Abstract
Objective
Females have a higher rate of mortality following burn injury, largely due to differences in sepsis-related mortality. The present study seeks to understand the underpinnings of the estrogen’s immunomodulatory effects in a murine model of burn injury and infection.
Methods
Gonad-intact and ovariectomized female mice were subjected to a 15% total body surface area scald injury and then inoculated with 3,000 CFU of Pseudomonas aeruginosa by topical application to the wound. Animals were sacrificed at 1, 2, or 7 days after injury. Tissue and whole blood were collected. Cultures were performed of all tissues to assess for bacteria content. Lungs were examined for histologic appearance and homogenates were analyzed for chemokines and myeloperoxidase activity.
Results
Mortality reached 95% by 3 days after injury for gonad intact mice, whereas in ovariectomized mice it was 76% at 7 days. Blood and tissue samples from gonad intact mice had significantly higher levels of Pseudomonas aeruginosa compared to ovariectomized mice. Histologic assessment of lungs demonstrated a similar overall cellularity in ovariectomized mice relative to gonad intact mice 1 day after injury, but increased neutrophil count in gonad intact mice. This correlated with chemotactic signaling as lung homogenates had lower levels of KC in ovariectomized mice (128.0±19.8 vs 48.3±5.7 pg/mg protein). Also, myeloperoxidase was significantly lower in lung homogenates of ovariectomized mice (1.12±0.34 vs 0.56±0.08 units/mg protein).
Conclusions
Ovariectomy confers an early, but brief survival advantage in female mice after burn injury and wound infection. This appears to be secondary to enhanced bacterial clearance
Keywords: Burn, Infection, Ovariectomy, Estrogen, Pulmonary, Survival
Introduction
An estimated 500,000 people seek medical care for burn injuries every year in the United States.1 Although only 10% require admission to the hospital, those that are admitted face significant risks related to infectious complications and mortality. In particular, female are at higher risk for both infections and mortality.2,3 This sex dimorphism has been attributed to the differing effects of estrogen and testosterone on immune responses.4 Although several studies have questioned the validity of a sexual dimorphism for trauma patients in general,5,6 the findings within the burn population has remained consistent across time and between different populations.7-10
Animal models of burn injury further support the hypothesis that sex-based differences in outcomes following injury are mediated, in part, by the sex hormones. In female rodents estrogen often has an inhibitory role on the inflammatory response after burn injury.11-13 These effects are mediated through estrogen-induced changes in innate immune cell responses following injury.4 Moreover, the effects can be mitigated through ovariectomy or administration of estrogen receptor antagonists.11,12 Previous studies have largely focused on either characterization of the immune response in relation to the hormones or the mechanism by which the immune response is altered, but little is understood about how this correlates with the sex-difference in mortality. The present study seeks to bridge this gap between our understanding of the immune response to burn injury and the increased risk of mortality through the use of a mouse model of burn injury and infection.
Methods
Animal Model
Eight to ten week old gonad intact and ovariectomized (OVX) female C57BL/6 mice were obtained from Harlan Laboratories one week prior to experimentation to allow them to acclimatize to the new environment. The animals were housed with food and water available ad libitum at the Loyola University Medical Center Animal Facility in rooms that were temperature and humidity controlled on a 12 hour light-dark cycle. All animal studies described were performed in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals, National Institutes of Health. The studies were approved by and followed the regulations of the Loyola University Chicago Animal Care and Use Committee.
Mice were anesthetized with pentobarbitol (50 mg/kg i.p.), their dorsum was shaved, and the animals were placed in a plastic template designed to expose 15% of the total body surface area as previously described.14 Full thickness scald injury was achieved by immersing the opening of the template with the animal’s dorsum exposed in a 95 °C water bath for 8 seconds. Immediately following injury, the mice were inoculated with 3,000 CFU of Pseudomonas aeruginosa (ATCC # 19660) by topical application to the wound bed. Topical application of this strain of bacteria has been previously shown to result in systemic inflammatory changes that are independent of the burn injury itself.15 As a control, sham animals were anesthetized, shaved, and immersed in room temperature water. Both sham and burn-injured animals were resuscitated with 1 ml of intraperitoneal saline and allowed to recover under a heat lamp.
