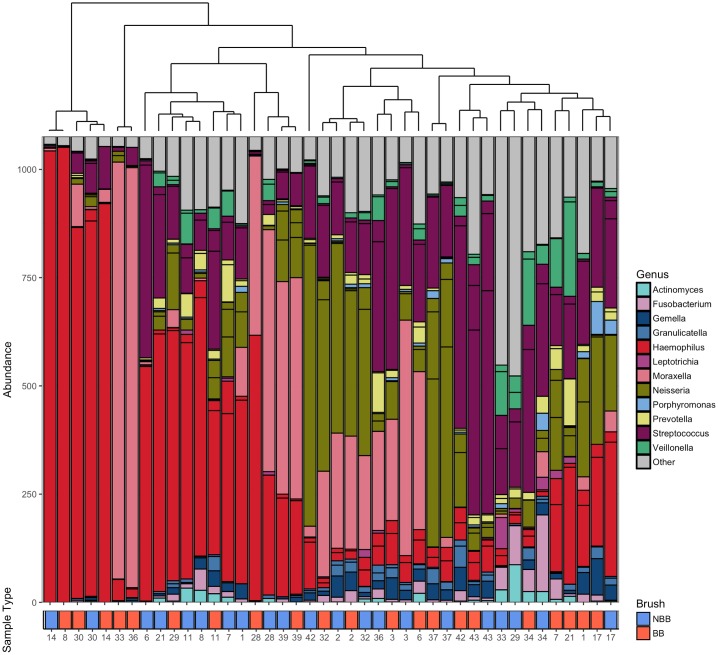
Considering community composition no significant differences were observed between the different sampling methods (Adonis: R2 = 0.012, P = 0.344). Hierarchical clustering using Bray-Curtis dissimilarity revealed that the samples clustered more closely between patients than within sampling groups (Fig 1).
Fig 1. Ordered bar chart of the top 20 OTUs present in both the blind and non-blind brushings.
Samples are ordered by a Bray Curtis dissimilarity hierarchical cluster shown by the top plot. Key to colours used for each genus is included. Identical patient numbers indicate samples were taken from the same individual. Sample type is indicated in the labelling beneath the graph with red indicating blind brush and blue indicating non-blind brush.
PBB versus healthy controls
The bacterial community of patients diagnosed with PBB (N = 24) was compared to healthy controls (N = 18). Samples were rarefied to 1,150 reads, resulting in the loss of 2 control patients. No difference in the bacterial abundance by qPCR was observed between the healthy controls and PBB patients (R2 = 0.021, P = 0.511) (Fig 2). Investigation into alpha diversity measures showed that PBB patients had a significantly lower diversity than the healthy controls (S2 Fig). This was seen across all measures; the Wilcoxon rank sum test, richness (W = 100.5, P = 0.001), Shannon-Weiner (W = 78, P < 0.001), Simpson’s reciprocal (W = 79, P < 0.001) and evenness (W = 84, P < 0.001).
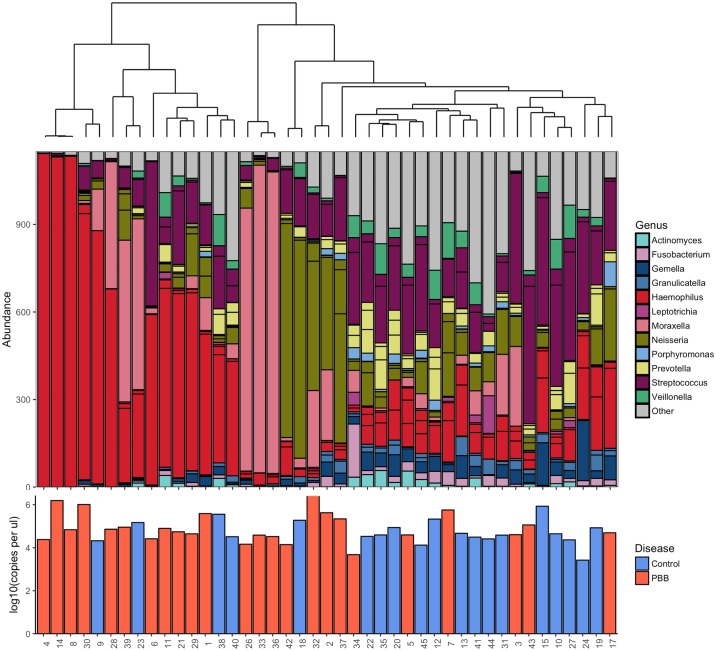
Fig 2. Ordered bar chart of the top 20 OTUs present in both the PBB and control subjects.
