Abstract
Pericytes are vascular mural cells embedded in the basement membrane of blood microvessels. They extend their processes along capillaries, pre-capillary arterioles, and post-capillary venules. The central nervous system (CNS) pericytes are uniquely positioned within the neurovascular unit between endothelial cells, astrocytes, and neurons. They integrate, coordinate, and process signals from their neighboring cells to generate diverse functional responses that are critical for CNS functions in health and disease including regulation of the blood-brain barrier permeability, angiogenesis, clearance of toxic metabolites, capillary hemodynamic responses, neuroinflammation, and stem cell activity. Here, we examine the key signaling pathways between pericytes and their neighboring endothelial cells, astrocytes, and neurons that control neurovascular functions. We also review the role of pericytes in different CNS disorders including rare monogenic diseases and complex neurological disorders such as Alzheimer's disease and brain tumors. Finally, we discuss directions for future studies.
Keywords: Pericytes, neurovascular functions, signal transduction pathways, neurological disorders
The neurovascular unit (NVU) is comprised of vascular cells (pericytes, vascular smooth muscle cells (VSMCs), endothelial cells), glial cells (astrocytes, microglia, oligodendrocytes), and neurons1–3. Pericytes are centrally positioned within the NVU between endothelial cells, astrocytes, and neurons (Figure 1a). They receive signals from their neighboring cells and generate functional responses that are essential for proper central nervous system (CNS) functioning2,4–6 (Figure 1b).
Figure 1. The multi-functional role of CNS pericytes at the neurovascular unit (NVU).
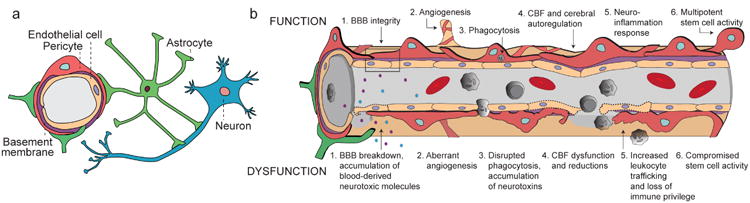
(a) A simplified NVU diagram showing the interactive cellular network at the level of brain capillaries that comprises vascular cells (e.g., pericytes and endothelial cells), glial cells (e.g., astrocytes), and neurons. Intricate cell-cell communication and signal transduction mechanisms of NVU cell types are highly controlled to regulate numerous functions in the CNS. (b) Under physiological conditions (top row), pericytes regulate 1) blood-brain barrier (BBB) integrity, i.e., tight/adherens junctions and transcytosis across the BBB; 2) angiogenesis, i.e. microvascular remodeling, stability, and architecture; 3) phagocytosis, i.e., clearance of toxic metabolites from the CNS; 4) cerebral blood flow (CBF) and capillary diameter; 5) neuroinflammation, i.e., leukocyte trafficking into the brain; and 6) multipotent stem cell activity. Pericyte dysfunction (bottom row) is characterized by 1) BBB breakdown causing leakage of neurotoxic blood-derived molecules into the brain (e.g., fibrinogen, thrombin, plasminogen, erythrocyte-derived free iron, and anti-brain antibodies); 2) aberrant angiogenesis; 3) impaired phagocytosis causing CNS accumulation of neurotoxins; 4) CBF dysfunction and ischemic capillary obstruction; 5) increased leukocyte trafficking promoting neuroinflammation; and 6) impaired stem cell-like ability to differentiate into neuronal and hematopoietic cells. Pericyte dysfunction is present in numerous neurological conditions and can contribute to the disease pathogenesis.
Endothelial cells form the blood-brain barrier (BBB) that sanctions entry of macromolecules, cells, and pathogens from blood into the CNS. Brain endothelium also regulates CNS transport of energy metabolites, nutrients, and ions and clearance of neurotoxic metabolites1,7. The BBB integrity is maintained chiefly by pericytes8–10. Endothelial tight junctions and lack of fenestrae contribute to a physical barrier5,7–11, which prevents transport of peptides and proteins into the brain12,13 unless they have specific carriers and/or receptors in brain endothelium14,15. The BBB integrity is vital for normal CNS functions as illustrated by rare genetic human diseases where specific gene defects in pericytes, endothelial cells, or astrocytes lead to NVU disruption and neurological disorders7. Pericyte degeneration and BBB breakdown are found in complex neurological disorders such as Alzheimer's disease (AD)1,3,16. Additionally, pericytes contribute to CNS tumor angiogenesis and growth5.
Here, we review the functions and signal transduction pathways in CNS pericytes in health and disease.
Pericytes: Characterization, function, and dysfunction
Characterization
Pericytes are embedded in the basement membrane of small blood vessels including capillaries, pre-capillary arterioles, and post-capillary venules2. Originally described by Charles Rouget, the term ‘pericytes’ was coined by Zimmermann in 1923 who proposed several subtypes of pericytes based on their morphology, location within the vascular network, and function17,18. Pericytes express several contractile and cytoskeletal proteins (e.g., α-smooth muscle actin (α-SMA), vimentin, desmin, myosin, nestin)5,6,19–21 and cell surface antigens (e.g., transmembrane chondroitin sulfate proteoglycan NG2, platelet-derived growth factor receptor-β (PDGFRβ), aminopeptidases A and N (CD13), regulator of G-protein signaling-5 (RGS5), CD146)5,18,21,22, some of which are also found on VSMCs2,21,22.
Recent studies of the cortical angioarchitecture in mice expressing fluorescent proteins under control of the NG2 and PDGFRβ promoters have identified several pericyte subpopulations including VSMC-pericyte hybrid on pre-capillary arterioles, thin strand helical mid-capillary pericyte, and mesh pericyte with a stellate morphology on post-capillary arterioles and venules19,20. Future single-cell RNA-seq and proteomic studies as used successfully to characterize subpopulations of cortical progenitor cells23 and drug resistant tumor cells24, may also contribute to better understanding of different pericyte subtypes.
Function
Pericytes regulate BBB permeability, angiogenesis, clearance, cerebral blood flow (CBF), neuroinflammation, and stem cell activity (Figure 1b).
BBB permeability
Pericytes control the expression of endothelial BBB tight and adherens junction proteins, the alignment of tight junction proteins, and bulk flow transcytosis of fluid-filled vesicles across the BBB8–10. The molecular pathways between endothelial cells and pericytes that can be manipulated to open the BBB ‘on demand’ for delivery of neuropharmaceuticals and/or to reverse BBB breakdown in neurological disorders7, remain at present, however, largely unknown.
Angiogenesis
Pericytes regulate angiogenesis, microvascular stability, and angioarchitecture during CNS development and vascular remodeling2,5,25.
Clearance
Pericytes can act as perivascular tissue macrophages to clear tissue debris and foreign proteins injected systemically and/or locally in the CNS2,9,10,26,27 and participate in clearance of Alzheimer's amyloid-β (Aβ) toxin, as shown in AD mice26.
Cerebral blood flow
VSMCs control dilation and constriction of arterioles and small brain arteries28,29. However, recent studies in cortical and cerebellar brain slices and retinal explants and in vivo studies of cortical, retinal, olfactory bulb, and ear microcirculation have demonstrated that capillaries contribute to hemodynamic responses19,30–34. It has been shown that pericytes regulate capillary tone and diameter6,18,35, as discussed in greater detail below. Some recent studies failed to show the role of pericytes in capillary blood flow regulation36, but the controversy has been attributed to a drift in pericyte definition, particularly renaming the mid-capillary pericytes into VSMCs19.
Neuroinflammation
Studies using transgenic pericyte-deficient mice have shown that pericytes control endothelial-mediated leukocyte adhesion and transmigration into the CNS37, and studies in wild type mice demonstrate enhanced leukocytes trafficking in microvascular regions lacking pericyte coverage38,39. The role of pericytes in neuroinflammation has also been suggested by in vitro studies40,41. Altogether, these findings suggest that immune activation of brain pericytes may contribute to communicating inflammatory signals within the NVU.
Stem cell activity
In vitro studies have suggested that cultured pericytes have multipotent stem cell potential17,42. Moreover, primary murine pericytes isolated from brain following ischemic stroke exhibited multipotential stem cell activity and differentiated into neural and vascular lineage cells42.
Dysfunction
Pericyte degeneration leads to BBB breakdown causing brain accumulation of blood-derived neurotoxic molecules10,43–45. Pericyte ischemic injury results in contractile rigor and obstruction of capillary blood flow30,46. Pericyte-specific genetic defects lead to primary familial brain calcification or Fahr's disease47,48. Pericytes degenerate and likely play a role in cerebrovascular dysfunction in complex neurological diseases such as AD26,49,50, amyotrophic lateral sclerosis (ALS)51, and type 2 diabetes mellitus (T2DM)-related microangiopathy and retinopathy52–54. Pericyte dysfunction has been also associated with human immunodeficiency virus (HIV)-related dementia55, epilepsy56, cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL)57, and brain cancer58,59.
Pericyte-endothelial signal transduction
Some of the key pathways between pericytes and endothelial cells are discussed below.
PDGF-BB/PDGFRβ pathway
Pathway characterization
The platelet-derived growth factor (PDGF) family has four ligands (A-D) and two receptors (α and β)59. PDGF receptor tyrosine kinases (PDGFR) form three active conformation dimers – αα ββ and αβ. The PDGF ligands differentially bind PDGFRs, specifically i) PDGFR-αα ligands are PDGF-AA, -CC, -AB, -BB; ii) PDGFR-αβ ligands are PDGF-AB, -BB, -CC, -DD; and iii) PDGFR-ββ ligands are PDGF-BB, -DD; the underlined ligands denote high affinity ligand-receptor interactions59. Here, we will focus on endothelial-secreted PDGF-BB and PDGFRβ, a receptor on pericytes5,60,61.
Precise spatial and temporal regulation of PDGF-BB signaling is achieved via its “retention motif,” a region of positively charged amino acids at the C terminus, which binds to negatively charged heparin sulfate proteoglycans of the extracellular matrix (ECM) resulting in retention of PDGF-BB that generates a concentration gradient5, as shown by in vitro studies. PDGF-BB binds to PDGFRβ causing non-covalent dimerization and autophosphorylation of the receptor on up to 13 cytoplasmic tyrosine (Y) residues, which activates PDGFRβ62 (Figure 2). Once activated, distinct phosphorylated Y residues of PDGFRβ are bound by specific Src homology 2 (SH2) domain-containing proteins, including phospholipase Cγ (PLCγ), Src family kinase (SFK), Grb2, phosphatidyl inositol 3-phosphate (PI3K), GTPase activating protein (GAP), SH2 tyrosine phosphatase (SHP-2) and Stat5, to induce downstream signaling, which promotes pericyte survival, proliferation, migration, and recruitment to the vessel wall2,62.
Figure 2. PDGF-BB/PDGFRp signaling in pericytes.