Animals were sacrificed at 1, 2, or 7 days after injury and infection. The cohort of animals sacrificed at 1 day after injury underwent explantation of the lungs with histology, chemokine, and myeloperoxidase analysis as described below. The second cohort of animals were sacrificed 2 days after injury and infection and underwent explantation of the lungs, liver, and spleen with testing for bacterial colonization performed as described below. Whole blood was also obtained via cardiac puncture for bacterial colonization studies. The third cohort of animals were followed for a week after injury and infection for derivation of a survival curve.
Bacterial Colonization
Lung, liver, and spleen were each homogenized in 1 mL of sterile PBS and 10-fold serial dilutions to 106 were plated on citrimide plates (BD Diagnostics, Sparks, MD). Heparinized whole blood (50 μL) was directly plated onto citrimide plates. Plates were incubated at 37 °C for 48 hours and colonies were counted to determine levels of bacterial colonization. Data are presented as colony forming units per 1 mg of tissue (lung, liver and spleen) or per 50 μL of whole blood.
Lung Histopathology
After sacrificing the animals, the upper left lobe was inflated with 10% formalin, fixed overnight and embedded in paraffin before sectioning (5 μm). Specimens were stained with hematoxylin and eosin as previously described.16 The sections were examined at X400 magnification in a blinded fashion. Overall cellularity (ie. % of the surface area covered by cells of any type) and neutrophil counts were determined by examining 10 high powered fields per animal.16 Cells with multilobular nuclei were counted as neutrophils. Neutrophil counts were reported as the mean number of neutrophils per one high powered (X400) field. Cellularity was determined by using the Java-based imaging program imageJ (National Institutes of Health, Bethesda, MD) as previously described.17 The histologic images were converted into a binary region of cellularity versus airspace and then analyzed for the percentage of the total area that was cellular. Cellularity is reported as the mean cellularity per animal.
Chemokine Levels
One lobe per mouse was homogenized in protease inhibitor cocktail (Roche Applied Sciences, Indianapolis, IN) as previously described.18 Samples were analyzed for macrophage inflammatory protein (MIP)-2 and KC content by ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Total protein content of the same aliquot of lung homogenate was determined by BioRad protein assay (BioRad Laboratories, Hercules, CA). Data are presented as pg of chemokine per mg of protein.
Myeloperoxidase Activity
Myeloperoxidase (MPO) activity was assessed as a surrogate for neutrophil activation. Lungs were homogenized in phosphate buffer containing 0.5% hexadecyl-trimethylammonium. The samples were sonicated and the supernatants cleared by centrifugation. They were incubated with o-dianisidine hydrochloride and hydrogen peroxide. Myeloperoxidase content was determined using optical density readings from the MPO standard (Sigma Aldrich, St. Louis, MO) which was run in parallel. Results were normalized to the total protein content of each sample. Data are listed as myeloperoxidase activity per mg of protein.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics, Version 22. Continuous variable are presented as mean ± standard error of the mean. Comparisons between gonad intact and OVX mice were made using a Student’s T-Test. Survival was assessed using a Cox proportional-hazards regression model. A p-value of <0.05 was considered statistically significant.
Results
Survival
Seven day survival was comparable between intact and OVX burn injured mice that were not also subjected to infection. For mice subjected to burn injury alone, the seven day survival was 93% for gonad intact mice and 94% for ovariectomized mice (Figure 1). In contrast, burn injury with concomitant Pseudomonas aeruginosa wound infection produces significant mortality compared to burn alone. Among the gonad intact mice, there were no surviving animals by 6 days after injury and infection. The seven day survival for ovariectomized mice subjected to the injury and infection was 24%. Additionally, there was a delay in mortality amongst the ovariectomized mice with greater survival in the first 48 hours. Of note, all animals survived the first six hours after injury.