Samples are ordered by a Bray Curtis dissimilarity hierarchical cluster, upper plot. Key to colours used for each genus is included. Patient numbers are detailed in the lower plot where disease status is also indicated by colour of the bars with red indicating PBB and blue indicates control subjects. Additionally lower bar plot indicates the log10 copies per μl as calculated by qPCR. No significant difference was found between the qPCR values between the PBB and control patients (R2 = 0.023, P = 0.445).
Differences in community composition were investigated using Bray-Curtis dissimilarity. Adonis showed significant differences in community composition (R2 = 0.082, P = 0.001). Hierarchical clustering using Bray-Curtis dissimilarity showed that the bacterial community present in healthy controls clustered separately from those with PBB (Fig 2).
DESeq2 was used to identify OTUs significantly associated with PBB. Haemophilus and Neisseria OTUs were identified as being significantly increased in patients with PBB (P < 0.001) (S3 Fig). This result was backed up using indicator species analysis between the PBB and control communities. Two OTUs were significantly associated with the PBB group, Haemophilus_3673 (P = 0.043) and Neisseria_4022 (P = 0.05). Haemophilus_3673 was a member of the top 20 most abundant OTUs observed. The control group had 35 OTUs significantly associated, 9 of which were included in the top 20 most abundant OTUs.
Comparing the results of standard clinical bacterial culture to the 16s rRNA gene sequencing results for the 24 PBB patients, 20 (83.33%) were culture positive. The four patients that were culture negative were, by sequencing, dominated either by Moraxella, Neisseria or Haemophilus OTUs. Whilst no patient cultured Neisseria, 5 of the patients were found to be dominated by a Neisseria OTU from the sequencing results. Seventeen (70.83%) of the 24 patients cultured Haemophilus influenzae, while only 9 (52.94%) of the same patients were found to be Haemophilus dominated by sequencing. Moraxella was cultured from 3 patients, in two of the three Moraxella was found to be the dominant organism by sequencing. Another 2 patients, however, were dominated by the Moraxella OTU despite not being positive on culture for the bacterium. Only fifteen of the 24 patients (62.5%) cultured the dominant organism that was identified by sequencing.
Neither parental smoking habits (R2 = 0.037, P = 0.145) or breastfeeding (R2 = 0.012, P = 0.846) were found to influence the bacterial community differences between patients with PBB and healthy controls.
To ascertain if wheeze explained any of the variation in the bacterial community observed between patients, wheeze diagnosis was investigated. No significant difference was observed between patients with and without wheeze (R2 = 0.048, P = 0.34) (S4 Fig). No control patients were diagnosed with wheeze.
Impact of respiratory virus on bacterial composition
PBB patients were tested for the presence of respiratory viruses. The presence of a virus (R2 = 0.166, P = 0.167) or number of different viruses present (R2 = 0.227, P = 0.095) in patients had no significant effect on the bacterial community composition. None of the 6 respiratory viruses showed any significant effect on the bacterial community composition, rhinovirus (R2 = 0.057, P = 0.266), respiratory syncytial virus (RSV) (R2 = 0.048, P = 0.406), Coronavirus (R2 = 0.027, P = 0.844), human metapneumovirus (HMP) (R2 = 0.052, P = 0.314), adenovirus (R2 = 0.008, P = 0.99), parainfluenza (R2 = 0.057, P = 0.259).