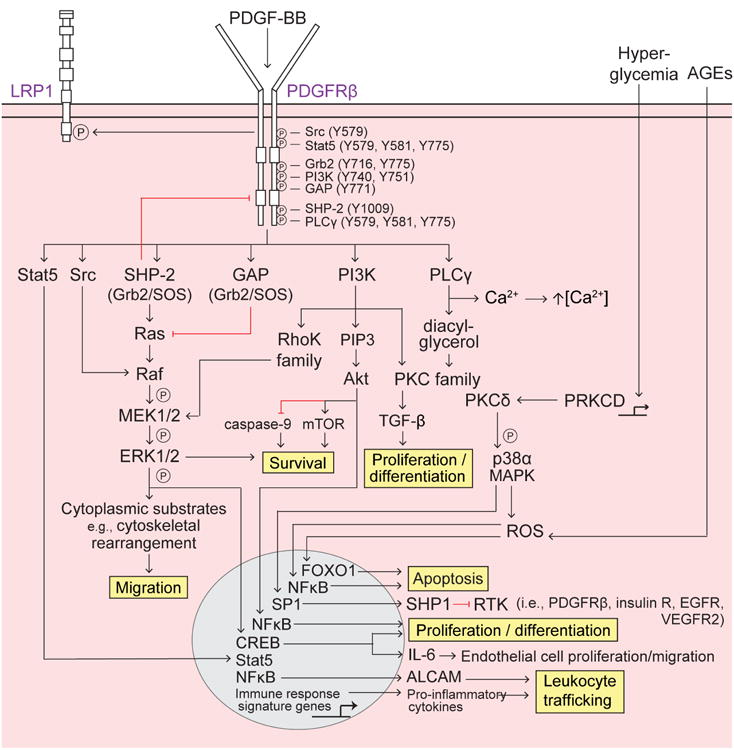
Platelet-derived growth factor-BB (PDGF-BB) secreted by endothelial cells binds to the PDGF receptor-β (PDGFRβ) on pericytes, causing receptor dimerization, autophosphorylation, and activation. Several Src homology 2 (SH2) domain-containing proteins (Src, Stat5, Grb2, phosphatidyl inositol 3-phosphate (PI3K), GTPase activating protein (GAP), SH2 tyrosine phosphatase (SHP-2), and phospholipase Cγ (PLCγ) bind to distinct phosphorylated (P) tyrosine (Y) residues SH2 domain-containing proteins bound to PDGFRβ differentially activate downstream signaling pathways to regulate pericyte survival, migration, apoptosis, proliferation and differentiation, as well as leukocyte trafficking, described as follows: Survival – promoted via PI3K-Akt activation of mammalian target of rapamycin (mTOR) and inhibition of caspase-9 and the SHP-2-mediated MAPK pathway; Migration – SHP-2-mediated MAPK pathway promotes cytoskeletal rearrangement and cell migration. Src activated Raf synergistically activates the MAPK pathway whereas GAP inhibition of Ras decreases MAPK signaling; Apoptosis – Extracellular advanced glycation endproducts (AGEs) induce intracellular reactive oxygen species (ROS) and FOXO1-mediated apoptosis, and PRKCD transcriptional expression of protein kinase C-δ (PKCδ) activates p38α MAPK to induce downstream production of ROS and mitochondrial cytochrome c release, resulting in apoptosis; Proliferation and differentiation – promoted by the PI3K pathway, specifically via PKC-TGF-β and PIP3-Akt transcriptional activation of NFκB; Leukocyte trafficking – PDGFRβ regulates pro-inflammatory responses, e.g., peripheral leukocyte trafficking into the CNS, via transcriptional expression of immune response signaling genes (e.g., cytokines and chemokines) and also via Akt-induced activation of NFκB and transcriptional expression of the novel activated leukocyte cell adhesion molecule (ALCAM).
RasGAP binding to phospho-PDGFRβ causes simultaneous binding of the Grb2-SOS1 complex to Ras that offsets the activation of Ras and reduces the downstream activity of the extracellular signal-regulated protein kinases 1 and 2 (Erk1/2), as shown in vitro63. Activation of Notch ligands and/or Notch receptors and activation of a Notch signal integrator transcription factor, CSL, can modulate PDGFRβ signaling independently of PDGF-BB, as shown using in vitro cultures and Notch3-deficient mice64 (Figure 2).
Functional importance
Developmental studies using Pdgfb and Pdgfrβ transgenic mouse models60,62 have shown that PDGF-BB/PDGFRβ signaling is important for endothelial-mesenchymal communication and CNS blood vessel development and stabilization5. Both Pdgfb and Pdgfrβ null mice are embryonic lethal and lead to development of CNS microvascular instability, endothelial hyperplasia, microaneurisms, and blood vessel ruptures with microhemorrhages, whereas deletion of the single allele does not result in an apparent vascular phenotype in the developing CNS.
In contrast, during postnatal development, adulthood and aging brain pericytes may fulfill a different regulatory role, as suggested by recent work in mouse models with partially disrupted PDGF-BB/PDGFRβ signaling due to either mutation in the PDGF-BB retention motif (Pdgfbret/ret) or deficient PDGFRβ signaling which both result in age-dependent BBB breakdown and accumulation of blood-derived neurotoxic proteins in the neuropil and brain interstitial fluid (ISF)8–10. Deficient PDGFRβ signaling also leads to microvascular reductions, which in parallel with BBB breakdown may contribute to secondary neurodegeneration10,43.
In animal models of diabetic retinopathy, hyperglycemia leads to diminished PDGFRβ signaling resulting in pericyte apoptosis52, whereas studies of tumor angiogenesis have shown that pericyte loss may lead to endothelial apoptosis65. PDGFRβ signal transduction in pericytes also mediates proinflammatory responses at the BBB by transcriptional regulation of several chemokines that promote endothelial expression of monocyte chemoattractant protein-1 (MCP-1), nitric oxide (NO), interleukins IL-1, IL-6, IL-12, and tumor necrosis factor-α (TNF-α), resulting in transvascular trafficking of macrophages and leukocytes into the brain, as shown in pericyte-deficient Pdgfrβ+/- mice37. Disrupted PDGF-BB/PDGFRβ signaling upregulates vascular endothelial growth factor (VEGF)-A that accelerates vascular abnormalities, as shown in vivo in Pdgfb and Pdgfrβ deficient mice66.
PDGF-BB/PDGFRβ signaling in neurological disorders
Dysfunction in the PDGF-BB/PDGFRβ signaling pathway contributes to various CNS pathophysiologies, as discussed below.
Fahr's disease
Fahr's disease is characterized by migraines, mood swings, motor symptoms (e.g., Parkinsonism), and dementia, and its etiology includes loss-of-function mutations in PDGFB and PDGFRβ genes47,48 implicating involvement of pericytes. PDGFB mutations in humans and Pdgfbret/ret pericyte-deficient transgenic mice with mutation in the retention motif of PDGF-BB9 lead to calcifications in capillaries and small microvessels occurring mainly in the basal ganglia, which correlates with the degree of pericyte deficiency and BBB breakdown as shown in the murine model of this disease47.
Loss-of-function mutations in SLC20A2 gene encoding the type III sodium-dependent phosphate transporter 2 (PiT-2) are also associated with Fahr's disease67,68, and likely involve changes in phosphate transport at the BBB that promote regional brain accumulation of inorganic phosphate that subsequently cause calcium phosphate deposition67.
Alzheimer's disease
Pericytes degenerate in AD, as shown by post-mortem brain tissue studies in humans50,69–71 and animal models of AD26,72,73. Moreover, plasma PDGF-BB levels are increased in AD patients74 and soluble PDGFRβ (sPDGFRβ) levels, reflecting pericyte injury75, are increased in cerebrospinal fluid (CSF) of subjects with mild dementia and transgenic AD and pericyte-deficient murine models73, suggesting dysfunction in PDGF-BB/PDGFRβ pathway compared to control subjects and in experimental models.
In transgenic mice, deficient Pdgfrβ signaling leads to pericyte loss causing BBB disruption and microvascular reductions followed by neurodegenerative changes independently of Aβ10. However, studies in mice overexpressing Aβ-precursor protein (APP) crossed with pericyte-deficient Pdgfrβ+/- mice, i.e., APPSw/0;Pdgfrβ+/- mice, indicate that defective PDGF-BB/PDGFRβ signaling leads to faulty Aβ clearance from brain ISF by diminishing low-density lipoprotein receptor-related protein 1 (LRP1)-mediated Aβ clearance on pericytes26. Compared to control APPSw/0 mice that develop a moderate pericyte loss26,72, APPSw/0;Pdgfrβ+/- mice have an earlier onset of cerebral amyloid angiopathy (CAA) and Aβ load, and increased Aβ40 and Aβ42 levels in the brain26. Interestingly, accelerated pericyte degeneration in APPSw/0;Pdgfrβ+/- mice also leads to tau pathology and neuronal loss, which is not normally seen in APPSw/0 mice26. These data suggest that a double vascular-Aβ hit is needed for the development of full-spectrum AD pathology in mice. Whether the same double-hit contributes to the pathogenesis of late onset AD in humans, which is characterized by pericyte degeneration50,69–72, is unclear at present.
Mutations in SORL1 and SORCS1-3 genes encoding proteins containing vacuolar protein sorting-10 (Vps10) domains, namely sorL1 (also known as sorLA) and sorCS1-3, are risk factors for sporadic AD76,77 and diabetes78. Under normal conditions, sorL1 and sorCS1-3 interact with the retromer complex to facilitate intracellular trafficking, recycling, sequestration, and metabolism of different proteins including APP79. Single nucleotide polymorphisms (SNPs) in these proteins have been suggested to promote either aberrant APP clearance and/or processing76. PDGF-BB binds to sorL1, sorCS1, and sorCS380,81, which may influence its interaction with and/or downstream signaling from PDGFRβ that in turn might lead to pericyte dysfunction and/or degeneration as seen in late-onset AD50,69–71,73. In addition to PDGF-BB, sorL1 binds other LRP1 ligands similarly to LRP181, which may influence LRP1-mediated BBB clearance82. More studies are needed to evaluate the effects of sorL1-PDGF-BB and sorCS1/3-PDGF-BB interactions on downstream PDGFRβ signaling in pericytes and whether these Vsps10 proteins can provide a molecular link between AD and diabetes pathogenesis.
Interestingly, presenilin-1 (PSEN1) and PSEN2 mutations, the most frequent cause of autosomal-dominant AD (ADAD)83,84, both result in reduced PDGFRβ mRNA and protein levels, reduced PDGF-BB binding sites, and reduced PDGFRβ activation and autophosphorylation that consequentially suppress the downstream MEK/ERK and PI3K/Akt signaling pathways, as shown in PSEN1 and PSEN2 knockout cells85. These changes may lead to pericyte degeneration and BBB disruption that has been reported in post-mortem brain tissue analysis of ADAD patients84 and PSEN1 transgenic mutants86. Elucidating the exact mechanism by which PSEN1 and PSEN2 mutations impair PDGF-BB/PDGFRβ signaling would importantly inform how pericyte and microvascular dysfunction contributes to ADAD pathophysiology.
Several dominantly inherited rare vasculotropic APP mutations within Aβ 21 to 23 residues (e.g., Dutch, Flemish, Iowa, Arctic) primarily affect the cerebrovascular system leading to BBB breakdown, CAA, and hemorrhages with recurrent strokes, as recently reviewed87. Although APP mutations lead to degeneration of mural cells, whether deficient PDGF-BB/PDGFRβ signaling might contribute to loss of pericytes, rupture of blood vessels, and/or BBB disruption is currently unknown.
A possible mechanism illustrating how deficient PDGF-BB/PDGFRβ pathway causing pericyte dysfunction and degeneration can contribute to dementia and AD pathology in late-onset and early inherited familial cases is illustrated in Figure 3.
Figure 3. Deficient PDGF-BB/PDGFRp signaling in pericytes: A possible role in promoting neuronal dysfunction and degeneration in Alzheimer's dementia.