Figure 1.
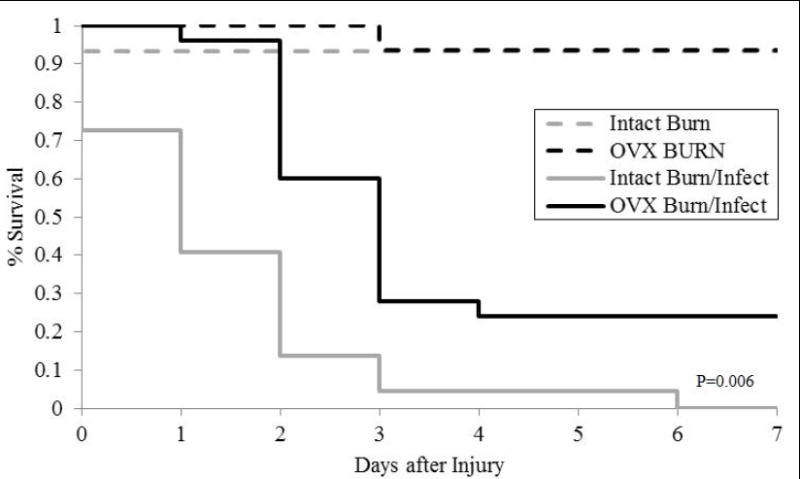
Survival following burn injury alone versus burn injury and wound infection among gonad intact and ovariecotmized (OVX) mice. N=15-25 per group. Comparison between groups were made using a Cox proportional-hazards regression model survival at seven days after injury. The P-value denotes significance for the gonad intact mice subjected to burn and infection relative to all other groups.
Bacterial Recovery
Given that infection of burn-injured skin is known to lead to systemic bacterial colonization and/or infection, bacterial recovery was performed as a surrogate for bacterial clearance. With the survival advantage between gonad intact and OVX mice being most pronounced at 48 hours after injury, this time point was utilized for bacterial recovery. Quantitative cultures for Pseudomonas aeruginosa demonstrated greater bacteria recovery from the liver, lung, spleen, and blood of gonad intact mice (Table 1). However, these differences could be attributed to the absence of any bacteria being recovered from the organs of some OVX animals. In particular, Pseudomonas aeruginosa was able to be recovered from the liver, lung, spleen, and blood of all gonad intact mice, but was only recovered from the liver in 25% of OVX mice, from the lung in 37% of OVX mice, from the spleen in 75% of OVX mice, and from the blood in 43% of OVX mice.
Table 1.
Bacterial content after burn and infection.
| Organ | Gonad Intact | OVX | P-value |
|---|---|---|---|
| Liver (CFU/1 mg tissue) | 4655 ± 1310 | 728 ± 245 | 0.014 |
| Lung (CFU/1 mg tissue | 786 ± 319 | 68 ± 23 | 0.013 |
| Spleen (CFU/1 mg tissue) | 3884 ± 1065 | 1036 ± 269 | 0.042 |
| Blood (CFU/50 μl whole blood) | 1577 ± 503 | 10 ± 3 | 0.003 |
Quantitative organ-specific bacteria recovery 48 hours after burn and infection for gonad intact and ovariectomized (OVX) mice. Data are presented as mean number of colony forming units of bacteria per 1 mg of tissue ± SEM. N=3-8 per group.Comparisons between groups were made using a Student’s T-Test.
Pulmonary Response
The lungs were used as a model distal organ for the immune response resulting in increased bacterial clearance for OVX mice. The combined insult of burn injury and infection resulted in increased cellularity and neutrophils to sham animals regardless of gonadal status (data not shown). When comparing gonad intact and OVX animals after burn and infection there was no significant difference in overall cellularity (30.7 ± 1.2% versus 32.0 ± 0.9%, P=0.210). However, there were more neutrophils in the gonad intact mice (1.01 ± 0.10 neutrophils per high powered field versus 0.45 ± 0.08 neutrophils per high powered field, P=0.002) (Figure 2).