Sampling of mothers, throat swabs versus nose swabs
Mothers of 16 children (11 PBB cases and 6 healthy controls) were sampled using both nose and throat swabs. To investigate if these sampling methods were comparable, samples were rarefied to 1,154 reads (4 samples were lost after rarefaction) after which non-metric multidimensional scaling (NMDS) using Bray-Curtis distance was used to investigate clustering based on bacterial community similarity (S5 Fig).
Notably samples from the same mother did not cluster together (S6 Fig). No significant difference in Shannon-Weiner (Z = -0.44, P = 0.7) or Simpson’s reciprocal (Z = -1.24, P = 0.24) diversity was observed between the two groups of samples nose versus throat swabs. There was however a significant difference in richness (Z = 2.58, P < 0.01) with throat swabs having more distinct OTUs than nose swabs. Additionally, bacterial abundance as determined by qPCR was significantly higher in throat swabs compared to nasal swabs (Z = 2.84, P < 0.01) (S6 Fig). Analysis by ADONIS confirmed these results, with 11% of the variation between samples being explained by the sample type (R2 = 0.11, P = 0.02).
Children and mothers
The bacterial community within the lung of children under the age of 2 years, both with (N = 11) and without a PBB diagnosis (N = 5), was compared to the bacterial communities present in the nose and throat of their mothers. Samples were rarefied to 1,067 reads. Neither nose or throat swabs of mothers were found to have significantly different bacterial richness compared to child communities using a Wilcoxon rank sum test, applied to independent samples (nose; richness, W = 111, P = 0.317, throat; richness, W = 164.5, P = 0.032). Significant differences in both Shannon-Weiner and Simpson’s reciprocal were however observed between the two groups (nose; Shannon-Weiner, W = 138, P = 0.019, Simpson’s reciprocal, W = 138, P = 0.019; throat; Shannon-Weiner, W = 177, P = 0.007, Simpson’s reciprocal, W = 185, P = 0.002). Adonis showed there was significant differences in community composition when comparing between different sampling methods, while controlling for sample family (R2 = 0.182, P < 0.001), indicting that the upper respiratory tract from mothers is not comparable to their children.
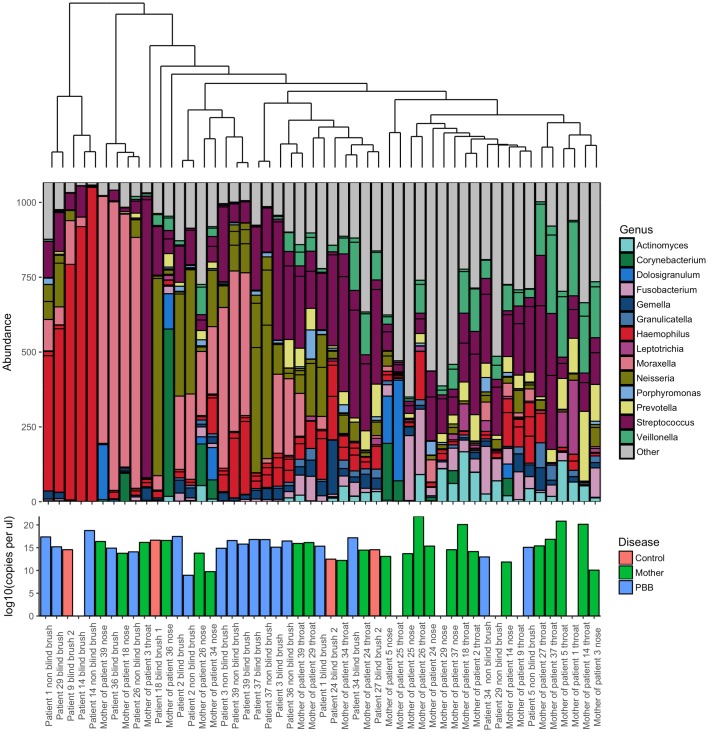
Bray-Curtis hierarchical clustering was used to detect any patterns of similarities in community composition between mothers and their children (Fig 3). Overall the samples collected from the mothers compared to those from the PBB children were significantly different, however there was a single exception. The bacterial communities present in the throat swab of the mother and the blind brush from the child of family 34 were found to have in common a high abundance of Streptococcus, as well as Veillonella and Neisseria (S6 Fig).