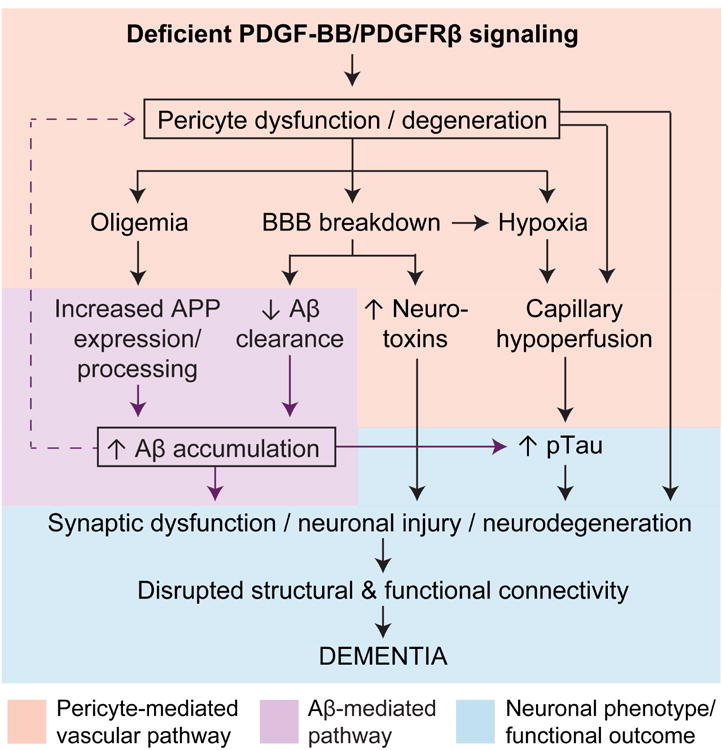
In the amyloid-β (Aβ)-independent pathway (pink box), a deficient PDGF-BB/PDGFRβ signaling leads to pericyte dysfunction and/or degeneration resulting in microvascular and cerebral blood flow (CBF) reductions and oligemia (brain hypoperfusion), from one hand, and blood-brain barrier (BBB) breakdown with accumulation of blood-derived toxic products in the brain, from the other. BBB breakdown leads to capillary edema contributing to capillary hypoperfusion and hypoxia. In the Aβ- pathway (purple box), oligemia leads to increased Aβ production, whereas BBB breakdown and deficient PDGFRβ signaling can both lead to faulty Aβ clearance, which in turn promotes Aβ accumulation in the brain. Synergistic action of Aβ-independent and Aβ pathway lead to accelerated tau hyperphosphorylation, formation of neurofibrillary tangles, synaptic dysfunction and loss, and neuronal degeneration, which altogether promotes behavioral deficits and dementia (blue box).
Amyotrophic lateral sclerosis
Microvascular pathology is present in sporadic and familial ALS cases carrying superoxide dismutase 1 (SOD1) mutations1,51. Pericyte degeneration in ALS patients coincides with BBB and blood-spinal cord-barrier (BSCB) breakdown in motor neuron dense regions of the spinal cord and motor cortex51. Consistently, ALS transgenic SOD1 mutants develop BSCB disruption, pericyte deficiency, and erythrocyte extravasation prior to motor neuron dysfunction43,51,88. Moreover, preventing BSCB breakdown delays the onset of motor neuron disorder in SOD1 mutants44. Whether ALS patients with the most frequent genetic cause of ALS, e.g., expanded hexanucleotide repeat of GGGGCC in a noncoding region of the C9Orf72 gene89, also develop pericyte degeneration and BBB breakdown and/or disrupted PDGF-BB/PDGFRβ signaling is presently unknown.
Type 2 diabetes mellitus and diabetic retinopathy
Hyperglycemia influences the downstream PDGFRβ signal transduction cascade to induce pericyte apoptosis, as shown in vivo in rats and in vitro in retinal cultures52–54 (Figure 2). Hyperglycemia-induced apoptosis in vivo can be prevented by inhibition of the PKCδ-p38α-SHP1 pathway52 and AGE-induced apoptosis by PDGFRβ downstream activation of Akt and NFκB54.
HIV-1-induced neurocognitive deficits
HIV-1-positive individuals can develop HIV-associated neurocognitive disorders (HAND) and HIV-associated dementia (HAD). Increased BBB permeability and pericyte loss, present in HAND and HAD, is thought to promote neurological impairments via increased transport of the HIV-1 viral neurotoxic protein, Tat101, into the brain55. Interestingly, Tat101 increases PDGF-BB expression and PDGF-BB/PDGFRβ signaling, specifically via MAPK and NFκB activation, resulting in elevated pericyte migration and deficiency55.
Brain cancer
The exact role of PDGFRβ signaling in pericytes in regulating growth and maturation of blood vessels in brain tumors remains still largely unexplored. Angiogenesis studies in the transgenic mouse model of pancreatic islet carcinogenesis (RIP1-Tag2) found that the broad spectrum receptor tyrosine kinase inhibitor SU6668, which preferentially targets PDGFRβ, leads to regression of blood vessels by detaching pericytes from tumor vessels that results in restricted tumor growth90. Similarly, SU6668 diminishes pericytes in xenograft tumors and restricts tumor growth91. Tumor-derived PDGFRP+ perivascular progenitor cells (PPCs) can differentiate into mature pericytes eliciting vascular stabilization, maturation, and survival65. Moreover, specific inhibition of PDGFRβ signaling eliminates PDGFRβ+ PPCs and mature pericytes around tumor vessels, leading to endothelial cell apoptosis in transgenic RIP1-Tag2 mice with pancreatic islet tumors65.
A recent study has shown that PDGFRβ regulates cell proliferation and invasion of medulloblastomas (MB) via JAG258, suggesting that PDGFRβ could be a potential therapeutic target for MB. It remains unclear, however, whether MB cells have the potential to differentiate into pericytes, as shown for some other types of brain neoplastic cells that can generate pericytes to control blood vessel function during tumor growth92,93.
TGF-P/TGFPR2 pathway
Pathway characterization
Transforming growth factor-(3 (TGF-β) is expressed in a latent form by pericytes, endothelial cells, neurons, and glia. Studies using in vitro primary co-cultures of endothelial cells and pericytes indicate that TGF-β activation at the BBB requires pericyte-endothelial cell interaction94, specifically formation of the gap junction connexin-43 hemichannels95. Activated TGF-β binds TGF-β receptor 2 (TGFβR2) on pericytes and endothelial cells2,5. TGF-β/TGFβR2 signals downstream via activin receptor-like kinase 5 (Alk5) in both pericytes and endothelial cells or Alk1 only in endothelial cells, as shown in primary bovine retinal cultures96 (Figure 4). In pericytes, Alk5-Smad2/3/4 signaling inhibits proliferation and promotes differentiation2,5 (Figure 4, left). For example, murine embryos with reduced TGFβR2-Alk5-Smad2/3 signaling exhibit enhanced pericyte proliferation97 and depleting TGF-β attenuates mural cell differentiation in murine embryonic mesenchymal cells and endothelial cell co-cultures98. In endothelial cells, Alk5 and Alk1 induce opposing effects via distinct downstream signal transduction events2,5,96. Specifically, in vitro studies in murine embryonic endothelial cells reveal that TGF-β/TGFβR2 signals downstream via i) Alk5-Smad2/3/4 to induce PDGF-BB expression and activate RBP-Jκ transcription factor to express N-cadherin to promote attachment; ii) Alk1-Smad1/5/8 to promote proliferation; and iii) PI3K-Akt pathway to promote survival and transcriptionally induce proliferation2,99,100 (Figure 4, right).
Figure 4. TGF-β/TGFβR2, Notch, VEGF-A/VEGFR2, Ang/Tie2 and MFSD2A signaling pathways.
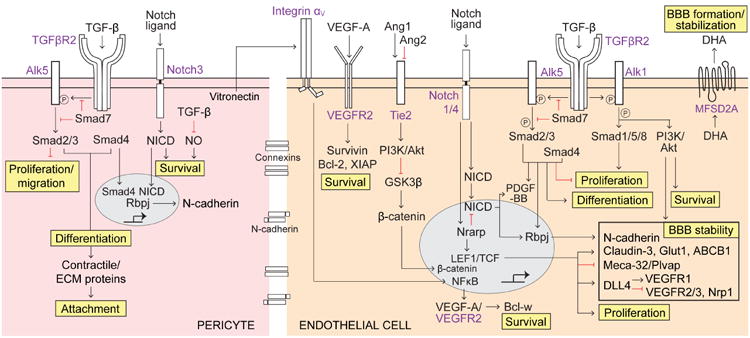
Pericytes (left, pink) – Tumor growth factor-β (TGF-β) secreted by endothelial cells and pericytes binds to TGF-β receptor 2 (TGFβR2) that phosphorylates activin-like kinase 5 (Alk5) (inhibited by Smad7) activating the downstream Smad signaling cascade. Activated Smad2/3 inhibits pericyte proliferation and migration. Recruitment of Smad4 to Smad2/3 complex transcriptionally promotes pericyte differentiation, expression of contractile/extracellular matrix (ECM) proteins, and pericyte attachment. TGF-β also inhibits nitric oxide (NO) generation promoting survival. Activated Notch3 receptor cleaves its Notch intracellular domain (NICD), which promotes survival. Notch-NICD pathway works cooperatively with TGF-β/TGFβR2-Smad-4 pathway to stimulate Rbpj-mediated expression of N-cadherin that increases blood-brain barrier (BBB) stability. Endothelial cells (right, orange) – VEGF-A/VEGFR2 autocrine/paracrine pathway promotes survival via increased expression of anti-apoptotic Bcl-2, Survivin, and X-linked inhibitor of apoptosis protein (XIAP). Vitronectin secreted by pericytes acts on integrin aV on endothelial cells resulting in NFκB-mediated transcriptional expression of VEGF-A and intracrine-mediated VEGF-A/VEGFR2-dependent Bcl-w expression promoting survival. Pericyte-derived Ang1 acts on endothelial Tie2 receptor, and endothelial-secreted Ang2 blocks Ang1 binding to Tie2, acting as a Tie2 antagonist. Ang1/Tie2 activates phosphatidyl inositol 3-phosphate (PI3K)/Akt pathway resulting in inhibition of glycogen synthase kinase 3β (GSK3P), an inhibitor of β-catenin; this leads to β-catenin nuclear translocation resulting in activation of TCF/LEF transcription factors that control expression of several proteins promoting BBB stability (shown in a box). Notch1/4 contributes to β-catenin-mediated BBB stability via the Notch-regulated ankyrin repeat protein (Nrarp), which increases β-catenin nuclear signaling by inhibiting LEF1 degradation and decreases Notch signaling via NICD destabilization. Additionally, Notch1/4-NICD pathway stimulates PDGF-BB expression and RBP-Jκ-mediated expression of N-cadherin contributing to BBB stability. As in pericytes, TGF-β/TGFβR2 pathway in endothelium similarly activates i) Alk5-Smad2/3/4 complex to transcriptionally promote differentiation, inhibit proliferation, and induce RBP-Jκ-mediated expression of N-cadherin, ii) Alk1-Smad1/5/8 to promote proliferation, and iii) Alk1-PI3K/Akt pathway to promote survival and BBB stability. The major facilitator superfamily domain-containing protein 2a (MFSD2A) facilitates luminal-to-abluminal transport of docosahexaenoic acid (DHA), an essential omega-3 fatty acid, and controls formation of the BBB; its expression depends on the presence of pericytes.
Functional importance
TGF-β/TGFβR2 signaling promotes cell differentiation, maturation, proliferation, migration, and attachment of endothelial cells and pericytes, as shown by in vitro studies of endothelial cells and pericytes of murine embryos or bovine retinas96–98 and confirmed in vivo in murine models99. Studies using transgenic mice with disrupted TGF-β/TGFβR2 downstream signaling, as for example Smad1, Smad2, and Smad4 knockout mice100,101, indicate that TGF-β/TGFβR2 downstream pathways regulate BBB formation, particularly endothelial-to-mesenchymal transition (EndMT) during normal development and vascular stabilization, and that aberrant TGF-β/TGFβR2 signaling within this pathway leads to development of brain hemorrhages99.
Recent studies have shown that the forkhead transcription factor Foxf2 is specifically expressed in brain pericytes, and that Foxf2 knockout embryos develop intracerebral hemorrhage, perivascular edema, thinning of the vascular basal lamina, an increase in luminal endothelial caveolae, and BBB breakdown97.
TGF-β/TGFβR2 signaling in neurological disorders
Neonatal intraventricular hemorrhage (IVH)
IVH is a major cause of premature infant mortality where hemorrhages are caused by vascular instability resulting from pericyte deficiency100. TGF-β signaling induces N-cadherin expression to promote BBB integrity, whereas regional disruption in TGF-β signaling leads to the development of IVH as shown in transgenic mice with endothelial cell-specific Smad4 knockout100. Mice are protected from IVH via perinatal glucocorticoid administration that increases TGF-β signaling, resulting in increased pericyte coverage102.