Figure 2.
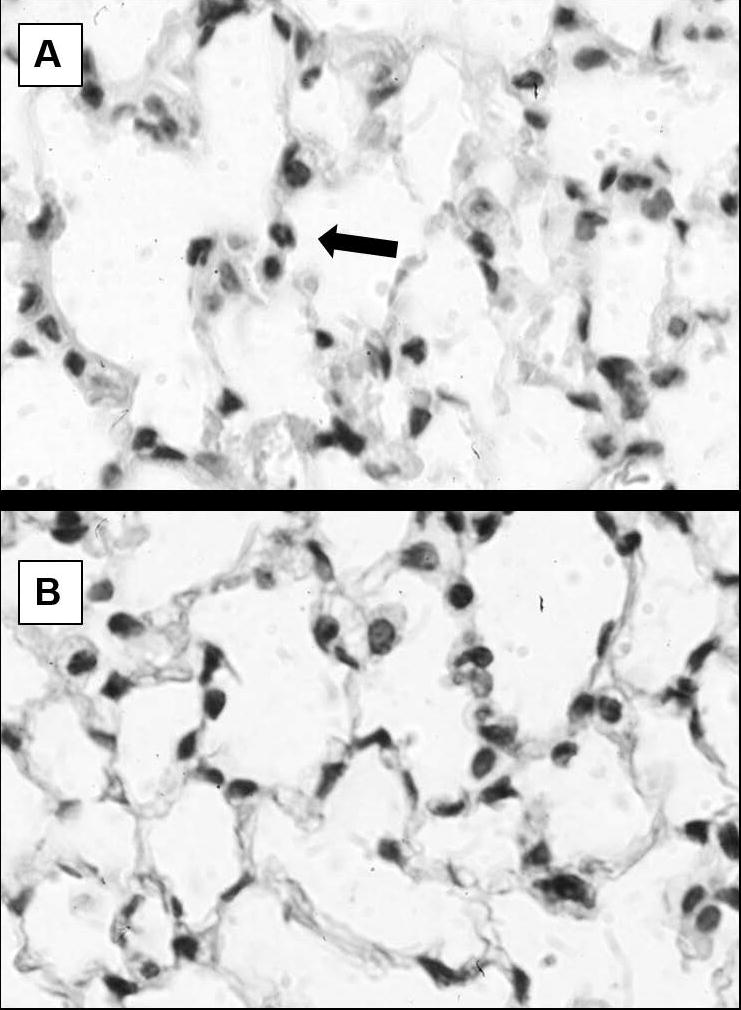
Histologic appearance of the lung 24 hours after burn and infection. Representative hematoxylin and eosin stained images are shown from gonad intact (A) and ovariectomized (B) mice. The images shown are at X1000 magnification with an arrow pointing to a neutrophil. There is no significant difference in the overall cellularity between gonad intact and OVX mice (30.7 ± 1.2% versus 32.0 ± 0.9%), but there was an increased number of neutrophils per high powered field in gonad intact mice compared to OVX mice (1.01 ± 0.10 neutrophils per high powered field versus 0.45 ± 0.08 neutrophils per high powered fields).
The chemokines KC and MIP-2 were assessed for neutrophil recruitment. Both KC and MIP-2 are potent attractants for neutrophils19 and levels of these peptides have been previously shown to correlate with the degree of pulmonary inflammation & neutrophil migration after burn injury.20 Pulmonary concentrations of KC were significantly higher in gonad intact mice (128.0 ± 19.8 pg/mg protein) compared to OVX mice (48.3 ± 5.7 pg/mg protein) after burn injury and infection (Table 2). No significant differences were noted in MIP-2 levels between gonad intact mice (42.7 ± 10.3 pg/mg protein) and OVX mice (33.5 ± 1.1 pg/mg protein) after burn injury and infection.