Fig 3. Ordered bar chart of the top 20 OTUs present in both the mother of study children less than 2 years old and their children.
This subgroup includes both healthy children (red) and those diagnosed with PBB (blue). Mothers are indicated in green. Samples are ordered by a Bray Curtis dissimilarity hierarchical cluster, shown above. Key to colours used for each genus is included. Lower bar plot indicates the log10 copies per μl as calculated by qPCR.
Discussion
This study provides the first insight into the composition of the bacterial community present within the lungs of both healthy infants and children and those with persistent bacterial bronchitis. Investigation into the bacterial community within the lungs of these children highlights the impact of PBB on the lung microbiota and provides insight into disease progression.
Across a range of pulmonary diseases, including COPD, non-CF bronchiectasis and cystic fibrosis, a reduction in the diversity and the appearance of dominant OTUs from potentially pathogenic genera has been observed [16–20]. It is perhaps unsurprising therefore, that in the present study a significant reduction in bacterial diversity associated with PBB was observed.
In this present study, Haemophilus, Neisseria, Streptococcus and Moraxella were all represented amongst the dominant OTUs in PBB samples, although DESeq2 and indicator species analysis showed that only a Haemophilus and a Neisseria OTU were significantly associated with PBB. This is likely to be due to the number of patients dominated in this particular sample set (Fig 3). Importantly in many cases the dominant organisms by sequencing were not those identified by culture, highlighting the potential limitations of traditional culture techniques the results of which typically dictate therapeutic strategies. It has been previously observed that conventional microbiology has the potential to miss the presence of potentially pathogenic organisms [16, 21].
Sequencing identified a Neisseria OTU as dominant in a number of subjects in this study yet culture failed to identify Neisseria in any patients. This failure to recognize organisms in chronic airways diseases may account in part for the ‘negative’ culture results from BAL and sputum samples that are frequently encountered in the presence of purulent secretions. This may be of particular importance when it comes to Neisseria species, which are commonly found to colonise the nasopharyngeal mucosa [22]. Despite many Neisseria species being considered non-pathogenic, they have been implicated in cases of pneumonia [23], periodontal disease [22] and COPD [24].
No significant differences in the bacterial community of PBB patients whose parents were smokers compared to non-smokers were found. However, due to the low number of individuals in this study we cannot exclude smoking having an effect on the bacterial communities of children. It would be important to expand this research to include a much larger sample set to investigate this further.
Wheeze is a common symptom associated with PBB [25], however in this dataset it was only diagnosed in a subset of children suffering from PBB and not in controls. No difference in the bacterial community was associated with a diagnosis of wheeze however, this may due to insufficient power.
In this study nose and throat samples were collected from mothers of children under 2 years old and compared with the results from the lower airways of their offspring. Nose swabs from mothers were significantly different from their own throat swabs, showing that there are major differences between these areas of the upper respiratory tract. Previous studies have shown that unlike the nose the bacterial community from the throat is more similar to that of the lower airways [11]. It was hypothesised that the bacterial community present in the respiratory tract of mothers and children would therefore potentially share similarities, due to their close proximity and shared environments when children are under 2 years of age. In the majority of cases however, the community composition of the mothers’ samples were significantly different from their children. Only a single case was observed where the maternal oropharyngeal community and their child’s lower airway bacterial communities were similar, and these samples were found to be dominated by the same organism.