Cerebral cavernous malformation (CCM)
CCM is characterized by raspberry-like lesions of microvessels that exhibit pericyte deficiency and enlarged endothelial cells with pinocytotic vesicles and poorly developed tight and adherens junctions, making the vessels prone to hemorrhaging99,103. This phenotype is present in sporadic and familial CCM including loss-of-function mutations in CCM1-3104. An analogous vascular phenotype develops in mice with endothelial cell-specific inducible ablation of Ccm1 (iCcm1ΔEC/EC)99,103 and Ccm2-399. Disrupted TGFβ signaling at the BBB is reported in studies using iCcm1ΔEC/EC mice, human brain tissue of CCM subjects, and cultured human brain vascular cells99,103 and contributes to CCM by inducing structural instability of capillaries. Human CCM3 also binds to paxillin, a scaffolding protein, and CCM3 and paxillin co-localize in mouse cerebral pericytes105.
Brain cancer
Several studies indicate the role of TGF-β signaling93,106,107 and pericytes93,108 in cancer growth and tumor-associated blood vessel function. For example, high TGF-β/Smad activity was found in aggressive, highly proliferative gliomas, which confers poor prognosis in patients with glioma106. The transcriptomic analysis of primary cultured patient-derived gliomas and human glioma biopsies indicate that the TGF-β/Smad pathway promotes proliferation through the induction of PDGF-B in gliomas with an unmethylated PDGF-B gene106.
More recent studies revealed that self-renewing tumorigenic glioma stem cells (GSCs) give rise to pericytes to support vessel formation and tumor growth92,93. GSCs are recruited to endothelial cells by the stromal-derived factor-1 (SDF-1), an alpha-chemokine that binds to G-protein-coupled CXCR4, and are induced to become pericytes predominantly by TGF-β93. TGF-β signaling has also been shown to regulate vascular phenotype in gliomas, and was sufficient to invoke many of the changes found in a gene signature associated with pathologically altered vessels in human glioblastoma (GBM) grade IV107.
Ischemic stroke
Multiple studies support the role of TGF-β/TGFβR2 signaling in mediating angiogenic responses to brain injury due to ischemia or hypoxia109, as TGF-β signaling stabilizes newly formed microvessels110.
Notch pathway
Pathway characterization
Pericytes express Notch3 and endothelial cells express Notch1/4 receptors (Figure 4). Notch ligands such as delta-like ligand 4 (Dll4) and Jagged, activate the receptor to induce cleavage of the Notch intracellular domain (NICD). NICD translocates to the nucleus and interacts with a Notch signal integrator transcription factor, the DNA-binding protein CSL (i.e., CBF-1, RBP-Jκ suppressor of hairless, Lag-1), to induce transcription of downstream target genes, as shown in Notch3 knockout mice111.
In endothelial cells, the Notch pathway is modulated by canonical Wnt signaling to influence vascular sprouting and remodeling112. The Wnt/β-catenin pathway in brain endothelium regulates differentiation of the brain vasculature, angiogenesis, and BBB integrity, as shown by in vitro studies using primary murine and rat brain microvascular endothelial cells and endothelial cells with conditional activation of β -catenin, and/or in vivo studies using Pdgfb-iCreERT2; βcat lox/lox mice, Pdgfb-iCreER; Nrarp, Ctnnblox mice, TOP-Gal Wnt reporter mice, and zebrafish (as reviewed112,113).
Functional importance
The Notch pathway regulates angiogenic sprouting and microvascular remodeling111. During sprouting angiogenesis, specialized endothelial tip cells lead the outgrowth of blood vessel sprouts towards gradients of VEGF-A114,115. Studies using Dll4+/- mice and mice with inducible endothelium-specific inactivation of Notch1 (VEcad-CreERT2/R26R/Notch1floxed/floxed mice) as well as Notch inhibition by γ -secretase inhibitors or activation with soluble Jagged-1 peptide, have demonstrated that Dll4-Notch signaling between endothelial cells within the angiogenic sprout serves to restrict tip-cell formation in response to VEGF116. This model offers not only an explanation for the dose-dependency and haploinsufficiency of the Dll4 gene117,118, but also suggests that γ-secretase inhibitors and/or modulators of Dll4 or Notch signaling developed originally for AD, could also be used as important pharmacological regulators of angiogenesis.
Notch signaling in neurological disorders
Cerebral cavernous malformation
Several studies using transgenic models, human tissue, and/or cultured brain vascular cells report CCM is associated with disruptions in TGFβ and Notch signaling at the BBB99,103.
Neonatal intraventricular hemorrhage
Similar to disrupted TGF-β signaling, dysfunction in Notch signaling is associated with IVH as observed in Rbpj (encodes the Notch-related RBP-Jκ transcription factor) knockout mice100.
Brain cancer
Activating Notch1 signaling in cultured GBM stem cells induces a vascularization switch and causes the GBM stem cells to differentiate into pericyte-like cells (PDGFRβ+, NG2+, α-SMA+) with upregulated expression of angiogenic factors including cytokines, matrix metalloproteinase-9 (MMP-9), and adhesion molecules92.
VEGF-A/VEGFR2 pathway
Pathway characterization
Pericytes (paracrine signaling) and endothelial cells (autocrine signaling) secrete VEGF-A that activates the VEGFR2 pathway to increase expression of anti-apoptotic Bcl-2, survivin, and X-linked inhibitor of apoptosis protein (XIAP), as shown by in vitro studies of human brain pericyte and endothelial cell co-cultures119 (Figure 4, right). For intracrine signaling, pericytes secrete vitronectin (in a PDGF-BB/PDGFRβ-dependent process) that activates integrin αV-NFκB signaling in endothelial cells to upregulate VEGF-A, which signals intracellularly via VEGFR2 to promote Bcl-w expression and cell survival, as shown by in vitro studies of human brain pericyte and endothelial cell co-cultures119 (Figure 4, right).
Functional importance
VEGF-A/VEGFR2 signal transduction promotes cell survival, angiogenesis, and vascular permeability2,119.
VEGF-A/VEGFR2 signaling in neurological disorders
Ischemic brain injury and hypoxia
In vivo studies in cats have shown that brain pericytes migrate away from capillaries rapidly following a hypoxic insult120 and pericyte-endothelial cell ratio in brain is reduced one week following hypobaric hypoxia in rodents121. During hypoxia, VEGF levels are upregulated in pericytes within 24 hours and upregulated in astrocytes after 4 days121. Inhibiting VEGF in chronic mild hypoxia (1% O2) promotes angiopoietin-1 (Ang1)-mediated endothelial cell tight junction stabilization, whereas severe hypoxia (0.1% O2) promotes apoptosis of endothelial cells, as shown in in vitro endothelial and pericyte co-cultures122. Hypoxia activates hypoxia-inducible factor (HIF)-1α that translocates to the nucleus and binds hypoxia-response elements (HRE) to induce target gene transcription that promotes angiogenesis by upregulating VEGF-A and MMPs, anaerobic metabolism, apoptosis, cell survival, and proliferation123.
Brain cancer
Inhibition of VEGF-induced angiogenesis suppresses growth of human GBM cells in vivo in mice124. Clinical trials with a monoclonal antibody against VEGF-A, i.e., Becacizumab, in GBM patients have shown it slows tumor growth but growth was inevitably recurrent125. Treatment with an anti-VEGF antibody was least effective in human subjects with GBM exhibiting heightened CBF and angiogenesis125. VEGF/VEGFR2 signaling can also function as a negative regulator of receptor tyrosine kinases including PDGFRβ in pericytes126 and mesenchymal epithelial transition (MET) receptor in GBM neoplastic cells127. Pericytes in vitro are also reported to express VEGFR2, and VEGF ablation in tumor cells disrupts PDGFRβ/VEGFR2 heterocomplex formation and downstream PDGFRβ signaling in pericytes, and increases tumor vessel maturation126. MET was more abundantly expressed in human GBM tissue of patients that exhibited more resistance to Becacizumab127, suggesting that VEGFR2's association with receptor tyrosine kinases in pericytes may play an important role in promoting cancer cell maturation and/or tumor growth.
Ang/Tie2 pathway
Pathway characterization
-Ang1, secreted by pericytes, binds to the endothelial-specific Tie2 receptor tyrosine kinase and activates downstream PI3K-Akt pathway in endothelial cells5. Angiopoietin-2 (Ang2), expressed by endothelial cells, was originally shown to inhibit Ang1-mediated phosphorylation of Tie2 as well as cellular responses during embryonic development5. Ang2 also has proangiogenic activities in adult tissues and cultured endothelial cells, independent of Ang1. For example, in the absence of Ang1, Ang2 binds endothelial Tie2 and activates PI3K-Akt pathway in cultured endothelial cells acting as a Tie2 agonist, but when Ang1 is present, Ang2 dose-dependently inhibits Ang1-induced Tie2 phosphorylation and endothelial cell survival acting as a Tie2 antagonist128. Ang2 also binds to endothelial integrins (i.e., αvβ3, αvβ5, α5β1) to induce phosphorylation of the integrin adaptor protein, focal adhesion kinase (FAK), and Rac1 activation, which promotes endothelial cell migration and sprouting, as demonstrated in cultured Tie2-silenced endothelial cells and in vivo human xenotransplanted endothelial cells in mice129.
Functional importance
Ang1 knockout mice exhibit pronounced angiogenic deficits in brain resulting in embryonic lethality, similar to Tie2 knockout mice130. Studies using in vivo assays demonstrate significantly attenuated vascular responses to histamine, bradykinin, and VEGF in Ang2-/- mice131. Recombinant Ang1 attenuates retinal vascular disruptions caused by pericyte loss132, whereas overexpressing Ang2 in the retina results in pericyte loss and aberrant retinal angiogenesis133. Furthermore, cytokine-induced intracellular calcium influx was impaired in Ang2 null endothelioma cells, consistent with reduced phospholipase activation in vivo131. Thus, Ang/Tie2 system has a critical role in regulating angiogenesis and vascular permeability, but Ang1 and Ang2 exert diverse effects on endothelial cell functions depending on experimental conditions and models.
Ang/Tie2 signaling in neurological disorders
Type 2 diabetes and ischemic stroke
In db/db transgenic model of diabetes, ischemic stroke leads to enhanced BBB breakdown associated with increased expression of Ang2, and decreased expression of Ang1, Tie2, and tight junction endothelial proteins134.
Brain cancer
Ang2 is upregulated in tumors and thought to denote the onset of angiogenic sprouting135.
MFSD2A pathway
Pathway characterization
The major facilitator superfamily domain-containing 2a (MFSD2A) is a sodium-dependent BBB transporter of long chain fatty acids that is expressed in the brain exclusively in the endothelium, as suggested by studies in mice and humans136,137. Studies in transgenic Mfsd2a null mice have shown that MFSD2A facilitates transport of docosahexaenoic acid (DHA), an essential omega-3 fatty acid, into the brain136. Its expression appears to depend on the presence of pericytes137, but the exact molecular pathway remains to be elucidated.
Functional importance
MFSD2A exhibits dual functions at the BBB by regulating the formation and maintenance of BBB integrity137 and delivery of essential fatty acids to the brain as shown in vitro in endothelial cell cultures and in vivo in murine models136,137.
MFSD2A signaling in neurological disorders
Microcephaly
Mfsd2a deficient mice develop a significantly reduced brain size, termed microcephaly136. Microcephaly syndrome in humans was recently shown to be caused by inactivating mutations in MFSD2A, and the syndrome's severity correlated with the degree of functional inactivation of the MFSD2A protein138,139.