Table 2.
Pulmonary inflammatory after burn and infection.
| Assay | Gonad Intact | OVX | P-value |
|---|---|---|---|
| KC (pg/mg protein) | 128.0 ± 19.8 | 48.3 ± 5.7 | <0.001 |
| MIP-2 (pg/mg protein) | 42.7 ± 10.3 | 33.5 ± 1.1 | 0.181 |
| MPO (units/mg protein) | 1.12 ± 0.34 | 0.56 ± 0.08 | 0.034 |
Pulmonary chemokine and myeloperoxidase levels 24 hours after burn and infection for gonad intact and ovariectomized (OVX) mice. Data are presented as mean chemokine (pg/mg protein) or myeloperoxidase (units/mg protein) level ± SEM. N=7-19 per group. Comparisons between groups were made using a Student’s T-Test.
MPO levels were assessed to determine if neutrophil activity was different between the groups. Similar to KC, MPO was significantly higher in gonad intact mice (1.12 ± 0.34 units/pg protein) compared to OVX mice (0.56 ± 0.08 units/pg protein). The increased MPO in gonad-intact mice relative to OVX mice was consistent with the histologic findings as well.
Discussion
Infection and sepsis remain a critical source of mortality amongst burn patients.21-23 The vast majority of deaths within the first 72 hours after injury are ascribed to hemodynamic instability and shock from the burn injury. Among the minority of deaths during the first 72 hours that are not attributable to hemodynamic instability, sepsis and pulmonary complications represented the next most common causes of death.23 As such, the pulmonary response to burn injury is an important component of survival.
The present study demonstrates that antecedent ovariectomy was able to improve early survival and that this was temporally associated with decreased systemic bacterial infection and differences in the immune response. In a series of experiments by Deitch and colleagues, ovariectomy was shown to result in increased neutrophil activity in both the periphery and pulmonary system three hours after burn injury.11,13 More specifically, there were higher levels of MPO in lung tissue & higher levels of CD11b expression and respiratory burst from circulating neutrophils. Although the present study did not directly examine this early time point after injury, an enhanced neutrophil response early after bacterial invasion would be expected to increase bacterial clearance, thereby resulting in less systemic infection. Myeloperoxidase is an important contributor to the neutrophil phagosome’s anti-bacteria effects and this increase in the initial hours after injury may have provided the necessary Pseudomonas aeruginosa killing to result in decrease bacterial counts two days later.24 However, further studies are needed to prove this association as the present study only examines later time points. Additionally, other neutrophil or non-neutrophil based mechanisms may be responsible for bacteria killing.24-27
Twenty-four hours after burn and topical infection, neutrophils were noted to have migrated into the pulmonary parenchyma in both gonad intact and OVX mice, although less were present in OVX mice. Similarly, levels of KC and MPO were significantly lower in the OVX mice. The lower levels of the neutrophil chemoattractant can, at least partially, account for the lower neutrophil counts. This suggests two possible scenarios. Under the first scenario, the lack of estrogen is impeding the immune response in the lung (in particular, the neutrophil-based response). However, if this were the case one might have expected the bacterial infection to be even greater in the OVX mice by the following day (the opposite of what was seen). Under the second scenario, bacterial clearance is enhanced in the absence of estrogen and either less neutrophils needed to be recruited or resolution of the inflammatory process is already occurring by 24 hours after injury and infection. Circumstantial evidence in support of this second scenario comes from acute inflammation models which demonstrated ovariectomy is associated with an enhanced pulmonary neutrophil recruitment and activation by 6 hours after in vivo antigen stimulation.28 The earlier neutrophil recruitment into the lungs and alveoli was shown to be mediated by increased intracellular adhesion molecule-1 (ICAM-1) and not through chemokine levels. This effect could be abrogated by exogenous estrogen supplementation to ovariectomized mice. These prior studies also suggest that the effect plateaus at an earlier time point, thus our present study examining differences at 24 hours after injury may be well after the peak physiologic response for OVX mice. Further studies looking at these earlier time points are needed in order to differentiate between these two different scenarios.