The inclusion of lower airway samples from healthy controls provides a challenge, particularly in paediatric studies, due to the invasive nature of the sampling methods required to access the lower respiratory tract. This has resulted in many paediatric studies either sampling the upper respiratory tract [26] or including “controls” with other respiratory indications [9, 11]. Hilty et al. observed a decrease in the Proteobacteria and an increase in Bacteroidetes in controls compared to asthmatics, although only 3 of their 7 controls had no respiratory symptoms [11]. The current study demonstrates that the data generated from a blind brushing via an ET tube is comparable to that obtained by visualization using bronchoscopy, the so-called non-blind brush, allowing the inclusion of healthy children attending the hospital for reasons unrelated to respiratory symptoms.
This has important implications for future studies of the lower airways microbiota, particularly those involving infants and children, as a non-invasive method will allow greater number of subjects to be studied and affords the opportunity of conducting longitudinal studies with more regular sampling.
Conclusion
In conclusion, the bacterial community within the lungs of children with PBB shows a significantly lower diversity than that observed in healthy children. This is due to the high levels of dominance of Haemophilus, Neisseria, Streptococcus and Moraxella. In many cases the dominant organisms by sequencing were not those identified by culture. This study is the first step in using next generation sequencing methods to increase our understanding of the bacterial community within the lungs of children with PBB. These methods have the potential to lead to quicker more effective treatments, reducing the risk of recurrence or disease progression.
Supporting information
This file includes supplementary methods and tables.
(DOCX)
(DOCX)
(DOCX)
Using a Wilcoxon paired sign rank test no significant difference was observed between sampling methods. Richness; Z = 1.843, P = 0.068, Shannon-Weiner; Z = -0.017, P = 1, Simpsons; Z = 0.261, P = 0.812, evenness; Z = -0.052, P = 0.973.
(TIF)
Using a Wilcoxon sign rank test significant differences in richness (W = 90.5, P = 0.001), Shannon-Weiner (W = 70, P < 0.001), Simpson’s reciprocal (W = 76, P < 0.001) and evenness (W = 65, P < 0.001) were observed between the two groups.
(TIF)
Grey points indicate OTUs with P > 0.001. Colours indicate OTU genus, while size indicates the sum of the reads in each OTU. Significant OTUs showed a more than 2.5 fold increase in abundance compared to controls.
(TIF)
Samples are ordered by a Bray Curtis dissimilarity hierarchical cluster upper plot with the lower plot indicating if patient has a diagnosis of wheeze. Key to colours used for each genus is included.
(TIF)
Nose swabs are indicated in blue, throat swabs are indicated in red. Mothers of PBB patients are indicated by triangles while the mothers of controls are shown by circles.
(TIF)
Rarefaction resulted in the removal of some samples due to low sequence numbers, the paired samples from these patients were removed to allow paired analysis. Samples are ordered by a Bray Curtis dissimilarity hierarchical cluster. Key to colours used for each genus is included. Log10 copies of 16S rRNA gene per μl of sample is shown in the lower plot with colour indicating sample type; blue being nasal swab and red being throat swab. Note: missing data 25 (Mother of Patient 25 throat) and 29 (Mother of patient 29 nose) samples had DNA concentrations below the detection limit of the qPCR assay.
(TIF)
Data Availability
Sequencing data generated during this study has been submitted to the European nucleotide archive (ENA), project number PRJEB18478, and is freely available. Data analysis scripts have been submitted to figshare, DOI: 10.6084/m9.figshare.4987016.
Funding Statement
The study was funded by the Wellcome Trust under WT097117 and WT096964. This project was funded and supported by the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London and the Sheffield Children’s Hospital Charity Research Fund. M.F. Moffatt and W.O.C.M. Cookson are Joint Wellcome Trust Senior Investigators.