Ephrin/Eph pathway
The ephrin receptor tyrosine kinases and their membrane-tethered ephrin ligands provide critical guidance cues at points of cell-to-cell contact140. Ephrin B receptors (EphB) and ephrin B ligands play a critical role in the regulation of developmental angiogenesis and pericyte-endothelial interactions during vascular assembly, as shown in vivo by genetic loss-of-function studies140. Ephrin-B ligands and EphB receptors mark angiogenic vessels in vivo in the developing murine retina141. Studies in transgenic Pdgfrb-Cre; Efnb2lox/lox mice reveal that ephrin-B2/EphB4 signaling controls pericyte directional migration and adhesion to maturing vessels142. Studies using a genetically engineered mouse in which the lacZ coding region substitutes and reports for the ephrin-B2 coding region, have shown that ephrin-B2 is expressed in brain pericytes and endothelial cells143. Moreover, EphB4 was shown to control blood vascular morphogenesis during postnatal angiogenesis144. Thus, ehprin-B2 and its receptor EphB4 can participate in vascular remodeling and in different aspects of NVU formation. The ephrin-B/EphB pathway is activated bidirectionally143, where ephrin-B binding to EphB receptors causes phosphorylation of both the ephrin-B transmembrane ligand and its EphB receptor141.
Other cell-cell communication pathways
Signaling between integrin α4β1 expressed by proliferating but not quiescent endothelial cells and VCAM-1 expressed by proliferating but not quiescent pericytes is critical for cell-cell adhesion events required for survival of endothelial and mural cells during vascularization, as shown both by in vitro and in vivo studies145.
Blocking N-cadherin function leads to defective pericyte adhesion, increased pericyte recruitment, and disturbed vascular morphogenesis as shown in chicken embryos146. Studies using in vitro pluripotent embryonic stem cells demonstrate that N-cadherin is required for the maturation of endothelial sprouts by interacting with pericytes147.
Endosialin is not expressed in the normal human adult brain148,149 or the adult mouse brain150, but is abundantly expressed in brain pericytes in the developing murine CNS150 and tumor vessel-associated pericytes149, suggesting that its expression in sites of active tissue remodeling and neovascularization might have implications for angiogenesis, tumor growth, and metastasis.
Pericyte-astrocyte signal transduction
CypA-NFκB-MMP-9 pathway
Pathway characterization
Astrocyte-secreted apolipoprotein E (APOE) interacts with the cell-surface LRP1 on pericytes that regulates, in an isoform-specific manner (i.e., APOE4 but not APOE2 and APOE3), activation of the proinflammatory BBB-degrading cyclophilin A (CypA)-NFκB-MMP-9 pathway11 (Figure 5).
Figure 5. Pericyte-astrocyte and pericyte-neuron signaling pathways.
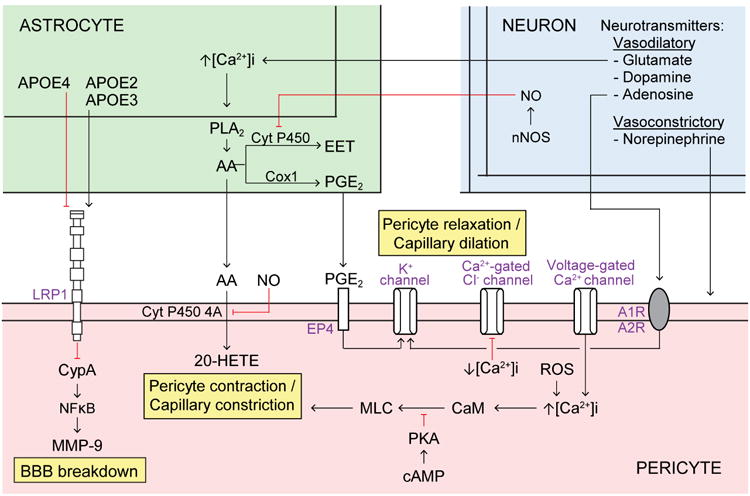
Astrocytes (top left, green) secrete apolipoprotein E (APOE) 2 and 3 that bind to pericyte lipoprotein LRP1 receptor (bottom, orange) to inhibit downstream CypA-NFκB-MMP-9 pathway. In contrast, APOE4 binds weakly to LRP1, which activates the pro-inflammatory CypA-NFκB-MMP-9 cascade leading to blood-brain barrier (BBB) breakdown. Astrocyte intracellular Ca2+ concentration ([Ca2+]i) increases in response to neuronal factors, for example glutamate, which promotes phospholipase A2 (PLA2)-mediated arachidonic acid (AA) generation. In astrocytes, AA is metabolized into prostaglandin E2 PGE2) via cyclooxtgenase-1 (Cox1), as well as into epoxyeicosotrienoic acids (EET) via cytochrome P450. Astrocytic AA is metabolized into 20-HETE in mural cells via membrane-bound cytochrome P450 4A, which promotes pericyte contraction. PGE2 from astrocytes binds to pericyte EP4 receptor, which alters K+ conductance and promotes pericyte relaxation. Nitric oxide (NO) generated by neurons inhibits cytochrome P450 in astrocytes and cytochrome P450 4A in pericytes to prevent AA to EET and AA to 20-HETE metabolism, respectively. In pericytes, increased cyclic adenosine monophosphate (cAMP) signals via protein kinase A (PKA) to inhibit myosin light chain (MLC) phosphorylation and prevent pericyte contraction. Additionally, pericyte [Ca2+]i increases in response to voltage-gated Ca2+ channels and reactive oxygen species (ROS). Increased [Ca2+]i in pericytes is shown to promote contraction, possibly via downstream signaling through calmodulin (CaM) and MLC kinase (MLCK) which phosphorylates MLC to induce contraction, as shown in VSMCs. Conversely, decreasing [Ca2+]i in pericytes inhibits Ca2+-gated Cl- channels which promotes relaxation. Furthermore, neurotransmitters promote pericyte, relaxation (e.g., glutamate, dopamine, and adenosine) or contraction (e.g., norepinephrine). For example, adenosine signals through adenosine A1 and A2 receptors (A1R, A2R) on pericytes to alter K+ conductance and promote pericyte relaxation.
Functional importance
Increased MMP-9 activity by APOE4, but not APOE2 and APOE3, in the vessel wall leads to degradation of endothelial tight junction and basement membrane proteins causing BBB breakdown11, which in turn leads to brain accumulation of blood-derived neurotoxic molecules and erythrocytes and secondary neurodegenerative changes.
CypA-NFκB-MMP-9 signaling in neurological disorders
Alzheimer's disease
The cerebrovascular contributions to dementia and AD are particularly salient in individuals carrying APOE4 gene, the major genetic risk factor for late-onset sporadic AD83. Increases in CypA and MMP-9 CSF levels were recently reported to correlate with BBB breakdown in human APOE4 carriers, but not age-matched APOE2 or APOE3 carriers that have an intact BBB49. Additionally, post-mortem analysis in APOE4-positive AD patients compared to non-carriers revealed increased CypA and MMP-9 protein levels in hippocampal and cortical pericytes as well as pericyte degeneration50,151.
Animal studies have demonstarted BBB breakdown in APOE4 transgenic mice, but not in APOE3 or APOE2 transgenic mice11,152,153, resulting in neuronal injury and neurodegeneration11.
Arachidonic acid (AA) pathway
Astrocytes have been proposed to regulate pericyte tone via some of the same signaling pathways as shown for astrocyte-mediated regulation of VSMCs tone6,30 (Figure 5). The arachidonic acid (AA) pathway has been shown to have a critical role in the regulation of pericyte tone and capillary diameter in studies using rat brain slices and retinal explants35. In vivo studies in the murine cortex have shown that AA is metabolized in astrocytes into prostaglandin E2 (PGE2) via cyclooxtgenase-1 (Cox1), which has been shown to regulate hemodynamic responses154. Studies using rat cerebellar slices and retinal explants have shown that PGE2 activates EP4 receptors in pericytes leading to pericyte relaxation after addition of glutamate to slices30. AA secreted by astrocytes is metabolized to 20-hydroxyeicosatetraenoic acid (20-HETE) in pericytes via membrane-bound cytochrome P450 4A, as shown in rat cerebral arterial microsomes and brain slices30,155. In turn, 20-HETE leads to contraction of pericytes in cerebral slices and retinal explants30,35. Although the role of the AA pathway in astrocytes in controlling pericyte tone and capillary diameter is supported by studies in brain slices, the in vivo evidence is still lacking.
[Ca2+]i-CaM-MLC pathway
Intracellular Ca2+, [Ca2+]i, increases in response to voltage-gated Ca2+ channels as shown in vitro in primary rat brain pericytes156 and isolated rat retinal microvascular pericytes157, and in response to ROS as shown in vitro in human microvascular pericytes158, ex vivo in rat cerebellar slices30, and murine pericytes in vivo following ischemic stroke46. An increase in extracellular [K+] activates voltage-gated Ca2+ channels resulting in [Ca2+]i increases and depolarization and contraction of primary rat brain pericytes156. Increased [Ca2+]i in pericytes is shown to promote contraction, possibly via downstream signaling through calmodulin (CaM) and myosin light chain kinase (MLCK) to phosphorylate myosin light chain (MLC) and induce contraction, by analogy to events described in VSMCs of isolated rat cerebral arteries159. Pericytes were recently shown to express Myl9, which encodes myosin light chain regulatory polypeptide 9, in single-cell RNA-seq analysis of the murine cortex160. Treating isolated rat retinal microvascular pericytes with a cyclic guanosine monophosphate (cGMP) analog inhibits voltage-gated Ca2+ channels, decreases [Ca2+]i, and inhibits Ca2+-gated Cl- channels, which functionally decreases whole-cell Ca2+ and Cl- currents and promotes pericyte relaxation157.
Pericytes-neuronal interactions
Neuronal innervation of pericytes covering brain capillaries is not as well understood as neuronal innervation of VSMCs surrounding arterioles and small arteries161 despite that capillaries are more numerous and more densely spaced than arterioles, as shown for example in the mouse cortex162. Moreover, the average distance between a neuron and a capillary is 8-23 μm in the mouse hippocampus, while the average distance between a neuron and an arteriole is 70-160 μm163 suggesting that pericytes and capillaries are well positioned to receive chemical transmitters from activated neurons and are likely to respond earlier to changes in neuronal activity than VSMCs.
Neurotrophic factors
Cultured human brain pericytes express low but comparable levels of neurotrophic factors as cultured astrocytes164, but the functional importance of these findings remains unknown. A recent study using a murine hypothalamic GT1-7 cell line has shown that only pericyte-derived media, but not astrocyte or VSMC media, increased the insulin-stimulated phosphorylation of Akt in GT1-7 cells and insulin-dependent tyrosine phosphorylation of insulin receptor β165, suggesting that pericytes rather than astrocytes and VSMCs can increase insulin sensitivity in hypothalamic neurons by releasing soluble factors. Given the strategic location of pericytes within the NVU, it is intriguing to speculate whether pericyte-derived molecules can be distributed within the NVU and reach their neuronal targets by para-arterial CSF-ISF flow166 or peri-vascular flow167.
Neurotransmitters
Studies using bovine retinal pericytes168 and ex vivo rat cerebellar slices and retinal explants30,35 have shown that norepinephrine leads to pericyte contraction and reduction of capillary diameter. Neurotransmitters that lead to pericyte relaxation include gamma-aminobutyric acid (GABA) as shown ex vivo in rat cerebellar slices35, adenosine as shown in vitro in rat retinal pericytes169, glutamate as shown ex vivo in rat cerebellar slices30, and dopamine as shown ex vivo in rat retinal pericytes170. Studies in rat cerebellar slices and retinal explants have also shown that glutamate suppresses pericyte contractility through PGE230,35 and that NO blocks 20-HETE-induced pericyte contraction by inhibiting AA conversion to 20-HETE resulting in capillary dilation30 (Figure 5).