The present results add to our growing understanding of the complex relationship between sex hormones and the immune response following burn injury. Although the results suggest an early benefit with regards to bacterial clearance and inflammation, an ovariectomy model is not practical for direct translation into a therapeutic strategy. Instead, these studies provide a foundational basis for ongoing research focusing on earlier time points for endocrine manipulation. Continual hormone blockade or elimination is likely too simplistic of a therapeutic strategy, as hinted at by the results of the later time points of the survival curve, rather the multitude of effects on different body systems need to be balanced.
In conclusion, ovariectomy improves bacterial clearance 48 hours following burn injury and topic infection and does so with less pulmonary neutrophil activation at 24 hours after injury. This is associated with an early survival benefit for the ovariectomized mice. These results are consistent with prior studies suggesting that estrogen may be inhibitory to neutrophil migration and activation during the initial hours after injury, however further studies at earlier time points are needed before a definitive conclusion is reached.
Acknowledgments
This work was supported by the National Institutes of Health AG018859 (EJK) and AA012034 (EJK), the Department of Defense W81XWH-07-1-0673 (EJK) and the Dr. Ralph and Marian C. Falk Medical Research Trust (EJK).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report
References
- 1.Healthcare Cost and Utilization Project. Introduction to the HCUP nationwide emergency department sample (NEDS), 2010. Agency for Healthcare Research and Quality; 2012. Retrieved from http://www.hcup-us.ahrq.gov/db/nation/neds/NEDS_Introduction_2010.jsp. [Google Scholar]
- 2.American Burn Association. National Burn Repository: 2014 Report [Google Scholar]
- 3.Summers JI, Ziembicki JA, Corcos AC, et al. Characterization of the sex dimorphism following severe thermal injury. J Burn Care Res. 2014;35:484–90. doi: 10.1097/BCR.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bird MD, Karavitis J, Kovacs EJ. Sex differences and estrogen modulation of the cellular immune response after injury. Cell Immunol. 2008;252:57–67. doi: 10.1016/j.cellimm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gannon CJ, Napolitano LM, Pasquale M, et al. A statewide population-based study of gender differences in trauma: validation of a prior single-institution study. J Am Coll Surg. 2002;195:11–8. doi: 10.1016/s1072-7515(02)01187-0. [DOI] [PubMed] [Google Scholar]
- 6.Magnotti LJ, Fischer PE, Zarzaur BL, et al. Impact of gender on outcomes after blunt trauma: a definitive analysis of more than 36,000 trauma patients. J Am Coll Surg. 2008;206:984–91. doi: 10.1016/j.jamcollsurg.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 7.Wasiak J, Lee SJ, Paul E, et al. Predictors of health status and health-related quality of life 12 months after severe burn. Burns. 2014;40:568–74. doi: 10.1016/j.burns.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Moore EC, Pilcher D, Bailey M, et al. Women are more than twice as likely to die from burns as men in Australia and New Zealand: an unexpected finding of the Burns Evaluation and Mortality (BEAM) Study. J Crit Care. 2014;29:594–8. doi: 10.1016/j.jcrc.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Fazeli S, Karami-Matin R, Kakaei N, et al. Predictive factors of mortality in burn patients. Trauma Mon. 2014;19:e14480. doi: 10.5812/traumamon.14480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGwin G, Jr, George RL, Cross JM, et al. Gender differences in mortality following burn injury. Shock. 2002;18:311–5. doi: 10.1097/00024382-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Deitch EA, Ananthakrishnan P, Cohen DB, et al. Neutrophil activation is modulated by sex hormones after trauma-hemorrhagic shock and burn injuries. Am J Physiol Heart Circ Physiol. 2006;291:H1456–65. doi: 10.1152/ajpheart.00694.2005. [DOI] [PubMed] [Google Scholar]