References
- 1.Craven V, Everard ML. Protracted bacterial bronchitis: reinventing an old disease. Arch Dis Child. 2013;98:72–76. doi: 10.1136/archdischild-2012-302760 [DOI] [PubMed] [Google Scholar]
- 2.Chang AB, Upham JW, Masters IB, Redding GR, Gibson PG, Marchant JM, Grimwood K. Protracted bacterial bronchitis: The last decade and the road ahead. Pediatr Pulmonol. 2016;51:225–242. doi: 10.1002/ppul.23351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishak A, Everard ML. Persistent and recurrent bacterial bronchitis—a paradigm shift in our understanding of chronic respiratory disease. Front Pediatr. 2017;9:19 doi: 10.3389/fped.2017.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnelly D, Critchlow A, Everard ML. Outcomes in children treated for persistent bacterial bronchitis. Thorax. 2007;62:80–84. doi: 10.1136/thx.2006.058933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickson RP, Erb-Downward JR, Martinez FJ, Huffnagle GB. The Microbiome and the Respiratory Tract. Annu Rev Physiol 2015;1–24. doi: 10.1146/annurev-physiol-021115-105238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Beck JM, Huffnagle GB, et al. Spatial variation in the healthy human lung microbiome and the adapted island model of lung biogeography. Ann Am Thorac Soc. 2015;12:821–830. doi: 10.1513/AnnalsATS.201501-029OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL, et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med. 2013;187:1067–1075. doi: 10.1164/rccm.201210-1913OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet. 2014;384:691–702. doi: 10.1016/S0140-6736(14)61136-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Der Gast CJ, Cuthbertson L, Rogers GB, Pope C, Marsh RL, Redding GJ, et al. Three clinically distinct chronic pediatric airway infections share a common core microbiota. Ann Am Thorac Soc. 2014;11:1039–1048. doi: 10.1513/AnnalsATS.201312-456OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsh RL, Kaestli M, Chang AB, Binks MJ, Pope CE, Hoffman LR, et al. The microbiota in bronchoalveolar lavage from young children with chronic lung disease includes taxa present in both the oropharynx and nasopharynx. Microbiome. 2016;4:37 doi: 10.1186/s40168-016-0182-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578 doi: 10.1371/journal.pone.0008578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. doi: 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.R Developement Core Team. R: A Language and Environment for Statistical Computing. R Found Stat Comput; 2015; [Google Scholar]
- 14.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550 doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Cáceres M, Legendre P. Associations between species and groups of sites: indices and statistical inference. Ecology. 2009;90:3566–3574. [DOI] [PubMed] [Google Scholar]
- 16.Duff RM, Simmonds NJ, Davies JC, Wilson R, Alton EW, Pantelidis P, et al. A molecular comparison of microbial communities in bronchiectasis and cystic fibrosis. Eur Respir J. 2013;41:991–993 doi: 10.1183/09031936.00052712 [DOI] [PubMed] [Google Scholar]
- 17.Mammen MJ, Sethi S. COPD and the microbiome. Respirology. 2016;21:590–599. doi: 10.1111/resp.12732 [DOI] [PubMed] [Google Scholar]
- 18.Rogers GB, van der Gast CJ, Cuthbertson L, Thomson SK, Bruce KD, Martin ML, et al. Clinical measures of disease in adult non-CF bronchiectasis correlate with airway microbiota composition. Thorax. 2013;68:731–7. doi: 10.1136/thoraxjnl-2012-203105 [DOI] [PubMed] [Google Scholar]