Recent in vivo studies have provided important evidence for neuronal control of capillary circulation by showing that capillaries dilate ahead of arterioles in the mouse cortex in response to whisker stimulation30.
Integrating pathways: Towards a systems biology approach
Recent studies also focused on modeling the BBB, NVU, and pericyte functions in blood vessels171–173. A co ch mputational model predicting disruption of blood vessel development incorporates endothelial, inflammatory, and mural cells (i.e. pericytes)172. A physical three-dimensional, multi-compartment, organotypic microphysiological system representative of the NVU – a NVU on a chip – recapitulates all critical barriers in the brain including BBB, brain-CSF and blood-CSF barrier171. It has also been suggested that in silico modeling might even more rapidly enhance our understanding of the NVU compared to in vitro cell-based modeling173, but requires a critical level of biological understanding to successfully bridge the logical connectivity of molecular pathways with the computational integration systems174.
Here, we schematically illustrate a multi-layered model of the NVU with: i) an interconnected system of pathways within endothelial cells, pericytes, and astrocytes (NVU cells layer); ii) converging points of key signaling pathways in CNS pericytes and between pericytes-endothelial cells and pericytes-astrocytes (Interactive signaling layer); and iii) potential impact of such convergent pathways to neurological disorders (Disorders layer) (Figure 6). Since all these processes maybe species, strain, disease, or context dependent, we provide Figure 6 as an all-inclusive data source and encourage the readers to explore and critically examine the specifics in the existing literature.
Figure 6. Integrated pathways between pericytes, endothelial cells and astrocytes within the neurovascular unit (NVU).
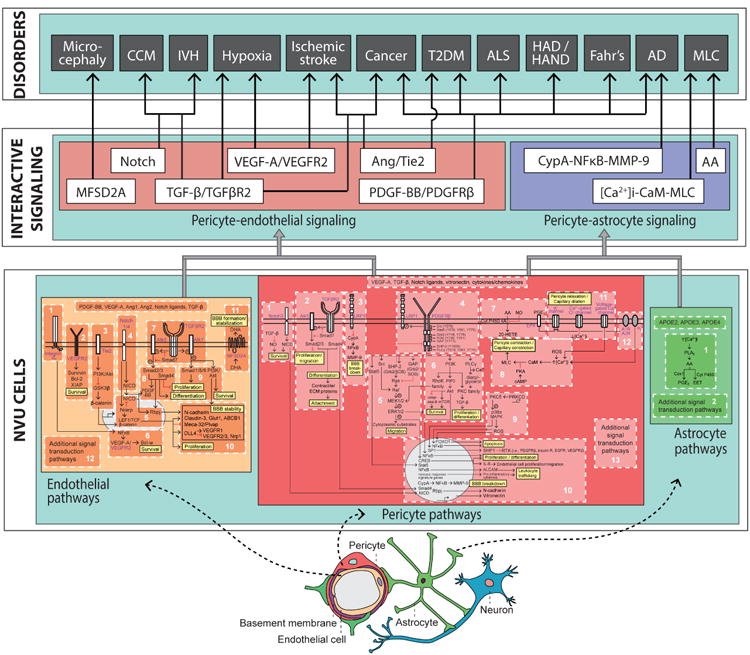
A proposed three-layered model of the NVU. The first layer, ‘NVU cells,’ is the foundational layer of cell type-specific systems, each of which consists of integrated modular molecular pathways. Here, we show the endothelial cell (yellow) partitioned into 11 pathways, the pericyte (pink) partitioned into 12 pathways, and the astrocyte (green) partitioned into 1 pathway. Each major signal transduction pathway within each NVU cell type also provides for the modular addition of new signaling pathways, denoted as “additional signal transduction pathways.” The second layer, ‘Interactive Signaling,’ instantiates the converging points of interactions of key signaling pathways between pericytes-endothelial cells and pericytes-astrocytes. The pericyte-endothelial signaling (coral box) at the second layer consists of 6 major signaling pathways: MFSD2A, Notch, TGF-β/TGFβR2, VEGF-A/VEGFR2, Ang/Tie2, and PDGF-BB/PDGFRβ. The pericyte-astrocyte signaling (purple box), also at the second layer, consists of 3 major signaling pathway: CypA-NFκB-MMP-9, arachidonic acid (AA), and [Ca2+]i-CaM-MLC. The third layer, ‘Disorders,’ proposes major signaling pathways of CNS pericytes with neighboring NVU cell types (i.e., endothelial cells and astrocytes) that are suggested to contribute to pericytes dysfunction in neurological disorders, including: Microcephaly, cerebral cavernous malformation (CCM), intraventricular hemorrhage (IVH), hypoxia, ischemic stroke, cancer, type 2 diabetes mellitus (T2DM), amyotrophic lateral sclerosis (ALS), HIV-associated dementia (HAD) and HIV-associated neurocognitive disorders (HAND), Fahr's disease, Alzheimer's disease (AD), and megalencephalic leukoencephalopathy with subcortical cysts (MLC).
Future directions
Several functions of pericytes such as capillary contractility, neuroinflammation, and multipotent stem cell activity remain still to be fully characterized. It is also unclear how each pericyte subtype contributes to pericyte function, as for example control of CBF versus control of BBB integrity. Developing novel genetic models with mural cell-specific ablation along the vascular tree combined with RNA-seq and proteomic analyses would greatly facilitate the study of CNS pericytes and other mural cell subpopulations.
Many studies have pointed towards pericytes as potentially an attractive cellular target in rare genetic neurological disorders such as Fahr's disease, IVH, CCM, and CADASIL. A growing body of evidence also indicates that targeting pericytes could be an important treatment option to control growth of brain tumors including GBM, as well as to improve vascular remodeling and stabilization of the BBB in AD and possibly other neurodegenerative diseases. The potential of targeting pericytes to open BBB on demand and/or to stabilize dysregulated CBF is another important area for future studies because of pericytes' emerging role in brain diseases affecting BBB and CBF such as stroke and AD.
The systems biology approach can facilitate addressing some emerging questions in the field as, for example, whether some of the pathways identified in rare monogenic neurological diseases caused by genetic defects in pericytes, endothelial cells, or astrocytes that lead to NVU disruption have converging points with complex neurological disorders such as sporadic AD, ALS, and others associated with pericyte and BBB dysfunction. Computational modeling of CNS pericytes combined with high-throughput screening of diverse libraries of compounds is also likely to advance discovery of novel therapeutics for neurological disorders based on correcting aberrant signaling and function of pericytes and their neighboring cell types within the NVU.
Acknowledgments
The work of B.V.Z. is supported by the National Institutes of Health grants R01AG023084, R01NS090904, R01NS034467, and R01AG039452 and the Cure for Alzheimer's Fund. We apologize to those authors whose original work we were not able to cite due to the limited length of this review.
Footnotes
Notes: The authors declare no conflict of interest.
References
- 1.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attwell D, et al. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010;2 doi: 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and Dysfunction of the Blood-Brain Barrier. Cell. 2015;163:1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armulik A, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 10.Bell RD, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell RD, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zlokovic BV, Begley DJ, Chain-Eliash DG. Blood-brain barrier permeability to leucine-enkephalin, D-alanine2-D-leucine5-enkephalin and their N-terminal amino acid (tyrosine) Brain Res. 1985;336:125–132. doi: 10.1016/0006-8993(85)90423-8. [DOI] [PubMed] [Google Scholar]
- 13.Zloković BV, Segal MB, Begley DJ, Davson H, Rakić L. Permeability of the blood-cerebrospinal fluid and blood-brain barriers to thyrotropin-releasing hormone. Brain Res. 1985;358199:191. doi: 10.1016/0006-8993(85)90963-1. [DOI] [PubMed] [Google Scholar]
- 14.Zloković BV, Lipovac MN, Begley DJ, Davson H, Rakić L. Transport of leucine-enkephalin across the blood-brain barrier in the perfused guinea pig brain. J Neurochem. 1987;49315:310. doi: 10.1111/j.1471-4159.1987.tb03431.x. [DOI] [PubMed] [Google Scholar]
- 15.Zlokovic BV. Cerebrovascular permeability to peptides: manipulations of transport systems at the blood-brain barrier. Pharm Res. 1995;12:1395–1406. doi: 10.1023/a:1016254514167. [DOI] [PubMed] [Google Scholar]
- 16.Zlokovic BV. Neurodegeneration and the neurovascular unit. Nat Med. 2010;16:1370–1371. doi: 10.1038/nm1210-1370. [DOI] [PubMed] [Google Scholar]
- 17.Dore-Duffy P. Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des. 2008;14:1581–1593. doi: 10.2174/138161208784705469. [DOI] [PubMed] [Google Scholar]
- 18.Krueger M, Bechmann I. CNS pericytes: concepts, misconceptions, and a way out. Glia. 2010;58:1–10. doi: 10.1002/glia.20898. [DOI] [PubMed] [Google Scholar]
- 19.Attwell D, Mishra A, Hall CN, O'Farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab. 2015;0271678X15610340 doi: 10.1177/0271678X15610340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartmann DA, et al. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics. 2015;2:041402. doi: 10.1117/1.NPh.2.4.041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Dijk CGM, et al. The complex mural cell: pericyte function in health and disease. Int J Cardiol. 2015;190:75–89. doi: 10.1016/j.ijcard.2015.03.258. [DOI] [PubMed] [Google Scholar]
- 22.Bondjers C, et al. Microarray analysis of blood microvessels from PDGF-B and PDGF-Rbeta mutant mice identifies novel markers for brain pericytes. FASEB J Off Publ Fed Am Soc Exp Biol. 2006;20:1703–1705. doi: 10.1096/fj.05-4944fje. [DOI] [PubMed] [Google Scholar]
- 23.Johnson MB, et al. Single-cell analysis reveals transcriptional heterogeneity of neural progenitors in human cortex. Nat Neurosci. 2015;18:637–646. doi: 10.1038/nn.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KT, et al. Single-cell mRNA sequencing identifies subclonal heterogeneity in anti-cancer drug responses of lung adenocarcinoma cells. Genome Biol. 2015;16:127. doi: 10.1186/s13059-015-0692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold TD, et al. Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking αVβ8-TGFβ signaling in the brain. Dev Camb Engl. 2014;141:4489–4499. doi: 10.1242/dev.107193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sagare AP, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Pieper C, Marek JJ, Unterberg M, Schwerdtle T, Galla HJ. Brain capillary pericytes contribute to the immune defense in response to cytokines or LPS in vitro. Brain Res. 2014;1550:1–8. doi: 10.1016/j.brainres.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 29.MacVicar BA, Newman EA. Astrocyte regulation of blood flow in the brain. Cold Spring Harb Perspect Biol. 2015;7 doi: 10.1101/cshperspect.a020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall CN, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai M, Nuttall A, Yang Y, Shi X. Visualization and contractile activity of cochlear pericytes in the capillaries of the spiral ligament. Hear Res. 2009;254:100–107. doi: 10.1016/j.heares.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamanishi S, Katsumura K, Kobayashi T, Puro DG. Extracellular lactate as a dynamic vasoactive signal in the rat retinal microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H925–934. doi: 10.1152/ajpheart.01012.2005. [DOI] [PubMed] [Google Scholar]
- 34.Chaigneau E, Oheim M, Audinat E, Charpak S. Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Proc Natl Acad Sci U S A. 2003;100:13081–13086. doi: 10.1073/pnas.2133652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill RA, et al. Regional Blood Flow in the Normal and Ischemic Brain Is Controlled by Arteriolar Smooth Muscle Cell Contractility and Not by Capillary Pericytes. Neuron. 2015;87:95–110. doi: 10.1016/j.neuron.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olson LE, Soriano P. PDGFRβ signaling regulates mural cell plasticity and inhibits fat development. Dev Cell. 2011;20:815–826. doi: 10.1016/j.devcel.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang S, et al. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voisin MB, Pröbstl D, Nourshargh S. Venular basement membranes ubiquitously express matrix protein low-expression regions: characterization in multiple tissues and remodeling during inflammation. Am J Pathol. 2010;176:482–495. doi: 10.2353/ajpath.2010.090510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jansson D, et al. A role for human brain pericytes in neuroinflammation. J Neuroinflammation. 2014;11:104. doi: 10.1186/1742-2094-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovac A, Erickson MA, Banks WA. Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. J Neuroinflammation. 2011;8:139. doi: 10.1186/1742-2094-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakagomi T, et al. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells Dayt Ohio. 2015;33:1962–1974. doi: 10.1002/stem.1977. [DOI] [PubMed] [Google Scholar]