- 12.Gregory MS, Duffner LA, Faunce DE, et al. Estrogen mediates the sex difference in post-burn immunosuppression. J Endocrinol. 2000;164:129–38. doi: 10.1677/joe.0.1640129. [DOI] [PubMed] [Google Scholar]
- 13.Ananthakrishnan P, Cohen DB, Xu DZ, et al. Sex hormones modulate distant organ injury in both a trauma/hemorrhagic shock model and a burn model. Surgery. 2005;137:56–65. doi: 10.1016/j.surg.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 14.Faunce DE, Gregory MS, Kovacs EJ. Effects of acute ethanol exposure on cellular immune responses in a murine model of thermal injury. J Leukoc Biol. 1997;62:733–40. doi: 10.1002/jlb.62.6.733. [DOI] [PubMed] [Google Scholar]
- 15.He LK, Liu LH, Hahn E, et al. The expression of cyclooxygenase and the production of prostaglandin E2 in neutrophils after burn injury and infection. J Burn Care Rehabil. 2001;22:58–64. doi: 10.1097/00004630-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Patel PJ, Faunce DE, Gregory MS, et al. Elevation in pulmonary neutrophils and prolonged production of pulmonary macrophage inflammatory protein-2 after burn injury with prior alcohol exposure. Am J Respir Cell Mol Biol. 1999;20:1229–37. doi: 10.1165/ajrcmb.20.6.3491. [DOI] [PubMed] [Google Scholar]
- 17.Chen MM, Bird MD, Zahs A, et al. Pulmonary inflammation after ethanol exposure and burn injury is attenuated in the absence of IL-6. Alcohol. 2013;47:223–239. doi: 10.1016/j.alcohol.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez CR, Hirano S, Cutro B, et al. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit Care Med. 2007;35:246–51. doi: 10.1097/01.CCM.0000251639.05135.E0. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi Y. Neutrophil infiltration and chemokines. Crit Rev Immunol. 2006;26:307–316. doi: 10.1615/critrevimmunol.v26.i4.20. [DOI] [PubMed] [Google Scholar]
- 20.Nomellini V, Faunce DE, Gomez CR, et al. An age-associated increase in pulmonary inflammation after burn injury is abrogated by CXCR2 inhibition. J Leukoc Biol. 2008;83:1493–1501. doi: 10.1189/jlb.1007672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Avignon LC, Hogan BK, Murray CK, et al. Contribution of bacterial and viral infections to attributable mortality in patients with severe burns: an autopsy series. Burns. 2010;36:773–9. doi: 10.1016/j.burns.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Williams FN, Herndon DN, Hawkins HK, et al. The leading causes of death after burn injury in a single pediatric burn center. Crit Care. 2009;13:R183. doi: 10.1186/cc8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swanson JW, Otto AM, Gibran NS, et al. Trajectories to death in patients with burn injury. J Trauma Acute Care Surg. 2012;74:282–8. doi: 10.1097/TA.0b013e3182788a1c. [DOI] [PubMed] [Google Scholar]
- 24.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunologic Reviews. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 25.Hirche TO, Benabid R, Deslee G, et al. Neutrophile elastase mediates innate host protection against Pseudomonas aeruginosa. J Immunol. 2008;181:4945–54. doi: 10.4049/jimmunol.181.7.4945. [DOI] [PubMed] [Google Scholar]
- 26.Young RL, Malcolm KC, Kret JE, et al. Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PLoS One. 2011;6:e23637. doi: 10.1371/journal.pone.0023637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnston LK, Rims CR, Gill SE, et al. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am J Respir Cell Mol Biol. 2012;47:417–26. doi: 10.1165/rcmb.2012-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Speyer CL, Rancilio NJ, McClintock SD, et al. Regulatory effects of estrogen on acute lung inflammation in mice. Am J Physiol Cell Physiol. 2005;288:C881–90. doi: 10.1152/ajpcell.00467.2004. [DOI] [PubMed] [Google Scholar]