- 19.Rogers GB, van der Gast CJ, Serisier DJ. Predominant pathogen competition and core microbiota divergence in chronic airway infection. ISME J. 2015;9:217–225. doi: 10.1038/ismej.2014.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sze MA, Dimitriu PA, Suzuki M, McDonough JE, Campbell JD, Brothers JF, et al. Host Response to the Lung Microbiome in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2015;192:438–445. doi: 10.1164/rccm.201502-0223OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickson RP, Erb-Downward JR, Prescott HC, Martinez FJ, Curtis JL, Lama VN, et al. Analysis of Culture-Dependent versus Culture-Independent Techniques for Identification of Bacteria in Clinically Obtained Bronchoalveolar Lavage Fluid. In: Doern G V, editor. J Clin Microbiol 2014;52:3605–3613. doi: 10.1128/JCM.01028-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vieira Colombo AP, Magalhães CB, Hartenbach FARR, Martins do Souto R, Maciel da Silva-Boghossian C. Periodontal-disease-associated biofilm: A reservoir for pathogens of medical importance. Microb Pathog 2016;94:27–34. doi: 10.1016/j.micpath.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 23.Vienne P, Ducos-Galand M, Guiyoule A, Pires R, Giorgini D, Taha M-K, et al. The Role of Particular Strains of Neisseria meningitidis in Meningococcal Arthritis, Pericarditis, and Pneumonia. Clin Infect Dis 2003;37:1639–1642. doi: 10.1086/379719 [DOI] [PubMed] [Google Scholar]
- 24.Cabrera-Rubio R, Garcia-Nunez M, Seto L, Anto JM, Moya A, Monso E, et al. Microbiome diversity in the bronchial tracts of patients with chronic obstructive pulmonary disease. J Clin Microbiol 2012;50:3562–3568. doi: 10.1128/JCM.00767-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kompare M, Weinberger M. Protracted Bacterial Bronchitis in Young Children: Association with Airway Malacia. J Pediatr 2012;160:88–92. doi: 10.1016/j.jpeds.2011.06.049 [DOI] [PubMed] [Google Scholar]
- 26.Biesbroek G, Tsivtsivadze E, Sanders EAM, Montijn R, Veenhoven RH, Keijser BJF, et al. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am J Respir Crit Care Med 2014;190:1283–1292. doi: 10.1164/rccm.201407-1240OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file includes supplementary methods and tables.
(DOCX)
(DOCX)
(DOCX)
Using a Wilcoxon paired sign rank test no significant difference was observed between sampling methods. Richness; Z = 1.843, P = 0.068, Shannon-Weiner; Z = -0.017, P = 1, Simpsons; Z = 0.261, P = 0.812, evenness; Z = -0.052, P = 0.973.
(TIF)
Using a Wilcoxon sign rank test significant differences in richness (W = 90.5, P = 0.001), Shannon-Weiner (W = 70, P < 0.001), Simpson’s reciprocal (W = 76, P < 0.001) and evenness (W = 65, P < 0.001) were observed between the two groups.
(TIF)
Grey points indicate OTUs with P > 0.001. Colours indicate OTU genus, while size indicates the sum of the reads in each OTU. Significant OTUs showed a more than 2.5 fold increase in abundance compared to controls.
(TIF)
Samples are ordered by a Bray Curtis dissimilarity hierarchical cluster upper plot with the lower plot indicating if patient has a diagnosis of wheeze. Key to colours used for each genus is included.
(TIF)
Nose swabs are indicated in blue, throat swabs are indicated in red. Mothers of PBB patients are indicated by triangles while the mothers of controls are shown by circles.
(TIF)
Rarefaction resulted in the removal of some samples due to low sequence numbers, the paired samples from these patients were removed to allow paired analysis. Samples are ordered by a Bray Curtis dissimilarity hierarchical cluster. Key to colours used for each genus is included. Log10 copies of 16S rRNA gene per μl of sample is shown in the lower plot with colour indicating sample type; blue being nasal swab and red being throat swab. Note: missing data 25 (Mother of Patient 25 throat) and 29 (Mother of patient 29 nose) samples had DNA concentrations below the detection limit of the qPCR assay.
(TIF)
Data Availability Statement
Sequencing data generated during this study has been submitted to the European nucleotide archive (ENA), project number PRJEB18478, and is freely available. Data analysis scripts have been submitted to figshare, DOI: 10.6084/m9.figshare.4987016.