- 43.Winkler EA, Sengillo JD, Bell RD, Wang J, Zlokovic BV. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2012;32:1841–1852. doi: 10.1038/jcbfm.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winkler EA, et al. Blood-spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc Natl Acad Sci U S A. 2014;111:E1035–1042. doi: 10.1073/pnas.1401595111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davalos D, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012;3:1227. doi: 10.1038/ncomms2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yemisci M, et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- 47.Keller A, et al. Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat Genet. 2013;45:1077–1082. doi: 10.1038/ng.2723. [DOI] [PubMed] [Google Scholar]
- 48.Nicolas G, et al. Mutation of the PDGFRB gene as a cause of idiopathic basal ganglia calcification. Neurology. 2013;80:181–187. doi: 10.1212/WNL.0b013e31827ccf34. [DOI] [PubMed] [Google Scholar]
- 49.Halliday MR, et al. Relationship between cyclophilin a levels and matrix metalloproteinase 9 activity in cerebrospinal fluid of cognitively normal apolipoprotein e4 carriers and blood-brain barrier breakdown. JAMA Neurol. 2013;70:1198–1200. doi: 10.1001/jamaneurol.2013.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halliday MR, et al. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer's disease. J Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.44. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winkler EA, et al. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol (Berl) 2013;125:111–120. doi: 10.1007/s00401-012-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geraldes P, et al. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Behl Y, Krothapalli P, Desta T, Roy S, Graves DT. FOXO1 plays an important role in enhanced microvascular cell apoptosis and microvascular cell loss in type 1 and type 2 diabetic rats. Diabetes. 2009;58:917–925. doi: 10.2337/db08-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alikhani M, Roy S, Graves DT. FOXO1 plays an essential role in apoptosis of retinal pericytes. Mol Vis. 2010;16:408–415. [PMC free article] [PubMed] [Google Scholar]
- 55.Niu F, Yao H, Zhang W, Sutliff RL, Buch S. Tat 101-mediated enhancement of brain pericyte migration involves platelet-derived growth factor subunit B homodimer: implications for human immunodeficiency virus-associated neurocognitive disorders. J Neurosci Off J Soc Neurosci. 2014;34:11812–11825. doi: 10.1523/JNEUROSCI.1139-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marchi N, Lerner-Natoli M. Cerebrovascular remodeling and epilepsy. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry. 2013;19:304–312. doi: 10.1177/1073858412462747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghosh M, et al. Pericytes are involved in the pathogenesis of CADASIL. Ann Neurol. 2015 doi: 10.1002/ana.24512. [DOI] [PubMed] [Google Scholar]
- 58.Wang F, et al. A microRNA-1280/JAG2 network comprises a novel biological target in high-risk medulloblastoma. Oncotarget. 2015;6:2709–2724. doi: 10.18632/oncotarget.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heldin CH. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun Signal CCS. 2013;11:97. doi: 10.1186/1478-811X-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 61.Winkler EA, Bell RD, Zlokovic BV. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol Neurodegener. 2010;5:32. doi: 10.1186/1750-1326-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tallquist MD, French WJ, Soriano P. Additive effects of PDGF receptor beta signaling pathways in vascular smooth muscle cell development. PLoS Biol. 2003;1:E52. doi: 10.1371/journal.pbio.0000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jurek A, Heldin CH, Lennartsson J. Platelet-derived growth factor-induced signaling pathways interconnect to regulate the temporal pattern of Erk1/2 phosphorylation. Cell Signal. 2011;23:280–287. doi: 10.1016/j.cellsig.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 64.Jin S, et al. Notch signaling regulates platelet-derived growth factor receptor-beta expression in vascular smooth muscle cells. Circ Res. 2008;102:1483–1491. doi: 10.1161/CIRCRESAHA.107.167965. [DOI] [PubMed] [Google Scholar]
- 65.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hellström M, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang C, et al. Mutations in SLC20A2 link familial idiopathic basal ganglia calcification with phosphate homeostasis. Nat Genet. 2012;44:254–256. doi: 10.1038/ng.1077. [DOI] [PubMed] [Google Scholar]
- 68.Lemos RR, et al. Update and Mutational Analysis of SLC20A2: A Major Cause of Primary Familial Brain Calcification. Hum Mutat. 2015;36:489–495. doi: 10.1002/humu.22778. [DOI] [PubMed] [Google Scholar]
- 69.Sengillo JD, et al. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer's disease. Brain Pathol Zurich Switz. 2013;23:303–310. doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 71.Baloyannis SJ, Baloyannis IS. The vascular factor in Alzheimer's disease: a study in Golgi technique and electron microscopy. J Neurol Sci. 2012;322:117–121. doi: 10.1016/j.jns.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 72.Park L, et al. Innate immunity receptor CD36 promotes cerebral amyloid angiopathy. Proc Natl Acad Sci U S A. 2013;110:3089–3094. doi: 10.1073/pnas.1300021110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Montagne A, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Björkqvist M, Ohlsson M, Minthon L, Hansson O. Evaluation of a previously suggested plasma biomarker panel to identify Alzheimer's disease. PloS One. 2012;7:e29868. doi: 10.1371/journal.pone.0029868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sagare AP, Sweeney MD, Makshanoff J, Zlokovic BV. Shedding of soluble platelet-derived growth factor receptor-β from human brain pericytes. Neurosci Lett. 2015;607:97–101. doi: 10.1016/j.neulet.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rogaeva E, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reitz C, et al. Meta-analysis of the association between variants in SORL1 and Alzheimer disease. Arch Neurol. 2011;68:99–106. doi: 10.1001/archneurol.2010.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kooner JS, et al. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat Genet. 2011;43:984–989. doi: 10.1038/ng.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reitz C. The role of intracellular trafficking and the VPS10d receptors in Alzheimer's disease. Future Neurol. 2012;7:423–431. doi: 10.2217/fnl.12.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hermey G, Sjøgaard SS, Petersen CM, Nykjaer A, Gliemann J. Tumour necrosis factor alpha-converting enzyme mediates ectodomain shedding of Vps10p-domain receptor family members. Biochem J. 2006;395:285–293. doi: 10.1042/BJ20051364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gliemann J, et al. The mosaic receptor sorLA/LR11 binds components of the plasminogen-activating system and platelet-derived growth factor-BB similarly to LRP1 (low-density lipoprotein receptor-related protein), but mediates slow internalization of bound ligand. Biochem J. 2004;381:203–212. doi: 10.1042/BJ20040149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer's amyloid β-peptide elimination from the brain. J Neurochem. 2010;115:1077–1089. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karch CM, Cruchaga C, Goate AM. Alzheimer's disease genetics: from the bench to the clinic. Neuron. 2014;83:11–26. doi: 10.1016/j.neuron.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Armstrong RA. Spatial correlations between beta-amyloid (Abeta) deposits and blood vessels in familial Alzheimer's disease. Folia Neuropathol Assoc Pol Neuropathol Med Res Cent Pol Acad Sci. 2008;46:241–248. [PubMed] [Google Scholar]
- 85.Kang DE, et al. Presenilins mediate phosphatidylinositol 3-kinase/AKT and ERK activation via select signaling receptors. Selectivity of PS2 in platelet-derived growth factor signaling J Biol Chem. 2005;280:31537–31547. doi: 10.1074/jbc.M500833200. [DOI] [PubMed] [Google Scholar]
- 86.Gama Sosa MA, et al. Age-related vascular pathology in transgenic mice expressing presenilin 1-associated familial Alzheimer's disease mutations. Am J Pathol. 2010;176:353–368. doi: 10.2353/ajpath.2010.090482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sweeney MD, Sagare AP, Zlokovic BV. Cerebrospinal fluid biomarkers of neurovascular dysfunction in mild dementia and Alzheimer's disease. J Cereb Blood Flow Metab. 2015;35:1055–1068. doi: 10.1038/jcbfm.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhong Z, et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci. 2008;11:420–422. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DeJesus-Hernandez M, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shaheen RM, et al. Tyrosine kinase inhibition of multiple angiogenic growth factor receptors improves survival in mice bearing colon cancer liver metastases by inhibition of endothelial cell survival mechanisms. Cancer Res. 2001;61:1464–1468. [PubMed] [Google Scholar]
- 92.Guichet PO, et al. Notch1 stimulation induces a vascularization switch with pericyte-like cell differentiation of glioblastoma stem cells. Stem Cells Dayt Ohio. 2015;33:21–34. doi: 10.1002/stem.1767. [DOI] [PubMed] [Google Scholar]
- 93.Cheng L, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–152. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hirschi KK, Burt JM, Hirschi KD, Dai C. Gap junction communication mediates transforming growth factor-beta activation and endothelial-induced mural cell differentiation. Circ Res. 2003;93:429–437. doi: 10.1161/01.RES.0000091259.84556.D5. [DOI] [PubMed] [Google Scholar]
- 96.Van Geest RJ, Klaassen I, Vogels IMC, Van Noorden CJF, Schlingemann RO. Differential TGF-{beta} signaling in retinal vascular cells: a role in diabetic retinopathy? Invest Ophthalmol Vis Sci. 2010;51:1857–1865. doi: 10.1167/iovs.09-4181. [DOI] [PubMed] [Google Scholar]
- 97.Reyahi A, et al. Foxf2 Is Required for Brain Pericyte Differentiation and Development and Maintenance of the Blood-Brain Barrier. Dev Cell. 2015;34:19–32. doi: 10.1016/j.devcel.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 98.Darland DC, D'Amore PA. TGF beta is required for the formation of capillary-like structures in three-dimensional cocultures of 10T1/2 and endothelial cells. Angiogenesis. 2001;4:11–20. doi: 10.1023/a:1016611824696. [DOI] [PubMed] [Google Scholar]
- 99.Maddaluno L, et al. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature. 2013;498:492–496. doi: 10.1038/nature12207. [DOI] [PubMed] [Google Scholar]
- 100.Li F, et al. Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev Cell. 2011;20:291–302. doi: 10.1016/j.devcel.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 101.Goumans MJ, Mummery C. Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol. 2000;44:253–265. [PubMed] [Google Scholar]
- 102.Vinukonda G, et al. Effect of prenatal glucocorticoids on cerebral vasculature of the developing brain. Stroke J Cereb Circ. 2010;41:1766–1773. doi: 10.1161/STROKEAHA.110.588400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schulz GB, et al. Cerebral Cavernous Malformation-1 Protein Controls DLL4-Notch3 Signaling Between the Endothelium and Pericytes. Stroke J Cereb Circ. 2015;46:1337–1343. doi: 10.1161/STROKEAHA.114.007512. [DOI] [PubMed] [Google Scholar]
- 104.Bergametti F, et al. Mutations within the programmed cell death 10 gene cause cerebral cavernous malformations. Am J Hum Genet. 2005;76:42–51. doi: 10.1086/426952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li X, et al. Molecular recognition of leucine-aspartate repeat (LD) motifs by the focal adhesion targeting homology domain of cerebral cavernous malformation 3 (CCM3) J Biol Chem. 2011;286:26138–26147. doi: 10.1074/jbc.M110.211250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bruna A, et al. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11:147–160. doi: 10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 107.Dieterich LC, et al. Transcriptional profiling of human glioblastoma vessels indicates a key role of VEGF-A and TGFβ2 in vascular abnormalization. J Pathol. 2012;228:378–390. doi: 10.1002/path.4072. [DOI] [PubMed] [Google Scholar]
- 108.Svensson A, Özen I, Genové G, Paul G, Bengzon J. Endogenous brain pericytes are widely activated and contribute to mouse glioma microvasculature. PloS One. 2015;10:e0123553. doi: 10.1371/journal.pone.0123553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Slevin M, et al. Serial measurement of vascular endothelial growth factor and transforming growth factor-beta1 in serum of patients with acute ischemic stroke. Stroke J Cereb Circ. 2000;31:1863–1870. doi: 10.1161/01.str.31.8.1863. [DOI] [PubMed] [Google Scholar]
- 110.Böttner M, Krieglstein K, Unsicker K. The transforming growth factor-betas: structure, signaling, and roles in nervous system development and functions. J Neurochem. 2000;75:2227–2240. doi: 10.1046/j.1471-4159.2000.0752227.x. [DOI] [PubMed] [Google Scholar]
- 111.Henshall TL, et al. Notch3 is necessary for blood vessel integrity in the central nervous system. Arterioscler Thromb Vasc Biol. 2015;35:409–420. doi: 10.1161/ATVBAHA.114.304849. [DOI] [PubMed] [Google Scholar]
- 112.Reis M, Liebner S. Wnt signaling in the vasculature. Exp Cell Res. 2013;319:1317–1323. doi: 10.1016/j.yexcr.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 113.Liebner S, Plate KH. Differentiation of the brain vasculature: the answer came blowing by the Wnt. J Angiogenesis Res. 2010;2:1. doi: 10.1186/2040-2384-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ruhrberg C, et al. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gerhardt H, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hellström M, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 117.Gale NW, et al. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A. 2004;101:15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Krebs LT, et al. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469–2473. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Franco M, Roswall P, Cortez E, Hanahan D, Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011;118:2906–2917. doi: 10.1182/blood-2011-01-331694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120G.onul E, et al. Early pericyte response to brain hypoxia in cats: an ultrastructural study. Microvasc Res. 2002;64:116–119. doi: 10.1006/mvre.2002.2413. [DOI] [PubMed] [Google Scholar]
- 121.Dore-Duffy P, Wang X, Mehedi A, Kreipke CW, Rafols JA. Differential expression of capillary VEGF isoforms following traumatic brain injury. Neurol Res. 2007;29:395–403. doi: 10.1179/016164107X204729. [DOI] [PubMed] [Google Scholar]
- 122.Al Ahmad A, Gassmann M, Ogunshola OO. Maintaining blood-brain barrier integrity: pericytes perform better than astrocytes during prolonged oxygen deprivation. J Cell Physiol. 2009;218:612–622. doi: 10.1002/jcp.21638. [DOI] [PubMed] [Google Scholar]
- 123.Engelhardt S, Patkar S, Ogunshola OO. Cell-specific blood-brain barrier regulation in health and disease: a focus on hypoxia. Br J Pharmacol. 2014;171:1210–1230. doi: 10.1111/bph.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim KJ, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 125.Kickingereder P, et al. MR Perfusion-derived Hemodynamic Parametric Response Mapping of Bevacizumab Efficacy in Recurrent Glioblastoma. Radiology. 2015;151172 doi: 10.1148/radiol.2015151172. [DOI] [PubMed] [Google Scholar]
- 126.Greenberg JI, et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lu KV, et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012;22:21–35. doi: 10.1016/j.ccr.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yuan HT, Khankin EV, Karumanchi SA, Parikh SM. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol. 2009;29:2011–2022. doi: 10.1128/MCB.01472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Felcht M, et al. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest. 2012;122:1991–2005. doi: 10.1172/JCI58832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Suri C, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 131.Benest AV, et al. Angiopoietin-2 is critical for cytokine-induced vascular leakage. PloS One. 2013;8:e70459. doi: 10.1371/journal.pone.0070459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Uemura A, et al. Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells. J Clin Invest. 2002;110:1619–1628. doi: 10.1172/JCI15621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feng Y, et al. Impaired pericyte recruitment and abnormal retinal angiogenesis as a result of angiopoietin-2 overexpression. Thromb Haemost. 2007;97:99–108. [PubMed] [Google Scholar]
- 134.Cui X, et al. Angiopoietin/Tie2 pathway mediates type 2 diabetes induced vascular damage after cerebral stroke. Neurobiol Dis. 2011;43:285–292. doi: 10.1016/j.nbd.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang L, et al. Tumor-derived vascular endothelial growth factor up-regulates angiopoietin-2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer. Cancer Res. 2003;63:3403–3412. [PubMed] [Google Scholar]
- 136.Nguyen LN, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 137.Ben-Zvi A, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alakbarzade V, et al. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet. 2015;47:814–817. doi: 10.1038/ng.3313. [DOI] [PubMed] [Google Scholar]
- 139.Guemez-Gamboa A, et al. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat Genet. 2015;47:809–813. doi: 10.1038/ng.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Augustin HG, Reiss Y. EphB receptors and ephrinB ligands: regulators of vascular assembly and homeostasis. Cell Tissue Res. 2003;314:25–31. doi: 10.1007/s00441-003-0770-9. [DOI] [PubMed] [Google Scholar]
- 141.Salvucci O, et al. EphrinB reverse signaling contributes to endothelial and mural cell assembly into vascular structures. Blood. 2009;114:1707–1716. doi: 10.1182/blood-2008-12-192294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Foo SS, et al. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 143.Gale NW, et al. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol. 2001;230:151–160. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- 144.Erber R, et al. EphB4 controls blood vascular morphogenesis during postnatal angiogenesis. EMBO J. 2006;25:628–641. doi: 10.1038/sj.emboj.7600949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Garmy-Susini B, et al. Integrin alpha4beta1-VCAM-1-mediated adhesion between endothelial and mural cells is required for blood vessel maturation. J Clin Invest. 2005;115:1542–1551. doi: 10.1172/JCI23445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gerhardt H, Wolburg H, Redies C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev Dyn Off Publ Am Assoc Anat. 2000;218:472–479. doi: 10.1002/1097-0177(200007)218:3<472::AID-DVDY1008>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 147.Tillet E, et al. N-cadherin deficiency impairs pericyte recruitment, and not endothelial differentiation or sprouting, in embryonic stem cell-derived angiogenesis. Exp Cell Res. 2005;310:392–400. doi: 10.1016/j.yexcr.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 148.Christian S, et al. Endosialin (Tem1) is a marker of tumor-associated myofibroblasts and tumor vessel-associated mural cells. Am J Pathol. 2008;172:486–494. doi: 10.2353/ajpath.2008.070623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Simonavicius N, et al. Endosialin (CD248) is a marker of tumor-associated pericytes in high-grade glioma. Mod Pathol Off J U S Can Acad Pathol Inc. 2008;21:308–315. doi: 10.1038/modpathol.3801006. [DOI] [PubMed] [Google Scholar]
- 150.MacFadyen J, Savage K, Wienke D, Isacke CM. Endosialin is expressed on stromal fibroblasts and CNS pericytes in mouse embryos and is downregulated during development. Gene Expr Patterns GEP. 2007;7:363–369. doi: 10.1016/j.modgep.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 151.Hultman K, Strickland S, Norris EH. The APOE ε4/ε4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer's disease patients. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2013;33:1251–1258. doi: 10.1038/jcbfm.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nishitsuji K, Hosono T, Nakamura T, Bu G, Michikawa M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J Biol Chem. 2011;286:17536–17542. doi: 10.1074/jbc.M111.225532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Alata W, Ye Y, St-Amour I, Vandal M, Calon F. Human apolipoprotein E ε4 expression impairs cerebral vascularization and blood-brain barrier function in mice. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2015;35:86–94. doi: 10.1038/jcbfm.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Takano T, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 155.Gebremedhin D, et al. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res. 2000;87:60–65. doi: 10.1161/01.res.87.1.60. [DOI] [PubMed] [Google Scholar]
- 156.Kamouchi M, et al. Calcium influx pathways in rat CNS pericytes. Brain Res Mol Brain Res. 2004;126:114–120. doi: 10.1016/j.molbrainres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 157.Sakagami K, Kawamura H, Wu DM, Puro DG. Nitric oxide/cGMP-induced inhibition of calcium and chloride currents in retinal pericytes. Microvasc Res. 2001;62:196–203. doi: 10.1006/mvre.2001.2343. [DOI] [PubMed] [Google Scholar]
- 158.Nakamura K, et al. Amiloride inhibits hydrogen peroxide-induced Ca2+ responses in human CNS pericytes. Microvasc Res. 2009;77:327–334. doi: 10.1016/j.mvr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 159.Mufti RE, et al. Intravascular pressure augments cerebral arterial constriction by inducing voltage-insensitive Ca2+ waves. J Physiol. 2010;588:3983–4005. doi: 10.1113/jphysiol.2010.193300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zeisel A, et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 161.Hamel E. Cholinergic modulation of the cortical microvascular bed. Prog Brain Res. 2004;145:171–178. doi: 10.1016/S0079-6123(03)45012-7. [DOI] [PubMed] [Google Scholar]
- 162.Blinder P, et al. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat Neurosci. 2013;16:889–897. doi: 10.1038/nn.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lovick TA, Brown LA, Key BJ. Neurovascular relationships in hippocampal slices: physiological and anatomical studies of mechanisms underlying flow-metabolism coupling in intraparenchymal microvessels. Neuroscience. 1999;92:47–60. doi: 10.1016/s0306-4522(98)00737-4. [DOI] [PubMed] [Google Scholar]
- 164.Shimizu F, et al. Pericyte-derived glial cell line-derived neurotrophic factor increase the expression of claudin-5 in the blood-brain barrier and the blood-nerve barrier. Neurochem Res. 2012;37:401–409. doi: 10.1007/s11064-011-0626-8. [DOI] [PubMed] [Google Scholar]
- 165.Takahashi H, et al. Brain pericyte-derived soluble factors enhance insulin sensitivity in GT1-7 hypothalamic neurons. Biochem Biophys Res Commun. 2015;457:532–537. doi: 10.1016/j.bbrc.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 166.Iliff JJ, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hawkes CA, et al. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol (Berl) 2011;121:431–443. doi: 10.1007/s00401-011-0801-7. [DOI] [PubMed] [Google Scholar]
- 168.Helbig H, et al. Membrane potentials in retinal capillary pericytes: excitability and effect of vasoactive substances. Invest Ophthalmol Vis Sci. 1992;33:2105–2112. [PubMed] [Google Scholar]
- 169.Li Q, Puro DG. Adenosine activates ATP-sensitive K(+) currents in pericytes of rat retinal microvessels: role of A1 and A2a receptors. Brain Res. 2001;907:93–99. doi: 10.1016/s0006-8993(01)02607-5. [DOI] [PubMed] [Google Scholar]
- 170.Wu DM, Kawamura H, Li Q, Puro DG. Dopamine activates ATP-sensitive K+ currents in rat retinal pericytes. Vis Neurosci. 2001;18:935–940. [PubMed] [Google Scholar]
- 171.Alcendor DJ, et al. Neurovascular unit on a chip: implications for translational applications. Stem Cell Res Ther. 2013;4(Suppl 1):S18. doi: 10.1186/scrt379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kleinstreuer N, et al. A computational model predicting disruption of blood vessel development. PLoS Comput Biol. 2013;9:e1002996. doi: 10.1371/journal.pcbi.1002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ruck T, Bittner S, Meuth SG. Blood-brain barrier modeling: challenges and perspectives. Neural Regen Res. 2015;10:889–891. doi: 10.4103/1673-5374.158342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ayyadurai VAS, Dewey CF. CytoSolve: A Scalable Computational Method for Dynamic Integration of Multiple Molecular Pathway Models. Cell Mol Bioeng. 2011;4:28–45. doi: 10.1007/s12195-010-0143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


